Abstract
Purpose
Our study aimed to examine the relationship between intelligence and health-related quality of life (HRQOL) in children (6-13 years old) diagnosed as having a brain tumor.
Materials and Methods
We administered a Korean version of the Wechsler Intelligence Scale for Children-III, the Pediatric Quality of Life Inventory, version 4.0 (PedsQL), the Korean version of the Parenting Stress Index-Short Form, and the Korean Version of the Parenting Sense of Competence (K-PSOC) scale before or after initial radiotherapy (T1) and after treatment termination (T2). In total, 13 patients completed both the T1 and T2 interviews.
Results
Scores significantly declined between T1 and T2 on the full-scale intelligence quotients (FIQ), verbal intelligence quotients (VIQ), performance intelligence quotients (PIQ), similarity and coding tests, as well as the K-PSOC, which measures parental anxiety. FIQ scores at T1 were correlated with the self-reported PedsQL total scores (r=0.739) and the parent proxy-report PedsQL scores for school functioning (r=0.706) at T2. Also, the FIQ scores at T2 were correlated with the self-reported PedsQL total scores (r=0.748) and scores for physical health (r=0.728) at T2.
Conclusion
The cognitive ability and intelligence level of the patients significantly declined between on and off treatment periods, and higher intelligence functioning at both on and off treatment was correlated with long-term higher HRQOL. Further investigations that monitor intelligence, HRQOL and parenting stress over a longer period, using a greater number of participants, are needed.
Keywords: Childhood brain tumors, intelligence, heath-related quality of life, parenting stress, prospective longitudinal study
INTRODUCTION
Brain tumors are the second most common form of childhood cancer, and account for approximately 20% of childhood cancer diagnoses.1 Due to the availability of aggressive combination therapies, including surgical resection, chemotherapy (CT), radiotherapy (RT), and peripheral blood stem cell transplantation, the prognosis for childhood brain tumors has improved considerably over the last several decades.2 Approximately 65% of all children treated for brain tumors now achieve long-term survival.3
However, due to the location of the tumors, the aggressive nature of the combined therapies, as well as the extended duration of treatment, recovery and rehabilitation periods, children treated for brain tumors are liable to experience long-term difficulties in many areas of their lives, both physically and mentally. Previous research suggests that children successfully treated for brain tumors may have difficulties reintegrating into normal life, maintaining peer relationships, and attaining normal academic milestones,4-6 and they are at greater risk of cognitive and mental difficulties compared to children who have been treated for other cancer variants.7,8 Parents of children with brain tumors likewise reported high levels of distress, post-traumatic stress, and a lower quality of life.9,10 They further reported that their elevated stress level continued for 5 years or more post-diagnosis.11
Pediatric health-related quality of life (HRQOL) is multidimensional, consisting at the minimum of physical, psychological (including emotional and cognitive), and social health dimensions, as delineated by the World Health Organization guidelines.12 HRQOL has emerged as an important health outcome in pediatric cancer clinical trials.13,14 Studies addressing the long-term survival of childhood brain tumor patients have reported that such individuals had a lower HRQOL than both their non-diagnosed peers and other childhood cancer survivors.15-17
Intellectual impairment is associated with a younger age at diagnosis, the site and extent of the tumor, the type of surgery, development of infection, increases in intracranial pressure and most significantly the use of RT.2,18 Impairment has been shown to significantly impact HRQOL by interfering with academic, vocational, and psycho-social functioning, and is related with the development of significant psychological and behavioral problems.19,20 Furthermore, lower intelligence quotients (IQ) seems to be a strong risk factor in poor HRQOL.18
Previous cross-sectional studies found that children with brain tumors had a lower HRQOL, and that the parents of children with brain tumors tended to experience stress related to management behavior and temperament of the patient, as well as their parent-child interactions compared to normal controls.17 However, only a few longitudinal studies examining the links between intelligence, HRQOL, and parenting stress, satisfaction and efficacy have been conducted in Korea.
We, therefore, aimed to compare intelligence, HRQOL, and parenting stress, satisfaction and efficacy in children with brain tumors between on and off treatment periods. We also aimed to examine the relationship between intelligence levels at on and off treatment and HRQOL at off-treatment periods in children with brain tumors. We hypothesized that intelligence would decline from on treatment to off treatment periods, and higher intelligence would be associated with a higher HRQOL. We also anticipated that HRQOL and parenting satisfaction and efficacy would increase, and parenting stress would decrease from on treatment to off treatment.
MATERIALS AND METHODS
Subjects
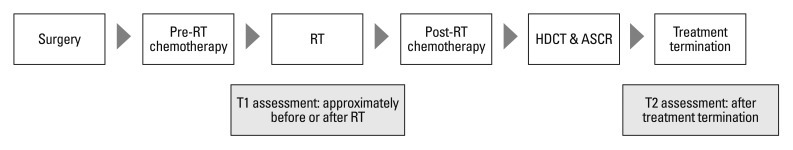
Between August 2007 and August 2010, 26 newly diagnosed brain tumor patients were enrolled at the Children's Cancer Unit at the Samsung Medical Center in Seoul, Republic of Korea. After undergoing surgical resection, the patients received two cycles of pre-RT chemotherapy, which consisted of cisplatin, etoposide, vincristine, and cyclophosphamide (cycle A), and carboplatin, etoposide, vincristine, and ifosphamide (cycle B), followed by cranio-spinal radiotherapy (CSRT) with 23.4 Gy and local RT with 30.6 Gy in cases of metastasis (M) of stage 0 or 1 or CSRT 30.6 Gy and local RT with 23.4 Gy in cases of metastasis of stage 2 or 3. After four cycles of post-RT chemotherapy (cycles A, B, A, and B), tandem double high dose chemotherapy with autologous stem cell rescue was performed. Intelligence, HRQOL, and parenting stress, satisfaction and efficacy scores were collected at interviews conducted approximately before or after initial radiotherapy (T1) and again after treatment termination (T2). Fig. 1 is a schematic diagram of the protocol.
Fig. 1.
Schema of the protocol. RT, radiotherapy; HDCT, high dose chemotherapy; ASCR, autologous stem cell rescue.
In total, 13 patients who completed both T1 and T2 interviews were included in this analysis.
Procedure
We gathered information concerning the patients' diagnoses, M stage, size of their residual tumor, and whether or not leptomeningeal seeding was present from their medical records.
We administered the Korean version of the Wechsler Intelligence Scale for Children-III (K-WISC-III)21 in order to assess intelligence; the Pediatric Quality of Life Inventory, version 4.0 (PedsQL)22,23 to assess HRQOL; the Korean version of the Parenting Stress Index-Short Form (K-PSI-SF)24 to assess parenting stress, and the Korean Version of the Parenting Sense of Competence scale (K-PSOC),24 which measures parenting self-esteem and two aspects of parents' self-reported competence, namely their feelings of satisfaction and efficacy in the parenting role.
The K-WISC-III (applicable for ages 6-16) consists of six verbal subtests: information, similarities, arithmetic, vocabulary, comprehension and digit span, and four performance subtests, namely picture completion, coding, picture arrangement, and block design. The full-scale intelligence quotients (FIQ) test is a measure of general cognitive ability; the verbal intelligence quotients (VIQ) test, is a measure of abstract verbal reasoning ability; and the performance intelligence quotients (PIQ) test is a measure of perceptual reasoning skills. For these, 10 scaled scores of subtests were calculated based on Korean age norms. Each test has an overall mean of 100 (50th percentile) and a standard deviation of 15. Scores of 90-109 are considered average, 80-89 are considered lower average, and 70-79 are considered as showing a borderline intellectual level. The subtests have a mean of 10 and a standard deviation of 3, with scores of 9-11 considered as average, 7-8 lower than average, and 5-6 borderline.
The PedsQL measures HRQOL over the month prior to the testing interview, and has four domains and 23 items: physical functioning (8 items), emotional functioning (5 items), social functioning (5 items), and school functioning (5 items). It generates a total score as a summary of all four domains. A 5-point Likert-type scale is utilized (0=never a problem; 1=almost never a problem; 2=sometimes a problem; 3=often a problem; 4=almost always a problem). Items are reverse-scored and linearly transformed to a 0 to 100 scale (0=100, 1=75, 2=50, 3=25, 4=0), so that higher scores indicate a higher HRQOL. Scale scores are computed as the sum of the items divided by the number of items answered. Age-specific versions of both the parent-reported format (applicable for ages 2-18) and the self-reported format (applicable for ages 5-18 years) are available.
Our study protocol was approved by our institutional review board, and written informed consent was obtained from each enrolled participant or, for minors, the participant's parents.
Statistical analysis
We analyzed the data using SPSS 17.0 software (version 17.0; SPSS, Chicago, IL, USA) for Windows.
We used the Mann-Whitney test and the Fisher's exact test to evaluate differences in clinical variables between patients included in the final analysis and patients excluded at T1.
We used the Wilcoxon signed-rank test for comparisons of differences in clinical variables between T1 and T2. Spearman's rank correlation was used to assess relationships between intelligence at T1 and T2 and HRQOL at T2.
The statistical significance level was set at p<0.05.
RESULTS
Of the 26 patients who were eligible for study, 5 patients died before T2 assessment, 1 patient withdrew following T1 assessment and 2 patients declined to participate in T2 assessment. Further to this, 5 patients who were under 6 years of age at T1 assessment had to have their intelligence function assessed not by the K-WISC-III but with the Korean version of the Wechsler Preschool and Primary Scales of Intelligence.25 They were therefore excluded because of the methodological need for testing to be the same for all participants.
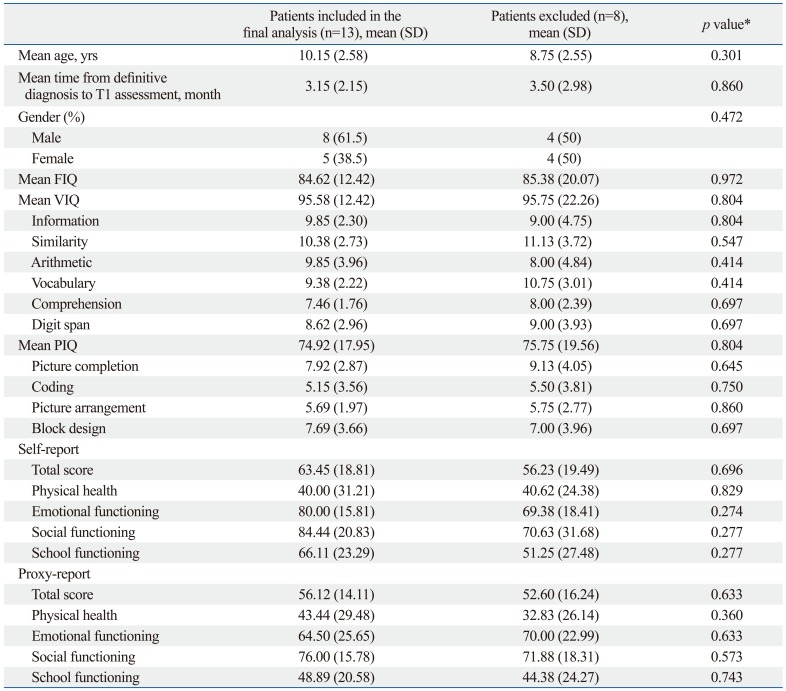
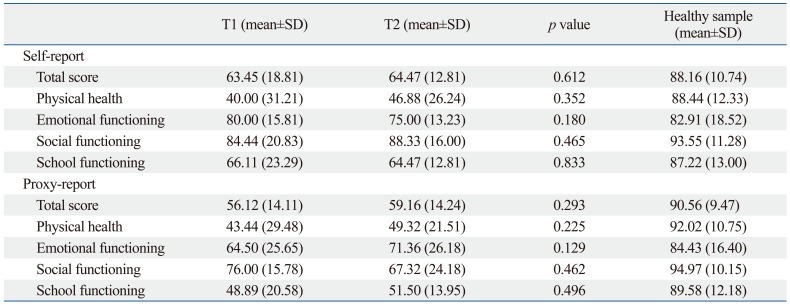
Comparing patients included in the final analysis with those who were not include, except for the 5 patients less than 6 years of age at T1 assessment, there were no significant differences in the mean age at diagnosis of brain tumors, the mean time from definitive diagnosis to T1 assessment, gender ratio, the mean intelligence scores, and mean PedsQL scores (Table 1).
Table 1.
Demographic Characteristics, Intelligence Test Scores and PedsQL Scores between Patients Included in the Final Analysis and Patients Excluded during On-Treatment
PedsQL, Pediatric Quality of Life Inventory, version 4.0; FIQ, full-scale intelligence quotients; VIQ, verbal intelligence quotients; PIQ, performance intelligence quotients.
*Mann-Whitney test or Fisher's exact test.
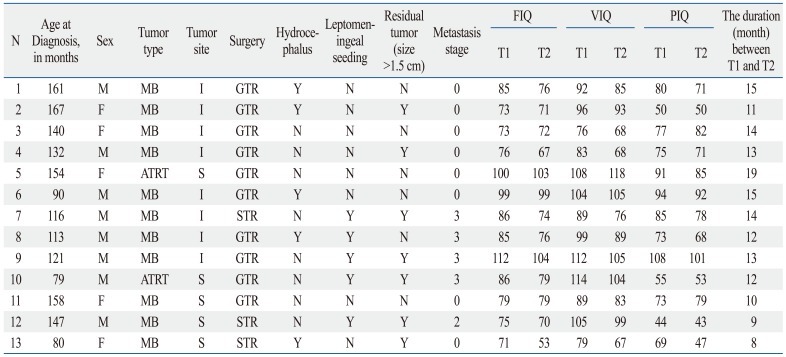
The mean age at diagnosis of brain tumors was 10.15±2.58 years. The mean time from definitive diagnosis to T1 assessment was 3.15±2.15 months, and the mean time from T1 assessment to T2 assessment was 12.69±2.90 months. At T1 assessment, 9 patients were pre-RT and 4 patients were post-RT. Table 2 lists the demographic information and the disease and treatment characteristics of the children involved.
Table 2.
Demographic Information and the Disease and Treatment Characteristics of Children with Brain Tumors
N, number; M, male; F, female; MB, medulloblastoma; ATRT, atypical teratoid rhabdoid tumor; I, infratentorial; S, supratentorial; GTR, gross total resection; STR, subtotal resection; Y, yes; N, no; FIQ, full-scale intelligence quotients; VIQ, verbal intelligence quotients; PIQ, performance intelligence quotients.
0: no evidence of gross subarachnoid hematogenous metastasis, 3: gross nodule seeding in the spinal subarachnoid space, T1: assessment during treatment, T2: assessment after treatment termination.
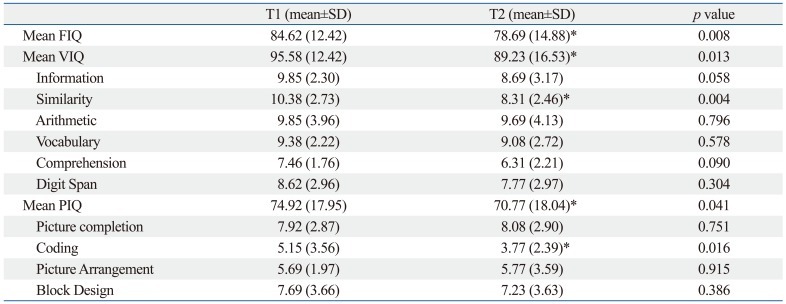
The mean FIQ, VIQ, PIQ, similarity, and coding scores significantly declined from T1 to T2 (Table 3). The mean PedsQL scores at T1 were not significantly different from those obtained at T2 (Table 4).
Table 3.
Comparisons between Intelligence Test Scores during On-Treatment and Off-Treatment Periods in Children with Brain Tumors
FIQ, full-scale intelligence quotients; VIQ, verbal intelligence quotients; PIQ, performance intelligence quotients; SD, standard deviation.
T1: assessment during treatment, T2: assessment after treatment termination.
*p<0.05, Wilcoxon signed rank test.
Table 4.
Comparison of the PedsQL Scores of Child Self-Assessment and Parental Proxy-Reporting at On-Treatment and Off-Treatment in Children with Brain Tumors
PedQL, Pediatric Quality of Life Inventory, version 4.0; SD, standard deviation.
T1: assessment during treatment, T2: assessment after treatment termination.
Healthy sample was derived from Kook and Varni.23
*p<0.05, Wilcoxon signed rank test.
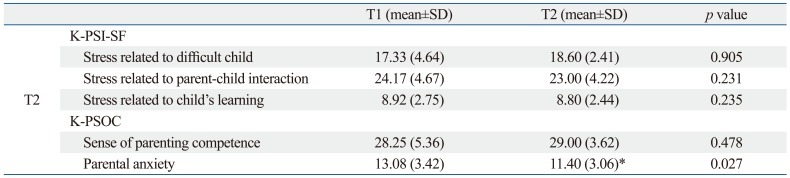
The mean K-PSI-SF and the K-PSOC scores for sense of parenting competence at T1 were not significantly different from those obtained at T2, but the mean K-PSOC score for parental anxiety significantly declined from T1 to T2 (Table 5).
Table 5.
Comparisons between the K-PSI-SF and K-PSOC Scores On and Off Treatment in Children with Brain Tumors
K-PSI-SF, Korean version of the Parenting Stress Index-Short Form; K-PSOC, Korean version of the Parenting Sense of Competence.
T1: assessment during treatment, T2: assessment after treatment termination.
*p<0.05, Wilcoxon signed rank test.
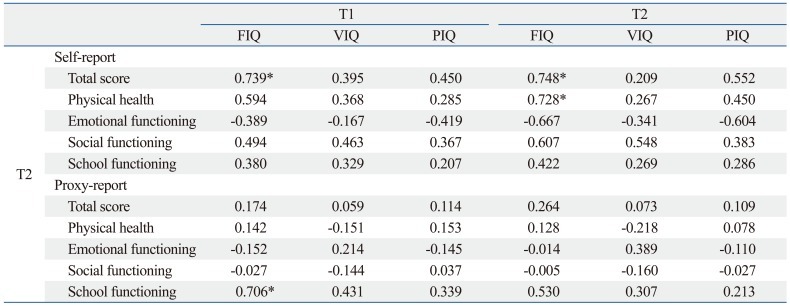
The mean FIQ scores at T1 were correlated with mean self-reported PedsQL total scores (r=0.739) and the mean parent proxy-report PedsQL scores for school functioning (r=0.706) at T2. Also, the mean FIQ scores at T2 were correlated with the mean self-reported PedsQL total scores (r=0.748) and scores for physical health (r=0.728) at T2 (Table 6).
Table 6.
Correlation of FIQ, VIQ and PIQ Scores at On- and Off-Treatment with the PedsQL Scores of Child Self-Assessment and Parental Proxy-Reports Off Treatment
FIQ, Full-Scale intelligence quotients; VIQ, verbal intelligence quotients; PIQ, performance intelligence quotients; PedQL, Pediatric Quality of Life Inventory, version 4.0.
T1: assessment during treatment, T2: assessment after treatment termination.
*p<0.05, Spearman correlation analysis.
DISCUSSION
The present study comprised an early stage investigation of the links between intelligence, HRQOL and parenting stress, satisfaction and efficacy in children diagnosed with brain tumors and their families. We compared intelligence, HRQOL and parenting stress, satisfaction and efficacy between on treatment and off treatment periods, and examined the relationship of intelligence at on and off treatment by assessing HRQOL in the off treatment period.
During on treatment, there were no significant differences in intelligence and HRQOL between patients included and patients excluded.
We found that the mean FIQ, VIQ, PIQ, similarity, and coding scores significantly declined from on treatment to off treatment. Due to risk factors such as age at diagnosis, the site and extent of the tumor, surgery type, infection, and increase in intracranial pressure and the use of radiotherapy, children with brain tumors are at greater risk of declining intelligence function, especially as demonstrated through performance tasks.20,26 These problems have been reported to extend into adulthood in long-term survivors of childhood cancer and can result in poor academic and vocational success, low self-esteem, and behavioral or emotional problems.8 Many studies on intelligence functioning in children with brain tumors have utilized various Wechsler scales; for subjects younger than 6 years, the primary scale should be used, whereas the children's scale should be used in children under the age of 16 years, after which the adult scale should be used.21,25 Due to scale differences, the comparison of results when using different scales becomes problematic.26 Our findings showed intelligence declines from on-treatment to off-treatment in brain tumors patients aged 6-16 years, assessed only by K-WISC-III. Reportedly, intellectual functioning declines quickly in the first few years after treatment termination, and then more gradually.27 Accordingly, longer-term monitoring of intellectual function in children with brain tumors is warranted.
In this study, there were no significant differences found between on-treatment HRQOLs and the off-treatment HRQOLs. However, both the on-treatment HRQOL and the off-treatment HRQOL scores were lower than those observed in a study of Korean healthy children (Table 4),23 and recent cross-sectional research by our group showed that children with brain tumors had lower HRQOL than a control group.17 Therefore, we and many other researchers have been interested in the progression of HRQOL levels after brain tumor diagnosis and treatment termination. Previous study results have differed from our findings: one longitudinal study reported significant improvement in HRQOL at 1, 6 and 12 months after diagnosis, but the authors of the aforementioned study did not specify whether the 12 months assessment was performed after termination of the treatment and whether all the individual participants received the same modality of treatment.16 Moreover, differences in the duration, amount and frequency of RT and CT, subjective responses to the impact of acute side effects, such as nausea, hair loss, and additional hospitalization, levels of individual concern about the long-term consequences of treatment, and trans-cultural and ethnic differences may result in different results in terms of how patients consider their quality of life.28,29 For the most successful long-term monitoring of HRQOL after the termination of brain tumor treatment, it is necessary that the studied patients receive the same therapeutic modality.
In this study, there were no significant differences between the level of parental stress at on and off treatment periods, but parental anxiety significantly declined from on treatment to off treatment. Recent cross-sectional research by our group showed that the parents of children with brain tumors tended to experience more parenting stress than the normal control group.17 Since much of the parental emotional and physical burden (e.g. care-giving, fear of child death, increased sense of helplessness and depression) created by child illness is relieved once the treatment process has completed, it is reasonable to consider that parental anxiety level is also likely to decrease after treatment has concluded.30-32 Despite this, even after treatment termination, parents of children with tumors often encounter long-term psychiatric sequelae, neuropsychological dysfunction, and other residual effects of the disease and its treatment, in addition to having an elevated risk of newly rising late effects or further distress created by disease recurrence that requires significant long-term follow-up and care.30,32 A significant number of parents still suffer from clinical parenting distress after five years,11 and as many as 5% to 10% of parents go on to develop post-traumatic stress disorder after their child's treatment has finished.32 Thus, careful monitoring of parental stress during off-treatment as well as on-treatment periods in cases of childhood brain tumor is needed.
In this study, decreased general cognitive ability in the on-treatment period was related to lower self-reported HRQOL and parent-rated HRQOL for school functioning in the off-treatment period, and a decreased general cognitive ability in off-treatment periods was related to lower self-reported HRQOL, specifically physical health off-treatment. In previous studies, decreased cognitive ability was associated with low school achievement, low mid-life income33 and poor physical complaints.34 Since the prognosis of childhood brain tumors has improved considerably in recent decades, HRQOL has received increasing attention in pediatric oncology. Accordingly, general studies have been conducted relating factors of HRQOL to IQ and have found a significant relationship;18 however, few studies have investigated the relationship of specific intelligence functions with specific domains of HRQOL.
The present study has several limitations. Firstly, the participant number was small due to the low prevalence of childhood brain tumors. It will be necessary to confirm these results in an expanded patient group in a future study. Secondly, even though a majority of the patients completed their T1 assessment before radiotherapy, there was still conflict in the ordering of T1 assessment that may have influenced study findings. Thirdly, we did not conduct a long-term follow-up after treatment termination, and thus, could not evaluate later effects.
Despite these limitations, our results nevertheless show that intelligence significantly declined between on-treatment and off-treatment and that higher intelligence functioning in both periods was positively correlated with a higher HRQOL off treatment. In other words, higher intelligence was associated with a better quality of life in the long term. Further investigations that monitor intelligence functioning, HRQOL and parenting stress over a more sustained period are needed.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Steliarova-Foucher E, Stiller C, Kaatsch P, Berrino F, Coebergh JW, Lacour B, et al. Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCISproject): an epidemiological study. Lancet. 2004;364:2097–2105. doi: 10.1016/S0140-6736(04)17550-8. [DOI] [PubMed] [Google Scholar]
- 2.Duffner PK. Risk factors for cognitive decline in children treated for brain tumors. Eur J Paediatr Neurol. 2010;14:106–115. doi: 10.1016/j.ejpn.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Peris-Bonet R, Martínez-García C, Lacour B, Petrovich S, Giner-Ripoll B, Navajas A, et al. Childhood central nervous system tumours--incidence and survival in Europe (1978-1997): report from Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2064–2080. doi: 10.1016/j.ejca.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Lannering B, Marky I, Lundberg A, Olsson E. Long-term sequelae after pediatric brain tumors: their effect on disability and quality of life. Med Pediatr Oncol. 1990;18:304–310. doi: 10.1002/mpo.2950180410. [DOI] [PubMed] [Google Scholar]
- 5.Mostow EN, Byrne J, Connelly RR, Mulvihill JJ. Quality of life in long-term survivors of CNS tumors of childhood and adolescence. J Clin Oncol. 1991;9:592–599. doi: 10.1200/JCO.1991.9.4.592. [DOI] [PubMed] [Google Scholar]
- 6.Zebrack BJ, Gurney JG, Oeffinger K, Whitton J, Packer RJ, Mertens A, et al. Psychological outcomes in long-term survivors of childhood brain cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2004;22:999–1006. doi: 10.1200/JCO.2004.06.148. [DOI] [PubMed] [Google Scholar]
- 7.Mulhern RK, Butler RW. Neurocognitive sequelae of childhood cancers and their treatment. Pediatr Rehabil. 2004;7:1–14. doi: 10.1080/13638490310001655528. [DOI] [PubMed] [Google Scholar]
- 8.Zeltzer LK, Recklitis C, Buchbinder D, Zebrack B, Casillas J, Tsao JC, et al. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2396–2404. doi: 10.1200/JCO.2008.21.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuemmeler BF, Mullins LL, Marx BP. Posttraumatic stress and general distress among parents of children surviving a brain tumor. Child Health Care. 2001;30:169–182. [Google Scholar]
- 10.Chien LY, Lo LH, Chen CJ, Chen YC, Chiang CC, Yu Chao YM. Quality of life among primary caregivers of Taiwanese children with brain tumor. Cancer Nurs. 2003;26:305–311. doi: 10.1097/00002820-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Wijnberg-Williams BJ, Kamps WA, Klip EC, Hoekstra-Weebers JE. Psychological adjustment of parents of pediatric cancer patients revisited: five years later. Psychooncology. 2006;15:1–8. doi: 10.1002/pon.927. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Constitution of the World Health Organization: Basic Document. Geneva, Switzerland: World Health Organization; 1948. [Google Scholar]
- 13.Varni JW, Burwinkle TM, Lane MM. Health-related quality of life measurement in pediatric clinical practice: an appraisal and precept for future research and application. Health Qual Life Outcomes. 2005;3:34. doi: 10.1186/1477-7525-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matza LS, Swensen AR, Flood EM, Secnik K, Leidy NK. Assessment of health-related quality of life in children: a review of conceptual, methodological, and regulatory issues. Value Health. 2004;7:79–92. doi: 10.1111/j.1524-4733.2004.71273.x. [DOI] [PubMed] [Google Scholar]
- 15.Speechley KN, Barrera M, Shaw AK, Morrison HI, Maunsell E. Health-related quality of life among child and adolescent survivors of childhood cancer. J Clin Oncol. 2006;24:2536–2543. doi: 10.1200/JCO.2005.03.9628. [DOI] [PubMed] [Google Scholar]
- 16.Penn A, Lowis SP, Hunt LP, Shortman RI, Stevens MC, McCarter RL, et al. Health related quality of life in the first year after diagnosis in children with brain tumours compared with matched healthy controls; a prospective longitudinal study. Eur J Cancer. 2008;44:1243–1252. doi: 10.1016/j.ejca.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 17.An KJ, Song MS, Sung KW, Joung YS. Health-related quality of life, activities of daily living and parenting stress in children with brain tumors. Psychiatry Investig. 2011;8:250–255. doi: 10.4306/pi.2011.8.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reimers TS, Mortensen EL, Nysom K, Schmiegelow K. Health-related quality of life in long-term survivors of childhood brain tumors. Pediatr Blood Cancer. 2009;53:1086–1091. doi: 10.1002/pbc.22122. [DOI] [PubMed] [Google Scholar]
- 19.Hocking MC, Hobbie WL, Deatrick JA, Lucas MS, Szabo MM, Volpe EM, et al. Neurocognitive and family functioning and quality of life among young adult survivors of childhood brain tumors. Clin Neuropsychol. 2011;25:942–962. doi: 10.1080/13854046.2011.580284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poggi G, Liscio M, Galbiati S, Adduci A, Massimino M, Gandola L, et al. Brain tumors in children and adolescents: cognitive and psychological disorders at different ages. Psychooncology. 2005;14:386–395. doi: 10.1002/pon.855. [DOI] [PubMed] [Google Scholar]
- 21.Kwak KJ, Park HW, Kim CT. A Study for the Standardization of Korean WISC-3 (1) Korean J Dev Psychol. 2002;15:19–33. [Google Scholar]
- 22.Varni JW, Limbers CA, Burwinkle TM. Parent proxy-report of their children's health-related quality of life: an analysis of 13,878 parents' reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:2. doi: 10.1186/1477-7525-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kook SH, Varni JW. Validation of the Korean version of the pediatric quality of life inventory 4.0 (PedsQL) generic core scales in school children and adolescents using the Rasch model. Health Qual Life Outcomes. 2008;6:41. doi: 10.1186/1477-7525-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin SJ, Chung MJ. Effects of stress, social support and efficacy on mothers' parenting behaviors. Korean J Child Stud. 1998;19:27–42. [Google Scholar]
- 25.Park HW, Kwak KJ, Park KB. The development of Korean version of WPPSI: the standardization study (1) Korean J Dev Psychol. 1996;9:60–70. [Google Scholar]
- 26.Saury JM, Emanuelson I. Cognitive consequences of the treatment of medulloblastoma among children. Pediatr Neurol. 2011;44:21–30. doi: 10.1016/j.pediatrneurol.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22:706–713. doi: 10.1200/JCO.2004.05.186. [DOI] [PubMed] [Google Scholar]
- 28.Schell LM. Culture as a stressor: a revised model of biocultural interaction. Am J Phys Anthropol. 1997;102:67–77. doi: 10.1002/(SICI)1096-8644(199701)102:1<67::AID-AJPA6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 29.Penn A, Lowis SP, Stevens MC, Hunt LP, Shortman RI, McCarter RJ, et al. Family, demographic and illness-related determinants of HRQL in children with brain tumours in the first year after diagnosis. Pediatr Blood Cancer. 2009;53:1092–1099. doi: 10.1002/pbc.22157. [DOI] [PubMed] [Google Scholar]
- 30.Hutchinson KC, Willard VW, Hardy KK, Bonner MJ. Adjustment of caregivers of pediatric patients with brain tumors: a cross-sectional analysis. Psychooncology. 2009;18:515–523. doi: 10.1002/pon.1421. [DOI] [PubMed] [Google Scholar]
- 31.Freeman K, O'Dell C, Meola C. Childhood brain tumors: parental concerns and stressors by phase of illness. J Pediatr Oncol Nurs. 2004;21:87–97. doi: 10.1177/1043454203262691. [DOI] [PubMed] [Google Scholar]
- 32.Rabineau KM, Mabe PA, Vega RA. Parenting stress in pediatric oncology populations. J Pediatr Hematol Oncol. 2008;30:358–365. doi: 10.1097/MPH.0b013e318168e76b. [DOI] [PubMed] [Google Scholar]
- 33.Spinks R, Arndt S, Caspers K, Yucuis R, McKirgan LW, Pfalzgraf C, et al. School achievement strongly predicts midlife IQ. Intelligence. 2007;35:563–567. [Google Scholar]
- 34.Hall NM, Kuzminskyte R, Pedersen AD, Ørnbøl E, Fink P. The relationship between cognitive functions, somatization and behavioural coping in patients with multiple functional somatic symptoms. Nord J Psychiatry. 2011;65:216–224. doi: 10.3109/08039488.2010.528024. [DOI] [PubMed] [Google Scholar]