Abstract
Purpose
Other than a single case report, no diffusion tensor tractography (DTT) studies of the precommissural fornix in the human brain have been conducted. In the current study, we attempted to visualize the precommissural fornix in the human brain using DTT.
Materials and Methods
We recruited 36 healthy volunteers for this study. Diffusion tensor images were scanned using a 1.5-T scanner, and the precommissural fornix was analyzed using Functional Magnetic Resonance Imaging of the Brain (FMRIB) software. Values of fractional anisotropy (FA), mean diffusivity (MD), and tract volume of the precommissural fornix were measured.
Results
The precommissural fornix originated from the hippocampal formation on each hemisphere as a crus; both crura were then joined to the body of the fornix. The body of the fornix continued anteriorly to the level just superior to the anterior commissure, where it divided into each column of the precommissural fornix. Each column descended anteriorly to the anterior commissure and terminated in the septal nuclei. Values of FA, MD, and tract volumes of the precommissural fornix did not differ between the right and left hemispheres (p>0.05).
Conclusion
We believe that the methodology and results of this study would be helpful to future research on the precommissural fornix and in the elucidation of the pathology of diseases involving the precommissural fornix.
Keywords: Diffusion tensor imaging, precommissural fornix, septal nucleus, hippocampus
INTRODUCTION
The fornix divides into the precommissural and postcommissural fornix around the anterior commissure. The precommissural fornix is the main neural pathway between hippocampal formation and septal nuclei, and contributes to central regulation of emotional behavior, motivation processes, and memory function.1-5 Many studies have reported on postcommissural fornix function and injury in the human brain;6-16 in contrast, little is known about the precommissural fornix, even though it has been explained in many "neuroanatomy" textbooks and atlases.17-19 This seems to be mainly ascribed to the fact that the precommissural fornix is not easily discriminated with adjacent structures on conventional brain MRI.
The recent development of diffusion tensor tractography (DTT), which is derived from diffusion tensor imaging (DTI), allows for visualization and localization of neural tracts at the subcortical level in three dimensions.20 Accordingly, DTT has been used for three-dimensional visualization of the architecture and assessment of the state of the postcommissural fornix, which is associated with cognitive function.12-15,21,22 However, except for one case report, no DTT study on the precommissural fornix in the human brain has been performed.23
In the current study, using DTT, we attempted to visualize the precommissural fornix in the human brain.
MATERIALS AND METHODS
Subjects
Thirty-six normal healthy subjects (21 males, 15 females; mean age, 30.92±8.27 years; range, 20 to 48) with no history of neurologic disease were recruited for the study. All participants provided written consent prior to participation in the study, which was approved by the institutional review board at our hospital.
Diffusion tensor image tractography
DTI were scanned using a 6-channel head coil on a 1.5 T Philips Gyroscan Intera MRI scanner (Philips, Ltd., Best, the Netherlands) with single-shot echo-planar imaging. For each of the 32 non-collinear diffusion sensitizing gradients, we acquired 67 contiguous slices parallel to the anterior commissure-posterior commissure line. Imaging parameters were as follows: acquisition matrix=96×96, reconstructed to matrix=128×128 matrix, field of view=221×221 mm2, repetition time=10726 ms, echo time =76 ms, parallel imaging reduction factor (SENSE factor)=2, echo-planar imaging factor=49 and b=1000 s/mm2, number of excitations=1, and a slice thickness of 2.3 mm (acquired isotropic voxel size 2.3×2.3×2.3 mm3).
Fiber tracking
Diffusion-weighted imaging data were analyzed using the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL; www.fmrib.ox.ac.uk/fsl). Head motion effect and image distortion by eddy current were corrected using affine multi-scale two-dimensional registration. Fiber tracking was performed using a probabilistic tractography method based on a multifiber model, and applied in the present study utilizing tractography routines implemented in FMRIB Diffusion (5000 streamline samples, 0.5 mm step lengths, curvature thresholds=0.2).24-26 The precommissural fornix and the post commissural fornix were determined by selection of fibers passing through seed and target regions of interest (ROI). For analysis of the precommissural fornix, we placed the seed ROI on the septal nucleus on the coronal image (Fig. 1).18,19 We defined the location of the septal nucleus as ventral to the septum pellucidum, superior to the hypothalamus, and below the rostrum of corpus callosum.18,19 The target ROI was placed on the crus of the fornix on the coronal image. For analysis of the postcommissural fornix, we placed the seed ROI on the mammillary body on the axial image. We defined the location of the mammillary body as dorsal to the hypothalamus and ventral to the interpeduncular fossa. The target ROI was placed on the crus of the fornix on the coronal image. Among the 5000 streamline samples generated from each seed voxel, tractography outputs from each individual subject were thresholded and binarized to include only those voxels through which at least one streamline sample passed. Values of fractional anisotropy (FA), mean diffusivity (MD), and tract volume of the precommissural and postcommissural fornix were measured.
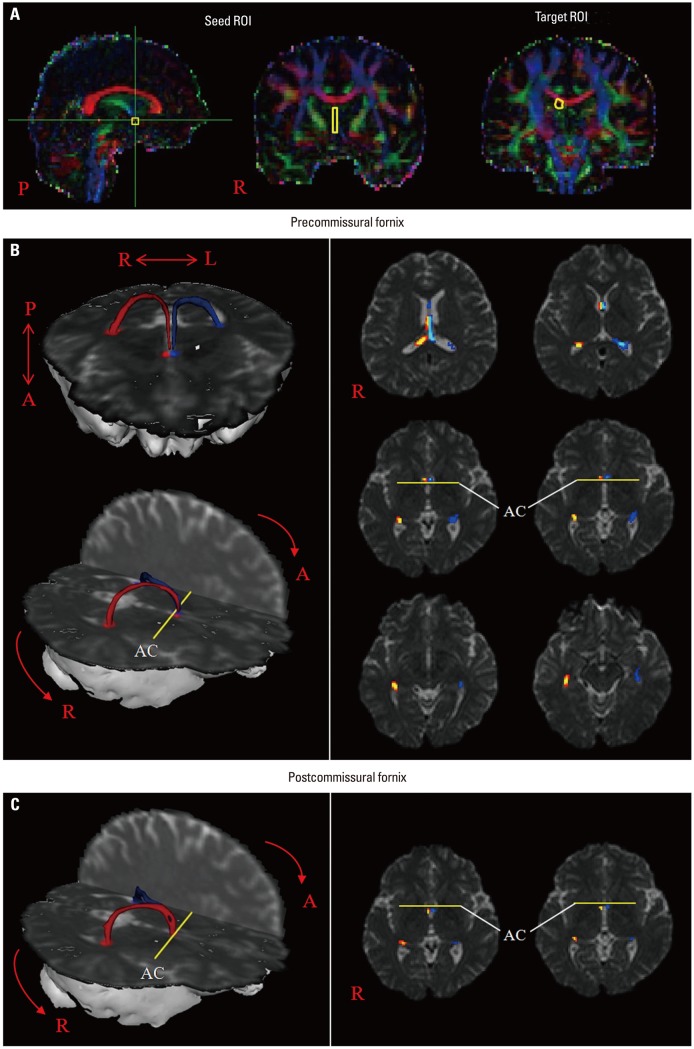
Fig. 1.
(A) Seed regions of interest (ROI) were placed in the septal nuclei (yellow rectangle). The target ROI was placed in the crus of the fornix (yellow circle). (B) The pathway of the precommissural fornix is shown at each level of the brain in a normal subject (42-year-old male). (C) The pathway of the postcommissural fornix is shown at the anterior commissure level in a normal subject (42-year-old male). AC, anterior commissure.
Two authors (Yeo SS and Seo JP) performed random analyses of the data to measure inter-observer and intra-observer variations. The consistency rates of analysis were moderate for the comparisons made by the two observers (K=0.654, p=0.000) and the two sets of analyses made by one analyser (Yeo SS) (K=0.654, p=0.000).
Statistical analysis
Data were analyzed using SPSS software (v.15.0; SPSS Inc., Chicago, IL, USA). Intra-observer and inter-observer reproducibility of the precommissural fornix was evaluated using kappa statistics. Values of FA, MD, and tract volume of the precommissural and postcommissural fornix were analyzed with an independent t-test for determination of variances between the right and left hemispheres. Statistical differences in DTI parameters between the precommissural and postcommissural fornix were determined by the independent t-test. Results were considered significant when the p-value was <0.05.
RESULTS
The precommissural fornix originated from the hippocampal formation on each hemisphere as a crus; both crura were joined to the body of the fornix. The body of the fornix continued anteriorly to the level just superior to the anterior commissure, where it divided into each column of the precommissural fornix. Each column descended anteriorly to the anterior commissure and terminated in the septal nuclei. The postcommissural fornix also originated from the hippocampal formation; however, the column passed from the posterior to the anterior commissure and terminated in the mammillary body (Fig. 1).
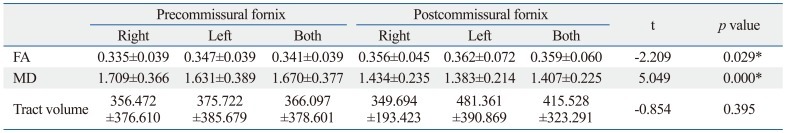
Values of FA, MD, and tract volumes of the precommissural and postcommissural fornix were not different between the right and left hemispheres (p>0.05). FA values of the postcommissural fornix were significantly higher than those of the precommissural fornix (p<0.05). MD values of the postcommissural fornix were significantly lower than those of the precommissural fornix (p<0.05). Tract volumes did not differ between the precommissural and postcommissural fornix (p>0.05) (Table 1).
Table 1.
Diffusion Tensor Parameters of the Precommissural Fornix and Postcommissural Fornix
FA, fractional anisotropy; MD, mean diffusivity.
Values represent mean (±standard deviation). MD×10-3 (mm2/s); Independent t-test was used to compare differences between the precommissural and postcommissural fornix.
*p<0.05.
DISCUSSION
In the current study, we used DTT for identification of the precommissural fornix in the human brain. The precommissural fornix, which is divided from the body of the fornix, descended anteriorly to the anterior commissure and terminated in the septal nuclei. By contrast, the postcommissural fornix passed posteriorly to the anterior commissure and terminated in the mammillary body. These results are compatible with those of previous studies and neuroanatomy textbooks.17-19,27,28 As for the DTI parameters, the FA value for the precommissural fiber was lower than that of the postcommissural fiber; in contrast, the MD value was for the precommissural fiber was higher than that of the postcommissural fiber, although there was no difference in the value for tract volume. The FA value represents the degree of directionality of microstructures, and the MD value indicates the magnitude of water diffusion.29-32 In contrast, the tract volume was determined by the number of voxels contained within the neural tract. Therefore, a decrement of FA value and increment of MD value without significant difference of tract volume may indicate less directionality of the precommissural fiber compared to the postcommissural fiber.
Several studies have reported on the anatomy of the precommissural fornix in the animal and human brain.23,27,28,33 In 1977, using an autoradiographic method in the rat brain, Swanson and Cowan27 found that the precommissural fornix originated solely in fields CA1-3 of the hippocampus proper and the subiculum, while the projection to the mammillary complex, which comprises a major part of the descending columns of the fornix, has its origin in the dorsal subiculum and the pre- and/or parasubiculum. In 2004, using T1-weighted 3D MRI, Watanabe, et al.28 demonstrated that the posterior hippocampal formation was connected to the dorsal part of the lateral septal nucleus via the fimbria and the precommissural fornix in the mouse brain. As for the human brain, many neuroanatomy textbooks and atlases have explained the pathway and function of the precommissural fornix.17-19,23,33 However, as for regular research studies, only two patients who showed injury of the precommissural fornix have been reported. In 2008, Vann, et al.33 reported on a patient who had tissue loss in the precommissural and postcommissural fornix following surgical removal of a cavernous angioma. In 2005, Rollins23 reported on a neonate with semilobar holoprosencephaly who showed an abnormal precommissural fornix on DTT. Therefore, to the best of our knowledge, this is the first original DTT study of the precommissural fornix in the human brain following Rollins's case report.23
In conclusion, we identified the precommissural fornix in the human brain using DTT. We believe that the methodology and results of this study would be helpful to future research on the precommissural fornix and in the elucidation of the pathology of diseases involving the precommissural fornix. However, several limitations of DTI should be considered in the interpretation of the results.34-37 First, DTI can underestimate the fiber tracts. DTI is a powerful anatomic imaging method that can demonstrate the gross fiber architecture; however, it is difficult to reflect all fibers. Second, regions of fiber complexity and crossing can prevent full reflection of the underlying fiber architecture by DTI. We suggest that additional studies on the clinical correlation and age-related changes of the precommissural fornix be undertaken.
ACKNOWLEDGEMENTS
This work was supported by the 2011 Yeungnam University Research Grant.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Brain Res Rev. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Brisch R, Bernstein HG, Dobrowolny H, Krell D, Stauch R, Trübner K, et al. A morphometric analysis of the septal nuclei in schizophrenia and affective disorders: reduced neuronal density in the lateral septal nucleus in bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2011;261:47–58. doi: 10.1007/s00406-010-0119-9. [DOI] [PubMed] [Google Scholar]
- 3.McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav Rev. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Henderson J, Greene E. Behavioral effects of lesions of precommissural and postcommissural fornix. Brain Res Bull. 1977;2:123–129. doi: 10.1016/0361-9230(77)90008-9. [DOI] [PubMed] [Google Scholar]
- 5.Thomas GJ. Delayed alternation in rats after pre- or postcommissural fornicotomy. J Comp Physiol Psychol. 1978;92:1128–1136. doi: 10.1037/h0077522. [DOI] [PubMed] [Google Scholar]
- 6.Park SA, Hahn JH, Kim JI, Na DL, Huh K. Memory deficits after bilateral anterior fornix infarction. Neurology. 2000;54:1379–1382. doi: 10.1212/wnl.54.6.1379. [DOI] [PubMed] [Google Scholar]
- 7.Poreh A, Winocur G, Moscovitch M, Backon M, Goshen E, Ram Z, et al. Anterograde and retrograde amnesia in a person with bilateral fornix lesions following removal of a colloid cyst. Neuropsychologia. 2006;44:2241–2248. doi: 10.1016/j.neuropsychologia.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Kuroki N, Kubicki M, Nestor PG, Salisbury DF, Park HJ, Levitt JJ, et al. Fornix integrity and hippocampal volume in male schizophrenic patients. Biol Psychiatry. 2006;60:22–31. doi: 10.1016/j.biopsych.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasuno F, Hirata M, Takimoto H, Taniguchi M, Nakagawa Y, Ikejiri Y, et al. Retrograde temporal order amnesia resulting from damage to the fornix. J Neurol Neurosurg Psychiatry. 1999;67:102–105. doi: 10.1136/jnnp.67.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korematsu K, Hori T, Morioka M, Kuratsu J. Memory impairment due to a small unilateral infarction of the fornix. Clin Neurol Neurosurg. 2010;112:164–166. doi: 10.1016/j.clineuro.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Moudgil SS, Azzouz M, Al-Azzaz A, Haut M, Gutmann L. Amnesia due to fornix infarction. Stroke. 2000;31:1418–1419. doi: 10.1161/01.str.31.6.1418. [DOI] [PubMed] [Google Scholar]
- 12.Hong JH, Jang SH. Degeneration of cingulum and fornix in a patient with traumatic brain injury: diffuse tensor tractography study. J Rehabil Med. 2010;42:979–981. doi: 10.2340/16501977-0603. [DOI] [PubMed] [Google Scholar]
- 13.Chang MC, Kim SH, Kim OL, Bai DS, Jang SH. The relation between fornix injury and memory impairment in patients with diffuse axonal injury: a diffusion tensor imaging study. NeuroRehabilitation. 2010;26:347–353. doi: 10.3233/NRE-2010-0572. [DOI] [PubMed] [Google Scholar]
- 14.Jang SH, Kim SH, Kim OL. Fornix injury in a patient with diffuse axonal injury. Arch Neurol. 2009;66:1424–1425. doi: 10.1001/archneurol.2009.242. [DOI] [PubMed] [Google Scholar]
- 15.Sugiyama K, Kondo T, Higano S, Endo M, Watanabe H, Shindo K, et al. Diffusion tensor imaging fiber tractography for evaluating diffuse axonal injury. Brain Inj. 2007;21:413–419. doi: 10.1080/02699050701311042. [DOI] [PubMed] [Google Scholar]
- 16.Hong JH, Jang SH. Left fornical crus injury and verbal memory impairment in a patient with head trauma. Eur Neurol. 2010;63:252. doi: 10.1159/000277477. [DOI] [PubMed] [Google Scholar]
- 17.Carpenter Malcolm B. Core Text of Neuroanatomy. 4th ed. Baltimore: Williams & Wilkins; 1991. pp. 373–375. [Google Scholar]
- 18.Afifi AK, Bergman RA. Functional Neuroanatomy: Text and Atlas. 2nd ed. New York: McGraw-Hill; 2005. pp. 287–288. [Google Scholar]
- 19.Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. 3rd ed. San Diego: Elsevier Academic Press; 2008. p. 127.p. 129.p. 131.p. 133. [Google Scholar]
- 20.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Takei K, Yamasue H, Abe O, Yamada H, Inoue H, Suga M, et al. Disrupted integrity of the fornix is associated with impaired memory organization in schizophrenia. Schizophr Res. 2008;103:52–61. doi: 10.1016/j.schres.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Zhuang L, Wen W, Trollor JN, Kochan NA, Reppermund S, Brodaty H, et al. Abnormalities of the Fornix in Mild Cognitive Impairment are Related to Episodic Memory Loss. J Alzheimers Dis. 2012;29:629–639. doi: 10.3233/JAD-2012-111766. [DOI] [PubMed] [Google Scholar]
- 23.Rollins N. Semilobar holoprosencephaly seen with diffusion tensor imaging and fiber tracking. AJNR Am J Neuroradiol. 2005;26:2148–2152. [PMC free article] [PubMed] [Google Scholar]
- 24.Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- 26.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 27.Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe T, Radulovic J, Spiess J, Natt O, Boretius S, Frahm J, et al. In vivo 3D MRI staining of the mouse hippocampal system using intracerebral injection of MnCl2. Neuroimage. 2004;22:860–867. doi: 10.1016/j.neuroimage.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 30.Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34:51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- 31.Neil JJ. Diffusion imaging concepts for clinicians. J Magn Reson Imaging. 2008;27:1–7. doi: 10.1002/jmri.21087. [DOI] [PubMed] [Google Scholar]
- 32.Stadlbauer A, Salomonowitz E, Strunk G, Hammen T, Ganslandt O. Quantitative diffusion tensor fiber tracking of age-related changes in the limbic system. Eur Radiol. 2008;18:130–137. doi: 10.1007/s00330-007-0733-8. [DOI] [PubMed] [Google Scholar]
- 33.Vann SD, Denby C, Love S, Montaldi D, Renowden S, Coakham HB. Memory loss resulting from fornix and septal damage: impaired supra-span recall but preserved recognition over a 24-hour delay. Neuropsychology. 2008;22:658–668. doi: 10.1037/a0012542. [DOI] [PubMed] [Google Scholar]
- 34.Lee SK, Kim DI, Kim J, Kim DJ, Kim HD, Kim DS, et al. Diffusion-tensor MR imaging and fiber tractography: a new method of describing aberrant fiber connections in developmental CNS anomalies. Radiographics. 2005;25:53–65. doi: 10.1148/rg.251045085. [DOI] [PubMed] [Google Scholar]
- 35.Parker GJ, Alexander DC. Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue. Philos Trans R Soc Lond B Biol Sci. 2005;360:893–902. doi: 10.1098/rstb.2005.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamada K. Diffusion tensor tractography should be used with caution. Proc Natl Acad Sci U S A. 2009;106:E14. doi: 10.1073/pnas.0812352106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamada K, Sakai K, Akazawa K, Yuen S, Nishimura T. MR tractography: a review of its clinical applications. Magn Reson Med Sci. 2009;8:165–174. doi: 10.2463/mrms.8.165. [DOI] [PubMed] [Google Scholar]