Abstract
BACKGROUND
During the seminiferous epithelial cycle, restructuring takes places at the Sertoli–Sertoli and Sertoli–germ cell interface to accommodate spermatogonia/spermatogonial stem cell renewal via mitosis, cell cycle progression and meiosis, spermiogenesis and spermiation since developing germ cells, in particular spermatids, move ‘up and down’ the seminiferous epithelium. Furthermore, preleptotene spermatocytes differentiated from type B spermatogonia residing at the basal compartment must traverse the blood–testis barrier (BTB) to enter the adluminal compartment to prepare for meiosis at Stage VIII of the epithelial cycle, a process also accompanied by the release of sperm at spermiation. These cellular events that take place at the opposite ends of the epithelium are co-ordinated by a functional axis designated the apical ectoplasmic specialization (ES)—BTB—basement membrane. However, the regulatory molecules that co-ordinate cellular events in this axis are not known.
METHODS
Literature was searched at http://www.pubmed.org and http://scholar.google.com to identify published findings regarding intercellular adhesion molecules (ICAMs) and the regulation of this axis.
RESULTS
Members of the ICAM family, namely ICAM-1 and ICAM-2, and the biologically active soluble ICAM-1 (sICAM-1) are the likely regulatory molecules that co-ordinate these events. sICAM-1 and ICAM-1 have antagonistic effects on the Sertoli cell tight junction-permeability barrier, involved in Sertoli cell BTB restructuring, whereas ICAM-2 is restricted to the apical ES, regulating spermatid adhesion during the epithelial cycle. Studies in other epithelia/endothelia on the role of the ICAM family in regulating cell movement are discussed and this information has been evaluated and integrated into studies of these proteins in the testis to create a hypothetical model, depicting how ICAMs regulate junction restructuring events during spermatogenesis.
CONCLUSIONS
ICAMs are crucial regulatory molecules of spermatogenesis. The proposed hypothetical model serves as a framework in designing functional experiments for future studies.
Keywords: ICAM, testis, spermatogenesis, ectoplasmic specialization, blood–testis barrier
Introduction
In mammalian tissues, including the seminiferous epithelium of the adult testis and the epididymis, cell–cell and cell–matrix interactions are indispensable to multiple cellular events, such as spermatogenesis in the testis, sperm maturation in the epididymis, and maintenance of cell/tissue homeostasis and survival (Dym and Cavicchia, 1978; Parvinen, 1982; Mruk and Cheng, 2004b; Hess and de Franca, 2008; Hermo et al., 2010a, b; Cyr, 2011; Ivanov, 2011; Shen et al., 2011; Dube and Cyr, 2012; Taddei et al., 2012) (Fig. 1). In some cellular processes, such as cell migration and morphogenesis during development, which alter cell adhesive states continuously (Gardel et al., 2010; Parsons et al., 2010), maintenance of cell adhesion to neighboring cells and/or to the extracellular matrix (ECM) is crucial to maintain tissue homeostasis and functionality. In the testis, spermatogenesis (which includes the cellular events of mitosis, meiosis, spermiogenesis and spermiation), which takes place in the epithelium of the seminiferous tubule, also involves extensive restructuring of cell junctions because developing germ cells must traverse the epithelium associated with their extensive morphological changes (Leblond and Clermont, 1952b; Fawcett, 1975; Setchell, 1978; de Kretser and Kerr, 1988). Germ cells per se, unlike other mammalian cells such as fibroblasts, macrophages, neutrophils and metastatic cancer cells, are non-motile: instead, they rely on Sertoli cells for their movement migrating from near the basement membrane to the luminal edge of the adluminal compartment via extensive morphological transformation, and can be defined into 14, 12 and 6 stages of the seminiferous epithelial cycle of spermatogenesis in the rat, mouse and human, respectively (Leblond and Clermont, 1952a; Clermont, 1963, 1972; Parvinen, 1982; de Kretser and Kerr, 1988; Mruk and Cheng, 2004b; Amann, 2008; Hess and de Franca, 2008; Mruk et al., 2008). Furthermore, preleptotene spermatocytes transformed from type B spermatogonia residing in the basal compartment must traverse the blood–testis barrier (BTB), which is created by Sertoli cell–cell junctions near the basement membrane (Dym and Fawcett, 1970; Fawcett et al., 1970; Fawcett, 1975; Setchell and Waites, 1975; Setchell, 2008; Mital et al., 2011). Thus, it is conceivable that extensive restructuring and turnover take place at the Sertoli cell–cell and Sertoli–germ cell interface during spermatogenesis.
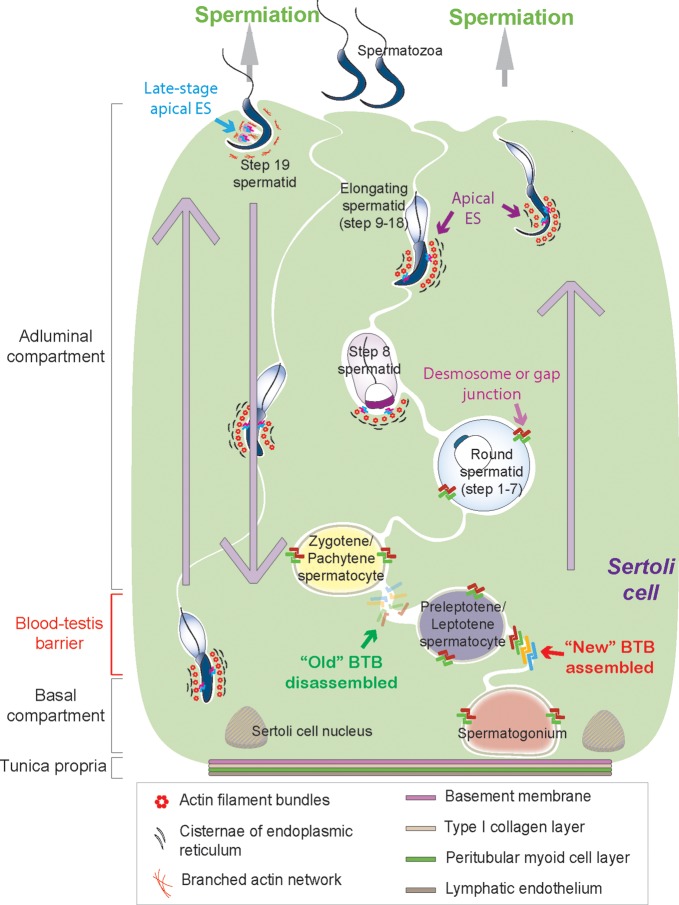
Figure 1.
A schematic diagram illustrating cellular events pertinent to transepithelial migration of developing germ cells during the epithelial cycle of spermatogenesis in the seminiferous epithelium of the adult rat testis. Adjacent Sertoli cells (green background, with the nucleus located near the basement membrane) extend from the tunica propria (consists of four layers: beginning with the basement membrane, type I collagen layer, the peritubular myoid cell layer and the lymphatic endothelium, from inside to outside of the seminiferous tubule) towards the tubule lumen in the rat testis. The seminiferous epithelium is divided by BTB into the basal and the adluminal (apical) compartments. Several co-existing junction types, namely tight junction (TJ), basal ES, and GJ, which together with the desmosome, constitute the BTB. However, these junctions undergo extensive restructuring at Stages VIII–IX to facilitate the transit of preleptotene spermatocytes residing at the basal compartment, crossing the BTB to enter the adluminal compartment while differentiating into leptotene spermatocytes to prepare for meiosis I/II. Before the ‘old’ BTB is being disassembled, Sertoli cells assemble a ‘new’ BTB behind transiting spermatocytes as shown in this diagram. Furthermore, developing germ cells, such as spermatogonia, spermatocytes and pre-step 8 spermatids attach to Sertoli cells via desmosomes and GJs, whereas elongating/elongated spermatids (step 8–19 spermatids) attach to Sertoli cells via the apical ES (typified by the presence of actin filament bundles sandwiched in between cisternae of endoplasmic reticulum and the apposed plasma members of the Sertoli cell and the spermatid) and must traverse the seminiferous epithelium moving from the basal to the adluminal compartment. Additionally, elongating spermatids also move ‘down’ towards the tunica propria, almost touching the Sertoli cell nuclei at Stage V of the epithelial cycle, before they move back to the adluminal compartment again and reach the luminal edge of the tubule lumen at Stages VI–VIII to prepare for spermiation (as shown by large gray arrows). At the same time of BTB restructuring at Stages VIII–IX, spermiation takes place at late Stage VIII to release the mature spermatozoa (step 19 spermatids) into the tubule lumen, with late-stage apical ES undergoing disintegration. In short, there are extensive restructuring at the Sertoli–Sertoli and Sertoli–germ cell interface during the seminiferous epithelial cycle of spermatogenesis.
In mammalian tissues, including the testis, cell adhesion is achieved via cell junctions, wherein cell adhesion molecules (CAMs) are crucial to cell adhesion in particular to elicit appropriate changes in cell adhesion in response to environmental stimuli (Byers et al., 1993a, b; Cheng and Mruk, 2002; Cavallaro and Dejana, 2011). CAMs are integral membrane proteins and each CAM is equipped with an extracellular, a transmembrane and a short cytoplasmic domain. In most cases, peripheral adaptors interact with the intracellular tail of CAMs and link them to the underlying cytoskeleton (Parsons et al., 2010; Gibson, 2011).
In mammalian cells, several major groups of CAMs have been identified, including cadherins, integrins and the immunoglobulin superfamily (IgSF) CAMs (Parsons et al., 2010; Cavallaro and Dejana, 2011; Gibson, 2011). In the testis, cadherins are the major components of the intercellular actin-based adherens junctions (AJs) connecting Sertoli–Sertoli and Sertoli–germ cells. They consist of classical cadherins, such as E-, P-, N- and VE cadherins, which associate with adaptors α-, β- or γ-catenin to constitute cell adhesion protein complexes. Cadherins/catenins are also one of the functional units of the actin-based cell–cell anchoring junctions known as ectoplasmic specialization (ES) (a testis-specific atypical AJ type) in the mammalian testis (note: the other functional units are the nectin-afadin and the integrin-laminin adhesion protein complexes) (Vogl et al., 1991, 1993, 2008; Cheng and Mruk, 2002, 2012 Wong et al., 2008; Yan et al., 2008). Cadherins are also components of desmosomes in the testis, which are intermediate filament-based cell–cell anchoring junctions, consisting of non-classical cadherins such as desmogleins and desmocollins (Russell and Peterson, 1985; Byers et al., 1993a; Lie et al., 2011; Mruk and Cheng, 2011, 2012). Recent studies have shown that there is extensive crosstalk between classical and non-classical cadherins in the testis, such as those at the BTB (Lie et al., 2011; Mruk and Cheng, 2011, 2012) (Fig. 1). In epithelial cells, integrins mainly connect ECM proteins (e.g. laminins, fibronectins and collagens) residing outside of a cell to the cytoskeleton within the cell (Luo et al., 2007; Weber et al., 2011). However, in the seminiferous epithelium of adult rat testes, integrins confer cell adhesion between the Sertoli and germ cells at the apical ES, as well as between the Sertoli cell and the basement membrane at the hemidesmosome (Cheng and Mruk, 2010). IgSF CAMs, probably the most populous family of cell surface molecules (with 765 members in humans), are a collection of integral membrane glycoproteins that possess at least one Ig-like domain in their extracellular region, capable of heterophilic and/or homophilic binding to integrins and/or among themselves (Brummendorf and Lemmon, 2001; Aricescu and Jones, 2007; Wang and Cheng, 2007; Cavallaro and Dejana, 2011). Its members include ICAM-1, sICAM-1, ICAM-2 and also CAR (coxsackievirus and adenovirus receptor) and nectin-3, which are also CAMs in the testis (see Table 1). Recent studies have shown that cell adhesion systems based on different CAMs are functionally inter-dependent rather than independent (Takai et al., 2008; Weber et al., 2011), illustrating considerable interplays between them. Besides direct heterophilic interactions between cadherins and integrins (Cepek et al., 1994; Whittard et al., 2002), as well as between integrins and IgSF CAMs, CAMs share common cytoskeletal networks (e.g. actin cytoskeleton), and there is accumulating evidence that they function beyond mere adhesion but are also involved in recruiting signaling molecules (e.g. non-receptor protein kinases, actin-binding proteins) and thus mediating multiple signal transduction cascades, regulating numerous cellular functions (Cavallaro and Dejana, 2011; Gibson, 2011).
Table I.
IgSF CAMs in the mammalian testis.
| IgSF CAM | KO male | KO phenotype | Cellular localization in the testis | Regulator (s) | Putative binding partner(s) | Reference(s) |
|---|---|---|---|---|---|---|
| Ceacam1 | Fertile | No apparent defects | SC/GC interface | Lauke et al. (2004); Kuespert et al. (2006) | ||
| Ceacam6 | SC; interstitial cells | Vimentin | Kurio et al. (2011) | |||
| Ceacam6-L | Apical ES | Kurio et al. (2008) | ||||
| Nectin-2 | Infertile | Disrupted spermatid morphogenesis; disorganized nectin-3 | BTB; apical ES (SC side) | Nectin-3 | Bouchard et al. (2000); Inagaki et al. (2006) | |
| Nectin-3 | Infertile | Disrupted spermatid morphogenesis; disappearing localization of nectin-2 at the apical ES | Apical ES (GC side) | Afadin; Nectin-1, -2; Necl-1, -2, -5; sGCβ1, ICAM-2 | Bouchard et al. (2000); Ozaki-Kuroda et al. (2002); Inagaki et al. (2006); Sarkar et al. (2006) | |
| VSIG1 | SC/GC interface (GC side) | ZO-1 | Kim et al. (2010) | |||
| JAM-A | Subfertile | Sperm motility defects | BTB; GC | Gonadotropin, CNP | P-glycoprotein, sGCβ1, Dynamin II | Lie et al. (2006); Sarkar et al. (2006); Xia et al. (2007); Shao et al. (2008); Tarulli et al. (2008); Su et al. (2009) |
| JAM-B | Fertile | No apparent defects | BTB; apical ES (SC side) | IL-1α, TGF-β2 | JAM-C | Gliki et al. (2004); Sakaguchi et al. (2006); Wang and Lui (2009) |
| JAM-C | Infertile | Lacked elongated spermatids; abnormal distribution of F-actin | Spermatocytes; round, elongating and elongated spermatids; apical ES (GC side) | Necl-2, Par3, Par6, CAR, JAM-B, Cdc42, PKCλ, PATJ | Gliki et al. (2004); Fujita et al. (2007); Mirza et al. (2006) | |
| JAM-D | Fertile | No apparent defects | GC | Nagamatsu et al. (2006) | ||
| Necl-2 | Infertile | Vacuoles in seminiferous epithelium; abnormal distribution of F-actin; disrupted spermatid morphogenesis | SC and spermatogonia/spermatocytes/elongating spermatids cell-cell interface; residual bodies | 4.1G, Necl-5, Par3, JAM-C | Fujita et al. (2006); Fujita et al. (2007); Terada et al. (2010); Maekawa et al. (2011) | |
| Necl-4 | 4.1G | Yang et al. (2011) | ||||
| Necl-5 | SC | Necl-2 | Wakayama et al. (2007) | |||
| ICAM-1 | Fertile | Granulocytosis | BTB; apical ES | IL-1, TNFα, IFN-γ, LPS, sICAM-1 | See Table II | Sligh et al. (1993); Riccioli et al. (1995); De Cesaris et al. (1998) |
| ICAM-2 | Fertile | Impaired angiogenesis; airway hyper-responsiveness | SC/GC interface | See Table II | Riccioli et al. (1995); De Cesaris et al. (1998); Gerwin et al. (1999); Huang et al. (2005) | |
| VCAM-1 | Fertile | No apparent defects | SC | IL-1, TNFα, IFN-γ, LPS | ICAM-1 | Gurtner et al. (1995); Riccioli et al. (1995); De Cesaris et al. (1998) |
| NCAM | Fertile | No apparent defects | Gonocyte/SC interface (immature SC side); Leydig cells | T3 | GFRα1 | Cremer et al. (1994); Laslett et al. (2000); Orth et al. (2000); Yang and Han (2010) |
| ALCAM | Fertile | Axon fasciculation defects; retinal dysplasias | Gonocyte/SC interface (gonocyte side); SC; myoid cells | sALCAM | McKinnon et al. (2000); Ohbo et al. (2003); Ikeda and Quertermous (2004) | |
| TCAM1 | Fertile | No apparent defects | SC and spermatocyte/round spermatid cell-cell interface | Sakatani et al. (2000); Nalam et al. (2010) | ||
| CAR | Embryonic death by E12 day | SC/GC interface (GC side), including BTB and apical ES | FSH, TNFα | JAM-C, Vinculin, β-Catenin, c-Src | Lie et al. (2006); Mirza et al. (2006); Mirza et al. (2007); Wang et al. (2007) | |
| Basigin | Infertile | Azoospermia; spermatogenesis arrested at the metaphase of the first meiosis; abnormal ES | SC, GC, Leydig cells | MCT1, MCT2, MMP-2 | Igakura et al. (1998); Toyama et al. (1999); Yuasa et al. (2001); Chen et al. (2010); Chen et al. (2011); Mannowetz et al. (2012) |
Ceacam, carcinoembryonic antigen-related cell adhesion molecule; JAM, junctional adhesion molecule; VSIG1, V-set and Ig domain containing 1; Necl, nectin-like molecule; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule; NCAM, neural cell adhesion molecule; ALCAM, activated leukocyte cell adhesion molecule; TCAM, testicular cell adhesion molecule; CAR, coxsackievirus and adenovirus receptor; LPS, lipopolysaccharide; IFN, interferon; JNK/SAPK, c-Jun N-terminal kinase/stress-activated protein kinase; T3, thyroid hormone; CNP, C-type natriuretic peptide; sGCβ1, soluble guanylate cyclase β1; MCT, monocarboxylate transporter; Par3, partitioning-defective protein 3; c-Src, cellular transforming protein of Rous sarcoma virus; apical ES, apical ectoplasmic specialization (a testis-specific actin filament rich-adherens junction, AJ); TGF, transforming growth factor; IL-1, interleukin 1; ZO-1, zonula occludens-1; PALS1, protein associated with Lin Seven 1; PATJ, PALS1-associated tight junction protein; MMP, matrix metalloproteinase; SC, Sertoli cell(s); GC, germ cell(s).
In this review, we focus on the roles of intercellular adhesion molecule (ICAM) family (Fig. 2) in junction dynamics with emphasis on junction restructuring events in the seminiferous epithelium of the testis; however, studies in other epithelia and/or endothelia are also discussed since this information is helpful to us to better understand the role of ICAMs in spermatogenesis. However, other adhesion proteins pertinent to the regulation of spermatogenesis in the testis are not discussed herein since they have recently been reviewed and discussed elsewhere, and readers can refer to these earlier excellent reviews (Setchell, 2008; Morrow et al., 2010; Mital et al., 2011; Pelletier, 2011; Franca et al., 2012). The ICAM family is a paradigmatic subgroup of the IgSF CAM family that are known to be expressed in a number of epithelial and endothelial cells, fibroblasts, neurons, leukocytes, platelets, erythrocytes, as well as testicular cells (Aricescu and Jones, 2007; Wang and Cheng, 2007; Kasprzak et al., 2012; Thomas and Baumgart, 2012) (Tables I and II). IgSF CAMs are known to take part in various biological processes with widely studied examples, such as neural cell adhesion molecule (NCAM) in neural development (Maness and Schachner, 2007) and ICAM-1 in transendothelial/transepithelial migration (TEM) of leukocytes during inflammation (Rahman and Fazal, 2009; Long, 2011). To date, several IgSF CAMs have been identified to be differentially expressed during spermatogenesis (see Table I) and it is increasingly clear that their loss and/or defects may cause infertility in both animals and humans (Bouchard et al., 2000; Yuasa et al., 2001; Maekawa et al., 2011). However, their roles at cell junctions and the underlying mechanism(s) that regulates cell adhesion in the testis during spermatogenesis remain elusive. Herein we provide an update on the possible roles of ICAM family members, in particular ICAM-1 and -2, during spermatogenesis based on recent findings in the field (Riccioli et al., 1995; De Cesaris et al., 1998; Xiao et al., 2012a, b). We also provide a hypothetical model regarding the mechanism(s) by which ICAMs regulate junction restructuring events during spermatogenesis. This model was prepared based on recent studies in the testis (Riccioli et al., 1995; De Cesaris et al., 1998; Xiao et al., 2012a, b) and other studies in the field that illustrate their roles in transmigration of leukocytes across the endothelial/epithelial barrier. This model thus serves as a framework upon which functional experiments can be designed. This review also sheds some lights on the role of ICAMs in fertility in men.
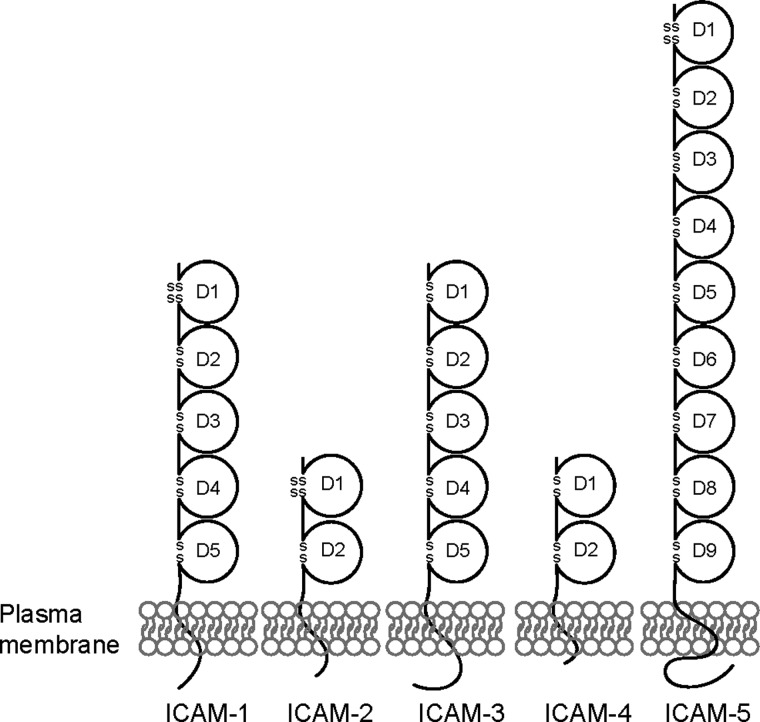
Figure 2.
Structure of ICAMs. Schematic drawing of the five ICAM family members illustrating different numbers of the extracellular Ig-like domains (D).
Table II.
Structure and function of ICAMs in mammalian cells/tissues.
| ICAMs | Structural featuresa | Cellular expression | Inducibility | Binding partnersb | Selected references |
|---|---|---|---|---|---|
| ICAM-1 (CD54) | 76–114 kDa, D1–D5, 8 N-glycans |
Broadly and differentially expressed: Sertoli cells, germ cells, epithelial cells, endothelial cells, leukocytes (low basal level), fibroblasts |
Highly inducible: TNFα, IFN-γ, IL-1α, IL-1β (α is more potent), LPS, phorbol ester, shear stress (by blood flow) |
Occludin, ZO-1, N-cadherin, β-catenin, αLβ2, αMβ2, αXβ2 integrins, (s)ICAM-1, VCAM-1, fibrinogen, CD9, CD43, SHP-2, Trio, PIP2, Actin, GAPDH, β-tubulin, α-actinin, cortactin, filamins, ezrin, moesin, PKCs, SGEF, Src, Pyk2 | Doulet et al. (2006); Federici et al. (1996); Pluskota et al. (2000); Muro et al. (2003); Millan et al. (2006); van Buul et al. (2007); Kanters et al. (2008); Di Lorenzo et al. (2011); van Rijssel et al. (2012); Xiao et al. (2012b) |
| ICAM-2 (CD102) | 55–60 kDa, D1–D2, 6 N-glycans |
Broadly and stably expressed: Sertoli cells, germ cells, epithelial cells, endothelial cells (high basal level), leukocytes, platelets |
Non-inducible: Down-regulated by TNFα/IL-1β in HUVECs |
Nectin-3, afadin, β1, αLβ2, αMβ2 integrins, (s)ICAM-2, DC-SIGN, actin, α-actinin, ezrin, radixin, moesin, PIP2, c-Src, Pyk2, annexin II |
Geijtenbeek et al. (2000a); Furutani et al. (2007); Yoon et al. (2008); Xiao et al. (2012b) |
| ICAM-3 (CD50) | 110–160 kDa, D1–D5, 15 N-glycans |
Specifically and stably expressed: Leukocytes (high basal level) |
Inducible: TNFα, retinoic acid, on endothelial cells (inflammation/ pathogenesis); down-regulated by PMA and Ca2+ ionophore |
αLβ2, αDβ2 integrins, DC-SIGN, (s)ICAM-3, ezrin, moesin, Lck, Fyn, Kidins220 |
Juan et al. (1994); Geijtenbeek et al. (2000b); Serrador et al., 2002; Jean-Mairet et al. (2011) |
| ICAM-4 (CD242, LW) | 42 kDa, D1–D2, 4 N-glycans |
Specifically expressed: Erythrocytes |
Not determined | αLβ2, αMβ2, αXβ2, α4β1, αVβ1, αIIbβ3, αVβ3, αVβ5 integrins | Spring et al. (2001); Hermand et al. (2003); Zennadi et al. (2004); Ihanus et al. (2007) |
| ICAM-5 (TLCN) | 130 kDa, D1–D9, 15 N-glycans |
Specifically expressed: Neurons |
Non-inducible | β1, αLβ2 integrins, (s)ICAM-5, α-actinin, ezrin, radixin, moesin, presenilin 1 and 2, UOL | Annaert et al. (2001); Nyman-Huttunen et al. (2006); Furutani et al. (2007); Conant et al. (2011); Tse et al. (2009) |
aStructural features of each ICAM in humans and/or rodents.
bLFA-1, leukocyte-specific β2-integrin subfamily consists of four family members: LFA-1, also known as CD11a/CD18 and αLβ2); Mac-1, macrophage-1 antigen, also known as CD11b/CD18 and αMβ2; p150, 95, also known as CD11c/CD18 and αXβ2, and CD11d/CD18 (αDβ2); CD, cluster of differentiation (according to the CD nomenclature of leukocyte antigens); D, Ig-like domain; N-glycans, potential N-glycosylation sites; LW, Landsteiner–Wiener (LW) blood group antigen; TLCN, telencephalin; TNFα, tumor necrosis factor α; IL-1, interleukin-1; IFN-γ, interferon-γ; LPS, lipopolysaccharide (a bacterial endotoxin); PMA, phorbol myristate acetate; SHP-2, Src-homology domain 2 (SH2)-containing phosphatase-2; DC-SIGN, dendritic cell-specific ICAM-3 grabbing non-integrin also known as cluster of differentiation 209 (CD209); PIP2, phosphatidylinositol 4,5-bisphosphate; GraP-DH, glyceraldehyde-3-phosphate dehydrogenase; PKC, protein kinase C; SGEF, RhoG-specific SH3-containing guanine-nucleotide exchange factor; Kidins220, kinase D-interacting substrate of 220 kDa; HUVECs, human umbilical vein endothelial cells; UOL, upstream of latency-associated-transcript, a late gene transcripts in Herpes simplex virus type 1 (HSV-1)-infected neurons; ZO-1, zonula occludens-1.
Methods
For this review, a search of the PubMed database at http://www.pubmed.org and http://scholar.google.com were performed to identify the current status of research on ICAM in both reproductive and non-reproductive fields. Articles in English from databases available in PubMed until October 2012 regarding the roles of IgSF CAMs in mammalian testes and ICAMs in cell adhesion dynamics were primarily considered. Individual searches were conducted using keywords and different combinations thereof. Keywords used for these searches include cell adhesion, cell junction, cell adhesion molecules, immunoglobulin superfamily CAMs, ICAM, soluble ICAM, human reproduction, testis, endothelial/epithelial barrier, BTB, seminiferous tubule, seminiferous epithelium, TEM of leukocytes and transmigratory cup. Journal articles were selected on the basis of relevance, quality and timeliness. We are indebted to many investigators in the field who have contributed significantly to the study of ICAMs in mammalian cell movement and early eminent investigators who laid the ground work in studying spermatogenesis, helping us to prepare this article for reproductive biologists interested in understanding the role of ICAMs in spermatogenesis. However, due to the space limit, many original references and important articles could not be cited, even though we have included several landmark earlier articles in this brief review. Also, every effort was made to cite the latest reviews on a specific subject by leading investigators in the field, so that readers can have access to many important original studies in these recent reviews.
Results
ICAMs and cell adhesion
Five ICAM family members, namely ICAM-1 to -5, are known to date, which possess different numbers of Ig-like C2-type domains in their extracellular region, ranging from two to nine (Fig. 2). Each of the Ig-like domains is derived from a separate exon and the deduced polypeptide sequence is differentially glycosylated (Fig. 2, Table II). Apart from the structural similarities, ICAMs differ greatly in tissue distribution and also in regulation. For example, ICAM-1 and -2 are ubiquitously expressed in mammalian cells and both of them (and also the only two of them) are expressed by Sertoli and germ cells in the rat testis, whereas ICAM-3 to -5 display highly restricted tissue/cellular distribution (Table II). Additionally, they are either inducible or non-inducible by different cytokines and other biomolecules (Table II). Human ICAM-1, -3, -4 and -5 genes were mapped to the chromosome region 19p13.2–13.3, with ICAM-2 at 17q23–25 (Hayflick et al., 1998). Genome-wide analysis in different populations has offered a genetic linkage of loci on human chromosome 19 to multiple diseases, reflecting the possibility that ICAMs could be useful diagnostic markers and therapeutic targets. For instance, the ICAM gene cluster on chromosome 19 has been identified as a susceptibility locus for breast and prostate cancer, as well as systemic lupus erythematosus (Kammerer et al., 2004; Kim et al., 2012).
ICAMs were first shown to be involved in leukocyte homotypic aggregation and leukocyte–endothelial cell adhesive interactions via their binding to β2-integrins (Harris et al., 2000). Each member of the leukocyte-specific β2-integrin subfamily (i.e. the CD11 clusters) is capable of binding to one or more member of the ICAM family at the cell–cell interface, and among them the leukocyte function-associated antigen 1 (LFA-1) recognizes all five ICAMs (Harris et al., 2000; Luo et al., 2007) (Table II). It is noted that integrins are pivotal cell adhesion receptors that mediate leukocyte activation and migration and their interactions with ICAMs in leukocyte adhesion were found to have various modulatory effects on the immune system, such as inducing the production of cytokines (e.g. interleukins) and/or activation of proteinases (e.g. matrix metalloproteinases (MMPs)) (Park et al., 2010; Costantini et al., 2011). Thus, much effort in the field has been devoted to understanding how ICAMs contribute to leukocyte recruitment and extravasation into target tissues in pathological conditions (e.g. inflammation) and ICAMs are generally accepted as co-stimulators of leukocyte activation. In the testis, β1-integrin, an adhesion molecule found at the apical ES, hemidesmosome and stem cell niche (Cheng et al., 2011a), was shown to interact with ICAM-2 at the apical ES (Xiao et al., 2012b). It has been demonstrated that activation of MMP-2 is needed for generation of biologically active laminin fragments from the α6β1-integrin–laminin-333 adhesion protein complex at the apical ES to prepare for spermiation (Cheng et al., 2011a). Along these lines of research, it is of interest to know whether ICAM-2 participates in the process, e.g. modulation of the MMP-2 action. Also, consistent with their roles as CAMs, ICAMs are actively engaged in remodeling of the actin cytoskeleton in effector cells (Vicente-Manzanares and Sanchez-Madrid, 2004; van Rijssel et al., 2012), but studies have implied that ICAMs may also associate with the microtubule- and intermediate filament-based cytoskeleton (Federici et al., 1996; Nieminen et al., 2006; Nejmeddine et al., 2009; Jean-Mairet et al., 2011). The cytoplasmic domain of several ICAMs has been shown to interact with various actin-binding and regulatory proteins. For instance, ICAM-1, -2, -3 and -5 were found to bind to ERM proteins, ezrin, radixin and moesin (Serrador et al., 2002; Doulet et al., 2006; Furutani et al., 2007). ICAM-1, -2 and -5 were found to bind to an actin cross-linker α-actinin (Nyman-Huttunen et al., 2006). ICAM-1 is also known to interact with cortactin, a substrate for Src that co-activates actin-related protein (Arp) 2/3 complex with neural Wiskott-Aldrich syndrome protein (N-WASP) to support branched actin filament (F-actin) polymerization to generate branched F-actin network (Allingham et al., 2007; Schnoor et al., 2011; van Rijssel et al., 2012) (Table II). Therefore, ICAMs can efficiently regulate F-actin reorganization in mammalian cells through their differential interactions with various actin-binding and regulatory proteins: cell surface distribution of ICAMs can also be controlled by the underlying cytoskeleton via linker proteins, such as ezrin and α-actinin (Nyman-Huttunen et al., 2006; Yoon et al., 2008). In the rat testis, both ICAM-1 and -2 were found to structurally interact with actin (Xiao et al., 2012a, b). Interestingly, ICAM-2 co-localized with F-actin at the apical ES throughout different stages of the epithelial cycle, except at stages VII–VIII shortly before spermiation (Xiao et al., 2012b), suggesting that ICAM-2 may play a role in F-actin reorganization at Stage VIII of the cycle to facilitate sperm release by causing disruption of F-actin bundles at the apical ES during spermiation. On the other hand, ICAMs also have the ability to activate signal transduction via ectodomain shedding, such as to generate soluble ICAMs, or clustering on plasma membrane to activate Ca2+- and/or Rho-mediated signaling, and/or recruit intracellular downstream partners, such as protein kinases and phosphatases, in response to external stimuli, which modulates the cytoskeleton to affect cell adhesion and migration (Etienne-Manneville et al., 2000; Pluskota et al., 2000; Thompson et al., 2002; Park et al., 2010). More notably, ICAM-1 clustering on endothelial cells was shown to recruit vascular cell adhesion molecule-1 (VCAM-1) (van Buul et al., 2010b) to the site to regulate cell adhesion, illustrating that ICAMs possess a broad signaling capacity. In the sections that follow, we elaborate on these points, e.g. findings concerning ICAM shedding and clustering in the regulation of cell adhesion, as well as the cooperative activities among ICAMs and the related cytoskeletal and signaling components during leukocyte TEM, and their implication in germ cell migration across the seminiferous epithelium.
Polymorphic and multifunctional molecules: soluble and multimeric ICAMs
Secretion and ectodomain shedding have been reported for several IgSF CAMs, such as JAMs (junctional adhesion molecules) and NCAM-L1 (Cavallaro and Dejana, 2011). It is therefore not surprising that in addition to the membrane-bound full-length ICAM (mICAM), different truncated forms of ICAMs have been found as normal physiological constituents of various biological fluids: they are known as soluble or circulating ICAM (sICAM/cICAM). Since sICAM-1, -2, -3 and -5 have been detected in serum of healthy humans (Mustjoki et al., 2001; Gahmberg et al., 2008; Lopez-Lerma and Estrach, 2009), it is conceivable that sICAM may function as essential biomolecules in mammalian cells/tissues. For instance, sICAM-4 was identified in spent media of COS-7 cells transfected with murine ICAM-4 cDNAs (Lee et al., 2003) and sICAM-1 was detected in mutant mice lacking mICAM-1 (van Den Engel et al., 2000). Also, the age-dependent expression profiles of mICAM-5 and sICAM-5 do not parallel each other during brain development (Gahmberg et al., 2008), indicating their differential roles. Additionally, considerably elevated levels of sICAM were detected in many pathological conditions, implying a crucial role of sICAM in immune surveillance (Gahmberg et al., 2008; Mendez et al., 2008; Lopez-Lerma and Estrach, 2009). Recently, a form of sICAM-1 with an apparent molecular weight (Mr) of ∼70 kDa was discovered in normal rat testis, which was also enriched in germ cells detected using a polyclonal antibody targeting the extracellular domains 2 and 3 of ICAM-1 (Xiao et al., 2012a) (Table II). By using this antibody, however, additional protein fragments with lower Mr were also observed in Sertoli and germ cells, suggesting other truncated forms of sICAM-1 may exist in the testis (Xiao et al., 2012a), but whether they have distinct biological functions in maintaining spermatogenesis remains to be investigated.
While sICAM was viewed as a molecular marker of inflammation, and might be involved in modulating immune responses such as the production of cytokines and proteinases, perhaps overlapping the function of or distinctly different from that of mICAM (Witkowska and Borawska, 2004; Gahmberg et al., 2008; Tse et al., 2009), the origin of sICAM and its significance remains to be elucidated. Nonetheless, it is generally accepted that proteolytic cleavage is the primary mechanism responsible for the ectodomain shedding of ICAM-1, such as via action of (i) serine proteases (e.g. elastase and cathepsin G) (Robledo et al., 2003; Mendez et al., 2008), (ii) MMPs [e.g. MMP-9, -13 (also known as collagenase-3) and -14 (also known as membrane type 1-MMP, i.e. MT1-MMP)] (Fiore et al., 2002; Sithu et al., 2007; Tarin et al., 2009), and/or (iii) zinc-dependent metalloproteinase (MP) [e.g. TNFα-converting enzyme (TACE, also known as a disintegrin and metalloproteinase-17, i.e. ADAM-17) (Tsakadze et al., 2006)]. sICAM-2, -3 and -5 are generated from the corresponding ICAMs via cleavage by MP, caspase-3 and -8, and MMP-2 and -9, respectively (Tian et al., 2007; Sato et al., 2009). These findings are useful since MMP-2 is known to be restricted to the apical ES in the rat testis, while MMP-9 is limited to the basal ES at the BTB (Cheng et al., 2011a), so it becomes desirable to determine whether ICAMs are substrates of MMPs in the testis. Moreover, it appears that the regulatory molecules (e.g. inflammatory mediators such as TNFα), needed to induce cleavage of ICAM extracellular domain, vary among cell types and this event can be modulated by multiple kinases [e.g. Src, p38 mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase], and phosphorylation of Tyr residues within the ICAM cytoplasmic domain can affect the shedding of the extracellular domain (Tsakadze et al., 2004; Sithu et al., 2007; Mendez et al., 2008). It is worth noting that the cytoskeletal association of mICAM may be crucial for its sensitivity to proteolytic cleavage, such as in ICAM-5 (Tian et al., 2007). Alternative RNA splicing can also be a mechanism that accounts for the generation of sICAM-1 and sICAM-4 (Lee et al., 2003; Mendez et al., 2008). It is noted that different cleaved products or splice variants of ICAMs have been identified, such as with or without deletion of one or more extracellular Ig-like repeats (Lee et al., 2003; Witkowska and Borawska, 2004; Tian et al., 2007).
sICAMs are biologically active molecules and their up-regulation by cytokines mirrors that of their corresponding mICAMs (Gahmberg et al., 2008; Mendez et al., 2008; Witkowska and Borawska, 2004). However, mICAM expression is often regulated at the transcriptional level by transcription factors, such as nuclear factor-κB, cAMP-response element-binding protein, E-twenty-six or the RUNX family in response to stimuli, whereas that of sICAM is regulated as a combined result of changes in the mICAM protein level and the proteolytic activity (Witkowska and Borawska, 2004; Hadad et al., 2011; Estecha et al., 2012). sICAM-1 and -4 have been suggested to compete for ligand binding with the native mICAMs present on the cell surface and thus reduce cell adhesion (Wang et al., 2005; Trinh-Trang-Tan et al., 2010). sICAM-1 was found to adversely affect endothelial barrier function, blocking leukocyte TEM (Otto et al., 2000; Yang et al., 2005; Clark et al., 2007; Sumagin and Sarelius, 2010). Indeed, overexpression of the aforementioned ∼70 kDa sICAM-1 containing all five Ig-like domains in Sertoli cells with an established barrier function in vitro was found to display a disruptive effect on the Sertoli cell barrier, which was a sharp contrast to ICAM-1 (Xiao et al., 2012a), illustrating that sICAM-1 and ICAM-1 can have antagonistic effects on the BTB integrity. Considering this and the relative abundance of sICAM-1 produced by germ cells, it is tempting to speculate that while ICAM-1 maintains the barrier function, sICAM-1 may be used by preleptotene spermatocytes to serve as a molecular ‘switch’ to ‘open up’ the BTB at or near their apical region to facilitate their passage at the site. sICAM may also induce the loss of cell adhesion between Sertoli and germ cells, in particular spermatocytes and round spermatids, since in vivo overexpression of the sICAM-1 in rat testes, besides perturbing the BTB, was also found to induce depletion of spermatocytes and round spermatids from the seminiferous epithelium (Xiao et al., 2012a). Cytokines (e.g. TNFα, TGF-βs and IL-1α) and androgens are known to mediate junction restructuring by triggering internalization and/or recycling of adhesion protein complexes at the BTB and apical ES, via the combined effects of protein kinases (e.g. Src family kinases, focal adhesion kinase (FAK)), proteases (e.g. MMPs) and actin-binding and regulatory proteins (e.g. Eps8, Arp3) and perhaps others (e.g. zyxin, vinulin, N-WASP, cortactin (Young et al., 2009a, b, 2012; Cheng and Mruk, 2012; Lie et al., 2012; Young and Vogl, 2012) and cytokines were shown to up-regulate ICAM-1 expression in mouse Sertoli cells (De Cesaris et al., 1998). Interestingly, a non-classical endocytosis pathway directed by ICAM-1 has been reported in endothelial cells, which involves Src- and Rho-dependent actin cytoskeleton remodeling (Muro et al., 2003; Serrano et al., 2012). However, it is not known whether ICAM-1 is involved in assisting intracellular protein trafficking in the seminiferous epithelium, such as the recycling of adhesion protein complexes from the ‘old’ BTB and/or apical ES to the ‘new’ sites (Fig. 1). It is likely that sICAM-1 is involved in TNFα-mediated protein endocytosis/junction disassembly in the testis (Xia et al., 2009), since TNFα treatment is known to increase endothelial sICAM-1 production which contributes to paracellular permeability (De Cesaris et al., 1998; Clark et al., 2007; Mendez et al., 2008). Nevertheless, it remains possible that sICAM-1 promotes TNFα action during spermatogenesis as shown in other cell systems, or, it is a bidirectional regulation that synergistically leads to disintegration of the BTB and apical ES (McCabe et al., 1993; Otto et al., 2000; Tsakadze et al., 2004). On another note, sICAM-5 was shown to stimulate the phosphorylation of cofilin (an actin-depolymerizing factor) to induce F-actin reorganization (Conant et al., 2011), whereas the remnant cytoplasmic tail remaining after cleavage might compete and disrupt the cellular anchorage of neighboring mICAM-5 to the actin cytoskeleton and consequently affect the intracellular signaling (Sithu et al., 2007; Tian et al., 2007). Furthermore, homophilic interactions between sICAMs and mICAMs have been demonstrated (Table II). It was found that the lowered level of mICAMs impeded cell survival (Sato et al., 2009; Trinh-Trang-Tan et al., 2010), but the correlations between different sICAMs in body fluids, as well as that between sICAM and its corresponding mICAM in pathological conditions, such as male infertility, have not been established and are probably more complicated than it was initially conceived (Mustjoki et al., 2001; Lopez-Lerma and Estrach, 2009; Cserti-Gazdewich et al., 2010; Xiao et al., 2012a).
As mentioned above, while the release of soluble ectodomains of ICAMs is capable of triggering signal transduction and mediating junction disassembly, the redistribution and/or clustering of ICAMs on the cell surface is equally critical in regulating cellular functions. For instance, ICAM-1 is known to dynamically inter-convert between monomers and dimers on cell surface and the latter assume a more potent and active role in cell adhesion (Miller et al., 1995; Reilly et al., 1995; Oh et al., 2011). Likewise, dimeric immobilized sICAM-1 binds more efficiently to LFA-1 versus monomeric sICAM-1 (Welder et al., 1993; Jun et al., 2001a, b). Structurally, ICAM dimers can further integrate to W-shaped tetramers which are apt for macro-clustering to form larger multimers in response to changes in environment, as well as undergo homophilic and/or heterophilic co-clustering with membrane partners on the adjacent cell surface (Jun et al., 2001a; Shaw et al., 2004; Yang et al., 2004; Millan et al., 2006). Studies have indicated that clustering may facilitate ICAM-1-mediated endocytosis and actin stress-fiber formation but do not necessarily lead to changes in vascular permeability (van Buul et al., 2002; Muro et al., 2003; Millan et al., 2006; Sumagin et al., 2011). Approach of leukocytes to an endothelium layer often suffices to induce clustering of endothelial ICAM-1, laying the foundation for successive leukocyte-endothelial cell interactions, which can be mimicked by cross-linking ICAM-1 on the plasma membrane with specific monoclonal antibodies (Millan et al., 2006; Muller, 2011). It has been found that cross-linking of ICAM-1 on diverse cell types transmits outside signals to inside of the cell (‘outside-in’ signaling), causing redistribution of ICAM-1 on the apical surface and establishes its association with the actin cytoskeleton (Hubbard and Rothlein, 2000; Amos et al., 2001; Rahman and Fazal, 2009). This is accompanied by increases in cytosolic Ca2+ and tyrosine phosphorylation of multiple cellular proteins, and an activation of numbers of signaling pathways (Rahman and Fazal, 2009). As a result of these signals, the F-actin cytoskeleton is rearranged and subsequently affects the cell–cell adhesion (Doulet et al., 2006; Wittchen, 2009). More intriguingly, ICAM-1 cross-linking has been shown to promote VCAM-1 expression and recruits VCAM-1 to the same sites (Lawson et al., 1999; van Buul et al., 2010b). Similarly, cross-linking of other ICAMs has been displayed to initiate and/or augment intracellular communication (Berney et al., 1999; Perez et al., 2002).
Apparently, the ‘outside-in’ signaling conveyed by ICAM clustering relies heavily on the short cytoplasmic tail, which interacts with a legion of cytoskeletal binding/regulatory proteins in the cell cytosol (Table II). Tailless ICAM-1 (i.e. sICAM-1) has been shown to fail in mediating intracellular signaling versus intact ICAM-1, and thus intact ICAM-1 is crucial in various biological activities, such as leukocyte TEM and virus infection (Pluskota et al., 2000; Greenwood et al., 2003; Lyck et al., 2003; Yang et al., 2005; Dermody et al., 2009; Sumagin and Sarelius, 2010). On the other hand, clustering of ICAM-1 induces better association between ICAM-1 and the underlying actin cytoskeleton and its disruption may create an ‘inside-out’ signal (Topp et al., 2003; van Buul et al., 2010a). The likely effects of ICAM-1 clustering-induced intracellular signaling in endothelial cells to facilitate leukocyte TEM are discussed below and are summarized in Fig. 3. It is worth noting that there remains a lack of evidence supporting the role of ICAM-1 clustering in signaling during spermatogenesis, which should be carefully evaluated in future studies. Nonetheless, the action of ICAM-mediated signaling function summarized herein can be the basis of designing future experiments.
Figure 3.
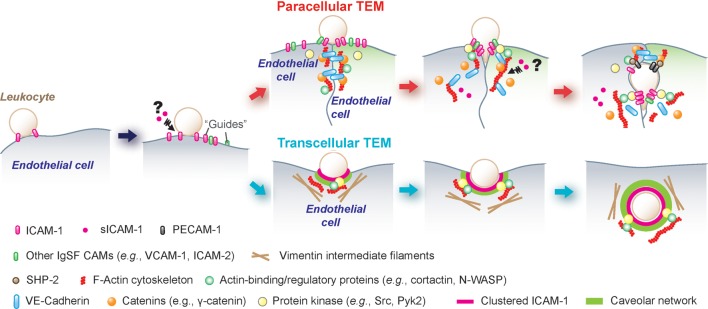
A schematic drawing illustrating the molecular mechanisms by which ICAM-1, sICAM-1 and ICAM-2 regulate TEM (transendothelial/transepithelial migration) of leukocytes via paracellular or transcellular pathways. Endothelial cells were shown in either light purple and green. Prior to leukocyte TEM, endothelial ICAM-1 and/or -2, besides acting as adhesion molecules, may serve as a guide for the movement of leukocytes on the endothelium. In paracellular TEM (see ‘red’ arrows), ICAM-1 clustering can recruit non-receptor protein tyrosine kinases (e.g. Src and Pyk2) to phosphorylate junctional protein complexes (e.g. VE-cadherin-catenin adhesion protein complex) and cause their disassociation to open up the endothelial junction. Also, Src recruitment can be used to phosphorylate cortactin (or other actin-binding and regulatory proteins), which results in an F-actin remodeling and/or re-organization to facilitate leukocyte TEM. In transcellular TEM (see ‘blue’ arrows), ICAM-1-mediated phagocytosis/endocytosis, either independent of or dependent on caveolar network, can be used for leukocyte transcellular TEM without perturbing the endothelial junctions.
ICAM in TEM
Throughout the seminiferous epithelial cycle of spermatogenesis, such as in the rat testis, developing germ cells, both spermatocytes and spermatids migrate progressively across the seminiferous epithelium from the basal to the adluminal compartment, including the passage of preleptotene spermatocytes across the BTB (Pelletier and Byers, 1992; Pelletier, 2001, 2011; Hermo et al., 2010b). Developing elongating spermatids that have reached and reside in the adluminal compartment at Stages III–IV also traverse the epithelium, returning to the epithelium near the tunica propria (Fig. 1), until almost ‘touching’ the Sertoli cell nuclei located near the basement membrane at Stage V of the epithelial cycle, before they move back to the adluminal compartment at Stages VI–VIII, illustrating extensive spermatid movement (Clermont, 1963, 1972; Russell, 1977). Yet, germ cells per se are non-motile cells, lacking the apparatus (e.g. lamellipodia) to ‘migrate’ on their own as do fibroblasts, macrophages, neutrophils, and other cells, but rely on the Sertoli cells where the extensive re-organization of actin and tubulin networks provides the protrusive force for their movement (Russell et al., 1989; Lee and Cheng, 2004; Vogl et al., 2008). Importantly, a large body of work is found in the literature delineating the involvement of ICAMs in leukocyte TEM, a process highly reminiscent of germ cell migration during spermatogenesis. We thought it pertinent to provide a brief and critical review herein since this information serves as the basis of some important future experiments.
In an immune response, circulating leukocytes leave the blood vessels by extravasation to reach the site of injury or infection. However, the vascular endothelium acts as a filtration blood–tissue barrier that regulates the leukocyte passage. As has been largely accepted, leukocyte extravasation is composed of a series of sequential steps, including initial capture/tethering, rolling along the vessel wall, arrest on the endothelium, adhesion strengthening and spreading, intravascular crawling, diapedesis/transendothelial migration and finally crossing the endothelial basement membrane to the interstitial tissue and arrival at the intended site. This multistep process demands drastic changes in cytoskeleton and efficient coupling of adhesion receptors on both leukocytes and endothelial cells (Wittchen, 2009; Fernandez-Borja et al., 2010; Nourshargh et al., 2010; Muller, 2011). ICAM-1 has been implicated in almost every course of leukocyte recruitment, including rolling, arrest, adhesion, crawling and TEM (Sumagin and Sarelius, 2007; Rahman and Fazal, 2009). For example, during the adhesive cell–cell interacting phase between leukocytes and endothelial cells prior to TEM, endothelial ICAM-1 and -2 have been proposed to serve as a guide or signal for leukocyte recognition of the sites for TEM, and help leukocyte switch from adhesion/crawling mode to TEM, as well as to optimize endothelial junctions for leukocyte access (Schenkel et al., 2004; Yang et al., 2005; Millan et al., 2006; Porter and Hall, 2009; Woodfin et al., 2009; Steiner et al., 2010; Sumagin and Sarelius, 2010). Leukocyte TEM has been shown to take place via both paracellular and transcellular routes. The use of the ‘paracellular’ route for TEM signifies a transient dissolution of inter-endothelial junctions to allow the passage of leukocytes, while in the ‘transcellular’ route leukocytes directly traverse the cytoplasm of an endothelial cell, without perturbing the endothelial junctions (Wittchen, 2009; Komarova and Malik, 2010; Muller, 2011).
Currently, the prevailing hypothesis of leukocyte TEM is as follows. Leukocyte TEM is launched and maintained by some specific endothelial structures enriched in ICAM-1, whose expression is rapidly induced upon activation by various inflammatory mediators (Table II). It was first reported that an actin-rich cup-like docking structure analogous to receptor-mediated phagosomes at the leukocyte–endothelial cell interface was found to be quickly vanished as the leukocyte TEM began (Barreiro et al., 2002). A similar ‘transmigratory cup’ was also found during transcellular and paracellular TEM; however, this ultrastructure enwrapped and followed leukocyte crossing all the way during the TEM process and appeared to require the involvement of the microtubule-based, besides the actin-based, cytoskeleton (Carman et al., 2003; Carman and Springer, 2004) (Fig. 3). Formation of these cup-like ultrastructures required dynamic redistribution/clustering of ICAM-1 at the leukocyte–endothelial cell–cell contact site, and involved the recruitment of multiple actin-binding proteins, such as ERM family proteins, α-actinin and vasodilator-stimulated phosphoprotein (an enhancer of Arp2/3/N-WASP-mediated branched actin nucleation to create a branched actin network, also found in phagosomes), as well as focal adhesion proteins such as vinculin, talin and paxillin (Barreiro et al., 2002; Doulet et al., 2006). Other CAMs, including VCAM-1, ICAM-2, platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31), JAM-A, and CD44, were also implicated in the processing of this cup-like docking ultrastructure (Barreiro et al., 2002, 2008; Carman et al., 2003; Carman and Springer, 2004; Doulet et al., 2006). In short, this cup-like docking ultrastructure serves as the receptor for leukocytes and the transmigration of leukocyte-docking/receptor-like ultrastructure across the endothelial barrier, either paracellularly or transcellularly, is analogous to a ‘giant’ phagosome, in which ICAM-1 summons all the necessary elements possibly via its redistribution/clustering ability at the site (Fig. 3).
It was also reported that the onset of TEM associated with the formation of a ring-like ultrastructure (Shaw et al., 2004) which was assembled via a spatiotemporal re-localization of LFA-1 on leukocytes that triggered a co-clustering of ICAM-1. During paracellular TEM, a leukocyte probably inserted a pseudopod into the ‘ring’ and squeezed across the endothelial junctions (Shaw et al., 2004) (Fig. 3). ICAM-1 was shown to be involved in the transient ‘opening’ of intercellular junctions, since clustering of ICAM-1 at the site recruits non-receptor protein tyrosine kinases (e.g. c-Src and Pyk2), which subsequently phosphorylate crucial components of the VE-cadherin-catenin adhesion protein complex, namely VE-cadherin, β- and γ-catenins, leading to a reversible loss of the junction integrity and thus ‘an opening’ in the endothelial tight junction (TJ) barrier for the passage of a leukocyte (Allport et al., 2000; Shaw et al., 2001; van Buul et al., 2007; Nottebaum et al., 2008; Turowski et al., 2008; Di Lorenzo et al., 2011; Pelletier, 2011; Alcaide et al., 2012). ICAM-1 clustering was also found to result in a dramatic remodeling of the actin cytoskeleton, via its interaction with the actin nucleation-promoting factor cortactin, which is phosphorylated by Src upon its association with ICAM-1 (Etienne-Manneville et al., 2000; Tilghman and Hoover, 2002; Yang et al., 2006; Schnoor et al., 2011). The net result of these changes in the ICAM-1-c-Src-cortactin pathway thus destabilizes the integrity of the endothelial TJ barrier to facilitate the transit of leukocytes. In the testis, ICAMs and Src/Pyk2 are also natural partners in regulating junction dynamics, in that the pathways involving either ICAM-1 or -2 were c-Src- and/or Pyk2 dependent (Xiao et al., 2012a, b). Collaboratively, in the TNFα-treated human Sertoli cells, ICAM-1 and c-Src were found to co-localize at the Sertoli cell–cell interface as shown in Fig. 4.
Figure 4.
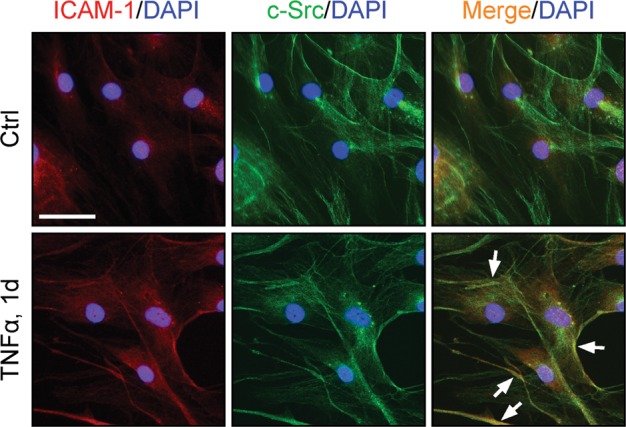
Co-localization of ICAM-1 with c-Src at the cell–cell interface of human Sertoli cells treated with TNFα. Human Sertoli cells were cultured as earlier described (Chui et al., 2011) at 0.05 × 105 cells/cm2 on fibronectin-coated glass coverslips in DMEM/F-12 containing 10 μg/ml insulin, 5 μg/ml human transferrin, 2.5 ng/ml epidermal growth factor and 100 μg/ml penicillin–streptomycin in a humidified atmosphere at 35°C with 95% air/5% CO2 (vol/vol) and immunostained for ICAM-1 [red fluorescence; rabbit polyclonal antibody from Novus Biologicals (NB100-81977)] and c-Src [green fluorescence; mouse monoclonal antibody from Santa Cruz Biotechnology (sc-8056)] was used at a 1:100 and 1:50 working dilution, respectively. Dual-labeled immunofluorescence analysis was performed essentially as earlier described (Xiao et al., 2011). In normal human Sertoli cells, ICAM-1 was mostly localized in the cell cytosol with relatively weak staining at the cell–cell interface but more c-Src was localized near the human Sertoli cell surface. However, following treatment of these human Sertoli cells with TNFα (10 ng/ml) for 1 day (1d), ICAM-1 was found to be redistributed and localized more to the human Sertoli cell–cell interface, co-localized with Src (‘white’ arrows indicate co-localized ICAM-1 and c-Src at the human Sertoli cell–cell interface). Cell nuclei (blue) were stained with DAPI (4′,6-diamidino-2-phenylindole). Bar = 100 μm, which applies to all other micrographs.
Besides the above-stated pathways for TEM, signaling pathways involving cytosolic Ca2+ flux [Etienne-Manneville et al., 2000), endothelial nitric oxide synthase (Kevil et al., 2004; Martinelli et al., 2009), other protein kinases (e.g. FAK and different protein kinase C isoforms (PKCs)] (Etienne et al., 1998; Rodriguez-Fernandez et al., 1999; Strell et al., 2010; Sumagin et al., 2011), small GTPases, including Rho/Rho associated protein kinase/Rac (Adamson et al., 1999; Barreiro et al., 2002; Thompson et al., 2002; Smith et al., 2003; van Buul et al., 2007), and other actin-binding proteins (e.g. filamins) (van Rijssel et al., 2012; Su et al., 2012) may also actively take part in this process. Furthermore, it was shown that PECAM-1 might block the ICAM-1-stimulated Src activation and cytoskeletal rearrangement via the tyrosine phosphatase SHP-2 in order to ‘re-seal’ the endothelial gaps following the passage of migrating leukocytes at the site (Wang et al., 2003; Couty et al., 2007), illustrating that the ICAM-Src interaction is crucial in regulating junction permeability.
Interestingly, there are two reports revealing that ICAM-1 might co-operate with caveolin-1 and/or vimentin to facilitate leukocyte transcellular transmigration (Millan et al., 2006; Nieminen et al., 2006) via an ICAM-1-caveolea-mediated transcytotic pathway. For instance, ICAM-1 was shown to co-cluster with caveolin-1 in actin-rich ‘ring-like’ invaginations in the endothelium, surrounding the invading leukocyte pseudopod and these ultrastructures were found to move together as a complex towards the basal plasma membrane (Fig. 3). Other studies have reported that ICAM-1 might bind indirectly to caveolin-1 via filamin (Kanters et al., 2008), and a vimentin intermediate filament-based cup-like ultrastructure around a migrating leukocyte in the transcellular route was also shown (Nieminen et al., 2006) (Fig. 3). Collectively, these findings illustrate the complexity of leukocyte TEM and the indispensability of ICAM-1 in these junctional rearrangements (Fig. 3). While germ cells are not motile cells per se, during spermatogenesis as they progressively ‘relocate’ across the seminiferous epithelium and move back-and-forth between the basal and the adluminal compartment, these findings will serve as a guide for future functional studies.
Transepithelial migration of germ cells in the testis
In the seminiferous tubule of mammalian testis where spermatogenesis takes place, Sertoli cells extend from the basement membrane towards the tubule lumen, and together with germ cells at different stages of their development, creating the seminiferous epithelium (Weber et al., 1983; Russell and Peterson, 1985; Russell et al., 1989; Griswold, 1995; Cheng and Mruk, 2002; Mruk and Cheng, 2004b; Vogl et al., 2008) (Fig. 1). During the translocation of developing germ cells from the basal to the adluminal compartment in the rat testis, germ cell contacts with Sertoli cells are mediated via either the desmosome, the gap junction, or the apical ES (Mruk and Cheng, 2004a; Yan et al., 2007; Wong et al., 2008). Specifically, spermatogonial stem cells, spermatogonia, spermatocytes and step 1–7 spermatids attach to Sertoli cells via desmosomes and communicate with each other via gap junctions (Lie et al., 2011; Mruk and Cheng, 2011), while the apical ES connects elongating/elongated spermatids (step 8–19 spermatids) to Sertoli cells (Russell and Peterson, 1985; Vogl et al., 2000; Toyama et al., 2003; Cheng and Mruk, 2010) (Fig. 1). Moreover, at Stage VIII of the seminiferous epithelial cycle, preleptotene spermatocytes residing in the basal compartment must traverse the BTB to the adluminal compartment while transforming to leptotene and then zygote spermatocytes prepare for meiosis (Russell, 1977). Yet the BTB is one of the tightest blood–tissue barriers in the mammalian body, constructed by apposed plasma membranes of adjacent Sertoli cells near the basement membrane (Fawcett et al., 1970; Fawcett, 1975; Setchell and Waites, 1975; Pelletier, 2011; Franca et al., 2012). It partitions the seminiferous epithelium into a basal and an adluminal compartment (Dym and Fawcett, 1970; Setchell, 1980; Pelletier, 2001, 2011; Mital et al., 2011; Cheng and Mruk, 2012; Franca et al., 2012). The BTB comprises multiple junction types, namely TJs, basal ESs, gap junctions (GJs) and desmosomes (Russell and Peterson, 1985; Vogl et al., 2008; Cheng and Mruk, 2012). Importantly, a unique ‘intermediate compartment’ mechanism involving massive cytoskeletal changes and endocytic vesicle-mediated protein endocytosis/recycling has been suggested, wherein the ‘old’ BTB above spermatocytes is disassembled immediately following the assembly of a ‘new’ BTB below, to facilitate the transmigration of preleptotene/leptotene spermatocytes at Stage VIII (Cheng and Mruk, 2012; Russell, 1977, 1978; Yazama, 2008) (Fig. 1). At the same time, spermiation happens near the tubule lumen, with the breakdown of apical ES to release the mature spermatozoa (Russell and Clermont, 1976; O'Donnell et al., 2011). It has been shown that complex adhesive interactions and extensive signaling cascades occur at the Sertoli–Sertoli and Sertoli–germ cell interface; (Russell and Clermont, 1976; Cheng and Mruk, 2012) these are reminiscent of the leukocyte TEM.
CAMs, such as N-cadherin at the BTB, and β1-integrin at the apical ES and hemidesmosome also take part in regulating junction turnover during spermatogenesis. They are essential constituents of the apical ES–BTB–hemidesmosome axis that co-ordinates the BTB restructuring and spermiation—the two cellular events taking place at the opposite ends of the Sertoli cell epithelium (Cheng and Mruk, 2010, 2012). Interestingly, ICAM-1 present on the surface of cultured mouse Sertoli cells was shown to be inducible by inflammatory mediators, such as TNFα, IL-1 and IFN-γ (Riccioli et al., 1995), and there are some novel findings in the field which characterized the role of ICAM family members at the cell junctions in the testis (Xiao et al., 2012a, b). In the following sections, we primarily focus on the role of ICAM-1 and -2 in the rat testis, which have been shown to regulate the dynamics of the BTB and the apical ES, respectively.
ICAM-1 in the testis: a multifunctional molecule in spermatogenesis
ICAM-1 was first shown to be expressed by mouse Sertoli cells cultured in vitro, residing on the Sertoli cell surface and it was up-regulated by treatment of these cells with IL-1, TNFα, lipopolysaccharide or interferon-γ; it was postulated that the interactions of ICAM-1 and the pro-inflammatory cytokines were involved in autoimmune pathologies and other immunological responses in the testis (Riccioli et al., 1995). Subsequent studies have shown that the TNFα-induced ICAM-1 expression is mediated via the JNK MAPK signaling pathway (De Cesaris et al., 1999). In the rat testis, ICAM-1, similar to the mouse testis (Riccioli et al., 1995), is expressed by Sertoli cells; however, ICAM-1 is also expressed by germ cells, and its expression in the seminiferous epithelium is stage specific (Xiao et al., 2012a). ICAM-1 is highly expressed in the basal region of the seminiferous epithelium including the BTB at Stages VIII–IX of the epithelial cycle (Xiao et al., 2012a), coinciding with the time of BTB restructuring. Using immunohistochemistry and a specific anti-ICAM-1 antibody, ICAM-1 has been shown to associate with most types of germ cells but it is most robust with elongating spermatids at Stages IX–XIII at the apical ES (Xiao et al., 2012a). More strikingly, by using co-immunoprecipitation and confocal microscopy, ICAM-1 has been found to partially co-localize with the TJ-protein occludin and a basal ES-protein N-cadherin (Xiao et al., 2012a). Consistent with its role as an integral membrane protein and a constituent component of the BTB, the overexpression of ICAM-1 in Sertoli cells exhibits a strengthening effect on the Sertoli cell barrier function. Figure 4 depicts the distribution of ICAM-1 in human Sertoli cells. Interestingly, the treatment of human Sertoli cells with TNFα was found to induce the re-distribution of ICAM-1 in these cells with ICAM-1 mostly found at the cell–cell interface and co-localized with c-Src (Fig. 4), suggesting c-Src may be used to alter the phosphorylation status of ICAM-1 at the Sertoli cell BTB, modulating its cell adhesive (or other) function at the site.
In our study, a soluble form of ICAM-1 that encompasses all five Ig-like domains was identified in the normal rat testis and enriched in germ cells (Xiao et al., 2012a). Subsequent analysis to overexpress this sICAM-1 in Sertoli cells in vitro was found to elicit an adverse effect on the Sertoli cell barrier (Xiao et al., 2012a). In short, sICAM-1 was found to down-regulate the expression of a number of BTB constituent proteins, such as N-cadherin, γ-catenin (also known as plakoglobin), as well as a GJ protein connexin 43 at the Sertoli cell BTB in vitro which mimicked the BTB in vivo (Xiao et al., 2012a), likely via a c-Src- and Pyk2-mediated pathway. In line with these observations, when the sICAM-1 construct was overexpressed in the rat testis in vivo, a down-regulation on the expression of N-cadherin and connexin 43 at the BTB was detected, concomitant with a disruption of the BTB function when the barrier integrity was assessed by a functional in vivo assay (Xiao et al., 2012a), analogous to findings in other epithelia/endothelia as discussed above. The F-actin network at the BTB was also severely damaged. Surprisingly, a loss of spermatocytes and round spermatids from the seminiferous epithelium was also observed (Xiao et al., 2012a), illustrating a primary loss of BTB function can lead to a secondary loss of adhesion function at the Sertoli–germ cell interface. It is noted that desmosomes at the site are the likely target of sICAM-1, since spermatocytes and round spermatids are anchored to Sertoli cells in the seminiferous epithelium via desmosomes. However, an evident upsurge in the expression of a desmosome protein desmoglein-2 was demonstrated by immunohistochemistry, despite significant destruction of the adhesion protein complexes between Sertoli and early germ cells, which may represent a physiological response in the testis to prevent further sICMA-1-induced germ cell loss. γ-Catenin, the only known common adaptor to both AJ (i.e. apical and basal ES in the testis) and desmosomes (Lewis et al., 1997), was found to be down-regulated at the Sertoli cell BTB site in vitro following the overexpression of sICAM-1 when examined by immunoblotting and immunofluorescence analysis (Xiao et al., 2012a). It was shown that in epithelial cells c-Src-induced tyrosine phosphorylation of γ-catenin would impede the association of γ-catenin with the AJ but the reverse was shown for desmosomes (Miravet et al., 2003). Therefore, it is important to assess the changes in the γ-catenin expression throughout the seminiferous epithelium, in particular at the sites of desmosomes and apical ESs. It is possible that c-Src-induced γ-catenin phosphorylation results in its dissociation with N-cadherin at the BTB, but a tighter association with the desmosomes at the Sertoli–germ cell interface.
During leukocyte paracellular TEM, the transient VE-cadherin-mediated gaps that form in the endothelial barrier to facilitate leukocyte migration in response to ICAM-1 clustering was shown to be resealed rather quickly, within 5 min (Shaw et al., 2001). Our findings by overexpressing sICAM-1 in the system that led to a severe BTB damage, however, suggest a mechanism that involves the degradation of several BTB components (e.g. the N-cadherin-γ-catenin protein complex) that takes hours before changes in the phenotypes were detected (Xiao et al., 2012a). Interestingly, presenilin-1, an intramembrane-cleaving protease involved in the degradation of the cadherin–catenin protein complex, was shown to be critical to the turnover of ICAM-5 in neurons via an autophagic degradative pathway (Kang et al., 2002; Marambaud et al., 2002; Esselens et al., 2004). In mouse testis, presenilin-1 is expressed by both spermatogonia and Sertoli cells (Dirami et al., 2001). Therefore, it remains to be examined if tailless ICAM-1 is working in concert with a similar intramembranous protein degradation pathway.
As noted above, there are emerging functional roles ascribed to ICAM-1 based on the studies on leukocyte TEM, some of which may be applicable to the transit of germ cells across the seminiferous epithelium during spermatogenesis. For instance, in addition to providing cell adhesive function in the seminiferous epithelium, ICAM-1 can modulate endocytic vesicle-mediated protein trafficking apparatus and cell signaling pathways involving c-Src and Pyk2 that act on the reorganization of actin cytoskeleton and Ca2+ inflow, which in turn affects the localization and/or distribution of TJ, basal ES, GJ and desmosome proteins. Furthermore, studies are needed to examine if macroclustering of ICAM-1 occurs in Sertoli cells, such as during the transit of preleptotene spermatocytes at the BTB, or if Sertoli–germ cell interactions would induce cleavage of mICAM-1 on the Sertoli or germ cell surface to generate sICAM-1 by MMPs, which could be used to mediate BTB ‘opening’ above the transiting preleptotene spermatocytes which are connected in clones via intercellular bridges (Fawcett, 1961). As depicted in Fig. 5, it is likely that during the transit of preleptotene spermatocytes at the BTB, ICAM-1 recruits TJ [e.g. occludin, claudins, zonula occludens-1 (ZO-1)], basal ES (e.g, N-cadherin, β-catenin, nectin-2, afadin) and GJ (e.g. connexin-43) proteins to assemble ‘new’ BTB at the basal region of the transiting spermatocytes, whereas sICAM-1 is being used to ‘disassemble’ the ‘old’ BTB at the apical region of the spermatocytes. It is also possible that ICAM-1 can recruit c-Src or Pyk2 to the site to confer proper phosphorylation of integral membrane proteins at the BTB (e.g. occludin, claudins). These kinases, in turn, can also regulate endocytic vesicle-mediated protein trafficking at the BTB (Cheng and Mruk, 2012), such as by inducing endocytosis, transcytosis and/or recycling of proteins at the BTB, so that integral membrane proteins can be recycled from the ‘old’ to the ‘new’ BTB site for junction assembly. In short, utilizing the antagonistic effects of ICAM-1 and sICAM-1 as demonstrated in in vitro and in vivo studies regarding their effects on the Sertoli cell TJ-permeability barrier function, the immunological barrier can be maintained during the transit of preleptotene spermatocytes at the BTB (Fig. 5).
Figure 5.
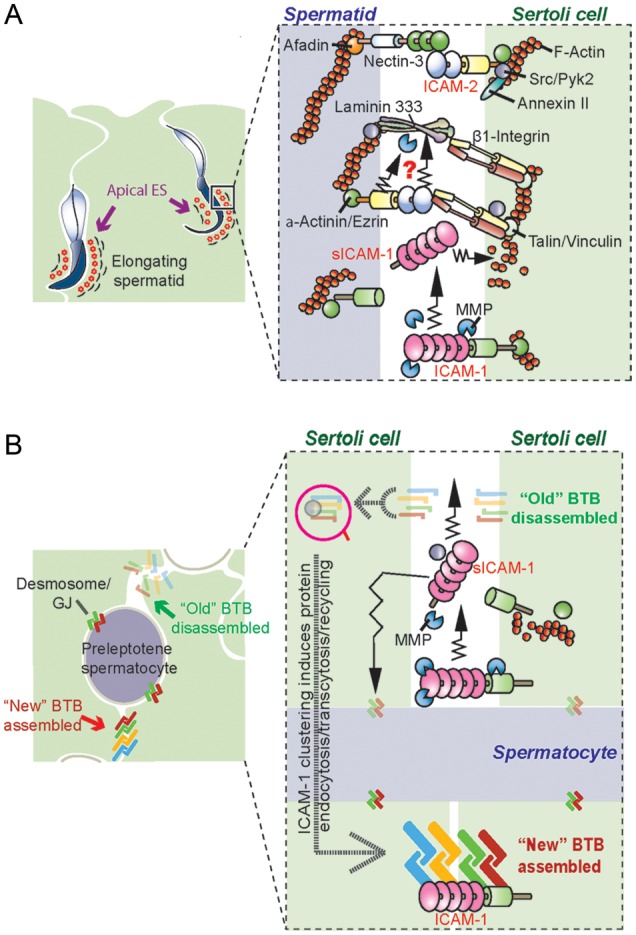
A schematic drawing illustrating a hypothetical model by which ICAM-1, sICAM-1 and ICAM-2 regulate the restructuring of the BTB to accommodate the transit of preleptotene spermatocytes across the BTB, and apical ES restructuring to facilitate spermatid movement across the seminiferous epithelium. In (A), sICAM-1 generated from ICAM-1 via cleavage by MMPs can modulate spermatid adhesion at the apical ES via its effects on F-actin organization. ICAM-2 can also form stable adhesion complex with nectin-3/afadin; however, ICAM-2 can also be involved in the degradation of the laminin-333 chains, generating the biologically active laminin fragments to induce apical ES degeneration at spermiation or to induce apical ES restructuring to facilitate spermatid transit across the seminiferous epithelium during spermiogenesis. In (B), cleavage of ICAM-1 by MMPs that generates sICAM-1 can facilitate the disruption of the ‘old’ BTB above the preleptotene spermatocytes in transit at the BTB, whereas the endocytosed adhesion protein complexes can be transcytosed and recycled to assemble ‘new’ BTB behind the transiting preleptotene spermatocytes, so that the integrity of the immunological barrier can be maintained. This model thus depicts the crucial physiological role of ICAM-1, sICAM-1 and ICAM-2 at the apical ES (A) and BTB (B) during the epithelial cycle of spermatogenesis.
ICAM-2: a functional molecule at the Sertoli–germ cell interface
In the rat testis, ICAM-2, analogous to ICAM-1, is expressed by the Sertoli and germ cells. However, its localization in the seminiferous epithelium is restricted to the Sertoli–germ cell interface, and is not found at the Sertoli cell–cell interface at the BTB (Xiao et al., 2012b). Subsequent analysis showed that ICAM-2 is an integrated component of the apical ES (Xiao et al., 2012b). Its expression at the apical ES displays highly restrictive spatiotemporal patterns during the epithelial cycle, which shifts from its diffuse localization surrounding the concave and convex sides of spermatid heads in early and late stages of the seminiferous epithelial cycle to the convex side of spermatid heads prior to spermiation (Xiao et al., 2012b). Similar to other epithelial cells, ICAM-2 associates with the actin cytoskeleton in the rat seminiferous epithelium. Surprisingly, it also physically interacts with nectin-3 (an IgSF CAM present in the testis, see Tables I and II), annexin II and c-Src (Xiao et al., 2012b). Annexin II is a Ca2+-dependent phospholipid-binding protein and a regulator of actin polymerization, and also a substrate for c-Src (Hayes et al., 2006; Hayes and Moss, 2009). Interestingly, during CdCl2-induced germ cell sloughing from the seminiferous epithelium and BTB disruption (Setchell and Waites, 1970; Wong et al., 2004; Siu et al., 2009; Elkin et al., 2010), the protein levels of ICAM-2, annexin II and c-Src were all up-regulated after ∼6–16 h of CdCl2 treatment, prior to extensive changes in the phenotypes in the seminiferous epithelium, such as germ cell exfoliation and BTB disruption (Xiao et al., 2012b), illustrating ICAM-2, together with annexin II and c-Src, may mediate cadmium-induced testis injury.
ICAM-2 is known to be constitutively expressed in endothelial and epithelial cells (Table II), and its deficiency causes reduced leukocyte TEM (Gerwin et al., 1999; Huang et al., 2006; Porter and Hall, 2009). Studies have proposed that ICAM-2 may serve as a ‘guide’ for leukocyte recognition of the endothelial TJ barrier before TEM (Porter and Hall, 2009; Woodfin et al., 2009). Thus, ICAM-2 may also function to direct the spermatid passage through the seminiferous epithelium via its interaction with nectin-3 and/or β1-intergrin (Xiao et al., 2012b).
It was shown that the lack of ICAM-2 in epithelia appeared to elicit cell apoptosis (Huang et al., 2005). In mammalian testes, ∼75% of germ cells undergo apoptosis during spermatogenesis to maintain the physiological homeostasis of the seminiferous epithelium (Billig et al., 1995; Shaha et al., 2010). Treatment of rats with CdCl2 is known to induce germ cell apoptosis, degeneration of the apical ES, leading to irreversible infertility. Current studies have indicated that CdCl2 may initially expedite branched actin polymerization activity, causing disruption of spermatid adhesion to Sertoli cells at the apical ES (Cheng et al., 2011b). During CdCl2 treatment, ICAM-2 was induced and associated less tightly with actin (Xiao et al., 2012b). Recent studies have shown that ICAM-2 may function as an actin stabilizer since it was associated with F-actin until just prior to spermiation (Xiao et al., 2012b). Indeed, ICAM-2 expression in tumor cells has been shown to suppress metastasis by establishing an ICAM-2/α-actinin-actin linkage (Yoon et al., 2008). Moreover, annexin II, an inhibitor of actin polymerization (Hayes et al., 2006; Hayes and Moss, 2009), was found to form a protein complex with ICAM-2 and c-Src (Xiao et al., 2012b). These three proteins in the rat testis were observed to be synchronously induced after CdCl2 treatment. It has been reported that c-Src phosphorylates adaptor protein(s) connected to ICAM-2, whose clustering would send out a survival signal that is sufficient to block TNFα-mediated apoptosis (Perez et al., 2002). Thus, it is logical to envision that activated c-Src may be recruited to the activated ICAM-2/annexin II complex at the early stage of CdCl2-induced apical ES damage in order to protect spermatids from being depleted from the epithelium.
Figure 5 also depicts the likely involvement of ICAM-2, an apical ES protein in the rat testis, in coordinating the release of sperm at spermiation during Stage VIII of the epithelial cycle. It is likely that ICAM-2, surrounding the entire head of the elongating spermatid at Stage X–V of the epithelial cycle, and via its association with nectin-3, afadin, β1-integrin, c-Src, Pyk2 and annexin II, are being used to stabilize the F-actin bundles at the apical ES (Fig. 5). Also, ICAM-1, appearing to be an apical ES protein as well, can also stabilize the apical ES integrity. However, the localization of ICAM-2 begins to ‘shift’ spatially to the convex side of the elongating spermatids at late Stages V–VIII, perhaps being used to facilitate re-organization of the underlying actin-based cytoskeleton to prepare for spermiation (Fig. 5). In short, ICAM-2 and ICAM-1/sICAM-1 likely serve as “molecular switches” to co-ordinate the events of spermiation and BTB restructuring at Stage VIII of the epithelial cycle as depicted in the hypothetical model shown in Fig. 5.
Concluding remarks
Herein, we have briefly reviewed the role of ICAMs in epithelial/endothelial physiology in particular ICAM-1/sICAM-1 and ICAM-2 in cell adhesion and cell movement. They likely serve as molecular ‘switches’, triggering changes in the organization of F-actin in Sertoli cells in the seminiferous epithelium, relating outside signals into the cell to elicit appropriate responses to stimuli, such as at different stages of the epithelial cycle, or changes in the environment via their effects on cell cytoskeleton, eliciting changes in cell adhesive function. Based on recent findings in the field regarding the role of ICAMs in leukocyte TEM and their effects on the Sertoli cell TJ-barrier function, a hypothetic model regarding the involvement of ICAM-1/sICAM-1 and ICAM-2 in regulating spermiation and BTB restructuring during the epithelial cycle, such as Stage VIII, is proposed. It is understood that this model (Fig. 5) will be updated when more data become available in the field. Nonetheless, this model will be useful to investigators to design functional experiments to understand the role of ICAMs in the apical ES–BTB–hemidesmosome functional axis during spermatogenesis.
Authors' roles
C.Y.C. conceived the ideas of preparing this review article; X.X. and C.Y.C. performed the literature search, research on the topic and critically evaluated findings in the field; X.X., D.D.M. and C.Y.C. critically discussed and evaluated the latest research on different aspects of ICAMs and testis function; D.D.M. and C.Y.C. provided the first draft of the hypothetical model; X.X. and C.Y.C. prepared the figures; X.X. and C.Y.C. co-wrote the paper and C.Y.C. performed the final editing of the manuscript.
Funding
This work was supported by grants from the National Institutes of Health (NICHD, R01 HD056034 to C.Y.C. and U54 HD029990, Project 5 to C.Y.C.).
Conflict of interest
None declared.
Acknowledgements
We are grateful to former and current members of our laboratory, in particular Drs. Elissa W.P. Wong and Pearl P.Y. Lie, who have contributed a lot of helpful comments and discussion during the preparation and writing of this review article in the past 4 years. We also wish to thank Dr Constance M. John at MandalMed, Inc. (San Francisco, CA) who provided us with some human Sertoli cells for our studies.
References
- Adamson P, Etienne S, Couraud PO, Calder V, Greenwood J. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a rho-dependent pathway. J Immunol. 1999;162:2964–2973. [PubMed] [Google Scholar]
- Alcaide P, Martinelli R, Newton G, Williams MR, Adam A, Vincent PA, Luscinskas FW. p120-Catenin prevents neutrophil transmigration independently of RhoA inhibition by impairing Src dependent VE-cadherin phosphorylation. Am J Physiol Cell Physiol. 2012;303:C385–C395. doi: 10.1152/ajpcell.00126.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179:4053–4064. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- Allport JR, Muller WA, Luscinskas FW. Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow. J Cell Biol. 2000;148:203–216. doi: 10.1083/jcb.148.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann RP. The cycle of the seminiferous epithelium in humans: a need to revisit? J Androl. 2008;29:469–487. doi: 10.2164/jandrol.107.004655. [DOI] [PubMed] [Google Scholar]
- Amos C, Romero IA, Schultze C, Rousell J, Pearson JD, Greenwood J, Adamson P. Cross-linking of brain endothelial intercellular adhesion molecule (ICAM)-1 induces association of ICAM-1 with detergent-insoluble cytoskeletal fraction. Arterioscler Thromb Vasc Biol. 2001;21:810–816. doi: 10.1161/01.atv.21.5.810. [DOI] [PubMed] [Google Scholar]
- Annaert WG, Esselens C, Baert V, Boeve C, Snellings G, Cupers P, Craessaerts K, De Strooper B. Interaction with telencephalin and the amyloid precursor protein predicts a ring structure for presenilins. Neuron. 2001;32:579–589. doi: 10.1016/s0896-6273(01)00512-8. [DOI] [PubMed] [Google Scholar]
- Aricescu AR, Jones EY. Immunoglobulin superfamily cell adhesion molecules: zippers and signals. Curr Opin Cell Biol. 2007;19:543–550. doi: 10.1016/j.ceb.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol. 2002;157:1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro O, Zamai M, Yanez-Mo M, Tejera E, Lopez-Romero P, Monk PN, Gratton E, Caiolfa VR, Sanchez-Madrid F. Endothelial adhesion receptors are recruited to adherent leukocytes by inclusion in preformed tetraspanin nanoplatforms. J Cell Biol. 2008;183:527–542. doi: 10.1083/jcb.200805076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berney SM, Schaan T, Alexander JS, Peterman G, Hoffman PA, Wolf RE, van der Heyde H, Atkinson TP. ICAM-3 (CD50) cross-linking augments signaling in CD3-activated peripheral human T lymphocytes. J Leukoc Biol. 1999;65:867–874. doi: 10.1002/jlb.65.6.867. [DOI] [PubMed] [Google Scholar]
- Billig H, Furuta I, Rivier C, Tapanainen J, Parvinen M, Hsueh AJ. Apoptosis in testis germ cells: developmental changes in gonadotropin dependence and localization to selective tubule stages. Endocrinology. 1995;136:5–12. doi: 10.1210/endo.136.1.7828558. [DOI] [PubMed] [Google Scholar]
- Bouchard MJ, Dong Y, McDermott BM, Jr, Lam DH, Brown KR, Shelanski M, Bellve AR, Racaniello VR. Defects in nuclear and cytoskeletal morphology and mitochondrial localization in spermatozoa of mice lacking nectin-2, a component of cell–cell adherens junctions. Mol Cell Biol. 2000;20:2865–2873. doi: 10.1128/mcb.20.8.2865-2873.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummendorf T, Lemmon V. Immunoglobulin superfamily receptors: cis-interactions, intracellular adapters and alternative splicing regulate adhesion. Curr Opin Cell Biol. 2001;13:611–618. doi: 10.1016/s0955-0674(00)00259-3. [DOI] [PubMed] [Google Scholar]
- Byers S, Jegou B, MacCalman C, Blaschuk O. Sertoli cell adhesion molecules and the collective organization of the testis. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater: Cache River Press; 1993a. pp. 461–476. [Google Scholar]
- Byers S, Pelletier RM, Suarez-Quain C. Sertoli cell junctions and the seminiferous epithelium barrier. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater: Cache River Press; 1993b. pp. 431–446. [Google Scholar]
- Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman CV, Jun CD, Salas A, Springer TA. Endothelial cells proactively form microvilli-like membrane projections upon intercellular adhesion molecule 1 engagement of leukocyte LFA-1. J Immunol. 2003;171:6135–6144. doi: 10.4049/jimmunol.171.11.6135. [DOI] [PubMed] [Google Scholar]
- Cavallaro U, Dejana E. Adhesion molecule signalling: not always a sticky business. Nat Rev Mol Cell Biol. 2011;12:189–197. doi: 10.1038/nrm3068. [DOI] [PubMed] [Google Scholar]
- Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- Chen H, Fok KL, Yu S, Jiang J, Chen Z, Gui Y, Cai Z, Chan HC. CD147 is required for matrix metalloproteinases-2 production and germ cell migration during spermatogenesis. Mol Hum Reprod. 2011;17:405–414. doi: 10.1093/molehr/gar013. [DOI] [PubMed] [Google Scholar]
- Chen L, Bi J, Nakai M, Bunick D, Couse JF, Korach KS, Nowak RA. Expression of basigin in reproductive tissues of estrogen receptor-α or -β null mice. Reprod. 2010;139:1057–1066. doi: 10.1530/REP-10-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli–germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol. 2010;6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. The blood–testis barrier and its implications for male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Lie PP, Mok KW, Cheng YH, Wong EW, Mannu J, Mathur PP, Yan HH, Mruk DD. Interactions of laminin beta3 fragment with beta1-integrin receptor: a revisit of the apical ectoplasmic specialization-blood–testis-barrier-hemidesmosome functional axis in the testis. Spermatogenesis. 2011a;1:174–185. doi: 10.4161/spmg.1.3.17076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Wong EW, Lie PP, Li MW, Su L, Siu ER, Yan HH, Mannu J, Mathur PP, Bonanomi M, et al. Environmental toxicants and male reproductive function. Spermatogenesis. 2011b;1:2–13. doi: 10.4161/spmg.1.1.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui K, Trivedi A, Cheng CY, Cherbavaz DB, Dazin PF, Huynh ALT, Mitchell JB, Rabinovich GA, Noble-Haeusslein LJ, John CM. Characterization and functionality of proliferative human Sertoli cells. Cell Transplant. 2011;20:619–635. doi: 10.3727/096368910X536563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PR, Manes TD, Pober JS, Kluger MS. Increased ICAM-1 expression causes endothelial cell leakiness, cytoskeletal reorganization and junctional alterations. J Invest Dermatol. 2007;127:762–774. doi: 10.1038/sj.jid.5700670. [DOI] [PubMed] [Google Scholar]
- Clermont Y. The cycle of the seminiferous epithelium in man. Am J Anat. 1963;112:35–51. doi: 10.1002/aja.1001120103. [DOI] [PubMed] [Google Scholar]
- Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–235. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- Conant K, Lonskaya I, Szklarczyk A, Krall C, Steiner J, Maguire-Zeiss K, Lim ST. Methamphetamine-associated cleavage of the synaptic adhesion molecule intercellular adhesion molecule-5. J Neurochem. 2011;118:521–532. doi: 10.1111/j.1471-4159.2010.07153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini C, Calzetti F, Perbellini O, Micheletti A, Scarponi C, Lonardi S, Pelletier M, Schakel K, Pizzolo G, Facchetti F, et al. Human neutrophils interact with both 6-sulfo LacNAc+ DC and NK cells to amplify NK-derived IFN{gamma}: role of CD18, ICAM-1, and ICAM-3. Blood. 2011;117:1677–1686. doi: 10.1182/blood-2010-06-287243. [DOI] [PubMed] [Google Scholar]
- Couty JP, Rampon C, Leveque M, Laran-Chich MP, Bourdoulous S, Greenwood J, Couraud PO. PECAM-1 engagement counteracts ICAM-1-induced signaling in brain vascular endothelial cells. J Neurochem. 2007;103:793–801. doi: 10.1111/j.1471-4159.2007.04782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff S, et al. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994;367:455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- Cserti-Gazdewich CM, Dzik WH, Erdman L, Ssewanyana I, Dhabangi A, Musoke C, Kain KC. Combined measurement of soluble and cellular ICAM-1 among children with Plasmodium falciparum malaria in Uganda. Malar J. 2010;9:233. doi: 10.1186/1475-2875-9-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr DG. Connexins and pannexins: coordinating cellular communication in the testis and epididymis. Spermatogenesis. 2011;1:325–338. doi: 10.4161/spmg.1.4.18948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cesaris P, Starace D, Riccioli A, Padula F, Filippini A, Ziparo E. Tumor necrosis factor-α induces interleukin-6 production and integrin ligand expression by distinct transduction pathways. J Biol Chem. 1998;273:7566–7571. doi: 10.1074/jbc.273.13.7566. [DOI] [PubMed] [Google Scholar]
- De Cesaris P, Starace D, Starace G, Filippini A, Stefanini M, Ziparo E. Activation of Jun N-terminal kinase/stress-activated protein kinase pathway by tumor necrosis factor α leads to intercellular adhesion molecule-1 expression. J Biol Chem. 1999;274:28978–28982. doi: 10.1074/jbc.274.41.28978. [DOI] [PubMed] [Google Scholar]
- de Kretser DM, Kerr JB. The cytology of the testis. In: Knobil E, Neill JB, Ewing LL, Greenwald GS, Markert CL, Pfaff DW, editors. The Physiology of Reproduction. New York: Raven Press; 1988. pp. 837–932. Vol. 1. [Google Scholar]
- Dermody TS, Kirchner E, Guglielmi KM, Stehle T. Immunoglobulin superfamily virus receptors and the evolution of adaptive immunity. PLoS Pathog. 2009;5:e1000481. doi: 10.1371/journal.ppat.1000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo A, Manes TD, Davalos A, Wright PL, Sessa WC. Endothelial reticulon-4B (Nogo-B) regulates ICAM-1-mediated leukocyte transmigration and acute inflammation. Blood. 2011;117:2284–2295. doi: 10.1182/blood-2010-04-281956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirami G, Ravindranath N, Achi MV, Dym M. Expression of Notch pathway components in spermatogonia and Sertoli cells of neonatal mice. J Androl. 2001;22:944–952. doi: 10.1002/j.1939-4640.2001.tb03434.x. [DOI] [PubMed] [Google Scholar]
- Doulet N, Donnadieu E, Laran-Chich MP, Niedergang F, Nassif X, Couraud PO, Bourdoulous S. Neisseria meningitidis infection of human endothelial cells interferes with leukocyte transmigration by preventing the formation of endothelial docking structures. J Cell Biol. 2006;173:627–637. doi: 10.1083/jcb.200507128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube E, Cyr DG. The blood–epididymis barrier and human male fertility. Adv Exp Med Biol. 2012;763:218–236. doi: 10.1007/978-1-4614-4711-5_11. [DOI] [PubMed] [Google Scholar]
- Dym M, Cavicchia J. Junctional morphology of the testis. Biol Reprod. 1978;18:1–15. doi: 10.1095/biolreprod18.1.1. [DOI] [PubMed] [Google Scholar]
- Dym M, Fawcett DW. The blood–testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970;3:308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- Elkin N, Piner J, Sharpe R. Toxicant-induced leakage of germ cell–specific proteins from seminiferous tubules in the rat: relatinship to blood–testis barrier integrity and prospects for biomonitoring. Toxicol Sci. 2010;117:439–448. doi: 10.1093/toxsci/kfq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esselens C, Oorschot V, Baert V, Raemaekers T, Spittaels K, Serneels L, Zheng H, Saftig P, De Strooper B, Klumperman J, et al. Presenilin 1 mediates the turnover of telencephalin in hippocampal neurons via an autophagic degradative pathway. J Cell Biol. 2004;166:1041–1054. doi: 10.1083/jcb.200406060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estecha A, Aguilera-Montilla N, Sanchez-Mateos P, Puig-Kroger A. RUNX3 regulates intercellular adhesion molecule 3 (ICAM-3) expression during macrophage differentiation and monocyte extravasation. PLoS ONE. 2012;7:e33313. doi: 10.1371/journal.pone.0033313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Manneville JB, Adamson P, Wilbourn B, Greenwood J, Couraud PO. ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J Immunol. 2000;165:3375–3383. doi: 10.4049/jimmunol.165.6.3375. [DOI] [PubMed] [Google Scholar]
- Etienne S, Adamson P, Greenwood J, Strosberg AD, Cazaubon S, Couraud PO. ICAM-1 signaling pathways associated with Rho activation in microvascular brain endothelial cells. J Immunol. 1998;161:5755–5761. [PubMed] [Google Scholar]
- Fawcett D. Ultrastructure and function of the Sertoli cell. In: Hamilton D, Greep R, editors. Handbook of Physiology. Washington, DC: American Physiological Society; 1975. pp. 21–25. [Google Scholar]
- Fawcett DW. Intercellular bridges. Exp Cell Res. 1961;8:174–187. doi: 10.1016/0014-4827(61)90347-0. [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Leak LV, Heidger PM. Electron microscopic observations on the structural components of the blood–testis barrier. J Reprod Fertil. 1970;(Suppl. 10):105–122. [PubMed] [Google Scholar]
- Federici C, Camoin L, Hattab M, Strosberg AD, Couraud PO. Association of the cytoplasmic domain of intercellular-adhesion molecule-1 with glyceraldehyde-3-phosphate dehydrogenase and beta-tubulin. Eur J Biochem. 1996;238:173–180. doi: 10.1111/j.1432-1033.1996.0173q.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Borja M, van Buul JD, Hordijk PL. The regulation of leucocyte transendothelial migration by endothelial signalling events. Cardiovasc Res. 2010;86:202–210. doi: 10.1093/cvr/cvq003. [DOI] [PubMed] [Google Scholar]
- Fiore E, Fusco C, Romero P, Stamenkovic I. Matrix metalloproteinase 9 (MMP-9/gelatinase B) proteolytically cleaves ICAM-1 and participates in tumor cell resistance to natural killer cell-mediated cytotoxicity. Oncogene. 2002;21:5213–5223. doi: 10.1038/sj.onc.1205684. [DOI] [PubMed] [Google Scholar]
- Franca LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Blood–tissue barriers: morphofunctional and immunological aspects of the blood–testis and blood–epididymal barriers. Adv Exp Med Biol. 2012;763:237–259. [PubMed] [Google Scholar]
- Fujita E, Kouroku Y, Ozeki S, Tanabe Y, Toyama Y, Maekawa M, Kojima N, Senoo H, Toshimori K, Momoi T. Oligo-astheno-teratozoospermia in mice lacking RA175/TSLC1/SynCAM/IGSF4A, a cell adhesion molecule in the immunoglobulin superfamily. Mol Cell Biol. 2006;26:718–726. doi: 10.1128/MCB.26.2.718-726.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita E, Tanabe Y, Hirose T, Aurrand-Lions M, Kasahara T, Imhof BA, Ohno S, Momoi T. Loss of partitioning-defective-3/isotype-specific interacting protein (par-3/ASIP) in the elongating spermatid of RA175 (IGSF4A/SynCAM)-deficient mice. Am J Pathol. 2007;171:1800–1810. doi: 10.2353/ajpath.2007.070261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani Y, Matsuno H, Kawasaki M, Sasaki T, Mori K, Yoshihara Y. Interaction between telencephalin and ERM family proteins mediates dendritic filopodia formation. J Neurosci. 2007;27:8866–8876. doi: 10.1523/JNEUROSCI.1047-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg CG, Tian L, Ning L, Nyman-Huttunen H. ICAM-5—a novel two-facetted adhesion molecule in the mammalian brain. Immunol Lett. 2008;117:131–135. doi: 10.1016/j.imlet.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol. 2010;26:315–333. doi: 10.1146/annurev.cellbio.011209.122036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TB, Krooshoop DJ, Bleijs DA, van Vliet SJ, van Duijnhoven GC, Grabovsky V, Alon R, Figdor CG, van Kooyk Y. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat Immunol. 2000a;1:353–357. doi: 10.1038/79815. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000b;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Gerwin N, Gonzalo JA, Lloyd C, Coyle AJ, Reiss Y, Banu N, Wang B, Xu H, Avraham H, Engelhardt B, et al. Prolonged eosinophil accumulation in allergic lung interstitium of ICAM-2 deficient mice results in extended hyperresponsiveness. Immunity. 1999;10:9–19. doi: 10.1016/s1074-7613(00)80002-3. [DOI] [PubMed] [Google Scholar]
- Gibson NJ. Cell adhesion molecules in context: CAM function depends on the neighborhood. Cell Adh Migr. 2011;5:48–51. doi: 10.4161/cam.5.1.13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliki G, Ebnet K, Aurrand-Lions M, Imhof BA, Adams RH. Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature. 2004;431:320–324. doi: 10.1038/nature02877. [DOI] [PubMed] [Google Scholar]
- Greenwood J, Amos CL, Walters CE, Couraud PO, Lyck R, Engelhardt B, Adamson P. Intracellular domain of brain endothelial intercellular adhesion molecule-1 is essential for T lymphocyte-mediated signaling and migration. J Immunol. 2003;171:2099–2108. doi: 10.4049/jimmunol.171.4.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MD. Interactions between germ cells and Sertoli cells in the testis. Biol Reprod. 1995;52:211–216. doi: 10.1095/biolreprod52.2.211. [DOI] [PubMed] [Google Scholar]
- Gurtner GC, Davis V, Li H, McCoy MJ, Sharpe A, Cybulsky MI. Targeted disruption of the murine VCAM1 gene: essential role of VCAM-1 in chorioallantoic fusion and placentation. Genes Dev. 1995;9:1–14. doi: 10.1101/gad.9.1.1. [DOI] [PubMed] [Google Scholar]
- Hadad N, Tuval L, Elgazar-Carmom V, Levy R. Endothelial ICAM-1 protein induction is regulated by cytosolic phospholipase A2alpha via both NF-kappaB and CREB transcription factors. J Immunol. 2011;186:1816–1827. doi: 10.4049/jimmunol.1000193. [DOI] [PubMed] [Google Scholar]
- Harris ES, McIntyre TM, Prescott SM, Zimmerman GA. The leukocyte integrins. J Biol Chem. 2000;275:23409–23412. doi: 10.1074/jbc.R000004200. [DOI] [PubMed] [Google Scholar]
- Hayes MJ, Moss SE. Annexin 2 has a dual role as regulator and effector of v-Src in cell transformation. J Biol Chem. 2009;284:10202–10210. doi: 10.1074/jbc.M807043200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MJ, Shao D, Bailly M, Moss SE. Regulation of actin dynamics by annexin 2. EMBO J. 2006;25:1816–1826. doi: 10.1038/sj.emboj.7601078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick JS, Kilgannon P, Gallatin WM. The intercellular adhesion molecule (ICAM) family of proteins. New members and novel functions. Immunol Res. 1998;17:313–327. doi: 10.1007/BF02786454. [DOI] [PubMed] [Google Scholar]
- Hermand P, Gane P, Huet M, Jallu V, Kaplan C, Sonneborn HH, Cartron JP, Bailly P. Red cell ICAM-4 is a novel ligand for platelet-activated alpha IIbbeta 3 integrin. J Biol Chem. 2003;278:4892–4898. doi: 10.1074/jbc.M211282200. [DOI] [PubMed] [Google Scholar]
- Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc Res Tech. 2010a;73:241–278. doi: 10.1002/jemt.20783. [DOI] [PubMed] [Google Scholar]
- Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 5: intercellular junctions and contacts between germ cells and Sertoli cells and their regulatory interactions, testicular cholesterol, and genes/proteins associated with more than one germ cell generation. Microsc Res Tech. 2010b;73:409–494. doi: 10.1002/jemt.20786. [DOI] [PubMed] [Google Scholar]
- Hess RA, de Franca LR. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- Huang MT, Mason JC, Birdsey GM, Amsellem V, Gerwin N, Haskard DO, Ridley AJ, Randi AM. Endothelial intercellular adhesion molecule (ICAM)-2 regulates angiogenesis. Blood. 2005;106:1636–1643. doi: 10.1182/blood-2004-12-4716. [DOI] [PubMed] [Google Scholar]
- Huang MT, Larbi KY, Scheiermann C, Woodfin A, Gerwin N, Haskard DO, Nourshargh S. ICAM-2 mediates neutrophil transmigration in vivo: evidence for stimulus specificity and a role in PECAM-1-independent transmigration. Blood. 2006;107:4721–4727. doi: 10.1182/blood-2005-11-4683. [DOI] [PubMed] [Google Scholar]
- Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med. 2000;28:1379–1386. doi: 10.1016/s0891-5849(00)00223-9. [DOI] [PubMed] [Google Scholar]
- Igakura T, Kadomatsu K, Kaname T, Muramatsu H, Fan QW, Miyauchi T, Toyama Y, Kuno N, Yuasa S, Takahashi M, et al. A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis. Dev Biol. 1998;194:152–165. doi: 10.1006/dbio.1997.8819. [DOI] [PubMed] [Google Scholar]
- Ihanus E, Uotila LM, Toivanen A, Varis M, Gahmberg CG. Red-cell ICAM-4 is a ligand for the monocyte/macrophage integrin CD11c/CD18: characterization of the binding sites on ICAM-4. Blood. 2007;109:802–810. doi: 10.1182/blood-2006-04-014878. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Quertermous T. Molecular isolation and characterization of a soluble isoform of activated leukocyte cell adhesion molecule that modulates endothelial cell function. J Biol Chem. 2004;279:55315–55323. doi: 10.1074/jbc.M407776200. [DOI] [PubMed] [Google Scholar]
- Inagaki M, Irie K, Ishizaki H, Tanaka-Okamoto M, Miyoshi J, Takai Y. Role of cell adhesion molecule nectin-3 in spermatid development. Genes Cells. 2006;11:1125–1132. doi: 10.1111/j.1365-2443.2006.01006.x. [DOI] [PubMed] [Google Scholar]
- Ivanov AI. Structure and regulation of intestinal epithelial tight junctions. Adv Exp Med Biol. 2011;763:132–148. doi: 10.1007/978-1-4614-4711-5_6. [DOI] [PubMed] [Google Scholar]
- Jean-Mairet RM, Lopez-Menendez C, Sanchez-Ruiloba L, Sacristan S, Rodriguez-Martinez M, Riol-Blanco L, Sanchez-Mateos P, Sanchez-Madrid F, Rodriguez-Fernandez JL, Campanero MR, et al. The neuronal protein Kidins220/ARMS associates with ICAM-3 and other uropod components and regulates T-cell motility. Eur J Immunol. 2011;41:1035–1046. doi: 10.1002/eji.201040513. [DOI] [PubMed] [Google Scholar]
- Juan M, Vinas O, Pino-Otin MR, Places L, Martinez-Caceres E, Barcelo JJ, Miralles A, Vilella R, de la Fuente MA, Vives J, et al. CD50 (intercellular adhesion molecule 3) stimulation induces calcium mobilization and tyrosine phosphorylation through p59fyn and p56lck in Jurkat T cell line. J Exp Med. 1994;179:1747–1756. doi: 10.1084/jem.179.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun CD, Carman CV, Redick SD, Shimaoka M, Erickson HP, Springer TA. Ultrastructure and function of dimeric, soluble intercellular adhesion molecule-1 (ICAM-1) J Biol Chem. 2001a;276:29019–29027. doi: 10.1074/jbc.M103394200. [DOI] [PubMed] [Google Scholar]
- Jun CD, Shimaoka M, Carman CV, Takagi J, Springer TA. Dimerization and the effectiveness of ICAM-1 in mediating LFA-1-dependent adhesion. Proc Natl Acad Sci USA. 2001b;98:6830–6835. doi: 10.1073/pnas.121186998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerer S, Roth RB, Reneland R, Marnellos G, Hoyal CR, Markward NJ, Ebner F, Kiechle M, Schwarz-Boeger U, Griffiths LR, et al. Large-scale association study identifies ICAM gene region as breast and prostate cancer susceptibility locus. Cancer Res. 2004;64:8906–8910. doi: 10.1158/0008-5472.CAN-04-1788. [DOI] [PubMed] [Google Scholar]
- Kang DE, Soriano S, Xia X, Eberhart CG, De Strooper B, Zheng H, Koo EH. Presenilin couples the paired phosphorylation of beta-catenin independent of axin: implications for beta-catenin activation in tumorigenesis. Cell. 2002;110:751–762. doi: 10.1016/s0092-8674(02)00970-4. [DOI] [PubMed] [Google Scholar]
- Kanters E, van Rijssel J, Hensbergen PJ, Hondius D, Mul FP, Deelder AM, Sonnenberg A, van Buul JD, Hordijk PL. Filamin B mediates ICAM-1-driven leukocyte transendothelial migration. J Biol Chem. 2008;283:31830–31839. doi: 10.1074/jbc.M804888200. [DOI] [PubMed] [Google Scholar]
- Kasprzak A, Surdacka A, Tomczak M, Konkol M. Role of high endothelial postcapillary venules and selected adhesion molecules in periodontal diseases: a review. J Periodontal Res. 2012 doi: 10.1111/j.1600-0765.2012.01492.x. (in press; oi:101111/j1600–0765201201492x) [DOI] [PubMed] [Google Scholar]
- Kevil CG, Orr AW, Langston W, Mickett K, Murphy-Ullrich J, Patel RP, Kucik DF, Bullard DC. Intercellular adhesion molecule-1 (ICAM-1) regulates endothelial cell motility through a nitric oxide-dependent pathway. J Biol Chem. 2004;279:19230–19238. doi: 10.1074/jbc.M312025200. [DOI] [PubMed] [Google Scholar]
- Kim E, Lee Y, Kim JS, Song BS, Kim SU, Huh JW, Lee SR, Kim SH, Hong Y, Chang KT. Extracellular domain of V-set and immunoglobulin domain containing 1 (VSIG1) interacts with sertoli cell membrane protein, while its PDZ-binding motif forms a complex with ZO-1. Mol Cells. 2010;30:443–448. doi: 10.1007/s10059-010-0138-4. [DOI] [PubMed] [Google Scholar]
- Kim K, Brown EE, Choi CB, Alarcon-Riquelme ME, Kelly JA, Glenn SB, Ojwang JO, Adler A, Lee HS, Boackle SA, et al. Variation in the ICAM1-ICAM4-ICAM5 locus is associated with systemic lupus erythematosus susceptibility in multiple ancestries. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2011-201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010;72:463–493. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- Kuespert K, Pils S, Hauck CR. CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol. 2006;18:565–571. doi: 10.1016/j.ceb.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurio H, Murayama E, Kaneko T, Shibata Y, Inai T, Iida H. Intron retention generates a novel isoform of CEACAM6 that may act as an adhesion molecule in the ectoplasmic specialization structures between spermatids and sertoli cells in rat testis. Biol Reprod. 2008;79:1062–1073. doi: 10.1095/biolreprod.108.069872. [DOI] [PubMed] [Google Scholar]
- Kurio H, Hatsuda H, Murayama E, Kaneko T, Iida H. Identification of CEACAM6 as an intermediate filament-associated protein expressed in Sertoli cells of rat testis. Biol Reprod. 2011;85:924–933. doi: 10.1095/biolreprod.111.092437. [DOI] [PubMed] [Google Scholar]
- Laslett AL, Li LH, Jester WF, Jr, Orth JM. Thyroid hormone down-regulates neural cell adhesion molecule expression and affects attachment of gonocytes in Sertoli cell-gonocyte cocultures. Endocrinology. 2000;141:1633–1641. doi: 10.1210/endo.141.5.7468. [DOI] [PubMed] [Google Scholar]
- Lauke H, Kilic N, Bozorgzad R, Fernando M, Neshat-Vahid S, Pottek T, Ergun S. Expression of carcinoembryonic antigen-related cell adhesion molecule-1 (CEACAM1) in normal human Sertoli cells and its up-regulation in impaired spermatogenesis. Mol Hum Reprod. 2004;10:247–252. doi: 10.1093/molehr/gah020. [DOI] [PubMed] [Google Scholar]
- Lawson C, Ainsworth M, Yacoub M, Rose M. Ligation of ICAM-1 on endothelial cells leads to expression of VCAM-1 via a nuclear factor-kappaB-independent mechanism. J Immunol. 1999;162:2990–2996. [PubMed] [Google Scholar]
- Leblond C, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann NY Acad Sci. 1952a;55:548–573. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- Leblond CP, Clermont Y. Spermiogenesis of rat, mouse, hamster, and guinea pig as revelaed by the periodic acid-fuchin sulfurous acid technique. Am J Anat. 1952b;90:167–210. doi: 10.1002/aja.1000900202. [DOI] [PubMed] [Google Scholar]
- Lee NPY, Cheng CY. Ectoplasmic specialization, a testis-specific cell-cell actin-based adherens junction type: is this a potential target for male contraceptive development. Hum Reprod Update. 2004;10:349–369. doi: 10.1093/humupd/dmh026. [DOI] [PubMed] [Google Scholar]
- Lee G, Spring FA, Parsons SF, Mankelow TJ, Peters LL, Koury MJ, Mohandas N, Anstee DJ, Chasis JA. Novel secreted isoform of adhesion molecule ICAM-4: potential regulator of membrane-associated ICAM-4 interactions. Blood. 2003;101:1790–1797. doi: 10.1182/blood-2002-08-2529. [DOI] [PubMed] [Google Scholar]
- Lewis JE, Wahl JK, III, Sass KM, Jensen PJ, Johnson KR, Wheelock MJ. Cross-talk between adherens junctions and desmosomes depends on plakoglobin. J Cell Biol. 1997;136:919–934. doi: 10.1083/jcb.136.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PP, Cheng CY, Mruk DD. The biology of the desmosome-like junction a versatile anchoring junction and signal transducer in the seminiferous epithelium. Int Rev Cell Mol Biol. 2011;286:223–269. doi: 10.1016/B978-0-12-385859-7.00005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PP, Cheng CY, Mruk DD. The biology of interleukin-1: emerging concepts in the regulation of the actin cytoskeleton and cell junction dynamics. Cell Mol Life Sci. 2012;69:487–500. doi: 10.1007/s00018-011-0760-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PP, Xia W, Wang CQ, Mruk DD, Yan HH, Wong CH, Lee WM, Cheng CY. Dynamin II interacts with the cadherin- and occludin-based protein complexes at the blood-testis barrier in adult rat testes. J Endocr. 2006;191:571–586. doi: 10.1677/joe.1.06996. [DOI] [PubMed] [Google Scholar]
- Long EO. ICAM-1: getting a grip on leukocyte adhesion. J Immunol. 2011;186:5021–5023. doi: 10.4049/jimmunol.1100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lerma I, Estrach MT. A distinct profile of serum levels of soluble intercellular adhesion molecule-1 and intercellular adhesion molecule-3 in mycosis fungoides and Sezary syndrome. J Am Acad Dermatol. 2009;61:263–270. doi: 10.1016/j.jaad.2009.03.041. [DOI] [PubMed] [Google Scholar]
- Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyck R, Reiss Y, Gerwin N, Greenwood J, Adamson P, Engelhardt B. T-cell interaction with ICAM-1/ICAM-2 double-deficient brain endothelium in vitro: the cytoplasmic tail of endothelial ICAM-1 is necessary for transendothelial migration of T cells. Blood. 2003;102:3675–3683. doi: 10.1182/blood-2003-02-0358. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Ito C, Toyama Y, Suzuki-Toyota F, Fujita E, Momoi T, Toshimori K. Localisation of RA175 (Cadm1), a cell adhesion molecule of the immunoglobulin superfamily, in the mouse testis, and analysis of male infertility in the RA175-deficient mouse. Andrologia. 2011;43:180–188. doi: 10.1111/j.1439-0272.2010.01049.x. [DOI] [PubMed] [Google Scholar]
- Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- Mannowetz N, Wandernoth P, Wennemuth G. Basigin interacts with both MCT1 and MCT2 in murine spermatozoa. J Cell Physiol. 2012;227:2154–2162. doi: 10.1002/jcp.22949. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, et al. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli R, Gegg M, Longbottom R, Adamson P, Turowski P, Greenwood J. ICAM-1-mediated endothelial nitric oxide synthase activation via calcium and AMP-activated protein kinase is required for transendothelial lymphocyte migration. Mol Biol Cell. 2009;20:995–1005. doi: 10.1091/mbc.E08-06-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SM, Riddle L, Nakamura GR, Prashad H, Mehta A, Berman PW, Jardieu P. sICAM-1 enhances cytokine production stimulated by alloantigen. Cell Immunol. 1993;150:364–375. doi: 10.1006/cimm.1993.1204. [DOI] [PubMed] [Google Scholar]
- McKinnon PJ, McLaughlin SK, Kapsetaki M, Margolskee RF. Extracellular matrix-associated protein Sc1 is not essential for mouse development. Mol Cell Biol. 2000;20:656–660. doi: 10.1128/mcb.20.2.656-660.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MP, Morris SB, Wilcoxen S, Du M, Monroy YK, Remmer H, Murphy H, Christensen PJ, Paine R., III Disparate mechanisms of sICAM-1 production in the peripheral lung: contrast between alveolar epithelial cells and pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L807–L814. doi: 10.1152/ajplung.00398.2007. [DOI] [PubMed] [Google Scholar]
- Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol. 2006;8:113–123. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- Miller J, Knorr R, Ferrone M, Houdei R, Carron CP, Dustin ML. Intercellular adhesion molecule-1 dimerization and its consequences for adhesion mediated by lymphocyte function associated-1. J Exp Med. 1995;182:1231–1241. doi: 10.1084/jem.182.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miravet S, Piedra J, Castano J, Raurell I, Franci C, Dunach M, Garcia de Herreros A. Tyrosine phosphorylation of plakoglobin causes contrary effects on its association with desmosomes and adherens junction components and modulates beta-catenin-mediated transcription. Mol Cell Biol. 2003;23:7391–7402. doi: 10.1128/MCB.23.20.7391-7402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza M, Hreinsson J, Strand ML, Hovatta O, Soder O, Philipson L, Pettersson RF, Sollerbrant K. Coxsackievirus and adenovirus receptor (CAR) is expressed in male germ cells and forms a complex with the differentiation factor JAM-C in mouse testis. Exp Cell Res. 2006;312:817–830. doi: 10.1016/j.yexcr.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Mirza M, Petersen C, Nordqvist K, Sollerbrant K. Coxsackievirus and adenovirus receptor is up-regulated in migratory germ cells during passage of the blood-testis barrier. Endocr. 2007;148:5459–5469. doi: 10.1210/en.2007-0359. [DOI] [PubMed] [Google Scholar]
- Mital P, Hinton BT, Dufour JM. The blood–testis and blood–epididymis barriers are more than just their tight junctions. Biol Reprod. 2011;84:851–858. doi: 10.1095/biolreprod.110.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow CMK, Mruk DD, Cheng CY, Hess RA. Claudin and occludin expression and function in the seminiferous epithelium. Philos Trans R Soc Lond B Biol Sci. 2010;365:1679–1696. doi: 10.1098/rstb.2010.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Cell–cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol Metab. 2004a;15:439–447. doi: 10.1016/j.tem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli–Sertoli and Sertoli–germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004b;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Desmosomes in the testis: moving into an unchartered territory. Spermatogenesis. 2011;1:47–51. doi: 10.4161/spmg.1.1.15443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. In search of suitable in vitro models to study germ cell movement across the blood-testis barrier. Spermatogenesis. 2012;2:6–10. doi: 10.4161/spmg.19878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J Cell Sci. 2003;116:1599–1609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- Mustjoki S, Alitalo R, Elonen E, Carpen O, Gahmberg CG, Vaheri A. Intercellular adhesion molecule-1 in extravasation of normal mononuclear and leukaemia cells. Br J Haematol. 2001;113:989–1000. doi: 10.1046/j.1365-2141.2001.02793.x. [DOI] [PubMed] [Google Scholar]
- Nagamatsu G, Ohmura M, Mizukami T, Hamaguchi I, Hirabayashi S, Yoshida S, Hata Y, Suda T, Ohbo K. A CTX family cell adhesion molecule, JAM4, is expressed in stem cell and progenitor cell populations of both male germ cell and hematopoietic cell lineages. Mol Cell Biol. 2006;26:8498–8506. doi: 10.1128/MCB.01502-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalam RL, Lin YN, Matzuk MM. Testicular cell adhesion molecule 1 (TCAM1) is not essential for fertility. Mol Cell Endocr. 2010;315:246–253. doi: 10.1016/j.mce.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejmeddine M, Negi VS, Mukherjee S, Tanaka Y, Orth K, Taylor GP, Bangham CR. HTLV-1-Tax and ICAM-1 act on T-cell signal pathways to polarize the microtubule-organizing center at the virological synapse. Blood. 2009;114:1016–1025. doi: 10.1182/blood-2008-03-136770. [DOI] [PubMed] [Google Scholar]
- Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8:156–162. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- Nottebaum AF, Cagna G, Winderlich M, Gamp AC, Linnepe R, Polaschegg C, Filippova K, Lyck R, Engelhardt B, Kamenyeva O, et al. VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J Exp Med. 2008;205:2929–2945. doi: 10.1084/jem.20080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- Nyman-Huttunen H, Tian L, Ning L, Gahmberg CG. alpha-Actinin-dependent cytoskeletal anchorage is important for ICAM-5-mediated neuritic outgrowth. J Cell Sci. 2006;119:3057–3066. doi: 10.1242/jcs.03045. [DOI] [PubMed] [Google Scholar]
- O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis. 2011;1:14–35. doi: 10.4161/spmg.1.1.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh HM, Kwon MS, Kim HJ, Jeon BH, Kim HR, Choi HO, Na BR, Eom SH, Song NW, Jun CD. Intermediate monomer-dimer equilibrium structure of native ICAM-1: implication for enhanced cell adhesion. Exp Cell Res. 2011;317:163–172. doi: 10.1016/j.yexcr.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Ohbo K, Yoshida S, Ohmura M, Ohneda O, Ogawa T, Tsuchiya H, Kuwana T, Kehler J, Abe K, Scholer HR, et al. Identification and characterization of stem cells in prepubertal spermatogenesis in mice small star, filled. Dev Biol. 2003;258:209–225. doi: 10.1016/s0012-1606(03)00111-8. [DOI] [PubMed] [Google Scholar]
- Orth JM, Jester WF, Li LH, Laslett AL. Gonocyte-Sertoli cell interactions during development of the neonatal rodent testis. Curr Top Dev Biol. 2000;50:103–124. doi: 10.1016/s0070-2153(00)50006-4. [DOI] [PubMed] [Google Scholar]
- Otto VI, Heinzel-Pleines UE, Gloor SM, Trentz O, Kossmann T, Morganti-Kossmann MC. sICAM-1 and TNF-alpha induce MIP-2 with distinct kinetics in astrocytes and brain microvascular endothelial cells. J Neurosci Res. 2000;60:733–742. doi: 10.1002/1097-4547(20000615)60:6<733::AID-JNR5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Ozaki-Kuroda K, Nakanishi H, Ohta H, Tanaka H, Kurihara H, Mueller S, Irie K, Ikeda W, Sakai T, Wimmer E, et al. Nectin couples cell-cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr Biol. 2002;12:1145–1150. doi: 10.1016/s0960-9822(02)00922-3. [DOI] [PubMed] [Google Scholar]
- Park JK, Park SH, So K, Bae IH, Yoo YD, Um HD. ICAM-3 enhances the migratory and invasive potential of human non-small cell lung cancer cells by inducing MMP-2 and MMP-9 via Akt and CREB. Int J Oncol. 2010;36:181–192. [PubMed] [Google Scholar]
- Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11:633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev. 1982;3:404–417. doi: 10.1210/edrv-3-4-404. [DOI] [PubMed] [Google Scholar]
- Pelletier R. The tight junctions in the testis, epididymis, and vas deferens. In: Cereijido M, Anderson J, editors. Tight Junctions. New York: CRC Press; 2001. pp. 599–628. [Google Scholar]
- Pelletier RM. The blood–testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem. 2011;46:49–127. doi: 10.1016/j.proghi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Pelletier R, Byers S. The blood–testis barrier and Sertoli cell junctions: structural considerations. Microsc Res Tech. 1992;20:3–33. doi: 10.1002/jemt.1070200104. [DOI] [PubMed] [Google Scholar]
- Perez OD, Kinoshita S, Hitoshi Y, Payan DG, Kitamura T, Nolan GP, Lorens JB. Activation of the PKB/AKT pathway by ICAM-2. Immunity. 2002;16:51–65. doi: 10.1016/s1074-7613(02)00266-2. [DOI] [PubMed] [Google Scholar]
- Pluskota E, Chen Y, D'Souza SE. Src homology domain 2-containing tyrosine phosphatase 2 associates with intercellular adhesion molecule 1 to regulate cell survival. J Biol Chem. 2000;275:30029–30036. doi: 10.1074/jbc.M000240200. [DOI] [PubMed] [Google Scholar]
- Porter JC, Hall A. Epithelial ICAM-1 and ICAM-2 regulate the egression of human T cells across the bronchial epithelium. FASEB J. 2009;23:492–502. doi: 10.1096/fj.08-115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Fazal F. Hug tightly and say goodbye: role of endothelial ICAM-1 in leukocyte transmigration. Antioxid Redox Signal. 2009;11:823–839. doi: 10.1089/ars.2008.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly PL, Woska JR, Jr, Jeanfavre DD, McNally E, Rothlein R, Bormann BJ. The native structure of intercellular adhesion molecule-1 (ICAM-1) is a dimer. Correlation with binding to LFA-1. J Immunol. 1995;155:529–532. [PubMed] [Google Scholar]
- Riccioli A, Filippini A, De Cesaris P, Barbacci E, Stefanini M, Starace G, Ziparo E. Inflammatory mediators increase surface expression of integrin ligands, adhesion to lymphocytes, and secretion of interleukin 6 in mouse Sertoli cells. Proc Natl Acad Sci USA. 1995;92:5808–5812. doi: 10.1073/pnas.92.13.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo O, Papaioannou A, Ochietti B, Beauchemin C, Legault D, Cantin A, King PD, Daniel C, Alakhov VY, Potworowski EF, et al. ICAM-1 isoforms: specific activity and sensitivity to cleavage by leukocyte elastase and cathepsin G. Eur J Immunol. 2003;33:1351–1360. doi: 10.1002/eji.200323195. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fernandez JL, Gomez M, Luque A, Hogg N, Sanchez-Madrid F, Cabanas C. The interaction of activated integrin lymphocyte function-associated antigen 1 with ligand intercellular adhesion molecule 1 induces activation and redistribution of focal adhesion kinase and proline-rich tyrosine kinase 2 in T lymphocytes. Mol Biol Cell. 1999;10:1891–1907. doi: 10.1091/mbc.10.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat. 1977;148:313–328. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- Russell L, Clermont Y. Anchoring device between Sertoli cells and late spermatids in rat seminiferous tubules. Anat Rec. 1976;185:259–278. doi: 10.1002/ar.1091850302. [DOI] [PubMed] [Google Scholar]
- Russell LD. The blood–testis barrier and its formation relative to spermatocyte maturation in the adult rat: a lanthanum tracer study. Anat Rec. 1978;190:99–111. doi: 10.1002/ar.1091900109. [DOI] [PubMed] [Google Scholar]
- Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol. 1985;94:177–211. doi: 10.1016/s0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- Russell LD, Saxena NK, Turner TT. Cytoskeletal involvement in spermiation and sperm transport. Tissue Cell. 1989;21:361–379. doi: 10.1016/0040-8166(89)90051-7. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T, Nishimoto M, Miyagi S, Iwama A, Morita Y, Iwamori N, Nakauchi H, Kiyonari H, Muramatsu M, Okuda A. Putative “stemness” gene jam-B is not required for maintenance of stem cell state in embryonic, neural, or hematopoietic stem cells. Mol Cell Biol. 2006;26:6557–6570. doi: 10.1128/MCB.00729-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakatani S, Takahashi R, Okuda Y, Aizawa A, Otsuka A, Komatsu A, Ono M. Structure, expression, and conserved physical linkage of mouse testicular cell adhesion molecule-1 (TCAM-1) gene. Genome. 2000;43:957–962. doi: 10.1139/g00-071. [DOI] [PubMed] [Google Scholar]
- Sarkar O, Xia W, Mruk DD. Adjudin-mediated junction restructuring in the seminiferous epithelium leads to displacement of soluble guanylate cyclase from adherens junctions. J Cell Physiol. 2006;208:175–187. doi: 10.1002/jcp.20651. [DOI] [PubMed] [Google Scholar]
- Sato H, Azuma Y, Higai K, Matsumoto K. Altered expression of glycoproteins on the cell surface of Jurkat cells during etoposide-induced apoptosis: shedding and intracellular translocation of glycoproteins. Biochim Biophys Acta. 2009;1790:1198–1205. doi: 10.1016/j.bbagen.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol. 2004;5:393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- Schnoor M, Lai FP, Zarbock A, Klaver R, Polaschegg C, Schulte D, Weich HA, Oelkers JM, Rottner K, Vestweber D. Cortactin deficiency is associated with reduced neutrophil recruitment but increased vascular permeability in vivo. J Exp Med. 2011;208:1721–1735. doi: 10.1084/jem.20101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrador JM, Vicente-Manzanares M, Calvo J, Barreiro O, Montoya MC, Schwartz-Albiez R, Furthmayr H, Lozano F, Sanchez-Madrid F. A novel serine-rich motif in the intercellular adhesion molecule 3 is critical for its ezrin/radixin/moesin-directed subcellular targeting. J Biol Chem. 2002;277:10400–10409. doi: 10.1074/jbc.M110694200. [DOI] [PubMed] [Google Scholar]
- Serrano D, Bhowmick T, Chadha R, Garnacho C, Muro S. Intercellular adhesion molecule 1 engagement modulates sphingomyelinase and ceramide, supporting uptake of drug carriers by the vascular endothelium. Arterioscler Thromb Vasc Biol. 2012;32:1178–1185. doi: 10.1161/ATVBAHA.111.244186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell B. Sermatogenesis. In: Finn C, editor. The Mammalian Testis. New York: Cornell University Press; 1978. pp. 181–232. [Google Scholar]
- Setchell BP. The Functional Significance of the Blood-testis Barrier. Journal of Andrology. 1980;1:3–10. [Google Scholar]
- Setchell BP. Blood-testis barrier, functional and transport proteins and spermatogenesis. Adv Exp Med Biol. 2008;636:212–233. doi: 10.1007/978-0-387-09597-4_12. [DOI] [PubMed] [Google Scholar]
- Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the ‘blood–testis barrier’ after injection of cadmium chloride in the rat. J Endocrinol. 1970;47:81–86. doi: 10.1677/joe.0.0470081. [DOI] [PubMed] [Google Scholar]
- Setchell BP, Waites GMB. The blood–testis barrier. In: Hamilton DW, Greep RO, editors. The Handbook of Physiology Section 7, Vol. V Male Reproductive System. Washington, D.C: American Physiological Society; 1975. pp. 143–172. [Google Scholar]
- Shaha C, Tripathi R, Mishra DP. Male germ cell apoptosis: regulation and biology. Philos Trans R Soc Lond B Biol Sci. 2010;365:1501–1515. doi: 10.1098/rstb.2009.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M, Ghosh A, Cooke VG, Naik UP, Martin-DeLeon PA. JAM-A is present in mammalian spermatozoa where it is essential for normal motility. Dev Biol. 2008;313:246–255. doi: 10.1016/j.ydbio.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol. 2001;167:2323–2330. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- Shaw SK, Ma S, Kim MB, Rao RM, Hartman CU, Froio RM, Yang L, Jones T, Liu Y, Nusrat A, et al. Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. J Exp Med. 2004;200:1571–1580. doi: 10.1084/jem.20040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283–309. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sithu SD, English WR, Olson P, Krubasik D, Baker AH, Murphy G, D'Souza SE. Membrane-type 1-matrix metalloproteinase regulates intracellular adhesion molecule-1 (ICAM-1)-mediated monocyte transmigration. J Biol Chem. 2007;282:25010–25019. doi: 10.1074/jbc.M611273200. [DOI] [PubMed] [Google Scholar]
- Siu E, Wong E, Mruk D, Porto C, Cheng C. Focal adhesion kinase is a blood–testis barrier regulator. Proc Natl Acad Sci USA. 2009;106:9298–9303. doi: 10.1073/pnas.0813113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sligh JE, Jr, Ballantyne CM, Rich SS, Hawkins HK, Smith CW, Bradley A, Beaudet AL. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1993;90:8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Bracke M, Leitinger B, Porter JC, Hogg N. LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. J Cell Sci. 2003;116:3123–3133. doi: 10.1242/jcs.00606. [DOI] [PubMed] [Google Scholar]
- Spring FA, Parsons SF, Ortlepp S, Olsson ML, Sessions R, Brady RL, Anstee DJ. Intercellular adhesion molecule-4 binds alpha(4)beta(1) and alpha(V)-family integrins through novel integrin-binding mechanisms. Blood. 2001;98:458–466. doi: 10.1182/blood.v98.2.458. [DOI] [PubMed] [Google Scholar]
- Steiner O, Coisne C, Cecchelli R, Boscacci R, Deutsch U, Engelhardt B, Lyck R. Differential roles for endothelial ICAM-1, ICAM-2, and VCAM-1 in shear-resistant T cell arrest, polarization, and directed crawling on blood–brain barrier endothelium. J Immunol. 2010;185:4846–4855. doi: 10.4049/jimmunol.0903732. [DOI] [PubMed] [Google Scholar]
- Strell C, Lang K, Niggemann B, Zaenker KS, Entschladen F. Neutrophil granulocytes promote the migratory activity of MDA-MB-468 human breast carcinoma cells via ICAM-1. Exp Cell Res. 2010;316:138–148. doi: 10.1016/j.yexcr.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Su L, Cheng CY, Mruk DD. Drug transporter, P-glycoprotein (MDR1), is an integrated component of the mammalian blood-testis barrier. Int J Biochem Cell Biol. 2009;41:2578–2587. doi: 10.1016/j.biocel.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W, Mruk DD, Cheng CY, Filamin A. A regulator of blood-testis barrier assembly during post-natal development. Spermatogenesis. 2012;2:73–78. doi: 10.4161/spmg.20223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumagin R, Sarelius IH. A role for ICAM-1 in maintenance of leukocyte-endothelial cell rolling interactions in inflamed arterioles. Am J Physiol Heart Circ Physiol. 2007;293:H2786–2798. doi: 10.1152/ajpheart.00720.2007. [DOI] [PubMed] [Google Scholar]
- Sumagin R, Sarelius IH. Intercellular adhesion molecule-1 enrichment near tricellular endothelial junctions is preferentially associated with leukocyte transmigration and signals for reorganization of these junctions to accommodate leukocyte passage. J Immunol. 2010;184:5242–5252. doi: 10.4049/jimmunol.0903319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumagin R, Kuebel JM, Sarelius IH. Leukocyte rolling and adhesion both contribute to regulation of microvascular permeability to albumin via ligation of ICAM-1. Am J Physiol Cell Physiol. 2011;301:C804–C813. doi: 10.1152/ajpcell.00135.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei ML, Giannoni E, Fiaschi T, Chiarugi P. Anoikis: an emerging hallmark in health and diseases. J Pathol. 2012;226:380–393. doi: 10.1002/path.3000. [DOI] [PubMed] [Google Scholar]
- Takai Y, Ikeda W, Ogita H, Rikitake Y. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu Rev Cell Dev Biol. 2008;24:309–342. doi: 10.1146/annurev.cellbio.24.110707.175339. [DOI] [PubMed] [Google Scholar]
- Tarin C, Gomez M, Calvo E, Lopez JA, Zaragoza C. Endothelial nitric oxide deficiency reduces MMP-13-mediated cleavage of ICAM-1 in vascular endothelium: a role in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:27–32. doi: 10.1161/ATVBAHA.108.169623. [DOI] [PubMed] [Google Scholar]
- Tarulli GA, Meachem SJ, Schlatt S, Stanton PG. Regulation of testicular tight junctions by gonadotrophins in the adult Djungarian hamster in vivo. Reprod. 2008;135:867–877. doi: 10.1530/REP-07-0572. [DOI] [PubMed] [Google Scholar]
- Terada N, Ohno N, Saitoh S, Saitoh Y, Komada M, Kubota H, Ohno S. Involvement of a membrane skeletal protein, 4.1G, for Sertoli/germ cell interaction. Reprod. 2010;139:883–892. doi: 10.1530/REP-10-0005. [DOI] [PubMed] [Google Scholar]
- Thomas S, Baumgart DC. Targeting leukocyte migration and adhesion in Crohn's disease and ulcerative colitis. Inflammopharmacol. 2012;20:1–18. doi: 10.1007/s10787-011-0104-6. [DOI] [PubMed] [Google Scholar]
- Thompson PW, Randi AM, Ridley AJ. Intercellular adhesion molecule (ICAM)-1, but not ICAM-2, activates RhoA and stimulates c-fos and rhoA transcription in endothelial cells. J Immunol. 2002;169:1007–1013. doi: 10.4049/jimmunol.169.2.1007. [DOI] [PubMed] [Google Scholar]
- Tian L, Stefanidakis M, Ning L, Van Lint P, Nyman-Huttunen H, Libert C, Itohara S, Mishina M, Rauvala H, Gahmberg CG. Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J Cell Biol. 2007;178:687–700. doi: 10.1083/jcb.200612097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman RW, Hoover RL. The Src-cortactin pathway is required for clustering of E-selectin and ICAM-1 in endothelial cells. FASEB J. 2002;16:1257–1259. doi: 10.1096/fj.01-0969fje. [DOI] [PubMed] [Google Scholar]
- Topp SA, Upadhya GA, Strasberg SM. Leukocyte adhesion to cold-preserved rat endothelial cells: role of actin disassembly and ICAM-1. Liver Transpl. 2003;9:1286–1294. doi: 10.1016/j.lts.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Toyama Y, Maekawa M, Kadomatsu K, Miyauchi T, Muramatsu T, Yuasa S. Histological characterization of defective spermatogenesis in mice lacking the basigin gene. Anat Histol Embryol. 1999;28:205–213. doi: 10.1046/j.1439-0264.1999.00194.x. [DOI] [PubMed] [Google Scholar]
- Toyama Y, Maekawa M, Yuasa S. Ectoplasmic specializations in the Sertoli cells: new vistas based on genetic defects and testicular toxicology. Anat Sci Int. 2003;78:1–16. doi: 10.1046/j.0022-7722.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- Trinh-Trang-Tan MM, Vilela-Lamego C, Picot J, Wautier MP, Cartron JP. Intercellular adhesion molecule-4 and CD36 are implicated in the abnormal adhesiveness of sickle cell SAD mouse erythrocytes to endothelium. Haematologica. 2010;95:730–737. doi: 10.3324/haematol.2009.017392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakadze NL, Sen U, Zhao Z, Sithu SD, English WR, D'Souza SE. Signals mediating cleavage of intercellular adhesion molecule-1. Am J Physiol Cell Physiol. 2004;287:C55–63. doi: 10.1152/ajpcell.00585.2003. [DOI] [PubMed] [Google Scholar]
- Tsakadze NL, Sithu SD, Sen U, English WR, Murphy G, D'Souza SE. Tumor necrosis factor-alpha-converting enzyme (TACE/ADAM-17) mediates the ectodomain cleavage of intercellular adhesion molecule-1 (ICAM-1) J Biol Chem. 2006;281:3157–3164. doi: 10.1074/jbc.M510797200. [DOI] [PubMed] [Google Scholar]
- Tse MC, Lane C, Mott K, Onlamoon N, Hsiao HM, Perng GC. ICAM-5 modulates cytokine/chemokine production in the CNS during the course of herpes simplex virus type 1 infection. J Neuroimmunol. 2009;213:12–19. doi: 10.1016/j.jneuroim.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turowski P, Martinelli R, Crawford R, Wateridge D, Papageorgiou AP, Lampugnani MG, Gamp AC, Vestweber D, Adamson P, Dejana E, et al. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci. 2008;121:29–37. doi: 10.1242/jcs.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Den Engel NK, Heidenthal E, Vinke A, Kolb H, Martin S. Circulating forms of intercellular adhesion molecule (ICAM)-1 in mice lacking membranous ICAM-1. Blood. 2000;95:1350–1355. [PubMed] [Google Scholar]
- van Buul JD, Voermans C, van den Berg V, Anthony EC, Mul FP, van Wetering S, van der Schoot CE, Hordijk PL. Migration of human hematopoietic progenitor cells across bone marrow endothelium is regulated by vascular endothelial cadherin. J Immunol. 2002;168:588–596. doi: 10.4049/jimmunol.168.2.588. [DOI] [PubMed] [Google Scholar]
- van Buul JD, Allingham MJ, Samson T, Meller J, Boulter E, Garcia-Mata R, Burridge K. RhoG regulates endothelial apical cup assembly downstream from ICAM1 engagement and is involved in leukocyte trans-endothelial migration. J Cell Biol. 2007;178:1279–1293. doi: 10.1083/jcb.200612053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buul JD, van Rijssel J, van Alphen FP, Hoogenboezem M, Tol S, Hoeben KA, van Marle J, Mul EP, Hordijk PL. Inside-out regulation of ICAM-1 dynamics in TNF-alpha-activated endothelium. PLoS ONE. 2010a;5:e11336. doi: 10.1371/journal.pone.0011336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buul JD, van Rijssel J, van Alphen FP, van Stalborch AM, Mul EP, Hordijk PL. ICAM-1 clustering on endothelial cells recruits VCAM-1. J Biomed Biotechnol. 2010b;2010:120328. doi: 10.1155/2010/120328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijssel J, Kroon J, Hoogenboezem M, van Alphen FP, de Jong RJ, Kostadinova E, Geerts D, Hordijk PL, van Buul JD. The Rho-guanine nucleotide exchange factor Trio controls leukocyte transendothelial migration by promoting docking structure formation. Mol Biol Cell. 2012;23:2831–2844. doi: 10.1091/mbc.E11-11-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Sanchez-Madrid F. Role of the cytoskeleton during leukocyte responses. Nat Rev Immunol. 2004;4:110–122. doi: 10.1038/nri1268. [DOI] [PubMed] [Google Scholar]
- Vogl A, Pfeiffer D, Redenbach D. Ectoplasmic (‘junctional’) specializations in mammalian Sertoli cells: influence on spermatogenic cells. Ann NY Acad Sci. 1991;637:175–202. doi: 10.1111/j.1749-6632.1991.tb27310.x. [DOI] [PubMed] [Google Scholar]
- Vogl A, Pfeiffer D, Redenbach D, Grove B. Sertoli cell cytoskeleton. In: Russell L, Griswold M, editors. The Sertoli Cell. Clearwater: Cache River Press; 1993. pp. 39–86. [Google Scholar]
- Vogl A, Pfeiffer D, Mulholland D, Kimel G, Guttman J. Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch Histol Cytol. 2000;63:1–15. doi: 10.1679/aohc.63.1. [DOI] [PubMed] [Google Scholar]
- Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Sai Y, Ito A, Kato Y, Kurobo M, Murakami Y, Nakashima E, Tsuji A, Kitamura Y, Iseki S. Heterophilic binding of the adhesion molecules poliovirus receptor and immunoglobulin superfamily 4A in the interaction between mouse spermatogenic and Sertoli cells. Biol Reprod. 2007;76:1081–1090. doi: 10.1095/biolreprod.106.058974. [DOI] [PubMed] [Google Scholar]
- Wang Q, Pfeiffer GR, II, Gaarde WA. Activation of SRC tyrosine kinases in response to ICAM-1 ligation in pulmonary microvascular endothelial cells. J Biol Chem. 2003;278:47731–47743. doi: 10.1074/jbc.M308466200. [DOI] [PubMed] [Google Scholar]
- Wang HW, Babic AM, Mitchell HA, Liu K, Wagner DD. Elevated soluble ICAM-1 levels induce immune deficiency and increase adiposity in mice. FASEB J. 2005;19:1018–1020. doi: 10.1096/fj.04-3094fje. [DOI] [PubMed] [Google Scholar]
- Wang CQ, Cheng CY. A seamless trespass: germ cell migration across the seminiferous epithelium during spermatogenesis. J Cell Biol. 2007;178:549–556. doi: 10.1083/jcb.200704061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CQ, Mruk DD, Lee WM, Cheng CY. Coxsackie and adenovirus receptor (CAR) is a product of Sertoli and germ cells in rat testes which is localized at the Sertoli-Sertoli and Sertoli-germ cell interface. Exp Cell Res. 2007;313:1373–1392. doi: 10.1016/j.yexcr.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lui WY. Opposite effects of interleukin-1α and transforming growth factor-β2 induce stage-specific regulation of junctional adhesion molecule-B gene in Sertoli cells. Endocr. 2009;150:2404–2412. doi: 10.1210/en.2008-1239. [DOI] [PubMed] [Google Scholar]
- Weber JE, Russell LD, Wong V, Peterson RN. Three-dimensional reconstruction of a rat stage V Sertoli cell: II. Morphometry of Sertoli—Sertoli and Sertoli—germ-cell relationships. Am J Anat. 1983;167:163–179. doi: 10.1002/aja.1001670203. [DOI] [PubMed] [Google Scholar]
- Weber GF, Bjerke MA, DeSimone DW. Integrins and cadherins join forces to form adhesive networks. J Cell Sci. 2011;124:1183–1193. doi: 10.1242/jcs.064618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welder CA, Lee DH, Takei F. Inhibition of cell adhesion by microspheres coated with recombinant soluble intercellular adhesion molecule-1. J Immunol. 1993;150:2203–2210. [PubMed] [Google Scholar]
- Whittard JD, Craig SE, Mould AP, Koch A, Pertz O, Engel J, Humphries MJ. E-cadherin is a ligand for integrin alpha2beta1. Matrix Biol. 2002;21:525–532. doi: 10.1016/s0945-053x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- Witkowska AM, Borawska MH. Soluble intercellular adhesion molecule-1 (sICAM-1): an overview. Eur Cytokine Netw. 2004;15:91–98. [PubMed] [Google Scholar]
- Wittchen ES. Endothelial signaling in paracellular and transcellular leukocyte transmigration. Front Biosci. 2009;14:2522–2545. doi: 10.2741/3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood–testis barrier dynamics: an in vivo study. J Cell Sci. 2004;117:783–798. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochem Biophys Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodfin A, Voisin MB, Imhof BA, Dejana E, Engelhardt B, Nourshargh S. Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A, and PECAM-1. Blood. 2009;113:6246–6257. doi: 10.1182/blood-2008-11-188375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Mruk DD, Cheng CY. C-type natriuretic peptide regulates blood-testis barrier dynamics in adult rat testes. Proc Natl Acad Sci USA. 2007;104:3841–3846. doi: 10.1073/pnas.0610100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Wong EW, Mruk DD, Cheng CY. TGF-beta3 and TNFalpha perturb blood–testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev Biol. 2009;327:48–61. doi: 10.1016/j.ydbio.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Mruk DD, Lee WM, Cheng CY. c-Yes regulates cell adhesion at the blood–testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes. Int J Biochem Cell Biol. 2011;43:651–665. doi: 10.1016/j.biocel.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Cheng CY, Mruk DD. Intercellular adhesion molecule (ICAM)-1 is a regulator of blood–testis barrier function. J Cell Sci. 2012a doi: 10.1242/jcs.107987. (in press; doi:10.1242/jcs107987; PMID:22976294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Cheng CY, Mruk DD. Intercellular adhesion molecule (ICAM)-2 is involved in apical ectoplasmic specialization dynamics during spermatogenesis in the rat. J Endocr. 2012b doi: 10.1530/JOE-12-0434. (in press; doi:10.1530/JOE-12-0434; PMID:23097088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HHN, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: a friend or a foe of spermatogenesis? BioEssays. 2007;29:36–48. doi: 10.1002/bies.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HHN, Mruk DD, Cheng CY. Junction restructuring and spermatogenesis: the biology, regulation, and implication in male contraceptive development. Curr Top Dev Biol. 2008;80:57–92. doi: 10.1016/S0070-2153(07)80002-0. [DOI] [PubMed] [Google Scholar]
- Yang Y, Han C. GDNF stimulates the proliferation of cultured mouse immature Sertoli cells via its receptor subunit NCAM and ERK1/2 signaling pathway. BMC Cell Biol. 2010;11:78. doi: 10.1186/1471-2121-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Jun CD, Liu JH, Zhang R, Joachimiak A, Springer TA, Wang JH. Structural basis for dimerization of ICAM-1 on the cell surface. Mol Cell. 2004;14:269–276. doi: 10.1016/s1097-2765(04)00204-7. [DOI] [PubMed] [Google Scholar]
- Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Kowalski JR, Yacono P, Bajmoczi M, Shaw SK, Froio RM, Golan DE, Thomas SM, Luscinskas FW. Endothelial cell cortactin coordinates intercellular adhesion molecule-1 clustering and actin cytoskeleton remodeling during polymorphonuclear leukocyte adhesion and transmigration. J Immunol. 2006;177:6440–6449. doi: 10.4049/jimmunol.177.9.6440. [DOI] [PubMed] [Google Scholar]
- Yang S, Weng H, Chen L, Guo X, Parra M, Conboy J, Debnath G, Lambert AJ, Peters LL, Baines AJ, et al. Lack of protein 4.1G causes altered expression and localization of the cell adhesion molecule nectin-like 4 in testis and can cause male infertility. Mol Cell Biol. 2011;31:2276–2286. doi: 10.1128/MCB.01105-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazama F. Continual maintenance of the blood–testis barrier during spermatogenesis: the intermediate compartment theory revisited. J Reprod Dev. 2008;54:299–305. doi: 10.1262/jrd.19169. [DOI] [PubMed] [Google Scholar]
- Yoon KJ, Phelps DA, Bush RA, Remack JS, Billups CA, Khoury JD. ICAM-2 expression mediates a membrane-actin link, confers a nonmetastatic phenotype and reflects favorable tumor stage or histology in neuroblastoma. PLoS One. 2008;3:e3629. doi: 10.1371/journal.pone.0003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JS, Vogl AW. Focal adhesion proteins zyxin and vinculin are co-distributed at tubulobulbar complexes. Spermatogenesis. 2012;2:63–68. doi: 10.4161/spmg.19391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JS, Guttman JA, Vaid KS, Vogl AW. Cortactin (CTTN), N-WASP (WASL), and clathrin (CLTC) are present at podosome-like tubulobulbar complexes in the rat testis. Biol Reprod. 2009a;80:153–161. doi: 10.1095/biolreprod.108.070615. [DOI] [PubMed] [Google Scholar]
- Young JS, Guttman JA, Vaid KS, Vogl AW. Tubulobulbar complexes are intercellular podosome-like structures that internalize intact intercellular junctions during epithelial remodeling events in the rat testis. Biol Reprod. 2009b;80:162–174. doi: 10.1095/biolreprod.108.070623. [DOI] [PubMed] [Google Scholar]
- Young JS, Takai YK, Kojic KL, Vogl AW. Internalization of adhesion junction proteins and their association with recycling endosome marker proteins in rat seminiferous epithelium. Reproduction. 2012;143:347–357. doi: 10.1530/REP-11-0317. [DOI] [PubMed] [Google Scholar]
- Yuasa J, Toyama Y, Miyauchi T, Maekawa M, Yuasa S, Ito H. Specific localization of the basigin protein in human testes from normal adults, normal juveniles, and patients with azoospermia. Andrologia. 2001;33:293–299. doi: 10.1046/j.1439-0272.2001.00448.x. [DOI] [PubMed] [Google Scholar]
- Zennadi R, Hines PC, De Castro LM, Cartron JP, Parise LV, Telen MJ. Epinephrine acts through erythroid signaling pathways to activate sickle cell adhesion to endothelium via LW-alphavbeta3 interactions. Blood. 2004;104:3774–3781. doi: 10.1182/blood-2004-01-0042. [DOI] [PubMed] [Google Scholar]