Abstract
Hepatocellular carcinoma (HCC) mostly develops in patients with advanced fibrosis; however, the mechanisms of interaction between a genotoxic insult and fibrogenesis are not well understood. This study tested a hypothesis that fibrosis promotes HCC via a mechanism that involves activation of liver stem cells. First, B6C3F1 mice were administered diethylnitrosamine (DEN; single ip injection of 1mg/kg at 14 days of age). Second, carbon tetrachloride (CCl4; 0.2ml/kg, 2/week ip starting at 8 weeks of age) was administered for 9 or 14 weeks to develop advanced liver fibrosis. In animals treated with DEN as neonates, presence of liver fibrosis led to more than doubling (to 100%) of the liver tumor incidence as early as 5 months of age. This effect was associated with activation of cells with progenitor features in noncancerous liver tissue, including markers of replicative senescence (p16), oncofetal transformation (Afp, H19, and Bex1), and increased “stemness” (Prom1 and Epcam). In contrast, the dose of DEN used did not modify the extent of liver inflammation, fibrogenesis, oxidative stress, proliferation, or apoptosis induced by subchronic CCl4 administration. This study demonstrates the potential role of liver stem-like cells in the mechanisms of chemical-induced, fibrosis-promoted HCC. We posit that the combination of genotoxic and fibrogenic insults is a sensible approach to model liver carcinogenesis in experimental animals. These results may contribute to identification of cirrhotic patients predisposed to HCC by analyzing the expression of hepatic progenitor cell markers in the noncancerous liver tissue.
Key Words: liver, fibrosis, cancer, mechanisms, genotoxic
The incidence of liver cancer, including hepatocellular carcinoma (HCC), continues to increase worldwide, most prominently in the developed countries in North America, Europe, and Oceania (Center and Jemal, 2011). Major etiological factors contributing to HCC are well established and include viral infections, excessive ethanol consumption, environmental carcinogens, and hemochromatosis (El-Serag and Rudolph, 2007; Farazi and DePinho, 2006). More recently, metabolic diseases related to insulin resistance collectively identified as nonalcoholic steatohepatitis have been recognized to be causally related to HCC as well (Della Corte and Colombo, 2012). Still, the understanding of HCC pathogenesis and the mechanisms of tumor development is incomplete (Fausto and Campbell, 2010; Vucur et al., 2010).
Animal models, most often rat or mouse, are commonly used to understand the molecular pathogenesis of HCC (Heindryckx et al., 2009), as well as to test for the cancer hazard potential of chemicals and drugs (Wells and Williams, 2009). Liver is the most common tissue for tumor development in experimental rodent studies of chronic exposure to xenobiotics (Hoenerhoff et al., 2011; Huff et al., 1991). However, the overwhelming majority of the positive (i.e., significant increases in the incidence of liver adenomas and carcinomas) chronic rodent cancer studies fail to produce liver fibrosis or cirrhosis. In cases when cirrhotic changes are observed in rodent liver in association with development of hepatocellular neoplasms (e.g., thioacetamide and N-nitrosomorpholine), high necrogenic doses were applied (Becker, 1983; Oh et al., 2002).
Lack of fibrosis and cirrhosis in most positive 2-year cancer bioassays in rodents is in stark contrast to human HCC where liver cirrhosis is both the most common histopathological feature observed in subjects with HCC and an important mechanism of hepatocarcinogenesis (Farazi and DePinho, 2006). In fact, the incidence of HCC in noncirrhotic human livers is estimated at only 15–20% of all cases, and in the livers that are devoid of any signs of chronic disease, the incidence is even lower at about 10% (Alkofer et al., 2011). Thus, among many limitations, chronic rodent cancer bioassays do not address this key feature of human HCC.
Although mechanistic studies of chemical carcinogenesis in the liver have been largely replaced in the past decade by the genetically modified mouse models (Lee et al., 2004), testing of chemicals for cancer hazard continues to be conducted in B6C3F1 mouse hybrid strain, rather than in genetically altered mouse models (Bucher, 1998). Given the importance of liver cirrhosis in the development of human HCC, integrative studies that evaluate the mechanisms of fibrogenesis, and how they relate to hepatocyte transformation and modulation of oncogenic signaling, are most relevant to better understanding of the pathogenesis of human disease.
These goals can be best achieved by animal models that, as much as possible, mimic molecular pathogenesis of liver disease leading to HCC development in humans, where a combination of etiological factors such as genotoxic injury and advanced fibrosis is likely to be in play (Fausto and Campbell, 2010). Accordingly, this study used a well-known model of liver carcinogenesis induced by a single injection of a low-dose genotoxic agent diethylnitrosamine (DEN) into 14-day-old male mice (Druckrey et al., 1964; Vesselinovitch and Mihailovich, 1983). Then, repeat dosing of the profibrogenic agent carbon tetrachloride (CCl4) was performed, starting at 8 weeks of age for up to 14 consecutive weeks. We observed that in mice initiated with DEN, chronic liver fibrosis led to dramatic potentiation of the liver tumor incidence where 100% of the animals had developed liver tumors at 5 months of age. In addition, we show that in DEN-treated animals, this promotional effect on liver carcinogenesis was associated with the induction of cancer stem cells in noncancerous liver tissue, rather than an effect on liver inflammation, fibrogenesis, oxidative stress, proliferation, or apoptosis.
Materials and Methods
Animals and treatments.
Timed pregnant B6C3F1/J mice (Jackson, Bar Harbor, ME) arrived about a week before delivery. Male pups were randomly assigned to treatment groups (Fig. 1A). Male mice were selected for these studies because male gender is a risk factor for human HCC (Jepsen et al., 2007). At 2 weeks of age, mice in Groups 1 and 3 were injected ip with sterile PBS (15 µl/g) vehicle. At the same age, mice in Groups 2 and 4 were injected ip with DEN (1mg/kg) in sterile saline (15 µl/g). Mice were maintained on animal chow with free access to food/water for the duration of the study. At 8 weeks of age (6 weeks after a single injection of DEN or vehicle as detailed above), mice in groups 1 and 2 were injected ip 2× weekly with sterile olive oil (15ml/kg), and mice in groups 3 and 4 were injected ip 2× weekly with CCl4 (0.2ml/kg) diluted in oil vehicle for up to 14 additional weeks. On the day of the last injection, mice were given drinking water containing bromodeoxyuridine (BrDU; 0.2g/l; Sigma, St Louis, MO) for 3 days and sacrificed at the end of BrDU treatment.
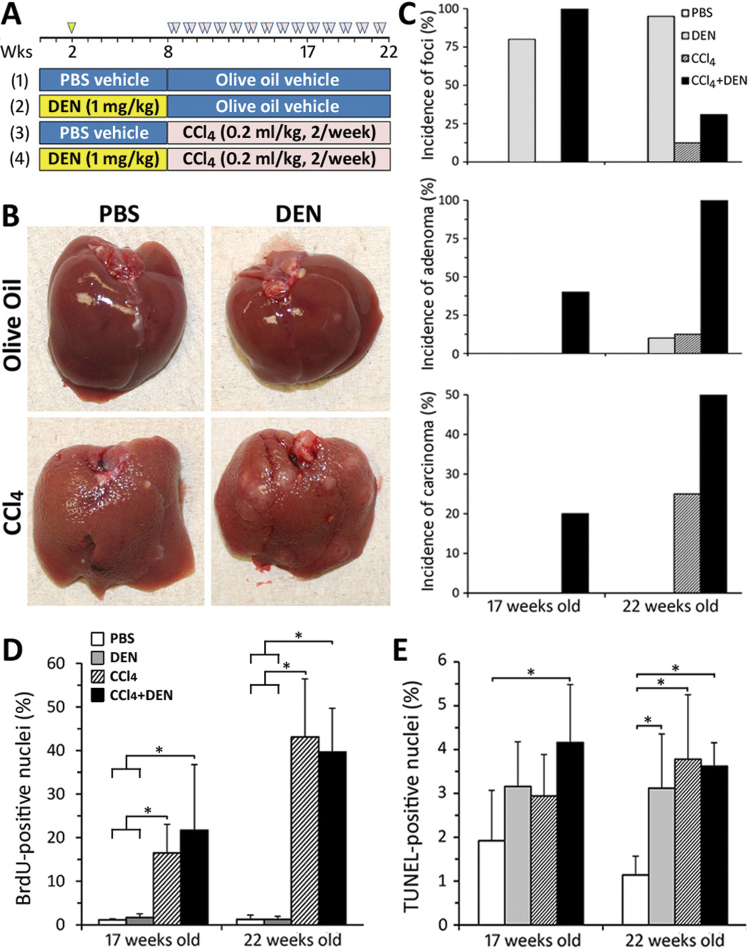
Fig. 1.
Experimental design, gross liver pathology, incidence of liver tumors, liver cell proliferation, and apoptosis. (A) Study design consisted of four groups (see Materials and Methods section) and two time points (17 and 22 weeks of age). (B) Representative photographs of the livers from the animals in each group at 22 weeks of age. (C) Incidence of the neoplastic (adenomas and carcinomas) and preneoplastic (foci) liver lesions. Mean ± SD values for percent BrDU- (D) or TUNEL- (E) positive nuclei for five randomly selected fields (devoid of foci, adenomas, or carcinomas) at ×200 are shown (n = 4–8 animals/group). Asterisks denote statistical significance from other groups (as indicated by the lines and brackets) at p < 0.05.
Two time points were considered. Five mice in each of the four groups were sacrificed at 17 weeks of age (9 weeks of CCl4). Remaining animals were sacrificed at 22 weeks of age (14 weeks of CCl4). Group sizes were larger (e.g., 26 animals in DEN + CCl4 group) for the 22 weeks of age time point to allow for more precise tumor incidence estimation. Body and wet liver weights were recorded, and blood was collected into heparin-containing syringes. All animal experiments were approved by the UNC Animal Care and Use Committee. Serum alanine aminotransferase, alkaline phosphatase, and albumin levels were determined using Vitro350 analyzer (Ortho-Clinical Diagnostic, Rochester, NY).
Liver histopathology.
The livers were examined macroscopically for masses by two certified veterinary pathologists independently and in a blinded fashion. All liver lobes were step-sectioned at 5–7mm to reveal macroscopically visible tumors. A section of the liver (including gross lesions) and duodenum was formalin-fixed and paraffin-embedded. Remaining liver tissue was stored at −80°C. Grossly visible tumors were separated from noncancerous liver tissue and frozen. Masson’s trichrome procedure was used to evaluate the degree of liver fibrosis with the degree of fibrosis assigned according to the following criteria: 0, no fibrotic changes; 1, slight fibrotic changes around the central vein and occasionally with thin bridging fibrosis; 2, thick bridging fibrosis and pseudolobule formation with dissecting nodules. Histopathological evaluation of the liver sections (scored using 0–3 scale), including the incidence and multiplicity of preneoplastic lesions (hepatocellular foci), was performed on the median and left liver lobes by veterinary pathologists.
Immunohistochemistry.
For paraffin-embedded liver sections (5 µm thick), we used Dako EnVision System (Dako, Carpinteria, CA) HRP kit. All primary antibodies were diluted in saline containing 1% bovine serum albumin. The primary antibodies used in these experiments: anti-BrDU (Dako; 1:200 dilution, 10min), rabbit anti-4-hydroxynonenal (HNE; Alpha Diagnostics, San Antonio, TX; 1:200, 30min), mouse anti-human alpha-smooth muscle actin (αSma; Dako; 1:100, 10min). Slides were counterstained with filtered Mayer’s hematoxylin (Sigma) for 5min. Apoptotic bodies in liver sections were detected by terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) of DNA fragments using an ApopTag Peroxidase In Situ Apoptosis Detection Kit (Serologicals Corporation, Norcross, GA). Quantitative analysis was performed using Image-ProVR Plus (Media Cybernetics, Silver Spring, MD) at 100× magnification. For αSma and 4-HNE, five fields of noncancerous tissue were randomly selected to calculate percent of positively stained area. For BrDU and TUNEL, 10 fields were selected at random, and liver parenchymal cells were counted (> 1000 cells per slide).
Frozen liver sections (10 μm) were fixed in acetone/methanol (1:1) at 4°C for 10min and incubated with 10% goat serum for 30min and then with 0.3% H2O2 and 0.065% sodium azide for 30min. The following primary antibodies (staining time 1h, room temperature) were used: rat anti-F4/80 (1:200), rat anti-CD68 (1:2000), rat anti-CD11b (1:500) were from AbD Serotec, Oxford, UK, and rat anti-MHC class II (1:1000, BD Pharmingen, San Diego, CA). Peroxidase-conjugated secondary antibody (Nichirei, Tokyo, Japan) and 3,3′-diaminobenzidine (Vector, Burlingame, CA) were employed for visualization. Positively stained cells (per millimeter square area) were counted in liver tissue, as well as in interlobular connective tissue, regions in three random fields using WinRoof software (Mitani, Fukui, Japan).
Quantification of genomic 8-oxo-deoxyguanine.
Capillary liquid chromatography tandem mass spectrometry was used for the detection of 8-oxo-deoxyguanine (8-oxo-dG) adducts in liver DNA isolated and processed as detailed in the study by Powell et al. (2006).
Quantitative real-time reverse transcription PCR.
RNeasy kit (Qiagen, Valencia, CA) was used to isolate mRNA from frozen liver. RNA concentration and quality were assessed using ND-1000 (NanoDrop, Wilmington, DE) and 2100 Bioanalyzer (Agilent, Santa Clara, CA), respectively. Reverse transcription was carried out using High Capacity cDNA RT kit (Applied Biosystems, Foster City, CA). Each sample was analyzed in duplicate using the Light-Cycler 480 (Roche, Indianapolis, IN). Primers were obtained from Applied Biosystems and are listed in Supplementary table 1. The relative amount of target gene mRNAs was normalized to internal control gene Gusb.
Statistical analyses.
Statistical significance was determined using GraphPad (San Diego, CA) Prism5. Quantitative values are expressed as mean ± SD unless otherwise noted. Statistical significance was evaluated using one-way ANOVA within each time point followed by the Bonferroni’s post hoc test. Statistical significance is indicated at any level below p < 0.05.
Results
We first studied whether chronic treatment with a profibrogenic agent (CCl4) affects carcinogenesis caused by DEN, a genotoxic agent. Animals in all four groups (Fig. 1A) survived until sacrifice at 17 (n = 5/group) or 22 weeks of age (n = 7–26/group). Serum markers of liver injury were evaluated (Table 1), and livers were examined both macro- and microscopically for presence of pre- and neoplastic lesions, as well as signs of tissue injury (Figs. 1B and C, Table 2). No liver injury or pre- or neoplastic lesions were found in the vehicle group at both time points.
Table 1.
Effects of DEN and CCl4 on Serum Markers of Liver Injury and Relative Liver Weight
| PBS | DEN | CCl4 | CCl4 + DEN | PBS | DEN | CCl4 | CCl4 + DEN | |
|---|---|---|---|---|---|---|---|---|
| Age at sacrifice (weeks) | 17 | 17 | 22 | 22 | ||||
| Number of animals/group | 5 | 5 | 7 | 20 | 8 | 26 | ||
| Serum albumin (g/dl) | 2.5±0.1 | 2.5±0.1 | 2.7±0.1 | 2.4±0.1 | 2.6±0.1 | 2.6±0.1 | 2.9±0.1 | 3.4±0.3a,b |
| Serum alanine aminotransferase (U/l) | 88±23 | 43±3 | 84±2 | 74±5 | 48±5 | 52±4 | 202±12a,b | 185±20a,b |
| Serum alkaline phosphatase (U/l) | 72±4 | 72±6 | 66±5 | 67±4 | 44±7 | 55±7 | 79±8a,b | 72±4a,b |
| Relative liver weight (%) | 4.6±0.1 | 4.8±0.2 | 5.3±0.1a | 5.2±0.1a | 5.2±0.2 | 4.9±0.2 | 6.4±0.2a,b | 7.2±0.3a,b,c |
Note. Mean ± SD values are shown.
aIndicates statistical significance of the differences (p < 0.05) from the corresponding (with respect to the time point) PBS-treated groups.
bIndicates statistical significance of the differences (p < 0.05) from the corresponding (with respect to the time point) DEN-treated groups.
cIndicates statistical significance of the differences (p < 0.05) from the corresponding (with respect to the time point) CCl4-treated groups.
Table 2.
Effects of DEN and CCl4 on Liver Histopathology
| PBS | DEN | CCl4 | CCl4 + DEN | PBS | DEN | CCl4 | CCl4 + DEN | |
|---|---|---|---|---|---|---|---|---|
| Age at sacrifice (weeks) | 17 | 17 | 22 | 22 | ||||
| Number of animals/group | 5 | 5 | 7 | 20 | 8 | 26 | ||
| Single cell necrosisa | ||||||||
| 1+ | 5 (19) | |||||||
| 2+ | 6 (75) | 7 (27) | ||||||
| 3+ | 1 (13) | 6 (23) | ||||||
| Total | 7 (88) | 18 (69) | ||||||
| Fibrosis with mononuclear cells infiltration | ||||||||
| 1+ | 5 (100) | 5 (100) | ||||||
| 2+ | 8 (100) | 26 (100) | ||||||
| 3+ | ||||||||
| Total | 5 (100) | 5 (100) | 8 (100) | 26 (100) | ||||
| Hypertrophy | ||||||||
| 1+ | 5 (100) | 5 (100) | ||||||
| 2+ | 8 (100) | 26 (100) | ||||||
| 3+ | ||||||||
| Total | 5 (100) | 5 (100) | 8 (100) | 26 (100) | ||||
| Increased mitosis | ||||||||
| 1+ | 5 (19) | |||||||
| 2+ | 8 (100) | 21 (81) | ||||||
| 3+ | ||||||||
| Total | 8 (100) | 26 (100) | ||||||
| Anisonucleosis | ||||||||
| 1+ | 5 (100) | 5 (100) | ||||||
| 2+ | 25 (96) | |||||||
| 3+ | 8 (100) | 1 (4) | ||||||
| Total | 5 (100) | 5 (100) | 8 (100) | 26 (100) | ||||
| Lipofuscin deposition | ||||||||
| 1+ | 5 (100) | 5 (100) | ||||||
| 2+ | 8 (100) | 26 (100) | ||||||
| 3+ | ||||||||
| Total | 5 (100) | 5 (100) | 8 (100) | 26 (100) | ||||
| Steatosis | ||||||||
| 1+ | 1 (20) | 2 (8) | ||||||
| 2+ | 4 (50) | 21 (80) | ||||||
| 3+ | 2 (25) | |||||||
| Total | 1 (20) | 6 (75) | 23 (88) | |||||
Note. aNumber of animals for each grade (% of total).
In animals that received only DEN (1mg/kg), a single nonnecrogenic dose to study initiation phase of carcinogenesis, no abnormalities in serum or liver markers of injury, or gross liver pathology, were observed (Tables 1 and 2, Fig. 1B); however, a marked increase in the incidence of liver foci (above 80% at both time points) and liver adenomas (10% only at 22 week of age group) was detected (Fig. 1C).
In CCl4 (0.2ml/kg) only–treated groups, a significant increase in liver to body weight ratio (Table 1) and progressively worsening (with time) changes in liver histopathology and serum markers (Tables 1 and 2) were detected. Specifically, single cell necrosis, ballooning degeneration of hepatocytes, and steatosis were observed in central and midlobular regions, often associated with fibrosis and inflammatory cell infiltration. Nonnecrotic hepatocytes exhibited hypertrophy with anisonucleosis. At 17 weeks of age group, no foci, adenomas, or carcinomas were observed. Liver foci and adenomas were found in 12.5% of mice at 22 weeks of age group, whereas the incidence of carcinomas was 25% (Fig. 1C).
Most pronounced pathological observations were detected in the livers of animals that were first initiated with DEN and then treated with repeat injections of CCl4. In 17-week-old animals, an increase in relative liver weight and mild liver histopathological changes were observed (Tables 1 and 2). All animals in this group had already developed liver foci, whereas 40 and 20% of animals manifested with liver adenomas and carcinomas, respectively (Fig. 1C). In 22-week-old animals, all animals in this group exhibited increases in relative liver weight and marked elevation of serum and histopathological markers of liver injury (Tables 1 and 2). All animals developed liver adenomas, and 50% also had liver carcinomas (Fig. 1C). It is of note that in both CCl4-treated groups, the severity of liver injury (single cell necrosis, ballooning degeneration and hypertrophy of hepatocytes, and fibrosis with inflammatory cells infiltration) in noncancerous tissue was of similar grade regardless of whether the animals were initiated with DEN or not (Tables 1 and 2).
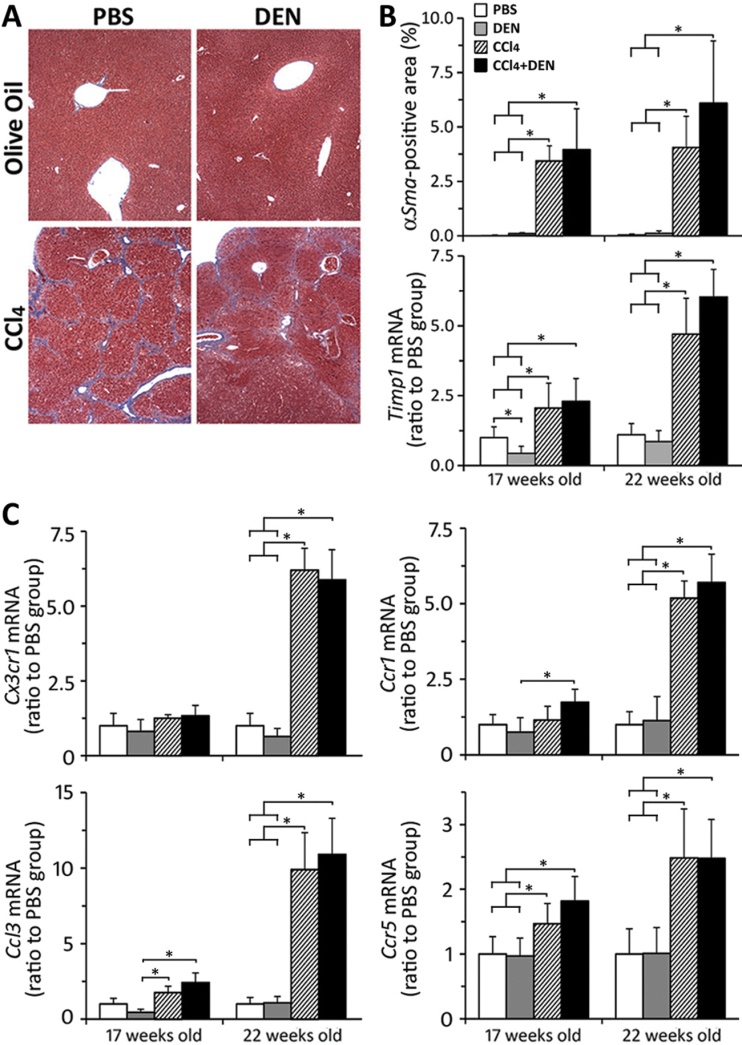
It is well established that continuous exposure to CCl4 leads to the development of severe hepatic fibrosis and compensatory cell proliferation (Stowell et al., 1951). Thus, we determined whether the marked increase in liver tumor incidence in DEN + CCl4 group may be due to combined effects on hepatocyte turnover or liver fibrosis. Cell proliferation, as assessed by BrDU labeling index (Fig. 1D) and mitotic figures (Table 2), was progressively elevated in animals that received CCl4, independent of prior initiation with DEN. Apoptosis, as measured by TUNEL-positive nuclei (Fig. 1E), was elevated up to threefold, but there was no difference between CCl4 and DEN + CCl4 groups at either time point. Liver fibrosis was assessed using Masson’s trichrome staining (Fig. 2A), immunostaining for αSma, and expression of Timp1 (Fig. 2B). Clear evidence of progressively worsening liver fibrosis was observed in CCl4-treated groups, whereas treatment with DEN had no additive effect.
Fig. 2.
Liver fibrogenesis markers. (A) Representative Mason’s trichrome stain sections (×100) from animals at 22 weeks of age. (B) Immunostaining of αSMA in liver in five randomly selected fields at ×200; liver expression of Timp1 (relative to levels in PBS group at each time point). Treatment groups are denoted as indicated in the graphical legend. Mean ± SD values are shown, n = 4–8 animals/group. (C) Liver expression of profibrogenic inflammatory cytokines. Treatment groups are denoted as indicated in the legend in panel B. Mean ± SD values are shown, n = 5 animals per group. Asterisks denote statistical significance from other groups (as indicated by the lines and brackets) at p < 0.05.
Recent studies have identified the key role of chemokines and chemokine receptors in promotion of hepatic fibrosis, including that caused by CCl4 (Aoyama et al., 2010; Seki et al., 2009). Accordingly, we have evaluated the changes in expression of key profibrogenic chemokines and their receptors in our model (Fig. 2C). Expression of Ccr1, Ccr5, Ccl3, and Cx3cr1 was markedly elevated in 22-week-old mice treated with CCl4, independent of prior initiation with DEN. Significant changes in expression of some chemokines were also evident in 17-week-old animals.
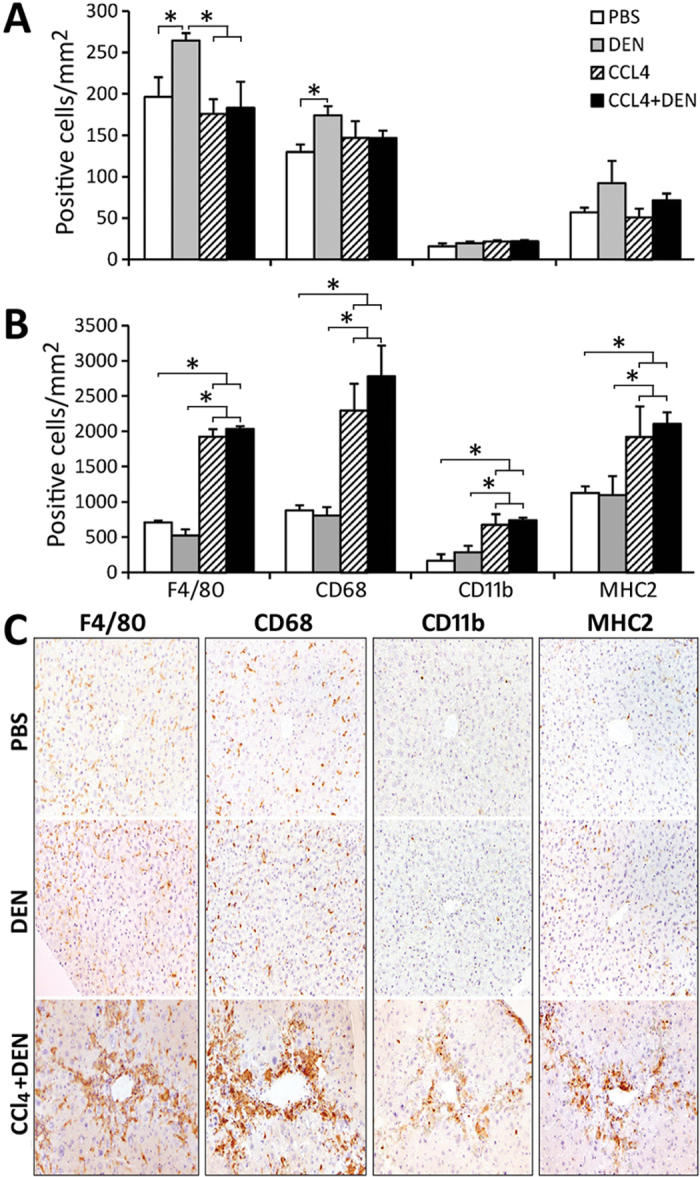
Both CCl4 and DEN are known to promote liver inflammation and oxidative stress, which are potential mechanisms involved in hepatic carcinogenesis. Thus, we evaluated whether these mechanisms may be responsible for the difference in liver tumor incidence between CCl4 and DEN + CCl4 groups. We performed detailed profiling of the populations of inflammatory cells in liver tissue and fibrotic areas in mice that were 22 weeks of age. In the liver tissue of control mice, F4/80-positive macrophages were predominant (Figs. 3A and C). These were located mainly in the sinusoids and exhibited cytoplasmic morphology characteristic of Kupffer cells. CD68-positive macrophages were the second most frequent inflammatory cell in the liver tissue, and the distribution pattern was similar to that of F4/80-positive macrophages. CD11b-positive small round cells with inverted vesicular nuclei and monocytic morphology were few in the normal tissue. There were some MHC2-positive macrophages in the sinusoids of liver tissue. In DEN-initiated mice, the number of F4/80- and CD68-positive sinusoidal macrophages was significantly increased compared with vehicle group. A much greater number of inflammatory cells of all types was present in nonparenchymal liver tissue (Figs. 3B and C), the predominant population being MHC2-positive macrophages with dendritic morphology that were located around portal blood vessels. The number of macrophages with all four immunophenotypes was significantly increased in the fibrotic lesions of CCl4 and DEN + CCl4 groups compared with vehicle and DEN-only groups; however, there was no significant difference between CCl4 and DEN + CCl4 groups. The macrophages in the fibrotic lesions varied in morphology (Fig. 3C), suggesting a heterogeneous population.
Fig. 3.
Macrophage subpopulations in liver tissue and fibrotic areas. Immunohistochemical analysis of macrophage markers F4/80, CD68, CD11b, and MHC2 was conducted at 22 weeks of age. Image analysis is presented for nonfibrotic/noncancerous liver tissue (A) or fibrotic and perivascular regions (B). Mean ± SD values are shown, n = 3 animals/group. Asterisks denote statistical significance from other groups (as indicated by the lines and brackets) at p < 0.05. Panel C shows representative microphotographs at ×100.
In all CCl4-treated animals at both time points, the histopathological analysis of liver (Table 2) revealed progressively worsening anisonucleosis, a morphological manifestation of nuclear injury characterized by variation in the size of the hepatocyte nuclei, an observation that has been associated with hepatic oxidative stress (Guzman et al., 2011). In addition, accumulation of lipofuscin granules, lipid-containing remnants of lysosomes considered to be a hallmark of lipid peroxidation in the liver, was also observed in these groups. DEN treatment had no effect on these phenotypes. To further evaluate the degree of oxidative stress (Table 3), we assessed lipid peroxidation through immunohistochemical detection of 4-HNE-adducted proteins, DNA oxidation from the number of 8-oxo-dG adducts, and expression of several key base-excision DNA repair genes (Rusyn et al., 2004). A significant increase in hepatic lipid peroxidation was observed in CCl4-treated groups at both time points. DEN + CCl4 groups had higher levels of 4-HNE positive staining than those in CCl4-only groups, albeit the difference was not significant (p > 0.05). No change in the number of 8-oxo-dG adducts was observed. Expression of Polβ was lower in CCl4 and DEN + CCl4 groups in 17- and 22-week-old animals compared with corresponding control groups. Expression of Ogg1 was decreased only in DEN + CCl4 group in 22-week-old animals.
Table 3 .
Effects of DEN and CCl4 on Oxidative Stress Markers
| PBS | DEN | CCl4 | CCl4 + DEN | PBS | DEN | CCl4 | CCl4 + DEN | |
|---|---|---|---|---|---|---|---|---|
| Age at sacrifice (weeks) | 17 | 17 | 22 | 22 | ||||
| 4-HNE positive area (%) | 4.1±1.7 | 5.8±3.5 | 19±8a,b | 30±6a,b | 5.4±2.3 | 10±3 | 13±7a | 26±11a,b |
| 8-oxo-dG/106 dG | 1.6±0.5 | 1.3±0.2 | 2.3±1.1 | 2.1±0.9 | 1.2±0.2 | 1.0±0.3 | 1.2±0.4 | 1.4±0.2 |
| Ogg1 mRNAc | 100±21 | 92±8 | 85±13 | 89±10 | 100±32 | 83±9 | 65±20 | 53±9b |
| Apex mRNAc | 100±22 | 88±14 | 160±97 | 140±25 | 100±17 | 126±33 | 82±30 | 84±34 |
| Polβ mRNAc | 100±10 | 84±11 | 48±26a | 36±8b | 100±25 | 110±14 | 48±11a | 43±28b |
Notes. Mean ± SD values are shown, n = 4–8 animals/group.
aStatistical significance (p < 0.05) from corresponding time point PBS groups is indicated.
bStatistical significance (p < 0.05) from corresponding time point DEN groups is indicated.
cLiver expression levels of base-excision DNA repair genes Ogg1 (8-oxoguanine DNA-glycosylase 1), Apex (apurinic/apyrimidinic endonuclease 1), and Polβ (polymerase (DNA directed), beta) are expressed as ratio to corresponding time point’s PBS group.
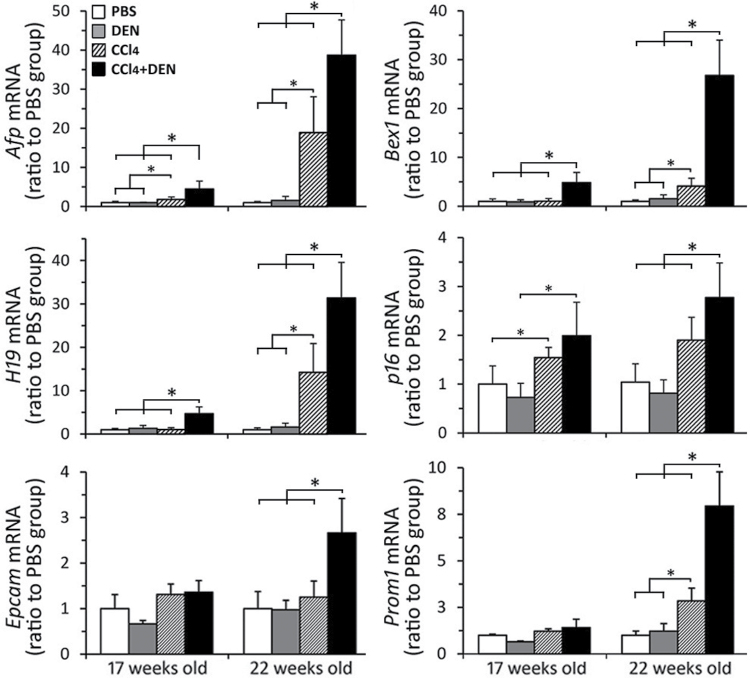
A dramatic increase in cell proliferation was observed in livers of CCl4- and DEN + CCl4–treated mice (Fig. 1D). Recent studies suggest that high proliferation due to hepatitis and cirrhosis leads to hepatocyte senescence and activation of hepatic progenitor cells, which in turn heightens the risk of neoplastic transformation (Oishi and Wang, 2011). The concept of liver cancer stem cells has gathered significant attention, and most of experimental evidence suggests that these cells derive from hepatic progenitor cells (Wang and Jacob, 2011). Accordingly, we have investigated the markers of oncofetal liver transformation and cancer stem cells (Fig. 4). Upregulation of Afp, H19, and Bex1, fetal stage–specific genes, is known to be present in liver tumors but has also been observed in noncancerous liver tissue (Inokuchi et al., 2010). In our study, increased expression of these genes was observed in noncancerous liver tissue in both CCl4-treated groups as early as 17 weeks of age. Notably, expression of these genes was significantly (two- to fivefold) greater in DEN + CCl4 groups compared with CCl4-only groups. To determine whether a decline in regenerative potential, indicative of hepatocyte senescence, is observed, we evaluated expression of p16, a cell-cycle inhibitor that is regarded as a key regulator of entry of cells into S-phase (Krishnamurthy et al., 2004). Indeed, expression of p16 was increased in DEN + CCl4 group at both 17 and 22 weeks of age and significantly different from that in other groups. To determine whether expression of cancer stem cell markers is associated with higher liver tumor incidence in this model, we considered Prom1 (CD133; Ma et al., 2007) and Epcam (Yamashita et al., 2009). Although no effect was observed in 17-week-old mice (all groups), expression of both genes was significantly higher (2- to 2.5-fold) in DEN + CCl4 group compared with CCl4-only and other groups at 22 weeks of age.
Fig. 4.
Markers of oncofetal transformation, senescence, and cancer stem cells. Liver gene expression of the genes indicated on the y-axis of each graph was evaluated as detailed in Materials and Methods section (see Supplementary table 1 for gene names and primers). Treatment groups are denoted as indicated in the legend in top left panel. Mean ± SD values are shown, n = 5 animals/group. Asterisks denote statistical significance from other groups (as indicated by the lines and brackets) at p < 0.05.
Discussion
Our current understanding of cancer etiology and pathogenesis in general, and liver carcinogenesis in particular, is based largely on the multistage model of clonal evolution (Pitot, 1977) and the concepts of the “mutator phenotype” (Loeb, 2001) and “hallmarks of cancer” (Hanahan and Weinberg, 2011). Much is known about the histopathological progression, molecular features, and genetic diversity of liver tumors (Farazi and DePinho, 2006; Nault and Zucman-Rossi, 2011). The role of cancer stem cells in tumor development has been also proposed (Marquardt and Thorgeirsson, 2010; Wang and Jacob, 2011). The field of experimental liver cancer research has generated much observational and molecular evidence for the above-mentioned conceptual models, including data on the modifying effects of various lifestyle factors (e.g., alcohol drinking), dietary exposures (e.g., aflatoxins), chronic viral infections (e.g., hepatitis viruses B and C), and advanced fibrosis and cirrhosis (e.g., CCl4). Specifically, CCl4 is a well-known “promoting” agent in rodent hepatocarcinogenesis, with most of the data demonstrating its ability to increase the incidence of liver tumors following initiation with a genotoxic chemical being published 20 or more years ago (Dragani et al., 1986).
Our study generated novel results as it allows better understanding of why patients with liver cirrhosis and etiological factors that may promote DNA damage (e.g., exposure to genotoxic agents, viral hepatitis, etc.) are more prone to development of HCC than patients with cirrhosis alone (El-Serag and Rudolph, 2007). Indeed, using a mouse model of HCC induced by a single injection of a genotoxic chemical followed by advanced liver fibrosis, we performed a comprehensive evaluation of the well-accepted mechanisms of liver carcinogenesis, such as cell proliferation, apoptosis, fibrogenesis, necrosis, inflammation, and oxidative stress. Although a clear increase in these molecular mechanisms of liver cancer was observed in animals treated with CCl4, the magnitude of the observed effects was largely unrelated to tumor incidence and not affected by DEN. This suggests that other more subtle molecular events may have a pronounced overall impact on tumor incidence, especially when coexposure to multiple agents takes place.
One mechanism that could be responsible for the observed acceleration of HCC development in this model is the potential for CCl4 to cause DNA damage. It cannot be unequivocally concluded that CCl4 is only fibrogenic and not genotoxic in this model. However, we reason that there is only a minor, if any, role of potential oxidative stress–derived DNA damage in the observed cocarcinogenesis effect. CCl4 is not a directly mutagenic agent, but it may contribute to DNA damage and mutations through indirect mechanisms resulting from oxidative and lipid peroxidative damage or damage occurring during necrosis or apoptosis (Eastmond, 2008). The key events in this process are expected to occur in a nonlinear fashion, and it is known that the relationship between CCl4 dose and carcinogenic response in the liver is nonlinear with a steep dose response (Nagano et al., 2007). We have used low doses of CCl4 compared with other published studies that evaluated subchronic liver toxicity in rodents using ip injections. For example, a dose that is 2.5-times higher (0.5ml/kg) was shown to only marginally affect liver oxidant/antioxidant balance (Cabré et al., 2000). In our study, neither hepatotoxicity was observed following 9 weeks of CCl4 administration (no elevation of serum enzymes or signs of liver necrosis) nor was there evidence for macrophage infiltration in nonfibrotic liver tissue (by immunohistochemical detection). Likewise, although lipid peroxidation was detected in CCl4-treated groups, compared with animals receiving oil injections, this effect was not associated with an increase in oxidative DNA damage (i.e., 8-oxo-dG adducts), and it was not concordant with the marked differences in tumor incidence between groups. Still, extensive genetic analysis (e.g., comparative genomic hybridization and exome sequencing) of the tumors in each group may be needed to assess whether CCl4 acts as a genotoxic agent or not in the present model.
The hierarchical cancer model posits that another key event in liver carcinogenesis that may be responsible for the observed differences is formation of cancer stem cells that occupy the apex of tumor formation and are responsible for tumor initiation, growth, and metastases (Marquardt and Thorgeirsson, 2010). Although the mechanisms of cancer stem cell involvement in the initiation and progression of HCC are a subject of intense research and debate, most accept their key role in HCC (Ji and Wang, 2012; Lee et al., 2012; Majumdar et al., 2012). Few studies, however, have evaluated cancer stem cells in the noncancerous liver tissue. In our study, the marked effect of liver fibrosis on genotoxic agent–initiated liver was associated with replicative senescence (e.g., overexpression of p16), oncofetal transformation (e.g., increased expression of Afp, H19, and Bex1), and increased “stemness” (e.g., increased expression of Prom1 and Epcam) of the noncancerous liver tissue. Indeed, all of these pathways have been linked to cancer stem cell–dependent events in hepatocarcinogenesis (Oishi and Wang, 2011; Wang and Jacob, 2011).
Expression of p16, a cell-cycle inhibitor that prevents CDK4/6-dependent phosphorylation of retinoblastoma protein that is controlling the entry of cells into S-phase (Chazal et al., 2002), is delicately balanced in mammalian tissues. Because of its role in proliferation, p16 is a molecular biomarker of cellular senescence, with its expression markedly increasing with age (Krishnamurthy et al., 2004). Interestingly, DNA damage from chemicals, UV or radiation, and telomere dysfunction and oxidative stress are known to result in increased expression of p16 (Li et al., 2011), which, in turn, triggers hepatocyte senescence and reactivation of hepatic progenitor cells (Yang et al., 2004).
The increased stemness of the noncancerous liver tissue in CCl4-treated animals is in line with recent evidence that there is an association between fibrosis and liver progenitor cell activation (Greenbaum and Wells, 2011). Prom1 was significantly induced in noncancerous and tumor samples in mice treated with CCl4 for 15 weeks (Fujii et al., 2010). Although the mechanisms that link progenitor cells and liver fibrosis have not been elucidated fully, Hedgehog, Wnt, and Notch have been identified as common pathways. Importantly, our observation of a much greater incidence of liver tumors in DEN + CCl4 group, compared with that in CCl4-alone group, suggests thatalthough fibrosis and hepatocarcinogenesis may share similar molecular pathways that result from adaptive responses during chronic liver injury, presence of DNA mutations, as a result of exposure to genotoxic agents, may tip the balance even more toward carcinogenesis. This concept is supported by a recent study that showed that a connection of differentiation and DNA damage responses in liver stem or progenitor cells allows for the formation of tumor-initiating cells (Kossatz et al., 2010). Increased expression of oncofetal genes in animals with the greatest incidence of liver tumors is another line of evidence that suggests that tissue regeneration pathways and carcinogenesis are not the same as several of marker genes tested are not upregulated in regenerating liver (Graveel et al., 2001). Reactivation of oncofetal genes is a common feature in both human HCC and in murine liver cancer induced by DEN (Braeuning et al., 2006; Graveel et al., 2001).
While we posit that markers of stemness in the noncancerous liver tissue are markers of early carcinogenesis, an additional explanation is that they may be a sign of poor hepatocyte regeneration. We (Sancho-Bru et al., 2012) and others (Katoonizadeh et al., 2006) have demonstrated that hepatic progenitor cells accumulate in patients with advanced liver cirrhosis and are associated with a poor outcome. The accumulation of these cells reflects poor hepatic regeneration and may be also linked to increased carcinogenesis. Thus, the results of this study may suggest that expression of hepatic progenitor cell markers in the noncancerous liver tissue could be explored in additional studies as a biomarker of predisposition for the development of HCC in cirrhotic subjects.
Another important outcome of this study is the model itself whereby it features the hallmarks of human liver cancer: chronic liver injury, inflammation, and fibrosis. This experimental design, incorporating chronic exposure to a relatively low dose of CCl4, compared with most other studies of CCl4-induced fibrosis or hepatocarcinogenesis (Dragani et al., 1986; Fujii et al., 2010), with an environmental chemical carcinogen may provide a useful model to further investigate the contribution of inflammation and fibrosis to liver cancer. Such “fibrotic” liver murine model may not only be more human relevant but also faster (HCCs and adenomas develop in as little as 17 weeks of age) than a traditional 2-year rodent cancer bioassay used widely to ascribe cancer hazard to chemicals and drugs. As much research effort and regulatory pressure is directed at the development of short-term transgenic mouse assays to supplant the need for most 2-year bioassays (Jacobson-Kram, 2010), the model characterized in our study may represent a more clinically relevant scenario. Indeed, as it is well recognized that most of the human HCCs are a result of multiple etiologies and require liver cirrhosis (Alkofer et al., 2011), the model described here is more likely, compared with several transgenic models currently being evaluated (Bucher, 1998), to be able to recapitulate fibrotic phenotype seen in humans.
Supplementary Data
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
Funding
National Institutes of Health (P42 ES005948, R01 ES015241, P30 ES010126).
Supplementary Material
Acknowledgments
The authors are grateful to Leonard Collins and Jessica Sorrentino for technical assistance.
References
- Alkofer B., Lepennec V., Chiche L. (2011). Hepatocellular cancer in the non-cirrhotic liver. J. Visc. Surg. 148, 3–11 [DOI] [PubMed] [Google Scholar]
- Aoyama T., Inokuchi S., Brenner D. A., Seki E. (2010). CX3CL1-CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology. 52, 1390–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker F. F. (1983). Thioacetamide hepatocarcinogenesis. J. Natl. Cancer Inst. 71, 553–558 [PubMed] [Google Scholar]
- Braeuning A., Jaworski M., Schwarz M., Köhle C. (2006). Rex3 (reduced in expression 3) as a new tumor marker in mouse hepatocarcinogenesis. Toxicology. 227, 127–135 [DOI] [PubMed] [Google Scholar]
- Bucher J. R. (1998). Update on national toxicology program (NTP) assays with genetically altered or “transgenic” mice. Environ. Health Perspect. 106, 619–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabré M., Camps J., Paternáin J. L., Ferré N., Joven J. (2000). Time-course of changes in hepatic lipid peroxidation and glutathione metabolism in rats with carbon tetrachloride-induced cirrhosis. Clin. Exp. Pharmacol. Physiol. 27, 694–699 [DOI] [PubMed] [Google Scholar]
- Center M. M., Jemal A. (2011). International trends in liver cancer incidence rates. Cancer Epidemiol. Biomarkers Prev. 20, 2362–2368 [DOI] [PubMed] [Google Scholar]
- Chazal M., Marionnet C., Michel L., Mollier K., Dazard J. E., Della Valle V., Larsen C. J., Gras M. P., Basset-Séguin N. (2002). P16(INK4A) is implicated in both the immediate and adaptative response of human keratinocytes to UVB irradiation. Oncogene. 21, 2652–2661 [DOI] [PubMed] [Google Scholar]
- Della Corte C., Colombo M. (2012). Surveillance for hepatocellular carcinoma. Semin. Oncol. 39, 384–398 [DOI] [PubMed] [Google Scholar]
- Dragani T. A., Manenti G., Della Porta G. (1986). Enhancing effects of carbon tetrachloride in mouse hepatocarcinogenesis. Cancer Lett. 31, 171–179 [DOI] [PubMed] [Google Scholar]
- Druckrey H., Steinhoff D., Preussmann R., Ivankovic S. (1964). [Induction of cancer by a single dose of methylnitroso-urea and various dialkylnitrosamines in rats]. Z. Krebsforsch. 66, 1–10 [PubMed] [Google Scholar]
- Eastmond D. A. (2008). Evaluating genotoxicity data to identify a mode of action and its application in estimating cancer risk at low doses: A case study involving carbon tetrachloride. Environ. Mol. Mutagen. 49, 132–141 [DOI] [PubMed] [Google Scholar]
- El-Serag H. B., Rudolph K. L. (2007). Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 132, 2557–2576 [DOI] [PubMed] [Google Scholar]
- Farazi P. A., DePinho R. A. (2006). Hepatocellular carcinoma pathogenesis: From genes to environment. Nat. Rev. Cancer. 6, 674–687 [DOI] [PubMed] [Google Scholar]
- Fausto N., Campbell J. S. (2010). Mouse models of hepatocellular carcinoma. Semin. Liver Dis. 30, 87–98 [DOI] [PubMed] [Google Scholar]
- Fujii T., Fuchs B. C., Yamada S., Lauwers G. Y., Kulu Y., Goodwin J. M., Lanuti M., Tanabe K. K. (2010). Mouse model of carbon tetrachloride induced liver fibrosis: Histopathological changes and expression of CD133 and epidermal growth factor. BMC Gastroenterol. 10, 79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveel C. R., Jatkoe T., Madore S. J., Holt A. L., Farnham P. J. (2001). Expression profiling and identification of novel genes in hepatocellular carcinomas. Oncogene. 20, 2704–2712 [DOI] [PubMed] [Google Scholar]
- Greenbaum L. E., Wells R. G. (2011). The role of stem cells in liver repair and fibrosis. Int. J. Biochem. Cell Biol. 43, 222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman G., Chennuri R., Voros A., Boumendjel R., Locante A., Patel R., Valyi-Nagy T. (2011). Nucleometric study of anisonucleosis, diabetes and oxidative damage in liver biopsies of orthotopic liver transplant recipients with chronic hepatitis C virus infection. Pathol. Oncol. Res. 17, 191–199 [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. (2011). Hallmarks of cancer: The next generation. Cell. 144, 646–674 [DOI] [PubMed] [Google Scholar]
- Heindryckx F., Colle I., Van Vlierberghe H. (2009). Experimental mouse models for hepatocellular carcinoma research. Int. J. Exp. Pathol. 90, 367–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenerhoff M. J., Pandiri A. R., Lahousse S. A., Hong H. H., Ton T. V., Masinde T., Auerbach S. S., Gerrish K., Bushel P. R., Shockley K. R., et al. (2011). Global gene profiling of spontaneous hepatocellular carcinoma in B6C3F1 mice: Similarities in the molecular landscape with human liver cancer. Toxicol. Pathol. 39, 678–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff J., Cirvello J., Haseman J., Bucher J. (1991). Chemicals associated with site-specific neoplasia in 1394 long-term carcinogenesis experiments in laboratory rodents. Environ. Health Perspect. 93, 247–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi S., Aoyama T., Miura K., Osterreicher C. H., Kodama Y., Miyai K., Akira S., Brenner D. A., Seki E. (2010). Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc. Natl. Acad. Sci. U.S.A. 107, 844–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson-Kram D. (2010). Cancer risk assessment approaches at the FDA/CDER: Is the era of the 2-year bioassay drawing to a close?. Toxicol. Pathol. 38, 169–170 [DOI] [PubMed] [Google Scholar]
- Jepsen P., Vilstrup H., Tarone R. E., Friis S., Sørensen H. T. (2007). Incidence rates of hepatocellular carcinoma in the U.S. and Denmark: Recent trends. Int. J. Cancer. 121, 1624–1626 [DOI] [PubMed] [Google Scholar]
- Ji J., Wang X. W. (2012). Clinical implications of cancer stem cell biology in hepatocellular carcinoma. Semin. Oncol. 39, 461–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoonizadeh A., Nevens F., Verslype C., Pirenne J., Roskams T. (2006). Liver regeneration in acute severe liver impairment: A clinicopathological correlation study. Liver Int. 26, 1225–1233 [DOI] [PubMed] [Google Scholar]
- Kossatz U., Breuhahn K., Wolf B., Hardtke-Wolenski M., Wilkens L., Steinemann D., Singer S., Brass F., Kubicka S., Schlegelberger B., et al. (2010). The cyclin E regulator cullin 3 prevents mouse hepatic progenitor cells from becoming tumor-initiating cells. J. Clin. Invest. 120, 3820–3833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy J., Torrice C., Ramsey M. R., Kovalev G. I., Al-Regaiey K., Su L., Sharpless N. E. (2004). Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 114, 1299–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Chu I. S., Mikaelyan A., Calvisi D. F., Heo J., Reddy J. K., Thorgeirsson S. S. (2004). Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat. Genet. 36, 1306–1311 [DOI] [PubMed] [Google Scholar]
- Lee T. K., Cheung V. C., Ng I. O. (2012). Liver tumor-initiating cells as a therapeutic target for hepatocellular carcinoma. Cancer Lett. (forthcoming). [DOI] [PubMed] [Google Scholar]
- Li J., Poi M. J., Tsai M. D. (2011). Regulatory mechanisms of tumor suppressor P16(INK4A) and their relevance to cancer. Biochemistry. 50, 5566–5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A. (2001). A mutator phenotype in cancer. Cancer Res. 61, 3230–3239 [PubMed] [Google Scholar]
- Ma S., Chan K. W., Hu L., Lee T. K., Wo J. Y., Ng I. O., Zheng B. J., Guan X. Y. (2007). Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 132, 2542–2556 [DOI] [PubMed] [Google Scholar]
- Majumdar A., Curley S. A., Wu X., Brown P., Hwang J. P., Shetty K., Yao Z. X., He A. R., Li S., Katz L., et al. (2012). Hepatic stem cells and transforming growth factor β in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 9, 530–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt J. U., Thorgeirsson S. S. (2010). Stem cells in hepatocarcinogenesis: Evidence from genomic data. Semin. Liver Dis. 30, 26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano K., Sasaki T., Umeda Y., Nishizawa T., Ikawa N., Ohbayashi H., Arito H., Yamamoto S., Fukushima S. (2007). Inhalation carcinogenicity and chronic toxicity of carbon tetrachloride in rats and mice. Inhal. Toxicol. 19, 1089–1103 [DOI] [PubMed] [Google Scholar]
- Nault J. C., Zucman-Rossi J. (2011). Genetics of hepatobiliary carcinogenesis. Semin. Liver Dis. 31, 173–187 [DOI] [PubMed] [Google Scholar]
- Oh J. Y., Jeong J. S., Kim Y. J., Nam K. J., Park B. H., Kwon E. Y., Kim Y. H., Hwang T. H. (2002). Ultrasonographic evidence of phenotypic instability during hepatocarcinogenesis in N-nitrosomorpholine-treated rats. Exp. Mol. Pathol. 73, 67–73 [DOI] [PubMed] [Google Scholar]
- Oishi N., Wang X. W. (2011). Novel therapeutic strategies for targeting liver cancer stem cells. Int. J. Biol. Sci. 7, 517–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitot H. C. (1977). The natural history of neoplasia. Newer insights into an old problem. Am. J. Pathol. 89, 402–411 [PMC free article] [PubMed] [Google Scholar]
- Powell C. L., Kosyk O., Ross P. K., Schoonhoven R., Boysen G., Swenberg J. A., Heinloth A. N., Boorman G. A., Cunningham M. L., Paules R. S., et al. (2006). Phenotypic anchoring of acetaminophen-induced oxidative stress with gene expression profiles in rat liver. Toxicol. Sci. 93, 213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusyn I., Asakura S., Pachkowski B., Bradford B. U., Denissenko M. F., Peters J. M., Holland S. M., Reddy J. K., Cunningham M. L., Swenberg J. A. (2004). Expression of base excision DNA repair genes is a sensitive biomarker for in vivo detection of chemical-induced chronic oxidative stress: Identification of the molecular source of radicals responsible for DNA damage by peroxisome proliferators. Cancer Res. 64, 1050–1057 [DOI] [PubMed] [Google Scholar]
- Sancho-Bru P., Altamirano J., Rodrigo-Torres D., Coll M., Millán C., José Lozano J., Miquel R., Arroyo V., Caballería J., Ginès P., et al. (2012). Liver progenitor cell markers correlate with liver damage and predict short-term mortality in patients with alcoholic hepatitis. Hepatology. 55, 1931–1941 [DOI] [PubMed] [Google Scholar]
- Seki E., De Minicis S., Gwak G. Y., Kluwe J., Inokuchi S., Bursill C. A., Llovet J. M., Brenner D. A., Schwabe R. F. (2009). CCR1 and CCR5 promote hepatic fibrosis in mice. J. Clin. Invest. 119, 1858–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell R. E., Lee C. S., Tsuboi K. K., Villasana A. (1951). Histochemical and microchemical changes in experimental cirrhosis and hepatoma formation in mice by carbon tetrachloride. Cancer Res. 11, 345–354 [PubMed] [Google Scholar]
- Vesselinovitch S. D., Mihailovich N. (1983). Kinetics of diethylnitrosamine hepatocarcinogenesis in the infant mouse. Cancer Res. 43, 4253–4259 [PubMed] [Google Scholar]
- Vucur M., Roderburg C., Bettermann K., Tacke F., Heikenwalder M., Trautwein C., Luedde T. (2010). Mouse models of hepatocarcinogenesis: What can we learn for the prevention of human hepatocellular carcinoma?. Oncotarget. 1, 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Jacob S. T. (2011). Role of cancer stem cells in hepatocarcinogenesis. Genome Med. 3, 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells M. Y., Williams E. S. (2009). The transgenic mouse assay as an alternative test method for regulatory carcinogenicity studies–implications for REACH. Regul. Toxicol. Pharmacol. 53, 150–155 [DOI] [PubMed] [Google Scholar]
- Yamashita T., Ji J., Budhu A., Forgues M., Yang W., Wang H. Y., Jia H., Ye Q., Qin L. X., Wauthier E., et al. (2009). EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 136, 1012–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Koteish A., Lin H., Huang J., Roskams T., Dawson V., Diehl A. M. (2004). Oval cells compensate for damage and replicative senescence of mature hepatocytes in mice with fatty liver disease. Hepatology. 39, 403–411 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.