Abstract
Leiomyoma are common tumors arising within the uterus that feature excessive deposition of a stiff, disordered extracellular matrix (ECM). Mechanical stress is a critical determinant of excessive ECM deposition and increased mechanical stress has been shown to be involved in tumorigenesis. Here we tested the viscoelastic properties of leiomyoma and characterized dynamic and static mechanical signaling in leiomyoma cells using three approaches, including measurement of active RhoA. We found that the peak strain and pseudo-dynamic modulus of leiomyoma tissue was significantly increased relative to matched myometrium. In addition, leiomyoma cells demonstrated an attenuated response to applied cyclic uniaxial strain and to variation in substrate stiffness, relative to myometrial cells. However, on a flexible pronectin-coated silicone substrate, basal levels and lysophosphatidic acid-stimulated levels of activated RhoA were similar between leiomyoma and myometrial cells. In contrast, leiomyoma cells plated on a rigid polystyrene substrate had elevated levels of active RhoA, compared to myometrial cells. The results indicate that viscoelastic properties of the ECM of leiomyoma contribute significantly to the tumor’s inherent stiffness and that leiomyoma cells have an attenuated sensitivity to mechanical cues. The findings suggest there may be a fundamental alteration in the communication between the external mechanical environment (extracellular forces) and reorganization of the actin cytoskeleton mediated by RhoA in leiomyoma cells. Additional research will be needed to elucidate the mechanism(s) responsible for the attenuated mechanical signaling in leiomyoma cells.
Keywords: Mechanotransduction, RhoA, Leiomyoma, Mechanical properties, Extracellular matrix, AKAP13, Myometrium, Uterine fibroids, Rho-kinase, ROCK
1. Introduction
Uterine leiomyomata are highly prevalent, fibrotic tumors of the uterus that disproportionally afflict African American women and are a public health concern (Day Baird et al., 2003; Walker and Stewart, 2005; Lee et al., 2007; Selo-Ojeme et al., 2008; Laughlin et al., 2009). Previously, we (Catherino et al., 2004; Leppert et al., 2004), and others (Wolanska et al., 1998; Mitropoulou et al., 2001; Wolanska et al., 2003; Behera et al., 2007) have shown the ECM of leiomyoma to be increased in amount and altered in composition, compared to the ECM of the uterine myometrium. In addition to a rich glycosaminoglycan (GAG) content (Wolanska et al., 1998; Wolanska et al., 2003), we observed that the ECM was structurally disordered, relative to adjacent myometrium (Catherino et al., 2004; Leppert et al., 2004). Furthermore, leiomyomata displayed an increased stiffness by unconfined compression in vitro (Rogers et al., 2008) and ultrasound elastography in vivo (Kiss et al., 2006; Stewart et al., 2011). Of note, leiomyomata possess an increased vascularity and fluid content relative to adjacent myometrium (Aleem and Predanic, 1995; Okuda et al., 2008). The increased fluid content is significant because fluid may contribute to the mechanical properties of the tumors and may explain the clinical response of leiomyoma to GnRH agonists and antagonist treatment (Chegini et al., 1996; McCarthy-Keith et al., 2011). Despite the remarkable stiffness of leiomyomata, their altered ECM structure and content, and increased water content, little is known about mechanical signaling in leiomyoma.
Mechanical signals are transmitted from the ECM scaffold via transmembrane receptors to the internal cytoskeleton in order to maintain an isometric state (for review, Alenghat and Ingber, 2002), Transmembrane receptors, such as the integrins and cadherins (Schwartz and DeSimone, 2008; Wang et al., 2009), respond to stretch (Kaneko et al., 2009), fluid shear stress (Lee et al., 2008), elevated hydrostatic pressure (Riou et al., 2007) and increased osmotic forces (Lunn and Rozengurt, 2004). Although tissues exist under mechanical tension, the resident cells react to, and may be protected from, external loads by the mechanical properties of the surrounding matrix (Tomasek et al., 2002) through secretion of ECM (Brown et al., 1998; Alexopoulos et al., 2005). Notably, increased ECM stiffness may contribute to tumorigenesis (Ingber, 2008; Butcher et al., 2009). For example, Paszek and colleagues demonstrated malignant transformation of mammary epithelial cells (MECs) correlated with increasing ECM stiffness, elevated compression forces, and higher tensional resistance mediated, in part, through increased active RhoA (Paszek and Weaver, 2004; Paszek et al., 2005). RhoA belongs to the Rho family of small GTPases that function as molecular switches to cycle between the inactive GDP-bound and active GTP-bound state (Ridley and Hall, 1992; for review: Wettschureck and Offermanns, 2002). Rho GTPases are activated by Rho-guanine nucleotide exchange factors (Rho-GEFs) and generate cytoskeletal tension via interaction with cytoskeletal filaments that attach to focal adhesion complexes that lead to activation of downstream effectors, including Rho-associated kinase (ROCK). Thus, Rho signaling might play an important role in leiomyoma stiffness, and possibly growth, but little is known about Rho-signaling in leiomyoma.
Recently, we observed that leiomyomata demonstrated increased beta-1 integrin expression and that inhibition of integrin signaling led to a reduction in levels of active RhoA (Malik et al., 2009). Furthermore, we found that the Rho-GEF Brx (AKAP13) was not only expressed at high levels in leiomyoma (Rogers et al., 2008), but AKAP13 was also involved in osmotic signaling (Kino et al., 2009) and osmotic signaling was altered in leiomyoma cells (McCarthy-Keith et al., 2011). Taken together, these observations suggest that altered mechanical signaling in leiomyoma involves RhoA and that altered viscoelastic properties contribute significantly to the increased stiffness characteristic of the tumors. Here we examined the biphasic mechanical properties of leiomyomata and characterized the response of leiomyoma cells to dynamic and static mechanical stresses.
2. Results
2.1. Leiomyoma tissue exhibit increased pseudo-dynamic modulus and peak stress
To assess the pseudo-dynamic modulus of myometrial and leiomyoma tissues, a measurement that takes into account the contribution of water and the structure of the ECM to the tissue’s viscoelastic properties, we used a porous stainless steel confined compression chamber (Fig. 1a, b). Surgically obtained leiomyoma tissue samples had mean pseudo-dynamic modulus of 202.7±27.8 megapascals (MPa) per millimeter (mm)/mm (mean±SEM; Fig. 2a). This was significantly more stiff than myometrial specimens (48.1±25.6 MPa per mm/mm, p<0.001; Fig. 2a). Furthermore, relative to paired myometrium, leiomyomata held a larger peak strain at 5% displacement (6.96±0.91 versus 1.35±0.70 MPa respectively, p-value<0.001; Fig. 2b). Comparing these data to our previous assessment of Young’s modulus (Rogers et al., 2008), we noted a much larger pseudo-dynamic modulus when the tissue’s viscoelastic mechanical properties were taken into account. Consistent with prior reports, the leiomyomata samples we analyzed contained more sulfated glycosaminoglycans (sGAG) per DNA content relative to myometrial specimens (Leiomyoma: 0.62±0.080 μg of sGAG per μg of DNA; Myometrium: 0.19±0.012 μg per μg, p<0.0001; Fig. 2c). A similar difference was noted for collagen (Leiomyoma: 246.7±26.2 μg of collagen per μg of DNA; Myometrium: 97.5±18.7 μg per μg, p<0.001; Fig. 2d), and the values resembled previously published data obtained with other methods (Berto et al., 2003).
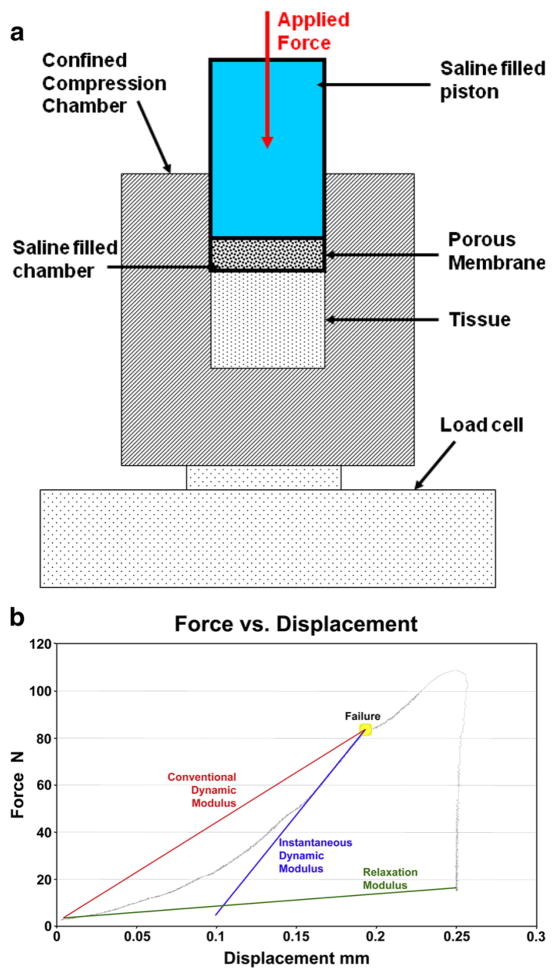
Fig. 1.
Apparatus and method used to quantify pseudo-dynamic modulus in myometrial and leiomyoma surgically obtained tissue samples. a: Schematic of the experimental confined compression apparatus with a porous membrane (40 micron pore size). A 5% constant displacement uniaxial load was applied to the myometrial and leiomyoma tissue. The confined compression chamber was smooth, rigid, and impermeable. b: Representative Force versus Displacement graph for a single leiomyoma specimen.
Fig. 2.
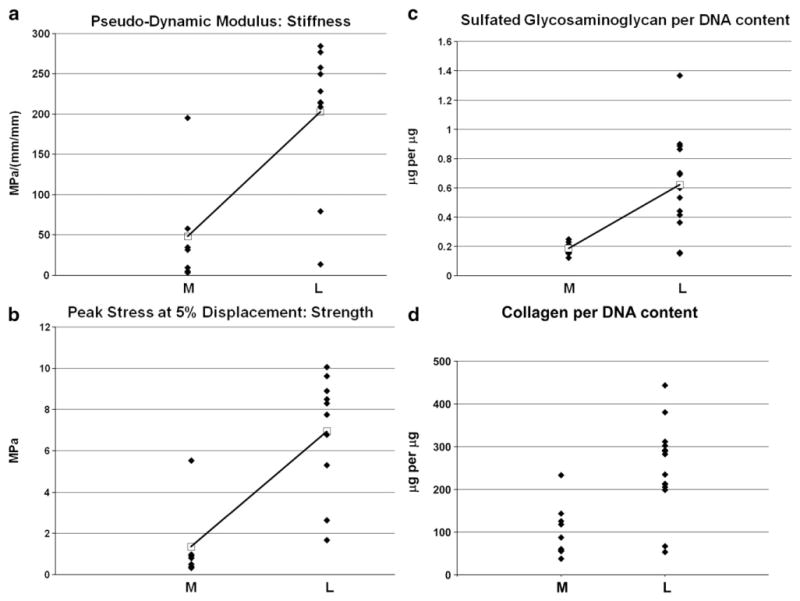
Leiomyoma tissue specimens have an increased pseudo-dynamic modulus compared to myometrial tissue samples. a: Summary of mechanical testing in matched surgical specimens. The pseudo-dynamic modulus (megapascals (MPa) per millimeter (mm) over mm, black diamonds) was increased in leiomyomata (L) surgical samples (n=10) relative to myometrium (M; n=7). Mean pseudo-dynamic moduli (open black squares) for myometrium and leiomyomata were 48.1±25.6 and 202.7±27.8 respectively (p<0.001). b: Leiomyoma surgical samples (n=10) held an increased peak stress (black diamonds) compared to myometrium (n=7). Mean peak stress (open black squares) for myometrium and leiomyomata were 1.35±0.70 and 6.96±0.91 respectively (p<0.001). c: Leiomyoma surgical samples (n=10) contained more sulfated glycosaminoglycan (sGAG) (DMMB assay) relative to matched myometrial samples, n=7: Leiomyoma=0.62±0.080 μg of sGAG per μg of DNA; Myometrium=0.19±0.012 μg per μg, p<0.0001. d: Leiomyoma surgical samples contained more collagen (Hydroxyproline assay) relative to matched myometrium: Leiomyoma=246.7±26.2 μg of collagen per μg of DNA; Myometrium=97.5±18.7 μg per μg, p<0.001. Values are reported as means±SEM. All statistical tests used a 2-tailed unpaired t-Test for unequal variance.
After normalizing the mechanical properties to biologic components and also to tissue sample weights, we performed correlation analyses (Spearman’s correlation for nonparametric data). Both the pseudo-dynamic modulus and the peak strain correlated with one another (p<0.001). The pseudo-dynamic modulus also correlated with both collagen and sGAG content (p<0.05). Of note, the peak stress correlated more strongly with the hydrophilic sGAG content (p=0.003) than with collagen content (p=0.011). Furthermore, neither mechanical property (pseudo-dynamic modulus or peak stress) correlated with dry weight. In sum, the mechanical properties strongly correlated with components of the matrix that reflect the viscoelastic properties of the tissue, and suggest that leiomyoma cells reside in a mechanically stiff microcellular environment. Furthermore, the results indicate that the molecular rearrangement of the ECM, including hydration, may play an important role in the stiffness of the tumors. These observations led us to question whether mechanical sensing might be altered in leiomyoma cells.
2.2. Leiomyoma cells have an attenuated response to applied cyclic strain
Previous studies have shown that airway smooth muscle cells (Deng et al., 2009) sense and respond to applied uniaxial cyclic strain in vitro by reorienting their actin cytoskeleton perpendicular to the axis of strain. To determine whether leiomyoma cells exhibit normal sensitivity and response to mechanical strain, we applied 8.9% uniaxial cyclic strain (1 Hz) to leiomyoma cells and compared their reorientation response to myometrial cells. Both cell types responded to mechanical strain (Fig. 3a & b). However, while 70% of myometrial cells reoriented their main axis perpendicular to the direction of strain, only 53% of leiomyoma cells exhibited this response (Fig. 3b). It was possible that the reduced re-orientation was due to constitutively elevated levels of the active Rho-kinase, ROCK. To test this possibility, we added the ROCK inhibitor, Y-27632. Similar to normal endothelial cells (Ghosh et al., 2008) reorganization of normal myometrial cells to mechanical strain was inhibited by Y-27632, consistent with the conclusion that the Y-compound was fully functional in the culture system. In contrast to reports of tumor-derived endothelial cells (Ghosh et al., 2008), treatment with the ROCK inhibitor prior to strain failed to increase the percentage of leiomyoma cells that oriented perpendicularly to applied strain. One interpretation of these findings is that leiomyoma cells have an impaired perception of mechanical strain.
Fig. 3.
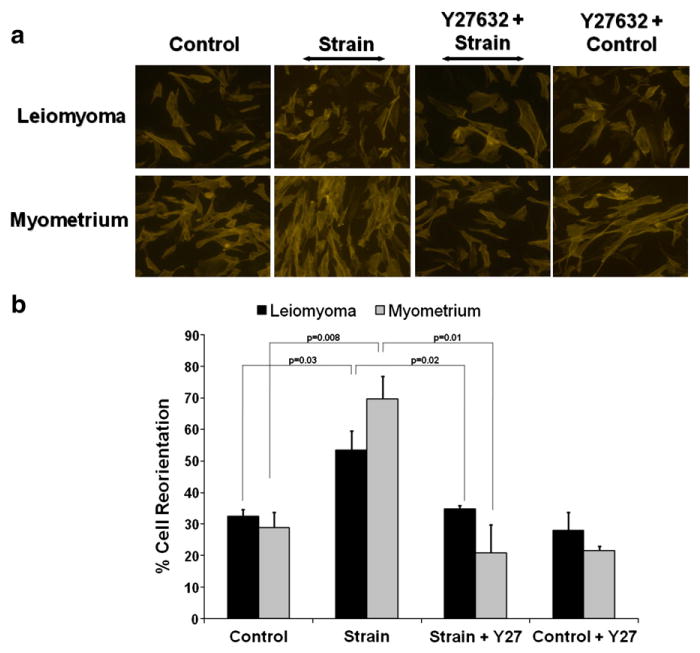
Response of myometrial and leiomyoma cells to cyclic uniaxial strain. a: Cytoimmunofluorescent images of leiomyoma and myometrial cells exposed to either no strain (control) or to 8.9% uniaxial cyclic strain (Strain) for 18 h at 1 Hz. Cells were cultured with or without pre-treatment of the ROCK inhibitor, Y-27632 (Y-27) (10 μM) for 30 min prior to strain or no strain (control). Actin stress fibers and nuclei were visualized by staining for Alexa Fluor-546 Phalloidin and DAPI, respectively. b: Quantitative computerized morphometric measurements of cellular reorientation in response to uniaxial strain with, or without, pre-treatment of Y-27632 (Y-27) for leiomyoma (black bars) or myometrial cells (grey bars). Results are shown as the percentage of cells aligned at 90°+/−30° relative to the direction of the applied strain. Data represent a mean of three independent experiments with a minimum of 45 cells measured per condition. Angular differences between unstrained and strained leiomyoma and myometrial cells differed significantly (p<0.05).
2.3. Response of leiomyoma cells to RhoA activation by mechanical and chemical stimulation
To examine the question of whether leiomyoma cells have impaired mechanical sensing in greater detail, we next quantified levels of active RhoA following either mechanical stimulation or treatment with lysophosphatidic acid (LPA), a known soluble activator of RhoA (Parizi et al., 2000). Basal levels of active RhoA were similar between leiomyoma or myometrial cells when cultured on a pronectin-coated flexible silicone substrate, but levels of RhoA were increased in leiomyoma cells cultured on polystyrene, compared to myometrial cells (Fig. 4a). LPA stimulation led to increased levels of active RhoA in both myometrial cells, and leiomyoma cells within 3 min on a flexible pronectin-coated substrate (Fig. 4b), and there were no significant differences between the cell types. In contrast, LPA stimulation of cells cultured on the stiff polystyrene substrate led to an increase in active RhoA in myometrial cells which also peaked at 3 min, but leiomyoma cells did not exhibit a significant increase over already elevated basal levels of active RhoA (Fig. 4c). When mechanically stimulated for 120 min on flexible pronectin-coated substrates, myometrial cells responded as expected with increased levels of active RhoA (Fig. 4d), whereas leiomyoma cells did not show an increase in active RhoA. We interpret these data to suggest that leiomyoma and myometrial cells are differentially affected by substrate stiffness, and these findings led us to examine how substrates of varying stiffness might differentially affect the two cell types.
Fig. 4.
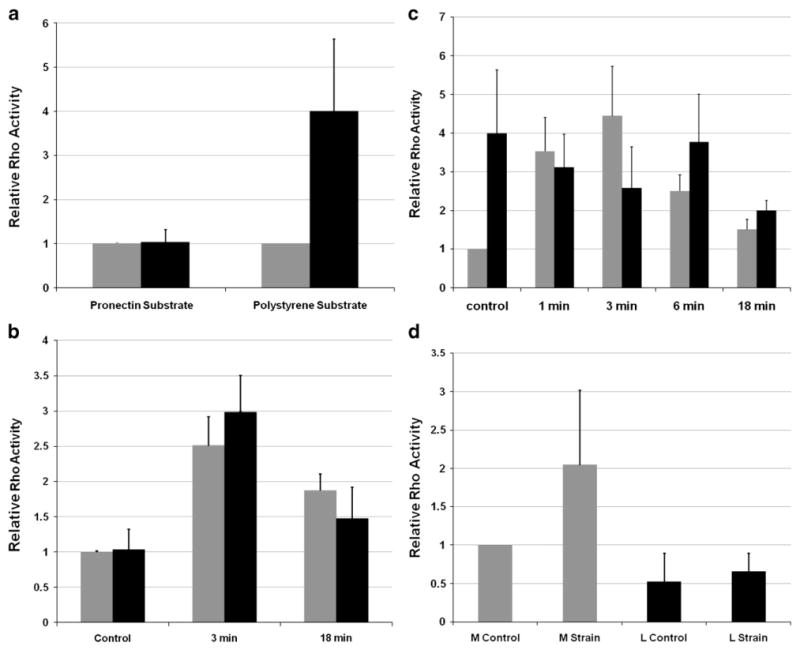
RhoA levels in leiomyoma and myometrial cells at baseline and in response to applied chemical or mechanical strain. a: Assessment of active RhoA in leiomyoma or myometrial cells cultured on flexible pronectin-coated substrate, or uncoated polystyrene. Y axis=relative level of active RhoA. Leiomyoma cells (black bars) demonstrated increased levels of activated RhoA relative to myometrial cells when cultured on polystyrene (p<0.05). b: Levels of active RhoA in myometrial (gray bars) or leiomyoma cells (black bars) cultured on flexible, pronectin-coated substrate untreated (control) or treated with a chemical activator or RhoA, lysophosphatidic acid (LPA), for minutes as indicated. Y axis= relative level of active RhoA. On the flexible, pronectin-coated substrate levels of activated RhoA in myometrial cells peaked at 3 min. c: Culture of leiomyoma (black bars) or myometrial cells (gray bars) on polystyrene either untreated (control) or treated with LPA for minutes as indicated. Y axis=relative level of active RhoA. Levels of active RhoA were significantly elevated in leiomyoma cells at baseline, and were less affected by LPA treatment. Data in a–c represent the average relative RhoA activation compared to myometrial control from three independent experiments. d: Quantification of active RhoA in myometrial (gray bars) or leiomyoma cells (black bars) to 2 h of applied uniaxial strain. Myometrial cells demonstrated a 2-fold increased active RhoA levels in response to uniaxial strain on pronectin-coated flexible silicone substrate (M Control versus M Strain). Leiomyoma cell active RhoA levels were attenuated and had a muted response (1.3 fold) to mechanical strain (L Control versus L Strain). The cell response was normalized to myometrial control activation of RhoA and reported as the mean±standard deviation from two independent experiments with 6 wells for each condition.
2.4. Leiomyoma cells respond abnormally to variation in substrate stiffness
Previous studies have shown that smooth muscle cells sense and respond to increasing substrate stiffness by increasing surface area (Engler et al., 2004), and that this response is an indirect measure of how efficiently cells sense and respond to ECM elasticity (Chicurel et al., 1998). As a third approach to assess the responsiveness of leiomyoma cells to mechanical cues, myometrial and leiomyoma cells were cultured for 22 h on polyacrylamide gels that varied in stiffness from 7 kPa (kilopascal) to 140 kPa (Fig. 5). At a baseline substrate of 7 kPa, myometrial cells were more rounded with a reduced surface area, versus leiomyoma cells, respectively (8298 pixels per cell±958 (SEM) vs 11554±768; p=0.02). On the most rigid substrate, myometrial cell surface area was increased as expected. In contrast, after 22 h leiomyoma cells had an attenuated increase in surface area (19464 pixels per cell±710 (SEM) versus 15730±556; p<0.001). When compared over the four different ECM substrates, myometrial cells responded to a greater degree to more rigid matrices by increasing cell spreading, indicating that leiomyoma cells did not sense, or were unable to respond to a change in their substrate elasticity (Fig. 5a and b; p=0.0042, two-way ANOVA).
Fig. 5.
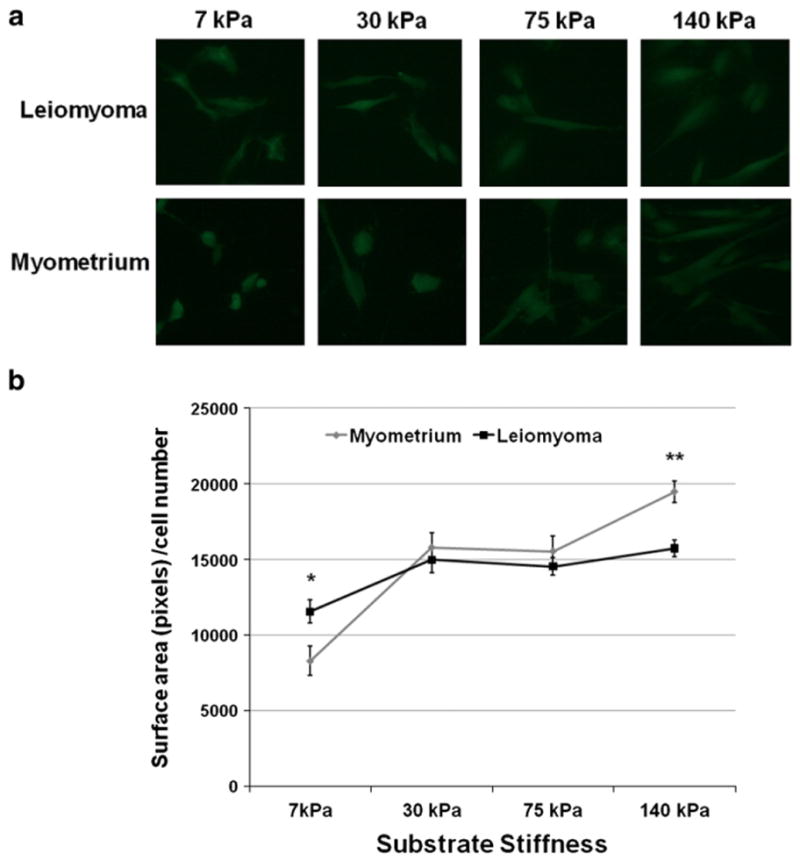
Response of myometrial or leiomyoma cells to substrates of varied stiffness. a: Leiomyoma and myometrial cells were cultured on collagen-coated polyacrylamide gels of varying stiffness, then treated with calcein AM and fluorescent images were obtained 22 h after plating for assessment of cell spreading. Stiffness as indicated. b: Mean surface area per cell was determined using ImageJ software as indicated for myometrial cells (gray line) or leiomyoma cells (black line). Myometrial cell spreading responded to the increased substrate stiffness more than leiomyoma cells. Values represent a mean of four independent experiments with a minimum of 45 cells measured per condition (** trend comparison: p<0.05).
3. Discussion
These studies demonstrate that the ECM microenvironment of leiomyoma cells is characterized by increased mechanical stress. Here we extend the results of our previous study (Rogers et al., 2008) to show that the viscoelastic properties of the ECM contributes substantially to the increased tissue stiffness of leiomyoma. Since the viscoelastic properties of the ECM are complex, it is possible that the interstitial fluid may alter the repulsive forces of the GAGs allowing them to collapse or inflate. Additional studies will be needed to discern how the complex ECM of leiomyoma and its molecular rearrangement contributes to the observed changes in viscoelasticity. Interestingly, in this environment characterized by increased stress, we noted that leiomyoma cells had an attenuated response to mechanical cues compared to myometrial cells as shown by: 1) reduced levels of active RhoA to acute strain; 2) failure to respond to cyclic stresses in a cell re-orientation assay; and 3) an attenuated response to substrates of varied stiffness. Leiomyoma cells did respond normally to LPA-mediated activation of RhoA, but only when the cells were cultured on a flexible substrate. Collectively, the flndings are consistent with the conclusion that mechanical signaling is attenuated in leiomyoma cells.
We noted a four-fold increase in both the pseudo-dynamic modulus and the peak strain in leiomyoma tissue relative to patient-matched myometrium (Fig. 2). Using a confined compression chamber with a porous paten, we observed a much higher modulus than in prior tests conducted on unconfined samples with a non-porous piston (Rogers et al., 2008). This increased modulus is, in part, likely explained by the contribution of both the fluid phase and solid phase of the tissue. Not only does the rich fluid component of leiomyoma contribute to its bulk (Okuda et al., 2008), but similar to articular cartilage (Cohen et al., 1998; Ateshian et al., 2003; Park et al., 2004), the fluid phase contributes to the viscoelastic properties of fibroids, contributing to large interstitial pressurized forces. For example, after testing bovine cartilage in a confined compression chamber, Soltz and Ateshian (Soltz and Ateshian, 2000) concluded that cartilage dynamic stiffness was derived primarily from flow-dependent viscoelasticity as predicted by the linear biphasic theory and that interstitial fluid pressurization is the fundamental mechanism of cartilage load support. Our findings support the notion that leiomyomata are tumors composed of large amounts of aberrant ECM (Malik et al., 2010) and that cells within the tumor continue to grow and proliferate (Peddada et al., 2008) while exposed to increased viscoelastic forces.
Changes in the mechanical properties of a tissue and the cellular microenvironment have been shown to contribute to tumor formation in other organ systems and in experimental models (Ingber, 2008; Butcher et al., 2009). The concept that changes in the cellular microenvironment could contribute to tumorigenesis were first suggested by experiments of Bischoff and Bryson (Bischoff and Bryson, 1964) where tumor formation was observed after implanting a rigid piece of metal or plastic, as opposed to the same material as a powder. Alterations to the ECM structure also appear to play a central role in tumor formation and in the tumor cell’s ability to sense and respond to the altered physical environment (Weaver et al., 1997; Paszek et al., 2005; Ghosh et al., 2008). The findings reported here, together with our previous data (Rogers et al., 2008), suggest that the mechanical properties of leiomyoma are a key feature of these tumors, and may contribute to their growth, but further studies will be needed to assess whether growth of a specific leiomyoma is correlated to its stiffness. One limitation of the studies presented is that the viscoelastic properties of a tissue are complex, especially in a tissue containing ECM consisting of numerous proteins and glycoproteins all of which may contribute to mechanical behavior. In this report, we have focused on characterization of the differences between leiomyoma and uterine muscle, especially differences in Rho signaling based on our prior report, but a more detailed assessment of the rheological differences between the cells such as reported for other tissue types (Stamenović, 2008) remains to be performed.
Notably, leiomyoma differ from other tumors in that some grow to several centimeters in size. Each uterine leiomyoma represents a monoclonal process, but within a single uterus different tumors arise from different cells, such that within a uterus multiple clones may be represented (Ligon and Morton, 2000). Within one uterus some tumors may grow, while others may undergo a reduction in size (Peddada et al., 2008). Recent reports of assessment of the elastic modulus in vivo (Stewart et al., 2011) may represent a clinical application of our findings to assess the stiffness in vivo and explore a possible correlation with growth or senescence of an individual leiomyoma.
The establishment of a tumor microenvironment by leiomyoma cells characterized by increased viscoelastic forces begs the question: is mechanical signaling altered in leiomyoma cells? The results indicate that myometrial cells responded to perturbation of the extracellular mechanical stresses as expected; but by three different measures of mechanosensing, leiomyoma cells appeared to have an attenuated response relative to myometrial cells. Specifically, leiomyoma cells failed to reorient perpendicularly to the applied uniaxial strain direction, had an attenuated RhoA activation response to uniaxial strain, and showed a diminished ability to change morphology in response to altered substrate stiffness. In contrast to these three observations which suggest an impaired response to extracellular mechanical cues, on the extremely rigid polystyrene plates with an estimated stiffness of 2–4 GPa (Paszek et al., 2005), leiomyoma cells demonstrated increased basal levels of active RhoA relative to myometrial cells. These observations could be considered contradictory. We interpret the increase in the basal levels of RhoA on the polystyrene substrate may reflect prior adaptation of the leiomyoma cells to a very stiff microenvironment. However, with each dynamic mechanical challenge, leiomyoma cells were not as adroit in their response, suggesting a fundamental alteration exists in communication between the external mechanical forces and the ability of the actin cytoskeleton to reorganize via RhoA. The findings suggest that mechanical signaling in leiomyoma cells is fundamentally altered, because in all 4 assays involving external mechanical cues, leiomyoma cells responded abnormally.
One plausible explanation for the seemingly contradictory results is that leiomyoma cells have become fundamentally adapted to their very stiff microenvironment, and are insensitive to more moderate and subtle mechanical cues. Stated differently, the cell response to mechanical stimulation could be down-regulated through feedback mechanisms, although the mechanisms responsible remain unknown. In support of this explanation, and contrary to the findings of Ghosh and colleagues (Ghosh et al., 2008) for capillary endothelial cells, the fundamental alteration in leiomyoma cells was not ROCK-dependent, as demonstrated by the finding that leiomyoma cells pre-treated Y27632 prior to uniaxial straining remained largely unchanged (Fig. 3). In further support of this explanation, leiomyoma cells contain increased levels of the Rho-GEF AKAP13 (Rogers et al., 2008), and knockdown of AKAP13 differentially affected leiomyoma cells, compared to myometrial cells (Owen et al., 2010). Thus, the results are consistent with the notion that leiomyoma cells have undergone a specific adaptation to their stiff microenvironment that is not ROCK-dependent, is associated with increased levels of Rho-GEF, and this adaptation persists in tissue culture. Additional experiments will be needed to unravel the specific changes associated with the mechanotransduction response of leiomyoma cells.
In conclusion, these results reveal that the increased stiffness and elastic moduli demonstrated in leiomyomata is accompanied by an altered mechanosensory response characterized by attenuated levels of active RhoA. A further understanding of mechanotransduction as it relates to leiomyomata may explain why some leiomyoma grow and others do not, and could help to guide future treatments for this very prevalent pelvic tumor.
4. Experimental procedures
4.1. Mechanical testing of leiomyoma and myometrial tissue
Specimens of leiomyoma and paired myometrium were collected from women undergoing hysterectomy for symptomatic leiomyoma in institutional review board-approved studies. Patient characteristics are described in Table 1. Surgical specimens were snap-frozen. Cylindrical specimens were precisely cut using a 5-mm punch biopsy (Miltex Inc., York, PA) and a 5-mm height cutting apparatus. Tissue was re-hydrated with normal saline approximately 2 min, weighed and then placed into the confined compression chamber attached to the Enduratec ElectroForce 3200 (Bose Corporation, Eden Prairie, MN) (Fig. 1a & b). Control experiments with varied re-hydrated times and frozen versus fresh tissue for both human fibroid tissue as well as beef muscle revealed no significant differences in tissue behavior for the tests conducted within the time frame used for the experiments. The saline filled stainless steel piston with a porous (40 micron pore size) stainless steel membrane (Small Parts Inc., Miramar, FL) was then placed adjacent to tissue (Fig. 1a). An initial 15 second ramp to 0.5 N was applied to the specimens to ensure proper contact between the tissue and the piston. After a 60 second relaxation cycle, a 5% displacement force was applied. Because the force generated under strain rate used in a conventional dynamic test was too large (exceeding 200 Newtons) we performed a pseudo-dynamic modulus test using a slow ramp (5% displacement in 4 s) which measured Young’s modulus (stress (MPa) per displacement [mm]) per tissue cross sectional area (mm) (Fig. 1). The peak strain and the relaxation modulus (Young’s modulus) at 5% displacement were measured. The pseudo-dynamic modulus and the peak strain at 5% displacement were measured during a 1200 second cycle (Fig. 1b). The tissue was then re-weighed, dried using a SpeedVac and vapor trap device (ThermoSavant, Waltham, MA) and then digested (0.56 U/ml papain, 2 mM L-cysteine, 2 mM EDTA, 55 mM NaCitrate, and 150 mM NaCl) at 60 °C overnight.
Table 1.
Patient characteristics.
| Patient | Age | Race | Fibroid Size (cm) | Location | LMP | Time of cycle | |
|---|---|---|---|---|---|---|---|
| 3 | 42 | West Indian, Caribbean | F1 | 3.5×4.0×3.5 | IM | 4 months Prior | Amennorrheic |
| F2 | 1.5×2.0 | IM | |||||
| 4 | 45 | Caucasian | F1 | 5.5×6.5×6 | IM | 2 days b/f surgery | Follicular |
| F2 | 2.5×1.5×2 | IM | |||||
| 5 | 47 | AA | F1 | 6.0×6.0×12.0 | SS | 41 days b/f surgery | Luteal |
AA = African American; b/f = before; LMP = last menstrual period; IM = intramural; SS = subserosal; F1 = Fibroid number 1; F2 = Fibroid number 2.
4.2. Biologic assays for tissue samples
The digested surgical specimens were then analyzed for DNA content, sulfated glycosylaminoglycan (GAG) and collagen content. DNA content was determined using a Picogreen assay kit (Picogreen; Invitrogen, Carlsbad, CA). Sulfated GAG content was determined with the 1,9-dimethylmethylene blue (DMMB) method (Farndale et al., 1986) and was normalized to a known quantity of chondroitin-4-sulphate. Collagen content was determined using a basic hydroxyproline assay described by Reddy and Enwemeka (Reddy and Enwemeka, 1996). PureCol (Sigma-Aldrich, St. Louis, MO) was used to generate standard curves.
4.3. Cell culture
Immortalized leiomyoma and myometrial cells which have been previously described and which retain features of the respective tissues (Malik et al., 2008) were cultured on polystyrene in culture medium composed of DMEM F12 (Invitrogen, Carlsbad, CA), 10% FBS, 1% glutamate, and 1% antibiotic mixture. Immortalized cells were used because primary cultures of leiomyoma cells do not retain features of the tumor in passage, and the immortalized cells strongly resemble in vivo tumors when compared using microarray analysis of ECM gene expression and other characteristics (Malik et al., 2008).
4.4. Mechanical strain application to leiomyoma and myometrial cells
Immortalized leiomyoma and myometrial cells were cultured on pronectin coated, flexible silicone substrates (Uniflex culture plates, Flex Cell International Hillsborough, NC) for 2 days to ~70–80% confluence. Both cell types were then exposed to a maximum of 8.9% uni-axial cyclic strain at a 1 Hertz sinusoidal waveform for 18 h using a custom manufactured loading device that uses commercial BioFlex plates and a computer-controlled vacuum stretch apparatus. The choice of 8.9% strain was empirically chosen based on prior data that suggested leiomyoma most strongly resemble tendon (Rogers et al., 2008) and prior reports using a similar strategy of comparison with tumor cells (Ghosh et al., 2008). Since the focus of application of mechanical strain was to examine fundamental differences in Rho signaling between cell types, and not a rheological assessment of cell characteristics, relaxation and recovery were not independently tested with the system. Control cells were cultured on the same pronectin-coated substrates placed in the same incubator and were positioned in the same strain apparatus, but did not receive applied strain. In some cyclic strain experiments, both cells types were treated with or without Y-27632, ROCK inhibitor, (10 μM) (Calbiochem EMD, San Diego, CA) for 30 min prior to application of strain.
4.5. Modulation of substrate stiffness
Porous polyacrylamide gels of increasing stiffness coated with type 1 Collagen (Invitrogen, Carlsbad, CA) were prepared as previously described (Wang and Pelham, 1998) with minor modifications as follows. Coverslips were treated with dichlorodimethylsilane (Sigma-Aldrich, St. Louis, MO) before using them to cover the 20 μL of gel solution that was applied to an activated bottom coverslip. During activation of the polyacrylamide surface and conjugation with type 1 Collagen, 400 μL of sulpho-SANPAH was used. Stiffness measurements of the gels were estimated based on the final acrylamide to Bis ratio as previously studied (Engler et al., 2004; Tse and Engler, 2010). Polyacryl-amide gels were allowed to equilibrate for 30–45 min in culture medium at 37 °C. To analyze the effects of varying substrate stiffness on cell spreading, cells were cultured on collagen-coated polyacrylamide gels of varying stiffness at a low density (20,000 to 40,000 cells/9.5 cm2) to minimize cell–cell interactions. Cells were treated with 2 μM calcein AM (Invitrogen, Carlsbad, CA) 22 h after plating and 30 min prior to obtaining fluorescent images for assessment of cell spreading.
4.6. RhoA activation assay
RhoA activity was determined by using the absorbance based G-LISA RhoA activation assay per manufacturer instructions (Cytoskeleton, Denver, CO). Immortalized leiomyoma and myometrial cells were cultured to ~50–60% confluence on either polystyrene or on the same pronectin coated culture dishes used in the reorientation experiments (Flex Cell International). Cells were serum starved for approximately 17 h and then treated with 10 μm Lyso PA (LPA or 1-oleoyl-2-hydroxy-sn-glycero-3-phosphate, Avanti Lipids, Albaster, AL) for increasing time periods. Cells were lysed in G-LISA cell lysis buffer at 4 °C and lysates were snap frozen in liquid nitrogen for subsequent assay. Immortalized leiomyoma and myometrial cells cultured on pronectin-coated flexible silicone substrates were serum starved for approximately 17 h (0.5% FBS media) and then exposed to 8.9% uniaxial cyclic strain for 2 h. Control cells of both cell types were under the same conditions, but did not receive strain. Immediately following the completion of strain, both strained and control cells were lysed in G-LISA cell lysis buffer at 4 °C and lysates were snap frozen in liquid nitrogen. All lysates were assayed for RhoA-GTP per the G-LISA RhoA activation assay. The signal indicating the level of RhoA-GTP was determined by a microplate spectrophotometer measuring absorbance at 490 nm. The absorbance was normalized to unstrained myometrial baseline (control) samples and all samples were reported as fold-increase over myometrial control. Data are representative replicates of three separate experiments.
4.7. Microscopy and image analysis
Mechanically strained cells fixed with 4% paraformaldehyde and live cells spreading on polyacrylamide gels were visualized and all images were taken with a Leica microscope at a 10X magnification and DFC320 camera at the same magnification for all conditions (Leica Microsystems Bannockburn, IL). For mechanical strain experiments, cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 in PBS 1X, blocked with 5% normal goat serum and 1% bovine serum albumin in PBS 1X, stained with Alexa Fluor-546 Phalloidin and DAPI (Invitrogen, Carlsbad, CA). Image analyses were performed using ImageJ software (National Institutes of Health Bethesda, MD). For cyclic strain experiments, fluorescent images were analyzed to determine the angle between the longest axis of the cell and the direction of the applied uni-axial cyclic strain. These results are reported as the percentage of cells aligned at 90°+/−30° relative to the direction of the applied strain and also as angular distribution profiles for cell populations obtained by the grouping of angles of individual cells into 20° intervals. For cell spreading studies, live cells cultured on polyacrylamide gels were treated with calcein AM and fluorescent images were obtained 22 h after plating for assessment of cell spreading. Fluorescent images were converted to 32-bit images and cell areas were measured using threshold imaging within ImageJ software. Results are reported as the mean surface area per cell.
4.8. Statistical tests
All data were obtained from replica experiments and are expressed as the mean (error bars=SEM). Statistical significance was determined by using Student’s t test two-sample assuming equal variance and assumed at p<0.05. An ANOVA was used to compare how the myometrial and leiomyoma cells respond to different substrate stiffness (Graph Pad Software Inc., La Jolla, CA). Spearman rank correlation analyses were performed for mechanically tested tissue samples to determine statistical dependence between the non-parametrically distributed results including: dry weight, DNA, sGAG, and collagen content, pseudo-dynamic modulus, and peak stress.
Supplementary Material
Acknowledgments
Financial support
This research was supported by Z01-HD-008737-10, Program in Reproductive and Adult Endocrinology, NICHD, NIH, Bethesda, MD and the Clinical Research Training Program (CRTP), a public-private partnership supported jointly by the NIH and Pfizer, Inc. (via a grant to the NIH Foundation from Pfizer Inc.).
The authors thank Dr. Alan DeCherney for critical support and guidance and Dr. Phyllis Leppert for helpful discussions and suggestions. Technical expertise and assistance was provided by Dr. Paul Driggers, Dr. Hisashi Koide, Dr. Tomoshige Kino and Catherine Guo.
Footnotes
Where the work was done: The Program in Reproductive and Adult Endocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Cartilage Biology and Orthopedics Branch, National Institute of Arthritis Musculo-skeletal Skin Diseases, National Institutes of Health, Bethesda, MD.
Conflict of interest
None.
Disclosure
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of Health and Human Services, the Department of Defense or the U.S. Government.
References
- Aleem FA, Predanic M. The hemodynamic effect of GnRH agonist therapy on uterine leiomyoma vascularity: a prospective study using transvaginal color Doppler sonography. Gynecol Endocrinol. 1995;9:253–258. doi: 10.3109/09513599509160454. [DOI] [PubMed] [Google Scholar]
- Alenghat FJ, Ingber DE. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci STKE. 2002;(119):pe6. doi: 10.1126/stke.2002.119.pe6. [DOI] [PubMed] [Google Scholar]
- Alexopoulos LG, Setton LA, Guilak F. The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomater. 2005;1:317–325. doi: 10.1016/j.actbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Ateshian GA, Soltz MA, Mauck RL, Basalo IM, Hung CT, Lai WM. The role of osmotic pressure and tension-compression nonlinearity in the frictional response of articular cartilage. Transp Porous Media. 2003;50:5–33. [Google Scholar]
- Behera MA, Feng L, Yonish B, Catherino W, Jung SH, Leppert P. Thrombospondin-1 and thrombospondin-2 mRNA and TSP-1 and TSP-2 protein expression in uterine fibroids and correlation to the genes COL1A1 and COL3A1 and to the collagen cross-link hydroxyproline. Reprod Sci. 2007;14:63–76. doi: 10.1177/1933719107309591. [DOI] [PubMed] [Google Scholar]
- Berto AGA, Sampaio LO, Franco CRC, Cesar RM, Michelacci YM. A comparative analysis of structure and spatial distribution of decorin in human leiomyoma and normal myometrium. Biochim Biophys Acta. 2003;1619:98–112. doi: 10.1016/s0304-4165(02)00446-4. [DOI] [PubMed] [Google Scholar]
- Bischoff F, Bryson G. Carcinogenesis through Solid State Surfaces. Prog Exp Tumor Res. 1964;5:85–133. doi: 10.1159/000385997. [DOI] [PubMed] [Google Scholar]
- Brown RA, Prajapati R, McGrouther DA, Yannas IV, Eastwood M. Tensional homeostasis in dermal fibroblasts: mechanical responses to mechanical loading in three-dimensional substrates. J Cell Physiol. 1998;175:323–332. doi: 10.1002/(SICI)1097-4652(199806)175:3<323::AID-JCP10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catherino WH, Leppert PC, Stenmark MH, Payson M, Potlog-Nahari C, Nieman LK, Segars JH. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosomes Cancer. 2004;40:204–217. doi: 10.1002/gcc.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chegini N, Rong H, Dou Q, Kipersztok S, Williams RS. Gonadotropin-releasing hormone (GnRH) and GnRH receptor gene expression in human myometrium and leiomyomata and the direct action of GnRH analogs on myometrial smooth muscle cells and interaction with ovarian steroids in vitro. J Clin Endocrinol Metab. 1996;81:3215–3221. doi: 10.1210/jcem.81.9.8784072. [DOI] [PubMed] [Google Scholar]
- Chicurel ME, Chen CS, Ingber DE. Cellular control lies in the balance of forces. Curr Opin Cell Biol. 1998;10:232–239. doi: 10.1016/s0955-0674(98)80145-2. [DOI] [PubMed] [Google Scholar]
- Cohen NP, Foster RJ, Mow VC. Composition and dynamics of articular cartilage: structure, function, and maintaining healthy state. J Orthop Sports Phys Ther. 1998;28:203–215. doi: 10.2519/jospt.1998.28.4.203. [DOI] [PubMed] [Google Scholar]
- Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- Deng L, Bosse Y, Brown N, Chin LY, Connolly SC, Fairbank NJ, King GG, Maksym GN, Pare PD, Seow CY, Stephen NL. Stress and strain in the contractile and cytoskeletal filaments of airway smooth muscle. Pulm Pharmacol Ther. 2009;22:407–416. doi: 10.1016/j.pupt.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86:617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Ghosh K, Thodeti CK, Dudley AC, Mammoto A, Klagsbrun M, Ingber DE. Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc Natl Acad Sci U S A. 2008;105:11305–11310. doi: 10.1073/pnas.0800835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE. Can cancer be reversed by engineering the tumor microenvironment? Semin. Cancer Biol. 2008;18:356–364. doi: 10.1016/j.semcancer.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko D, Sasazaki Y, Kikuchi T, Ono T, Nemoto K, Matsumoto H, Toyama Y. Temporal effects of cyclic stretching on distribution and gene expression of integrin and cytoskeleton by ligament fibroblasts in vitro. Connect Tissue Res. 2009;50:263–269. doi: 10.1080/03008200902846270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T, Takatori H, Manoli I, Wang Y, Tiulpakov A, Blackman MR, Su YA, Chrousos GP, DeCherney AH, Segars JH. Brx mediates the response of lymphocytes to osmotic stress through the activation of NFAT5. Sci Signal. 2009;2:ra5. doi: 10.1126/scisignal.2000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss MZ, Hobson MA, Varghese T, Harter J, Kliewer MA, Hartenbach EM, Zagzebski JA. Frequency-dependent complex modulus of the uterus: preliminary results. Phys Med Biol. 2006;51:3683–3695. doi: 10.1088/0031-9155/51/15/006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin SK, Baird DD, Savitz DA, Herring AH, Hartmann KE. Prevalence of uterine leiomyomas in the first trimester of pregnancy: an ultrasound-screening study. Obstet Gynecol. 2009;113:630–635. doi: 10.1097/AOG.0b013e318197bbaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Ozminkowski RJ, Carls GS, Wang S, Gibson TB, Stewart EA. The direct and indirect cost burden of clinically significant and symptomatic uterine fibroids. J Occup Environ Med. 2007;49:493–506. doi: 10.1097/JOM.0b013e31805f6cf2. [DOI] [PubMed] [Google Scholar]
- Lee DY, Yeh CR, Chang SF, Lee PL, Chien S, Cheng CK, Chiu JJ. Integrin-mediated expression of bone formation-related genes in osteoblast-like cells in response to fluid shear stress: roles of extracellular matrix, Shc, and mitogen-activated protein kinase. J Bone Miner Res. 2008;23:1140–1149. doi: 10.1359/jbmr.080302. [DOI] [PubMed] [Google Scholar]
- Leppert PC, Baginski T, Prupas C, Catherino WH, Pletcher S, Segars JH. Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil Steril. 2004;82:1182–1187. doi: 10.1016/j.fertnstert.2004.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon AH, Morton CC. Genetics of uterine leiomyomata. Genes Chromosomes Cancer. 2000;28:235–245. [PubMed] [Google Scholar]
- Lunn JA, Rozengurt E. Hyperosmotic stress induces rapid focal adhesion kinase phosphorylation at tyrosines 397 and 577. Role of Src family kinases and Rho family GTPases. J Biol Chem. 2004;279:45266–45278. doi: 10.1074/jbc.M314132200. [DOI] [PubMed] [Google Scholar]
- Malik M, Webb J, Catherino WH. Retinoic acid treatment of human leiomyoma cells transformed the cell phenotype to one strongly resembling myometrial cells. Clin Endocrinol. 2008;69:462–470. doi: 10.1111/j.1365-2265.2008.03207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik M, Jardine D, Owen CM, McCarthy-Keith D, Segars JH, Catherino WH. Human leiomyoma cell proliferation and extracellular matrix expression is inhibited by integrin signaling. Fertil Steril. 2009;92(Suppl):S2. [Google Scholar]
- Malik M, Norian J, McCarthy-Keith D, Britten J, Catherino WH. Why leiomyomas are called fibroids: the central role of extracellular matrix in symptomatic women. Semin Reprod Med. 2010;28:169–179. doi: 10.1055/s-0030-1251475. [DOI] [PubMed] [Google Scholar]
- McCarthy-Keith DM, Malik M, Britten J, Segars J, Catherino WH. Gonadotropin-releasing hormone agonist increases expression of osmotic response genes in leiomyoma Q4 cells. Fertil Steril. 2011;95:2383–2387. doi: 10.1016/j.fertnstert.2011.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitropoulou TN, Theocharis AD, Stagiannis KD, Karamanos NK. Identification, quantification and fine structural characterization of glycosaminoglycans from uterine leiomyoma and normal myometrium. Biochimica. 2001;83:529–536. doi: 10.1016/s0300-9084(01)01281-0. [DOI] [PubMed] [Google Scholar]
- Okuda S, Oshio K, Shinmoto H, Tanimoto A, Asada H, Fujii T, Yoshimura Y, Kuribayashi S. Semiquantitative assessment of MR imaging in prediction of efficacy of gonadotropin-releasing hormone agonist for volume reduction of uterine leiomyoma: initial experience. Radiology. 2008;248:917–924. doi: 10.1148/radiol.2483071288. [DOI] [PubMed] [Google Scholar]
- Owen CM, Norian JM, Guo XC, Malik M, Catherino WH, Segars JH. Leiomyoma cells show attenuated mechanosensing, but increased dependence on Rho-GEF activation compared to myometrial cells. Fertil Steril. 2010;94(Suppl):S76. [Google Scholar]
- Parizi M, Howard EW, Tomasek JJ. Regulation of LPA-promoted myofibroblast contraction: role of Rho, myosin light chain kinase, and myosin light chain phosphatase. Exp Cell Res. 2000;254:210–220. doi: 10.1006/excr.1999.4754. [DOI] [PubMed] [Google Scholar]
- Park S, Hung CT, Ateshian GA. Mechanical response of bovine articular cartilage under dynamic unconfined compression loading at physiological stress levels. Osteoarthr Cartil. 2004;12:65–73. doi: 10.1016/j.joca.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. J Mammary Gland Biol Neoplasia. 2004;9:325–342. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Peddada SD, Laughlin SK, Miner K, Guyon JP, Haneke K, Vahdat HL, Semelka RC, Kowalik A, Armao D, Davis B, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci U S A. 2008;105:19887–19892. doi: 10.1073/pnas.0808188105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–229. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Riou S, Mees B, Esposito B, Merval R, Vilar J, Stengel D, Ninio E, van Haperen R, de Crom R, Tedgui A, Lehoux S. High pressure promotes monocyte adhesion to the vascular wall. Circ Res. 2007;100:1226–1233. doi: 10.1161/01.RES.0000265231.59354.2c. [DOI] [PubMed] [Google Scholar]
- Rogers R, Norian J, Malik M, Christman G, Abu-Asab M, Chen F, Korecki C, Iatridis J, Catherino WH, Tuan RS, Dhillon N, Leppert P, Segars JH. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol. 2008;198(474):e1–e11. doi: 10.1016/j.ajog.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 2008;20:551–556. doi: 10.1016/j.ceb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selo-Ojeme D, Lawal O, Shah J, Mandal R, Pathak S, Selo-Ojeme U, Samuel D. The incidence of uterine leiomyoma and other pelvic ultrasonographic findings in 2,034 consecutive women in a north London hospital. J Obstet Gynaecol. 2008;28:421–423. doi: 10.1080/01443610802149863. [DOI] [PubMed] [Google Scholar]
- Soltz MA, Ateshian GA. Interstitial fluid pressurization during confined compression cyclical loading of articular cartilage. Ann Biomed Eng. 2000;28:150–159. doi: 10.1114/1.239. [DOI] [PubMed] [Google Scholar]
- Stamenović D. Rheological behavior of mammalian cells. Cell Mol Life Sci. 2008;65:3592–3605. doi: 10.1007/s00018-008-8292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart EA, Taran FA, Chen J, Gostout BS, Woodrum DA, Felmlee JP, Ehman RL. Magnetic resonance elastography of uterine leiomyomas: a feasibility study. Fertil Steril. 2011;95:281–284. doi: 10.1016/j.fertnstert.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Tse JR, Engler AJ. Curr Protoc Cell Biol. Vol. 47. John Wiley & Sons; Hoboken, NJ: 2010. Preparation of hydrogel substrates with tunable mechanical properties; pp. 10.16.1–16. [DOI] [PubMed] [Google Scholar]
- Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–1592. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- Wang YL, Pelham RJ., Jr Preparation of a flexible, porous polyacrylamide substrate for mechanical studies of cultured cells. Methods Enzymol. 1998;298:489–496. doi: 10.1016/s0076-6879(98)98041-7. [DOI] [PubMed] [Google Scholar]
- Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettschureck N, Offermanns S. Rho/Rho-kinase mediated signaling in physiology and pathophysiology. J Mol Med. 2002;80:629–638. doi: 10.1007/s00109-002-0370-2. [DOI] [PubMed] [Google Scholar]
- Wolanska M, Sobolewski K, Drozdzewicz M, Bankowski E. Extracellular matrix components in uterine leiomyoma and their alteration during the tumour growth. Mol Cell Biochem. 1998;189:145–152. doi: 10.1023/a:1006914301565. [DOI] [PubMed] [Google Scholar]
- Wolanska M, Sobolewski K, Cechowska-Pasko M, Jaworski S. The activities of some glycosaminoglycan-degrading enzymes in uterine leiomyomas. Eur J Obstet Gynecol Reprod Biol. 2003;110:73–78. doi: 10.1016/s0301-2115(03)00110-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.