Abstract
Adipose-derived stem cells (ASCs) are an abundant, readily available population of multipotent progenitor cells that reside in adipose tissue. Isolated ASCs are typically expanded in monolayer on standard tissue culture plastic with a basal medium containing 10% fetal bovine serum. However, recent data suggests that altering the monolayer expansion conditions by using suspension culture plastic, adding growth factors to the medium, or adjusting the seeding density may affect the self-renewal rate, multipotency, and lineage-specific differentiation potential of the ASCs. We hypothesized that variation in any of these expansion conditions would influence the chondrogenic potential of ASCs. ASCs were isolated from human liposuction waste tissue and expanded through two passages with different tissue culture plastic, feed medium, and cell seeding densities. Once expanded, the cells were cast in an agarose gel and subjected to identical chondrogenic culture conditions for 7 days, at which point cell viability, radiolabel incorporation, and gene expression were measured. High rates of matrix synthesis upon chondrogenic induction were mostly associated with smaller cells, as indicated by cell width and area on tissue culture plastic, and it appears that expansion in a growth factor supplemented medium is important in maintaining this morphology. All end-point measures were highly dependent on the specific monolayer culture conditions. These results support the hypothesis that monolayer culture conditions may “prime” the cells or predispose them towards a specific phenotype and thus underscore the importance of early culture conditions in determining the growth and differentiation potential of ASCs.
Keywords: Adult stem cells, Differentiation, Mesenchymal stem cells, Adipose stem cells, Progenitor cells, Chondrogenesis
INTRODUCTION
Adipose-derived stem cells (ASCs) are a highly abundant, easily accessible population of stem cells residing in adipose tissue (Aust et al. 2004; Gimble and Guilak 2003a; Guilak et al. 2006; Zuk et al. 2001). Under controlled in vitro conditions, ASCs can be induced to synthesize various tissue-specific matrix proteins of both mesodermal and ectodermal lineages (Awad et al. 2004; De Ugarte et al. 2003; Dragoo et al. 2003; Erickson et al. 2002; Estes et al. 2006a; Estes et al. 2006b; Gimble and Guilak 2003b; Guilak et al. 2006; Halvorsen et al. 2000; Huang et al. 2004; Mizuno et al. 2002; Safford et al. 2002; Wall et al. 2007; Wang et al. 2005; Wickham et al. 2003). The ability to differentiate the ASCs along various mesodermal lineages persists even after extended passaging (Estes et al. 2006b; Wall et al. 2007; Zuk et al. 2001). In the presence of potent morphogens such as transforming growth factor beta (TGF-β), insulin-like growth factor (IGF), and bone morphogenetic proteins (BMPs), ASCs have the capacity to produce several chondrocytic markers including aggrecan, collagen type II, chondroitin sulfate and link protein in a time dependent manner (Awad et al. 2003; Awad et al. 2004; Dragoo et al. 2003; Dragoo et al. 2005; Erickson et al. 2002; Estes et al. 2006a; Estes et al. 2006b; Zuk et al. 2002; Zuk et al. 2001). In two separate clonal analyses, the differentiation potential along multiple lineages was investigated (Guilak et al. 2006; Zuk et al. 2002). Though somewhat different in methodology, these studies confirmed, at the clonal level, the ability of ASCs to be induced along adipogenic, osteogenic, chondrogenic, and neurogenic lineages in controlled in vitro biochemical environments. Taken together, these papers demonstrate the substantial effects of the specific isolation and expansion conditions used, as 81% of the ASCs in the Guilak et al. study differentiated along at least one lineage as compared to 6% in the Zuk et al. study. One important difference in the Guilak study was the use of suspension culture plastic in lieu of standard tissue culture plastic during passage 0. This difference in tissue culture methodology and the increased differentiation potential observed in the Guilak et al. study suggest a potential link between the cell surface substrate and the ultimate differentiation potential of the cells.
It is also known that culture conditions can affect the differentiation capacity of bone marrow derived mesenchymal stem cells (MSCs). In this regard, cell shape and self-renewal rate in culture have been implicated in assessing adult MSC differentiation potential. Sekiya and colleagues demonstrated that, in general, cells that were thin and spindle-shaped possessed the highest propensity for multilineage differentiation (Sekiya et al. 2002). This same group also demonstrated that plating at low densities allows for the detection of a distinct population of rapidly self-renewing cells that have a higher capacity for multilineage differentiation (Colter et al. 2000; Colter et al. 2001). In contrast to studies involving MSCs, studies with ASCs suggest a high plating density is required to maintain self-renewal and differentiation potential (Erickson et al. 2002; Halvorsen et al. 2001a; Halvorsen et al. 2001b). To our knowledge, the effect of initial seeding density on the chondrogenic differentiation potential of ASCs has yet to be prospectively investigated; however, in one study, low seeding density proved to be more favorable for osteogenic and adipogenic differentiation (Lee et al. 2004). Further, the use of certain growth factors during monolayer expansion in both MSC and ASC populations may help to maintain the stem cells' ability to differentiate along various lineages (Coleman et al. 2007; Estes et al. 2006b; Jiang et al. 2002; Mastrogiacomo et al. 2001; Reyes et al. 2002; Reyes et al. 2001; Schwartz et al. 2002; Tsutsumi et al. 2001). MSCs grown on fibronectin-coated tissue culture plastic in the presence of epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) with low concentrations of fetal bovine serum (FBS) demonstrated high proliferation rates and the ability to maintain multipotent characteristics (Reyes et al. 2002; Reyes et al. 2001; Schwartz et al. 2002). EGF has also been linked to cell survival in mesenchymal stem cells (Fan et al. 2007). Other studies have shown that media containing basic fibroblastic growth factor (bFGF or FGF-2) in MSC culture maintain telomere length and result in a prolonged retention of differentiation potential (Bianchi et al. 2003; Yanada et al. 2006). The osteogenic and proliferative potential of ASCs can also be preserved by expanding the cells in the presence of bFGF (Quarto and Longaker 2006). For chondrogenic activity, several studies have confirmed the ability of bFGF, while in monolayer culture and also during induction, to significantly enhance the chondrogenic potential of MSCs and ASCs (Chiou et al. 2006; Coleman et al. 2007; Mastrogiacomo et al. 2001; Tsutsumi et al. 2001). It has also been shown that ASCs grown in the presence of both bFGF and EGF along with TGF-β maintain telomerase activity throughout 9 passages while maintaining the potential for chondrogenic differentiation (Estes et al. 2006b).
From these previous studies, it is readily apparent that monolayer cell expansion methods play a critical role in determining the phenotype and differentiation potential of the stem cells. The objective of this study was therefore to investigate the influence of initial cell seeding density, the use of growth factors, and the type of tissue culture plastic on the differentiation potential of ASCs. We specifically hypothesized that any modification to “standard” ASC monolayer culture conditions would significantly influence the ability of the cells to be chondrogenically induced in 3-D agarose culture. To this end, ASCs were subjected to a variety of monolayer expansion conditions and then chondrogenically induced for 7 days in a 3-D agarose culture system. The effects of the monolayer cell culture were monitored by examining the cell morphology and rate of expansion at passage 1 (P1). The phenotype following 7 days of chondrogenic induction was assessed by quantitative real-time polymerase chain reaction (qPCR) analysis examining not only chondrogenic genes such as collagen II and aggrecan, but also genes associated with phenotypes of osteogenic, adipogenic, and neurogenic lineages. 35S-sulfate and 3H-proline incorporation were assessed as measures of cell biosynthesis in 3-D culture, and total DNA content was used to determine cell number.
MATERIALS AND METHODS
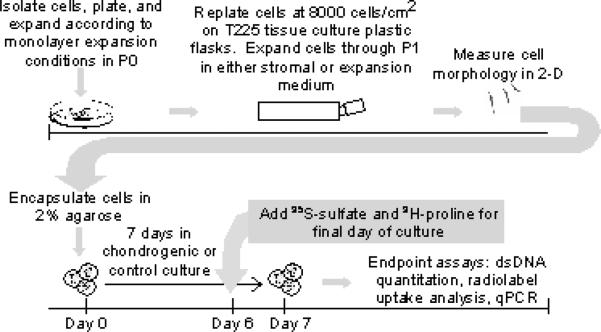
The experimental methods (described in detail in this section) for this study involve two phases. The cells were expanded in monolayer through the various specified cell culture conditions and were then placed in identical control and chondrogenic 3-D culture conditions over a 7 day period (Figure 1).
Figure 1.
Diagram of experimental design
Cell Culture
ASCs were isolated by collagenase digestion of lipoaspirates (Aust et al. 2004)obtained from plastic surgery waste tissue from a 48 year old female with a body mass index (BMI) of 32.7. All protocols were performed in concordance with the Duke University Institutional Review Board. The cells were then divided into 5 different monolayer expansion conditions according to three factors: cell density, medium and tissue culture plastic (Table 1). Briefly, the cells were cultured on suspension culture plastic in expansion medium at either high or low density or on tissue culture plastic in stromal medium at either high or low density. These four conditions were compared to the “standard” expansion protocol used in our laboratory, cells plated on tissue culture plastic with expansion medium at high density (i.e., TEHI). Plating the cells on suspension culture plastic with stromal medium at either high or low density was not used as expansion conditions as the cells will readily senesce under these parameters (unpublished data). Since ASC number after tissue digestion cannot be directly quantified due to the presence of other cell types and undigested tissue, high and low densities were defined by ten-fold differences in the amount of lipoaspirate tissue plated on the culture dishes (8 ml and 0.8 ml for high and low densities respectively). The cells were plated on either suspension culture 150 mm dishes (Corning Life Sciences, Corning, NY, Cat. no. 430597) or tissue culture treated 150 mm dishes (Corning Life Sciences, Cat. no. 430599). Stromal medium consisted of DMEM/F12 (Cambrex Bio Science, Walkersville, MD), 10% fetal bovine serum (FBS, Hyclone, Logan UT) and 1% penicillin-streptomycin- fungizone (Gibco, Grand Island, NY). Expansion medium consisted of stromal medium, 0.25 ng/ml TGF-β1 (R&D Systems, Minneapolis, MN), 5 ng/ml EGF (Roche Diagnostics, Indianapolis, IN), and 1 ng/ml bFGF (Roche Diagnostics). After 24 hours, non-adherent cells were washed off, and the medium was replaced every other day. At P0, the cells were allowed to proliferate to ~90% confluence before the cells were trypsinized (0.05% trypsin/EDTA, Gibco) and replated at 8,000 cells/cm2 in T225 tissue culture treated flasks (Corning Life Sciences, Cat. no. 431082) with the appropriate media for all groups. Throughout P0 and P1, cell morphology was captured with differential contrast imaging, and a minimum of 10 representative cells on each image were analyzed using the Zeiss LSM Image Browser and Adobe Photoshop. The doubling rate at P1 was calculated by determining the number of doublings in culture divided by the number of days in culture (i.e., doublings/day = (ln(final cell number/starting cell number)/ln(2))/number of days in culture)).
Table 1.
Monolayer expansion conditions at P0
| Group Name | Culture Plastic | Medium | Cell Density |
|---|---|---|---|
| SEHI | Suspension | Expansion | High |
| SELO | Suspension | Expansion | Low |
| TEHI | Tissue | Expansion | High |
| TSHI | Tissue | Stromal | High |
| TSLO | Tissue | Stromal | Low |
Construct Fabrication
At ~90% confluence in P1, cells from each respective group were mixed in sterile 2% (w/v) type VII low gelling point agarose (Sigma-Aldrich Co., St. Louis, MO) to obtain a homogenous cell distribution (5×106 cells/ml) and then injected into a custom mold (1.8 mm thick, Ø25 mm) for gelation at room temperature. A Ø6 mm biopsy punch was then used to create discs that were placed in individual wells of a 24 well plate with 1 ml of chondrogenic medium containing DMEM-HG (Gibco), 10% fetal bovine serum (Hyclone), 1% penicillin-streptomycin (Gibco), 100 nM dexamethasone (Sigma), 10 ng/ml TGF-β1 (R & D Systems), 37.5 μg/ml L-ascorbic acid 2-phosphate (Sigma), and 1% ITS+ premix (Collaborative Biomedical, Becton–Dickinson, Bedford, MA), which contains human recombinant insulin, human transferrin (12.5 mg each), selenous acid (12.5 mg), BSA (2.5 g), and linoleic acid (10.7 mg). The cultures were maintained at 37° C and 5% CO2 for 7 days with the chondrogenic medium exchanged every other day.
DNA, 3H-proline, and 35S-sulfate assays
For the last 24 hours of culture, 10 μCi/ml of 3H-proline and 5 μCi/ml of 35S-sulfate were added to each of the different cultures in order to quantify total protein and sulfated glycosaminoglycan synthesis, respectively. After the agarose discs were digested in papain (125 μg/ml), DNA content (per agarose disc) was quantified using the PicoGreen fluorescent double-stranded DNA (dsDNA) assay (Invitrogen, Eugene, OR). Radiolabel incorporation was quantified using a scintillation analyzer (Packard, Meriden, CT) after the addition of Bio-Safe II scintillation mixture (Research Products International, Mount Prospect, IL), and the resulting data were normalized to dsDNA content.
RNA isolation and real-time PCR
β-agarase (Cambrex Bio Science Rockland, Inc.; Rockland, ME) was used to digest the polysaccharide backbone of the agarose prior to RNA isolation. Briefly, 600 μl of a 1× solution of RNAsecure (Ambion; Austin, TX) was heated to 60° C for 10 minutes to activate RNase inhibitors. The solution was then cooled to 45° C, at which time the β-agarase buffer at 1× (Cambrex Bio Science Rockland, Inc.) was added to each tube along with the agarose construct. Following 15 minutes of incubation at 45° C, the buffer solution was removed and the tubes were heated to 70° C to melt the agarose. Once the agarose was molten, the tubes were cooled to 45° C, and 3 μl of β-agarase (supplied at 1U/ml) was added to each tube to effectively allow for the digestion of the polysaccharide backbone (incubation time ≥ 1 hour). Total RNA was isolated using the Aurum Total RNA Mini Kit (Bio-Rad Laboratories, Inc., Hercules, CA) according to the manufacturer's protocol. Once isolated, cDNA was made from total RNA by reverse transcriction using iScript reverse transcriptase (Bio-Rad Laboratories, Inc.). Using commercially-available primer-probes from Applied Biosystems (Foster City, CA), real-time quantitative PCR (ABI Prism 7000 Sequence Detection System; Applied Biosystems) was used to compare transcript levels for eight different genes: 18S rRNA (endogenous control, assay ID Hs99999901_s1), aggrecan (AGC1, assay ID Hs00153936_m1), type I collagen (COL1A1, assay ID Hs00164004_m1), type II collagen (COL2A1, assay ID Hs00156568_m1), leptin (LEP, assay ID Hs00174877_m1), osteopontin (SPP1, assay ID Hs00167093_m1), Sox 9 (SOX9, assay ID Hs00165814_m1) and beta 3 tubulin (TUBB3, assay ID Hs00801390_s1). The delta delta Ct method was used for relative quantification of gene expression to compare the effects of the 5 different monolayer culture conditions on ASC gene expression (Livak and Schmittgen 2001). Briefly, the following equation was used to determine ΔΔCt:
Fold differences in gene expression were determined by calculating 2-ΔΔCt.
Statistical Analysis
Analysis of variance (ANOVA) was used with Fisher's protected least significant difference (PLSD) post hoc test (α = 0.05) to determine statistical significance.
RESULTS
Monolayer expansion conditions had a significant effect on the doubling rate of the cells at P1. The TEHI group (e.g., Tissue culture plastic, Expansion medium, High seeding density) resulted in the fastest doubling rate of 0.405 doublings/day, which was statistically greater (p < 0.05) than all other groups except for the SEHI group (e.g. Suspension culture plastic, Expansion medium, High seeding density) (Figure 2). The various culture conditions also had a significant influence on the morphology of the cells in 2-D monolayer (Figures 3 and 4). Expansion of the cells in expansion medium resulted in thinner, spindle-shaped cells, which effectively occupied significantly less projected surface area (p < 0.05) on the plates (Figure 4a) and had thinner cell bodies (p < 0.05) (Figure 4b). Viability in 3-D agarose culture over the 7 day induction period was assessed for each monolayer condition group by comparing day 7 dsDNA to day 0 starting quantity dsDNA (Figure 5a). If originally plated on non-treated tissue culture plastic, the smaller, narrower cells of the expansion media groups exhibited a 59% (high density) and 69% (low density) decrease in dsDNA as compared to day 0. However, if the cells were initially plated and expanded on tissue culture plastic, a moderate 23% decrease in dsDNA over the 7 day 3-D culture period was observed. Conversely, the cells which became large in culture (plated in stromal medium at either high or low densities on tissue culture plastic) continued to proliferate once in agarose, resulting in a net increase in dsDNA over the 7 day culture period with a 77% and 58% increase, respectively.
Figure 2.
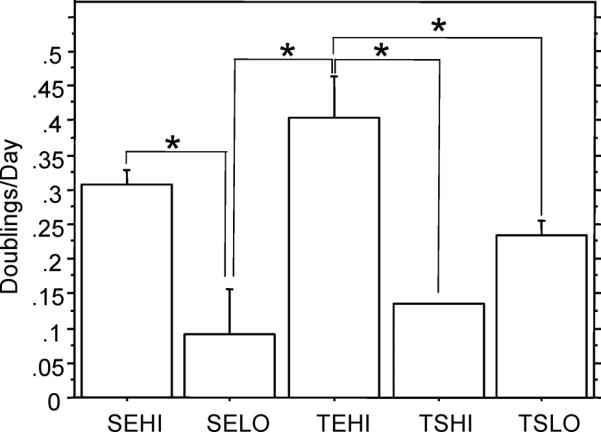
Doublings per day in passage 1 (*, p < 0.05) (Data presented as mean ± S.E.M.) SEHI: Suspension culture plastic, Expansion medium, High density; SELO: Suspension culture plastic, Expansion medium, Low density; TEHI: Tissue culture plastic, Expansion medium, High density; TSHI: Tissue culture plastic, Stromal medium, High density; TSLO: Tissue culture plastic, Stromal medium, Low density
Figure 3.
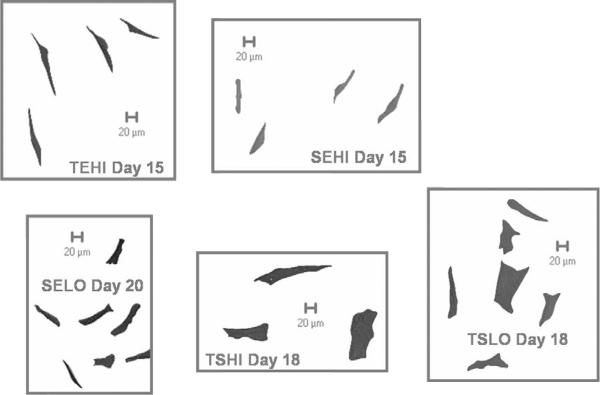
Representative cell morphology from the different culture conditions at the end of passage 1 (P1). SEHI: Suspension culture plastic, Expansion medium, High density; SELO: Suspension culture plastic, Expansion medium, Low density; TEHI: Tissue culture plastic, Expansion medium, High density; TSHI: Tissue culture plastic, Stromal medium, High density; TSLO: Tissue culture plastic, Stromal medium, Low density
Figure 4.
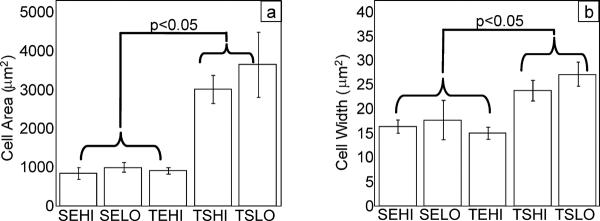
(a) Cell area on final culture day before chondrogenic induction and (b) Cell width on final culture day before chondrogenic induction, n ≥ 10 cells analyzed per flask for each respective condition. (Data presented as mean ± S.E.M.) SEHI: Suspension culture plastic, Expansion medium, High density; SELO: Suspension culture plastic, Expansion medium, Low density; TEHI: Tissue culture plastic, Expansion medium, High density; TSHI: Tissue culture plastic, Stromal medium, High density; TSLO: Tissue culture plastic, Stromal medium, Low density
Figure 5.
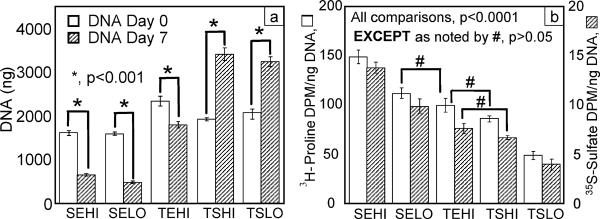
(a) dsDNA quantity in agarose disks at day 0 and day 7 (b) rate of radiolabel incorporation for final 24 hours of chondrogenic culture normalized to day 7 dsDNA, n ≥ 5. (Data presented as mean ± S.E.M.) SEHI: Suspension culture plastic, Expansion medium, High density; SELO: Suspension culture plastic, Expansion medium, Low density; TEHI: Tissue culture plastic, Expansion medium, High density; TSHI: Tissue culture plastic, Stromal medium, High density; TSLO: Tissue culture plastic, Stromal medium, Low density
Cell metabolic activity as measured by 3H-proline and 35S-sulfate incorporation over the final 24 hours of 3-D culture was also significantly affected by initial monolayer expansion conditions (Figure 5b). Low initial plating density resulted in significantly less radiolabel uptake (p <0.0001) as compared to high initial plating density for both the suspension culture plastic, expansion medium group (i.e., SEHI vs. SELO) and the tissue culture plastic, stromal medium group (i.e., TSHI vs. TSLO). Decreases of 26% and 28% (SEHI vs. SELO) and 43% and 40% (TSHI vs. TSLO) in normalized 3H-proline and 35S-sulfate incorporation, respectively, were observed. In addition, cells expanded on tissue culture plastic in expansion medium (i.e. TEHI) exhibited a significantly higher radiolabel incorporation rate compared to the TSLO group (p < 0.0001), but not to the TSHI condition.
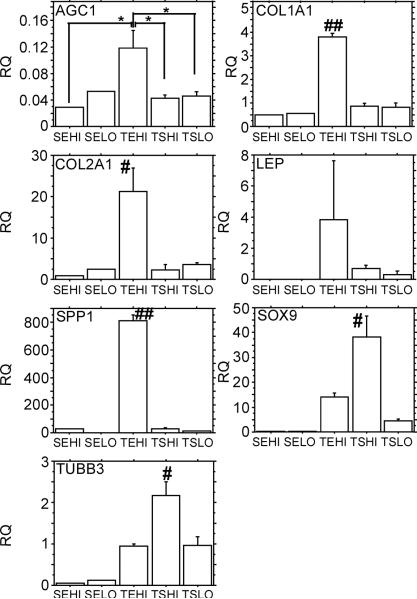
For relative quantification of all the genes studied, two calibrators were used, either the day 0 cells for each monolayer condition (Figure 6), or the TSHI group at day 7 (Figure 7).
Figure 6.
AGC1, COL1A1, COL2A1, LEP, SPP1, SOX9, and TUBB3 (corrected with the housekeeping gene, 18s) gene expression results at the end of the 7 day 3-D agarose culture. Relative Quantitation (RQ) to day 0 control cells (also corrected with the housekeeping gene, 18s) from the same monolayer expansion conditions. n≥ 4 constructs per condition. Values are the mean ± SEM. ** = P < 0.0001; * = P < 0.05; ## = P < 0.0001 relative to all other conditions and # = P < 0.05 relative to all other conditions unless otherwise noted. SEHI: Suspension culture plastic, Expansion medium, High density; SELO: Suspension culture plastic, Expansion medium, Low density; TEHI: Tissue culture plastic, Expansion medium, High density; TSHI: Tissue culture plastic, Stromal medium, High density; TSLO: Tissue culture plastic, Stromal medium, Low density
Figure 7.
AGC1, COL1A1, COL2A1, LEP, SPP1, SOX9, and TUBB3 (corrected with the housekeeping gene, 18s) gene expression results at the end of the 7 day 3-D agarose culture. Relative Quantitation (RQ) to day 7 TSHI induced cells (also corrected with the housekeeping gene, 18s). n≥ 4 constructs per condition. Values are the mean ± SEM. ** = P < 0.0001; * = P < 0.05; ## = P < 0.0001 relative to all other conditions and # = P < 0.05 relative to all other conditions unless otherwise noted. SEHI: Suspension culture plastic, Expansion medium, High density; SELO: Suspension culture plastic, Expansion medium, Low density; TEHI: Tissue culture plastic, Expansion medium, High density; TSHI: Tissue culture plastic, Stromal medium, High density; TSLO: Tissue culture plastic, Stromal medium, Low density
ASCs cultured on tissue culture plastic at high density in expansion medium or stromal medium (i.e., TEHI and TSHI) demonstrated the most profound effects on transcript levels for all analyzed genes, relative to day 0 controls. Under chondrogenic culture conditions, AGC1 transcript levels were downregulated in all conditions ranging from an 8.47 (TEHI) to 34.48 (SEHI) fold decrease. Unlike AGC1 levels, COL1A1 transcript levels were increased with the TEHI condition, resulting in a 3.8 fold increase relative to day 0 cells cultured in the same condition. However, only the TEHI condition resulted in an increase in COL1A1 expression; whereas all other conditions resulted in a statistically significant decrease in COL1A1 gene expression (p < 0.0001). COL2A1 expression showed a similar trend in that the TEHI condition resulted in ASCs with the largest potential for increased COL2A1 levels under chondrogenic conditions. Approximately 10 fold higher expression levels were observed in TEHI cells than in cells plated in the TSHI condition (i.e., the same condition with stromal medium substituted for expansion medium). This level of increased COL2A1 expression in the TEHI condition was statistically greater than all other monolayer expansion conditions (p < 0.05); though all conditions except the SEHI condition demonstrated an ability to support upregulation of COL2A1 under chondrogenic conditions once cast in agarose. A similar pattern of differences in the cell culture conditions was also noted with osteopontin (SPP1) expression, in which the TEHI condition resulted in an 809 fold increase relative to day 0 cells in the same condition, which was also statistically greater than all the other conditions (p < 0.0001). Differing from the trends noted in the results for the previous analyzed genes, ASCs plated in the TSHI condition demonstrated the largest increase in SOX9 transcript, with a 38 fold increase relative to day 0 cells in the same condition (p < 0.05 relative to all other conditions). SOX9 transcript levels were also elevated in the other two conditions in which the ASCs were plated on tissue culture plastic and were downregulated when the cells were plated on suspension culture plastic prior to being encapsulated in agarose. The TSHI condition was also the only condition that resulted in an upregulation of β3-tubulin (TUBB3) expression at 2.18 fold higher than day 0 cells from the same condition (p < 0.05 relative to all other conditions). No statistically significant differences were noted in expression levels for the various culture conditions for leptin (LEP) expression.
Different trends were noted when comparing transcript levels using the TSHI group (e.g., Tissue culture plastic, Stromal medium, High seeding density) as the calibrator. ASCs plated in expansion medium at low density on suspension culture plastic (i.e., SELO) demonstrated a 540 fold increase in AGC1 expression relative to the TSHI group after 7 days in agarose in chondrogenic conditions, which was significantly greater than all other conditions (p < 0.0001). COL1A1 transcript levels were increased approximately 2 fold in all conditions relative to the cells isolated and cast in agarose from the TSHI condition, though this increase was not significant in the SEHI monolayer expansion condition. For COL2A1 transcript analysis, only cells originating from the TEHI condition resulted in a statistically significant increase (6.22 fold increase) in transcript levels relative to cells obtained from the TSHI culture condition (p < 0.05). Cells plated in expansion medium at high density on both tissue culture plastic and suspension culture plastic (i.e., SEHI and TEHI) demonstrated significantly higher osteopontin (SPP1) expression levels relative to all other conditions (p < 0.0001) once cast in agarose and chondrogenically induced over the 7 day period. Specifically, ASCs cultured at high density in expansion medium increased SPP1 expression 41.5 fold (suspension culture plastic) and 16.42 fold (tissue culture plastic) as compared to the stromal medium and tissue culture plastic group. When comparing SOX9 transcript levels in the various cell culture conditions, only the TSLO condition demonstrated an ability to significantly increase SOX9 transcript levels relative to the TSHI condition (2.75 fold increase, p < 0.01). Plating the ASCs on suspension culture plastic at both high and low densities resulted in significant 6.37 and 9.79 fold increases respectively in β3-tubulin (TUBB3) compared to cells plated in the TSHI condition; these increased expression levels were significant relative to all other conditions (p < 0.05). As was observed when using the respective day 0 cells as the calibrator, no statistically significant differences were noted in expression levels for the various culture conditions for leptin (LEP) expression.
DISCUSSION
The findings of this study demonstrate that the initial monolayer culture conditions used to expand ASCs play a critical role in determining their differentiation capacity. The 5 different monolayer culture conditions resulted in cells that responded in a distinctly different fashion when chondrogenically induced in 3-D culture. While no clear trend was noted toward any specific lineage, it was apparent that changes in monolayer culture expansion conditions had a profound effect on all end-point assays, including viability, biosynthesis rates, and gene expression analyses. Several studies using bone marrow derived MSCs have shown that the initial monolayer culture conditions affect the phenotype of the stem cells (Jiang et al. 2002; Reyes et al. 2002; Reyes et al. 2001; Schwartz et al. 2002; Sekiya et al. 2002); to our knowledge, this is the first report of the effects of the environment on the phenotype and ultimate differentiation potential of ASCs. It is important to recognize that this study was conducted using cells from one donor and may not be universally representative of differences that might be obtained from other donors' cells.
In terms of viability and metabolic activity, the results can be divided into three different groups: (1) monolayer culture conditions which resulted in a significant loss in DNA content while maintaining a high rate of matrix synthesis (i.e., SEHI and SELO), (2) monolayer culture conditions which resulted in a minimal loss of DNA content while retaining a high rate of matrix synthesis (i.e., TEHI) and (3) monolayer culture conditions which resulted in a significant increase in DNA with low matrix synthesis (i.e., TSHI and TSLO). In the TSHI and TSLO group, culturing the cells in monolayer on tissue culture plastic in the presence of stromal medium increased dsDNA content by approximately 70% over 7 days of chondrogenic induction. However, this increase in dsDNA content appears to be at the expense of matrix biosynthesis as indicated by the approximate 70% decrease in 3H-proline and 35S-sulfate incorporation rates, as compared to the group with the highest biosynthesis rates, SEHI. The opposite trends were noted in the SEHI and SELO grouping suggesting perhaps that a compromise to maintain dsDNA content while invoking high matrix biosynthesis rates may be achieved by culturing the cells on Tissue culture plastic, with Expansion medium at a High initial seeding density (i.e., TEHI).
Cell morphology on the final day of monolayer culture, as measured by cell area and width of the elongated cell body (Figure 4), was correlated to both DNA and radiolabel uptake data (Figure 5). Cells which became large in monolayer culture exhibited the highest DNA content once in agarose, but also exhibited the lowest matrix synthesis rates. Conversely, a significant percentage of cells which were smaller, narrower, and substantially more spindle-shaped appeared to die during chondrogenic induction, with the remaining cells exhibiting relatively high biosynthetic activity. The connection between stem cell size and function is consistent with bone marrow derived MSCs, which have also been shown to possess more “stemness” when the MSCs are small and rapidly dividing (Sekiya et al. 2002). It appears from these data that cell expansion in a supplemented medium is important to maintain the small, spindle-shaped morphology associated with higher synthesis of matrix, as the three expansion medium groups were significantly smaller and narrower than the two stromal medium groups. Cells plated in expansion medium at high densities also replicated at a greater rate during monolayer expansion than the other groups (Figure 2), though these data alone did not correlate with DNA content or metabolic activity after 7 days in 3-D culture.
Gene expression data using the day 0 cells from each monolayer condition as the calibrator (Figure 6) provides a measure for how the cells from each group responded to chondrogenic induction in 3-D agarose culture. All groups except SEHI showed an upregulation in COL2AI and all groups cultured on tissue culture plastic showed elevated SOX9 transcript levels, a critical transcription factor related to chondrogenesis (Lefebvre et al. 1997). Cells expanded at high density on tissue culture plastic using expansion medium (TEHI) demonstrated an increased expression of COL1A1 and showed the largest increase in COL2A1 expression. The TEHI group also showed a large increase (809 fold) in osteopontin. Calibrating the gene expression data by chondrogenically induced day 7 cells expanded with typical monolayer conditions (tissue culture plastic, stromal medium, high density) allows for a comparison of chondrogenically induced cells across different monolayer conditions. The considerable difference seen in the results depending on the calibrator used indicates that some aspects of the gene expression profiles were already significantly altered at the end of monolayer culture. For example, upregulation of aggrecan in SELO was not significant when compared to its day 0 control but showed a 540 fold increase in expression when compared to the TSHI group after 7 days in agarose, indicating that much of this relative upregulation in the SELO condition occurred during monolayer expansion. When compared to TSHI, both cell groups plated at high density in expansion medium (SEHI and TEHI) showed large increases in osteopontin upregulation, suggesting that these monolayer conditions may favor osteogenesis.
The most commonly used chondrogenic cocktail for ASCs (TGF-β1 and dexamethasone) was used for this study (Afizah et al. 2007; Mehlhorn et al. 2006; Winter et al. 2003). However, genes not associated with chondrogenesis, per se, were upregulated. The TEHI condition, as has been discussed, resulted in an approximate 800 fold increase in osteopontin gene expression, an early marker for osteogenesis. In like fashion, substituting stromal medium for expansion medium (i.e., TSHI) resulted in the upregulation of a neuronal marker, TUBB3. In this light, it should be noted that the chondrogenic induction conditions used in this study may not be optimal, as recent studies have shown that ASCs may be more potently induced by BMPs than by TGF-β1 and dexamethasone (Estes et al. 2006a; Estes et al. 2006b; Hennig et al. 2007). The monolayer conditions used in this study may “prime” cells in such a way that they would respond differently to BMP-mediated chondrogenic induction as compared to TGF-β1 induction. Regardless, these findings demonstrate that specific monolayer expansion conditions significantly contribute to the performance of ASCs in 3-D chondrogenic culture.
Without performing a clonal analysis of cells obtained in each respective group, it is impossible to predict the ultimate differentiation potential of cells obtained from each culture condition. Nonetheless, the data in this study suggest that using expansion medium for ASC monolayer culture is desirable as this medium results in consistent small, spindle shaped cell morphology (Figure 4), rapid expansion profiles (Figure 2), and high metabolic activity (Figure 5). In terms of assessing which monolayer culture condition was most successful in promoting chondrogenesis, three positive gene transcript markers for chondrogenesis were employed: collagen 2 (COL2A1), the primary collagen found in articular cartilage; aggrecan (AGC1), a critical negatively charged aggregating proteoglycan found in articular cartilage; and SOX9, a potent chondrogenic transcription factor. In this study, culturing the cells in the TEHI condition resulted in the highest levels of COL2A1 transcript (Figures 6 & 7). AGC1 expression, however, was repressed across all groups. This result is most likely a function of the morphogens used to promote chondrogenesis, and is not reflective of the ability of the monolayer expansion conditions to support AGC1 expression. AGC1 expression along with proteoglycan accumulation is observed after culture in expansion medium and induction with BMP-6 (Estes et al. 2006a; Estes et al. 2006b). SOX9 was also upregulated after monolayer culture in expansion medium on tissue culture plastic. Taken together, these data suggest that culturing ASCs in expansion medium on tissue culture plastic (i.e. TEHI) provides ASCs that have the greatest chondrogenic inducibility across all groups studied.
It is well known that cultured chondrocytes readily dedifferentiate in monolayer culture with increasing expression levels of COL1A1 across passage and decreases in COL2A1 across passage (Darling and Athanasiou 2005a; Darling and Athanasiou 2005b; Valcourt et al. 2003). Cultured chondrocytes will attach and maintain their native rounded morphology when plated on tissue culture plastic but will then slowly flatten and occupy larger amounts of surface area in monolayer culture. This “switch” to a fibroblastic phenotype, more occupied surface area, and more cell processes is associated with the presence of F-actin stress fibers in cells (Mallein-Gerin et al. 1991). This increase in F-actin stress fibers results in greater surface area, thereby giving the appearance of cells with wide cell bodies, much like what was observed with ASCs cultured in stromal medium. This process of cell flattening coincides with a loss of the chondrogenic phenotype. Much in the same way, the ASCs cultured in stromal medium had more cell processes and larger projected surface area on tissue culture plastic (Figures 3 and 4) and demonstrated less chondrogenic differentiation potential. While the dedifferentiation process is not fully understood, the data suggest that the cell shape and the presence of F-actin stress fibers, regardless of cell type, in monolayer culture might be predictive of the ultimate ability to obtain a chondrogenic phenotype
It should also be noted that our results may be indicative of the enrichment for distinct sub-populations of cells that are either in the process of differentiation or predispose them to becoming chondrogenic (or other lineages) under the proper chemical stimuli. For this initial study, immunophenotyping of the cells prior to chondrogenic differentiation was not performed as our goal was to assess the effect the environment had on the heterogeneous cell population. Nonetheless, characterizing the immunophenotype of the cells that are primed or predisposed toward differentiation might lend insight into a sub-population niche that, when expanded, will be more chondrogenic. Regardless of the starting cell population, however, an abundant supply of cells capable of regenerating tissue is required, which likely necessitates in vitro expansion of a progenitor cell population while maintaining a robust differentiation potential. While previous studies have largely focused on the conditions required for differentiation to the desired tissue lineage, the results of this study suggest that stem cell expansion conditions may play a critical role in the ultimate differentiation potential of adult stem cells. In this regard, our findings suggest that the early monolayer culture conditions of progenitor cells play a significant role in the downstream differentiation potential of the cells and may act in synergy with the differentiation conditions to optimally induce the desired phenotype.
ACKNOWLEDGMENTS
We would like to thank Steven Kreuzer and Dr. Beverley Fermor for their contributions to the initial stages of this study. This study was supported in part by NIH grants UL1RR024128, AG15768, AR50245, AR48182, and GM08555, the Coulter Foundation, and by a NSF Graduate Fellowship.
REFERENCES
- Afizah H, Yang Z, Hui JH, Ouyang HW, Lee EH. A comparison between the chondrogenic potential of human bone marrow stem cells (BMSCs) and adipose-derived stem cells (ADSCs) taken from the same donors. Tissue Eng. 2007;13(4):659–66. doi: 10.1089/ten.2006.0118. [DOI] [PubMed] [Google Scholar]
- Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, Sen A, Willingmyre GD, Gimble JM. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6(1):7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25(16):3211–22. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- Bianchi G, Banfi A, Mastrogiacomo M, Notaro R, Luzzatto L, Cancedda R, Quarto R. Ex vivo enrichment of mesenchymal cell progenitors by fibroblast growth factor 2. Exp Cell Res. 2003;287(1):98–105. doi: 10.1016/s0014-4827(03)00138-1. [DOI] [PubMed] [Google Scholar]
- Chiou M, Xu Y, Longaker MT. Mitogenic and chondrogenic effects of fibroblast growth factor-2 in adipose-derived mesenchymal cells. Biochem Biophys Res Commun. 2006;343(2):644–52. doi: 10.1016/j.bbrc.2006.02.171. [DOI] [PubMed] [Google Scholar]
- Coleman RM, Case ND, Guldberg RE. Hydrogel effects on bone marrow stromal cell response to chondrogenic growth factors. Biomaterials. 2007;28(12):2077–86. doi: 10.1016/j.biomaterials.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000;97(7):3213–8. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A. 2001;98(14):7841–5. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005a;23(2):425–32. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Darling EM, Athanasiou KA. Retaining zonal chondrocyte phenotype by means of novel growth environments. Tissue Eng. 2005b;11(3–4):395–403. doi: 10.1089/ten.2005.11.395. [DOI] [PubMed] [Google Scholar]
- De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174(3):101–9. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- Dragoo JL, Choi JY, Lieberman JR, Huang J, Zuk PA, Zhang J, Hedrick MH, Benhaim P. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21(4):622–9. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;290(2):763–9. doi: 10.1006/bbrc.2001.6270. [DOI] [PubMed] [Google Scholar]
- Estes BT, Wu AW, Guilak F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 2006a;54(4):1222–32. doi: 10.1002/art.21779. [DOI] [PubMed] [Google Scholar]
- Estes BT, Wu AW, Storms RW, Guilak F. Extended passaging, but not aldehyde dehydrogenase activity, increases the chondrogenic potential of human adipose-derived adult stem cells. J Cell Physiol. 2006b;209(3):987–95. doi: 10.1002/jcp.20808. [DOI] [PubMed] [Google Scholar]
- Fan VH, Tamama K, Au A, Littrell R, Richardson LB, Wright JW, Wells A, Griffith LG. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells. 2007;25(5):1241–51. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003a;5(5):362–9. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Guilak F. Differentiation potential of adipose derived adult stem (ADAS) cells. Curr Top Dev Biol. 2003b;58:137–60. doi: 10.1016/s0070-2153(03)58005-x. [DOI] [PubMed] [Google Scholar]
- Guilak F, Lott KE, Awad HA, Cao Q, Hicok KC, Fermor B, Gimble JM. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206(1):229–37. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- Halvorsen YC, Wilkison WO, Gimble JM. Adipose-derived stromal cells--their utility and potential in bone formation. Int J Obes Relat Metab Disord. 2000;24(Suppl 4):S41–4. doi: 10.1038/sj.ijo.0801503. [DOI] [PubMed] [Google Scholar]
- Halvorsen YD, Bond A, Sen A, Franklin DM, Lea-Currie YR, Sujkowski D, Ellis PN, Wilkison WO, Gimble JM. Thiazolidinediones and glucocorticoids synergistically induce differentiation of human adipose tissue stromal cells: biochemical, cellular, and molecular analysis. Metabolism. 2001a;50(4):407–13. doi: 10.1053/meta.2001.21690. [DOI] [PubMed] [Google Scholar]
- Halvorsen YD, Franklin D, Bond AL, Hitt DC, Auchter C, Boskey AL, Paschalis EP, Wilkison WO, Gimble JM. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 2001b;7(6):729–41. doi: 10.1089/107632701753337681. [DOI] [PubMed] [Google Scholar]
- Hennig T, Lorenz H, Thiel A, Goetzke K, Dickhut A, Geiger F, Richter W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211(3):682–91. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- Huang JI, Zuk PA, Jones NF, Zhu M, Lorenz HP, Hedrick MH, Benhaim P. Chondrogenic potential of multipotential cells from human adipose tissue. Plast Reconstr Surg. 2004;113(2):585–94. doi: 10.1097/01.PRS.0000101063.27008.E1. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh K, Bae YC, Jung JS. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14(4–6):311–24. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. 1997;17(4):2336–46. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mallein-Gerin F, Garrone R, van der Rest M. Proteoglycan and collagen synthesis are correlated with actin organization in dedifferentiating chondrocytes. Eur J Cell Biol. 1991;56(2):364–73. [PubMed] [Google Scholar]
- Mastrogiacomo M, Cancedda R, Quarto R. Effect of different growth factors on the chondrogenic potential of human bone marrow stromal cells. Osteoarthritis Cartilage. 2001;9(Suppl A):S36–40. doi: 10.1053/joca.2001.0442. [DOI] [PubMed] [Google Scholar]
- Mehlhorn AT, Niemeyer P, Kaiser S, Finkenzeller G, Stark GB, Sudkamp NP, Schmal H. Differential expression pattern of extracellular matrix molecules during chondrogenesis of mesenchymal stem cells from bone marrow and adipose tissue. Tissue Eng. 2006;12(10):2853–62. doi: 10.1089/ten.2006.12.2853. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Zuk PA, Zhu M, Lorenz HP, Benhaim P, Hedrick MH. Myogenic differentiation by human processed lipoaspirate cells. Plast Reconstr Surg. 2002;109(1):199–209. doi: 10.1097/00006534-200201000-00030. discussion 210–1. [DOI] [PubMed] [Google Scholar]
- Quarto N, Longaker MT. FGF-2 inhibits osteogenesis in mouse adipose tissue-derived stromal cells and sustains their proliferative and osteogenic potential state. Tissue Eng. 2006;12(6):1405–18. doi: 10.1089/ten.2006.12.1405. [DOI] [PubMed] [Google Scholar]
- Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109(3):337–46. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie CM. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98(9):2615–25. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- Safford KM, Hicok KC, Safford SD, Halvorsen YD, Wilkison WO, Gimble JM, Rice HE. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002;294(2):371–9. doi: 10.1016/S0006-291X(02)00469-2. [DOI] [PubMed] [Google Scholar]
- Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109(10):1291–302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20(6):530–41. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- Tsutsumi S, Shimazu A, Miyazaki K, Pan H, Koike C, Yoshida E, Takagishi K, Kato Y. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun. 2001;288(2):413–9. doi: 10.1006/bbrc.2001.5777. [DOI] [PubMed] [Google Scholar]
- Valcourt U, Gouttenoire J, Aubert-Foucher E, Herbage D, Mallein-Gerin F. Alternative splicing of type II procollagen pre-mRNA in chondrocytes is oppositely regulated by BMP-2 and TGF-beta1. FEBS Lett. 2003;545(2–3):115–9. doi: 10.1016/s0014-5793(03)00510-6. [DOI] [PubMed] [Google Scholar]
- Wall ME, Bernacki SH, Loboa EG. Effects of Serial Passaging on the Adipogenic and Osteogenic Differentiation Potential of Adipose-Derived Human Mesenchymal Stem Cells. Tissue Eng. 2007;13(6) doi: 10.1089/ten.2006.0275. [DOI] [PubMed] [Google Scholar]
- Wang DW, Fermor B, Gimble JM, Awad HA, Guilak F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J Cell Physiol. 2005;204(1):184–91. doi: 10.1002/jcp.20324. [DOI] [PubMed] [Google Scholar]
- Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res. 2003;(412):196–212. doi: 10.1097/01.blo.0000072467.53786.ca. [DOI] [PubMed] [Google Scholar]
- Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber RM, Ewerbeck V, Richter W. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003;48(2):418–29. doi: 10.1002/art.10767. [DOI] [PubMed] [Google Scholar]
- Yanada S, Ochi M, Kojima K, Sharman P, Yasunaga Y, Hiyama E. Possibility of selection of chondrogenic progenitor cells by telomere length in FGF-2-expanded mesenchymal stromal cells. Cell Prolif. 2006;39(6):575–84. doi: 10.1111/j.1365-2184.2006.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]