Abstract
Purpose/Objectives
To evaluate the safety and efficacy of reflexology, a complementary therapy that applies pressure to specific areas of the feet.
Design
Longitudinal, randomized clinical trial.
Setting
Thirteen community-based medical oncology clinics across the midwestern United States.
Sample
A convenience sample of 385 predominantly Caucasian women with advanced-stage breast cancer receiving chemotherapy and/or hormonal therapy.
Methods
Following the baseline interview, women were randomized into three primary groups: reflexology (n = 95), lay foot manipulation (LFM) (n = 95), or conventional care (n = 96). Two preliminary reflexology (n = 51) and LFM (n = 48) test groups were used to establish the protocols. Participants were interviewed again postintervention at study weeks 5 and 11.
Main Research Variables
Breast cancer–specific health-related quality of life (HRQOL), physical functioning, and symptoms.
Findings
No adverse events were reported. A longitudinal comparison revealed significant improvements in physical functioning for the reflexology group compared to the control group (p = 0.04). Severity of dyspnea was reduced in the reflexology group compared to the control group (p < 0.01) and the LFM group (p = 0.02). No differences were found on breast cancer–specific HRQOL, depressive symptomatology, state anxiety, pain, and nausea.
Conclusions
Reflexology may be added to existing evidence-based supportive care to improve HRQOL for patients with advanced-stage breast cancer during chemotherapy and/or hormonal therapy.
Implications for Nursing
Reflexology can be recommended for safety and usefulness in relieving dyspnea and enhancing functional status among women with advanced-stage breast cancer.
Women with advanced-stage breast cancer represent 38% of all women diagnosed with breast cancer each year, and they experience the burden of unmanaged symptoms resulting from the disease and its treatment (Grabsch et al., 2006; National Cancer Institute, 2011). Unmanaged symptoms lead to reduced health-related quality of life (HRQOL) (Cella et al., 2007; McMillan & Small, 2002). Although conventional medicine provides standard symptom care (primarily through pharmaceutical means), more than 80% of women with breast cancer turn to complementary and alternative medicine (CAM) for symptom management (Boon, Olatunde, & Zick, 2007). Among CAM therapies, reflexology is one specific choice reported by women with breast cancer (Lengacher et al., 2006).
Symptom management is critical during chemotherapy treatment, and women often experiment with CAM therapies without adequate basis for their safety and efficacy (Deyo, 2001; Weiger et al., 2002). This research is the first large-scale study to evaluate reflexology for safety and efficacy in relation to HRQOL outcomes for women with advanced-stage breast cancer undergoing chemotherapy and/or hormonal therapy. Safety outcomes included adherence to protocol and self-reported adverse events. Efficacy outcomes were physical functioning and emotional and physical symptom severity.
Literature Review
Although the current state of the science is not based on numerous large-scale trials, findings suggest the potential benefit of reflexology as supportive care for physical and emotional symptoms among patients with cancer. A systematic review by Ernst (2009) included the following three cancer studies, which used reflexology. In Stephenson, Weinrich, and Tavakoli (2000), use of reflexology significantly decreased anxiety in patients with breast and lung cancer. In Hodgson (2000), patients with cancer receiving palliative care demonstrated significant improvement in quality of life (QOL), including ease of breathing, following reflexology. Finally, no change was reported in depression or anxiety in a sample of patients with cancer receiving palliative care in Ross et al. (2002).
A second review by Wilkinson, Lockhart, Gambles, and Storey (2008) included an additional study with hospitalized patients with metastatic cancer and demonstrated an immediate postintervention reduction in pain. Finally, a systematic review by Kim, Lee, Kang, Choi, and Ernst (2010) examined the use of reflexology for patients with breast cancer receiving surgery. A randomized clinical trial (RCT) within that review (Sharp et al., 2012) reported significant improvement in QOL and mood. Three additional nonrandomized trials (Chang, Tsai, Chang, & Tsao, 2007; Park, Yoo, & Lee, 2006; Yang, 2005) noted improvement in pain, fatigue, sleep, and mood.
Apart from the systematic reviews, three RCTs of reflexology (Quattrin et al., 2006; Stephenson, Swanson, Dalton, Keefe, & Engelke, 2007; Tsay, Chen, Chen, Lin, & Lin, 2008) have focused on populations of patients with cancer. All three reported a significant improvement in anxiety, and Stephenson et al. (2007) and Tsay et al. (2008) reported lower pain. Among those studies, sample sizes were low and ranged from 15–44 patients per group. The dose also was not consistent and ranged from one to three sessions of reflexology for the intervention group, with a range of 15–30 minutes per session. Therefore, no consistent reflexology protocol has been tested with adequate sample sizes to date, and the current study aims to fill that gap.
The current study was guided by a modified version of Wilson and Cleary’s (1995) HRQOL model, which was adapted by Ferrans, Zerwic, Wilbur, and Larson (2005) (the model is available from the authors by request). Conceptually, HRQOL has four central components: biologic, symptoms, functioning, and general health perceptions, as well as factors that influence the central components (i.e., characteristics of the individual). Overall HRQOL is defined as subjective well-being related to how happy or satisfied someone is with life (Wilson & Cleary, 1995). The biologic component is described as a continuum of ideal molecular, cellular, and organ function on one end and serious life-threatening pathology on the other. The women in the current study were receiving chemotherapy and/or hormonal therapy for advanced-stage breast cancer, posing a serious threat to their biologic processes. The symptom component is defined as the patient’s perception of abnormal physical or emotional states. Symptoms assessed included dyspnea, nausea, fatigue, pain, anxiety, and depressive symptomatology. Functioning is defined as the ability to perform in multiple domains (i.e., role, physical, and social) (Wilson & Cleary, 1995). Physical functioning was the focus of the current study and was measured with the SF-36® (Ware, Snow, Kosinski, & Gandek, 1993). General health perception is a synthesis of components in the model, plus subjective factors. Characteristics of the individual were operationalized as individual demographics.
The hypothesized mechanism of action within the model is that reflexology affects biologic pathways that have a positive impact on the symptom component of the model, which, in turn, affects functioning and general health perception. Foot reflexology involves applying pressure to specific areas of the feet called reflexes and is based on the premise that stimulation of those reflexes create a nerve pathway connecting to specific organs, glands, and systems of the body (Stephenson et al., 2007). The underlying assumption of reflexology therapy is that by stimulating reflexes, the body is better able to achieve states of balance, adaptation, and strength and restore homeostasis (Byers, 2001). The specific reflexology protocol tested in the current study was designed by an experienced reflexology practitioner. The protocol stimulates reflexes that are associated with concerns and symptoms of breast cancer and its treatment: nervous system, lung and diaphragm, breast or chest, kidney and adrenal, spleen, and intestinal. However, to date, the specific mechanism of action remains unknown, and future research will need to isolate the effects of specific reflexes.
The protocol reflexes were selected according to the symptoms presented by patients with breast cancer in treatment (Grabsch et al., 2006); therefore, the symptom and functioning components of HRQOL were chosen as the primary outcomes (Cella et al., 2007). Those outcomes were assessed during the trial, which tested the value of four consecutive weeks of reflexology or lay foot manipulation (LFM) superficially similar to reflexology versus conventional care alone. The study was a three-group longitudinal RCT with reflexology, LFM, and a standard care control group. In addition, two preliminary test groups (one for reflexology and one for LFM) were used to help establish the protocol. The value of four consecutive weeks of reflexology or LFM compared to conventional care alone was examined for the outcomes of breast cancer–specific QOL, physical functioning, and symptoms.
The following research questions were posed. Is reflexology a safe supportive care therapy to use for women with advanced-stage breast cancer during chemotherapy? Do outcome variable means differ among study groups at weeks 5 and 11? Finally, if improvements in mean breast cancer–specific QOL and physical functioning exist at weeks 5 and 11, are they mediated by a reduction in symptoms?
Methods
Setting
Thirteen medical oncology settings in the midwestern United States enrolled patients with breast cancer who were on chemotherapy and/or hormonal therapy. All sites were outpatient urban or rural settings, and most were associated with a medical center. Providers at each site supported the study and facilitated enrollment. A nurse at each site enrolled patients and entered data electronically onto the study server. Recruitment took place from 2006–2010. Human subjects approval was obtained from Michigan State University and all enrollment sites. Reflexology or LFM sessions were conducted primarily in women’s homes. Other settings included an oncology clinic and integrative therapy centers.
Participants
Inclusion criteria were being aged 21 years or older; having a diagnosis of stage III or IV breast cancer, metastasis, or recurrence; being able to perform basic activities of daily living; being cognitively intact and without a documented diagnosis of mental illness; being able to speak and understand English; having access to a telephone; being able to hear normal conversation; receiving chemotherapy at intake into the study; and having a score of 11 or lower on the Palliative Prognostic Score (Pirovano et al., 1999), which indicates a 30% probability of having a life expectancy of at least three months. Exclusion criteria were receiving hospice care at intake, residing in a nursing home or similar care facility, being bedridden, regularly using CAM similar to those used in the protocol (e.g., reflexology, foot massage, pedicure with massage), and participating in an experimental chemotherapy protocol.
The trial was powered at 80% to detect a medium effect size of 0.4 in pair-wise comparisons of reflexology and LFM groups, as well as reflexology and control groups. At the time of planning the current study, literature on the effects of reflexology was limited. Therefore, a medium effect size of 0.4 was used for planning purposes. That effect size exceeds 0.33, which often is used as a cutoff for clinical significance (Sloan, 2005). The sample size requirement was 100 women per group, so that 300 (after attrition) were available for analysis. As this was the first large-scale study to test reflexology with breast cancer, test protocols needed to be run during the early phase of the study. The available research resources were sufficient to accrue 286 women into the three primary trial arms at baseline.
In total, 595 eligible women were approached by specially trained nurse recruiters, and 451 (76%) consented. The leading reasons for refusal were lack of interest and being too busy. Consistent with the demographic makeup of the participating sites in the midwestern United States, 84% were Caucasian with a mean age of 56 years (see Table 1). Thirty-three women with stage I or II breast cancer listed in the charts had staging at the time of initial diagnosis with a later recurrence or metastasis, but were not restaged in the medical record. Therefore, all study participants had advanced-stage breast cancer, defined as stages III and IV, or stages I and II with recurrence or metastasis.
Table 1.
Demographic and Clinical Characteristics
| Characteristic | Reflexology (N = 95)
|
Lay Foot Manipulation (N = 95)
|
Control (N = 96)
|
p | |||
|---|---|---|---|---|---|---|---|
| X̄ | SD | X̄ | SD | X̄ | SD | ||
| Age (years) | 55.3 | 9.4 | 54.8 | 11.2 | 57.3 | 11.8 | 0.25 |
|
| |||||||
| Characteristic | n | % | n | % | n | % | p |
|
| |||||||
| Race | 0.62 | ||||||
| Caucasian | 80 | 84 | 75 | 79 | 83 | 86 | |
| Other | 14 | 15 | 15 | 16 | 11 | 11 | |
| Missing | 1 | 1 | 5 | 5 | 2 | 2 | |
| Employed | 0.34 | ||||||
| Yes | 38 | 40 | 33 | 35 | 29 | 30 | |
| No | 56 | 59 | 62 | 65 | 67 | 70 | |
| Missing | 1 | 1 | – | – | – | – | |
| Education | 0.06 | ||||||
| High school or less | 17 | 18 | 25 | 26 | 32 | 33 | |
| Some college or more | 77 | 81 | 70 | 74 | 64 | 67 | |
| Missing | 1 | 1 | – | – | – | – | |
| Marital status | 0.86 | ||||||
| Married or partnered | 61 | 64 | 63 | 66 | 60 | 63 | |
| Not married | 33 | 35 | 31 | 33 | 35 | 36 | |
| Missing | 1 | 1 | 1 | 1 | 1 | 1 | |
| Stage of cancer | 0.38 | ||||||
| I | 7 | 7 | 7 | 7 | 4 | 4 | |
| II | 9 | 9 | 13 | 14 | 20 | 21 | |
| III | 35 | 37 | 33 | 35 | 27 | 28 | |
| IV | 44 | 48 | 42 | 44 | 44 | 46 | |
| Missing | – | – | – | – | 1 | 1 | |
| Recurrent disease | 0.66 | ||||||
| Yes | 30 | 32 | 35 | 37 | 30 | 31 | |
| No | 65 | 68 | 60 | 63 | 66 | 69 | |
| Metastasis | 0.69 | ||||||
| Yes | 76 | 80 | 78 | 82 | 74 | 77 | |
| No | 19 | 20 | 17 | 18 | 22 | 23 | |
Note. Because of rounding, not all percentages total 100.
Randomization
Following consent and baseline data collection, women were randomized using the computerized minimization technique (McEntegart, 2003; Scott, McPherson, Ramsay, & Campbell, 2002; Taves, 1974). Minimization is a procedure that is superior to stratified randomization because it allocates patients to groups when they become available and works well when stratification would have resulted in small counts (e.g., when the recruitment site is small) (Senn, 2007). With minimization procedure, the algorithm is run centrally at the main study office, which ensures random allocation and concealment. The algorithm uses the history of all prior randomizations to allocate each patient while balancing the groups with respect to selected factors. Balancing factors in the current study were recruitment sites, levels of pain and fatigue, and goal of therapy. Pain and fatigue variables were dichotomized into low and high levels according to published cutoffs (Cleeland, 1990; Mendoza et al., 1999), and the goal of therapy was at four levels (curative, maintenance, palliative, and uncertain).
Intervention
The study comprised three groups: reflexology, LFM, and standard care control. Test protocols were first run and labeled as test reflexology (n = 51) and test LFM (n = 48). Following testing, the reflexology protocol was established to include 30 minutes of stimulation to the nine essential breast cancer–specific reflexes while using reflexology-specific deep thumb–walking pressure. With that technique, the reflexologist exerts firm downward pressure with his or her thumb, and then inches forward across the specific reflex (Byers, 2001). The LFM protocol was designed to appear superficially similar to reflexology, but did not include deep thumb–walking pressure and avoided direct stimulation to the nine breast cancer–specific reflexes. Women in the reflexology and LFM groups were blinded to their group assignment. The success of blinding was evaluated during the final interview when women were asked to guess their group assignment and then were unblinded. Those in the LFM and control groups were offered a complimentary reflexology session in appreciation for participating in the study.
The intervention comprised four weekly 30-minute sessions of either reflexology or LFM. All three groups received conventional medical care during the study. Reflexology providers were certified reflexologists through the Ingham method of reflexology (Byers, 2001) and trained by the study’s lead reflexologist, who had 22 years of practice and teaching experience. The nine reflexes and expected effects of stimulation were presented in Flynn, Bush, Sikorskii, Mukherjee, and Wyatt (2011). LFM providers were lay women who were naive to reflexology and trained in LFM protocol by the study education coordinator.
Both types of providers were instructed in a standard set of interpersonal skills that facilitated appropriate interaction with women while minimizing unnecessary social support and dialogue. All providers were required to perform with at least a 90% agreement to the established criteria as judged by the lead reflexologist and education coordinator. Fidelity checks were conducted quarterly throughout the study and followed recommendations from the National Institutes of Health as established by the Treatment Fidelity Workgroup (Bellg et al., 2004). Details on intervention fidelity were provided in Wyatt, Sikorskii, Rahbar, Victorson, and Adams (2010).
Data Collection
Nurse recruiters entered information into a database that had a Web interface to accommodate data collection with the participating sites. For consenting women, data included enrollment criteria and demographic and clinical characteristics listed in the chart. For those who did not agree to participate, reasons for refusal were collected.
Interviewers were blinded to group assignment. They entered outcome data electronically into the study database as they were collected from women via telephone interviews in three waves. Wave 1 data were collected between consent and randomization. Wave 2 data collection was done five weeks after the randomization for all groups, which was one week after the intervention. Similarly, wave 3 data collection was performed 11 weeks after the randomization for all groups, which was six weeks after the intervention, to evaluate sustained effects.
After each session (or date of the missed session), the providers filled out a standardized session form that included reasons for a missed session and any comments or concerns expressed by the women. Adverse events included any foot concerns (e.g., infection, sores, worsening of peripheral neuropathy) that could possibly be attributed to reflexology or LFM. Adverse events documented via the session form and other elements of protocol fidelity were monitored and discussed during quarterly meetings of the data and safety monitoring committee, which comprised investigators and representatives from recruitment sites. Those data were tracked for all women by the project manager of the study. Completion of three of the four sessions was categorized as adherent.
Self-report of adverse events was queried weekly by the reflexologists on the standardized session forms and entered into the electronic database. Reflexologists and LFM providers recorded women’s comments and concerns about the respective protocol (e.g., whether foot manipulation was painful, comfortable, or too long).
Safety was measured by adherence data and self-reports of adverse events. Outcomes of breast cancer–specific HRQOL, physical functioning, and symptoms, corresponding with the conceptual model, were measured during three telephone interviews: preintervention, immediately postintervention, and six weeks postintervention.
Instruments
The physical function subscale of the SF-36 (Ware et al., 1993) has 10 items. Total scores range from 0–100, with higher scores reflecting better functioning. The scale has established content and construct validity, as well as internal consistency reliability for the subscales and substantial clinical validity. In the current study, Cronbach alpha for the physical functioning subscale at baseline was 0.91. The physical functioning subscale measures limitations in vigorous activities (e.g., an aerobic exercise program), moderate activities (e.g., vacuuming), lifting groceries, climbing one or several flights of stairs, bending (e.g., kneeling, stooping), walking one or several blocks, walking more than one mile, and bathing or dressing oneself (Ware et al., 1993).
The Functional Assessment of Cancer Therapy–Breast (FACT-B) scale, version 4 (Cella & Bonomi, 1994), covers five areas of HRQOL: physical, emotional, social, functional, and other breast cancer–specific concerns. Items on the five subscales are rated on a five-point scale ranging from 0 (not at all) to 4 (very much). Test-retest reliability ranged from 0.82–0.92 in a sample with various cancer diagnoses. In the current study, subscale and total scores were evaluated. Cronbach alphas for the subscales at baseline ranged from 0.68 for the breast cancer–specific concerns subscale to 0.85 for the physical subscale. In addition, specific symptom items were used, such as nausea from the physical subscale and dyspnea from the other concerns subscale. Higher scores on the items, subscales, and total represent better outcomes (i.e., better function or lower symptom severity).
The Brief Fatigue Inventory (BFI) (Mendoza et al., 1999) consists of nine items. The first three ask respondents to rate the severity of fatigue right now and at its usual and worst levels during the past 24 hours. Answers are selected on a scale from 0 (no fatigue) to 10 (as bad as you can imagine). The remaining six items assess how fatigue interfered with activities of daily living. The single item of severity of fatigue at its worst was used in this analysis, where higher scores represent worse severity. Severity of fatigue at its worst was shown to be highly related to HRQOL outcomes (Mendoza et al., 1999).
The Brief Pain Inventory–Short Form (Cleeland, 1990) includes four items measuring the severity of pain in the past 24 hours on a scale from 0 (no pain) to 10 (pain as bad as you can imagine). Seven additional items measure the extent that pain interferes with daily activities. The single item of severity of pain at its worst was used in this analysis, with higher scores reflecting worse pain.
The Center of Epidemiologic Studies–Depression (CES-D) scale (Radloff & Locke, 1986) measures the state of a person’s depressive symptomatology. The four subscales within the 20-item measure are interpersonal, depressed affect, positive affect, and somatic activity. A total score ranging from 0–60 was used in this study, with higher scores reflecting higher depressive symptomatology. Cronbach alpha at baseline was 0.91.
State anxiety was measured with the State-Trait Anxiety Inventory (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983), which comprises 20 items that evaluate feelings of apprehension, tension, nervousness, and worry. Scores range from 20–80, with higher scores reflecting higher levels of anxiety. In the current study, Cronbach alpha at baseline was 0.95. Concurrent, convergent, divergent, and construct validity have been established (Spielberger et al., 1983).
Data Analysis
Data were stored on the study server at the investigators’ university. Access to the server was password protected. The server had firewalls and protection from unauthorized entry, and data were backed up daily. All study staff had protection of human subjects certification.
Principles of intent-to-treat were followed for the primary analysis. All participants were analyzed as randomized, regardless of adherence to the protocol (e.g., number of foot sessions completed). Those who completed at least one of two postintervention interviews were included. Characteristics of those who dropped out and reasons for attrition were compared by study group using chi-square tests or analysis of variance as appropriate to ensure absence of bias because of missing values.
Analysis of variance and chi-square tests were used to assess the group equivalence at baseline. Variables that differed across groups at baseline despite randomization were used as covariates in all analyses. Using linear mixed effects (LME) models, longitudinal analyses were conducted for FACT-B scores, the physical functioning subscale of the SF-36, severity of fatigue, pain, dyspnea, nausea, CES-D, and state anxiety while adjusting for baseline values. The LME model is a generalization of the classical analysis of repeated measures, which allows for data missing at random, time-varying covariates, and a structured covariance matrix (McCullagh & Nelder, 1989; McCulloch, Searle, & Neuhaus, 2008). With LME modeling, patients who completed the interview at week 5, week 11, or both were included in the analysis. The mixed procedure in SAS®, version 9.2, was used to implement LME models. Additive group effects that corresponded to average differences between each group and control (referent group) were of main interest. In addition, the group-by-time interaction was explored to determine whether differences among groups remained the same as time progressed. Least squares or adjusted means were calculated from the LME models and plotted.
To determine whether improvements in functioning were mediated by a reduction in severity of specific symptoms, the approach proposed by Baron and Kenny (1986) and further developed by others (MacKinnon, 1994; MacKinnon, Krull, & Lockwood, 2000) was used. Symptom severity was added as a time-varying covariate to the LME models for FACT-B scores and physical functioning, and the significance of the coefficient for the group variable provided a test for mediation. All statistical tests were two-sided. No interim efficacy analyses were planned or performed. All outcomes were prespecified; therefore, a 5% significance level was used in hypothesis testing.
Results
A significantly different distribution for recruitment location among groups existed because some sites were added during the study, whereas other sites remained open throughout the study. Among the 13 recruitment sites, enrollment numbers ranged from 2 (1%) to 64 (25%). No difference existed between women who received the intervention in their home versus the clinic. In addition, despite randomization, differences were found at baseline among the three primary groups on CES-D (p = 0.02) and anxiety (p < 0.01). To ensure that postintervention differences were not the result of baseline differences on recruitment location, baseline CES-D and anxiety scores were used as covariates in all analyses.
No difference in attrition existed by study group (data not shown). Therefore, the missing-at-random mechanism was implemented in the LME modeling, and 83 women in the reflexology group, 83 women in the LFM group, and 77 women in the control group were analyzed.
Reflexology as Supportive Care
Three women in the reflexology group and five women in the LFM group dropped out after the baseline interview. Those women were never assigned a provider nor had any sessions scheduled. Of the remaining women, 82 of 92 (89%) in the reflexology group and 81 of 90 (90%) in the LFM group completed all four sessions. More than 92% in both of the active groups received at least three of the four sessions, which was considered a full dose. No adverse events were reported on the standardized session forms. The main reason for missed foot sessions was unavailability of the woman on a scheduled date (e.g., because of hospitalization).
Differences in Outcome Variable Means Among Groups
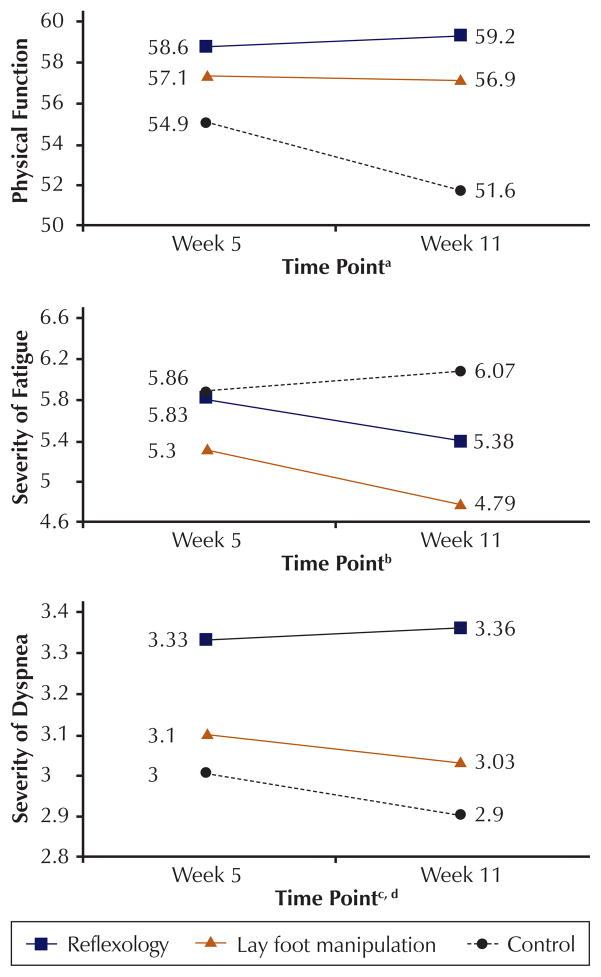
Unadjusted means of outcomes at weeks 5 and 11 are presented in Table 2. However, the intervention effect cannot be appropriately estimated from unadjusted means because differences existed at baseline among the three primary groups, and the means do not reflect missing data. Results of the longitudinal model that adjusted for baseline differences and accounted for repeated measures are presented in Table 3. No differences were found on breast cancer–specific QOL, depressive symptomatology, anxiety, pain, and nausea. The significant differences among study groups were limited to physical function, dyspnea, and fatigue. Participants in the reflexology group reported statistically significant reductions in mean dyspnea severity compared to the control group (p < 0.01) and the LFM group (p = 0.02). In addition, a mean improvement was found for physical functioning for the reflexology group compared to the control group (p = 0.04). Compared to control, participants in the LFM group reported significantly lower scores on fatigue severity (p < 0.01). To illustrate those findings and the beta coefficients in Table 3, the adjusted means of physical functioning, dyspnea, and fatigue at weeks 5 and 11 are displayed in Figure 1. The standard derivations as adjusted for the inclusion of covariates and longitudinal design were 17.39 for physical function, 0.91 for dyspnea, and 2.58 for fatigue. Therefore, the adjusted effect sizes for reflexology versus control were estimated to be 0.21 at week 5 and 0.44 at week 11 for physical function, and 0.36 at week 5 and 0.51 at week 11 for dyspnea. For severity of fatigue, the adjusted effect sizes for LFM versus control were estimated to be 0.22 at week 5 and 0.5 at week 11.
Table 2.
Descriptive Statistics for Outcomes at Baseline, Week 5, and Week 11
| Characteristic | Range | Reflexology (N = 95)
|
Lay Foot Manipulation (N = 95)
|
Control (N = 96)
|
|||
|---|---|---|---|---|---|---|---|
| X̄ | SD | X̄ | SD | X̄ | SD | ||
| Physical functiona | 0–100 | ||||||
| Baseline | 55.8 | 27 | 58 | 26.4 | 55.4 | 28.3 | |
| Week 5 | 58.1 | 27 | 61.8 | 26.8 | 53.8 | 27.1 | |
| Week 11 | 58.8 | 26.4 | 62.7 | 28.5 | 51.9 | 26.5 | |
| Fatigueb | 0–10 | ||||||
| Baseline | 5.6 | 2.9 | 5.9 | 2.8 | 5.6 | 2.9 | |
| Week 5 | 5.9 | 2.8 | 5.4 | 3 | 6 | 2.8 | |
| Week 11 | 5.4 | 2.7 | 4.7 | 2.9 | 5.9 | 3.1 | |
| Dyspneaa | 0–4 | ||||||
| Baseline | 3.1 | 1.2 | 2.9 | 1.4 | 3.1 | 1.2 | |
| Week 5 | 3.3 | 1 | 3 | 1.2 | 3 | 1 | |
| Week 11 | 3.3 | 0.9 | 3 | 1.3 | 2.9 | 1.1 | |
| CES-D scaleb | 0–60 | ||||||
| Baseline | 17.8 | 11.3 | 15.3 | 11.3 | 13.4 | 9.9 | |
| Week 5 | 16.6 | 10.5 | 13.5 | 10.8 | 12.4 | 9.2 | |
| Week 11 | 13.1 | 10.3 | 12.7 | 10.2 | 13.4 | 10.4 | |
| Anxietyb | 0–60 | ||||||
| Baseline | 39.6 | 13.1 | 34.9 | 11.1 | 34.3 | 12.6 | |
| Week 5 | 37.7 | 13.3 | 32.5 | 10.2 | 34.1 | 10.6 | |
| Week 11 | 35.4 | 11.1 | 33.3 | 10.7 | 34.1 | 12.1 | |
| FACT-B totala | 0–180 | ||||||
| Baseline | 95.3 | 19.1 | 93.1 | 20.6 | 96.7 | 19.4 | |
| Week 5 | 96 | 20.4 | 98 | 19.3 | 99.4 | 19 | |
| Week 11 | 101.1 | 18.3 | 99.7 | 21.5 | 100.4 | 18.7 | |
| Painb | 0–10 | ||||||
| Baseline | 3.76 | 2.98 | 3.87 | 3.1 | 3.95 | 3.2 | |
| Week 5 | 4 | 3.1 | 3.4 | 3.1 | 4.3 | 3.1 | |
| Week 11 | 3.2 | 3.1 | 3.2 | 3 | 3.9 | 3.1 | |
| Nauseaa | 0–4 | ||||||
| Baseline | 3.29 | 1.04 | 3.03 | 1.27 | 3.14 | 1.14 | |
| Week 5 | 3.5 | 0.9 | 3.4 | 1 | 3.4 | 1 | |
| Week 11 | 3.5 | 1 | 3.6 | 1 | 3.3 | 1 | |
Higher scores indicate better outcomes.
Lower scores indicate better outcomes.
CES-D—Center of Epidemiologic Studies–Depression; FACT-B—Functional Assessment of Cancer Therapy–Breast scale, version 4
Note. Sample sizes were: baseline (reflexology: N = 95; lay foot manipulation: N = 95; control: N = 96), week 5 (reflexology: N = 75; lay foot manipulation: N = 76; control: N = 71), and week 11 (reflexology: N = 71; lay foot manipulation: N = 67; control: N = 63).
Table 3.
Longitudinal Model for Outcomes
| Outcome and Group | Estimate (Beta) | SEB | p |
|---|---|---|---|
| FACT-B total | |||
| Reflexology | 0.387 | 2.194 | 0.86 |
| LFM | 0.43 | 2.158 | 0.84 |
| Control | 0 | – | Referent |
| Physical function | |||
| Reflexology | 5.527 | 2.728 | 0.04* |
| LFM | 3.666 | 2.722 | 0.18 |
| Control | 0 | – | Referent |
| Fatigue severity | |||
| Reflexology | −0.335 | 0.381 | 0.38 |
| LFM | −0.889 | 0.378 | 0.02* |
| Control | 0 | – | Referent |
| Fatigue interference with ADL | |||
| Reflexology | −2.832 | 2.01 | 0.16 |
| LFM | −3.695 | 1.99 | 0.06 |
| Control | 0 | – | Referent |
| Dyspnea | |||
| Reflexology | 0.39 | 0.13 | < 0.01** |
| LFM | 0.113 | 0.129 | 0.38 |
| Control | 0 | – | Referent |
| CES-D scale | |||
| Reflexology | −0.487 | 1.21 | 0.69 |
| LFM | −0.231 | 1.205 | 0.85 |
| Control | 0 | – | Referent |
| State anxiety | |||
| Reflexology | −0.886 | 1.259 | 0.48 |
| LFM | −1.622 | 1.255 | 0.2 |
| Control | 0 | – | Referent |
| Nausea | |||
| Reflexology | 0.212 | 0.124 | 0.089 |
| LFM | 0.164 | 0.123 | 0.182 |
| Control | 0 | – | Referent |
| Pain severity | |||
| Reflexology | −0.287 | 0.389 | 0.46 |
| LFM | −0.559 | 0.385 | 0.148 |
| Control | – | – | Referent |
p < 0.05;
p < 0.01
ADL—activities of daily living; CES-D—Center of Epidemiologic Studies–Depression; FACT-B—Functional Assessment of Cancer Therapy–Breast scale, version 4; LFM—lay foot manipulation; SEB—standard error of beta
Figure 1. Adjusted Means of Physical Function, Severity of Fatigue, and Severity of Dyspnea.
aAverage difference over time (week 5 and week 11) between reflexology and control was significant (p = 0.04).
b Average difference over time (week 5 and week 11) between lay foot manipulation and control was significant (p < 0.01).
c Average difference over time (week 5 and week 11) between reflexology and control was significant (p < 0.01).
d Average difference over time (week 5 and week 11) between reflexology and lay foot manipulation was significant (p = 0.02).
Note. Higher scores reflect better physical function, higher fatigue severity, and lower dyspnea severity.
Improvements Mediated by Symptom Reduction
No group differences in breast cancer–specific QOL were found; therefore, only physical functioning was examined. The effect of reflexology on physical functioning was no longer significant when dyspnea severity was added as a time-varying covariate to the model (data not shown). Therefore, the improvement in physical functioning caused by reflexology was completely mediated by the reduction in dyspnea severity.
Discussion
In a systematic review of reflexology among patients with breast cancer, Kim et al. (2010) concluded that none of the studies reviewed had assessed or reported on safety and that those data must be noted in future studies. The current study was the first to have a data and safety monitoring committee, which quarterly reviewed the data for adverse events and incorporated a mechanism for reporting adverse events through a standardized session form. Among a vulnerable sample of women with advanced-stage breast cancer, no adverse events were reported. In addition, rates of reflexology and LFM session completion were high, which adds to the study’s feasibility and credibility; therefore, either can be used with confidence in future studies and practice, with consideration of routine precautions such as open sores and painful foot neuropathy.
Most of the efficacy findings of the current study were supported by prior literature, but depressive symptoms and anxiety were exceptions. Unlike other reflexology studies that reported improvements in emotional functioning and symptoms (Ernst, 2009; Quattrin et al., 2006; Stephenson et al., 2007; Tsay et al., 2008), the current study’s findings did not support those prior results.
Dyspnea
The findings identified dyspnea as a symptom affected by reflexology, which, in turn, improved physical functioning. Those results are supported by Gupta, Grutsch, and Lis (2008), who reported a strong association between dyspnea and QOL among a sample of 954 patients with cancer, of whom the most common site was breast. Additional support for that positive impact on breathing was found among a small convenience sample of patients with cancer receiving palliative care (Hodgson, 2000). Another possibility is that when women are less short of breath, they are more willing and able to be active. In future work, metastatic locations should be assessed to determine why dyspnea was the symptom most associated with physical functioning and how to better design targeted interventions. In a systematic review of evidence-based approaches to symptom management in advanced-stage cancer, only opioids and nonpharmacologic treatments (e.g., use of a fan) were included for dyspnea (Dy & Apostol, 2010). In a breast cancer–specific review of reflexology, Kim et al. (2010) concluded that existing evidence does not show reflexology to be effective in breast cancer care. Reflexology may now be suggested as a supportive care intervention for women with advanced-stage breast cancer, particularly when dyspnea is a symptom of concern.
Fatigue
This study is the first to demonstrate significant improvement in fatigue using LFM; however, in contrast to the reflexology group, no improvement in physical functioning occurred in the LFM group versus control group. As argued by Cleeland (2007), improvements in symptoms are noteworthy even in the absence of improved physical functioning. LFM providers may have been close enough to some reflexes to have an adequate impact, and that proximity to the exact location may have been adequate. That premise suggests that LFM modality may be a valuable addition to supportive care for patients with cancer.
Those findings support the therapeutic effects of reflexology, as well as the usefulness of LFM. Stephenson et al. (2007) reported the benefits of providing reflexology via lay friend or family members of 86 hospitalized patients with metastatic cancer and found an immediate decrease in pain intensity and anxiety as a result of LFM. Wyatt, Sikorskii, Siddiqi, and Given (2007) also successfully involved lay family members in a feasibility study of reflexology among a sample of 100 patients with solid tumors undergoing chemotherapy. The total time needed to train a family member in the protocol was about three hours, including two hours for initial training plus a one-hour booster session. Future research could employ a reflexologist to teach a lay family member to deliver the foot sessions at home and measure outcomes over time. That would make reflexology more available to patients and provide a way for family members to participate in supportive care with this therapeutic skill. No adverse events related to reflexology or LFM were found; therefore, patients could have access to a session from lay friends or family members whenever they experience symptoms or lowered functioning.
Pain and Nausea
In contrast to other studies, significant effects were not found for pain. Reasons for those differences may be that Tsay (2008) investigated pain in a different population (i.e., postoperative patients with gastric and liver cancer) and Stephenson et al. (2007) used lay providers rather than certified reflexologists. The current study also was the first to evaluate the effects on nausea. Although trends toward improvement were noted, the designated significance level was not reached.
Limitations
Although the current study is the largest single therapy trial of reflexology to date among a homogeneous sample, the results can be generalized only to women with advanced-stage breast cancer who match the inclusion criteria. Future studies should seek a more diverse sample. Because of limited research resources, the final three trial groups were slightly below the numbers needed for the projected power. A third potential limitation related to the safety data, which were collected by the reflexologist or LFM providers at each session. Participants may have tended to not report adverse events to the provider, but the fact that none were reported during the entire study may neutralize that concern. A measure of expectancy could have enhanced the findings by correlating the women’s expectancy with outcomes. Reflexology may or may not be readily available in all rural locations; however, many cancer centers where patients seek care have information on local CAM providers. Finally, a higher dose of reflexology may have affected more outcomes.
Conclusions
The current study found reflexology and LFM were safe among even the most fragile patients with advanced-stage breast cancer and contribute to improvements in physical function, dyspnea, and fatigue, but do not affect depressive symptoms, anxiety, pain, and nausea. Future research could explore cost factors, including the average cost of a reflexology session ($45 per half hour in many midwestern locations). In addition, research efforts should consider potential physiologic mechanisms of action through biomarkers and the potential for involving lay partners in this therapy for patients with breast cancer as supportive care during chemotherapy. The long-term objective of this research is to help clarify which CAM therapies have a sound scientific basis for safety and efficacy. The current study informs clinicians and patients on which CAM therapies are transferable to community-based cancer centers and home care programs to improve HRQOL for patients receiving treatment.
Implications for Nursing
Reflexology can be safely used by patients with cancer who are undergoing chemotherapy. In addition, aspects of quality of life can be improved with reflexology delivered by either certified reflexologists or lay providers.
Acknowledgments
The authors gratefully acknowledge Barbara Brower, CRR, for guiding the protocol training for the study interventionists.
This work was supported by a grant (#R01 CA104883) from the National Institutes of Health (NIH), National Cancer Institute. The Branch Reflexology Institute and Biostatistics, Epidemiology, and Research Design (BERD) Core of the Center for Clinical and Translational Sciences (CCTS) is primarily funded by an NIH Clinical and Translational Science Award grant (UL1 RR024148), awarded to the University of Texas Health Science Center at Houston in 2006. Wyatt can be reached at gwyatt@msu.edu, with copy to editor at ONFEditor@ons.org
Contributor Information
Gwen Wyatt, College of Nursing, Michigan State University in East Lansing.
Alla Sikorskii, Department of Statistics and Probability, both at Michigan State University in East Lansing.
Mohammad Hossein Rahbar, Division of Epidemiology and Biostatistics in the School of Public Health and the Biostatistics, Epidemiology, and Research Design Core Center for Clinical and Translational Sciences at the University of Texas Health Science Center at Houston.
David Victorson, Department of Medical Social Sciences in the Feinberg School of Medicine at Northwestern University in Chicago, IL.
Mei You, College of Nursing at Michigan State University.
References
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory MG, Czajkowski S. Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the NIH Behavior Change Consortium. Health Psychology. 2004;23:443–451. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- Boon HS, Olatunde F, Zick SM. Trends in complementary/alternative medicine use by breast cancer survivors: Comparing survey data from 1998 and 2005. BMC Women’s Health. 2007;7:4. doi: 10.1186/1472-6874-7-4. Retrieved from http://www.biomedcentral.com/content/pdf/1472-6874-7-4.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers DC. Better health with foot reflexology: The Ingham method of reflexology. St. Petersburg, FL: Ingham; 2001. (Rev. ed.) [Google Scholar]
- Cella DF, Bonomi AE. Functional Assessment of Cancer Therapy (FACT) scales and the Functional Assessment of HIV Infection (FAHI) scale [version 3] Chicago, IL: Rush Cancer Institute; 1994. [Google Scholar]
- Cella DF, Wagner L, Cashy J, Hensing TA, Yount S, Lilenbaum RC. Should health-related quality of life be measured in cancer symptom management clinical trials? Lessons learned using the Functional Assessment of Cancer Therapy. Journal of the National Cancer Institute Monographs. 2007;37:53–60. doi: 10.1093/jncimonographs/lgm009. [DOI] [PubMed] [Google Scholar]
- Chang EW, Tsai YY, Chang TW, Tsao CJ. Quality of sleep and quality of life in caregivers of breast cancer patients. Psycho-Oncology. 2007;16:950–955. doi: 10.1002/pon.1167. [DOI] [PubMed] [Google Scholar]
- Cleeland CS. Assessment of pain in cancer: Measurement issues. Advances in Pain Research and Therapy. 1990;16:47–55. [Google Scholar]
- Cleeland CS. Symptom burden: Multiple symptoms and their impact as patient-reported outcomes. Journal of the National Cancer Institute Monographs. 2007;37:16–21. doi: 10.1093/jncimono graphs/lgm005. [DOI] [PubMed] [Google Scholar]
- Deyo RA. A key medical decision maker: The patient. BMJ. 2001;323:466–467. doi: 10.1136/bmj.323.7311.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dy SM, Apostol CC. Evidence-based approaches to other symptoms in advanced cancer. Cancer Journal. 2010;16:507–513. doi: 10.1097/PPO.0b013e3181f45877. [DOI] [PubMed] [Google Scholar]
- Ernst E. Is reflexology an effective intervention? A systematic review of randomised controlled trials. Medical Journal of Australia. 2009;191:263–266. doi: 10.5694/j.1326-5377.2009.tb02780.x. [DOI] [PubMed] [Google Scholar]
- Ferrans CE, Zerwic JJ, Wilbur JE, Larson JL. Conceptual model of health-related quality of life. Journal of Nursing Scholarship. 2005;37:336–342. doi: 10.1111/j.1547-5069.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- Flynn LL, Bush TR, Sikorskii A, Mukherjee R, Wyatt G. Understanding the role of stimulation in reflexology. European Journal of Cancer Care. 2011;20:686–696. doi: 10.1111/j.1365-2354.2011.01268.x. [DOI] [PubMed] [Google Scholar]
- Grabsch B, Clarke D, Love A, McKenzie DP, Snyder RD, Bloch S, Kissane DW. Psychological morbidity and quality of life in women with advanced breast cancer: A cross-sectional survey. Palliative and Supportive Care. 2006;4:47–56. doi: 10.1017/ S1478951506060068. [DOI] [PubMed] [Google Scholar]
- Gupta D, Grutsch JF, Lis CG. Comparison of two quality of life instruments for cancer patients: The Ferrans and Powers Quality of Life Index and the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire C30. Journal of the Society for Integrative Oncology. 2008;6:13–18. [PubMed] [Google Scholar]
- Hodgson H. Does reflexology impact on cancer patients’ quality of life? Nursing Standard. 2000;14(31):33–38. doi: 10.7748/ns2000.04.14.31.33.c2817. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee MS, Kang JW, Choi DY, Ernst E. Reflexology for the symptomatic treatment of breast cancer: A systematic review. Integrative Cancer Therapies. 2010;9:326–330. doi: 10.1177/1534735410387423. [DOI] [PubMed] [Google Scholar]
- Lengacher CA, Bennett MP, Kip KE, Gonzalez JR, Jacobsen P, Cox CE. Relief of symptoms, side effects, and psychological distress through use of complementary and alternative medicine in women with breast cancer. Oncology Nursing Forum. 2006;33:97–104. doi: 10.1188/06.ONF.97-104. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP. Analysis of mediating variables in prevention and intervention research. In: Cazares A, Beatty LA, editors. Scientific methods for prevention intervention research. Washington, DC: U.S. Department of Health and Human Services; 1994. pp. 127–153. [PubMed] [Google Scholar]
- MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding, and suppression effect. Prevention Science. 2000;1:173–181. doi: 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh P, Nelder J. Generalized linear models. 2. London, England: Chapman and Hall; 1989. [Google Scholar]
- McCulloch CE, Searle SR, Neuhaus JM. Generalized, linear, and mixed models. 2. Hoboken, NJ: John Wiley and Sons; 2008. [Google Scholar]
- McEntegart DJ. The pursuit of balance using stratified and dynamic randomization techniques: An overview. Drug Information Journal. 2003;37:293–308. [Google Scholar]
- McMillan SC, Small BJ. Symptom distress and quality of life in patients with cancer newly admitted to hospice home care. Oncology Nursing Forum. 2002;29:1421–1428. doi: 10.1188/02.ONF.1421-1428. [DOI] [PubMed] [Google Scholar]
- Mendoza T, Wang S, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Surveillance Epidemiology and End Results (SEER) stat fact sheets: Breast. 2011 Retrieved from http://seer.cancer.gov/statfacts/html/breast.html.
- Park JW, Yoo HR, Lee HS. Effects of foot reflex zone massage on patients’ pain and sleep satisfaction following mastectomy. Journal of Korean Academic Society of Home Care Nursing. 2006;13:54–60. [Google Scholar]
- Pirovano M, Maltonia M, Nanni O, Mariani M, Indelli M, Zaninetta G, Piva L. A new palliative prognostic score: A first step for the staging of terminally ill cancer patients. Journal of Pain and Symptom Management. 1999;17:231–239. doi: 10.1016/s0885-3924(98)00145-6. [DOI] [PubMed] [Google Scholar]
- Quattrin R, Zanini A, Buchini S, Turello D, Annunziata MA, Vidotti C, Brusaferro S. Use of reflexology foot massage to reduce anxiety in hospitalized cancer patients in chemotherapy treatment. Journal of Nursing Management. 2006;14:96–105. doi: 10.1111/ j.1365-2934.2006.00557.x. [DOI] [PubMed] [Google Scholar]
- Radloff LS, Locke BZ. The community mental health assessment survey and the CED-D scale. In: Weissman MM, Meyers JK, Ross CE, editors. Community surveys of psychiatric disorders. New Brunswick, NJ: Rutgers University Press; 1986. [Google Scholar]
- Ross CSK, Hamilton J, Macrae G, Docherty C, Gould A, Cornbleet MA. A pilot study to evaluate the effect of reflexology on mood and symptom rating of advanced cancer patients. Palliative Medicine. 2002;16:544–545. doi: 10.1191/0269216302pm597xx. [DOI] [PubMed] [Google Scholar]
- Scott NW, McPherson GC, Ramsay CR, Campbell MK. The method of minimization for allocation to clinical trials: A review. Controlled Clinical Trials. 2002;23:662–674. doi: 10.1016/s0197-2456(02)00242-8. [DOI] [PubMed] [Google Scholar]
- Senn S. Statistical issues in drug development. 2. Chichester, England: John Wiley and Sons; 2007. [Google Scholar]
- Sharp DM, Walker MB, Chaturvedi A, Upadhyay S, Hamid A, Walker AA, Walker LG. A randomised, controlled trial of the psychological effects of reflexology in early breast cancer. European Journal of Cancer. 2012;46:312–322. doi: 10.1016/j.ejca.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Sloan JA. Clinical significance of patient-reported questionnaire data: Another step toward consensus. Journal of Clinical Epidemiology. 2005;58:1217–1219. doi: 10.1016/j.jclinepi.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Mind Garden; 1983. [Google Scholar]
- Stephenson N, Swanson M, Dalton J, Keefe FJ, Engelke M. Partner-delivered reflexology. Oncology Nursing Forum. 2007;34:127–132. doi: 10.1188/07.ONF.127-132. [DOI] [PubMed] [Google Scholar]
- Stephenson N, Weinrich S, Tavakoli A. The effects of foot reflexology on anxiety and pain in patients with breast and lung cancer. Oncology Nursing Forum. 2000;27:67–72. [PubMed] [Google Scholar]
- Taves DR. Minimization: A new method of assigning patients to treatment and control groups. Clinical Pharmacology and Therapeutics. 1974;15:443–453. doi: 10.1002/cpt1974155443. [DOI] [PubMed] [Google Scholar]
- Tsay S, Chen H, Chen S, Lin H, Lin K. Effects of reflexotherapy on acute postoperative pain and anxiety among patients with digestive cancer. Cancer Nursing. 2008;31:109–115. doi: 10.1097/01.NCC.0000305694.74754.7b. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Snow KK, Kosinski M, Gandek B. SF-36 health survey: Manual and interpretation guide. Boston, MA: Health Institute, New England Medical Center; 1993. [Google Scholar]
- Weiger WA, Smith M, Boon H, Richardson MA, Kaptchuk TJ, Eisenberg DM. Advising patients who seek complementary and alternative medical therapies for cancer. Annals of Internal Medicine. 2002;137:889–903. doi: 10.7326/0003-4819-137-11-200212030-00010. [DOI] [PubMed] [Google Scholar]
- Wilkinson S, Lockhart K, Gambles M, Storey L. Reflexology for symptom relief in patients with cancer. Cancer Nursing. 2008;31:354–360. doi: 10.1097/01.NCC.0000305756.58615.81. [DOI] [PubMed] [Google Scholar]
- Wilson IB, Cleary PD. Linking clinical variables with health related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273:59–65. doi: 10.1001/jama.273.1.59. [DOI] [PubMed] [Google Scholar]
- Wyatt G, Sikorskii A, Rahbar MH, Victorson D, Adams L. Intervention fidelity: Aspects of complementary and alternative medicine research. Cancer Nursing. 2010;33:331–342. doi: 10.1097/NCC.0b013e3181d0b4b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt G, Sikorskii A, Siddiqi A, Given CW. Feasibility of a reflexology and guided imagery intervention during chemotherapy: Results of a quasi-experimental study. Oncology Nursing Forum. 2007;34:635–642. doi: 10.1188/07.ONF.635-642. [DOI] [PubMed] [Google Scholar]
- Yang JH. The effects of foot reflexology on nausea, vomiting and fatigue of breast cancer patients undergoing chemotherapy. Taehan Kanho Hakhoe Chi. 2005;35:177–185. doi: 10.4040/jkan.2005.35.1.177. [DOI] [PubMed] [Google Scholar]