Summary
Spontaneous waves of activity propagating across large cortical areas may play important roles in sensory processing and circuit refinement. However, whether these waves are in turn shaped by sensory experience remains unclear. Here we report that visually evoked cortical activity reverberates in subsequent spontaneous waves. Voltage-sensitive dye imaging in rat visual cortex showed that following repetitive presentation of a given visual stimulus, spatiotemporal activity patterns resembling the evoked response appear more frequently in the spontaneous waves. This effect is specific to the response pattern evoked by the repeated stimulus, and it persists for several minutes without further visual stimulation. Such wave-mediated reverberation could contribute to short-term memory and help to consolidate the transient effects of recent sensory experience into long-lasting cortical modifications.
Introduction
An essential prerequisite for perception and cognition is the ability of neural circuits to sustain the representation of a sensory event for some period of time after the event occurs. Such sustained neural representation may underlie short-term perceptual memory such as iconic memory (Averbach and Sperling, 1961) and priming (Tulving and Schacter, 1990). Hebb postulated that reverberating activity in cell assemblies could serve as a mechanism for short-term memory and help induce stable circuit modifications underlying long-term memory (Hebb, 1949). Although this conjecture has been highly influential, there has been little direct evidence for reverberating activity in early sensory circuits.
In the neocortex, spontaneous activity in the absence of sensory input often exhibits non-random spatiotemporal patterns (Abeles and Gerstein, 1988; Fiser et al., 2004; Kenet et al., 2003; Tsodyks et al., 1999), some of which propagate as waves across large cortical areas (Amzica and Steriade, 1995; Ermentrout and Kleinfeld, 2001; Ferezou et al., 2006; Luczak et al., 2007; Petersen et al., 2003; Prechtl et al., 1997; Rubino et al., 2006; Sanchez-Vives and McCormick, 2000). Such spontaneous network activity can exert strong influences on sensory evoked responses (Arieli et al., 1996; Fiser et al., 2004; Tsodyks et al., 1999) and play important roles in activity-dependent circuit modifications (Katz and Shatz, 1996; Weliky, 2000). Recent studies also suggested that many spontaneous activity patterns correspond closely to sensory evoked responses (Kenet et al., 2003), raising the possibility that the spatiotemporal properties of spontaneous activity can be shaped by sensory experience. However, little is known about how sensory stimulation affects spontaneous activity, and what the time courses of these effects are.
In this study, we used voltage-sensitive dye (VSD) imaging (Grinvald and Hildesheim, 2004) to examine the spatiotemporal patterns of spontaneous and evoked activity in the visual cortex of anesthetized rats. We found that both spontaneous and visually-evoked activity manifest as propagating waves (Benucci et al., 2007; Xu et al., 2007). Interestingly, following repetitive presentation of a given visual stimulus, the evoked activity pattern reverberated in the subsequent spontaneous waves for several minutes. Such a rapid, stimulus-induced modification of spontaneous activity in an early sensory circuit could contribute to short-term memory and facilitate long-term perceptual learning.
Results
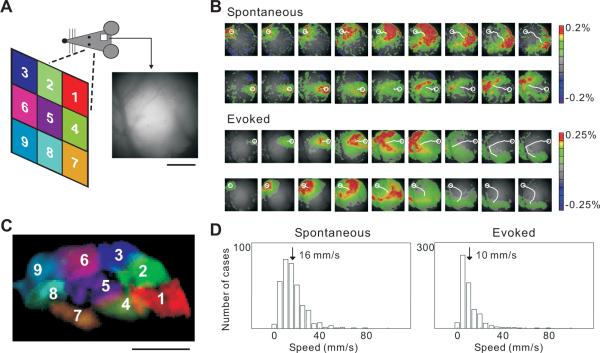
We used VSD imaging to measure the spontaneous and evoked patterns of activity in rat visual cortex (Figure 1A) (see Experimental Procedures). In the absence of visual stimulation, spontaneous activity was initiated at random locations at a frequency of 0.5 – 4 Hz and propagated as waves across several millimeters of the cortical surface (Figure 1B, upper panel, Movie S1). These waves propagated in various directions (Figure 1B, white lines) with a mean speed of 16 ± 4.3 mm/s (SD, Figure 1D), comparable to the spontaneous waves observed in several sensory cortical areas in vivo (Ferezou et al., 2006; Luczak et al., 2007; Petersen et al., 2003; Xu et al., 2007). We also measured the cortical responses to a set of visual stimuli, each of which was a bright square (15–20° visual angle) briefly flashed (50ms) at one of nine positions in the contralateral visual field (Figure 1A). Each stimulus also evoked a wave of activity (Figure 1B, lower panel, Movie S2) (Benucci et al., 2007; Ferezou et al., 2006; Prechtl et al., 1997; Roland et al., 2006; Xu et al., 2007), which was initiated 50–100 ms following the stimulus onset and propagated at a mean speed of 10 ± 4.7 mm/s (SD, Figure 1D). However, unlike the spontaneous waves with random initiation sites and variable propagation directions (Figure 1B and 2A), the evoked waves were much more reproducible. For each of the nine stimulus positions, the wave was initiated at a fixed cortical location organized retinotopically (Figure 1C), and the propagation path (Figure 1B, white lines) was highly reproducible across trials (Figure S1).
Figure 1.
Spontaneous and evoked waves in rat visual cortex
(A) Schematic illustration of visual stimulation and cranial window for VSD imaging. Positions for visual stimulation are marked with numbers and colors (left). For the image of the visual cortex (right), the left-right axis corresponds to anterior-posterior axis of the cortex; the upper-lower axis corresponds to lateral-medial. This imaging area contains mostly V1 and a small portion of V2 (lower left region). Scale bar, 1 mm.
(B) Examples of spontaneous and visually evoked waves, shown in 20 ms intervals. VSD signal was color coded (warm color indicates depolarization), with scale bar shown on the right. Top, two examples of spontaneous waves. Bottom, waves evoked by stimuli at positions 1 & 9 (average of 2 trials; 7 frames following stimulus onset but before response onset were omitted). White circle, initiation site measured as center of mass of VSD signal in the first frame. White line, wave trajectory by connecting the centers of mass of consecutive frames. Across experiments the mean amplitude was not significantly different between spontaneous and evoked waves (p > 0.9, Mann-Whitney U test; evoked: ΔF/F = 0.231 ± 0.046%, SD; spontaneous: ΔF/F = 0.234 ± 0.051%).
(C) Initiation sites of waves evoked by the nine stimuli (retinotopy), with colors and numbers corresponding to (A). Scale bar, 1mm.
(D) Distributions of the instantaneous wave speed, measured as the distance in the center of mass of ΔF/F between consecutive frames divided by the inter-frame interval (10 ms). For each wave, up to 18 instantaneous speeds were measured. Shown are data from 9 rats; in each rat, the stimuli were flashed at multiple positions to measure the evoked waves.
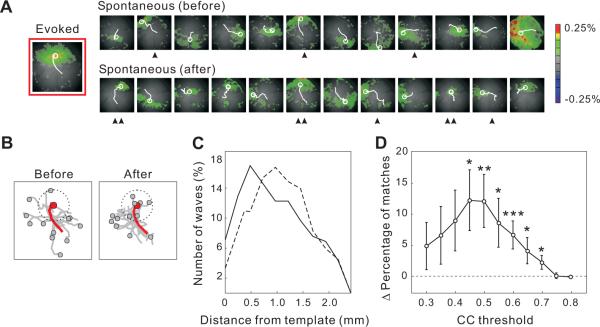
Figure 2.
Effect of repeated visual stimulation at a given position on spontaneous waves
(A) Spontaneous waves immediately before and after training (10.24 s/session). Each image shows the initial frame of a wave. Initiation site and propagation path are indicated by a white circle and a line. Left, Evoked wave in response to the training stimulus. Spontaneous waves well matched to the template are indicated; ▲, CC>0.6; ▲▲, CC>0.7.
(B) Superposition of the initiation sites and propagation paths for all spontaneous waves before and after training (gray) and for the training-evoked wave (red). Dotted circle indicates a 0.6 mm radius from the initiation site of the training-evoked wave.
(C) Distribution of the distance between the initiation sites of the spontaneous waves and the evoked template before (dashed) and after (solid) training (30 training experiments, 9 rats).
(D) Difference in the percentage of matched waves before and after training plotted as a function of the CC threshold (*, p < 0.05; **, p < 0.02; ***, p < 0.01, same data as in C). Error bar, ± SEM.
Consistent with a previous finding in the visual cortex (Kenet et al., 2003), some of the spontaneous waves resembled visually evoked responses (Figure 2A, indicated by ▲ and ▲▲). This similarity could reflect the intrinsic cortical connectivity or the long-term impact of previous visual experience. We wondered whether repeated visual stimulation could leave an immediate memory trace in the subsequent spontaneous activity. We presented 50 flashes (0.6 Hz) at one of the nine stimulus positions (referred to as the “training stimulus”) and compared the spontaneous waves before and after the training with respect to their similarity to the training-evoked wave. Immediately after the training, we found an increase in the number of spontaneous waves that resembled the training-evoked wave in initiation site and propagation path (Figure 2A and 2B). As shown in the population summary in Figure 2C, training induced a significant increase in the proportion of spontaneous waves with initiation sites close to that of the evoked wave (< 600 μm, p < 0.005, Wilcoxon signed rank test, n = 30).
To further quantify the similarity between the spontaneous and evoked waves, we used the evoked wave as a spatiotemporal template and computed the correlation coefficients (CCs) between the spontaneous activity and this template. A spontaneous wave was considered to be a “match” to the evoked template if the CC was above a given threshold (Figure S2; Experimental Procedures). When we compared the percentage of matched spontaneous waves before and after training, we found a significant increase over a range of CC thresholds (Figure 2D). At a threshold of 0.5, the percentage of matched waves increased from 37.5% to 49.5% (a difference of 12.0 ± 4.2%, SEM, p < 0.02, Wilcoxon signed rank test, n = 30). In contrast, control analyses using spatially randomized or rotated (by 180°) spontaneous activity (“surrogate data”) showed no significant increase in the percentage of matches at any CC threshold (Figure S3A, S3B; p > 0.4, Wilcoxon signed rank test). Thus, the observed increase in the percentage of matched waves cannot be accounted for by non-specific changes in the amplitude, frequency, or the spatial extent of the propagation of spontaneous waves, since these changes would all be preserved in the spatially rotated surrogate data. Such an effect of repeated visual stimuli on spontaneous cortical activity is reminiscent of the notion of network reverberation (Hebb, 1949; Lorente de No, 1938), in which activity patterns evoked by sensory stimuli continue to reverberate in the neural circuits after termination of the stimulus.
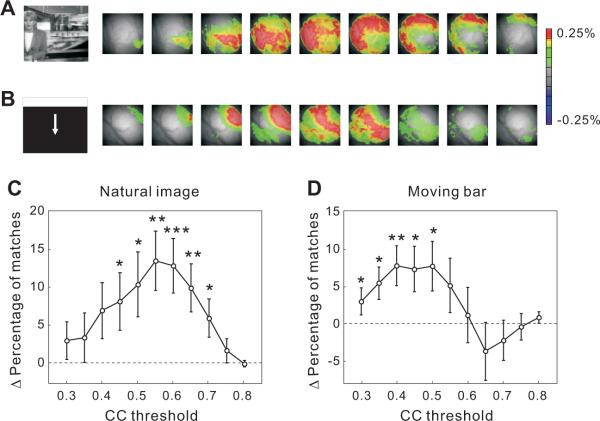
We also tested the effects of more complex training stimuli (natural images and moving bars) on the subsequent spontaneous activity. The natural images contain luminance patterns that are more complex and spatially distributed than the flashed squares, while the moving bars sweep across a large area of the visual field with different temporal structure from both the flashed squares and natural images (Experimental Procedures). Both types of stimuli evoked propagating waves of activity that were qualitatively similar to those elicited by the flash square stimuli (Figures 3A, 3B and S4). After training, we also found similar changes in the subsequent spontaneous activity (Figure 3C and 3D). At a CC threshold of 0.5, the increases in the percentage of matches were 10.2 ± 4.2% (p < 0.03, Wilcoxon signed rank test, n = 38) for natural images and 7.7 ± 3.3% (p < 0.04, n = 49) for moving bars. This suggests that the reverberation effect is not limited to simple, spatially localized stimuli but is general to a broad range of visual inputs.
Figure 3.
Effect of repeated presentation of natural images and moving bars on spontaneous waves
(A) Example of a wave evoked by a natural image, shown in 20 ms intervals. Left, The natural image used for stimulation. VSD signal was color coded as in Figure 1 (average of 3 trials; 7 frames following stimulus onset but before response onset were omitted).
(B) Example of a wave evoked by a moving bar, shown in 20 ms intervals (average of 3 trials; 7 frames following stimulus onset but before response onset were omitted).
(C) Difference in the percentage of matched spontaneous waves before and after training with natural images plotted as a function of CC threshold (*, p < 0.05; **, p < 0.005; ***, p < 0.001, 38 training experiments, 5 rats).
(D) Same as (A), for moving bars as training stimuli (49 training experiments, 19 rats).
To serve as a potential mechanism for short-term memory, an important requirement for the reverberating activity is its specificity to the recent sensory stimulus. We thus tested whether repeated stimulation at one position also resulted in an increase in the percentage of matches to the responses elicited by stimuli at other positions. Since in some cases the response patterns evoked by different stimuli were similar (e.g., Figure 4A, middle and right positions in top row), which could mask the stimulus specificity of the effect, we first measured the dissimilarity between each untrained template and the trained template as 1– CC(Templatetrained, Templateuntrained) (Figure 4B). We then plotted the change in the percentage of matches between the spontaneous waves and each evoked template as a function of its dissimilarity to the trained template. As shown in Figure 4C, we found increases in the percentage of matches for some of the untrained templates, but this effect decreased with the dissimilarity to the trained template, reaching the baseline at a dissimilarity of 0.8. Thus, the effect of training does not spread to untrained templates that are dissimilar to the trained template. Moreover, when we presented the same number of flashes but randomly distributed over the nine positions, we found no significant increase in the percentage of matches between the spontaneous waves and any of the evoked templates (Figure S3C, p > 0.2, Wilcoxon signed rank test, n = 19). Together, these results indicate that the reverberation effect is specific to the repeated stimulus and is not a general consequence of recent visual stimulation.
Figure 4.
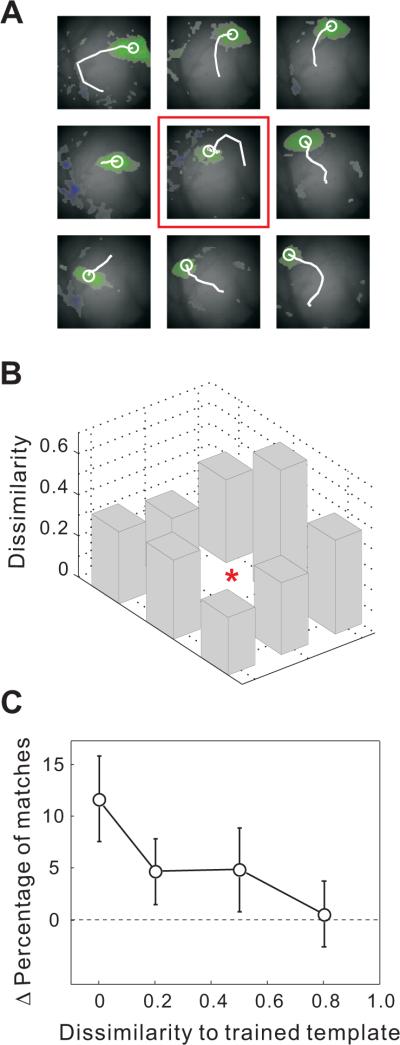
Specificity of the effect to the training pattern after 50 repeats of flashed squares at 0.6 Hz
(A) Initiation sites and trajectories of waves evoked by the nine stimuli in an example experiment. Red box indicates response to the training stimulus.
(B) Dissimilarity (1-CC) between the wave evoked by each non-training stimulus and that evoked by the training stimulus for the experiment shown in (A). Red star, dissimilarity for the training stimulus (=0).
(C) Change in the percentage of matches between spontaneous waves and all evoked templates plotted against the dissimilarity between the untrained and trained templates. Data are from the same 30 experiments as in Figure 2. Error bar, ± SEM.
Finally, we examined the time course for the induction and persistence of the effect. Figure 5A shows the increase in the percentage of matches immediately after training as a function of the number of training flashes. The magnitude of the effect increased with the number of repeats. To measure the persistence of the effect, we measured the increase in the number of matches at different time periods following training. As shown in Figure 5B, following 50 flashes, the effect decayed with a time constant of 0.8 min and reached the baseline within ~3 min. Increasing the amount of training to 125 flashes did not significantly augment the initial magnitude of the effect, but it increased the decay time constant to 14.4 min. Thus, the reverberation effect can last for several minutes, with its persistence depending on the amount of training.
Figure 5.
Induction and decay time course of the effect induced by flashed squares
(A) Change in the percentage of matches immediately after training vs. number of training stimuli (10 repeats: 24 training experiments, 8 rats; 25 repeats: 28 experiments, 8 rats; 50 repeats: 30 experiments, 9 rats; 125 repeats: 44 experiments, 16 rats).
(B) Persistence of ΔCC induced by 50 (gray) and 125 (black) training stimuli (30 and 44 training experiments for gray and black lines, respectively).
Discussion
In this study, we found that repeated visual stimulation causes a significant increase in the percentage of spontaneous waves that are similar to the cortical response evoked by the training stimulus. This effect is specific to the training stimulus, and it lasts for several minutes after training, consistent with the notion of reverberating activity in neuronal circuits (Hebb, 1949; Lorente de No, 1938). Reactivation of learning-related neural activity, thought to contribute to memory consolidation, has been shown in the hippocampus (Louie and Wilson, 2001; Nadasdy et al., 1999; Wilson and McNaughton, 1994), neocortex (Ji and Wilson, 2007; Ribeiro et al., 2004), and birdsong circuits (Dave and Margoliash, 2000) during sleep. Our study shows that visual stimuli can leave an immediate trace in the spontaneous activity in the first stage of cortical processing. Given its specificity (Figure 4) and time course (Figure 5B), such reverberation provides a potential mechanism for learning effects such as short-term visual memory (Phillips, 1974) and visual priming (Tulving and Schacter, 1990).
A related phenomenon has been described recently in cat V1 using single-unit recordings (Yao et al., 2007), in which the temporal patterns of spontaneous activity of individual cells were found to become more similar to the visually evoked response after repeated stimulation. In the current study, VSD imaging of cortical population activity has revealed that spontaneous and visually-evoked activity manifest as waves (Figure 1) and that the increased similarity between the spontaneous and evoked activity after repeated stimulation is at least partly due to a shift in the initiation sites of the spontaneous waves towards that of the training-evoked wave (Figure 2C). These observations provide important clues to the mechanisms for cortical reverberation. Furthermore, whereas the temporal reverberation shown in the previous study occurs periodically at the repetition rate of the training stimulus (Yao et al., 2007), the effect observed in the current study is not periodic, thus representing a more general form of reverberation. In addition to the mammalian visual cortex, reactivation of odorant-evoked spatial activity patterns has been shown in the honeybee antennal lobe using Ca2+ imaging (Galan et al., 2006). This suggests that reverberation exists in multiple sensory modalities in both vertebrate and invertebrate animals. Of course, a significant limitation of all these studies is that they were performed in anesthetized animals. It would be important for future studies to examine the contributions of these reactivated patterns in early sensory areas to perception and cognition in awake behaving animals.
The observed changes in spontaneous cortical activity could reflect changes in the earlier stages of the visual pathway (the retina and thalamus) and/or within the visual cortex. At the cellular level, the reverberation may be mediated by short-term changes in synaptic efficacy (Zucker and Regehr, 2002) or in the intrinsic membrane properties (Zhang and Linden, 2003) of the neurons activated by the repeated visual stimulation. Some of these modifications can last for hundreds of seconds, comparable to the time course of the reverberation (Figure 5). In particular, changes in these cellular properties at the initiation site of the evoked template may increase the probability of initiation of the spontaneous waves at this location (Figure 2C), which could in turn affect the wave trajectory. Although in this study we have examined the reverberation effect with limited sets of training stimuli, the above mechanisms could also support reverberation of more complex visual experience by operating on neurons and synapses that are preferentially activated by other features of the stimuli (e.g., orientations of local edges).
The reverberation effect may also involve longer-lasting forms of synaptic plasticity, since the correlated activation of a large number of neurons during waves is likely to be conducive to long-term synaptic modifications (Bi and Poo, 2001; Weliky, 2000). In this context, the relatively short persistence of the reverberation effect may reflect depotentiation or de-depression caused by the ongoing spontaneous activity in the cortex (Zhou and Poo, 2004). Interestingly, we found that increasing the number of training stimuli markedly prolonged the duration of the effect (Figure 5), suggesting that recurring visual stimuli can convert transient reverberations into more stable cortical modifications. In fact, reverberation of the evoked activity patterns is likely to not only reflect the stimulus-induced circuit modifications, but to also take part in their consolidation. Long-term strengthening of the connections within specific cell assemblies may explain the spontaneous occurrence of activity patterns that resemble sensory evoked responses even in the absence of training (Kenet et al., 2003). These spontaneous reactivations may in turn improve cortical response reliability in future encounters with the familiar stimuli (Galan et al., 2006; Stopfer and Laurent, 1999; Yao et al., 2007). Such dynamic, reciprocal interactions between spontaneous and sensory-evoked activity are likely to play important roles in cortical development and function. Note that in some cases, spontaneous and evoked activity exhibit distinct spatiotemporal features (Prechtl et al., 1997; Xu et al., 2007). It would be interesting for future studies to examine whether and how repeated visual training affects these properties.
Our findings also highlight the importance of cortical waves in network reverberation. Waves are natural modes of information propagation in large neuronal networks (Ermentrout and Kleinfeld, 2001), and they are thought to play important roles in memory consolidation (Marshall et al., 2006). Recent studies have revealed the prevalence of waves among sensory cortical areas (Amzica and Steriade, 1995; Ermentrout and Kleinfeld, 2001; Ferezou et al., 2006; Jancke et al., 2004; Lee et al., 2005; Luczak et al., 2007; Petersen et al., 2003; Prechtl et al., 1997; Roland et al., 2006; Sanchez-Vives and McCormick, 2000), but their contributions to sensory processing remain poorly understood. Our study shows that spontaneous waves can mediate the reverberation of visually evoked activity patterns in an early sensory area. Such reverberation may serve as a carrier for short-term memory and promote long-lasting modifications of cortical circuitry underlying perceptual learning.
Experimental Procedures
Surgical procedures and preparation
Animal use procedures were approved by the Animal Care and Use Committee at the University of California, Berkeley. Adult Long-Evans rats (250–400g) were anesthetized with pentobarbital (i.p., 60–70mg/kg initially, maintained at 3mg/hr). Eye movements were prevented by suturing the sclera to a sterile metal ring around the eye. The body temperature was maintained at 37°C with a regulated heating pad. A 5×5 mm2 craniotomy and durotomy were performed over the visual cortex. Voltage-sensitive dye RH1838 or RH1691 was dissolved in saline (1mg/ml) and topically applied to the exposed cortex for 2 hr. The cortex was then washed to remove unbound dye and covered with 1.5% agarose and a glass cover slip.
A total of 53 rats were used in this study. Each animal was used for multiple training experiments. The experiments with 50 repeats of a flashed square were performed on 9 rats, and 19 additional rats were used to study the effects of 10 repeats (8 rats), 25 repeats (8 rats) and 125 repeats (16 rats) of the flashed square. The experiments with the moving bar (19 rats) and random stimuli (6 rats) were conducted on some of these same animals and on 11 additional rats. The effects of natural scenes were tested on 5 additional rats. For the remaining 9 rats, no training experiments were performed, but the spontaneous and evoked waves observed in these rats were used to measure the wave speed (Figure 1D).
Image acquisition and processing
Voltage-sensitive dye signals were collected with a high-speed MiCam Ultima camera (Brainvision Inc.) with a 3×3 mm2 field of view (100×100 pixels). The imaging area was 1.5 to 4.5 mm from the midline and −5.5 to −8.5mm from Bregma, containing mostly V1 and a small portion of V2 (lower left region in Figure 1A). Light from a tungsten lamp (12V, 100W, Olympus) was filtered by a 630 ± 30 nm interference filter and reflected down onto the cortex by a 655 nm dichroic mirror (Chroma Technology). Fluorescence signals from the stained cortex were filtered with a 695 nm long-pass filter.
Images were acquired with MiCam Ultima Software (Brainvision Inc.) at 10ms/frame and analyzed with the BV_Analyser software (Brainvision Inc.). To measure spontaneous and evoked activity, we calculated ΔF/F as (F(x, y, t) – F(x, y, t0))/F(x, y, t0), where F(x, y, t) represents the fluorescence signal at location x, y and time t, and t0 represents time of the first frame in each recording session. We then applied a 2D spatial filter (boxcar, 5×5 pixels) to the image in each frame. Bleaching was then corrected using a temporal high pass filter at 0.5 Hz. Subsequent analyses were performed in Matlab (MathWorks Inc.) using custom written software.
Visual stimulation
Visual stimuli were generated with a PC computer containing a NVIDIA GeForce 6600 graphics board and presented with a XENARC 700V LCD monitor (19.7cm × 12.1cm, 960×600 pixels, 60 Hz, maximum luminance 40 cd m−2) placed 10 cm from the contralateral eye.
Each training experiment consists of a single recording session (1,024 frames, 10.24 s) of spontaneous activity before training, the training period, and one or more sessions of spontaneous activity after the training. Spontaneous cortical activity was recorded while the stimulation monitor was dark. During training, three types of stimuli were used: (1) a bright square (15–20° visual angle) briefly flashed (50 ms, 40 cd m−2) at one of the 3×3 positions (Figure 1A), (2) a bright horizontal bar (11 × 52°, maximum luminance) sweeping across 52° of the visual field at a velocity of 310 °/s (this velocity was chosen arbitrarily), and (3) an image of a natural scene presented for 50 ms (mean luminance 17.8 cd m−2, mean contrast 53%). For the natural scene stimuli, we randomly selected 22 images from a natural scene database (van Hateren and Ruderman, 1998) and used the center patch of each image (64×64 pixels, 52×52°) for the experiments. As shown in Figures 3A and S4, these image patches contain luminance patterns that are more complex and spatially distributed than the flashed squares, and in some cases the initiation site of the evoked wave was not easily predictable from the luminance distribution in the image (Figure S4).
For each training experiment, we presented 10–125 repeats of the stimulus at 0.6 Hz. For an experiment with 125 repeats (Figure 5), we either presented all the flashes in one block, or divided them into 5 blocks of 25 flashes each, separated by 40 s. When multiple training experiments were run on the same animal, consecutive experiments were separated by at least 10 min, and different training stimuli were used in order to minimize the potential interference between successive experiments. Each experiment was therefore considered an independent measure of the effect of training, and they were pooled together in the population data. Within each animal, the same training stimulus was never used more than two (flashed squares and natural images) or three (moving bars) times. The reverberation effect was measured by the difference in the percentage of matched spontaneous waves before and after training in each experiment.
Data analysis
To measure the instantaneous wave speed (Figure 1D), we computed the center of mass of ΔF/F in each frame. Wave speed was defined as the distance in the center of mass between consecutive frames divided by the inter-frame interval (10ms). Up to 18 measures of instantaneous speed were made for each wave. Note that during each wave, the activity spreads as well as propagates. Thus the speed estimated from the center of mass is likely to be slower than the speed of the wave front (Xu et al., 2007).
To measure the similarity between the spontaneous activity and each evoked response (150 – 300 ms), we averaged the spatiotemporal signals in response to 2–4 trials of each visual stimulus and used it as a sliding template to identify similar patterns in spontaneous activity (Figure S2). The Pearson correlation coefficient (CC) between the template and the segment of spontaneous recording centered at time t, CC(t), was computed for each spontaneous recording session (10.24 s). The effect of repeated visual stimulation on the spontaneous waves was quantified in the following steps:
-
(i)
We computed the spatial average of ΔF/F for each frame (f1, f2, …, f1024) and used 70% quantile of f in each spontaneous session as the threshold (Figure S2C, dashed line) to select the time periods corresponding to spontaneous waves (gray shading). We found that 70% quantile allows reliable identification of the waves, but results are similar for thresholds between 50% and 75% quantile.
-
(ii)
For each spontaneous wave, we computed the peak CC value, which is the CC at the optimal temporal alignment between the spontaneous and evoked waves (Figure S2D, dot). A “match” is defined as a spontaneous wave with a peak CC value above a given CC threshold (Figure S2D, dashed line).
-
(iii)
The number of matches was normalized by the total number of waves in each session (to eliminate the effect of wave frequency). The difference in the percentage of matches before and after training was plotted as a function of the CC threshold (Figures 2D, 3C, 3D and S3).
Supplementary Material
Acknowledgements
We thank Jianyoung Wu, Weifeng Xu, and Kentaroh Takagaki for technical help. This work was supported by HHMI Predoctoral Fellowship (to N.C.) and a grant from the National Eye Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abeles M, Gerstein GL. Detecting spatiotemporal firing patterns among simultaneously recorded single neurons. J Neurophysiol. 1988;60:909–924. doi: 10.1152/jn.1988.60.3.909. [DOI] [PubMed] [Google Scholar]
- Amzica F, Steriade M. Short- and long-range neuronal synchronization of the slow (< 1 Hz) cortical oscillation. J Neurophysiol. 1995;73:20–38. doi: 10.1152/jn.1995.73.1.20. [DOI] [PubMed] [Google Scholar]
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- Averbach E, Sperling G. Short term storage of information in vision. In: Cherry C, editor. Information Theory. Butterworth; London: 1961. pp. 196–211. [Google Scholar]
- Benucci A, Frazor RA, Carandini M. Standing waves and traveling waves distinguish two circuits in visual cortex. Neuron. 2007;55:103–117. doi: 10.1016/j.neuron.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Dave AS, Margoliash D. Song replay during sleep and computational rules for sensorimotor vocal learning. Science. 2000;290:812–816. doi: 10.1126/science.290.5492.812. [DOI] [PubMed] [Google Scholar]
- Ermentrout GB, Kleinfeld D. Traveling electrical waves in cortex: insights from phase dynamics and speculation on a computational role. Neuron. 2001;29:33–44. doi: 10.1016/s0896-6273(01)00178-7. [DOI] [PubMed] [Google Scholar]
- Ferezou I, Bolea S, Petersen CC. Visualizing the cortical representation of whisker touch: voltage-sensitive dye imaging in freely moving mice. Neuron. 2006;50:617–629. doi: 10.1016/j.neuron.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Fiser J, Chiu C, Weliky M. Small modulation of ongoing cortical dynamics by sensory input during natural vision. Nature. 2004;431:573–578. doi: 10.1038/nature02907. [DOI] [PubMed] [Google Scholar]
- Galan RF, Weidert M, Menzel R, Herz AV, Galizia CG. Sensory memory for odors is encoded in spontaneous correlated activity between olfactory glomeruli. Neural Comput. 2006;18:10–25. doi: 10.1162/089976606774841558. [DOI] [PubMed] [Google Scholar]
- Grinvald A, Hildesheim R. VSDI: a new era in functional imaging of cortical dynamics. Nat Rev Neurosci. 2004;5:874–885. doi: 10.1038/nrn1536. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior. Wiley; New York: 1949. [Google Scholar]
- Jancke D, Chavane F, Naaman S, Grinvald A. Imaging cortical correlates of illusion in early visual cortex. Nature. 2004;428:423–426. doi: 10.1038/nature02396. [DOI] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A. Spontaneously emerging cortical representations of visual attributes. Nature. 2003;425:954–956. doi: 10.1038/nature02078. [DOI] [PubMed] [Google Scholar]
- Lee SH, Blake R, Heeger DJ. Traveling waves of activity in primary visual cortex during binocular rivalry. Nat Neurosci. 2005;8:22–23. doi: 10.1038/nn1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente de No R. Analysis of the activity of the chains of internuncial neurons. J Neurophysiol. 1938;1:207–244. [Google Scholar]
- Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- Luczak A, Bartho P, Marguet SL, Buzsaki G, Harris KD. Sequential structure of neocortical spontaneous activity in vivo. Proc Natl Acad Sci U S A. 2007;104:347–352. doi: 10.1073/pnas.0605643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci U S A. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips WA. Distinction between Sensory Storage and Short-Term Visual Memory. Perception & Psychophysics. 1974;16:283–290. [Google Scholar]
- Prechtl JC, Cohen LB, Pesaran B, Mitra PP, Kleinfeld D. Visual stimuli induce waves of electrical activity in turtle cortex. Proc Natl Acad Sci U S A. 1997;94:7621–7626. doi: 10.1073/pnas.94.14.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S, Gervasoni D, Soares ES, Zhou Y, Lin SC, Pantoja J, Lavine M, Nicolelis MA. Long-lasting novelty-induced neuronal reverberation during slow-wave sleep in multiple forebrain areas. PLoS Biol. 2004;2:E24. doi: 10.1371/journal.pbio.0020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland PE, Hanazawa A, Undeman C, Eriksson D, Tompa T, Nakamura H, Valentiniene S, Ahmed B. Cortical feedback depolarization waves: a mechanism of top-down influence on early visual areas. Proc Natl Acad Sci U S A. 2006;103:12586–12591. doi: 10.1073/pnas.0604925103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino D, Robbins KA, Hatsopoulos NG. Propagating waves mediate information transfer in the motor cortex. Nat Neurosci. 2006;9:1549–1557. doi: 10.1038/nn1802. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Stopfer M, Laurent G. Short-term memory in olfactory network dynamics. Nature. 1999;402:664–668. doi: 10.1038/45244. [DOI] [PubMed] [Google Scholar]
- Tsodyks M, Kenet T, Grinvald A, Arieli A. Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science. 1999;286:1943–1946. doi: 10.1126/science.286.5446.1943. [DOI] [PubMed] [Google Scholar]
- Tulving E, Schacter DL. Priming and human memory systems. Science. 1990;247:301–306. doi: 10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- van Hateren JH, Ruderman DL. Independent component analysis of natural image sequences yields spatio-temporal filters similar to simple cells in primary visual cortex. Proc Biol Sci. 1998;265:2315–2320. doi: 10.1098/rspb.1998.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weliky M. Correlated neuronal activity and visual cortical development. Neuron. 2000;27:427–430. doi: 10.1016/s0896-6273(00)00053-2. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Xu W, Huang X, Takagaki K, Wu JY. Compression and reflection of visually evoked cortical waves. Neuron. 2007;55:119–129. doi: 10.1016/j.neuron.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Shi L, Han F, Gao H, Dan Y. Rapid learning in cortical coding of visual scenes. Nat Neurosci. 2007;10:772–778. doi: 10.1038/nn1895. [DOI] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Poo MM. Reversal and consolidation of activity-induced synaptic modifications. Trends Neurosci. 2004;27:378–383. doi: 10.1016/j.tins.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.