Abstract
The semi-synthetic vitamin E derivative alpha-tocopheryloxyacetic acid (α-TEA) induces tumor cell apoptosis and may offer a simple adjuvant supplement for cancer therapy if its mechanisms can be better understood. Here we report that α-TEA also triggers tumor cell autophagy and that it improves cross-presentation of tumor antigens to the immune system. α-TEA stimulated both apoptosis and autophagy in murine mammary and lung cancer cells and inhibition of caspase-dependent apoptosis enhanced α-TEA-induced autophagy. Cell exposure to α-TEA generated double membrane-bound vesicles indicative of autophagosomes, which efficiently cross-primed antigen-specific CD8+ T cells. Notably, vaccination with dendritic cells pulsed with α-TEA-generated autophagosomes reduced lung metastases and increased the survival of tumor-bearing mice. Taken together, our findings suggest that both autophagy and apoptosis signaling programs are activated during α-TEA-induced tumor cell killing. We suggest that the ability of α-TEA to stimulate autophagy and enhance cross-priming of CD8+ T cells might be exploited as an adjuvant strategy to improve stimulation of anti-tumor immune responses.
Keywords: α-TEA, autophagy, cross-presentation, tumor immunity
INTRODUCTION
Alpha-tocopheryloxyacetic acid (α-TEA) is a stable semi-synthetic ether derivative of naturally occurring vitamin E (alpha-tocopherol). Although vitamin E has been pursued as an anti-cancer agent because of its anti-oxidant properties, the available epidemiologic data as well as in vivo studies in experimental animal tumor models have demonstrated a limited role for vitamin E in cancer prevention or control (1–3) and may even be harmful as shown in the aborted SELECT clinical trial that examined the effect of high dose vitamin E on prostate cancer (4). α-TEA is derived from vitamin E by a chemical modification which involves the replacement of the hydroxyl group at the number 6 carbon of the phenolic ring of the chroman head by an acetic acid residue linked by an ether bond (Supplemental Figure S1) (5). This modification renders α-TEA, in contrast to vitamin E, redox silent but active against tumors of various origins (5–7). The presence of a non-cleavable ether bond ensures the stability of α-TEA allowing it to be delivered via the oral route in a biologically active form. We reported for the first time, that when incorporated into mouse chow, and supplied to mice in the diet, α-TEA significantly inhibited the growth of transplanted and spontaneously-arising metastatic breast cancers and dramatically reduced the incidence of spontaneous lung metastases before and after primary tumor establishment without overt toxicity (7, 8).
Reports from numerous laboratories including our own have demonstrated that apoptosis is a primary mode of α-TEA-induced tumor cell death (7–10), a process that is initiated by mitochondrial depolarization followed by release of cytochrome c to the cytosol and activation of the caspase execution pathway [Reviewed in (11)]. However, the observation that the anti-tumor activity of α-TEA cannot be completely blocked using pan or caspase-specific inhibitors (12, 13) suggests the involvement of additional pathway(s) in α-TEA-mediated tumor cell killing.
Autophagy is normally a protective survival mechanism employed by cells undergoing various forms of stress, including chemotherapy to sequester, process and recycle damaged cellular organelles and mis-folded and long-lived proteins to provide nutrients to the cell [Reviewed in Maiuri (14)]. It has recently become clear, that apoptosis and autophagy are not mutually exclusive events [Reviewed in (15)] and that both could lead to cell death (14, 16). The formation of autophagosomes involves 3 major steps: The first (initiation stage) is the de novo formation of an isolation membrane which is regulated by the mammalian target of rapamycin (mTOR) and the Beclin-1 (Atg6)/class III phospohoinsitol-3 kinase (PI3K) complex. The second stage involves elongation and expansion of the phagophore to enclose cytosolic components including damaged organelles and mis-folded proteins and requires the conjugation of Atg5 to Atg12. The final stage is the formation of a mature autophagosome ready for fusion with lysosomal vesicles, which requires conversion of soluble LC3-I (Atg8) to the membrane-bound form LC3-II (17).
During autophagy, cellular components including viral or endogenous tumor-associated antigens (TAA) become available for cross-presentation by professional antigen presenting cells (APC) to prime antigen- or tumor-specific T cell responses (14, 18). Although autophagy is known to play an essential role in major histocompatibility complex (MHC)-class II-restricted antigen presentation (19), only recently has its role in MHC class I-restricted stimulation of CD8+ T cells (cross-presentation) become appreciated (18, 20).
In this study, we investigated whether α-TEA stimulates tumor cell autophagy and enhances antigen cross-presentation by dendritic cells (DC). We demonstrate that α-TEA induces tumor cell autophagy and that the α-TEA-derived autophagosome-enriched supernatant fraction (α-TAGS) stimulates efficient antigen cross-presentation. We describe here a novel mechanism of immune activation by α-TEA that involves the stimulation of tumor cell autophagy and enhanced cross-priming of CD8+ T cells.
Materials and Methods
Preparation of α-tocopheryloxyacetic acid
Alpha-TEA [(2,5,7,8-tetramethyl-(2R-(4R,8R,12-trimethyltridecyl) chroman-6-yloxy) acetic acid)] was synthesized using a combination of previously described methods (2, 5) and vesiculated α-TEA (Vα-TEA) was generated as previously described (8).
Mice
Six to 8-week old female BALB/c and C57BL/6 mice were purchased from Harlan Laboratories (Indianapolis, IN). OT-I TCR transgenic breeders were purchased from the Jackson Laboratory. CT-TCR transgenic breeders were kindly provided by Dr. Jill E. Slansky (University of Colorado Denver School of Medicine, Denver, CO) (21). All mice were maintained and used in accordance with the Principles of Animal Care (NIH publication No. 85-23) and all studies were approved by the EACRI Institutional Animal Care and Use Committee.
Tumor Cell Lines and Cell Culture
The poorly immunogenic and highly metastatic 4T1 tumor cell line was kindly provided by Dr. Fred Miller of the Michigan Cancer Foundation (Detroit, MI). The Lewis Lung carcinoma (3LL) cell line was kindly provided by Dr. Lea Eisenbach of the Weizman Institute of Science (Rehovot, Israel).
DNA Construction and Transduction of Cell Lines
3LL or 4T1 tumor cells were transduced with lentiviral vectors that either expressed the ubiquitin-methionine green fluorescence protein-ovalbulmin (Ub-M-GFP-OVA) antigen or LC3-GFP as previously described (18). Ub-M-GFP-OVA antigen- or LC3-GFP-expressing cells were enriched for GFP positivity by fluorescence activated cell sorting. LC3-GFP was transiently expressed in 3LL tumor cells after transfection using Lipofectamine-LTX (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions.
4T1 tumor cells stably transduced with Atg12-specific short hairpin RNA (shRNA) (4T1-Atg12shRNA) and scrambled (control) shRNA (4T1-CTRLshRNA) were generated previously. Atg12-specific shRNA expression is doxycycline inducible and co-expressed with red fluorescent protein (RFP) which was monitored by epifluoresence. Atg12 gene knockdown was determined by western immunoblotting. The transduced cells (4T1-LC3-GFP, 4T1-OVA-GFP, 4T1-Atg12shRNA, 4T1-CTRLshRNA and 3LL-OVA-GFP) were maintained in culture medium containing 2 µg/mL puromycin (Sigma-Aldrich, St Louis, MO).
Quantification of LC3-GFP Punctae
4T1-LC3-GFP or 3LL-LC3-GFP cells were cultured overnight on Lab-Tek II chamber slides (Thermo Fisher Scientific, Rockford, IL). The cells were then treated with Vα-TEA or 100 mM trehalose (Sigma) with the addition of 10 nM bafilomycin A1 (Sigma) for the last 2 h of incubation. Cells were fixed and the fraction of punctate positive cells per field of view was determined by counting punctate positive and total number of cells in 12 to 30 random high powered-fields (100X) of view per treatment by epifluoresence microscopy.
Apoptosis Assay
Tumor cells were pre-treated with 50 µM zVAD-fmk (Sigma) for 2 h before the addition of 40 µM Vα-TEA. Non-adherent and adherent cells were collected using 10 mM EDTA, pooled and stained using the APC-Annexin-V/7AAD apoptosis detection kit (BD Pharmingen, San Diego, CA) according to the manufacturer’s instructions. Cells positively staining with APC-Annexin-V were considered to be apoptotic.
Clonogenic Cell Survival Assay
4T1-Atg12shRNA or 4T1-CTRLshRNA tumor cells (1.25×105) were cultured with 2 µg/mL doxycycline (Sigma) for 72 h to induce Atg12 knockdown and were then treated with 60 µM Vα-TEA. After 24 h, non-adherent and adherent cells were collected (300×g, 5 min). Cell number and viability were determined by trypan blue dye exclusion and 1.25×1022.5×1025×102103 and 104 viable cells (trypan blue negative) from each treatment group were plated in triplicate (100 mm dishes) and incubated (7% CO237°C) for 7 days. The resulting colonies were methanol-fixed, stained with Giemsa (Sigma) and counted. The surviving cell fraction was determined as previously described (7).
Generation and Collection of α-TEA-Generated Autophagosome-Enriched Supernatant Fraction (α-TAGS
Tumor cells were treated with 20 µM (4T1) or 80 µM (3LL) Vα-TEA for 24 h. The culture supernatant was collected and cleared of dead cells and cell debris (300×g, 10 min). To obtain the autophagosome enriched fraction (α-TAGS), the supernatant containing the crude large vesicles was centrifuged at 10,000×g for 15 min. The α-TAGS pellet was re-suspended in PBS and protein content was determined by bicinchoninic acid (BCA) protein assay (Thermo Scientific). For the control cells [vehicle (PBS)-treated] in which cells were not dying, culture medium was collected from twice as many cells. To block autophagy, cells were pretreated with 10 mM 3-Methyladenine (3-MA, Sigma) for 16 h before α-TEA treatment.
Transmission Electron Microscopy
α-TAGS was prepared from 4T1 or 3LL tumor cells treated with 20 µM or 80 µM Vα-TEA respectively for 24 h and prepared for transmission electron microscopy (TEM) as described in the supplemental methods. Microscopy was performed at 60 kV on a Philips Morgagne TEM (FEI Inc.), equipped with a CCD camera and images were collected at original magnifications of 1,000–37,000×.
Western Blot Analysis
To detect LC3 conversion, 3LL or 4T1 tumor cells were treated with 40 or 60 µM Vα-TEA or 400 nM rapamycin (Sigma) for 16 h with the addition of bafilomycin A1 (Sigma) for the last 2 h of culture. Cell lysates were prepared using Complete Lysis-M Buffer (Roche Applied Sciences) containing protease inhibitors. For in vivo LC3 conversion, 4T1-tumor-bearing mice received α-TEA in the diet (~6mg/mouse/day) for 5 days. Subsequently, tumors were resected and lysed using CelLytic Mammalian Tissue Lysis Reagent (Sigma) containing protease inhibitors and a rotor-stator homogenizer. In order to detect Beclin-1 and cleavage of nuclear poly (ADP-ribose) polymerase (PARP), tumor cells were pre-incubated with zVAD-fmk (80 µM) for 2 h before treatment with α-TEA (80 µM) for 16 h. To detect Atg12, 4T1-Atg12shRNA and 4T1-CTRLshRNA were cultured with 2 µg/mL doxycycline for 72 h before α-TEA treatment. All lysates were clarified (14,000×g, 15 min, 4°C) and protein content was determined by BCA (Thermo Scientific). After electrophoretic separation, proteins were detected by western immunoblotting.
To detect endogenous LC3 in α-TAGS, α-TAGS was lysed in RIPA buffer and clarified. Protein content was determined by BCA (Thermo Scientific) and equal amounts (5 µg) of protein were electrophoretically separated and LC3 was detected by western immunoblotting and quantified using Image-J software (22).
Cross-Presentation Assay
For the in vitro cross-presentation assay, splenic dendritic cells (DCs) were generated as previously described (23). OT-I splenocytes were CFSE-labeled (5 µM) and co-incubated with antigen-loaded DCs (20 µg α-TAGS/1×106 DC; 6 h) in 10 mL tissue culture flat tubes (Techno Plastic Products AG, Trasadingen, Switzerland) for 4 or 6 days. CFSE dilution was measured by flow cytometry as previously described (18). In order to determine in vivo cross-presentation, α-TAGS (20 µg protein) was injected into both inguinal lymph nodes of naïve C57BL/6 mice. On the same day, 3×106 CFSE-labeled Thy1.1+ OT-I T cells were adoptively transferred by intravenous injection. Lymph nodes were collected 5 days later, and single cell suspensions were prepared and CFSE dilution of Thy1.1+ CD8+ cells was determined by flow cytometry. Data were acquired and analyzed using BD CellQuest Pro software (BD Biosciences, San Jose, CA).
IFN-γ ELISA
4T1-Atg12shRNA and 4T1-CTRLshRNA cells were incubated with 2 µg/mL doxycycline for 72 h before preparation of α-TAGS as described above. DCs (3 × 106) were pulsed with 20 µg α-TAGS for 6 hours, and washed three times. T cells (3 × 106) isolated from CT-TCR mice were then co-incubated with the DC for 48 h and IFN-γ secretion was detected by ELISA (eBioscience).
Generation of Dendritic Cells and Animal Vaccination Studies
For the 4T1 tumor model experiments, bone marrow-derived DC (24) were either left untreated [non-pulsed-DC, npDC], pulsed overnight with 40 µg α-TAGS per 1×106 DC (α-TAGS-DC) or tumor cell freeze-thaw (3 cycles) lysate (Lysate-DC) at a ratio of 3 tumor cell equivalents per DC. Subsequently, the DC were matured by adding 200 U/mL TNF-α (Peprotech) for 24 h. 4T1 tumor cells (5 × 104) were injected sub-cutaneously (s.c.) into the right mammary fat pad of female BALB/c mice. The mice then received s.c. vaccinations of 1 × 106 α-TAGS-DC, Lysate-DC or npDC on days 7, 9 and 11 post-tumor injection. Mice injected subcutaneously with α-TAGS (40 µg) were included as controls. For the 3LL experiments, DC were left untreated (npDC), or pulsed with either 20 µg α-TAGS per 3×106 DC (α-TAGS-DC) or tumor cell freeze-thaw lysate (Lysate-DC) in the presence of 200 ng/ml IFN-γ (Peprotech). 3LL tumor cells (2×105) were injected intravenously into female C57BL/6 mice. On day 3 post-tumor injection, the mice were vaccinated subcutaneously with 3×106 α-TAGS-DC, Lysate-DC, npDC or 20 µg α-TAGS. Visible metastatic nodules in the lungs were enumerated on day 28.
Statistical Analysis
Statistical significance of differences among data sets was assessed by Student’s t-test for pair wise comparisons or for comparisons of multiple groups by one-way analysis of variances (ANOVA) with Tukey’s HSD test. Survival was assessed according to Kaplan and Meier including log-rank test. All analyses were performed using Prism software (GraphPad, San Diego, CA). Probability values (P) of ≤ 0.05 were considered indicative of significant differences.
RESULTS
α-TEA stimulates autophagy in tumor cells
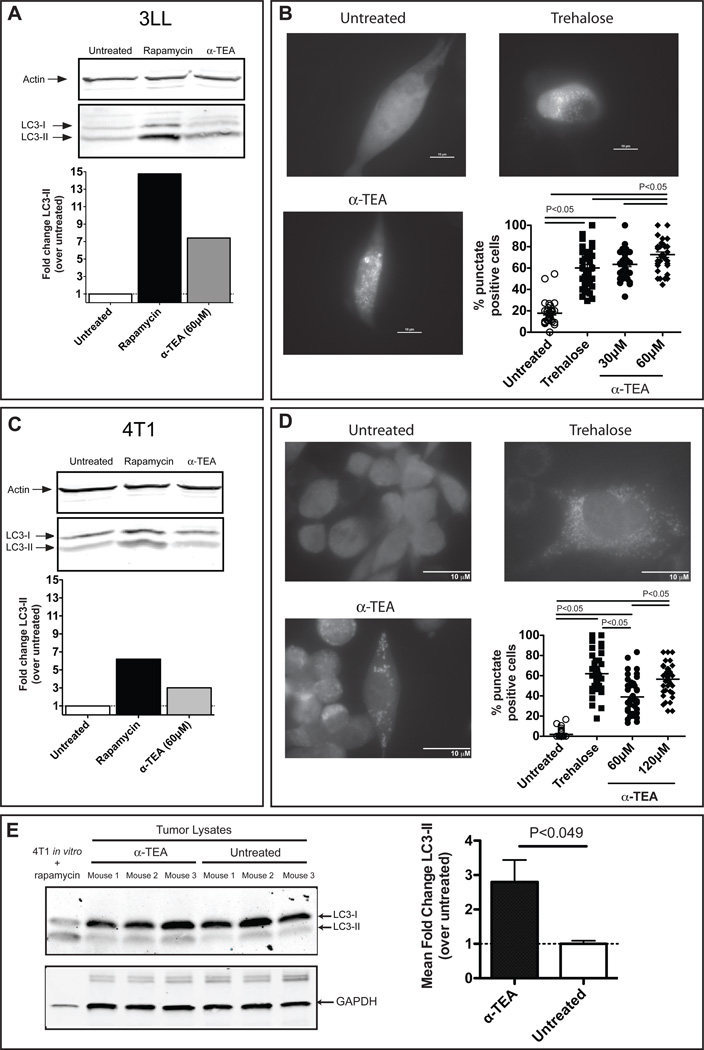
To assess whether α-TEA stimulates autophagy in tumor cells, Lewis Lung carcinoma (3LL) and murine mammary tumor (4T1) cells were treated with α-TEA and the levels of LC3-I and LC3-II were determined by western immunoblotting. Induction of autophagy is associated with conversion of the cytosolic LC3-I to the membrane-bound LC3-II (17). The data show that α-TEA induced a 7-fold increase in LC3-II conversion in α-TEA-treated 3LL tumor cells (Figure 1A). LC3-II conversion in α-TEA-treated 4T1 tumor cells was less robust and was ~3-fold higher than vehicle (PBS)-treated cells (Figure 1C). As with α-TEA, 4T1 tumor cells were also less responsive than 3LL tumor cells to rapamycin, a known autophagy inducer. To confirm autophagy induction in α-TEA-treated tumor cells, we assessed the formation of LC3-GFP punctae in LC3-GFP transgene-expressing 3LL and 4T1 cells. α-TEA treatment caused a dose-dependent increase in LC3 punctae formation indicative of autophagy (25) in both 3LL (Figure 1B) and 4T1 (Figure 1D) tumor cells. Punctae formation in α-TEA-treated 3LL cells was similar or higher than punctae formation induced by the known autophagy inducer trehalose (Figure 1B). α-TEA treatment of 4T1 cells also significantly increased punctae formation to levels comparable to trehalose (Figure 1D). In order to determine if α-TEA induces autophagy in vivo4T1 tumor-bearing animals received an oral dose of α-TEA (6mg/day) that we have previously shown to reduce tumor growth and prolong survival (2, 7). LC3 conversion in the tumor was determined after 5 days of α-TEA treatment and we found approximately three-fold increased LC3-II levels in 4T1 mammary tumors from α-TEA-treated mice compared to untreated mice (Figure 1E). Taken together, these three lines of evidence indicate that α-TEA induced autophagy in tumor cells.
Figure 1. Alpha-TEA induces tumor cell autophagy.
(A) 3LL tumor cells were treated with α-TEA for 16 h. LC3-I to LC3-II conversion was determined by western-immunoblotting. Actin was used as loading control. Bar graphs indicate fold change of LC3-II (normalized to actin) over untreated cells. (B) Accumulation of LC3-II-GFP punctae. 3LL cells transiently expressing a LC3-GFP fusion protein (3LL-LC3-GFP) were treated with α-TEA or trehalose for 16 h with the addition of bafilomycin-A1 for the last 2 h of treatment. Formation of punctae was determined by epifluorescence microscopy and the proportion of punctate positive cells per field of view was determined. Representative pictures (100× magnification) of untreated, trehalose- and α-TEA (30 µM)-treated cells. (C) Increased conversion of LC3-I to LC3-II in 4T1 tumor cells after α-TEA treatment was determined as in (A). (D) Formation of LC3-II-GFP punctae was determined as in (B) in 4T1 mammary tumor cells stably expressing a LC3-GFP fusion protein (4T1-LC3-GFP). Representative pictures (100× magnification) of untreated, trehalose- and α-TEA (60 µM)-treated cells. Data are from 3 independent experiments. (E) 4T1 mammary tumor-bearing mice (n=3 per group) received α-TEA in the diet for 5 days. Subsequently, tumors were resected and LC3-I to LC3-II conversion was determined by western-immunoblotting. 4T1 tumor cells treated in vitro with rapamycin were used as positive control. GAPDH was used as loading control. The bar graph represents mean fold change±SD of LC3-II bands (normalized to GAPDH) from 3 mice per group.
Autophagy precedes apoptosis during α-TEA treatment
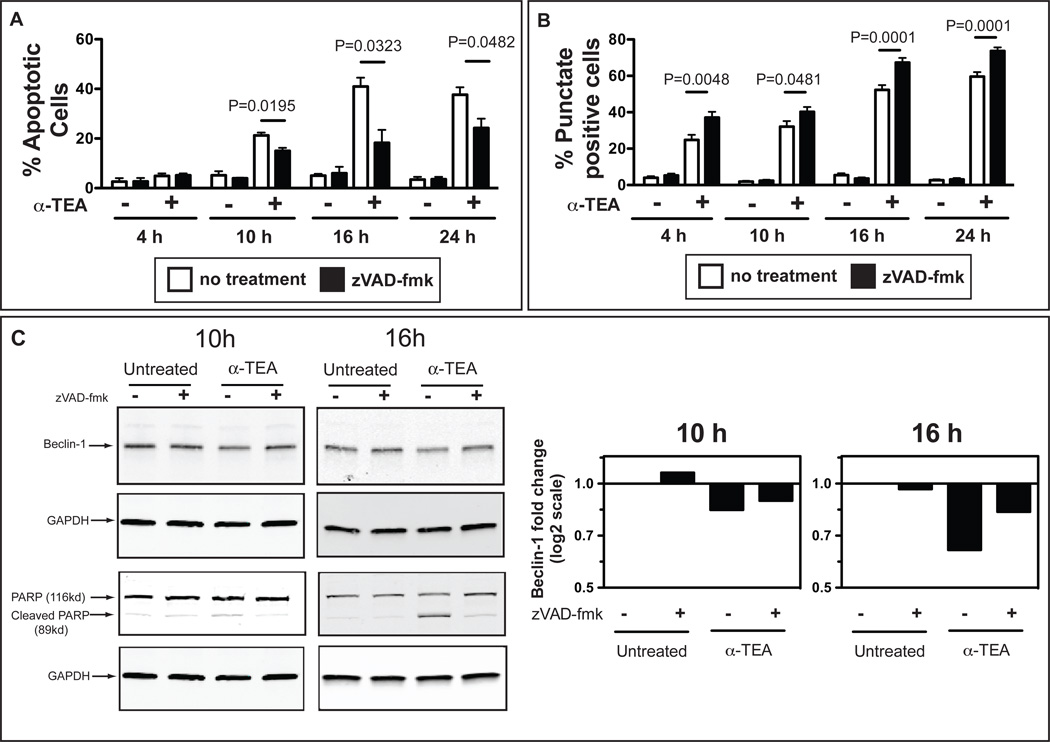
Based on the documented property of α-TEA as a potent apoptosis-inducer (7, 8, 12, 13, 26), we compared the kinetics of apoptosis and autophagy induction during α-TEA treatment. The data (Figure 2A and 2B) show that α-TEA-induced autophagy precedes apoptosis. At 4 h of α-TEA treatment, when no change in the percentage of apoptotic cells is observed compared to untreated cells (Figure 2A), the percentage of punctate positive cells, indicative of autophagy is ~25% (Figure 2B); peak levels of apoptosis and autophagy are observed at 16 h of α-TEA treatment. Our observation that α-TEA-stimulates autophagy together with a recent report that treatment of stressed cells with the pan-caspase inhibitor zVAD-fmk enhanced autophagy (27, 28), prompted us to evaluate the effect of apoptosis inhibition on α-TEA-induced autophagy. For this purpose, 4T1-LC3-GFP cells were treated with α-TEA in the presence of the pan-caspase inhibitor zVAD-fmk. α-TEA induced caspase-dependent apoptosis, which reached a maximum at 16 h, was inhibited ~55% by zVAD-fmk. In contrast, increase in the percentage of LC3-GFP punctate positive cells, which was detected as early as 4 h after α-TEA treatment (Figure 2B) was enhanced by zVAD-fmk treatment. It has recently been reported that apoptosis induction may inhibit autophagy via regulation of Beclin-1 levels (27, 28). Therefore, we determined Beclin-1 protein levels in α-TEA-treated tumor cells. The data (Figure 2C) demonstrate a modest reduction in Beclin-1 protein in α-TEA-treated tumor cells compared to untreated cells (Figure 2C). Pre-treatment with zVAD-fmk, appeared to restore Beclin-1 expression (Figure 2C).
Figure 2. Effect of pan-caspase inhibition on α-TEA-induced tumor autophagy.
(A) 4T1-LC3-GFP cells were pre-incubated with the pan-caspase inhibitor zVAD-fmk for 2 h and then treated with α-TEA for 4, 10, 16 or 24 h in the presence of zVAD-fmk. Apoptosis was determined by flow cytometry after staining with 7-AAD and Annexin-V-PE. (B) 4T1-LC3-GFP cells were treated as in (A) and formation of LC3-GFP punctae was determined by epifluorescence microscopy. Proportion of punctate positive cells per field of view (100× magnification) was determined. Data are from 3 independent experiments. (C) 4T1 tumor cells were treated with α-TEA for 10 h or 16 h in the presence of zVAD-fmk. PARP cleavage and Beclin-1 levels were determined by western-immunoblotting. GAPDH was used as loading control. Bar graphs indicate fold change of Beclin-1 (normalized to GAPDH) over untreated cells.
α-TEA-induced autophagy decreases tumor cell survival
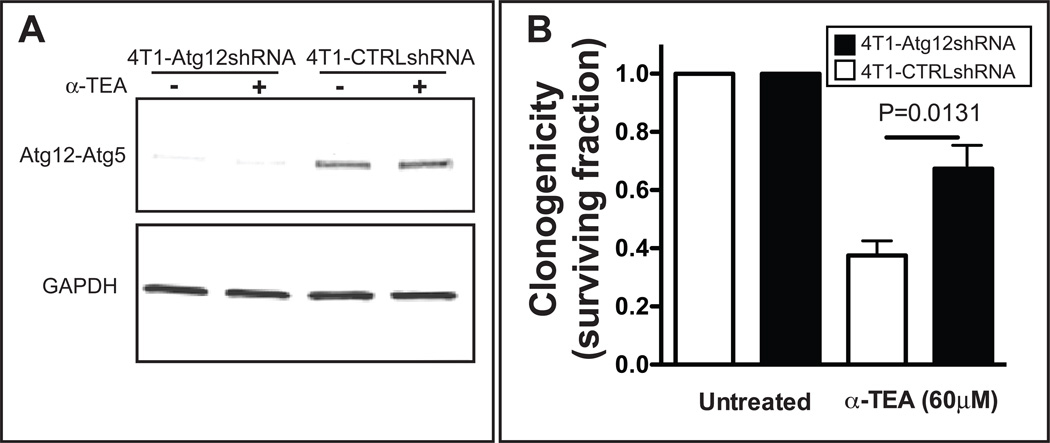
Since autophagy can either be protective or lead to tumor cell death (14, 29), we assessed the impact of α-TEA treatment on tumor cell survival during autophagy inhibition. This was achieved by doxycycline-induced shRNA knockdown of the autophagy pathway gene, Atg12Figure 3A), which is essential for the membrane extension step that leads to autophagosomal vesicle formation (14). Tumor cell survival was assessed using a long-term clonogenicity assay, which provides the most accurate assessment of cell death. Alpha-TEA treatment concurrent with Atg12 knockdown significantly increased colony formation of α-TEA-treated cells (p>0.0131) suggesting that autophagy contributed to α-TEA-mediated tumor cell death (Figure 3B).
Figure 3. α-TEA-induced autophagy decreases tumor cell survival.
(A) The autophagy pathway was inhibited by doxycycline-inducible shRNA knockdown of Atg12 protein expression in 4T1 tumor cells (4T1-Atg12shRNA). 4T1-CTRLshRNA cells that express a non-specific shRNA were included as control. After 72 h doxycycline incubation, Atg12 protein levels were determined by western immunoblot detection of the Atg12-Atg5 complex using an Atg12-specific antibody. (B) The cells were then treated with α-TEA (60 µM) for 24 h. Non-adherent and adherent cells were collected and the surviving fraction (SF) was determined after 7 days of culture without α-TEA. Mean SF±SD from four independent experiments is shown.
α-TEA-generated autophagosome-enriched supernatant fraction (α-TAGS) is an efficient antigen carrier for cross-presentation
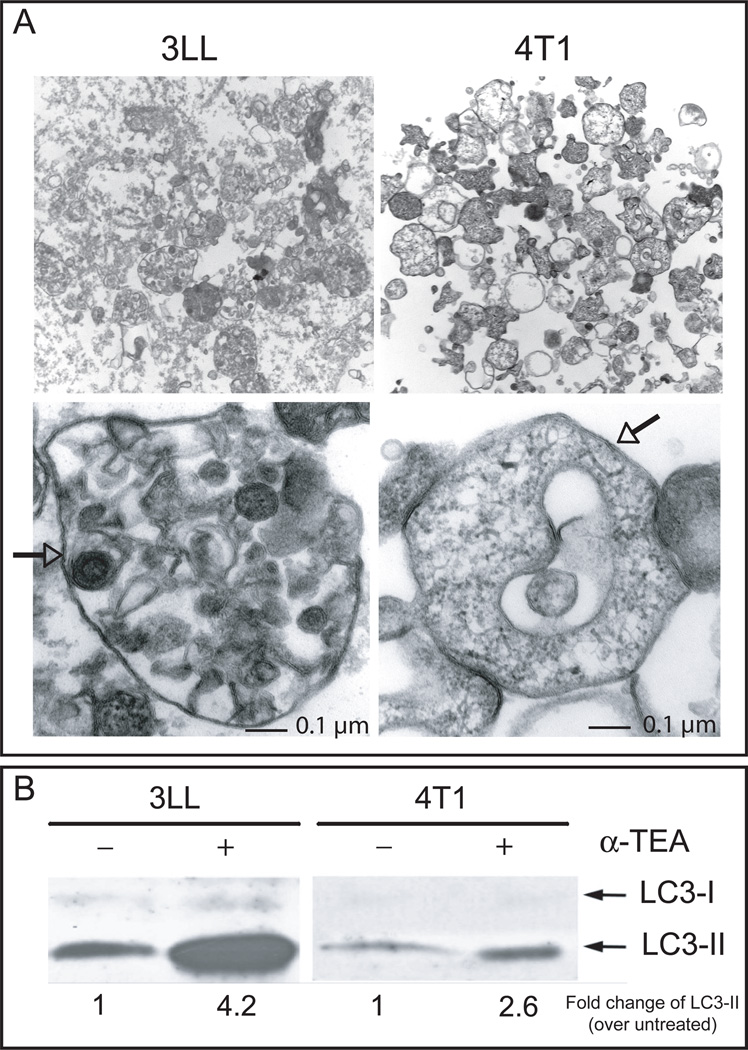
Given that we (18, 30) and others (19, 31) have previously shown that autophagy induction in tumor cells can play a pivotal role in cross-presentation of tumor-associated antigen, we hypothesized that α-TEA-generated autophagosomes augment antigen-specific CD8+ T cell activation. We first determined if α-TEA treatment of 4T1 and 3LL tumor cells resulted in release of autophagosomes. Examination of the pre-cleared, high-speed (10,000×g) pellet fraction revealed the presence of double membrane structures (Figure 4A) typical of autophagosomes (25). In addition, immunoblot analysis showed that the autophagosome-enriched fraction (α-TAGS) contained elevated amounts of LC3-II (Figure 4B).
Figure 4. The high-speed (10,000 × g) supernatant fraction from α-TEA-treated tumor cells contains autophagosomes.
(A) 3LL or 4T1 tumor cells were treated with 80 µM or 20 µM α-TEA for 24 h or 48 h, respectively. The cell culture supernatant was collected and dead cells and debris were removed by centrifugation at 300×g for 10 min. Subsequently the low-speed supernatant was centrifuged at 10,000×g for 15 min and the high-speed pellet was examined by transmission electron microscopy. Double membrane vesicles (arrows) with a variety of morphology were present. Their sizes ranged between 100 nm and 1 µm. Images were collected at original magnifications of 1,000–37,000×. (B) The high-speed fractions from untreated and α-TEA-treated 3LL or 4T1 cells were solubilized in lysis buffer and equal amounts of protein (5 µg) were electrophoretically separated and the autophagosome marker, LC3-II was detected by western immunoblotting.
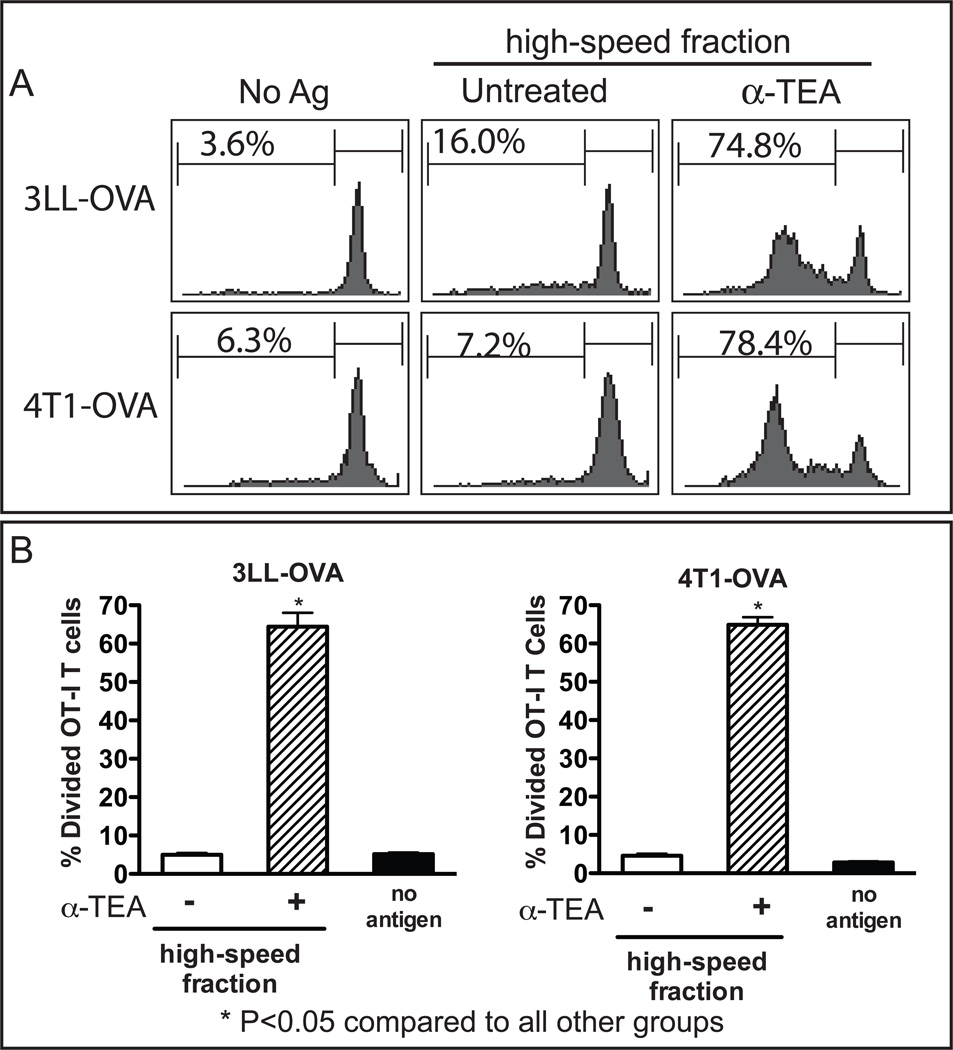
In order to determine whether α-TAGS is an antigen carrier and can stimulate cross-presentation to CD8+ T cells, we pulsed dendritic cells (DC) with α-TAGS collected from 3LL or 4T1 tumor cells that stably express the OVA protein and tested their ability to stimulate OVA-specific transgenic OT-I CD8+ T cells (Figure 5A). DCs pulsed with α-TAGS from 3LL-OVA and 4T1-OVA cells, stimulated vigorous OT-I CD8+ T cell proliferation with over 60% of T cells undergoing cell division (Figure 5A and 5B). In contrast, the equivalent high-speed fraction from α-TEA-treated parental 4T1 or 3LL tumor cells (data not shown) or untreated OVA-expressing (4T1-OVA, 3LL-OVA) cells did not significantly stimulate OT-I T cell proliferation compared to un-pulsed DC (Figure 5B). Furthermore, α-TAGS from OVA-expressing tumor cells stimulated proliferation of OT-I CD8+ T cells in an α-TEA-dose-dependent manner. Peak T cell stimulation was achieved using 3LL and 4T1-derived α-TAGS after treatment with 80 µM and 20 µM α-TEA, respectively (data not shown). This finding suggests that α-TAGS is an efficient carrier of tumor antigens for cross-presentation.
Figure 5. α-TEA-generated autophagosome-enriched supernatant fraction (α-TAGS) stimulates cross-presentation.
3LL and 4T1 tumor cells that stably express the OVA peptide (3LL-OVA, 4T1-OVA), were treated with α-TEA and the cell culture supernatant was subjected to centrifugal fractionation. The high-speed α-TEA-generated autophagosome-enriched supernatant fraction (α-TAGS) was re-suspended in culture medium and pulsed onto DCs for 6 h. The DCs were then washed and co-incubated with CFSE-labeled OVA-specific TCR transgenic OT-I CD8+ T cells. Un-pulsed DCs (no antigen) were included as controls. Proliferation of OT-I CD8+ T cells was assessed by determination of CFSE dilution using flow cytometry. (A) Representative histograms of percent proliferating OT-I CD8+ T cells are shown. (B) Mean percent divided cells ± SD is shown for 3 to 5 independent experiments.
Tumor cell autophagy plays an important role in α-TAGS activation of CD8+ T cells
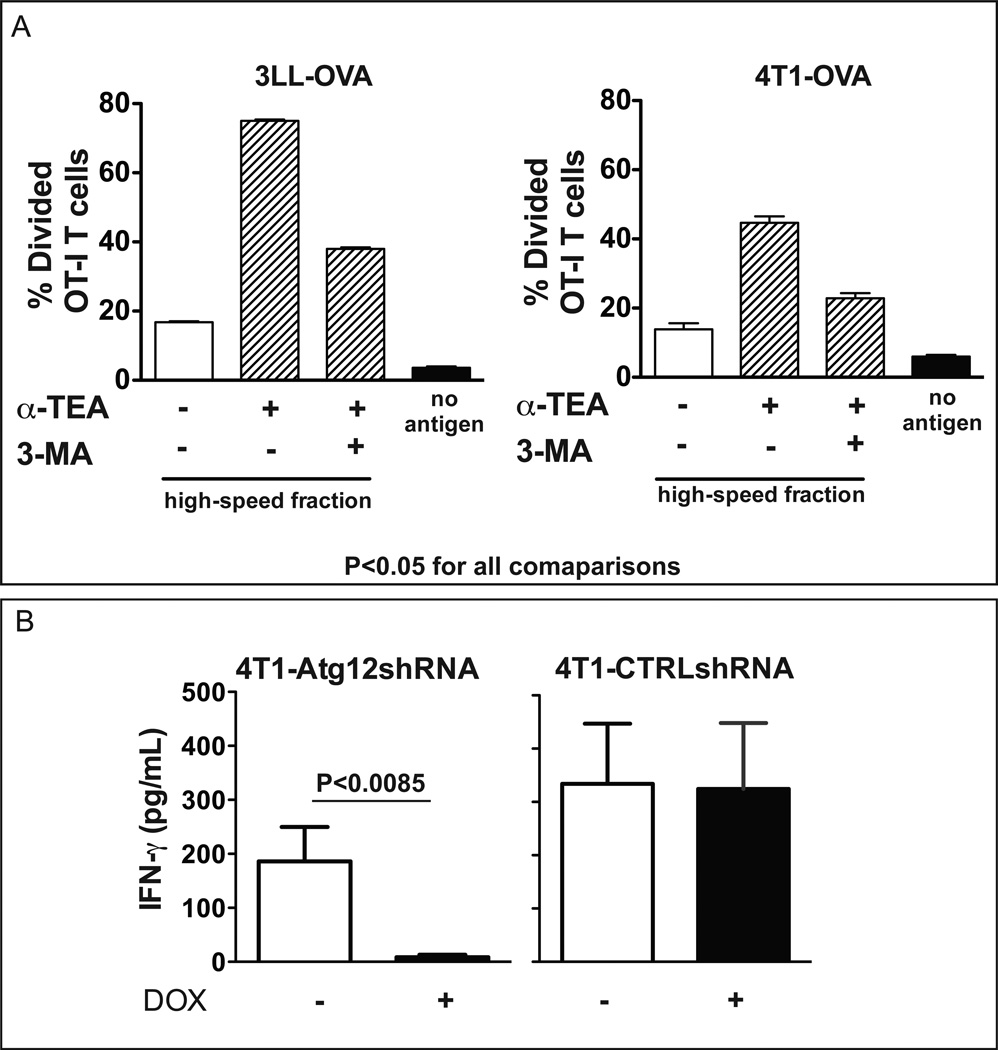
We next confirmed the role of autophagy in cross-presentation of α-TAGS by blocking tumor cell autophagy using 3-Methyladenine (3-MA), which inhibits class III phosphoinositide 3-kinase (PI3K) required for initiation of autophagy. Inhibition of autophagy by 3-MA significantly inhibited OT-I CD8+ T cell activation by α-TAGS obtained from both 3LL and 4T1 tumor cells (Figure 6A). To further consolidate the role of autophagy in CD8+ T cell activation, α-TAGS was collected from doxycycline-treated 4T1-Atg12shRNA tumor cells in which the autophagy pathway was inhibited by RNA interference-mediated knockdown of Atg12. Activation of CT-TCR CD8+ T, that are specific for the AH1 peptide derived from the envelope (GP70) of an endogenous ecotropic murine leukemia virus in 4T1 tumor cells (21), by α-TAGS-pulsed DC was monitored by measuring IFN-γ levels in the culture supernatant. Inhibition of autophagy by Atg12 gene knockdown dramatically diminished IFN-γ production by CT-CTR CD8+ T cells (Figure 6B). In contrast, CT-CTR CD8+ T cells stimulated with DCs pulsed with α-TAGS isolated from cells expressing non-silencing shRNA (4T1-CTRLshRNA) stimulated equivalent amounts of IFN-γ in the absence or presence of doxycycline (Figure 6B).
Figure 6. Inhibition of autophagy before α-TEA treatment reduces cross-presentation by α-TAGS.
(A) The autophagy pathway was inhibited in 3LL-OVA and 4T1-OVA cells by overnight incubation with the autophagy inhibitor 3-Methyladenine (3-MA). Subsequently, α-TAGS was prepared and a cross-presentation assay was performed. Mean percent divided OT-I CD8+ T cells ± SD is shown from 3 independent experiments. (B) α-TAGS was collected from 4T1-Atg12shRNA cells in which the autophagy pathway was inhibited by doxycycline-induced shRNA knockdown of Atg12 protein. α-TAGS was pulsed onto DCs. The DCs were washed and co-incubated for 48 h with CT-TCR T cells specific for the 4T1 endogenous AH1 antigen. α-TAGS from 4T1-CTRLshRNA cells that express a non-specific shRNA were included as control. IFN-γ in the supernatant was detected by ELISA and mean IFN-γ levels ± SD from two independent experiments are shown.
Anti-tumor efficacy of α-TAGS-pulsed DC vaccination
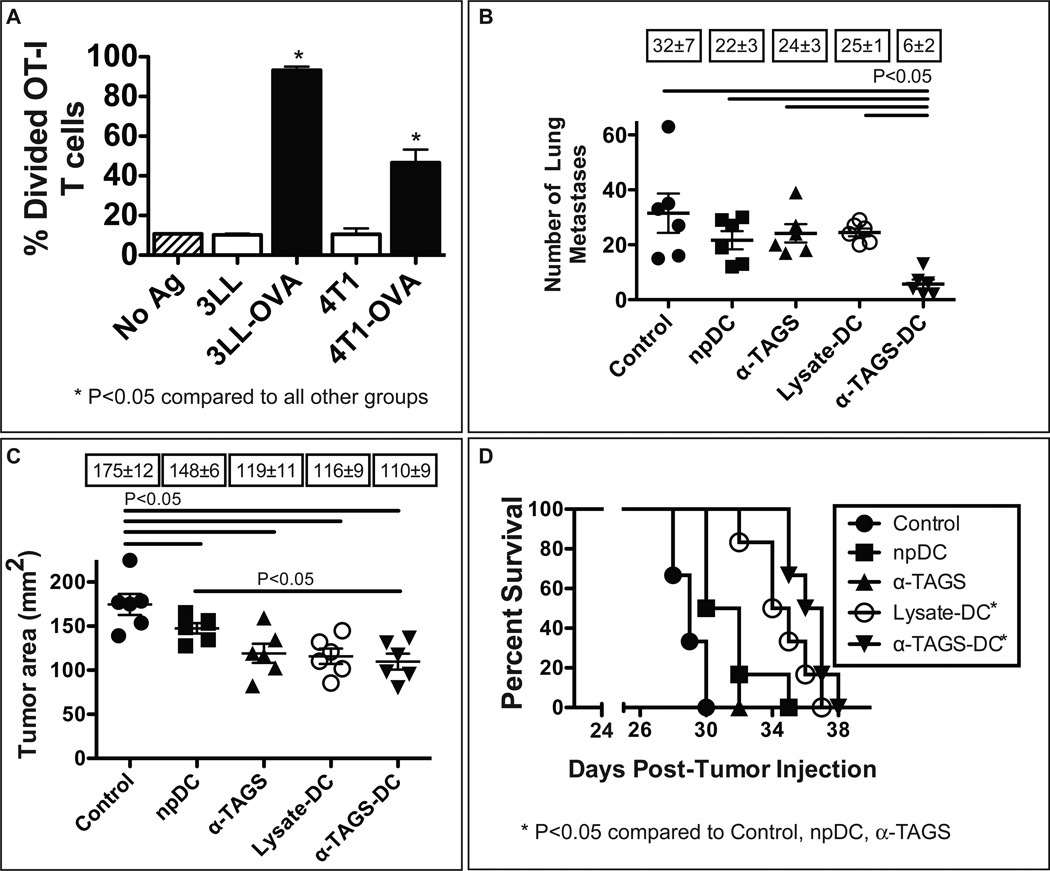
Following our findings that α-TEA induces tumor cell autophagy and that autophagosomes derived from α-TEA-treated tumor cells are antigen carriers that stimulate cross-presentation in vitrowe wanted to first evaluate whether α-TAGS can induce antigen-specific CD8+ T cell proliferation in vivo. We found that α-TAGS isolated from 3LL-OVA and 4T1-OVA cells that was injected into the inguinal lymph nodes of naïve mice induced proliferation of over 90% and 50% of adoptively transferred OT-I CD8+ T cells respectively, suggesting α-TAGS was efficiently cross-presented to CD8+ T cells in vivo (Figure 7A). We next determined the impact of α-TAGS-pulsed DC (α-TAGS-DC) vaccination on the growth of experimental 3LL lung metastases. Mice were injected intravenously with 3LL tumor cells, received α-TAGS-DC vaccinations 3 days later, and visible lung metastases were enumerated 28 day post-tumor injection (Figure 7B). The results show that α-TAGS-DC vaccination significantly reduced the number of lung metastases ~5-fold (P<0.05) compared to no treatment (Control). Furthermore, the number of lung metastases in the α-TAGS-DC vaccination group was significantly lower (P<0.05) compared to the DC alone (npDC), α-TAGS alone or DC pulsed with freeze-thaw tumor cell lysate (Lysate-DC) groups. After these encouraging results, we wanted to determine if α-TAGS-DC vaccination is also efficacious against established tumors. For this purpose, 4T1 mammary tumor-bearing mice received three subcutaneous α-TAGS-DC injections. The data (Figure 7C) show that α-TAGS-DC treatment reduced the mean tumor size by 37% to 110 mm2 from 175 mm2 mean tumor size in untreated mice. Lysate-DC and α-TAGS vaccination resulted in 34% and 32% tumor size reduction respectively; npDC vaccination had a minimal effect on tumor growth (148 mm2). In addition, α-TAGS-DC treatment significantly (P<0.05) prolonged the median survival to 37 days post-tumor injection compared to 29 days median survival of control mice (Figure 7D). Injections of npDC or α-TAGS alone had no effect on survival (median survival in both groups was 31 days). Lysate-DC vaccination prolonged the median survival to 35 days.
Figure 7. α-TAGS induces CD8+ T cell immune responses in vivo and DC pulsed with α-TAGS show therapeutic anti-tumor effect.
(A) In vivo cross-presentation of α-TAGS antigens. α-TAGS from 3LL-OVA, 3LL, 4T1-OVA, and 4T1 tumor cells were injected into both inguinal lymph nodes of naïve C57BL/6 or BALB/c mice. CFSE-labeled Thy1.1+ OT-I splenocytes were adoptively transferred by i.v. injection and proliferation of OT-I CD8+ T cells in the lymph nodes was analyzed by flow cytometry 5 days post-T cell transfer. Data represent mean ± SEM of 3 mice per group. (B) To determine the effect of DC pulsed with α-TAGS on 3LL lung metastases, C57BL/6 mice were i.v. injected with 3LL tumor cells. Three days later, the mice received s.c vaccination with un-pulsed DC (npDC), DC pulsed with α-TAGS derived from 3LL tumor cells (α-TAGS-DC), DC pulsed with freeze-thaw tumor cell lysate (Lysate-DC) or α-TAGS alone. Numbers of lung metastases of individual mice (n = 6/group) on day 28 post-tumor injection. Boxed numbers indicate means of lung metastases ± SEM. (C) To assess the effect of DC pulsed with α-TAGS on 4T1 tumor growth, BALB/c mice with established 4T1 mammary tumors (~13 mm2), received s.c. injections of npDC, α-TAGS-DC, Lysate-DC or α-TAGS alone on days 7, 9 and 11 post-tumor injection. Individual tumor areas on day 28 post-tumor injection. Boxed numbers indicate mean tumor areas ± SEM. (D) Kaplan-Meier analysis of survival.
DISCUSSION
In this study we investigated α-TEA-induced tumor cell autophagy and its role in the enhancement of the anti-tumor immune response. We demonstrate for the first time that in addition to its well-described induction of tumor cell apoptosis, α-TEA induces autophagy in lung and mammary tumor cells and that autophagy-mediated cell death is partially responsible for the cytotoxic properties of α-TEA.
Autophagy is considered a pro-survival mechanism for recycling damaged organelles and proteins by cells undergoing various forms of stress including nutrient deprivation. However, recent evidence suggests that under certain circumstances and depending on the stressor, extensive and irreversible cellular damage can occur culminating in autophagic cell death (32, 33). The contribution of autophagy to cell death has been documented in studies in which inhibition of the autophagy pathway genes, Atg5, Atg6 or Atg7 by RNAi resulted in increased cell survival (34–36). Our finding that shRNA knockdown of Atg12 increased the clonogenicity of α-TEA-treated tumor cells is supportive of the role of autophagy in α-TEA-mediated tumor cell death.
Recently, it has become increasingly clear that autophagy and apoptosis are not independently regulated and that cross-talk may exist between both pathways (14, 15, 37). The simultaneous induction of autophagy and apoptosis by α-TEA in our study has also been reported for other anti-cancer drugs including paclitaxel (38), mitoxantrone (39), oxaliplatin (39), melphalan (38), arsenic trioxide (40), imatinib (41), and the proteasome inhibitor MG132 (42). In our study, we found that autophagy preceded apoptosis during α-TEA treatment as has been previously reported for other stress triggers including growth factor starvation and apoptosis-inducing agents (27, 43). However, unlike previous reports, which demonstrated autophagy inhibition in association with progressive apoptosis (27, 43), we showed that α-TEA-induced autophagy remained high over time. The finding in our study that blockade of apoptosis using the pan-caspase inhibitor, zVAD-fmk further increased the formation of LC3-II punctates in α-TEA-treated tumor cells, suggested cross-talk between both pathways during α-TEA-mediated tumor cell cytotoxicity. Interestingly, in our study, α-TEA treatment resulted in only a very modest decrease in Beclin-1 protein without detectable Beclin-1 cleavage products (data not shown). In the studies by Wirawan et al. (27) and Zhu et al. (43) where progressive apoptosis correlated with autophagy inhibition, caspase-dependent cleavage of Beclin-1 was observed. The discrepancy between their findings and ours could be due, in part, to differences in the cell lines and stress triggers used in the studies. Our results suggest that autophagy and apoptosis induction by α-TEA occur in parallel and that Beclin-1 is likely not involved in regulation of cross-talk between both pathways.
We also show here for the first time that the autophagosome-enriched fraction (α-TAGS), generated by treating tumor cells in vitro with α-TEA, was an efficient tumor antigen carrier. DC pulsed with α-TAGS derived from OVA-expressing tumor cells stimulated antigen cross-presentation evidenced by enhanced proliferation of OVA-specific OT-I CD8+ T cells. This CD8+ T cell activation could be partially blocked with 3-MA suggesting that autophagy is essential for efficient antigen cross-presentation. Cross-presentation of an endogenous tumor associated antigen (GP70) was also induced by α-TAGS and was inhibited by short hairpin RNA knockdown of Atg12, a gene product essential for autophagy. Thus, we show in our study by both pharmacological and genetic inhibition of autophagy, that α-TEA-induced autophagy is necessary for α-TAGS-mediated stimulation of cross-presentation. The improved antigen cross-presentation by α-TAGS-pulsed DC (α-TAGS-DC) observed in vitrowas also evident in vivo as intra-nodally injected α-TAGS derived from 3LL-OVA and 4T1-OVA tumor cells induced proliferation of adoptively transferred OT-I CD8+ T cells, suggesting that α-TAGS-associated tumor antigens are cross-presented in vivo. More importantly, α-TAGS-DC vaccination was significantly more efficacious than tumor lysates-pulsed DC (Lysate-DC) at reducing the incidence of experimental lung metastasis in the 3LL tumor model. In contrast, α-TAGS-DC vaccination was less effective at suppressing established 4T1 tumors and its ability to prolong animal survival was comparable to that of Lysate-DC that have long been recognized to induce tumor specific anti-tumor immune responses (44). Taken together, these results suggest that tumor antigens in α-TAGS were cross-presented in vivo to stimulate an anti-tumor immune response. These results are also in agreement with our earlier report (2) showing that α-TEA-treatment of tumor-bearing mice enhanced the anti-tumor immune response and resulted in increased cytokine secretion and tumor specific cytotoxicity and that the anti-tumor efficacy of α-TEA treatment was partially dependent on a functional T cell response (2).
It has recently been reported that cytoreductive chemotherapeutics such as gemcitabine (45) and doxorubicin (46) can have immune-stimulatory effects and the emerging consensus is that these effects depend on the specific cytotoxic mechanisms of a given drug (47). Here we provide evidence for the first time that α-TEA induces tumor cell autophagy and that the autophagosome-enriched fraction stimulated tumor-specific cross-presentation that was dependent on a functional autophagy pathway. Due to its ability to stimulate autophagy and enhance cross-priming of CD8+ T cells, α-TEA chemotherapy may have clinical relevance as a new strategy for generating tumor-associated antigens for cross-presentation by endogenous DC. Combining α-TEA therapy with immune modulators such as anti-CTLA-4, anti-OX40 or anti-4-1BB, which promote T cell expansion, effector function, and survival could be an effective strategy for enhancing the anti-tumor response. Some of these agents either have already gained Food and Drug Administration approval [anti-CTLA-4 (48)] or are in various stages of clinical testing [anti-OX40 (49) (NCT01416844), anti-4-1BB (50) (NCT01471210), anti-programmed death ligand-1 (NCT00729664)], anti-programmed death-1 (NCT00730639)] and could potentially be more effective when combined with α-TEA. Plans are underway at our institute to test the safety and tolerability of α-TEA in a first-in-human phase I trial in patients with advanced cancer.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Edwin Walker and the staff of the Immune Monitoring Laboratory at the EACRI for their technical assistance. We thank Dr. Eric Barklis, Mike Webb and the Oregon Health and Science University Electron Microscopy Core Facility for electron microscopy imaging.
GRANT SUPPORT
This study was supported by grants 5R01CA120552 to ETA, R01CA10724375 and R21CA141278 to H.-M.H., 2R01 CA111421 and 1R01CA150925 to AT from the National Institutes of Health.
REFERENCES
- 1.Kline K, Lawson KA, Yu W, Sanders BG. Vitamin E and breast cancer prevention: current status and future potential. Journal of Mammary Gland Biology and Neoplasia. 2003;8:91–102. doi: 10.1023/a:1025787422466. [DOI] [PubMed] [Google Scholar]
- 2.Hahn T, Jagadish B, Mash EA, Garrison K, Akporiaye ET. Alpha-tocopheryloxyacetic acid: a novel chemotherapeutic that stimulates the anti-tumor immune response. Breast Cancer Research. 2011;13:R4. doi: 10.1186/bcr2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimmick GG, Bell RA, Bostick RM. Vitamin E and breast cancer: a review. Nutrition and Cancer. 1997;27:109–117. doi: 10.1080/01635589709514511. [DOI] [PubMed] [Google Scholar]
- 4.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA : the journal of the American Medical Association. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawson KA, Anderson K, Menchaca M, Atkinson J, Sun L, Knight V, et al. Novel vitamin E analogue decreases syngeneic mouse mammary tumor burden and reduces lung metastasis. Molecular Cancer Therapeutics. 2003;2:437–444. [PubMed] [Google Scholar]
- 6.Jia L, Yu W, Wang P, Sanders BG, Kline K. In vivo and in vitro studies of anticancer actions of alpha-TEA for human prostate cancer cells. Prostate. 2008;68:849–860. doi: 10.1002/pros.20750. [DOI] [PubMed] [Google Scholar]
- 7.Hahn T, Szabo L, Gold M, Ramanathapuram L, Hurley LH, Akporiaye ET. Dietary Administration of the Proapoptotic Vitamin E Analogue {alpha}-Tocopheryloxyacetic Acid Inhibits Metastatic Murine Breast Cancer. Cancer Research. 2006;66:9374–9378. doi: 10.1158/0008-5472.CAN-06-2403. [DOI] [PubMed] [Google Scholar]
- 8.Hahn T, Fried K, Hurley LH, Akporiaye ET. Orally active {alpha}-tocopheryloxyacetic acid suppresses tumor growth and multiplicity of spontaneous murine breast cancer. Molecular Cancer Therapeutics. 2009;8:1570–1578. doi: 10.1158/1535-7163.MCT-08-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kline K, Yu W, Sanders BG. Vitamin E: mechanisms of action as tumor cell growth inhibitors. Journal of Nutrition. 2001;131:161S–163S. doi: 10.1093/jn/131.1.161S. [DOI] [PubMed] [Google Scholar]
- 10.Neuzil J, Tomasetti M, Mellick AS, Alleva R, Salvatore BA, Birringer M, et al. Vitamin E analogues: a new class of inducers of apoptosis with selective anti-cancer effects. Current Cancer Drug Targets. 2004;4:355–372. doi: 10.2174/1568009043332943. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong JS. Mitochondrial membrane permeabilization: the sine qua non for cell death. Bioessays. 2006;28:253–260. doi: 10.1002/bies.20370. [DOI] [PubMed] [Google Scholar]
- 12.Jia L, Yu W, Wang P, Li J, Sanders BG, Kline K. Critical roles for JNK, c-Jun, and Fas/FasL-Signaling in vitamin E analog-induced apoptosis in human prostate cancer cells. Prostate. 2008;68:427–441. doi: 10.1002/pros.20716. [DOI] [PubMed] [Google Scholar]
- 13.Wang P, Yu W, Hu Z, Jia L, Iyer VR, Sanders BG, et al. Involvement of JNK/p73/NOXA in vitamin E analog-induced apoptosis of human breast cancer cells. Mol Carcinog. 2008;47:436–445. doi: 10.1002/mc.20400. [DOI] [PubMed] [Google Scholar]
- 14.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 15.Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966–975. doi: 10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- 16.Eisenberg-Lerner A, Kimchi A. The paradox of autophagy and its implication in cancer etiology and therapy. Apoptosis. 2009;14:376–391. doi: 10.1007/s10495-008-0307-5. [DOI] [PubMed] [Google Scholar]
- 17.Eskelinen EL. New insights into the mechanisms of macroautophagy in mammalian cells. Int Rev Cell Mol Biol. 2008;266:207–247. doi: 10.1016/S1937-6448(07)66005-5. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Wang LX, Yang G, Hao F, Urba WJ, Hu HM. Efficient cross-presentation depends on autophagy in tumor cells. Cancer Research. 2008;68:6889–6895. doi: 10.1158/0008-5472.CAN-08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munz C. Enhancing immunity through autophagy. Annual Review of Immunology. 2009;27:423–449. doi: 10.1146/annurev.immunol.021908.132537. [DOI] [PubMed] [Google Scholar]
- 20.Kepp O, Tesniere A, Schlemmer F, Michaud M, Senovilla L, Zitvogel L, et al. Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis. 2009;14:364–375. doi: 10.1007/s10495-008-0303-9. [DOI] [PubMed] [Google Scholar]
- 21.Jordan KR, McMahan RH, Oh JZ, Pipeling MR, Pardoll DM, Kedl RM, et al. Baculovirus-infected insect cells expressing peptide-MHC complexes elicit protective antitumor immunity. Journal of Immunology. 2008;180:188–197. doi: 10.4049/jimmunol.180.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasband WS. ImageJ. 1997-2011 [cited; Available from: http://imagej.nih.gov/ij/
- 23.He Y, Pimenov AA, Nayak JV, Plowey J, Falo LD, Jr, Huang L. Intravenous injection of naked DNA encoding secreted flt3 ligand dramatically increases the number of dendritic cells and natural killer cells in vivo. Human Gene Therapy. 2000;11:547–554. doi: 10.1089/10430340050015734. [DOI] [PubMed] [Google Scholar]
- 24.Kobie JJ, Wu RS, Kurt RA, Lou S, Adelman MK, Whitesell LJ, et al. Transforming growth factor beta inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Research. 2003;63:1860–1864. [PubMed] [Google Scholar]
- 25.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiwary R, Yu W, Sanders BG, Kline K. alpha-TEA cooperates with MEK or mTOR inhibitors to induce apoptosis via targeting IRS/PI3K pathways. British Journal of Cancer. 2011;104:101–109. doi: 10.1038/sj.bjc.6606019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wirawan E, Vande Walle L, Kersse K, Cornelis S, Claerhout S, Vanoverberghe I, et al. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 2010;1:e18. doi: 10.1038/cddis.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho DH, Jo YK, Hwang JJ, Lee YM, Roh SA, Kim JC. Caspase-mediated cleavage of ATG6/Beclin-1 links apoptosis to autophagy in HeLa cells. Cancer Letters. 2009;274:95–100. doi: 10.1016/j.canlet.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Thorburn A. Studying autophagy's relationship to cell death. Autophagy. 2008;4:391–394. doi: 10.4161/auto.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Wang LX, Pang P, Twitty C, Fox BA, Aung S, et al. Cross-presentation of tumor associated antigens through tumor-derived autophagosomes. Autophagy. 2009;5:576–577. doi: 10.4161/auto.5.4.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munz C. Autophagy and antigen presentation. Cell Microbiol. 2006;8:891–898. doi: 10.1111/j.1462-5822.2006.00714.x. [DOI] [PubMed] [Google Scholar]
- 32.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gozuacik D, Kimchi A. Autophagy and cell death. Curr Top Dev Biol. 2007;78:217–245. doi: 10.1016/S0070-2153(06)78006-1. [DOI] [PubMed] [Google Scholar]
- 34.Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nature Cellular Biology. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 36.Pyo JO, Jang MH, Kwon YK, Lee HJ, Jun JI, Woo HN, et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. Jpirnal of Biological Chemistry. 2005;280:20722–20729. doi: 10.1074/jbc.M413934200. [DOI] [PubMed] [Google Scholar]
- 37.Djavaheri-Mergny M, Maiuri MC, Kroemer G. Cross talk between apoptosis and autophagy by caspase-mediated cleavage of Beclin 1. Oncogene. 2010;29:1717–1719. doi: 10.1038/onc.2009.519. [DOI] [PubMed] [Google Scholar]
- 38.Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE, et al. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010;29:5299–5310. doi: 10.1038/onc.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 40.Qian W, Liu J, Jin J, Ni W, Xu W. Arsenic trioxide induces not only apoptosis but also autophagic cell death in leukemia cell lines via up-regulation of Beclin-1. Leuk Res. 2007;31:329–339. doi: 10.1016/j.leukres.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Basciani S, Vona R, Matarrese P, Ascione B, Mariani S, Cauda R, et al. Imatinib interferes with survival of multi drug resistant Kaposi's sarcoma cells. FEBS Lett. 2007;581:5897–5903. doi: 10.1016/j.febslet.2007.11.069. [DOI] [PubMed] [Google Scholar]
- 42.Yang W, Monroe J, Zhang Y, George D, Bremer E, Li H. Proteasome inhibition induces both pro- and anti-cell death pathways in prostate cancer cells. Cancer Letters. 2006;243:217–227. doi: 10.1016/j.canlet.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 43.Zhu Y, Zhao L, Liu L, Gao P, Tian W, Wang X, et al. Beclin 1 cleavage by caspase-3 inactivates autophagy and promotes apoptosis. Protein Cell. 2010;1:468–477. doi: 10.1007/s13238-010-0048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fields RC, Shimizu K, Mule JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proceedings of the National Academy of Sciences USA. 1998;95:9482–9487. doi: 10.1073/pnas.95.16.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Research. 2003;63:4490–4496. [PubMed] [Google Scholar]
- 46.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. Journal of Experimental Medicine. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140:798–804. doi: 10.1016/j.cell.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 48.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nature Reviews Immunology. 2004;4:420–431. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 50.Cheuk AT, Mufti GJ, Guinn BA. Role of 4-1BB:4-1BB ligand in cancer immunotherapy. Cancer Gene Therapy. 2004;11:215–226. doi: 10.1038/sj.cgt.7700670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.