Abstract
Background
Dental amalgam is approximately 50% metallic mercury and releases mercury vapor into the oral cavity, where it is inhaled and absorbed. Maternal amalgams expose the developing fetus to mercury vapor. Mercury vapor can be toxic, but uncertainty remains whether prenatal amalgam exposure is associated with neurodevelopmental consequences in offspring.
Objective
To determine if prenatal mercury vapor exposure from maternal dental amalgam is associated with adverse effects to cognition and development in children.
Methods
We prospectively determined dental amalgam status in a cohort of 300 pregnant women recruited in 2001 in the Republic of Seychelles to study the risks and benefits of fish consumption. The primary exposure measure was maternal amalgam surfaces present during gestation. Maternal occlusal points were a secondary measure. Outcomes were the child’s mental (MDI) and psychomotor (PDI) developmental indices of the Bayley Scales of Infant Development-II (BSID-II) administered at 9 and 30 months. Complete exposure, outcome, and covariate data were available on a subset of 242 mother-child pairs.
Results
The number of amalgam surfaces was not significantly (p>0.05) associated with either PDI or MDI scores. Similarly, secondary analysis with occlusal points showed no effect on the PDI or MDI scores for boys and girls combined. However, secondary analysis of the 9 month MDI was suggestive of an adverse association present only in girls.
Conclusion
We found no evidence of an association between our primary exposure metric, amalgam surfaces, and neurodevelopmental endpoints. Secondary analyses using occlusal points supported these findings, but suggested the possibility of an adverse association with the MDI for girls at 9 months. Given the continued widespread use of dental amalgam, we believe additional prospective studies to clarify this issue are a priority.
Keywords: Amalgam, mercury, methylmercury, prenatal, neurodevelopment, co-exposure
1. Introduction
The safety of dental amalgam has been the subject of heated debate since its introduction into the practice of dentistry over a century and a half ago. Dental amalgam is approximately 50% metallic mercury by weight and when present in the oral cavity, exposes an individual to mercury vapor (Hg0) released from the restoration’s surface (WHO, 1991). For individuals not occupationally exposed to Hg0, amalgam restorations are the primary source of exposure. Chronic exposure to elevated levels of Hg0 is known to result in neurotoxicity consisting of various sensory, motor, cognitive and personality disturbances. Presently, the lowest level at which such associations occur is unknown. Reviews focused on low level Hg0 exposure from dental amalgam restorations suggest that adverse health effects are unlikely to occur in adults (Brownawell et al., 2005; CETS, 1997; European Commission, 2004; Health Canada, 2004; WHO, 1997). However, the most recent extensive review of amalgam safety concluded that there was insufficient evidence to evaluate risk from in utero exposure to Hg0 from maternal amalgam, and from co-exposure to Hg0 and methylmercury (MeHg) (Brownawell et al., 2005). This gap in knowledge continues to be highlighted by numerous agencies (European Commission, 2004; FDA, 2009; Health Canada, 2004).
Against this backdrop, the presence and placement of dental amalgam restorations remain substantial in all age groups worldwide, including children and women of child-bearing age. There are clear benefits to the use of dental amalgams such as their low cost, durability, and ease of placement. In 2009 the FDA estimated nearly 900 million existing amalgam restorations were in place in the US (FDA, 2009). Moreover, current utilization of amalgam by US practitioners is still considerable, especially for restoration of posterior teeth (DeRouen et al., 2010; Makhija et al., 2011; Nascimento et al., 2010). Estimates suggest 50 million additional amalgam restorations were placed in the US in 2009 (about one third of restorations placed), with approximately 1.8 million amalgams placed in pregnant and lactating women (FDA, 2009). These amalgam restorations are in addition to the tens of millions of amalgams already in place in this potentially sensitive population.
Mercury vapor readily crosses the placental barrier and an increased number of maternal amalgams has been associated with elevated levels of mercury in the placenta (Ask et al., 2002), amniotic fluid (Luglie et al., 2005), cord blood (Palkovicova et al., 2008), the kidney and liver of the fetus (Drasch et al., 1994), and the kidney and cerebral cortex of older infants (Drasch et al., 1994). Despite concerns, there remains a paucity of scientific data to adequately assess whether there are health risks to the developing human fetus from prenatal exposure to Hg0 from maternal dental amalgams. We recently reported an adverse association of maternal amalgams present during gestation on Seychellois boys’ performance at 66 months of age on the Letter-Word Identification subtest of the Woodcock-Johnson Tests of Achievement (Watson et al., 2011). Uncertainties associated with the retrospective nature of the exposure assessment and inconsistencies in the findings, however, limited our ability to draw firm conclusions.
Besides Hg0, exposure to MeHg is common and occurs principally through fish consumption (WHO, 1991). Experimental studies in animals have suggested that co-exposure to Hg0 and MeHg resulted in more adverse findings than either exposure alone (Fredriksson et al., 1996). These findings led us to hypothesize that a study of the Seychelles Child Development Nutrition Study (SCDNS) might be more likely to identify adverse Hg0 associations if any were present. Consequently, we prospectively studied mothers and children enrolled in the SCDNS. The original aim of this longitudinal cohort study was to examine potential confounding effects of beneficial nutrients associated with maternal fish consumption against possible adverse effects of prenatal MeHg exposure.
2. Methods
2.1 Subjects
The SCDNS Cohort is a well-characterized group of mother-infant pairs residing in the Republic of Seychelles (Bonham et al., 2008; Davidson et al., 2008; Strain et al., 2008). The study is prospective, double-blind, and longitudinal. Enrollment, inclusion, and exclusion criteria were reported previously (Davidson et al., 2008). Data on amalgam, covariates and at least one outcome were available on 242 mothers. The study was reviewed and approved by the institutional review boards of the University of Rochester, Rochester, NY, and the Ministry of Health, Republic of Seychelles.
2.2 Determination of Maternal Dental Amalgam Status (Hg0 Exposure)
The total number of amalgam surfaces present in the mother during gestation was the primary metric of Hg0 exposure. We determined this by examining the mothers shortly before or just after the birth of her child, and reviewing the historic dental records maintained by the national dental service. The total amalgam surfaces metric has been widely used in numerous studies (Bellinger et al., 2006; Bellinger et al., 2007; DeRouen et al., 2006; Factor-Litvak et al., 2003; Luglie et al., 2005; Kingman et al., 1998; Kingman et al., 2005; Maserejian et al., 2008; Pesch et al., 2002) and includes all surfaces of amalgam available for release of Hg0. The first author independently examined approximately 5% of subjects for amalgam status reliability and there was 100% agreement.
We also determined a secondary metric, an occlusal point score (Watson et al., 2011). Several studies have demonstrated significantly enhanced release of Hg0 from amalgams during brushing and chewing, which likely results from perturbation of the occlusal surface of the amalgam restoration (Abraham et al., 1984; Gay et al., 1979; Sallsten et al., 1996; Vimy and Lorscheider, 1985). To further refine occlusal exposure, we estimated the area of each occlusal surface by assigning a score of 1 point for small size occlusal amalgams such as pits, 2 points for medium size such as on premolars, and 3 points for large size on molars, based on a modification of the ‘amalgam points’ scoring system developed by Olstad and colleagues (Olstad et al., 1987).
2.3 Metrics of Other Environmental Toxin Exposures
Prenatal MeHg exposure was determined by assessing the average concentration of total mercury (THg) in the longest available segment of maternal hair representing growth during gestation. Infant brain THg levels have been shown to correlate well with maternal hair THg (Cernichiari et al., 1995). Exposures to lead and PCBs have been shown to be low in children and mothers in Seychelles (Davidson et al., 1998) and were not assessed in this cohort.
2.4 Developmental Assessment
The primary developmental endpoints were the mental developmental index (MDI) and psychomotor developmental index (PDI) of the Bayley Scales of Infant Development II (BSID-II). The BSID-II was administered at ages 9 and 30 months by specially trained evaluators. Reliabilities for BSID-II testing were high as reported previously (Davidson et al., 2008).
2.5 Maternal Nutrition
Total omega-3 (n-3) and omega-6 (n-6) polyunsaturated fatty acids (PUFA) were measured as total lipids (including phospholipids) in maternal serum samples taken at 28 weeks and at delivery as described previously (Strain et al., 2008).
2.6 Statistical Analysis
The primary analysis used linear regression models to examine the cross-sectional covariate-adjusted relationships between amalgam surfaces and MDI and PDI at 9 and 30 months each in separate models. These models were fit both with and without adjustment for prenatal MeHg, n-3 and n-6 PUFA. Each model was first fit with and then without a sex by amalgam interaction, since we previously found differential associations by sex (Watson et al., 2011). Secondary analyses were carried out using the same set of models with occlusal points replacing amalgam surfaces as the exposure metric.
All regression models were adjusted for covariates known or predicted to influence neurodevelopmental outcomes as in earlier analyses (Strain et al., 2008). These covariates were sex, family status at 9 months (0 if living with both parents, 1 if not), maternal age at birth, birth weight, maternal intelligence [assessed by the Matrices subtest of the Kaufman Brief Intelligence Test (K-BIT)], socioeconomic status (SES) (measured by the Hollingshead Four-Factor SES), and the Pediatric Review of Children’s Environmental Support and Stimulation (PROCESS). All covariates were treated as continuous variables except for sex and family status.
Model assumptions were checked using standard methods, including checking for constant variance, nonlinearity, and normally distributed residuals (Weisberg, 2005). Outliers and influential observations were examined. Checks of model assumptions indicated that the models were reasonable for our data and there were no unduly influential observations or extreme outliers. We used a two tailed p value of <0.05 to determine significance.
3. Results
There were 242 subjects available for analysis. The cohort consisted of 228 of the 229 study subjects reported previously by Davidson et al. (2008) [one subject was missing amalgam data] and there were 14 additional subjects who had adequate covariate data for these analyses. A comprehensive summary of the 242 subjects by sex is given in Table 1. Over 80% of the mothers (196 out of 242) had at least one amalgam restoration present while pregnant (mean 8.5 amalgam surfaces or 13.5 occlusal points among mothers with amalgams). There was a high correlation between amalgam surfaces and occlusal points (r = 0.93). The means of outcomes and covariates by prenatal mercury exposure categories (Hg0 and MeHg) are presented in Table 2. The correlation was very low between amalgam surfaces and prenatal MeHg (r = 0.10), maternal n-3 PUFA (r = 0.03) and maternal n-6 PUFA (r = 0.01). Below, we report detailed results from models which adjust for MeHg and PUFA, however, in all cases, results from models that did not adjust for MeHg and PUFA were similar.
Table 1.
Summary statistics for both sexes, and separately for boys (n=120) and girls (n=122)
| All Subjects | Boys | Girls | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | mean | SD | min | max | mean | SD | mean | SD | P-value | |
| MDI, at 9 months | 239 | 102.86 | 8.32 | 72 | 122 | 102.58 | 8.41 | 103.12 | 8.25 | 0.62 |
| PDI, at 9 months | 238 | 105.78 | 10.27 | 68 | 141 | 104.77 | 9.49 | 106.78 | 10.93 | 0.13 |
| MDI, at 30 months | 241 | 85.08 | 9.54 | 56 | 115 | 83.25 | 8.58 | 86.87 | 10.10 | < 0.001 |
| PDI, at 30 months | 238 | 89.85 | 13.78 | 50 | 123 | 86.05 | 13.09 | 93.53 | 13.47 | < 0.001 |
| Amalgam Surfaces > 0 | 196 | 8.49 | 6.27 | 1 | 28 | 8.65 | 6.98 | 8.35 | 5.57 | 0.74 |
| Occlusal Points > 0 | 196 | 13.49 | 8.61 | 2 | 40 | 13.72 | 9.68 | 13.27 | 7.55 | 0.72 |
| Maternal MeHg (ppm) | 242 | 5.60 | 3.65 | 0.19 | 18.49 | 5.51 | 3.69 | 5.69 | 3.62 | 0.70 |
| Omega-3 PUFA (mg/mL) | 242 | 0.03 | 0.01 | 0.01 | 0.06 | 0.03 | 0.01 | 0.03 | 0.01 | 0.23 |
| Omega-6 PUFA (mg/mL) | 242 | 1.22 | 0.21 | 0.66 | 1.72 | 1.23 | 0.23 | 1.20 | 0.18 | 0.35 |
| Living with 2 parents (%) | 242 | 50 | 50 | 0 | 100 | 48 | 50 | 52 | 50 | 0.44 |
| Maternal age (years) | 242 | 27.82 | 5.95 | 16 | 43 | 27.88 | 6.24 | 27.76 | 5.68 | 0.87 |
| Maternal intelligence (K-BIT) | 242 | 86.26 | 13.96 | 48 | 117 | 85.84 | 14.65 | 86.67 | 13.29 | 0.64 |
| SES (Mod. Hollingshead) | 242 | 33.96 | 10.81 | 13 | 63 | 34.15 | 11.25 | 33.77 | 10.40 | 0.79 |
| Home environment (PROCESS) | 242 | 151.88 | 14.78 | 113 | 190 | 151.73 | 15.11 | 152.03 | 14.51 | 0.88 |
| Child's birth weight (kg) | 242 | 3.24 | 0.49 | 1.65 | 4.45 | 3.33 | 0.48 | 3.14 | 0.48 | < 0.001 |
P- values indicate sex differences. MDI = Mental Developmental Index; PDI = Psychomotor Developmental Index; PROCESS = Pediatric Review of Children’s Environmental Support and Stimulation; K-BIT = Kaufman Brief Intelligence Test; SES = Socioeconomic status; PUFA = polyunsaturated fatty acids
Table 2.
Means of Outcomes and Covariates according to prenatal mercury exposure categories and amalgam status
| Prenatal MeHg Exposure (ppm) | Prenatal Mercury Vapor Exposure Number of Amalgams |
|||||
|---|---|---|---|---|---|---|
| 0–3 | 3–6 | 6–9 | 9+ | 0 | 1+ | |
| Sample Size (n) | 71 | 74 | 52 | 45 | 46 | 196 |
| MDI, at 9 months | 103.87 | 102.65 | 100.67 | 104.07 | 101.40 | 103.20 |
| PDI, at 9 months | 107.15 | 105.33 | 102.78 | 107.73 | 104.83 | 106.01 |
| MDI, at 30 months | 84.89 | 85.20 | 84.87 | 85.44 | 86.56 | 84.74 |
| PDI, at 30 months | 91.33 | 89.43 | 87.94 | 90.44 | 89.07 | 90.04 |
| Omega-3 PUFA (mg/mL) | 0.03 | 0.03 | 0.04 | 0.04 | 0.03 | 0.03 |
| Omega-6 PUFA (mg/mL) | 1.17 | 1.24 | 1.24 | 1.23 | 1.21 | 1.22 |
| Sex (Girl %) | 49 | 47 | 52 | 56 | 43 | 52 |
| Maternal age (years) | 27.72 | 26.68 | 28.44 | 29.16 | 26.37 | 28.16 |
| Child's birth weight (kg) | 3.17 | 3.25 | 3.28 | 3.27 | 3.16 | 3.25 |
| Home environment (PROCESS) | 148.83 | 153.08 | 151.79 | 154.84 | 151.59 | 151.95 |
| Maternal intelligence (K-BIT) | 84.30 | 86.00 | 88.40 | 87.31 | 86.91 | 86.11 |
| Living with 2 parents (%) | 56 | 45 | 46 | 53 | 41 | 52 |
| SES (Mod. Hollingshead) | 33.17 | 34.51 | 34.12 | 34.13 | 30.61 | 34.75 |
Amalgam means are presented as 0 or 1+ rather than separately for points and surfaces since the subjects with no occlusal points are the same as those with no occlusal surfaces. MDI = Mental Developmental Index; PDI = Psychomotor Developmental Index; PROCESS = Pediatric Review of Children’s Environmental Support and Stimulation; K-BIT = Kaufman Brief Intelligence Test; SES = Socioeconomic status; PUFA = polyunsaturated fatty acids
3.1 Primary Analysis with Amalgam Surfaces Metric
3.1.1 Mental Developmental Index
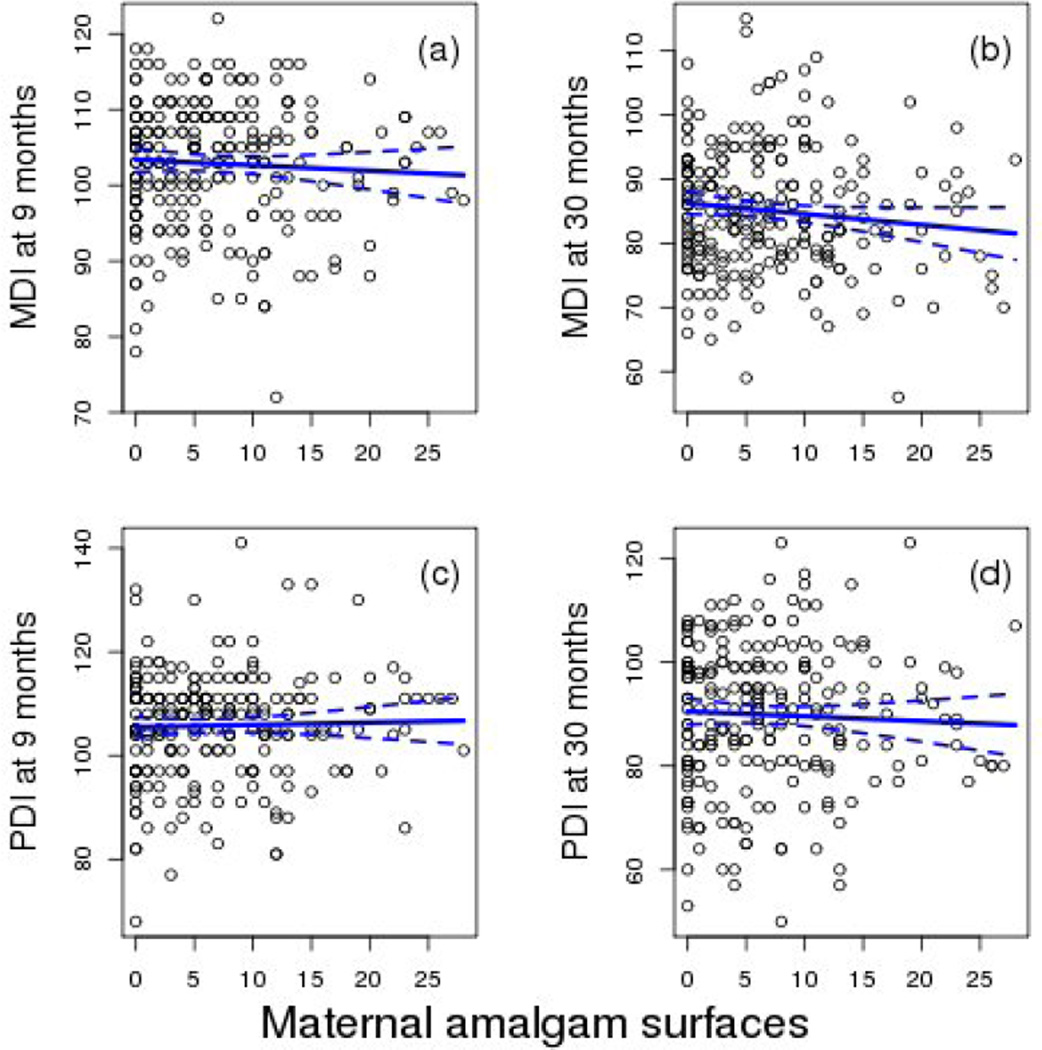
The overall model for MDI at 9 months was not significant and none of the a priori selected covariates were significant predictors of the MDI. At 30 months the model was significant and child’s sex and PROCESS were significant predictors (Table 3). The interaction of sex and amalgam surfaces was not significant at either age. Amalgam surfaces were not a significant predictor at either 9 or 30 months in models that adjusted for MeHg and PUFA [Table 3; Figs. 1(a) and 1(b)].
Table 3.
Adjusted regression coefficients, 95% confidence intervals and p-values from cross sectional models for the Bayley MDI at 9 and 30 months with Amalgam Surfaces.
| 9 Months (model p = 0.182) | 30 Months (model p = 0.0004) | |||||
|---|---|---|---|---|---|---|
| Beta | 95% CI | P-value | Beta | 95% CI | P-value | |
| Primary Exposure Metric | ||||||
| Amalgam Surfaces | −0.07 | (−0.24, 0.1) | 0.408 | −0.17 | (−0.35, 0.02) | 0.075 |
| Covariates | ||||||
| Sex (Girl) | 0.99 | (−1.16, 3.14) | 0.367 | 3.93 | (1.57, 6.29) | 0.001 |
| Maternal MeHg (ppm) | −0.10 | (−0.41, 0.2) | 0.505 | −0.12 | (−0.46, 0.22) | 0.482 |
| Omega-3 (mg/mL) | 49.29 | (−85.41, 183.99) | 0.474 | 13.85 | (−133.19, 160.89) | 0.854 |
| Omega-6 (mg/mL) | −3.43 | (−9.02, 2.15) | 0.230 | −1.95 | (−8.05, 4.14) | 0.530 |
| Maternal age (years) | 0.02 | (−0.17, 0.21) | 0.837 | 0.01 | (−0.2, 0.22) | 0.944 |
| Child's birth weight (kg) | 2.07 | (−0.16, 4.3) | 0.070 | 2.06 | (−0.38, 4.51) | 0.099 |
| Home environment (PROCESS) | 0.06 | (−0.02, 0.14) | 0.120 | 0.19 | (0.1, 0.27) | < 0.001 |
| Maternal intelligence (K-BIT) | 0.04 | (−0.04, 0.12) | 0.319 | 0.01 | (−0.08, 0.1) | 0.783 |
| Living with 2 parents | −1.44 | (−3.56, 0.69) | 0.186 | 0.76 | (−1.57, 3.09) | 0.523 |
| SES (Mod. Hollingshead) | 0.07 | (−0.04, 0.17) | 0.230 | −0.01 | (−0.13, 0.1) | 0.811 |
Model results are based on n=239 (at 9 months), n=241 (at 30 months) subjects. MDI = Mental Developmental Index; PDI = Psychomotor Developmental Index; PROCESS = Pediatric Review of Children’s Environmental Support and Stimulation; K-BIT = Kaufman Brief Intelligence Test; SES = Socioeconomic status; PUFA = polyunsaturated fatty acids
Figure 1.
The association at 9 and 30 months between the total number of maternal amalgam surfaces and the MDI and PDI. Data points are observed values for all subjects and the lines show the slope and 95% confidence interval for the slope adjusted for other model covariates. There were no significant associations with maternal amalgam surfaces and any outcome (Tables 3 and 4). 1(a). Association between amalgam surfaces and MDI at 9 months. 1(b). Association between amalgam surfaces and MDI at 30 months. 1(c). Association between amalgam surfaces and PDI at 9 months. 1(d). Association between amalgam surfaces and PDI at 30 months.
3.1.2 Psychomotor Developmental Index
At 9 and 30 months the models were significant, but the interaction of sex and amalgam surfaces was not significant at either age. Amalgam surfaces for both sexes together were not significantly predictive of the PDI in any model [Table 4; Figs. 1(c) and 1(d)]. At 9 months the child’s sex, birth weight, PROCESS and n-3 PUFA significantly predicted the PDI. At 30 months only the child’s sex significantly predicted the PDI. As reported previously, prenatal n-3 PUFA were positively associated with the PDI at 9 months (Strain et al., 2008).
Table 4.
Adjusted regression coefficients, 95% confidence intervals and p-values from cross sectional models for the Bayley PDI at 9 and 30 months with Amalgam Surfaces.
| 9 Month ( model p = 0.007) | 30 Month ( model p = 0.002) | |||||
|---|---|---|---|---|---|---|
| Beta | 95% CI | P-value | Beta | 95% CI | P-value | |
| Primary Exposure Metric | ||||||
| Amalgam Surfaces | 0.04 | (−0.16, 0.25) | 0.675 | −0.09 | (−0.37, 0.18) | 0.503 |
| Covariates | ||||||
| Sex (Girl) | 3.23 | (0.63, 5.83) | 0.016 | 7.97 | (4.51, 11.43) | <0.001 |
| Maternal MeHg (ppm) | −0.23 | (−0.6, 0.14) | 0.223 | −0.46 | (−0.95, 0.04) | 0.070 |
| Omega-3 (mg/mL) | 211.44 | (48.71, 374.17) | 0.012 | 79.30 | (−136.13, 294.72) | 0.471 |
| Omega-6 (mg/mL) | −1.12 | (−7.87, 5.62) | 0.744 | −4.04 | (−12.95, 4.87) | 0.375 |
| Maternal age (years) | 0.03 | (−0.2, 0.26) | 0.791 | 0.31 | (0, 0.62) | 0.050 |
| Child's birth weight (kg) | 4.70 | (2.01, 7.39) | 0.001 | 1.94 | (−1.65, 5.53) | 0.291 |
| Home environment (PROCESS) | 0.10 | (0.01, 0.2) | 0.040 | 0.05 | (−0.07, 0.18) | 0.424 |
| Maternal intelligence (K-BIT) | −0.03 | (−0.12, 0.07) | 0.596 | 0.03 | (−0.1, 0.16) | 0.680 |
| Living with 2 parents | −0.66 | (−3.23, 1.91) | 0.617 | 1.32 | (−2.1, 4.75) | 0.449 |
| SES (Mod. Hollingshead) | −0.04 | (−0.17, 0.09) | 0.502 | 0.05 | (−0.12, 0.23) | 0.550 |
Model results are based on n=238 (at 9 months), n=238 (at 30 months) subjects. MDI = Mental Developmental Index; PDI = Psychomotor Developmental Index; PROCESS = Pediatric Review of Children’s Environmental Support and Stimulation; K-BIT = Kaufman Brief Intelligence Test; SES = Socioeconomic status; PUFA = polyunsaturated fatty acids
3.2 Secondary Analysis with the Occlusal Points Metric
3.2.1 Mental Developmental Index
As in the primary analysis, the overall model at 9 months was not significant (p=0.08). However, the model did show a significant occlusal points by sex interaction (p=0.05) and an adverse association in girls only (p = 0.03; slope = −0.20, 95% CI: −0.38, −0.02). The overall model at 30 months was significant, but neither the occlusal points by sex interaction (p=0.39), nor the association with occlusal points for both sexes combined were significant (p = 0.07).
3.2.2 Psychomotor Developmental Index
As in the primary analyses, all models were significant, but occlusal points were not a significant predictor of the PDI in any model (data not shown).
4. Discussion
We believe this to be the first prospective study to comprehensively examine neurodevelopmental outcomes in children exposed prenatally to their mother’s dental amalgam. The total number of amalgam surfaces present in the mother’s teeth during gestation was chosen a priori as the primary exposure measure based on its extensive use and validation in prior research studies. We found no statistically significant association between amalgam surfaces and developmental outcomes, and no significant sex by amalgam surfaces interactions.
We also explored the use of a secondary amalgam exposure metric, occlusal points. This metric was chosen based on several reports suggesting occlusal surfaces are the principal source of stimulated release of Hg0 from amalgam restorations and exposure. Compared to total amalgam surfaces (which include the sides and biting surfaces of a restoration), occlusal surfaces (only the biting surfaces) correlate better with Hg0 concentrations in intraoral air (Vimy and Lorscheider, 1985) and with cumulative urinary mercury excretion, the most common biomarker used to monitor Hg0 exposure (Maserejian et al., 2008). The number of occlusal surfaces is also known to be positively correlated with mercury levels in human autopsy brain samples (Eggleston et al., 1987; Guzzi et al., 2006). To roughly take into account the relative size of an occlusal surface, we assigned a point value from 1 to 3. Whether this metric correlates more accurately with Hg0 exposure from amalgam restorations compared to other metrics is not presently known. Using the occlusal points metric of exposure, the overall 9-month MDI model remained non-significant, however, the sex by occlusal points interaction was significant and there was an adverse association only in girls. In the 30 month MDI models (which were significant using either amalgam metric) the adverse association was not significant using either the amalgam surfaces metric (p = 0.08) or occlusal points (p = 0.07).
The disparity of outcomes associated with the different chemical forms of mercury exposure (i.e. Hg0 vs. MeHg) was of interest. Previous analyses of this cohort reported that prenatal MeHg exposure may have an adverse association with the PDI after adjusting for PUFA (Davidson et al., 2008; Strain et al., 2008; Stokes-Riner et al., 2011). Conversely, the current study suggests that prenatal exposure to Hg0 from dental amalgam (occlusal points) might be associated with MDI deficits in girls. Previously described sex differences to the adverse effects of Hg have suggested that males are more sensitive. Disparate sensitivity or selective targeting of specific domains within the developing brain by the different mercury species could explain these differences (Clarkson and Magos, 2006; Counter and Buchanan, 2004). However, humans chronically exposed to subacute levels of Hg0 typically develop symptoms associated with ‘psychomotor’ disturbances, including erethism and tremor, although possible mild cognitive changes and memory loss can manifest (WHO, 1991). Elimination of Hg0 exposure has been shown to reduce tremor and erethism with time, therefore the lack of association of maternal amalgams with the PDI, could be explained by the fact that most of the children’s direct exposure to Hg0 had ended at birth. On the other hand, it is possible that our significant adverse association between Hg0 exposure and the MDI in girls at 9 months of age using the secondary amalgam exposure metric (occlusal points), may represent a spurious finding. This interpretation is supported by the lack of association between occlusal points and the MDI at 30 months, there being no sex interaction at 30 months, and the absence of adverse associations in the combined sexes.
Humans are most commonly exposed to MeHg through the consumption of fish and seafood. Maternal fish intake in SCDNS mothers during gestation averaged 76g/day (Bonham et al., 2009), resulting in mean prenatal mercury exposure of 5.6 ppm. This level of exposure is approximately 12 times higher than reported (0.47 ppm) for female participants of childbearing age in the 1999–2000 U.S. National Health and Nutrition Examination Survey (McDowell et al., 2004). Experimental studies on rats suggest concurrent exposure to MeHg and Hg0 elevates total mercury in the brain of the offspring greater than seen with individual exposures (Fredriksson et al., 1996). In another study in rats, both organic and inorganic mercury in the pup brain were strongly influenced by maternal exposure to MeHg, and only in dams with no exposure to MeHg was Hg0 associated with higher pup brain levels (Ishitobi et al., 2010). Prenatal exposure of SCDNS cohort children to elevated levels of MeHg, along with concurrent Hg0 exposure from maternal dental amalgam provided ideal conditions to examine for any potential increased risk of adverse outcomes from co-exposure to both forms of mercury. Contrary to our hypothesis that we would find increased neurodevelopmental risk with co-exposure, MeHg did not confound the effects of Hg0 exposure in any model. One possible explanation for this finding is that consumption of fish is also the primary source of PUFA that are essential for growth and development of the fetal brain (Innis, 2008). Our earlier studies in this cohort suggested that the benefits of PUFA may outweigh or mask any possible adverse effects of prenatal MeHg exposure (Davidson et al., 2008; Strain et al., 2008). However, there is currently no scientific evidence to suggest beneficial PUFA from consumption of fish modify the effects of Hg0 exposure. Our finding of similar associations both in the presence and absence of adjustment for PUFA suggests that PUFA exposure from fish consumption does not confound the effects of Hg0 exposure. Moreover, we found that the beneficial association of n-3 PUFA on PDI at 9 months (Strain et al., 2008) persists when Hg0 exposure is taken into account (Table 4).
While not taken into account in this report, it should be noted that ocean fish consumed in Seychelles are also a rich source of the nutritionally essential element selenium (Se) (Robinson and Shroff, 2004). Diets enriched in Se have been shown to be protective of MeHg toxicity (Ralston, 2008). Optimal intake of Se is required to preserve the activity of selenoenzymes, which play a crucial role in the prevention and reversal of oxidative damage, especially in the brain. Mercury has a high binding affinity for Se, and MeHg in particular has been shown to be an irreversible inhibitor of these protective selenoenzymes (Ralston and Raymond, 2010). The overall lack of confounding of Hg0 by MeHg may therefore be related to the favorable dietary excess of Se to MeHg associated with ocean fish consumption in Seychelles.
Our study has several strengths. It utilized a well-defined cohort, and standard neurodevelopmental assessments. Also, there were significant statistical associations with covariates known to influence child developmental outcomes, suggesting there was adequate power to detect associations if they were present. The study also has limitations. Although the BSID-II is normed and its utility at 9 and 30 months should be equal, in general testing sensitivity increases as children mature. The BSID-II is considered the developmental ‘gold standard’ for this age group, its predictive validity to IQ and development at later ages is limited (Aylward, 2004).
In our previous retrospective study of maternal dental amalgam in a different Seychellois cohort at 66 months age, we found no association of amalgam surfaces with any of six neurodevelopmental outcomes (Watson et al., 2011). However, using maternal occlusal points in that study we found one adverse association in boys on the Woodcock-Johnson (W-J) Letter Word Identification test and beneficial associations on girl’s test scores for the General Cognitive Index (GCI) of the McCarthy Scales of Children’s Abilities, total Preschool Language Scale, and the W-J Applied Problems subtest. These inconsistencies, the retrospective determination of maternal amalgam status during gestation, and the statistical probability of chance findings given the multitude of comparisons led us to conclude that those data did not support the hypothesis that prenatal dental amalgam was associated with adverse neurodevelopmental outcomes.
While the findings of this study are intriguing, we do not consider them confirmatory of an adverse association of maternal dental amalgam. Further evaluation of these children at a later age, when the sensitivity of the testing is increased, may provide insight regarding the consistency and persistence of any associations. Clearly, comparable studies on larger cohorts of children are also warranted.
5. Conclusion
In this prospective study of prenatal vapor exposure we found no evidence for an association between our primary exposure metric, amalgam surfaces, and neurodevelopmental endpoints. However, secondary analyses using occlusal points as the exposure metric suggested an adverse association with the MDI at 9 months in girls only. Given the continued widespread use of dental amalgam, we believe additional prospective studies to clarify this issue are a priority.
Acknowledgements
This research was supported by NIEHS and NIDCR (R01-ES-015578, R01-ES-010219, P30-ES-001247and T32-ES-007271) and by the Ministry of Health, Victoria, Mahé, Republic of Seychelles. The funding organizations did not have a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. We are especially grateful to members of the Oral Health Directorate, Ministry of Health, Seychelles (Elisabeth Arissol, Marie Helene Dogley, Agnes Elizabeth, Helena Elizabeth, Kathleen Ernesta, Dr. Harold Pothin, and Dr. Eric Van Hollebeke). At the University of Rochester, we thank Jean Reeves, MS, the senior health project coordinator; Joanne Janciuras, AS, the senior programmer; and Margaret Langdon, BS, for technical assistance in mercury analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare there are no conflicts of interest.
References
- Abraham JE, Svare CW, Frank CW. The effect of dental amalgam restorations on blood mercury levels. J Dent Res. 1984;63(1):71–73. doi: 10.1177/00220345840630011801. [DOI] [PubMed] [Google Scholar]
- Anonymous. Reports from the conseil d'evaluation des technologies de la sante du quebec (CETS). The safety of dental amalgam: A state-of-the-art review. Int J Technol Assess Health Care. 1997;13(4):639–642. [PubMed] [Google Scholar]
- Ask K, Akesson A, Berglund M, Vahter M. Inorganic mercury and methylmercury in placentas of Swedish women. Environ Health Perspect. 2002;110(5):523–526. doi: 10.1289/ehp.02110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward GP. Prediction of function from infancy to early childhood: Implications for pediatric psychology. J Pediatr Psychol. 2004;29(7):555–564. doi: 10.1093/jpepsy/jsh057. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Trachtenberg F, Barregard L, Tavares M, Cernichiari E, Daniel D, et al. Neuropsychological and renal effects of dental amalgam in children: A randomized clinical trial. JAMA. 2006;295(15):1775–1783. doi: 10.1001/jama.295.15.1775. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Trachtenberg F, Daniel D, Zhang A, Tavares MA, McKinlay S. A dose-effect analysis of children's exposure to dental amalgam and neuropsychological function: The New England children's amalgam trial. J Am Dent Assoc. 2007;138(9):1210–1216. doi: 10.14219/jada.archive.2007.0345. [DOI] [PubMed] [Google Scholar]
- Bonham MP, Duffy EM, Robson PJ, Wallace JM, Myers GJ, Davidson PW, et al. Contribution of fish to intakes of micronutrients important for fetal development: A dietary survey of pregnant women in the republic of Seychelles. Public Health Nutr. 2009;12(9):1312–1320. doi: 10.1017/S136898000800387X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham MP, Duffy EM, Wallace JM, Robson PJ, Myers GJ, Davidson PW, Clarkson TW, Shamlaye CF, Strain JJ. Habitual fish consumption does not prevent a decrease in LCPUFA status in pregnant women (the Seychelles Child Development Nutrition Study) Prostaglandins Leukot Essent Fatty Acids. 2008 Jun;78(6):343–350. doi: 10.1016/j.plefa.2008.04.005. Epub 2008 Jun 26. Erratum in: Prostaglandins Leukot Essent Fatty Acids. 2011 Dec;85(6):407. PMID: 18585023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownawell AM, Berent S, Brent RL, Bruckner JV, Doull J, Gershwin EM, et al. The potential adverse health effects of dental amalgam. Toxicol Rev. 2005;24(1):1–10. doi: 10.2165/00139709-200524010-00001. [DOI] [PubMed] [Google Scholar]
- Cernichiari E, Myers GJ, Ballatori N, Zareba G, Vyas J, Clarkson T. The biological monitoring of prenatal exposure to methylmercury. Neurotoxicology. 2007;28(5):1015–1022. doi: 10.1016/j.neuro.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds.[see comment] Crit Rev Toxicol. 2006;36(8):609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- Counter SA, Buchanan LH. Mercury exposure in children: A review. Toxicol Appl Pharmacol. 2004;198(2):209–230. doi: 10.1016/j.taap.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Cox C, Axtell C, Shamlaye C, Sloane-Reeves J, et al. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: Outcomes at 66 months of age in the Seychelles child development study. JAMA. 1998;280(8):701–707. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Strain JJ, Myers GJ, Thurston SW, Bonham MP, Shamlaye CF, et al. Neurodevelopmental effects of maternal nutritional status and exposure to methylmercury from eating fish during pregnancy. Neurotoxicology. 2008;29(5):767–775. doi: 10.1016/j.neuro.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRouen TA, Cunha-Cruz J, Hilton TJ, Ferracane J, Berg J, Zhou L, et al. What's in a dental practice-based research network? characteristics of northwest PRECEDENT dentists, their patients and office visits. J Am Dent Assoc. 2010;141(7):889–899. doi: 10.14219/jada.archive.2010.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRouen TA, Martin MD, Leroux BG, Townes BD, Woods JS, Leitao J, et al. Neurobehavioral effects of dental amalgam in children: A randomized clinical trial. JAMA. 2006;295(15):1784–1792. doi: 10.1001/jama.295.15.1784. [DOI] [PubMed] [Google Scholar]
- Drasch G, Schupp I, Hofl H, Reinke R, Roider G. Mercury burden of human fetal and infant tissues. Eur J Pediatr. 1994;153(8):607–610. doi: 10.1007/BF02190671. [DOI] [PubMed] [Google Scholar]
- Eggleston DW. Dental amalgam: A review of the literature. Compendium. 1989;10(9):500–505. [PubMed] [Google Scholar]
- European Commission, Health and Consumer Protection Directorate-General, Scientific Committee on Emerging and Newly Identified Health Risks. [accessed 14 June 2012];The safety of dental amalgam and alternative dental restoration materials for patients and users. 2004 Available at: http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_016.pdf.
- Factor-Litvak P, Hasselgren G, Jacobs D, Begg M, Kline J, Geier J, et al. Mercury derived from dental amalgams and neuropsychologic function. Environ Health Perspect. 2003;111(5):719–723. doi: 10.1289/ehp.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson A, Dencker L, Archer T, Danielsson BR. Prenatal coexposure to metallic mercury vapour and methylmercury produce interactive behavioural changes in adult rats. Neurotoxicol Teratol. 1996;18(2):129–134. doi: 10.1016/0892-0362(95)02059-4. [DOI] [PubMed] [Google Scholar]
- Gay DD, Cox RD, Reinhardt JW. Chewing releases mercury from fillings. Lancet. 1979;1(8123):985–986. doi: 10.1016/s0140-6736(79)91773-2. [DOI] [PubMed] [Google Scholar]
- Guzzi G, Grandi M, Cattaneo C, Calza S, Minoia C, Ronchi A, et al. Dental amalgam and mercury levels in autopsy tissues: Food for thought. Am J Forensic Med Pathol. 2006;27(1):42–45. doi: 10.1097/01.paf.0000201177.62921.c8. [DOI] [PubMed] [Google Scholar]
- Health Canada. Mercury and human health. [accessed 14 June 2012];It’s Your Health. 2008 (updated March 2009). Available at: http://www.hc-sc.gc.ca/hl-vs/alt_formats/pacrb-dgapcr/pdf/iyh-vsv/environ/merc2008-eng.pdf.
- Innis SM. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008;1237:35–43. doi: 10.1016/j.brainres.2008.08.078. [DOI] [PubMed] [Google Scholar]
- Ishitobi H, Stern S, Thurston SW, Zareba G, Langdon M, Gelein R, et al. Organic and inorganic mercury in neonatal rat brain after prenatal exposure to methylmercury and mercury vapor. Environ Health Perspect. 2010;118(2):242–248. doi: 10.1289/ehp.0900956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingman A, Albers JW, Arezzo JC, Garabrant DH, Michalek JE. Amalgam exposure and neurological function. Neurotoxicology. 2005;26(2):241–255. doi: 10.1016/j.neuro.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Kingman A, Albertini T, Brown LJ. Mercury concentrations in urine and whole blood associated with amalgam exposure in a US military population. J Dent Res. 1998;77(3):461–471. doi: 10.1177/00220345980770030501. [DOI] [PubMed] [Google Scholar]
- Luglie PF, Campus G, Chessa G, Spano G, Capobianco G, Fadda GM, et al. Effect of amalgam fillings on the mercury concentration in human amniotic fluid. Arch Gynecol Obstet. 2005;271(2):138–142. doi: 10.1007/s00404-003-0578-6. [DOI] [PubMed] [Google Scholar]
- Makhija SK, Gordan VV, Gilbert GH, Litaker MS, Rindal DB, Pihlstrom DJ, et al. Practitioner, patient and carious lesion characteristics associated with type of restorative material: Findings from the dental practice-based research network. J Am Dent Assoc. 2011;142(6):622–632. doi: 10.14219/jada.archive.2011.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maserejian NN, Trachtenberg FL, Assmann SF, Barregard L. Dental amalgam exposure and urinary mercury levels in children: The New England children's amalgam trial. Environ Health Perspect. 2008;116(2):256–262. doi: 10.1289/ehp.10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell MA, Dillon CF, Osterloh J, Bolger PM, Pellizzari E, Fernando R, et al. Hair mercury levels in U.S. children and women of childbearing age: Reference range data from NHANES 1999–2000. Environ Health Perspect. 2004;112(11):1165–1171. doi: 10.1289/ehp.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento MM, Gordan VV, Qvist V, Litaker MS, Rindal DB, Williams OD, et al. Reasons for placement of restorations on previously unrestored tooth surfaces by dentists in the dental practice-based research network. J Am Dent Assoc. 2010;141(4):441–448. doi: 10.14219/jada.archive.2010.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olstad ML, Holland RI, Wandel N, Pettersen AH. Correlation between amalgam restorations and mercury concentrations in urine. J Dent Res. 1987;66(6):1179–1182. doi: 10.1177/00220345870660061701. [DOI] [PubMed] [Google Scholar]
- Palkovicova L, Ursinyova M, Masanova V, Yu Z, Hertz-Picciotto I. Maternal amalgam dental fillings as the source of mercury exposure in developing fetus and newborn. J Expo Sci Environ Epidemiol. 2008;18(3):326–331. doi: 10.1038/sj.jes.7500606. [DOI] [PubMed] [Google Scholar]
- Pesch A, Wilhelm M, Rostek U, Schmitz N, Weishoff-Houben M, Ranft U, et al. Mercury concentrations in urine, scalp hair, and saliva in children from germany. J Expo Anal Environ Epidemiol. 2002;12(4):252–258. doi: 10.1038/sj.jea.7500228. [DOI] [PubMed] [Google Scholar]
- Ralston N. Selenium health benefit values as seafood safety criteria. Ecohealth. 2008;5:442–455. doi: 10.1007/s10393-008-0202-0. [DOI] [PubMed] [Google Scholar]
- Ralston N, Raymond L. Dietary selenium’s protective effects against methylmercury toxicity. Toxicology. 2010;278:112–123. doi: 10.1016/j.tox.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Robinson J, Shroff J. Observations on the levels of total mercury (Hg) and selenium (Se) in species common to the artisanal fisheries of Seychelles. Seychelles Med Dent J. 2004;7(1):56–60. doi: 10.1016/j.neuro.2020.09.017. [DOI] [PubMed] [Google Scholar]
- Sallsten G, Thoren J, Barregard L, Schutz A, Skarping G. Long-term use of nicotine chewing gum and mercury exposure from dental amalgam fillings. J Dent Res. 1996;75(1):594–598. doi: 10.1177/00220345960750011301. [DOI] [PubMed] [Google Scholar]
- Stokes-Riner A, Thurston SW, Myers GJ, Duffy EM, Wallace J, Bonham M, et al. A longitudinal analysis of prenatal exposure to methylmercury and fatty acids in the Seychelles. Neurotoxicol Teratol. 2011;33(2):325–328. doi: 10.1016/j.ntt.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain JJ, Davidson PW, Bonham MP, Duffy EM, Stokes-Riner A, Thurston SW, et al. Associations of maternal long-chain polyunsaturated fatty acids, methyl mercury, and infant development in the Seychelles Child Development Nutrition Study. Neurotoxicology. 2008;29(5):776–782. doi: 10.1016/j.neuro.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration, Department of Health and Human Services. Dental devices: classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental amalgam, mercury, and amalgam alloy—final rule. Fed Regist. 2009;74(148):38685–38714. [PubMed] [Google Scholar]
- Vimy MJ, Lorscheider FL. Intra-oral air mercury released from dental amalgam. J Dent Res. 1985;64(8):1069–1071. doi: 10.1177/00220345850640080901. [DOI] [PubMed] [Google Scholar]
- Watson GE, Lynch M, Myers GJ, Shamlaye CF, Thurston SW, Zareba G, et al. Prenatal exposure to dental amalgam: Evidence from the Seychelles child development study main cohort. J Am Dent Assoc. 2011;142(11):1283–1294. doi: 10.14219/jada.archive.2011.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg S. Applied Linear Regression. 3rd ed. Hoboken, New Jersey: Wiley; 2005. [Google Scholar]
- World Health Organization. Environmental Health Criteria 118: Inorganic Mercury. Geneva, Switzerland: 1991. [Google Scholar]
- World Health Organization. WHO consensus statement on dental amalgam. 1997 [PubMed]