Abstract
Objectives
Intracranial hemorrhage (ICH) after acute stroke thrombolysis is associated with poor outcomes. Previous investigations of the relationship between pre-existing antiplatelet use and the safety of intravenous (IV) thrombolysis have been limited by low event rates. The objective of this study was to determine whether pre-existing antiplatelet therapy increased the risk of ICH following acute stroke thrombolysis. The primary hypothesis was that antiplatelet use would not be associated with radiographic evidence of ICH after controlling for relevant confounders.
Methods
Consecutive cases of thrombolysis patients treated in the emergency department (ED) were identified using multiple methods. Retrospective data were collected from four hospitals from 1996 to 2004, and 24 distinct hospitals from 2007 to 2010 as part of a cluster randomized trial. The same chart abstraction tool was used during both time periods, and data were subjected to numerous quality control checks. Hemorrhages were classified using a pre-specified methodology: ICH was defined as presence of hemorrhage in radiographic interpretations of follow-up imaging (primary outcome). Symptomatic ICH (sICH) was defined as radiographic ICH with associated clinical worsening. A multivariable logistic regression model was constructed to adjust for clinical factors previously identified to be related to post-thrombolysis ICH. Sensitivity analyses were conducted where the unadjusted and adjusted results from this study were combined with those of previously published external studies on this topic via meta-analytic techniques.
Results
There were 830 patients included, with 47% having documented pre-existing antiplatelet treatment. The mean age was 69 years (SD ± 15 years), and the cohort was 53% male. The unadjusted proportion of patients with any ICH was 15.1% without antiplatelet use, and 19.3% with antiplatelet use (absolute risk difference 4.2%, 95% CI = −1.2% to 9.6%); for sICH this was 6.1% without antiplatelet use and 9% with antiplatelet use (absolute risk difference 3.1%, 95% CI = −1% to 6.7%). After adjusting for confounders, antiplatelet use was not significantly associated with radiographic ICH (odds ratio 1.1, 95% CI = 0.8 to 1.7), or sICH (odds ratio 1.3, 95% CI = 0.7 to 2.2). In patients 81 years and older, there was a higher risk of radiographic ICH (absolute risk difference 11.9%, 95% CI = 0.1% to 23.6%). The meta-analyses combined the findings of this investigation with previous similar work and found increased unadjusted risks of radiographic ICH (absolute risk difference 4.9%, 95% CI = 0.7% to 9%) and sICH (absolute risk difference 4%, 95% CI = 2.3% to 5.6%). The meta-analytic adjusted odds ratio of sICH for antiplatelet use was 1.6 (95% CI = 1.1 to 2.4).
Conclusions
The authors did not find that pre-existing antiplatelet use was associated with post-thrombolysis ICH or sICH in this cohort of community treated patients. Pre-existing tobacco use, younger age, and lower severity were associated with lower odds of sICH. The meta-analyses demonstrated small, but statistically significant increases in the absolute risk of radiographic ICH and sICH, along with increased odds of sICH in patients with pre-existing antiplatelet use.
INTRODUCTION
Treatment of acute stroke with tissue plasminogen activator (tPA) reduces disability in appropriately selected patients.1,2 Concerns over safety, specifically symptomatic intracranial hemorrhage (sICH), have contributed to relatively limited use of this treatment and slow universal acceptance in emergency medicine.3 To address these concerns and improve patient outcomes, the development of prediction rules to select patients who have a lower risk of post-tPA sICH is a high priority. To date, such decision rules have had limited discriminatory ability.4,5
In the setting of ST-segment elevation myocardial infarction, the combination of thrombolytics with a variety of anti-platelet agents and anticoagulants is safe and efficacious.6 The same has not been established in stroke. An early randomized controlled thrombolysis trial in acute stroke found that the combination of aspirin and streptokinase increased the risk of sICH compared to streptokinase alone.7 Subsequent stroke tPA trials treatment protocols mandated subjects not receive anti-platelet treatment until 24 hours post-treatment.1,2,8 Following demonstration of tPA efficacy in the NINDS tPA stroke study, this restriction on post-treatment anti-platelet use became part of the U.S. Food and Drug Administration-approved package insert. The same trials did not, however, exclude patients with pre-existing antiplatelet use from study entry, despite the relatively long duration of effect of most antiplatelet agents (aspirin, clopidogrel).
Because patients with prior antiplatelet use have been included in stroke thrombolysis trials and post-marketing surveillance observational studies, several retrospective investigations have provided conflicting conclusions regarding the relationship between anti-platelet use and ICH.9–16 Unadjusted analyses typically show a higher absolute risk of ICH in patients with existing antiplatelet use, but adjusted analyses accounting for age, severity, and other potential confounders have been conflicting. Therefore, our objective was to determine whether pre-existing antiplatelet therapy was associated with ICH following tPA treatment for ischemic stroke, with particular exploration of the effects of age, as many studies have restricted thrombolysis patients to those younger than 80 years. The primary hypothesis was that antiplatelet use would not be associated with radiographic evidence of ICH after controlling for relevant confounders. Our secondary hypothesis was that antiplatelet use would not be associated with sICH.
METHODS
Study Design
This was a secondary, retrospective, observational study using data collected in two studies of tPA use in stroke in Michigan: the Safety of Intravenous Thrombolysis in Four Emergency Departments (SIT-ED) project, and the Increasing Stroke Treatment through Interventional Behavior Change Tactics (INSTINCT) trial, which have been previously described.17,18 The University of Michigan institutional review board (IRB) and all applicable local IRBs approved the protocols for SIT-ED and INSTINCT.
Study Setting and Population
The SIT-ED project was a retrospective case series of all stroke patients receiving tPA within four hospitals; INSTINCT was a cluster randomized trial including all cases of stroke tPA treatment within 24 randomly selected community hospitals.
All stroke patients aged 18 years or greater receiving IV tPA were identified from all 28 hospitals during the study periods. Cases were identified by several methods, including ED logs, institutional stroke registries, pharmacy medication administration records, and hospital billing records. Clinical data were only available from the index hospitalizations; however, full records of the initial hospitalizations were obtained for cases that were transferred from an INSTINCT hospital to an institution not participating in the trial.
Study Protocol
Data were collected from four hospitals associated with the SIT-ED project (1996 to 2004), and 24 additional community hospitals located in the Lower Peninsula of Michigan from the INSTINCT trial (2007–2010). The general characteristics of the hospitals from both studies are summarized in Table 1. The same data collection instrument and quality control processes were used for both studies. Trained clinical abstractors collected all the data from source documents, double review of key data elements was conducted, and agreement was excellent, with agreement of 99% for key data elements (thrombolysis inclusion criteria, presence of ICH). The abstractors were not aware of this study hypothesis when collecting data, as this investigation was designed after the completion of data analysis for both the SIT-ED project and the INSTINCT trial.
Table 1.
Characteristics of the Study Hospitals
| SIT-ED Hospital Characteristics | ||||||
|---|---|---|---|---|---|---|
| Hospital | Location | Urban/Rural | Inpatient Beds | Adult ED Volume | Teaching Hospital | Neurology |
| 1 | Ann Arbor | Urban | 792 | 50,918 | Yes | Yes |
| 2 | Ypsilanti | Urban | 529 | 65,259 | Yes | Yes |
| 3 | Flint | Urban | 463 | 54,707* | Yes | Yes |
| 4 | Jackson | Rural | 411 | 52,500 | No | Yes |
| INSTINCT Hospital Characteristics | N (%) | |||||
| Inpatient Beds | ||||||
| < 100 | 4 (17) | |||||
| 101 – 250 | 11 (46) | |||||
| 251 – 500 | 6 (25) | |||||
| > 500 | 3 (13) | |||||
| Annual ED Volume (adult) | ||||||
| < 20,000 | 7 (29) | |||||
| 20,001 – 40,000 | 8 (33) | |||||
| 40,001 – 60,000 | 8 (33) | |||||
| 60,001 – 80,000 | 1 (4) | |||||
| General | ||||||
| Teaching hospital | 11 (46) | |||||
| Neurology available | 22 (92) | |||||
| Primary stroke center | 8 (33) | |||||
| Urban | 8 (33) | |||||
Characteristics of the hospitals included from 1996–2004 (SIT-ED) and 2007–2010 (INSTINCT).
Different data collection strategies were used to summarize hospital data.
This hospital reported combined adult and pediatric volume.
Patient-level data including age, sex, stroke risk factors, and self-reported ethnicity were obtained from the medical record. Time of onset or time last known well was recorded when it was explicitly stated in the medical record. When not specifically documented, the National Institutes of Health Stroke Scale (NIHSS) score was estimated using a previously described method.19 Pre-existing use of antiplatelet agents was a binary field in the data collection instrument and was determined by study team abstractors. The last recorded blood pressure prior to treatment and first recorded blood glucose were abstracted from the ED record as pre-treatment variables. Post-treatment protocol deviations were defined as antiplatelet or anticoagulant use within 24 hours of tPA treatment, or blood pressure not treated when out of range for two or more measurements at least 30 minutes apart.
Hemorrhages were classified using a prespecified methodology: ICH was defined as presence of hemorrhage in radiographic interpretations (primary outcome of this investigation) of any follow-up neuroimaging (CT or MRI) obtained during initial hospitalization. The timing of follow-up neuroimaging was at the discretion of the treating teams; the reports of all neuroimaging studies obtained during the first 10 days of the initial hospitalization were reviewed. The main secondary outcome was sICH, defined as radiographic ICH with associated clinical worsening based on retrospective review of hospital chart (NINDS tPA trial definition).
Sample Size
Prior to conducting the analysis, we hypothesized that a clinically meaningful increase in the radiographic ICH rate would be from 17% to 25.3% (50% relative increase). Using a two-sided binomial difference in proportions, with a significance level (alpha) of 0.05 and 80% power, a sample size of 377 patients per group (with and without anti-platelets) was estimated. To detect a 25% relative increase (range 17% to 21.25%), 1341 patients per group would be needed; to detect a 100% relative increase (range 17% to 34%), 101 patients per group would be needed.
Data Analysis
Demographics and clinical characteristics are summarized using descriptive statistics (means with standard deviation [SD], medians with interquartile ranges [IQR], or proportions as appropriate). The raw proportions for the primary outcome for patients with and without pre-existing antiplatelet use and the 95% confidence interval (CI) for the difference were calculated. A multivariable logistic regression model was fitted to adjust for clinical factors previously identified to be related to post-thrombolysis ICH with all variables selected a priori. In addition to our variable of interest, pre-existing antiplatelet use, the model included age, NIHSS, pretreatment systolic blood pressure, onset to treatment interval, pre-treatment blood glucose, tobacco use, and presence of post-treatment protocol violations. Continuous variables other than onset to treatment interval were divided into quartiles to allow for potential non-linear associations between the selected covariates and the outcome. Due to fewer sICH events, the multivariable model for this endpoint was constructed with continuous variables dichotomized.
In addition, a propensity score was calculated using logistic regression for each case to provide a single summary measure of the likelihood that any individual was on an antiplatelet agent given his or her age, sex, race, and vascular risk factor profile.20 This was then added as a covariate to the final models measuring the association between ICH and antiplatelet use. The Cochran-Armitage test was used to assess for a trend in the rate of ICH over time; this was performed because neuroimaging use has changed over the time period. Specifically, magnetic resonance imaging (MRI) has become more common and may detect more ICH.21 In addition, the risk difference for ICH comparing those with antiplatelet use to those without was calculated within each age quartile. SAS version 9.2 (SAS Corporation, Cary, NC) was used for analysis. We fitted the final main outcome model using two alternative techniques to account for clustering within hospitals – specifically hierarchical logistic regression with a random intercept for hospital, and a logistic regression using a generalized estimating equation (GEE) accounting for clustering within hospitals. In addition, we calculated the radiographic and symptomatic ICH rates based on whether the patient had a follow-up CT versus MRI.
Sensitivity Analysis
In order to put the current investigation in the context of the previous understanding of the topic, we performed a structured literature review and used meta-analytic techniques to quantify how this investigation has updated our knowledge. To identify relevant studies, a PubMed search was conducted using the following search logic (thrombolys* OR alteplase OR tPA) AND stroke AND platelet aggregation inhibitors AND (cerebral hemorrhage OR intracranial hemorrhages). Studies were included if they reported the unadjusted proportions of patients with radiographic and/or sICH in accordance with the PRISMA statement.22 Studies that reported a subset of our four summary measures (unadjusted risk difference for radiographic ICH, unadjusted risk difference for sICH, adjusted odds ratio [aOR] for radiographic ICH, and aOR for sICH) were included in only the relevant analyses. For studies that used multiple definitions of sICH, the NINDS definition (any ICH plus any increased NIHSS or death within a week) was used, as this definition is conservative (generally assigning the highest proportion of hemorrhages to the symptomatic category) and has been most commonly reported across studies.1 The DerSimonian and Laird random-effects model was used to calculate a pooled estimate for the probability of radiographic and symptomatic hemorrhage following tPA for cases with and without pre-existing antiplatelet use. In addition, estimates of the aOR for the association relating anti-platelet use to radiographic and sICH from the current and previous investigations were also combined using the DerSimonian and Laird method. R version 2.12.2 and R package meta (R Project, Institute for Statistics and Mathematics, WU Wein, Vienna, Austria), which includes functions for performing meta-analyses of studies reporting proportions and odds ratios, were used to conduct this portion of the analysis.23 In the main analyses, patients without follow-up neuroimaging were assumed to be free of ICH, as the clinical teams may have elected to not perform repeat imaging in patients who were doing well.
RESULTS
Participants
A total of 830 patients were included (SIT-ED, n = 273; INSTINCT, n = 557). The clinical characteristics of the patients are summarized in Table 2. A total of 388 patients (47%) had documented histories of taking anti-platelet agents. Patients with documented antiplatelet use had higher burdens of stroke and cardiovascular risk factors. Severity and age were also higher in the antiplatelet group.
Table 2.
Characteristics of the Study Population
| Characteristics | Antiplatelet Use | No Antiplatelet Use | Difference | 95% Cl | ||
|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | |||
| Age (years) | 74 | (12.6) | 65 | (16) | 8.1 | (6.2 to 10.1)* |
| Onset to treatment (minutes) (n=780) | 148 | (40.8) | 151 | (53.9) | −2.7 | (−9.5 to 4.1) |
| Pre-treatment | ||||||
| Blood glucose (mg/DL)(n=820) | 137 | (55.1) | 129 | (42.9) | 8.5 | (1.8 to 15.2)* |
| Systolic blood pressure (mm Hg)(n=826) | 151 | (21.7) | 147 | (22) | 4.4 | (1.4 to 7.4)* |
| Diastolic blood pressure (mm Hg)(n=826) | 78 | (16.6) | 79 | (15.3) | −0.8 | (−3 to 1.4) |
| NIHSS | 13 | (5.7) | 12 | (5.8) | 0.8 | (0.1 to 1.6)* |
| Demographics | Percentage | (N) | Percentage | (N) | Difference, % | 95% Cl |
| Female | 47.7 | (185) | 46.2 | (204) | 1.5 | (−5.3 to 8.3 |
| Non-Hispanic white | 80.9 | (314) | 78.3 | (346) | 2.6 | (−2.9 to 8.2) |
| African American | 7.7 | (30) | 10.0 | (44) | −2.2 | (−6.1 to 1.7) |
| Other/unknown | 11.1 | (43) | 11.5 | (51) | −0.5 | (−4.8 to 3.9) |
| Prior stroke | 27.6 | (107) | 9 | (40) | 18.5 | (13.3 to 23.7)* |
| Prior TIA | 18.3 | (71) | 8,6 | (38) | 9.7 | (5.1 to 14.3)* |
| Diabetes mellitus | 28.6 | (111) | 18.8 | (83) | 9.8 | (4.1 to 15.6)* |
| Hypertension | 86.9 | (337) | 62.4 | (276) | 24.4 | (18.4 to 30.4)* |
| Hyperlipidemia | 61.1 | (237) | 31.2 | (138) | 29.9 | (23.1 to 36.6)* |
| Coronary artery disease | 50.8 | (197) | 16.1 | (71) | 34.7 | (28.3 to 41.1)* |
| Congestive heart failure | 17 | (66) | 7.9 | (35) | 9.1 | (4.6 to 13.5)* |
| Atrial fibrillation | 31.4 | (122) | 14.5 | (64) | 17 | (11.3 to 22.6)* |
| Valvular heart disease | 9.8 | (38) | 4.5 | (20) | 5.3 | (1.8 to 8.7)* |
| Smoking within 1 year | 18.8 | (73) | 30.1 | (133) | −11.3 | (−17.2 to −5.4) |
| Post-treatment deviations | ||||||
| Anti-platelet given within 24 hours (n=829) | 10.3 | (40) | 11.5 | (51) | −1 | (−5.5 to 3.1) |
| Anticoagulant given within 24 hours | 5.4 | (21) | 6.6 | (29) | −1.1 | (−4.4 to 2.1) |
| Blood pressure maintained < 180/105 mm Hg (n=816) | 21.4 | (83) | 15.8 | (70) | 5.6 | (0.3 to 10.8)* |
N = count; NIHSS = National Institutes of Health Stroke Scale; TIA = transient ischemic attack.
Items with confidence intervals excluding 0.
Difference in means or binomial difference in proportions with 95% CI reported calculated by subtracting no antiplatelet use group from antiplatelet group
Outcome data
Radiographic ICH was observed in 67 patients (15.2%) without pre-existing antiplatelet use, and 75 patients (19.3%) with pre-existing antiplatelet use. The absolute difference was 4.2% (95% CI = −1.2% to 9.6%). Symptomatic ICH was observed in 27 patients (6.1%) without and 35 patients (9%) with pre-existing antiplatelet use; the absolute difference was 3.1% (95% CI = −1% to 6.7%).
Main results
In the fully adjusted model, there was no association of pre-existing antiplatelet use with the primary outcome, radiographic ICH (aOR 1.13, 95% CI = 0.76 to 1.68). Increasing severity, older age, and higher baseline blood glucose were associated with any radiographic ICH. No association between antiplatelet use was seen with sICH (see Table 3). Patients who had a history of tobacco use had lower odds of sICH in the fully adjusted analysis. When the propensity score was added to these models, no meaningful change in the estimated associations was observed (data not shown). In addition, the hierarchical model and the GEE did not demonstrate a substantial effect of clustering within hospital (data not shown).
Table 3.
Fully adjusted models for any radiographic ICH and symptomatic ICH
| Characteristics | Model 1: Any ICH | Model 2: slCH | |||
|---|---|---|---|---|---|
| aOR | (95% Cl) | Category for Model 2 | aOR | (95% Cl) | |
| Pre-existing antiplatelet use | 1.13 | (0.76–1.68) | 1.26 | (0.72–2.19) | |
| Post tPA treatment protocol deviations | 1.00 | (0.65–1.52) | 1.57 | (0.90–2.75) | |
| History of tobacco use | 0.70 | (0.40–1.24) | 0.32 | (0.11–0.94) | |
| Systolic blood pressure (10 mmHg increase) | 1.07 | (0.97–1.17) | 1.09 | (0.96–1.25) | |
| Onset to treatment time in minutes | 1.00 | (0.995–1.004) | 0.997 | (0.99–1.004) | |
|
| |||||
| Baseline NIHSS | |||||
| 1 – 6 | 0.29 | (0.15–0.55) | 1–11 | 0.53 | (0.30–0.95) |
| 7 – 11 | 0.33 | (0.19–0.55) | |||
| 12 – 15 | 0.72 | (0.44–1.17) | 12+ | ref | |
| 16+ | ref | ||||
|
| |||||
| Age in Years | |||||
| 20 – 57 | 0.41 | (0.20–0.83) | 20–70 | 0.52 | (0.27–1.001) |
| 58 – 70 | 0.80 | (0.47–1.37) | |||
| 71 – 70 | 0.83 | (0.51–1.37) | 71+ | ref | |
| 81+ | ref | ||||
|
| |||||
| Baseline Blood Glucose (mg/DL) | |||||
| 59 – 101 | 0.50 | (0.28–0.91) | 59–118 | 1.00 | (0.58–1.73) |
| 102 – 118 | 0.69 | (0.41–1.16) | |||
| 119 – 144 | 0.83 | (0.50–1.39) | 119+ | ref | |
| 145+ | ref | ||||
Final models evaluating the relationship between intracranial hemorrhage (ICH) and antiplatelet use. sICH = symptomatic ICH; aOR = adjusted odds ratio; NIHSS = National Institutes of Health Stroke Scale; TIA = transient ischemic attack.
Age, severity, and glucose were divided into quartiles (Model 1) or dichotomized (Model 2) to allow for non-linear effects. Model 1 fit was satisfactory (c-statistic 0.704, Hosmer and Lemeshow Goodness of Fit Test failed to reject hypothesis of lack of fit p = 0.76) as was the fit of Model 2 (c-statistic 0.719, Hosmer and Lemeshow Goodness of Fit Test p = 0.14).
Secondary results
There was no increase in radiographic or sICH over time (see Data Supplement S1). In the results stratified by age, there was an observed increased risk of radiographic ICH in patients 81 and older (11.9% absolute increase) who were taking antiplatelet agents compared to those who were not (Table 4). In 312 patients with follow-up MRI, 12 had sICH (3.9%), and 41 had radiographic ICH (13.1%). In the group of 468 patients without follow-up MRI, there were 413 with one or more follow-up CT and 55 with no follow up neuroimaging; in this group there were 50 sICH (9.7%), and 101 radiographic ICH (19.5%). Of the 55 without follow-up neuroimaging, only eight had clinical worsening compared to arrival at the time of hospital discharge.
Table 4.
Any ICH and symptomatic ICH stratified by age
| Age, yrs | Overall Severity | Antiplatelet Use | No-Antiplatelet Use | Absolute Risk Increase | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NIHSS | (IQR) | No ICH | ICH | ICH% | No ICH | ICH | ICH% | RD, % | (95% Cl) | |
| Any ICH | ||||||||||
| 20–57 | 10 | (7–15) | 51 | 5 | 8.9 | 128 | 11 | 7.9 | 1.0 | (−7.5 to 9.5) |
| 58–70 | 11 | (7–15) | 79 | 12 | 13.2 | 106 | 22 | 17.2 | −4.0 | (−13.7 to 5.7) |
| 71–80 | 13 | (9–17) | 96 | 21 | 17.9 | 68 | 18 | 20.9 | −3.0 | (−13.9 to 8.0) |
| 81+ | 14 | (9–18) | 87 | 37 | 29.8 | 73 | 16 | 18.0 | 11.9 | (0.1 to 23.6)* |
| Symptomatic ICH | ||||||||||
| 20–57 | 10 | (7–15) | 56 | 0 | 0.0 | 135 | 4 | 2.9 | −2.9 | (−7.3 to 1.5) |
| 58–70 | 11 | (7–15) | 86 | 5 | 5.5 | 120 | 8 | 6.3 | −0.8 | (−7.1 to 5.6) |
| 71–80 | 13 | (9–17) | 107 | 10 | 8.5 | 79 | 7 | 8.1 | 0.4 | (−7.3 to 8.1) |
| 81+ | 14 | (9–18) | 104 | 20 | 16.1 | 81 | 8 | 9.0 | 7.1 | (−2.1 to 16.3) |
RD = risk difference; NIHSS = National Institutes of Health Stroke Scale; IQR = interquartile range.
Binomial difference in proportions with 95% CI reported calculated by subtracting no antiplatelet use group from antiplatelet group; items with confidence intervals excluding 0 indicated with *. Stroke severity within each stratum summarized with median NIHSS and IQR.
Sensitivity analysis
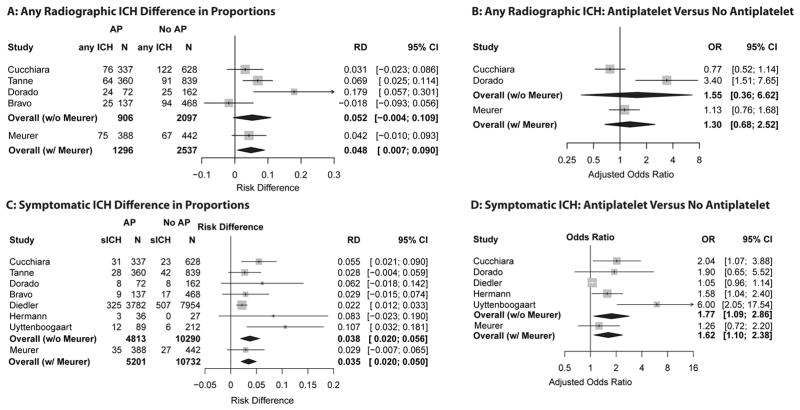
The pooled estimate for the excess risk in antiplatelet treated thrombolysis patients across all seven identified studies in the meta-analysis was 4.9% (95% CI = 0.7% to 9%) for any ICH, and 4% (95% CI = 2.3% to 5.6%) for sICH. (The PRISMA flowchart and table of included studies are available in Data Supplement S2). The pooled adjusted odds ratio for the association of antiplatelet use with any ICH was 1.3 (95% CI = 0.7 to 2.5) and with sICH was 1.6 (95% CI = 1.1 to 2.4). The results of the individual studies in the meta-analysis are included in Figure 1.
Figure 1. Meta-analyses with and without current investigation for risk difference and adjusted odds ratios.
Results of meta-analyses. AP = antiplatelet, RD = risk difference, OR = odds ratio, ICH = intracranial hemorrhage, sICH = symptomatic ICH. Risk difference is the absolute excess proportion of patients with ICH in patient with documented pre-stroke antiplatelet use versus those without. For example, in panel A, 4.9% more antiplatelet patients had radiographic evidence of ICH versus those not receiving antiplatelets when estimates across all studies were combined.
DISCUSSION
In this cohort of 830 tPA-treated stroke patients, we found no significant association between pre-thrombolytic antiplatelet use and post-thrombolytic risk of either radiographic or sICH. Unadjusted analyses identified a trend towards increased risk for both radiographic and sICH; however, after adjusting for important confounders (age, stroke severity, and hyperglycemia) no significant relationship was observed. These confounders have previously been associated with increased ICH risk in multivariable analysis.24
Our data also suggest that the risk of radiographic ICH increases with age, since the point estimates for the unadjusted absolute risk difference increase across the age strata, and the fully adjusted model shows possible excess risk in the group aged 81 years and older. This is an interesting area of potential future investigation, as the majority of patients included in the meta-analyses were from the Safe Implementation of Thrombolysis in Stroke International Stroke Thrombolysis Register (SITS-ISTR), which restricted inclusion to ages 18 to 80 years.12,15
In addition, the potential protective effect of pre-existing tobacco use against symptomatic hemorrhage has been re-demonstrated in this analysis. Nicotine increases plasminogen activator inhibitor-1 (PAI-1) activity (which de-activates therapeutic tPA treatment), although in clinical practice better recanalization has been observed in myocardial infarction and stroke in smokers.25,26 Since sICH may represent an end process of brain damage in situations where recanalization does not occur, one potential mechanism for the reduction of sICH among smokers may be better recanalization. It may also reflect the presence of the higher levels of PAI-1 in smokers, decreasing the effective half-life of exogenous tPA, thereby limiting the potential damage as parts of the ischemic penumbra progress to frank infarction and disruption of the blood brain barrier. Nicotine may have promise as an agent to enhance the acute effectiveness and safety of tPA, although it may cause post-ischemic brain inflammation when administered continuously for days after experimental stroke.27 Further studies are warranted, including translational animal models of thrombolysis-associated hemorrhage.
In order to put the current investigation in the context of the previous understanding of the topic, we performed a structured literature review and used meta-analytic techniques to quantify how this investigation has updated our knowledge. Overall, the point estimates for the radiographic and sICH proportions and aOR from the current investigation are congruent with the prior work in this area; however, the overall meta-analysis did estimate a small but statistically increased adjusted odds of sICH for patients who were on anti-platelet agents.
LIMITATIONS
This study has several important limitations. First, we do not have 90-day functional outcomes on the patients in the study, limiting our ability to determine what contribution the ICH had on the ultimate clinical outcome. Since many hemorrhages occur in patients with large strokes (high NIHSS) whose expected outcomes are poor, it is difficult to separate out the independent effect of ICH from the influence of a large ischemic stroke. Another important limitation of our investigation is our reliance on retrospective medical record review. For this reason our primary outcome was radiographic evidence of hemorrhage, which is easier to determine objectively from the medical record. This measure may be of more limited clinical utility, since small radiographic hemorrhages have not demonstrated impairment of ultimate functional outcome.28 The current study was powered to identify a relatively large effect on ICH. Another limitation is the potential confounding effects of new stroke therapies over the time course of the study, which spanned 14 years. It is plausible that greater coexisting use of medications affecting platelet inhibition, such as statins or selective serotonin reuptake inhibitors, may complicate this relationship. To the best of our knowledge, no studies to date have had adequate numbers of events to detect any synergistic effects of these agents with antiplatelet use on increasing ICH.29,30 In addition, increasing numbers of patients are on alternate antiplatelet agents, particularly clopidogrel, either alone or in combination. Our current investigation collected antiplatelet use as a dichotomous variable, thus precluding inference regarding dosing and combinations. While subgroup analyses have been done in some of the prior evaluations, the majority of included patients have been on aspirin alone. Current estimates of the potential interaction between clopidogrel or combination treatments with stroke thrombolysis are imprecise; however, it does appear that some increased risk exists.11 It is important to note that studies focusing on this have not contained enough patients on alternative regimens to allow generalizable conclusions for clinical practice. In addition, the anti-platelet group had a higher burden of vascular risk factors, which is not surprising; however, when we adjusted for residual confounding by vascular risk factors using propensity scores, the associations between antiplatelet use and hemorrhage were unchanged. Another consideration is the variable use of imaging and increasing use of MRI. The sensitivity of MRI versus CT for intracranial blood is an area of controversy in the radiologic literature, and over this time period it is unlikely that CT or MRI had substantially different performance.31 Our finding that ICH did not increase over time, despite the likely increased popularity of MRI, lends credence to this, although going forward this may need to be examined as maximally heme-sensitive sequences on MRI (gradient echo) are becoming more commonly used. In addition, we found the rates of radiographic ICH to actually be lower when follow-up MRI was performed. Finally, the context analyses were designed to place the current results in perspective based on other published data on the topic; as with any form of meta-analysis, heterogeneity exists across studies, centers, and regions of the world.
The results of this study, and its combination with other existing knowledge on this topic, are applicable to clinical situations that emerge routinely. Since the adjusted increased odds of symptomatic hemorrhage in patients receiving antiplatelets is consistently small, it is likely, at least with respect to aspirin use, that any increased risk from stroke thrombolysis in patients receiving aspirin is of questionable importance. Other clinical variables, most notably stroke severity and age, appear to be much more important.
CONCLUSIONS
We found no significant increases in either radiographic or symptomatic intracranial hemorrhage following thrombolysis in patients receiving antiplatelet agents in the INSTINCT and SIT-ED studies. The meta-analyses increased the precision of estimates for the unadjusted proportion and adjusted odds of intracranial hemorrhage, and showed that antiplatelet agents may minimally increase symptomatic intracranial hemorrhage. Future work should focus on the association of antiplatelet use prior to thrombolysis on functional outcomes, the development of selection strategies to triage high risk patients to treatments, and the discovery of agents that can inhibit the development of post-thrombolysis intracranial hemorrhage.
Supplementary Material
Acknowledgments
This trial was supported by grant number R01-NS-050372 from the National Institute of Neurological Disorders and Stroke of the United States National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH
Footnotes
Presentations: Society for Academic Emergency Medicine Annual Meeting, Chicago, IL, May 2012, and the International Stroke Conference, New Orleans, LA, February 2012
Disclosures: The authors have no disclosures or conflicts of interest to report
References
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Eng J Med. 2008;359(13):1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 3.Brown DL, Barsan WG, Lisabeth LD, Gallery ME, Morgenstern LB. Survey of emergency physicians about recombinant tissue plasminogen activator for acute ischemic stroke. Ann Emerg Med. 2005;46(1):56–60. doi: 10.1016/j.annemergmed.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 4.Lou M, Safdar A, Mehdiratta M, et al. The HAT score. Neurology. 2008;71(18):1417–23. doi: 10.1212/01.wnl.0000330297.58334.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cucchiara B, Kasner S, Tanne D, et al. Validation assessment of risk scores to predict postthrombolysis intracerebral haemorrhage. Int J Stroke. 2011;6(2):109–11. doi: 10.1111/j.1747-4949.2010.00556.x. [DOI] [PubMed] [Google Scholar]
- 6.Kushner FG, Hand M, Smith SC, Jr, et al. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (Updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (Updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54(23):2205–41. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Multicentre Acute Stroke Trial--Italy. Randomised controlled trial of streptokinase, aspirin, and combination of both in treatment of acute ischaemic stroke. Lancet. 1995;346(8989):1509–14. [PubMed] [Google Scholar]
- 8.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II) Lancet. 1998;352(9136):1245–51. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 9.Bravo Y, Martí-Fàbregas J, Cocho D, et al. Influence of antiplatelet pre-treatment on the risk of symptomatic intracranial haemorrhage after intravenous thrombolysis. Cerebrovasc Dis. 2008;26(2):126–33. doi: 10.1159/000139659. [DOI] [PubMed] [Google Scholar]
- 10.Tanne D, Kasner SE, Demchuk AM, et al. Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice. Circulation. 2002;105(14):1679–85. doi: 10.1161/01.cir.0000012747.53592.6a. [DOI] [PubMed] [Google Scholar]
- 11.Cucchiara B, Kasner SE, Tanne D, et al. Factors associated with intracerebral hemorrhage after thrombolytic therapy for ischemic stroke. Stroke. 2009;40(9):3067–72. doi: 10.1161/STROKEAHA.109.554386. [DOI] [PubMed] [Google Scholar]
- 12.Diedler J, Ahmed N, Sykora M, et al. Safety of intravenous thrombolysis for acute ischemic stroke in patients receiving antiplatelet therapy at stroke onset. Stroke. 2010;41(2):288–94. doi: 10.1161/STROKEAHA.109.559724. [DOI] [PubMed] [Google Scholar]
- 13.Dorado L, Millán M, De La Ossa NP, et al. Influence of antiplatelet pre-treatment on the risk of intracranial haemorrhage in acute ischaemic stroke after intravenous thrombolysis. Eur J Neurol. 2010;17(2):301–6. doi: 10.1111/j.1468-1331.2009.02843.x. [DOI] [PubMed] [Google Scholar]
- 14.Uyttenboogaart M, Koch MW, Koopman K, Vroomen PC, De Keyser J, Luijckx GJ. Safety of antiplatelet therapy prior to intravenous thrombolysis in acute ischemic stroke. Arch Neurol. 2008;65(5):607–11. doi: 10.1001/archneur.65.5.noc70077. [DOI] [PubMed] [Google Scholar]
- 15.Wahlgren N, Ahmed N, Eriksson N, et al. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe Implementation of Thrombolysis in Stroke-MOnitoring STudy (SITS-MOST) Stroke. 2008;39(12):3316–22. doi: 10.1161/STROKEAHA.107.510768. [DOI] [PubMed] [Google Scholar]
- 16.Hermann A, Dzialowski I, Koch R, Gahn G. Combined anti-platelet therapy with aspirin and clopidogrel: risk factor for thrombolysis-related intracerebral hemorrhage in acute ischemic stroke? J Neurologic Sci. 2009;284(1–2):155–7. doi: 10.1016/j.jns.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Scott PA. Enhancing community delivery of tissue plasminogen activator in stroke through community–academic collaborative clinical knowledge translation. Emerg Med Clin N Am. 2009;27(1):115–36. doi: 10.1016/j.emc.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott PA, Frederiksen SM, Kalbfleisch JD, et al. Safety of intravenous thrombolytic use in four emergency departments without acute stroke teams. Acad Emerg Med. 2010;17:1062–71. doi: 10.1111/j.1553-2712.2010.00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke. 2000;31(4):858–62. doi: 10.1161/01.str.31.4.858. [DOI] [PubMed] [Google Scholar]
- 20.D’Agostino RB. Tutorial in biostatistics: propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 21.Kidwell CS, Chalela JA, Saver JL, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA. 2004;292(15):1823–30. doi: 10.1001/jama.292.15.1823. [DOI] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Schwarzer G. Meta: Meta-Analysis with R. [Accessed Dec 2, 2012];R package version 2.0–0. Available at: http://cran.r-project.org/web/packages/meta/index.html.
- 24.Lansberg MG, Albers GW, Wijman CAC. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: a review of the risk factors. Cerebrovasc Dis. 2007;24(1):1–10. doi: 10.1159/000103110. [DOI] [PubMed] [Google Scholar]
- 25.Ovbiagele B, Saver JL. The smoking–thrombolysis paradox and acute ischemic stroke. Neurology. 2005;65(2):293–5. doi: 10.1212/01.wnl.0000168163.72351.f3. [DOI] [PubMed] [Google Scholar]
- 26.Zidovetzki R, Chen P, Fisher M, Hofman FM, Faraci FM. Nicotine increases plasminogen activator inhibitor-1 production by human brain endothelial cells via protein kinase C–associated pathway (Editorial Comment) Stroke. 1999;30(3):651–5. doi: 10.1161/01.str.30.3.651. [DOI] [PubMed] [Google Scholar]
- 27.Bradford ST, Stamatovic SM, Dondeti RS, Keep RF, Andjelkovic AV. Nicotine aggravates the brain postischemic inflammatory response. Am J Physiol. 2011;300(4):H1518–29. doi: 10.1152/ajpheart.00928.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berger C, Fiorelli M, Steiner T, et al. Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic? Stroke. 2001;32(6):1330–5. doi: 10.1161/01.str.32.6.1330. [DOI] [PubMed] [Google Scholar]
- 29.Engelter ST, Soinne L, Ringleb P, et al. IV thrombolysis and statins. Neurology. 2011;77(9):888–95. doi: 10.1212/WNL.0b013e31822c9135. [DOI] [PubMed] [Google Scholar]
- 30.Kharofa J, Sekar P, Haverbusch M, et al. Selective serotonin reuptake inhibitors and risk of hemorrhagic stroke. Stroke. 2007;38(11):3049–51. doi: 10.1161/STROKEAHA.107.491472. [DOI] [PubMed] [Google Scholar]
- 31.Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369(9558):293–8. doi: 10.1016/S0140-6736(07)60151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.