Abstract
Neuronal signaling consumes much of the brain energy, mainly through the restoration of the membrane potential (MP) by ATP-consuming ionic pumps. We have reported that, compared with waking, ATP levels increase during the initial hours of natural slow-wave sleep, a time with prominent EEG delta oscillations (0.5-4.5Hz). We have hypothesized that there is a delta oscillation-ATP increase coupling, since, during delta waves, neurons exhibit a prolonged hyperpolarizing phase followed by a very brief phase of action potentials. However, direct proof of this hypothesis is lacking, and rapid changes in EEG/ neuronal activity preclude measurement in the naturally sleeping brain. Thus, to induce a uniform state with pure delta oscillations and one previously shown to be accompanied by a similar pattern of neuronal activity during delta waves as natural sleep, we used ketamine-xylazine treatment in rats. We here report that, with this treatment, the high energy molecules ATP and ADP increased in frontal and cingulate cortices, basal forebrain, and hippocampus compared with spontaneous waking. Moreover, the degree of ATP increase positively and significantly correlated with the degree of EEG delta-activity. Supporting the hypothesis of decreased ATP consumption during delta activity, the ATP-consuming Na+-K+-ATPase mRNA levels were significantly decreased whereas the mRNAs for the ATP-producing cytochrome c oxidase (COX) subunits COX III and COX IVa were unchanged. Taken together, these data support the hypothesis of a cortical delta oscillation-dependent reduction in ATP consumption, thus providing the brain with increased ATP availability, and likely occurring because of reduced Na+-K+-ATPase related energy consumption.
Keywords: ATP, Brain Energy Metabolism, Ketamine-Xylazine, Slow-Wave-Activity, Na+-K+-ATPase, NREM Sleep
1. Introduction
Neuronal information processing is metabolically expensive. Recent work indicates that some 87% of brain energy consumption is proportional to neuronal firing rates, predominantly consumed in the restoration of membrane potential (MP) after postsynaptic potentials (PSPs), whereas only 13% is used to maintain the resting potential (Attwell and Gibb, 2005). This increase in brain energy consumption with PSPs is a function of the increased activity of the sodium-potassium pump (Na+-K+-ATPase) which is activated by the influx of sodium ions and efflux of potassium ions accompanying PSP or action potential depolarizations. In contrast, periods of decreased neuronal activity, as observed during spontaneous sleep or some forms of anesthesia might reduce brain energy demand and conserve brain energy (Dworak et al., 2007, 2010, 2011a,b). These periods of reduced neuronal activity are characterized by delta oscillations (0.5–4.5 Hz), with each delta wave comprised of a prolonged (0.2-0.8s) hyperpolarization followed by a brief burst of action potentials (Steriade et al., 1993a, Steriade et al., 1993b, Steriade and McCarley, 2005). Overall, neuronal activity decreases during delta oscillations since the long-duration “down” state of hyperpolarization and neuronal inactivity outweighs that the much shorter “up” state with activity (Vyazovskiy et al., 2009).
In a previous study we showed a surge in ATP during the initial hours of spontaneous non-rapid eye movement (NREM) sleep; the degree of ATP increase showed a strong correlation with the degree of EEG delta activity during this time (Dworak et al., 2010). We hypothesized that there was a coupling of delta oscillation-ATP increase, based on the lessened neuronal activity during delta waves. However, testing of this hypothesis during spontaneous, natural NREM sleep was not feasible, due to the variation in delta intensity of time periods sufficiently long for measurement of ATP. We thus turned to ketamine-xylazine (KX) anesthesia to induce a uniform state with pure delta oscillations. During KX anesthesia, cortical, thalamocortical and nucleus reticularis thalamic neurons show discharges coincident with to EEG delta oscillations (1-4 Hz) (Contreras and Steriade, 1997), as do these neurons during the delta oscillations of natural sleep (see review in Steriade and McCarley, 2005).
To facilitate comparison with the natural sleep findings, the present KX study measured ATP in the same 4 brain regions where we previously measured ATP increases during the delta-rich initial hours of NREM sleep. The basal forebrain (BF) predominantly contains wake-active neurons that increase firing during wake and sleep deprivation and has been implicated in the homeostatic sleep response (Basheer et al., 2004). The frontal cortex (FC) and cingulate cortex (CCX) receive projections from BF neurons (Jones 2004, Szymusiak 1995, Zaborszky and Duque 2003). Morevoer, the role of FC in sleep regulation is supported by a recent paper, which showed that A1 receptor-mediated inhibition of acetylcholine release in FC decreased wakefulness, and promoted slow EEG activity (Van Dort et al., 2009). Both FC and CCX are involved in attention and cognition. Hippocampus (HIPP) is critical in learning and memory, functions that are facilitated with sleep. Moreover, to corroborate and elaborate further the ATP results, we also measured the energy-related compounds of phosphocreatine (PCr), creatine (Cr), adenosine-diphosphate (ADP), adenosine-monophosphate (AMP) and adenosine (AD). To provide a measure of whether any KX treatment increases in ATP were the result of decreased utilization and/or increased production, we examined mRNA for the key ATP utilizing enzyme, Na+-K+-ATPase, and the ATP-producing cytochrome c oxidase (COX) subunits COX III and COX IVa.
2. Materials and Methods
2.1. Animals and electroencephalogram (EEG) electrode implantation
All experiments were conducted in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care and Use Committee at Boston VA Healthcare system, Harvard University and U.S. National Institute of Health. Male Sprague-Dawley rats (350-400 g) were housed in 12 h light-dark cycle (lights on 7AM to 7PM) with food and water provided ad libitum. For EEG recordings, electrodes were implanted epidurally over the frontal (primary motor, AP = +2.0; ML = 2.0) and parietal (retrosplenial, AP = -4.0; ML = 1.0) cortices under general anesthesia. EMG recording electrodes were implanted into nuchal muscles.
2.2. Ketamine-Xylazine administration and EEG measurements
EEG was monitored 7 days post-surgery for a 24h period. The EEG/EMG signals were amplified and sampled at 104Hz. EEG recordings (acquisition using Grass Gamma v4.3) were scored using the Rat Sleep Stager (version 3.2) in 10sec epochs manually.
On the experimental day, the rats were injected (i.p.) with KX (ketamine 100mg/kg, xylazine 5mg/kg,) or saline (injection controls) at 8:30PM. The level of anesthesia was maintained for 90 minutes and controlled by the EEG pattern and hind limb pinching reflex tests. EEG, heart rate and body temperature were controlled throughout the whole anesthesia period and body temperature was maintained constant (36.0 ± 0.11° C) using an adjustable heating pad. The EEG pattern and loss of responsiveness to the hind limb pinching reflex tests remained stable during 90 minutes of KX anesthesia and no additional dose of KX was necessary in all rats. The Saline injected rats were awake most of the time and served as controls.
2.3. EEG spectral analysis
For EEG power spectral analysis, the EEG power density was calculated in 0.2 Hz intervals in the 0.2-25.0 Hz range. A Hanning window was applied to the data before the transform, and artifacts were removed. The fast Fourier transform was applied to the EEG signal at 5 s intervals. The resulting frequency resolution was 0.2 Hz, and the lowest two frequency bins, 0.2 and 0.4 Hz, were discarded due to the sensitivity of these bins to noise. Frequency bins were averaged for 0.6 Hz bins and each value was expressed as a relative percentage of the total. Spectral analysis of average power was conducted across four frequency ranges: delta (0.5– 4.5 Hz), theta (4.5– 8.5 Hz), alpha (8.5–12.5 Hz), and sigma (12.5–15.5 Hz).
2.4. Brain tissue collection and measurements of high energy ATP
After 90 minutes of KX or saline treatment the rats were decapitated and the brain regions were rapidly (80.0±9.0 sec) dissected on dry-ice (-78.5°C) out of 2mm thick coronal slices (frontal cortex (FC), Bregma 4.2 to 2.2; cingulate cortex (CCX) and basal forebrain (BF), Bregma -0.26 to -1.2, entire hippocampus) and stored at -80° C.
Determination of ATP was performed as described previously (Dworak et al., 2010). Briefly, a luciferin-luciferase based Assay (Lundin and Thore, 1975, McElroy and DeLuca, 1983) was performed using a commercial ATP Assay system with bioluminescence detection kit (Enliten, Promega). Weighed tissue samples were homogenized in 5% trichloroacetic acid (TCA) and transferred to 1.5 ml Eppendorf tubes. The samples were centrifuged at 5000 rpm in the cold for 5 min and the supernatant were transferred to a fresh tube. Samples (10μl) were neutralized with Tris Acetate buffer (490 μl) adjusted to a pH value of 7.75. The luciferase reagent was added immediately before measurement in the luminometer (Flexstation III, Molecular Devices, Sunnyvale, CA), as described by the supplier. The absolute concentration was calculated per mg wet tissue weight using known ATP standards provided in the kit. This method gave highly reproducible results.
It should be noted that measurements from tissue samples include both, the intra- and extracellular concentrations of various metabolites. However, intracellular ATP concentrations are much higher (1.000 – 10.000 fold) than the low extracellular concentrations (~10nM) (Pegg et al., 2010). Thus, changes in brain tissue ATP concentrations reflect primary intracellular ATP concentrations and only to a small extend the extracellular levels.
2.5. Reversed-phase high performance liquid chromatography (RP-HPLC)
For further determination of brain energy metabolism, we measured the concentrations of the following energy transferring molecules, nucleotides and nucleosides using reversed phase high performance liquid chromatography and UV-detection: phosphocreatine (PCr), creatine (Cr), adenosine-diphosphate (ADP), adenosine-monophosphate (AMP) and adenosine (AD). A C-18, 150 × 3.9 mm, 4μm particle size column (Waters), was equilibrated with a mobile phase containing 14mM H2KO4P and 3mM tetrabutylammonium bisulfate (Sigma) adjusted to pH 5.4 with 2M KOH. A gradient method was generated using a second mobile phase (70% methanol). TCA-supernatants were neutralized with 2M K2HPO4. Quantitative analysis of both standards and samples was performed at 210 nm wavelengths for PCr and Cr and 254nm wavelength for AD, AMP, and ADP and calculated to the known standards as described earlier Helzberg et al., 1987, Dworak et al., 2007, 2010)
RNA extraction and real-time PCR
RNA extraction and RT-PCR was performed as described earlier (Chen et al., 2006). Briefly, RNA extraction was performed using TRIzol® reagent. The tissue was homogenized in 500 μL of TRizol followed by addition of 100 μL of chloroform. After mixing for 2min at room temperature the samples were centrifuged at 12 000 g for 15 min at 4° C and the supernatant mixed with 1 μL of glycogen and 250 μL of isopropyl alcohol. The RNA precipitate was collected by centrifugation at 12000 g for 15 min at 4° C. The pellets were washed twice with 75% ethanol, air-dried and resuspended in 50 μL RNase-free water. For RT-PCR (performed in duplicates), RNA (2 μL) was reverse transcribed using superscript II. RT-PCR were performed using TaqMan primer (Applied Biosystems) for rat Na+-K+-ATPase α1 (Rn01533986_m1), cytochrome oxidase (COX) subunits, COX III (Rn03296820_s1) and COX IVa (Rn00665001_gi). 18S RNA (Cat. No.4333760F) served as the internal control. The relative quantification was performed using the comparative threshold cycle method (ΔΔCt method) (Livak and Schmittgen, 2001).
3. Results
3.1.Effects of KX on EEG-activity
KX administration produced immobilization, loss of the righting reflex, and, within 5 minutes from injection, an EEG pattern dominated by slow waves that persisted until the end of the experiment (Fig. 1A). EEG power spectral analysis showed a significant increase in the EEG delta-range (0.5-4.5 Hz) and clamped the slow oscillation at a particular frequency between 1.5 – 3.0 Hz (Fig. 1B).
Figure 1. Ketamine-xylazine induced changes in EEG activity and delta activity.
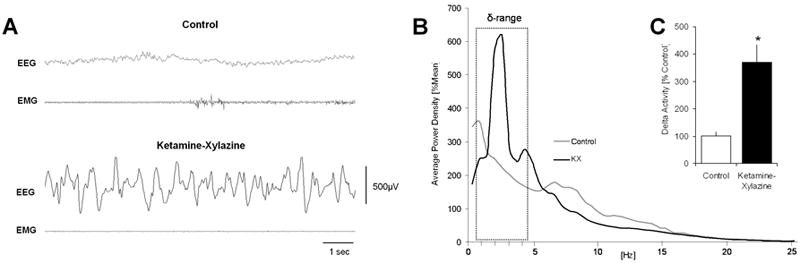
(A) EEG changes after acute administration of ketamine-xylazine (KX). KX treatment (100mg/kg, 5mg/kg, i.p.) produced an EEG pattern dominated by slow waves compared with the EEG of saline injected controls recorded at the same time of day. Controls predominantly showed low amplitude, high frequency wake EEG. (B) Average EEG power spectra in KX and saline treated control rats (n = 6). Note the KX-induced high values of spectral power in the δ-frequency range, predominantly between 1.5 and 3.0 Hz. (C) KX treated animals showed a significant increase in total EEG δ-activity (n = 7, P = 0.002) when compared to saline treated controls.
EEG analysis showed a significant increase (370.88 ± 51.34%, n = 7, p=0.002) in total EEG delta-activity when compared to the same time period on a baseline day with saline treatment (101.03 ± 13.34%, n = 7) (Fig. 1C).
3.2. Effects of KX treatment on brain ATP concentrations
There was a significant increase in μmol/g of tissue ATP concentrations at the end of the experimental period in all 4 brain regions (Fig. 2A) of KX injected rats when compared with saline controls (ANOVA H=42.162, df (7), P=0.001) (Fig. 2B). EEG delta-activity measured over the 90 min KX-treatment period showed a significant positive correlation (Pearson) in all brain regions with ATP levels, measured at the end of 90 min (Fig. 2C; FC (R = 0.860, P = 0.0007), BF (R = 0.650, P = 0.02), CCX (R = 0.945, P = 0.00004), HIPP (R = 0.484, P = 0.132)). Taken together, these results indicate that brain ATP concentrations are significantly increased after KX treatment and correlate positively with EEG-delta activity.
Figure 2. Ketamine-xylazine induced delta-oscillations correlate with brain regional ATP-concentrations.

(A) Coronal slices with red indicated sampled regions in frontal cortex (FC), basal forebrain (BF), cingulate cortex (CCX) and hippocampus (HIPP) (Paxinos and Watson, 2005). (B) Percent increase in ATP concentrations in 4 brain regions after KX. ATP concentrations increased significantly (ANOVA H=42.162, df (7), P=0.001) in FC, BF, CCX and HIPP after KX treatment when compared to saline treated controls. (C) Correlation between ATP concentrations in μmol/g tissue for 4 brain regions and cortical EEG delta activity. Note the strong positive correlations between ATP levels and EEG delta activity. Closed circles = values after KX. Open circles = control values (saline treatment).
3.3.HPLC results for AD, AMP, ADP, Cr and PCr
As shown in Fig. 3 brain energy concentrations increased with KX treatment when compared to control rats. The levels of ADP increased significantly in FC (+ 13.49%, P < 0.05), BF (+37.18%, P < 0.05), CCX (+ 37.95%, P < 0.05) and HIPP: (+ 24.53%, P < 0.05; ANOVA and Student-Newman-Keuls post-hoc analysis) after KX (Fig. 3A). Changes in PCr and Cr were limited to FC (Cr -34.67%, P < 0.05) and CCX (PCr + 61.50%, P < 0.05). AMP and AD concentrations decreased significantly (AMP levels: FC: -32.80%, P < 0.05; BF: -17.44%, P < 0.05; CCX: -25.10%, P < 0.05; and AD levels: FC: -24.45%, P < 0.05; CCX: -22.23%, P < 0.05) (Fig. 3). The concentrations of tissue AD in BF and HIPP did not change after KX treatment.
Figure 3. Ketamine-xylazine (KX) treatment increases brain energy levels.
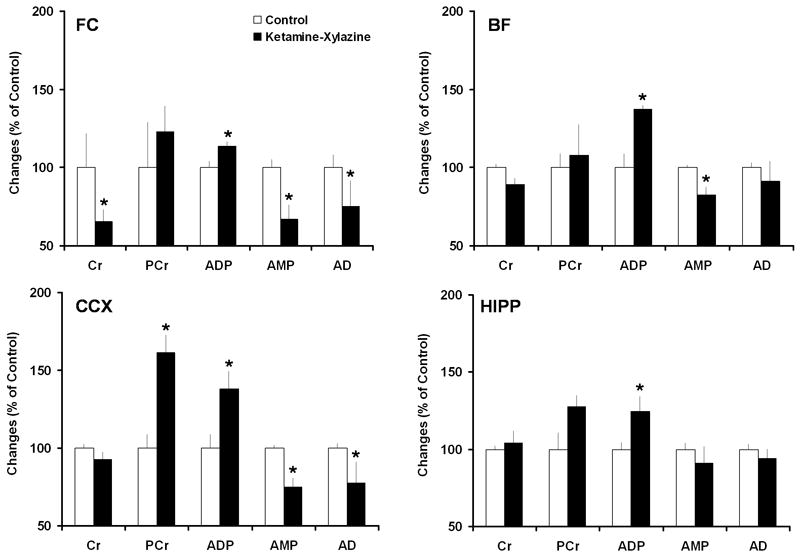
Increased brain energy levels after KX treatment. The concentrations of ADP increased significantly in all tested brain regions. PCr and Cr changed only in FC and CCX. Consistent with the ATP and ADP increases, there were significant decreases in AMP and AD concentrations in FC, BF and CCX. Statistical analysis was performed using ANOVA followed by Student-Newman-Keuls post-hoc analysis. *P < 0.05 versus control treatment.
3.4. Decreased mRNA expression of energy-consuming enzymes but not energy-producing enzymes after KX treatment
Since the most prominent effects of KX treatment on the energy metabolites were observed in the cortex, it was here that we investigated changes in the mRNA expression of the ATP-consuming enzyme, Na+-K+-ATPase and two subunits of the ATP- synthesizing protein complex COX, COX III and COX IVa after KX treatment. Both, FC and CCX showed a significant decrease in the mRNA levels of Na+-K+-ATPase (FC: -28.23%, P = 0.013; CCX: -75.88%, P = 0.041) (Fig. 3B); COX III and COX IVa mRNA expression remained unchanged (COX III, FC: -17.57%, P = 0.234; CCX: -29.11%, P=0.249; COX IVa FC: -9.70 %; P = 0.594; CCX: -28.87%; P = 0.279) (Fig. 4).
Figure 4. Ketamine-xylazine (KX) treatment decreases mRNA expression of energy-consuming enzymes but not energy-producing enzymes.
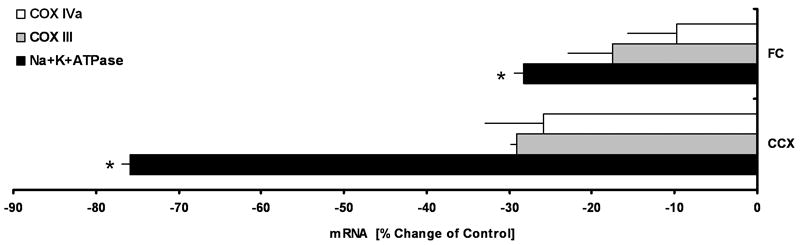
(A) Quantitative gene expression analysis by real-time RT-PCR of energy-producing and energy-consuming enzymes. KX treatment resulted in a significant decrease in the expression of Na+-K+-ATPase mRNA. (FC: P = 0.013; CCX: P = 0.041). No significant changes were observed in the expression of COX III and COX IVa. ABBREVIATIONS: creatine (Cr), phosphocreatine (PCr), adenosine-diphosphate (ADP), adenosine-monophosphate (AMP), adenosine (AD), cytochrome c oxidase (COX), sodium-potassium-ATPase (Na+-K+-ATPase).
Discussion
In the present study we used KX treatment in rats to induce a uniform state characterized by delta oscillations. With this treatment, the high energy molecules ATP and ADP increased in frontal and cingulate cortices, basal forebrain, and hippocampus. Moreover, the degree of ATP increase significantly correlated with the level of increase in EEG delta-activity in both KX-treated animals and their saline-injected controls. In contrast to ATP levels, ATP-consuming Na+-K+-ATPase mRNA levels were significantly decreased whereas the mRNAs for the ATP-producing cytochrome c oxidase (COX) subunits COX III and COX IVa were unchanged. Together, these data support the hypothesis that cortical delta oscillation are coupled to ATP increase which, in part, results from decreased Na+-K+-ATPase dependent ATP consumption.
We see these findings as highly relevant to two major streams of recent neuroscience interest, delta oscillations and brain energy usage. First, delta oscillations have become of widespread interest because of their relevance to sleep processes of homeostasis and synaptic reorganization (Borbely, 2001, Tononi and Cirelli, 2006), to human cognitive processes (Kirov et al., 2009, Walker, 2009), to descriptions of delta-wave associated neural activity (McCarley, 2005), and to anesthesia (Franks, 2008). Second, brain energy usage has become an important topic for the fields of behavioral state control (Dworak et al., 2010, Petit et al., 2010, Nikonova et al., 2010) and of investigations of the relationship of neuronal activity to energy metabolism (Magistretti 2009, Dworak et al., 2010). We believe our findings provide a confluence of these streams of interest, by relating delta oscillations to neural energy changes.
Our data are in agreement with previous observations showing that neuronal signaling is metabolically expensive (Attwell and Laughlin, 2001, Attwell and Gibb, 2005) and that periods of neuronal slowdown increase the availability of high energy molecules such as ATP (Dworak et al., 2007, 2010, 2011a,b).
Energy metabolism is highly organized within cells and tightly coupled to neuronal activity (Ames, 2000, Dhar and Wong-Riley, 2009, Magistretti, 2009). Postsynaptic potentials and associated ATP-consuming ion pumping dominate the energy requirements for neuronal signaling (Alle et al., 2009, Magistretti, 2009). Because glutamate synapses represent at least 80% of cortical synapses, most PSPs are excitatory (Buzsaki et al., 2007). In contrast to the sustained discharge activity in waking, the prolonged hyperpolarization and consequent quiescence of cortical neurons that occurs during the long-lasting “down” states of delta-oscillations, in contrast to the very short-duration discharges during the “up” states of delta-oscillations (Steriade and McCarley, 2005), will lead to an overall reduction in energy consumption. This reduction is due to both fewer action potentials and consequently fewer PSPs generated in target neurons.
The levels of intracellular ATP have been shown to vary in many systems (Dworak et al., 2011). The ATP levels show circadian variations in liver and heart (Kaminsky et al., 1984) and decrease with sleep deprivation in wake-associated brain regions (Dworak et al., 2011b). Five-fold increase in ATP is reported during ascorbate-induced differentiation of osteoblasts (Komarova et al., 2000). Induction of anorectic activity by steroidal glycoside causes a two fold increase in hypothalamic ATP (MacLean and Luo, 2004). Intracellular ATP levels drop from 4-8mM to 2-3mM during muscle fatigue (Harris DA, 1996). The energy charge of the adenylate pool: ([ATP]+1/2[ADP]/([ATP]+[ADP]+[AMP]) has been proposed as a control parameter in the regulatory interactions by which biological homeostasis is maintained (Atkinson and Walton, 1967). Decrease in ATP concentration result in increases in ADP and more so in AMP, but without drastic changes in the overall energy charge. In normal brain tissue the energy charge is closer to 0.85 (Derr and Zieve, 1972) and is regulated by direct participation of adenine nucleotides in energy converting processes such as glycolysis, oxidative phosphorylation and in many energy-expending biosynthetic pathways such as those of amino acids, nucleic acids, protein, fatty acids and cholesterol synthesis (Thompson and Atkinson, 1971). Ketamine has been shown to alter the glucose utilization and thus the regional metabolism in brain (Crosby et al., 1982). Here we report chages in the regional levels of ATP during ketamine-induced increase in delta activity.
That the cortical regions of FC and CCX show the most significant correlation between KX and NREM delta (Fig. 2C) is in agreement with the cortical predominance of NMDA-receptors and the HCN1 channels (Jarvis et al., 1987; Monteggia et al., 2000; Notomi and Shigemoto, 2004). The observation showing no change in AD concentrations in the BF tissue suggests that intracellular adenosine levels are unaffected by KX, since AD from homogenized tissue predominantly represents intracellular AD and thus do not do not contradict the somnogenic role of extracellular adenosine (Porkka-Heiskanen et al., 1997, Basheer et al., 2004).
Our studies evaluated whether an increased energy production or reduced energy consumption, or both, during KX anesthesia accounted for the observed increase in brain energy. Among the diverse metabolic activities of neurons, ion pumping requires by far the most ATP (Erecinska and Silver, 1989). The most prominent ion pump in terms of energy requirements is ATP- consuming Na+-K+-ATPase, known to be highly expressed in the brain (Klodos and Ottolenghi, 1975). A decrease in the expression of the Na+-K+-ATPase suggests a decreased use of the pump during KX-induced slow wave oscillation.
We observed no evidence for increased energy production, since mRNA expression for COX III and COX IVa did not change significantly thus indicating a lack of inhibition of mitochondrial oxidative phosporylation. This may be a consequence of only minimal changes in the ATP/ADP ratio, known to be involved in the regulation of cytochrome c oxidase (Arnold and Kadenbach, 1997, 1999, Ludwig et al., 2001), since both the levels of ATP and ADP increased significantly with KX treatment.
Ketamine easily crosses the blood-brain-barrier and binds the NMDA receptor-ion channel. At sub-anesthetic doses (2.5-10mg/kg) ketamine acts as antidepressant and has been shown to enhance wakefulness-related cortical gamma oscillations in rat (Pinault, 2008). On the contrary, higher doses of Ketamine (50-100mg/kg), inhibits both NMDA receptors and the HCN1 pacemaker channels (Thomson et al., 1985, Oshima and Richards, 1988). Inhibition of both NMDA receptor and HCN1 channels is suggested to depress excitatory synaptic transmission, and enhance cortical synchronization and hypnosis, respectively. On the other hand, xylazine is a muscle relaxant and minimizes the effects produced by ketamine alone, such as tremors, muscle rigidity, and excitement during recovery (Wright, 1982). Acting as an agonist for alpha 2-noradrenoreceptor, xylazine inhibits noradrenergic neurotransmission, thus further reducing the excitatory noradrenergic influence in brain (Oria et al, 2008). There is similarity between the EEG patterns seen in slow-wave sleep and those seen in the maintenance period of general anesthesia (Brown et al.,2010). Even if the EEG pattern with KX-anesthesia is not identical but similar with the EEG pattern of natural SWS, recent data suggest that the occurrence of natural and pharmacologically induces slow-wave activity might share common functions (Nelson et al., 2010). One hour of anesthesia reduced significantly the SWA rebound expected following 4 h of sleep deprivation in rats and suggests that anesthesia slow waves may displace for sleep slow waves (Nelson et al.,2010). Imaging studies have shown obvious parallels between the anesthetized brain and the brain during deep NREM sleep (Boly et al., 2008). The neurophysiologic similarities during anesthesia and natural sleep might share common functional consequences, supported by recent observations indicating that a recovery process similar to that occurring during natural sleep also takes place during anesthesia and that anesthesia induced slow waves may substitute for sleep slow waves during recovery from sleep deprivation (Tung et al., 2004, Tung and Mendelson, 2004).
These functional similarities might be, at least in part, based on the increased energy availability associated with prolonged periods of reduced neuronal activity. High ATP levels could favor structural changes through supporting the synthesis of plasticity related proteins by favoring anabolic processes (Hoeffer and Klann,2010, Dennis et al., 2001). The close association between brain ATP concentrations and the AMP-activated protein kinase (AMPK), known to be a sensor of cellular energy status and regulator of anabolic and catabolic processes, links energy metabolism and anabolic processes at the molecular level (Hardie, 2007). This regulatory pathway might play an important role in the regulation of anabolism and catabolism in the brain during different behavioral states depending on the energy availability (Dworak et al., 2010). Further studies are needed to examine the functional implications of the increased brain energy availability.
In conclusion, our data suggest that a cortical delta oscillation-dependent reduction in energy consumption provides the brain with increased energy availability, likely due to reductions in ATP-consuming Na+-K+-ATPase and indicate that periods of reduced neuronal activity provide the brain with increased energy availability.
Highlights.
ATP and ADP levels increase during ketamine-xylazine anesthesia in rat brain regions.
The degree of ATP increase positively correlated with EEG delta-activity.
ATP-consuming Na+/K+-ATPase mRNA levels were significantly decreased with ketamine-xylazine treatment.
Cortical delta oscillation-dependent reduction in energy consumption leads to increased ATP.
Acknowledgments
We gratefully acknowledge Farzana Pervin Nipa for technical assistance, and Diane Ghera and Dewayne Williams for help with animal care. This work was supported by awards from the Department of Veterans Affairs Medical Research Service Award to RB, a Deutsche Forschungsgemeinschaft fellowship (DW66/1-1) to MD, and the National Institute of Mental Health (NIMH39683) to RWM.
Footnotes
Conflict of Interest: The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alle H, Roth A, Geiger JR. Energy-efficient action potentials in hippocampal mossy fibers. Science. 2009;325:1405–1408. doi: 10.1126/science.1174331. [DOI] [PubMed] [Google Scholar]
- Ames A., 3rd CNS energy metabolism as related to function. Brain Res Brain Res Rev. 2000;34:42–68. doi: 10.1016/s0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- Arnold S, Kadenbach B. Cell respiration is controlled by ATP, an allosteric inhibitor of cytochrome-c oxidase. Eur J Biochem. 1997;249:350–354. doi: 10.1111/j.1432-1033.1997.t01-1-00350.x. [DOI] [PubMed] [Google Scholar]
- Arnold S, Kadenbach B. The intramitochondrial ATP/ADP-ratio controls cytochrome c oxidase activity allosterically. FEBS Lett. 1999;443:105–108. doi: 10.1016/s0014-5793(98)01694-9. [DOI] [PubMed] [Google Scholar]
- Atkinson DE, Walton GM. Adenosine triphosphate conservation in metabolic regulation. Rat liver citrate cleavage enzyme. J Biol Chem. 1967;242:3239–3241. [PubMed] [Google Scholar]
- Attwell D, Gibb A. Neuroenergetics and the kinetic design of excitatory synapses. Nat Rev Neurosci. 2005;6:841–849. doi: 10.1038/nrn1784. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Boly M, Phillips C, Tshibanda L, Vanhaudenhuyse A, Schabus M, Dang-Vu TT, Moonen G, Hustinx R, Maquet P, Laureys S. Intrinsic brain activity in altered states of consciousness: how conscious is the default mode of brain function? Ann N Y Acad Sci. 2008;1129:119–129. doi: 10.1196/annals.1417.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely AA. From slow waves to sleep homeostasis: new perspectives. Arch Ital Biol. 2001;139:53–61. [PubMed] [Google Scholar]
- Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Kaila K, Raichle M. Inhibition and brain work. Neuron. 2007;56:771–783. doi: 10.1016/j.neuron.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Thakkar MM, Winston S, Bolortuya Y, Basheer R, McCarley RW. REM sleep changes in rats induced by siRNA-mediated orexin knockdown. Eur J Neurosci. 2006;24:2039–2048. doi: 10.1111/j.1460-9568.2006.05058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Steriade M. State-dependent fluctuations of low-frequency rhythms in cortico-thalamic network. Neuroscience. 1997;76:25–38. doi: 10.1016/s0306-4522(96)00392-2. [DOI] [PubMed] [Google Scholar]
- Crosby G, Crane AM, Sokoloff L. Local changes in cerebral glucose utilization during ketamine anesthesia. Anesthesiology. 1982;56:437–443. doi: 10.1097/00000542-198206000-00005. [DOI] [PubMed] [Google Scholar]
- Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294:1102–1105. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- Derr RF, Zieve L. Adenylate energy charge: relation to guanylate energy charge and the adenylate kinase equilibrium constant. Biochem Biophys Res Commun. 1972;49:1385–1390. doi: 10.1016/0006-291x(72)90492-5. [DOI] [PubMed] [Google Scholar]
- Dhar SS, Wong-Riley MT. Coupling of energy metabolism and synaptic transmission at the transcriptional level: role of nuclear respiratory factor 1 in regulating both cytochrome c oxidase and NMDA glutamate receptor subunit genes. J Neurosci. 2009;29:483–492. doi: 10.1523/JNEUROSCI.3704-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworak M, Diel P, Voss S, Hollmann W, Struder HK. Intense exercise increases adenosine concentrations in rat brain: implications for a homeostatic sleep drive. Neuroscience. 2007;150:789–795. doi: 10.1016/j.neuroscience.2007.09.062. [DOI] [PubMed] [Google Scholar]
- Dworak M, McCarley RW, Kim T, Kalinchuk AV, Basheer R. Sleep and brain energy levels: ATP changes during sleep. J Neurosci. 2010;30:9007–9016. doi: 10.1523/JNEUROSCI.1423-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworak M, McCarley RW, Kim T, Kalinchuk AV, Basheer R. Replies to commentaries on ATP changes during sleep. Sleep. 2011a;34:841–843. doi: 10.5665/SLEEP.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworak M, Kim T, McCarley RW, Basheer R. Sleep, brain energy levels and food intake. Somnology. 2011b;15:111–117. doi: 10.1007/s11818-011-0524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Silver IA. ATP and brain function. J Cereb Blood Flow Metab. 1989;9:2–19. doi: 10.1038/jcbfm.1989.2. [DOI] [PubMed] [Google Scholar]
- Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- Harris DA. Cellular ATP. Principles of Medical Biol. 1996;4:1–47. [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Helzberg JH, Brown MS, Smith DJ, Gore JC, Gordon ER. Metabolic state of the rat liver with ethanol: comparison of in vivo 31phosphorus nuclear magnetic resonance spectroscopy with freeze clamp assessment. Hepatology. 1987;7:83–88. doi: 10.1002/hep.1840070118. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Murphy DE, Williams M. Quantitative autoradiographic localization of NMDA receptors in rat brain using [3H]CPP: comparison with [3H]TCP binding sites. Eur J Pharmacol. 1987;14:149–152. doi: 10.1016/0014-2999(87)90423-7. [DOI] [PubMed] [Google Scholar]
- Jones BE. Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Prog Brain Res. 2004;145:157–169. doi: 10.1016/S0079-6123(03)45011-5. [DOI] [PubMed] [Google Scholar]
- Kaminsky YG, Kosenko EA, Kondrashova MN. Analysis of the circadian rhythm in energy metabolism of rat liver. Int J Biochem. 1984;16:629–639. doi: 10.1016/0020-711x(84)90032-6. [DOI] [PubMed] [Google Scholar]
- Kirov R, Weiss C, Siebner HR, Born J, Marshall L. Slow oscillation electrical brain stimulation during waking promotes EEG theta activity and memory encoding. Proc Natl Acad Sci U S A. 2009;106:15460–15465. doi: 10.1073/pnas.0904438106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klodos I, Ottolenghi P. Large-scale preparation of Na, K-ATPase from ox brain. Anal Biochem. 1975;67:397–403. doi: 10.1016/0003-2697(75)90311-5. [DOI] [PubMed] [Google Scholar]
- Komarova SV, Ataullakhanov FI, Globus RK. Bioenergetics and mitochondrial transmembrane potential during differentiation of cultured osteoblasts. Am J Physiol Cell Physiol. 2000;279:C1220–1229. doi: 10.1152/ajpcell.2000.279.4.C1220. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ludwig B, Bender E, Arnold S, Huttemann M, Lee I, Kadenbach B. Cytochrome C oxidase and the regulation of oxidative phosphorylation. Chembiochem. 2001;2:392–403. doi: 10.1002/1439-7633(20010601)2:6<392::AID-CBIC392>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Lundin A, Thore A. Analytical information obtainable by evaluation of the time course of firefly bioluminescence in the assay of ATP. Anal Biochem. 1975;66:47–63. doi: 10.1016/0003-2697(75)90723-x. [DOI] [PubMed] [Google Scholar]
- MacLean DB, Luo LG. Increased ATP content/production in the hypothalamus may be a signal for energy-sensing of satiety: studies of the anorectic mechanism of a plant steroidal glycoside. Brain Res. 2004;1020:1–11. doi: 10.1016/j.brainres.2004.04.041. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ. Neuroscience. Low-cost travel in neurons. Science. 2009;325:1349–1351. doi: 10.1126/science.1180102. [DOI] [PubMed] [Google Scholar]
- McElroy WD, DeLuca MA. Firefly and bacterial luminescence: basic science and applications. J Appl Biochem. 1983;5:197–209. [PubMed] [Google Scholar]
- Monteggia LM, Eisch AJ, Tang MD, Kaczmarek LK, Nestler EJ. Cloning and localization of the hyperpolarization-activated cyclic nucleotide-gated family in rat brain. Brain Res Mol Br Res. 2000;81:129–139. doi: 10.1016/s0169-328x(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Faraguna U, Tononi G, Cirelli C. Effects of anesthesia on the response to sleep deprivation. Sleep. 2010;33:1659–1667. doi: 10.1093/sleep/33.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonova EV, Naidoo N, Zhang L, Romer M, Carter JR, Scharf MT, Galante RJ, Pack AI. Changes in components of energy regulation in mouse cortex with increases in wakefulenss. Sleep. 2010;33:889–900. doi: 10.1093/sleep/33.7.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol. 2004;471:241–276. doi: 10.1002/cne.11039. [DOI] [PubMed] [Google Scholar]
- Oria M, Chatauret N, Raquer N, Córdoba J. A new method for measuring motor evoked potentials in the awake rat:effects of anesthetics. J Neurotrauma. 2008;25:266–275. doi: 10.1089/neu.2007.0393. [DOI] [PubMed] [Google Scholar]
- Oshima E, Richards CD. An in vitro investigation of the action of ketamine on excitatory synaptic transmission in the hippocampus of the guinea-pig. Eur J Pharmacol. 1988;148:25–33. doi: 10.1016/0014-2999(88)90450-5. [DOI] [PubMed] [Google Scholar]
- Pegg CC, He C, Stronic AR, Kattner KA, Wang CX. Technique for collection of cerebrospinal fluid from the cisterna magna in rat. J Neurosci Methods. 187:8–12. doi: 10.1016/j.jneumeth.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Petit JM, Tobler I, Kopp C, Morgenthaler F, Borbély AA, Magistretti PJ. Metabolic response of the cerebral cortex following gentle sleep deprivation and modafinil administration. Sleep. 2010;33:901–908. doi: 10.1093/sleep/33.7.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 2008;63:730–735. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Amzica F, Nunez A. Cholinergic and noradrenergic modulation of the slow (approximately 0.3 Hz) oscillation in neocortical cells. J Neurophysiol. 1993a;70:1385–1400. doi: 10.1152/jn.1993.70.4.1385. [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Curro Dossi R, Nunez A. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993b;13:3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCarley RW. Synchronized brain oscillations leading to neuronal plasticity during wakign and sleep states. In: Steriade M, McCarley RW, editors. Brain Control of Wakefulness and Sleep. 2. Chapter 7. Kluwer Academic/Plenum Publishers; NY: 2005. pp. 255–380. [Google Scholar]
- Szymusiak R. Magnocellular nuclei of the basal forebrain: substrates of sleep and arousal regulation. Sleep. 1995;18:478–500. doi: 10.1093/sleep/18.6.478. [DOI] [PubMed] [Google Scholar]
- Thompson FM, Atkinson DE. Response of nucleoside diphosphate kinase to the adenylate energy charge. Biochem Biophys Res Commun. 1971;45:1581–1585. doi: 10.1016/0006-291x(71)90201-4. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC, Lodge D. An N-methylaspartate receptor-mediated synapse in rat cerebral cortex: a site of action of ketamine? Nature. 1985;313:479–481. doi: 10.1038/313479a0. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Tung A, Bergmann BM, Herrera S, Cao D, Mendelson WB. Recovery from sleep deprivation occurs during propofol anesthesia. Anesthesiology. 2004;100:1419–1426. doi: 10.1097/00000542-200406000-00014. [DOI] [PubMed] [Google Scholar]
- Tung A, Mendelson WB. Anesthesia and sleep. Sleep Med Rev. 2004;8:213–225. doi: 10.1016/j.smrv.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Van Dort CJ, Baghdoyan HA, Lydic R. Adenosine A(1) and A(2A) receptors in mouse prefrontal cortex modulate acetylcholine release and behavioral arousal. J Neurosci. 2009;29:871–881. doi: 10.1523/JNEUROSCI.4111-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP. The role of slow wave sleep in memory processing. J Clin Sleep Med. 2009;5:S20–26. [PMC free article] [PubMed] [Google Scholar]
- Wright M. Pharmacologic effects of ketamine and its use in veterinary medicine. J Am Vet Med Assoc. 1982;180:1462–1471. [PubMed] [Google Scholar]
- Zaborszky L, Duque A. Sleep-wake mechanisms and basal forebrain circuitry. Front Biosci. 2003;8:d1146–1169. doi: 10.2741/1112. [DOI] [PubMed] [Google Scholar]


