Background: The molecular mechanisms underlying GLP-1 secretion induced by amino acids from intestinal L cells are not fully understood.
Results: The l-amino acid-sensing GPRC6A receptor is expressed in the clonal L cell GLUTag. Activation of GPRC6A by l-ornithine evoked GLP-1 secretion.
Conclusion: GLUTag cells respond to amino acids via the GPRC6A receptor.
Significance: A new pathway for GLP-1 secretion induced by amino acids in GLUTag cells was identified.
Keywords: Amino Acid, Endocrinology, G Protein-coupled Receptor (GPCR), Imaging, Secretion, GPRC6A, Glucagon-like Peptide 1, Intestinal L Cells
Abstract
Although amino acids are dietary nutrients that evoke the secretion of glucagon-like peptide 1 (GLP-1) from intestinal L cells, the precise molecular mechanism(s) by which amino acids regulate GLP-1 secretion from intestinal L cells remains unknown. Here, we show that the G protein-coupled receptor (GPCR), family C group 6 subtype A (GPRC6A), is involved in amino acid-induced GLP-1 secretion from the intestinal L cell line GLUTag. Application of l-ornithine caused an increase in intracellular Ca2+ concentration ([Ca2+]i) in GLUTag cells. Application of a GPRC6A receptor antagonist, a phospholipase C inhibitor, or an IP3 receptor antagonist significantly suppressed the l-ornithine-induced [Ca2+]i increase. We found that the increase in [Ca2+]i stimulated by l-ornithine correlated with GLP-1 secretion and that l-ornithine stimulation increased exocytosis in a dose-dependent manner. Furthermore, depletion of endogenous GPRC6A by a specific small interfering RNA (siRNA) inhibited the l-ornithine-induced [Ca2+]i increase and GLP-1 secretion. Taken together, these findings suggest that the GPRC6A receptor functions as an amino acid sensor in GLUTag cells that promotes GLP-1 secretion.
Introduction
Glucagon-like peptide-1 (GLP-1)3 secretion from intestinal L cells in vivo occurs in response to various dietary components, including glucose, fatty acids, and amino acids. Whereas the mechanisms underlying glucose- and fatty acid-induced GLP-1 secretion are partially understood, the mechanisms underlying amino acid-induced GLP-1 secretion are less clear.
Glucose-induced GLP-1 secretion is thought to be critically dependent on electrogenic uptake of this nutrient via the sodium-dependent glucose transporter-1 (SGLT-1), directly depolarizing the plasma membrane and triggering action potentials, eventually opening voltage-gated Ca2+ channels (1–3). The subsequent rise in cytosolic Ca2+ triggers fusion of GLP-1-containing vesicles. Consistent with this mechanism, SGLT1 knock-out mice lack glucose-triggered Ca2+-responses and GLP-1 secretion (4, 5).
Fatty acids by contrast are thought to act through G protein-coupled receptors (GPCRs) (6). GPR40 (also known as FFAR1), for example, which is abundantly expressed in intestinal L cells, is predominantly coupled to the Gq protein, which activates phospholipase Cγ (PLCγ) upon ligand binding to the receptor. The activation of GPR40 in intestinal L cells results in increased [Ca2+]i via inositol trisphosphate (IP3)-mediated release from the endoplasmic reticulum and subsequent increased secretion of GLP-1. Consistent with an important role of this receptor in L cells, GPR40 knock-out mice display attenuated GLP-1 secretion in response to dietary fat (7).
Amino acids in digested food have also been found to stimulate GLP-1 secretion (8–10). l-Glutamine in particular was found to be a potent secretagogue in the GLUTag cell line and murine L cells in primary culture (11, 12).
l-Glutamine-triggered GLP-1 secretion has been shown to involve sodium-dependent electrogenic uptake; however, additional molecular mechanisms must exist, given the fact that glutamine and asparagine trigger comparable sodium-dependent Ca2+ responses, but glutamine is superior as a secretagogue (11, 12). These differences are not simply explained by mitochondrial metabolism of l-glutamine, as inhibition of this pathway by 6-diazo-5-oxo-l-norleucine (DON) had no effect on l-glutamine-induced GLP-1 secretion (11, 12). l-Glutamine- and other amino acid-induced GLP-1 secretion in intestinal L cells is therefore thought to be regulated by amino acid-sensing receptors as yet unidentified.
In the present study, we hypothesized that amino acid-sensing GPCRs might be involved in GLP-1 secretion. By analogy to fatty acid sensing, we speculated that such GPCRs might couple with the Gq protein to activate PLCγ, increasing first intracellular IP3 ([IP3]i) and subsequently [Ca2+]i. Activation of such receptors in intestinal L cells could induce GLP-1 secretion via increased [Ca2+]i. GPCRs known to respond to extracellular amino acid levels involve various family C members, such as the calcium-sensing receptor (CaR), GPRC6A, and the T1R1/T1R3 heterodimer (also known as the umami receptor) (13).
Here, we provide the first direct evidence showing that the GPRC6A receptor is expressed in the clonal intestinal L cell line GLUTag. Application of the GPRC6A receptor ligand, l-ornithine, a basic l-amino acid, provoked an increase in [Ca2+]i as well as GLP-1 secretion. The effects of l-ornithine on [Ca2+]i and GLP-1 secretion were suppressed by application of a GPRC6A receptor antagonist. Furthermore, the depletion of endogenous GPRC6A by a specific small interfering RNA (siRNA) significantly inhibited l-ornithine-induced GLP-1 secretion from GLUTag cells. These findings indicate that GLUTag cells respond to extracellular amino acids via the GPRC6A receptor.
EXPERIMENTAL PROCEDURES
Chemicals and Expression Vectors
l-Ornithine, l-arginine, l-lysine, l-phenylalanine, l-tryptophan, diazoxide, and nifedipine were purchased from WAKO (Osaka, Japan). Calindol was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). U-73122, 2-aminoethoxydiphenyl borate (2-APB), EDTA, and DDA were purchased from Sigma-Aldrich. Stealth small interfering RNAs (siRNAs) for the GPRC6A receptor (Gprc6a-MSS210013: 5′-UCCAGAUGAUUUCACGACAGGUGUC-3′) were purchased from Invitrogen. Expression vectors encoding green fluorescent protein (GFP)-tagged tissue-type plasminogen activator (tPA-GFP), Venus-tagged brain-derived neurotrophic factor (BDNF-Venus), Venus-tagged neuropeptide Y (NPY-Venus), and GFP-tagged growth hormone (GH) were constructed as described previously (14–17).
Cell Culture and Transfection
GLUTag cells (kindly provided by Dr. Daniel Drucker, Toronto) and STC-1 cells (kindly provided by Dr. Douglas Hanahan, San Francisco) were cultured in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum. Lipofectamine 2000 reagent (Invitrogen) was used for transfection, according to the manufacturer's instructions.
RNA Isolation and RT-PCR Analysis
Total RNA from GLUTag, STC-1, and mouse small intestine was isolated and treated with RNase-free DNase (Promega, Madison, WI). cDNA was synthesized using Prime Script (Takara Bio Inc., Shiga, Japan). For PCR amplification of GPRC6A (NM_153071), the forward primer 5′-CGGGATCCAGACGACCACAAATCCAG-3′ and the reverse primer 5′-CCAAGCTTGATTCATAACTCACCTGTGGC-3′ (amplicon size 363 bp) were used (18). For CaR (NM_013803), the forward primer 5′-AGCAGGTGACCTTCGATGAGT-3′ and the reverse primer 5′-ACTTCCTTGAACACAATGGAGC-3′ (amplicon size 100 bp) were used. For taste receptors type-1 member-1 (T1R1; NM_031867), the forward primer 5′-CTGCCAAAGGACAGAATCCTC-3′ and the reverse primer 5′-GAACCGCATGGCTTGGAAG-3′ (amplicon size 178 bp) were used. For taste receptors type-1 member-3 (T1R3; NM_031872), the forward primer 5′-TGGGGGCCTCTTTGTGTCT-3′ and the reverse primer 5′-TGGGTTGTGTTCTCTGGTTGA-3′ (amplicon size 117 bp) were used. For glyceraldehyde 3-phosphate dehydrogenase (GAPDH, NM_008084), the forward primer 5′-CCATCACCATCTTCCAGGAG-3′ and the reverse primer 5′-TTCAGCTCTGGGATGACCTT-3′ (amplicon size 457 bp) were used.
Quantitative RT-PCR of Fluorescence-activated Cell Sorter (FACS)-sorted Primary Intestinal L Cells
Intestinal L cells were obtained from a transgenic mouse in which cells expressing proglucagon were labeled by the yellow fluorescence protein (Venus). Venus-positive cells were sorted by flow cytometry, as described previously (2). Total RNA from FACS-purified L cells was isolated using a microscale RNA isolation kit (Ambion) and reverse-transcribed according to standard protocols. Quantitative RT-PCR was performed with a 7900 HT Fast Real-Time PCR system (Applied Biosystems), as described previously (2).
Measurement of Intracellular Ca2+ Concentration
Changes in the intracellular Ca2+ concentration ([Ca2+]i) were measured using Fluo-3 acetoxymethylester (Sigma). Cells on coverslips were loaded with 5 μm Fluo-3 for 30 min at 37 °C in Ringer buffer (RB). Cells were then mounted in a chamber and placed on the stage of an Olympus IX-71 inverted microscope (Olympus, Tokyo, Japan). Fluo-3-loaded cells were excited at 480 nm at 5-s intervals using a xenon lamp, and emission signals at 515 nm were detected with an electron multiplying charge-coupled device (EM-CCD) camera (C9100-02; Hamamatsu Photonics, Hamamatsu, Japan).
Immunocytochemistry
GLUTag cells were plated onto poly-l-lysine-coated coverslips. The cells were transfected with tPA-GFP using Lipofectamine 2000. After 2 days of transfection, the cells were washed in phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 20 min at room temperature. The cells were then sequentially reacted with an anti-GLP-1 antibody (1:200 dilution; Yanaihara Institute Inc., Shizuoka, Japan) and anti-rabbit Alexa Fluor 568-conjugated secondary antibody (1:1,000 dilution; Invitrogen). Confocal images were obtained using a Nipkow disk-type confocal microscope (CSU-10, Yokogawa, Tokyo, Japan).
Total Internal Reflection Fluorescence (TIRF) Imaging of tPA-GFP Release
To monitor the release of tPA-GFP at the single vesicle level, we used a TIRF microscope similar to that described previously (14, 19, 20). Imaging was performed in modified RB (130 mm NaCl, 3.6 mm KCl, 0.5 mm NaH2PO4, 0.5 mm MgSO4, 1.5 mm CaCl2, 10 mm HEPES, 2 mm NaHCO3, 5 mm glucose, pH 7.4). Stimulation with amino acids was achieved by perfusion of either 1 mm l-ornithine, or 1 mm l-ornithine plus 10 μm U-73122, 2-APB, or calindol.
To analyze the TIRF imaging data, single exocytotic events were selected manually, and the average fluorescence intensity of an individual vesicle in a 0.7 μm × 0.7 μm square placed over the vesicle center was calculated (14, 19, 20). To distinguish between fusion events and retreated vesicles, we focused on fluorescence changes just before the disappearance of fluorescent signals. A fusion event was demonstrated by a rapid transient increase in fluorescence intensity (to a peak intensity 2.5 times greater than the original fluorescence intensity within 1 s). By contrast, vesicle movements were shown by the fluorescence intensity gradually decreasing to the background level, as described previously (21). The numbers of fusion events during a 20-min period and of plasma membrane-docked vesicles were counted manually.
Measurement of tPA-GFP Release and GLP-1
GLUTag cells were plated at a density of 3.0 × 105 cells in 6-well plates, and after 2 days in culture, the cells were transfected with tPA-GFP using Lipofectamine 2000. After 2 days of transfection, the cells were preincubated in RB for 30 min and then incubated for 2 h in RB with or without the addition of 1 mm l-ornithine, or 1 mm l-ornithine plus various antagonists. The amount of tPA-GFP secreted into the RB was measured by detecting fluorescence using a spectrophotofluorometer from 460 nm to 650 nm (F-7000; Hitachi, Tokyo, Japan), as described previously (14). To measure the amount of secreted GLP-1 into the RB, we used an enzyme-linked immunosorbent assay (ELISA) kit for GLP-1 (Millipore).
RNA Interference of GPRC6A
GLUTag cells were plated onto poly-l-lysine-coated coverslips. The cells were transfected using 20 pmol/μl GPRC6A siRNAs using Lipofectamine 2000, according to the manufacturer's instructions. Imaging and secretion experiments were conducted 2 days after transfection.
Statistics
Data are reported as the mean ± S.E. Means were compared by ANOVA followed by a Newman-Keuls test or a Dunnett's test using Prism software (GraphPad software, La Jolla, CA).
RESULTS
Expression of Amino Acid Receptors in GLUTag, STC-1, and Small Intestine
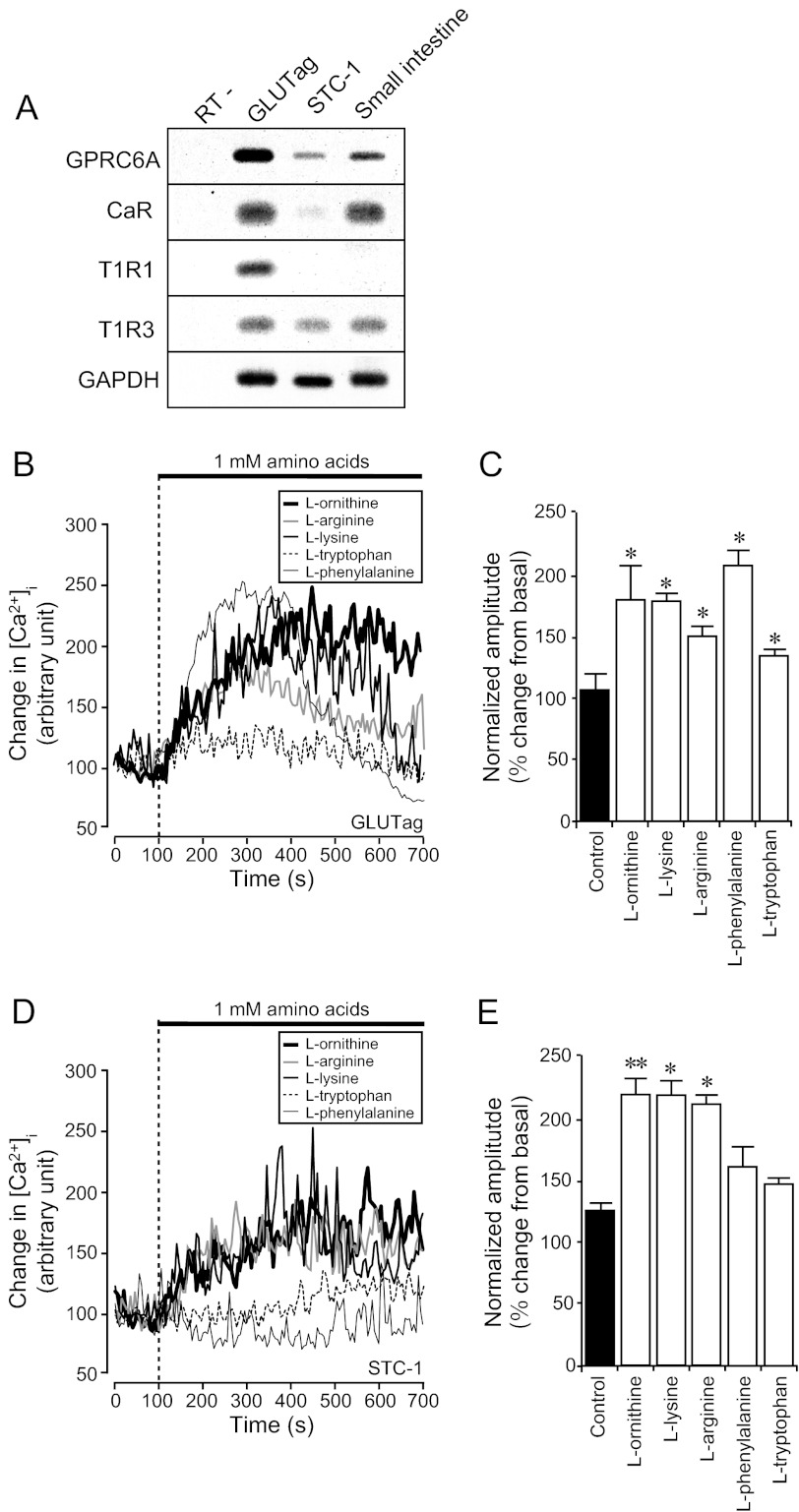
Previous studies have suggested that intestinal L cells respond to changes in the extracellular concentration of amino acids (11, 12). To clarify the underlying mechanism, we first examined whether GLUTag cells expressed known amino acid-sensing receptors, including the CaR (22), taste receptors type-1 (T1R) (23), and GPRC6A (24). These receptors are closely related to metabotropic glutamate receptors (mGluRs) and the GABAB receptor and are thought to be l-amino acid-sensing receptors (25). RT-PCR analysis demonstrated that GLUTag cells expressed mRNAs for GPRC6A, CaR, T1R1, and T1R3 (Fig. 1A). By contrast, another clonal intestinal L cell line, STC-1, expressed only mRNAs for GPRC6A and T1R3, but not T1R1. In the mouse small intestine, we detected mRNAs for GPRC6A, CaR, and T1R3 but were unable to detect T1R1 (Fig. 1A). Although expressed at lower levels than in the GLUTag cell line, we were able to detect GPRC6A expression in FACS-purified mouse primary intestinal L cells by quantitative RT-PCR. Expression of the receptor was enriched in the L cell population compared with non-L cells from the same sorts (supplemental Fig. S1). Because T1R3 requires T1R1 to function as an amino acid sensor (i.e. the umami taste receptor), we focused on GPRC6A and CaR as possible amino acid sensors in the small intestine.
FIGURE 1.
Expression of amino acid receptors in intestinal L cell lines and effect of l-amino acids on [Ca2+]i changes. A, expression of amino acid receptor mRNAs in GLUTag, STC-1, and mouse small intestine analyzed by RT-PCR. B and D, typical time course of [Ca2+]i during application of various l-amino acids to GLUTag cells (B) and to STC-1 cells (D). Stimuli were applied at the times indicated by the dotted line. Basal fluorescence intensity is normalized to 100. C and E, normalized amplitude calculated from the peak amplitude of fluorescence intensity induced by 1 mm l-amino acids. Basal fluorescence intensity is normalized to 100. Data are shown as mean ± S.E. (error bars; n = 8 cells in each condition). *, p < 0.05; **, p < 0.01.
l-Ornithine Increases the Intracellular Ca2+ Concentration in GLUTag and STC-1 Cells
Whereas CaR favors aromatic l-amino acids such as l-tryptophan and l-phenylalanine, GPRC6A and T1R1/T1R3 favor basic l-amino acids (l-ornithine, l-lysine, and l-arginine) and sulfur- or hydroxyl-containing l-amino acids (l-cysteine, l-threonine, and l-serine), respectively (26). To examine whether and which l-amino acids stimulate GLUTag cells, we used the Ca2+-sensing dye, Fluo-3, to monitor the [Ca2+]i following application of different amino acids. l-Ornithine, l-arginine, and l-lysine strongly induced [Ca2+]i responses in GLUTag cells, consistent with activation of GPRC6A. We next applied the CaR agonists, l-tryptophan and l-phenylalanine. l-Phenylalanine significantly elevated [Ca2+]i, whereas l-tryptophan induced only a small [Ca2+]i response (Fig. 1, B and C). These results suggest that various extracellular amino acids elevate [Ca2+]i in intestinal GLUTag cells and that both GPRC6A and CaR are strong candidate receptors.
We further examined whether amino acids similarly provoke [Ca2+]i elevations in another clonal intestinal L cell model, STC-1. STC-1 cells responded to l-ornithine, l-arginine, and l-lysine, but not to l-tryptophan or l-phenylalanine, suggesting that they may utilize GPRC6A but not CaR. These results are consistent with the apparently low expression levels of CaR in this cell line (Fig. 1, A, D, and E).
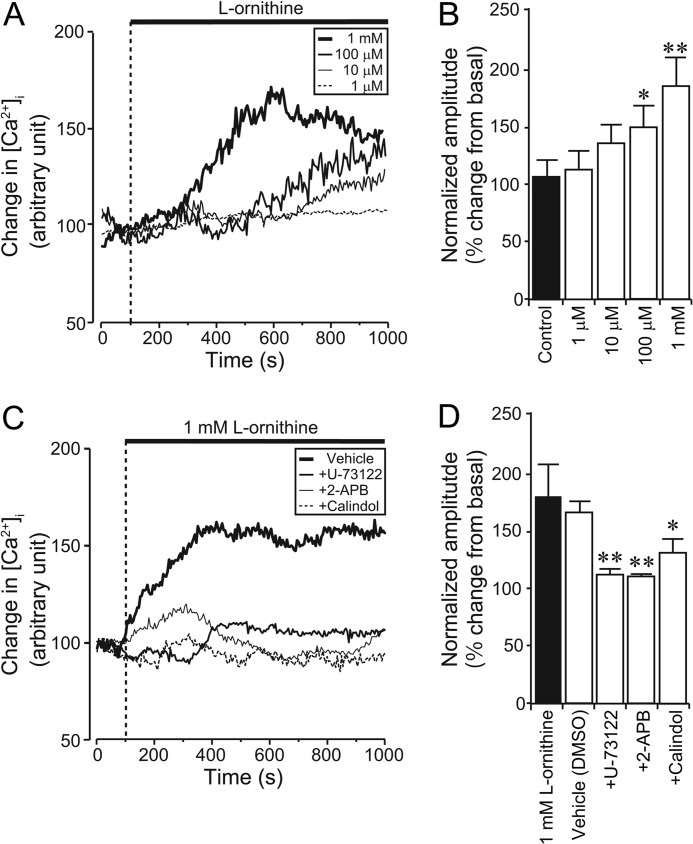
As l-ornithine is a potent and specific stimulant of GPRC6A (27), we further utilized l-ornithine for analyzing GPRC6A function in GLUTag cells. Application of a range of concentrations of l-ornithine significantly induced an increase in [Ca2+]i (Fig. 2A), and the amplitude of the [Ca2+]i changes increased in a dose-dependent manner (Fig. 2B).
FIGURE 2.
Effect of l-ornithine on [Ca2+]i changes in GLUTag cells. A, typical time course showing [Ca2+]i during application of various concentrations of l-ornithine to GLUTag cells. Stimuli were applied at the times indicated by the dotted line. Basal fluorescence intensity is normalized to 100. B, l-ornithine provoking [Ca2+]i elevation in a dose-dependent manner. Normalized amplitude was calculated from the peak amplitude induced by various concentrations of l-ornithine (n = 7 cells in each condition). C, typical time course of [Ca2+]i during the application of a PLC inhibitor (U-73122), an IP3 receptor inhibitor (2-APB), or a GPRC6A antagonist (calindol) together with 1 mm l-ornithine. Note that application of U-73122, 2-APB, or calindol significantly inhibited in the l-ornithine-induced [Ca2+]i increase. D, normalized amplitude calculated from the peak amplitude of fluorescence intensity induced by 1 mm l-ornithine plus U-73122, 2-APB, or calindol (n = 6 cells in each condition). Data are shown as mean ± S.E. (error bars). *, p < 0.05; **, p < 0.01. DMSO, dimethyl sulfoxide.
GPRC6A is coupled to the Gq protein, which activates PLCγ (28) and elevates [Ca2+]i via IP3-mediated release from the endoplasmic reticulum. We therefore examined the effects of PLC and IP3 receptor inhibitors (U-73122 and 2-APB, respectively) on [Ca2+]i responses induced by l-ornithine. Co-administration of either U-73122 or 2-APB with 1 mm l-ornithine significantly suppressed the l-ornithine-induced [Ca2+]i increase. The GPRC6A antagonist calindol (29) also abolished l-ornithine-evoked [Ca2+]i changes (Fig. 2, C and D). These results suggest that the binding of extracellular l-ornithine to GPRC6A increases [Ca2+]i via an IP3-mediated pathway in GLUTag cells.
l-Ornithine Triggers GLP-1 Secretion in GLUTag Cells
To examine whether the l-ornithine-induced [Ca2+]i changes stimulate GLP-1 secretion from GLUTag cells, we observed the dynamics of single GLP-1 secretion events in live GLUTag cells by TIRF microscopy (14, 19, 20, 30). Preferentially, the visualization of GLP-1-containing secretory vesicles with this technique would employ fluorescent protein-tagged GLP-1. However, as GLP-1 is generated by post-translational processing of the proglucagon peptide precursor (31), it is technically difficult to label GLP-1 directly with GFP. To overcome this problem, we investigated the localization of a number of fluorescent protein (FP)-tagged peptide hormones (i.e. tPA, BDNF, NPY, and GH (14–17)) after transient transfection. (Fig. 3A and supplemental Fig. S2). As the targeting efficiency of tPA-GFP to GLP-1-containing vesicles in GLUTag cells was higher than that of the other FP-tagged peptides (supplemental Fig. S2 and supplemental Table S1), we selected tPA-GFP as a surrogate maker for GLP-1-containing vesicles for further studies.
FIGURE 3.
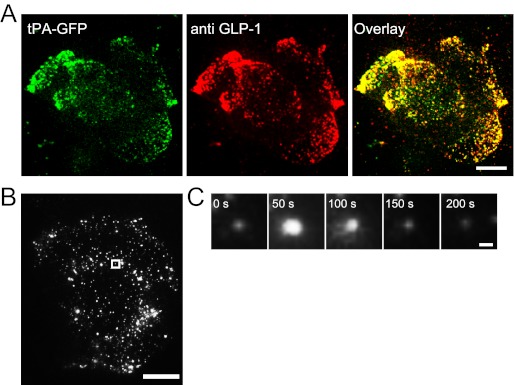
Co-localization of tPA-GFP in GLP-1-containing vesicles in GLUTag cells. A, typical confocal image of paraformaldehyde-fixed GLUTag cells showing the distribution of tPA-GFP (green) and GLP-1 (red). Scale bar, 10 μm. B, typical TIRF image of tPA-GFP-expressing GLUTag cells. The position of the vesicle before exocytosis is outlined by a square. Scale bar, 10 μm. C, five sequential TIRF images showing exocytotic release of tPA-GFP after application of 1 mm l-ornithine. Scale bar, 1 μm.
We analyzed the behavior of tPA-GFP-expressing vesicles near the plasma membrane in GLUTag cells by TIRF microscopy (14, 19, 20). Overexpression of tPA-GFP in GLUTag cells produced a highly punctate pattern of fluorescence observable under the TIRF microscope (Fig. 3B). After application of 1 mm l-ornithine, tPA-GFP fluorescent spots suddenly brightened and then dimmed (Fig. 3, B and in the 50-s panel of C). The fluorescent dynamics of this fusion protein are considered a result of a fast reduction in pH-dependent quenching, followed by the release and loss of the fluorescent protein into the extracellular space, indicative of a vesicular fusion event. The results suggested that release of secretory vesicles can be monitored by using tPA-GFP in GLUTag cells.
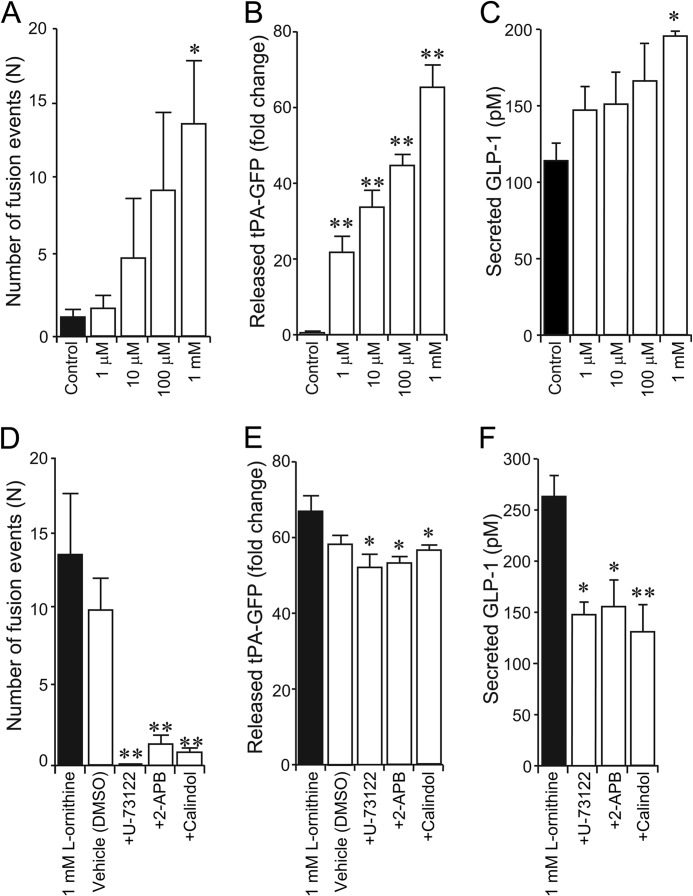
During application of l-ornithine, the total number of tPA-GFP fusion events in GLUTag cells increased significantly in a dose-dependent manner (Fig. 4A). To confirm whether tPA-GFP fusion events represent vesicle release, we stimulated the cells for 2 h, collected the medium, and measured the fluorescence intensity of tPA-GFP released into the medium using a spectrophotofluorometer. The fluorescence index (FI), representing the amount of tPA-GFP, was calculated by integrating the FI of the medium at wavelengths from 460 to 650 nm. The FI for secreted tPA-GFP was significantly increased in a dose-dependent manner (Fig. 4B) compared with the FI of medium from unstimulated cells. The concentration of GLP-1 secreted from cells incubated with l-ornithine was also measured by ELISA. Application of l-ornithine significantly and dose-dependently increased GLP-1 secretion compared with unstimulated control cells (Fig. 4C).
FIGURE 4.
Effect of l-ornithine on GLP-1 secretion. A, number of exocytotic events during application of l-ornithine at various concentrations (n = 5 cells in each condition). B, spectrophotofluorometer measurement of fluorescence intensity of released tPA-GFP into the recording medium after application of various concentrations of l-ornithine. The FI value of medium from control cells without stimulation (Control) was normalized to 1 (n = 3 trials in each condition). C, ELISA measurement of the amount of secreted GLP-1 after application of various concentrations of l-ornithine (n = 3 trials in each condition). D, number of exocytotic events during application of a PLC inhibitor (U-73122), an IP3 receptor inhibitor (2-APB), or a GPRC6A antagonist (calindol) together with l-ornithine (n = 7 cells in each condition). DMSO, dimethyl sulfoxide. E, spectrophotofluorometer measurement of fluorescence intensity of released tPA-GFP into the recording medium after application of U-73122, 2-APB, or calindol together with l-ornithine (n = 3 trials in each condition). The FI value of control cells without stimulation was normalized to 1 (n = 3 trials in each condition). F, ELISA measurement of the amount of secreted GLP-1 after application of U-73122, 2-APB, or calindol together with l-ornithine (n = 3 trials in each condition). Data are shown as mean ± S.E. (error bars). *, p < 0.05; **, p < 0.01.
To further confirm a role for PLC and IP3 as downstream mediators of GPRC6A receptor activation by l-ornithine, we employed pharmacological inhibitors of this signal transduction pathway. Co-administration of U-73122, 2-APB, or calindol with 1 mm l-ornithine significantly decreased the number of l-ornithine-induced tPA-GFP fusion events (Fig. 4D), the FI for tPA-GFP (Fig. 4E), and the amount of secreted GLP-1 (Fig. 4F). These results are consistent with the observed [Ca2+]i changes shown in Fig. 2 and suggest that the binding of extracellular l-ornithine to GPRC6A increases GLP-1 exocytosis in a dose-dependent manner via an IP3-mediated pathway in GLUTag cells.
GPRC6A Is Involved in l-Ornithine-induced GLP-1 Secretion from GLUTag Cells
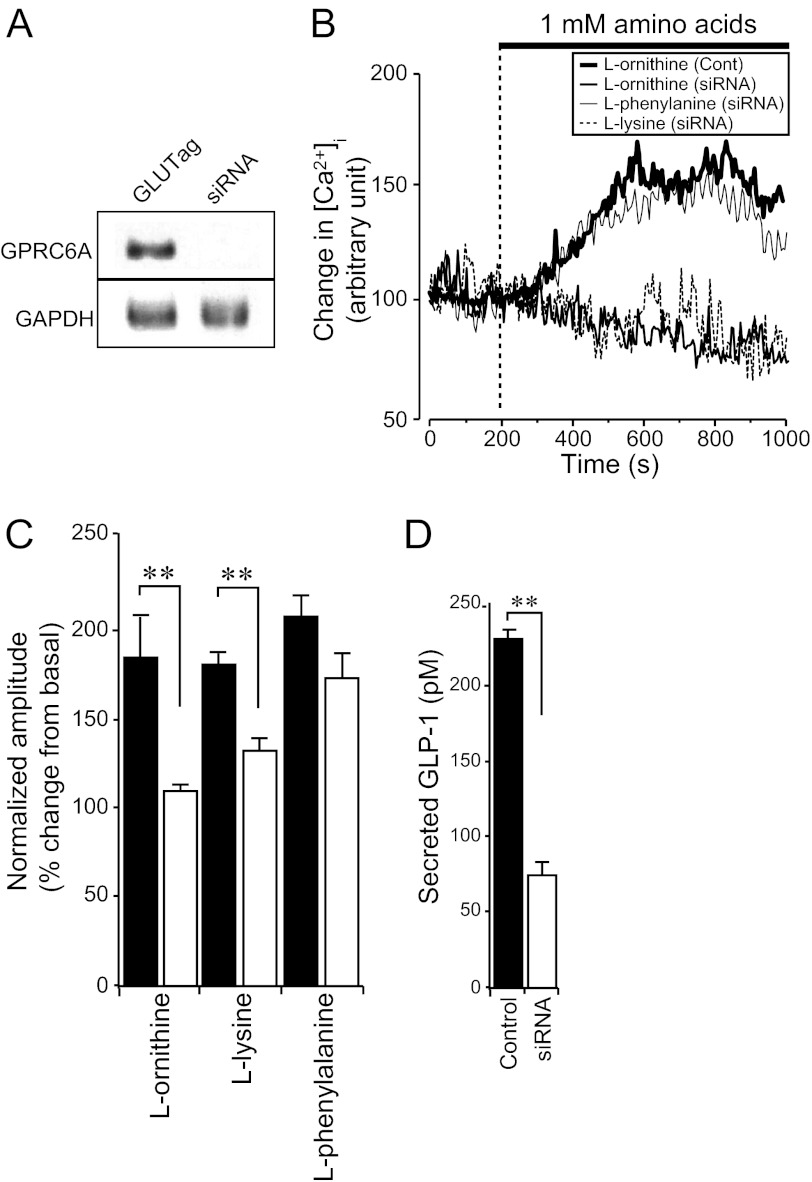
The results described above provide pharmacological evidence that extracellular l-ornithine increases [Ca2+]i and GLP-1 secretion via GPRC6A. We next depleted endogenous GPRC6A by expressing siRNAs targeting sequences in GLUTag cells, to further investigate whether the l-ornithine-induced [Ca2+]i increase was mediated by GPRC6A. RT-PCR analysis confirmed that when GPRC6A siRNAs were applied to GLUTag cells, the expression of GPRC6A was significantly suppressed (Fig. 5A). Depletion of GPRC6A by siRNA significantly inhibited l-ornithine- and l-lysine-induced [Ca2+]i increases (Fig. 5, B and C). By contrast, the CaR ligand, l-phenylalanine, induced a [Ca2+]i increase even when GPRC6A expression was depleted (Fig. 5, B and C). The results suggest that these amino acids selectively stimulate different receptors in GLUTag cells. To confirm further the effect of GPRC6A siRNA on l-ornithine-induced GLP-1 secretion in GLUTag cells, we measured the amount of secreted GLP-1 by ELISA. When GPRC6A siRNAs were transfected into GLUTag cells, the amount of l-ornithine-induced GLP-1 secretion was significantly decreased (Fig. 5D).
FIGURE 5.
Depletion of endogenous GPRC6A reduces l-ornithine-induced [Ca2+]i changes and GLP-1 secretion from GLUTag cells. A, RT-PCR analysis of the effect of GPRC6A siRNA on the expression of GPRC6A in GLUTag cells. The expression of GPRC6A mRNA was reduced by specific siRNAs. B, typical time course of the [Ca2+]i during application of 1 mm l-amino acids in GLUTag cells (Cont) or GPRC6A-depleted GLUTag cells (siRNA). C, normalized amplitude calculated from the peak amplitude of fluorescence intensity induced by 1 mm l-amino acids in GLUTag cells (filled bars) or GPRC6A-depleted GLUTag cells (open bars). Basal fluorescence intensity is normalized to 100 (n = 8 cells in each groups). D, amount of secreted GLP-1 after application of l-ornithine in GLUTag cells (filled bars) or GPRC6A-depleted GLUTag cells (open bars) measured by ELISA. Note that GPRC6A siRNA expression inhibited the amount of l-ornithine-induced GLP-1 secretion (n = 3 trials in each condition). Data are shown as mean ± S.E. (error bars). **, p < 0.01.
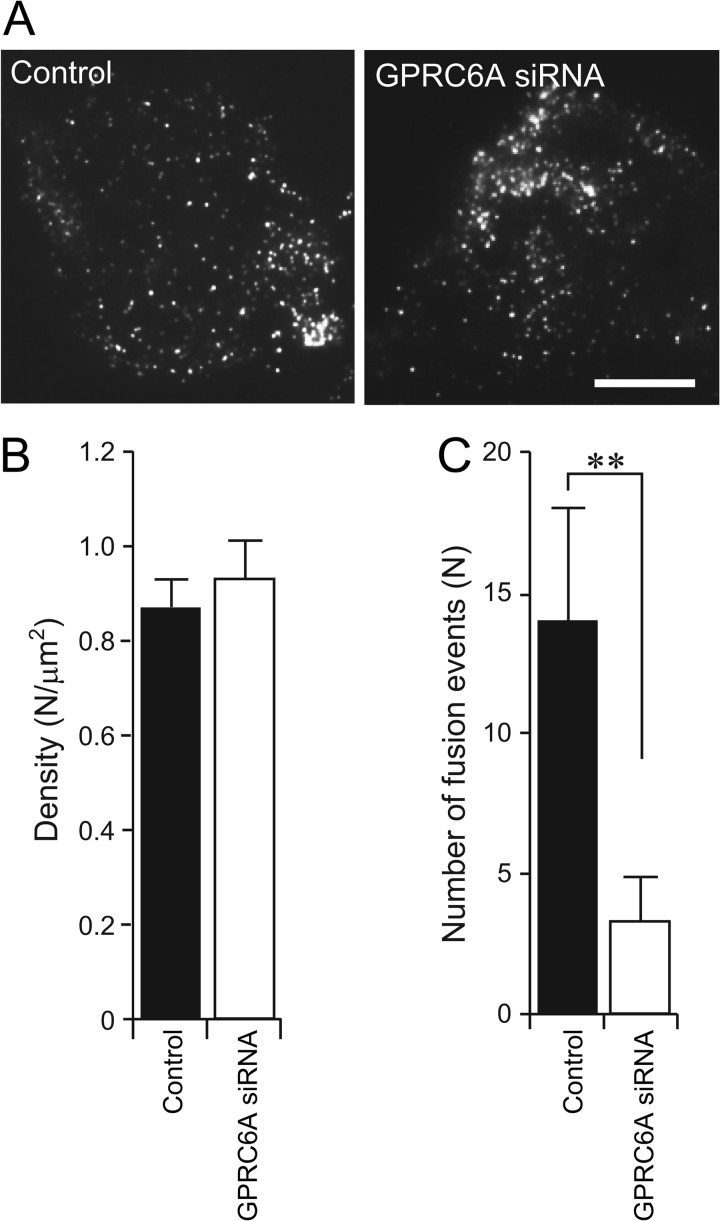
We also observed the effect of l-ornithine on the exocytosis of GLP-1 in GPRC6A-depleted GLUTag cells by TIRF microscopy. Although the depletion of endogenous GPRC6A by siRNA had no effect on the density of plasma membrane-docked tPA-GFP vesicles (Fig. 6, A and B), the depletion of GPRC6A significantly inhibited the number of tPA-GFP fusion events (Fig. 6C). Thus, these data strongly support the idea that GPRC6A is not involved in the transport and docking steps but in the exocytotic fusion step, via an IP3-mediated pathway in GLUTag cells.
FIGURE 6.
Depletion of endogenous GPRC6A reduces the number of fusion events from GLUTag cells. A, typical TIRF images of the plasma membrane-docked tPA-GFP-containing vesicles in untreated control (Control) or GPRC6A-depleted (GPRC6A siRNA) GLUTag cells. Scale bar, 10 μm. B, density of plasma membrane-docked vesicles (n = 5 cells in each group). Note that depletion of endogenous GPRC6A had no effect on the density of plasma membrane-docked vesicles. C, reduction of l-ornithine-induced fusion events (n = 5 cells in each group) by depletion of GPRC6A. Data are shown as mean ± S.E. (error bars). **, p < 0.01.
Contribution of Mitochondrial Metabolism and cAMP-mediated Pathways for l-Ornithine-induced GLP-1 Secretion
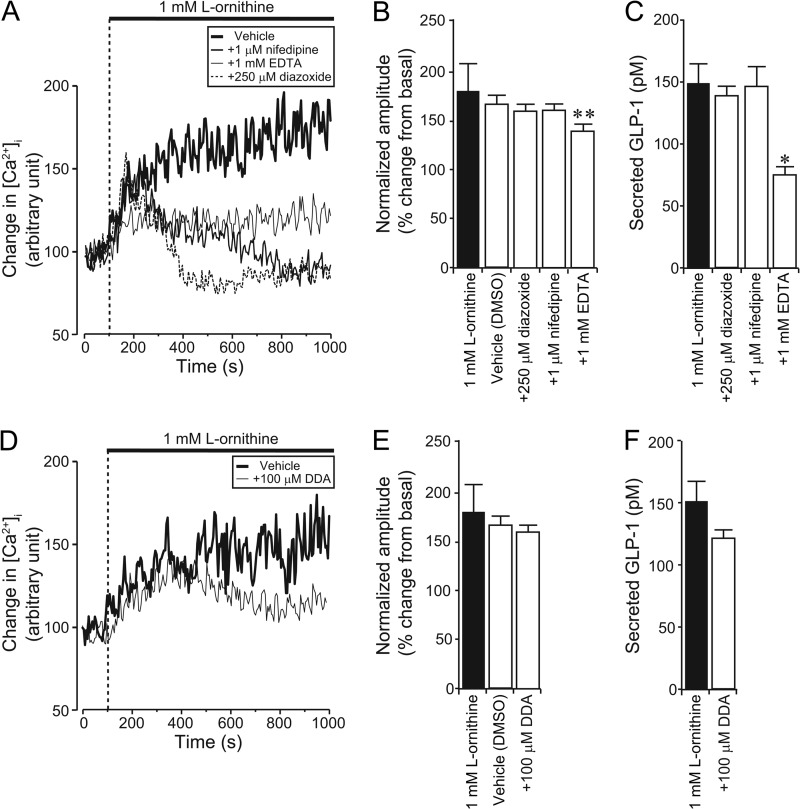
In a final set of experiments, we examined possible additional pathways underlying l-ornithine-induced GLP-1 secretion. l-Ornithine could act as a substrate for mitochondrial metabolism, and as GLUTag cells have been shown to express ATP-sensitive potassium (KATP) channels (32), a subsequent increase in the ATP/ADP ratio and depolarization could contribute to the observed l-ornithine responses. Application of EDTA to deplete extracellular Ca2+ ions significantly suppressed l-ornithine-induced [Ca2+]i responses. Whereas application of the KATP channel opener diazoxide or the L-type voltage-gated Ca2+ channel blocker nifedipine suppressed the late phase of l-ornithine-induced [Ca2+]i changes (Fig. 7A), they were without effect on the peak response (Fig. 7B); and only EDTA, but not diazoxide or nifedipine, inhibited l-ornithine-induced GLP-1 secretion (Fig. 7C).
FIGURE 7.
Effect of KATP channel opener, L-type Ca2+ channel blocker, adenylyl cyclase inhibitor, or chelation of extracellular Ca2+ ions on l-ornithine-induced [Ca2+]i increase and GLP-1 secretion in GLUTag cells. A and D, typical time course showing [Ca2+]i changes during application of a KATP channel opener (diazoxide), an L-type calcium channel blocker (nifedipine), chelation of extracellular Ca2+ ions (EDTA) (A) or adenylyl cyclase inhibitor (DDA) together with l-ornithine (D). Basal fluorescence intensity is normalized to 100. B and E, normalized amplitude calculated from the peak amplitude of fluorescence intensity induced by 1 mm l-ornithine plus diazoxide, nifedipine, EDTA (B), or DDA (E) (n = 7 cells in each condition). DMSO, dimethyl sulfoxide. Note that application of EDTA significantly inhibited the peak l-ornithine-induced [Ca2+]i increase, whereas diazoxide, nifedipine, and DDA had no effect. C and F, amount of secreted GLP-1 after application of diazoxide, nifedipine, EDTA (C), or DDA (F) together with l-ornithine measured by ELISA (n = 3 trials in each condition). Note that application of EDTA significantly inhibited l-ornithine-induced GLP-1 secretion, whereas diazoxide, nifedipine, and DDA had no effect. Data are shown as mean ± S.E. (error bars).*, p < 0.05; **, p < 0.01.
It has been reported that l-glutamine-triggered GLP-1 secretion involves elevation of cytosolic cAMP (11, 12), possibly downstream of an unidentified Gs-coupled GPCR. Thus, it is conceivable that l-ornithine could also additionally recruit Gs-coupled pathways. To test this hypothesis, we investigated the effect of the adenylyl cyclase inhibitor, DDA. We found that DDA had no effect on either l-ornithine-induced [Ca2+]i elevation or GLP-1 secretion in GLUTag cells (Fig. 7, D–F).
DISCUSSION
In the present study, we identified GPRC6A and CaR as functional l-amino acid-sensing receptors in the GLUTag intestinal L cell line (Fig. 1). Furthermore, we detected expression of GPRC6A in FACS-sorted mouse primary small intestinal L cells (supplemental Fig. S1). GLUTag cells also expressed T1R1 and T1R3. Another clonal intestinal L cell line STC-1 and small intestinal tissue expressed T1R3 but not T1R1. Because T1R1 is necessary to form the amino acid sensing umami taste receptor complex with T1R3, it is unlikely that intestinal L cell responses to amino acids are dominated by this pathway. T1R3 also forms a glucose-sensing complex with T1R2 (i.e. the sweet taste receptor), which senses sugars and synthetic sweeteners. Some studies have shown that the sweet taste receptor together with key signal transduction elements (i.e. gustducin, PLC, and transient receptor potential m5) are expressed in human and mouse small intestine and colon (33) and that these receptors might be involved in GLP-1 secretion in response to glucose and artificial sweeteners (34, 35). However, the importance of the T1R2/T1R3 heterodimer in L cell stimulation remains controversial (2, 36, 37). As no clear role has yet been assigned to homomeric T1R3, the importance of this receptor in enteroendocrine cells awaits further research.
Amino acid-induced GLP-1 secretion from L cells has previously been attributed to activation of mitochondrial metabolism, plasma membrane depolarization, or elevation of cAMP (11, 12, 38). However, in view of the breadth of amino acids that trigger GLP-1 release, it has also been suggested that unidentified amino acid-sensing receptors may play a role (11, 12). Gs mediated elevation of cAMP does not appear necessary for l-ornithine-induced Ca2+ entry or secretion, as responses were maintained in the presence of the adenylate cyclase inhibitor DDA. Peak l-ornithine-induced [Ca2+]i changes and GLP-1 secretion were unaffected by opening KATP channels or blocking L-type Ca2+ channels (Fig. 7), supporting the idea that the Ca2+ response is independent of membrane depolarization and Ca2+ influx via L-type Ca2+ channels. Responses were, however, impaired by the PLC inhibitor U-73122 or the IP3 receptor blocker 2-APB, supporting the idea that a Gq mediated pathway is recruited by ornithine in GLUTag cells. A recent report implicated the CaR, also predominantly Gq-coupled, in amino acid-stimulated GLP-1 secretion (36). This receptor is, however, unlikely to underlie the majority of the results reported here. Our data clearly demonstrate that the GPRC6A antagonist, calindol, inhibited l-ornithine-induced GLP-1 secretion from GLUTag cells (Fig. 4F), and depletion of endogenous GPRC6A in GLUTag cells by specific siRNAs inhibited l-ornithine-induced GLP-1 secretion (see Figs. 5 and 6). Thus, our results suggest that GPRC6A is involved in amino acid-induced GLP-1 secretion from GLUTag cells.
GLP-1 is generated by the processing of proglucagon-derived peptides (31), and it is therefore technically difficult to directly label GLP-1 with a fluorescent protein. To overcome this problem, an expression vector encoding Venus fused to human growth hormone (hGH-Venus) was used previously to label GLP-1-containing vesicles in the STC-1 cell line (39). When we first used rat GH-GFP (rGH-GFP) for visualizing GLP-1-containing vesicles in GLUTag cells, we did not observe co-localization of rGH-GFP with GLP-1-containing vesicles (supplemental Fig. S2). We therefore tested other hormones fused to FPs (i.e. GFP and Venus), which may be predicted to co-localize with GLP-1-containing vesicles. When we compared plasmids encoding mouse BDNF-Venus, human NPY-Venus, or rat tPA-GFP, to analyze the targeting efficiency to GLP-1-containing vesicles by confocal microscopy (supplemental Fig. S2), we found that tPA-GFP showed the highest co-localization efficiency with GLP-1-containing vesicles in GLUTag cells (Fig. 3, supplemental Fig. S2, and supplemental Table S1). tPA-GFP was therefore used in subsequent studies as a surrogate for endogenous GLP-1. Results obtained using this probe demonstrated a clear regulation of the final steps of vesicular exocytosis downstream of GPRC6A. No obvious changes in vesicle density close to the membrane were detected after receptor knock-down, suggesting that signals arising from GPRC6A activation do not play a major role in vesicular transport and recruitment.
In the present study, we largely used the GLUTag L cell line, because the population of L cells in the small intestine is small, and it is difficult to purify living L cells away from neighboring enterocytes, Paneth cells and goblet cells. GPRC6A expression was, however, detectable in primary FACS-purified L-cell populations. Secretion experiments from mixed primary cultures of the entire mouse small intestine did not reveal l-ornithine-triggered GLP-1 release (supplemental Fig. S3), but this might be explained if GPRC6A is only expressed in a restricted subset of native L-cells. A recent study has shown that GPRC6A-null mice exhibit a mild metabolic syndrome phenotype characterized by hyperglycemia, decreased serum insulin levels, glucose intolerance, and insulin resistance (18). By contrast, an alternative study found no differences in basal insulin or glucose levels, glucose tolerance, or insulin sensitivity in another GPRC6A-null mouse model compared with WT littermates (40). This discrepancy could reflect the relative composition of glucose, fatty acids, and amino acids in different mouse diets. Thus, further studies are required to elucidate the importance of GPRC6A-dependent amino acid-induced GLP-1 secretion in vivo.
Acknowledgments
We thank Dr. D. Drucker (University of Toronto) and Dr. D. Hanahan (University of California) for the GLUTag cells and STC-1 cells, respectively, and Dr. G. A. Rutter and members of the Tsuboi Laboratory at the University of Tokyo for valuable discussion.
This work was supported in part by Grants-in-aid for Scientific Research 24-8221 (to M. O.), 22790197 (to T. K.), and 24790207 (to T. T.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; by the Nestlé Nutrition Council, Japan (to T. T.), the JGC-S Scholarship Foundation (to T. K.), the Mishima Kaiun Memorial Foundation (to T. T.), the Suntory Foundation for Life Sciences (to T. T.), and the Takeda Science Foundation (to T. T.).

This article contains supplemental Figs. S1–S3, Table S1, and additional references.
- GLP-1
- glucagon-like peptide-1
- 2-APB
- 2-aminoethoxydiphenyl borate
- BDNF
- brain-derived neurotrophic factor
- CaR
- calcium-sensing receptor
- DDA
- 2′,5′-dideoxyadenosine
- FI
- fluorescence index
- FP
- fluorescent protein
- GH
- growth hormone
- GPCR and GPR
- G protein-coupled receptor
- NPY
- neuropeptide Y
- PLCγ
- phospholipase Cγ
- RB
- Ringer buffer
- T1R
- taste receptors type-1
- TIRF
- total internal reflection fluorescence
- tPA
- tissue-type plasminogen activator.
REFERENCES
- 1. Gribble F. M., Williams L., Simpson A. K., Reimann F. (2003) A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 52, 1147–1154 [DOI] [PubMed] [Google Scholar]
- 2. Reimann F., Habib A. M., Tolhurst G., Parker H. E., Rogers G. J., Gribble F. M. (2008) Glucose sensing in L cells: a primary cell study. Cell Metab. 8, 532–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diakogiannaki E., Gribble F. M., Reimann F. (2012) Nutrient detection by incretin hormone-secreting cells. Physiol. Behav. 106, 387–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gorboulev V., Schürmann A., Vallon V., Kipp H., Jaschke A., Klessen D., Friedrich A., Scherneck S., Rieg T., Cunard R., Veyhl-Wichmann M., Srinivasan A., Balen D., Breljak D., Rexhepaj R., Parker H. E., Gribble F. M., Reimann F., Lang F., Wiese S., Sabolic I., Sendtner M., Koepsell H. (2012) Na+-d-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61, 187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parker H. E., Wallis K., le Roux C. W., Wong K. Y., Reimann F., Gribble F. M. (2012) Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. Br. J. Pharmacol. 165, 414–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reimann F., Tolhurst G., Gribble F. M. (2012) G-protein-coupled receptors in intestinal chemosensation. Cell Metab. 15, 421–431 [DOI] [PubMed] [Google Scholar]
- 7. Edfalk S., Steneberg P., Edlund H. (2008) Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 57, 2280–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herrmann C., Göke R., Richter G., Fehmann H. C., Arnold R., Göke B. (1995) Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion 56, 117–126 [DOI] [PubMed] [Google Scholar]
- 9. Elliott R. M., Morgan L. M., Tredger J. A., Deacon S., Wright J., Marks V. (1993) Glucagon-like peptide-1 (7–36) amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J. Endocrinol. 138, 159–166 [DOI] [PubMed] [Google Scholar]
- 10. Hall W. L., Millward D. J., Long S. J., Morgan L. M. (2003) Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br. J. Nutr. 89, 239–248 [DOI] [PubMed] [Google Scholar]
- 11. Reimann F., Williams L., da Silva Xavier G., Rutter G. A., Gribble F. M. (2004) Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia 47, 1592–1601 [DOI] [PubMed] [Google Scholar]
- 12. Tolhurst G., Zheng Y., Parker H. E., Habib A. M., Reimann F., Gribble F. M. (2011) Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology 152, 405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bjarnadóttir T. K., Fredriksson R., Schiöth H. B. (2005) The gene repertoire and the common evolutionary history of glutamate, pheromone (V2R), taste (1) and other related G protein-coupled receptors. Gene 362, 70–84 [DOI] [PubMed] [Google Scholar]
- 14. Aoki R., Kitaguchi T., Oya M., Yanagihara Y., Sato M., Miyawaki A., Tsuboi T. (2010) Duration of fusion pore opening and the amount of hormone released are regulated by myosin II during kiss-and-run exocytosis. Biochem. J. 429, 497–504 [DOI] [PubMed] [Google Scholar]
- 15. Lochner J. E., Honigman L. S., Grant W. F., Gessford S. K., Hansen A. B., Silverman M. A., Scalettar B. A. (2006) Activity-dependent release of tissue plasminogen activator from the dendritic spines of hippocampal neurons revealed by live-cell imaging. J. Neurobiol. 66, 564–577 [DOI] [PubMed] [Google Scholar]
- 16. Nagai T., Ibata K., Park E. S., Kubota M., Mikoshiba K., Miyawaki A. (2002) A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20, 87–90 [DOI] [PubMed] [Google Scholar]
- 17. Kawashima R., Ikematsu K., Abe Y., Sato M., Tsuruya S., Nakasono I., Fukushima H., Inoue K., Tsuboi T. (2010) Effect of glucocorticoid on the biosynthesis of growth hormone-containing secretory granules in pituitary cells. Biochem. Biophys. Res. Commun. 400, 225–229 [DOI] [PubMed] [Google Scholar]
- 18. Pi M., Chen L., Huang M. Z., Zhu W., Ringhofer B., Luo J., Christenson L., Li B., Zhang J., Jackson P. D., Faber P., Brunden K. R., Harrington J. J., Quarles L. D. (2008) GPRC6A-null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS One 3, e3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oya M., Suzuki H., Watanabe Y., Sato M., Tsuboi T. (2011) Amino acid taste receptor regulates insulin secretion in pancreatic β-cell line MIN6 cells. Genes Cells 16, 608–616 [DOI] [PubMed] [Google Scholar]
- 20. Sato M., Mori Y., Matsui T., Aoki R., Oya M., Yanagihara Y., Fukuda M., Tsuboi T. (2010) Role of the polybasic sequence in the Doc2α C2B domain in dense-core vesicle exocytosis in PC12 cells. J. Neurochem. 114, 171–181 [DOI] [PubMed] [Google Scholar]
- 21. Tsuboi T., Ravier M. A., Parton L. E., Rutter G. A. (2006) Sustained exposure to high glucose concentrations modifies glucose signaling and the mechanics of secretory vesicle fusion in primary rat pancreatic β-cells. Diabetes 55, 1057–1065 [DOI] [PubMed] [Google Scholar]
- 22. Brown E. M., Gamba G., Riccardi D., Lombardi M., Butters R., Kifor O., Sun A., Hediger M. A., Lytton J., Hebert S. C. (1993) Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 366, 575–580 [DOI] [PubMed] [Google Scholar]
- 23. Nelson G., Chandrashekar J., Hoon M. A., Feng L., Zhao G., Ryba N. J., Zuker C. S. (2002) An amino-acid taste receptor. Nature 416, 199–202 [DOI] [PubMed] [Google Scholar]
- 24. Wellendorph P., Bräuner-Osborne H. (2004) Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene 335, 37–46 [DOI] [PubMed] [Google Scholar]
- 25. Conigrave A. D., Hampson D. R. (2010) Broad-spectrum amino acid-sensing class C G-protein coupled receptors: molecular mechanisms, physiological significance and options for drug development. Pharmacol. Ther. 127, 252–260 [DOI] [PubMed] [Google Scholar]
- 26. Wellendorph P., Bräuner-Osborne H. (2009) Molecular basis for amino acid sensing by family C G-protein-coupled receptors. Br. J. Pharmacol. 156, 869–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Christiansen B., Hansen K. B., Wellendorph P., Bräuner-Osborne H. (2007) Pharmacological characterization of mouse GPRC6A, an l-α-amino-acid receptor modulated by divalent cations. Br. J. Pharmacol. 150, 798–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wellendorph P., Hansen K. B., Balsgaard A., Greenwood J. R., Egebjerg J., Bräuner-Osborne H. (2005) Deorphanization of GPRC6A: a promiscuous l-α-amino acid receptor with preference for basic amino acids. Mol. Pharmacol. 67, 589–597 [DOI] [PubMed] [Google Scholar]
- 29. Faure H., Gorojankina T., Rice N., Dauban P., Dodd R. H., Bräuner-Osborne H., Rognan D., Ruat M. (2009) Molecular determinants of noncompetitive antagonist binding to the mouse GPRC6A receptor. Cell Calcium 46, 323–332 [DOI] [PubMed] [Google Scholar]
- 30. Sato M., Kitaguchi T., Numano R., Ikematsu K., Kakeyama M., Murata M., Sato K., Tsuboi T. (2012) The small GTPase Cdc42 modulates the number of exocytosis-competent dense-core vesicles in PC12 cells. Biochem. Biophys. Res. Commun. 420, 417–421 [DOI] [PubMed] [Google Scholar]
- 31. Drucker D. J. (2003) Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol. Endocrinol. 17, 161–171 [DOI] [PubMed] [Google Scholar]
- 32. Reimann F., Gribble F. M. (2002) Glucose-sensing in glucagon-like peptide-1-secreting cells. Diabetes 51, 2757–2763 [DOI] [PubMed] [Google Scholar]
- 33. Bezençon C., le Coutre J., Damak S. (2007) Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem. Senses 32, 41–49 [DOI] [PubMed] [Google Scholar]
- 34. Jang H. J., Kokrashvili Z., Theodorakis M. J., Carlson O. D., Kim B. J., Zhou J., Kim H. H., Xu X., Chan S. L., Juhaszova M., Bernier M., Mosinger B., Margolskee R. F., Egan J. M. (2007) Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. U.S.A. 104, 15069–15074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Margolskee R. F., Dyer J., Kokrashvili Z., Salmon K. S., Ilegems E., Daly K., Maillet E. L., Ninomiya Y., Mosinger B., Shirazi-Beechey S. P. (2007) T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc. Natl. Acad. Sci. U.S.A. 104, 15075–15080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mace O. J., Schindler M., Patel S. (2012) The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J. Physiol. 590, 2917–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mace O. J., Lister N., Morgan E., Shepherd E., Affleck J., Helliwell P., Bronk J. R., Kellett G. L., Meredith D., Boyd R., Pieri M., Bailey P. D., Pettcrew R., Foley D. (2009) An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestine. J. Physiol. 587, 195–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Neu J., Shenoy V., Chakrabarti R. (1996) Glutamine nutrition and metabolism: where do we go from here? FASEB J. 10, 829–837 [DOI] [PubMed] [Google Scholar]
- 39. Ohara-Imaizumi M., Aoyagi K., Akimoto Y., Nakamichi Y., Nishiwaki C., Kawakami H., Nagamatsu S. (2009) Imaging exocytosis of single glucagon-like peptide-1-containing granules in a murine enteroendocrine cell line with total internal reflection fluorescent microscopy. Biochem. Biophys. Res. Commun. 390, 16–20 [DOI] [PubMed] [Google Scholar]
- 40. Smajilovic S., Clemmensen C., Johansen L. D., Wellendorph P., Holst J. J., Thams P. G., Ogo E., Brauner-Osborne H. (2013) The l-α-amino acid receptor GPRC6A is expressed in the islets of Langerhans but is not involved in l-arginine-induced insulin release. Amino Acids 44, 383–390 [DOI] [PubMed] [Google Scholar]