Background: NADPH oxidase-generated lyso-PS enhances efferocytosis by macrophages.
Results: Recruited, viable lyso-PShigh neutrophils are readily cleared by macrophages in vitro and in vivo via G2A signaling. Lyso-PShigh neutrophils also program macrophages to a highly efferocytic resolving state.
Conclusion: Lyso-PS in/on exudate neutrophils orchestrates early removal of neutrophils and subsequent macrophage programming.
Significance: Neutrophil-generated lyso-PS regulates tissue neutrophilia of acute inflammation.
Keywords: Inflammation, Lysophospholipid, Macrophages, Neutrophil, Phagocytosis, Efferocytosis, Lysophosphatidylserine, Macrophage Programming
Abstract
Resolution of neutrophilia characteristic of acute inflammation requires cessation of neutrophil recruitment and removal of tissue neutrophils. Based on in vitro studies, a role in these events was hypothesized for oxidant-generated lysophosphatidylserine (lyso-PS) on recruited neutrophils signaling via the G2A receptor on macrophages. Peritoneal exudate neutrophils harvested from wild type (WT) mice had 5-fold more lyso-PS (lyso-PShigh) than those of gp91phox−/− (lyso-PSlow) mice. Ex vivo engulfment of lyso-PShigh neutrophils (95% viable) by WT peritoneal macrophages was quantitatively similar to UV-irradiated apoptotic blood neutrophils, although the signaling pathway for the former was uniquely dependent on macrophage G2A. In contrast, lyso-PSlow neutrophils were poorly engulfed unless presented with exogenous lyso-PS. Enhanced clearance of lyso-PShigh neutrophils was also seen in vivo following their adoptive transfer into inflamed peritonea of WT but not G2A−/− mice, further supporting a requirement for signaling via G2A. To investigate downstream effects of lyso-PS/G2A signaling, antibody blockade of G2A in WT mice reduced macrophage CD206 expression and efferocytosis during peritonitis. Conversely, adoptive transfer of lyso-PShigh neutrophils early in inflammation in gp91phox−/− mice led to accelerated development of efferocytichigh and CD206high macrophages. This macrophage reprogramming was associated with suppressed production of pro-inflammatory mediators and reduced neutrophilia. These effects were not seen if G2A was blocked or lyso-PSlow neutrophils were transferred. Taken together, the results demonstrate that oxidant-generated lyso-PS made by viable tissue neutrophils is an endogenous anti-inflammatory mediator working in vivo to orchestrate the “early” and rapid clearance of recruited neutrophils as well as the reprogramming of “resolving” macrophages.
Introduction
Acute inflammation results in brisk neutrophil recruitment with a time course and robustness dependent on the nature of the insult and production of danger signals and neutrophil chemoattractants. When the danger is passed, e.g. microbes neutralized, signals for neutrophil recruitment subside abruptly curtailing further recruitment. Clearance of recruited neutrophils is then essential to return tissues to their normal function, and clearance must be timely before these short lived cells disintegrate and spill their phlogistic contents (1, 2). The “turn off” of neutrophil recruitment and the “turn on” of neutrophil removal are often simplistically represented as sequential processes but, in fact, occur simultaneously. Indeed, the critical accumulation of neutrophils in tissues at any point in time is the net sum of both the processes of recruitment and removal (3).
Signals for suppression of recruitment and for neutrophil removal are poorly understood but are likely highly orchestrated. The removal of dying cells by macrophages in a process known as efferocytosis (4) is touted as key to both. The paradigm holds that apoptotic neutrophils are phagocytosed, and the process is actively anti-inflammatory with the production of factors such as PGE2,2 IL-10, and TGFβ by macrophages that, in turn, suppress the production of pro-inflammatory mediators driving neutrophil recruitment (5, 6). Efferocytosis is generally highly efficient such that apoptotic neutrophils and other cells rarely accumulate in the absence of defects in the process (7, 8). Conversely, dysregulation of efferocytosis is often associated with chronic inflammation and autoimmunity (9, 10).
Neutrophil-generated oxidants, in addition to microbial killing and inciting inflammation, may also play somewhat paradoxical anti-inflammatory roles (11, 12). Indeed, deficient functioning of the NADPH oxidase is associated with exaggerated inflammation in human and murine models of chronic granulomatous disease (CGD) (13–15). We have recently shown that lysophosphatidylserine (lyso-PS) made in an NADPH oxidase-dependent manner in activated live as well as aged (apoptotic) neutrophils signals to macrophages via the G-protein-coupled receptor G2A for enhanced clearance in vitro (16, 17). As such, we hypothesized that lyso-PS-driven clearance would be a key mechanism for control of neutrophil numbers in vivo during acute inflammation. Using neutrophils recruited to the peritoneum in a well characterized model of sterile peritonitis, it was shown that lyso-PS, a downstream product of the NADPH oxidative burst, drives the “early” recognition and clearance of viable and nonapoptotic neutrophils by macrophages via the process of efferocytosis, reprograms macrophages to a “resolving” state, and suppresses production of pro-inflammatory cytokines, including those implicated in neutrophil recruitment. These findings support the hypothesis that lyso-PS plays a pivotal role in calibration of tissue neutrophilia.
EXPERIMENTAL PROCEDURES
Materials
All lipids were purchased from Avanti Polar Lipids (Alabaster, AL). Amiloride was from Sigma. Zymosan, annexin V Alexa 488, and anti-F4/80 were from Invitrogen. Anti-G2A M-20 and normal goat IgG were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Ly6G (1A8), anti-CD115, anti-CD45.1, anti-CD45.2, isotype control antibodies, and cell proliferation dye eFluor®450 (PBSE) were from eBioscience (San Diego). Anti-CD16/32 (Fc-block) was from BD Biosciences. Anti-CD206 was from Biolegend (San Diego). DeadEndTM Colorimetric TUNEL system was from Promega (Madison, WI). IL-6 and KC ELISAs were from ElisaTech (Aurora, CO).
Animals
Male and female C57BL/6 (WT CD45.2), B6.SJL-PtprcaPep3b/BoyJ (WT CD45.1), and gp91phox−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and were generated from a breeding colony at National Jewish Health (Denver, Colorado). G2A−/− mice were a generous gift from Dr. Katherine Hedrick (University of Virginia) and were bred in-house. All animals received care in accordance with the guidelines of the Institutional Animal Care and Use Committee and were maintained on food and water ad libitum. Mice between the ages of 8 and 12 weeks were used for in vitro and in vivo experiments and were age- and gender-matched for all experiments.
Induction and Assessment of Sterile Peritonitis
Mice were injected intraperitoneally with 1 mg of zymosan (in 1 ml of PBS), and peritoneal cells were harvested by lavage with ice-cold sterile Hanks' balanced salt solution supplemented with 1 mm EDTA and 10 mm HEPES (pH 7.2) at the times indicated. Cell counts and cytospins were done to determine cell differentials and absolute numbers. Efferocytosis was determined as described below, and apoptotic cells were determined by visual inspection of morphology or by TUNEL staining. Cells were also stained and analyzed by flow cytometry as described below. Cell-free lavage supernatants were analyzed where indicated for pro-inflammatory cytokines. In some experiments, mice were injected intraperitoneally with either goat IgG isotype or anti-G2A antibody (100 μg/mouse dialyzed against PBS) 18 h post-zymosan injection to test the role of lyso-PS signaling via G2A during resolution of inflammation. Where exudate neutrophils were to be adoptively transferred (below), 100 μg of either IgG isotype control or anti-G2A antibody was injected intraperitoneally 2 h prior to neutrophil transfer.
Preparation of Murine-recruited Neutrophils for Phagocytosis Assays and Adoptive Transfer
Murine exudate neutrophils were harvested from either WT (CD45.2 or CD45.1) or gp91phox−/− mice 6–10 h after induction of zymosan-induced peritonitis. Characterization of exudate neutrophils included measurement of lyso-PS by LC/MS/MS as described previously (16, 17), as well as surface PS exposure detected by annexin V binding and propidium iodide staining (as a test for permeability) and flow cytometry according to the manufacturer's instructions.
For in vitro phagocytosis assays, exudate neutrophils, with or without exogenous lyso-PS (below), were resuspended at 2 × 107 cells/ml in DMEM supplemented with 10% FBS and were added to macrophages as described below. For in vivo phagocytosis assays, exudate neutrophils were suspended in PBS or lyso-PS liposomes (below) at 1 × 107 cells/ml, and 0.5 ml was injected intraperitoneally into recipient mice at the indicated times. In some cases, exudate neutrophils were first labeled with 10 μm PBSE according to the manufacturer's instructions. In experiments to test the effects of adoptively transferred neutrophils on downstream macrophage programming, exudate neutrophils were resuspended at 2 × 107 cells/ml in PBS, and 0.5 ml was injected intraperitoneally into recipient mice at the indicated times.
Lyso-PS Vesicle Preparation and Loading of Neutrophils
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine small unilamellar vesicles containing 1-oleoyl-2-hydroxy-sn-glycero-3-phosphoserine (lyso-PS) at 30 mol % were prepared as described previously for in vitro phagocytosis assays (16, 17). For in vivo phagocytosis assays, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine and lyso-PS (10 mg/kg at 30 mol %) were evaporated to dryness under nitrogen and resuspended in 0.5 ml of PBS by vigorous vortexing. Small unilamellar vesicles were then created by sonication in a water bath sonicator and mixed with exudate neutrophils for 30 min prior to adoptive transfer. Under these conditions, ∼20% of the lyso-PS was incorporated into the neutrophils as measured by LC/MS/MS.
In Vitro Phagocytosis Assays
Resident peritoneal MΦ were used for in vitro phagocytosis assays. RPMΦ were isolated from mice using 5 ml of sterile Hanks' balanced salt solution (Cellgro; Kansas City, MO) supplemented with 10 mm HEPES (pH 7.2) and 1 mm EDTA to lavage the peritoneum following euthanization with CO2. Resident peritoneal cells were collected, centrifuged at 1,000 rpm for 10 min at 4 °C, and plated at 4 × 105 cells/well in a 24-well tissue culture plate in DMEM supplemented with 10% heat-inactivated FBS (Atlanta Biologicals; Lawrenceville, GA), 2 mm l-glutamine, 100 μg/ml streptomycin, and 100 units/ml penicillin. Macrophages were allowed to adhere for 2 h at 37 °C in a 10% CO2-humidified incubator at which time nonadherent cells were removed, and macrophages were cultured for an additional 48 h before use in phagocytosis assays.
Apoptotic human neutrophils were also prepared as targets for in vitro phagocytosis. Neutrophils were obtained from normal, healthy donors in accordance with a protocol reviewed and approved by the Institutional Review Board. Using endotoxin-free reagents and plasticware, human neutrophils were isolated by the plasma Percoll method as described previously (18). The neutrophils were suspended at 5 × 106/ml in a HEPES buffer (137 mm NaCl, 2.7 mm KCl, 2 mm MgCl2, 5 mm glucose, 1 mm CaCl2, 10 mm HEPES (pH 7.4)) supplemented with 0.05% fatty acid-free bovine serum albumin and UV-irradiated for 5 min on a trans-illuminator followed by incubation at 37 °C for 2 h. Under these conditions UV-irradiated neutrophils were greater than 50% apoptotic as determined by nuclear morphology and annexin V staining, and >95% propidium iodide-negative as determined by flow cytometry. Although isolated human neutrophils have variable amounts of lyso-PS at base line, their lyso-PS content does not change during UV irradiation-induced apoptosis, and signaling via G2A has not been demonstrated (16, 17).
For these in vitro studies, target cells (2 × 106 per well), either murine exudate neutrophils or UV-irradiated apoptotic human neutrophils, with or without liposomes (100 nmol of total lipid), in 100 μl of DMEM supplemented with 10% FBS were added simultaneously to macrophages. Prior studies have shown that lyso-PS liposomes added either prior to or simultaneously with UV-irradiated apoptotic Jurkat cells or carboxylate-modified beads (apoptotic cell mimics) were equally effective at enhancing engulfment by macrophages (16, 17). For antibody blocking experiments, 10 μg/ml anti-G2A or isotype control antibody was added for 30 min before target cells and liposomes were added. The macrophages and target cells were co-cultured for 60 min at 37 °C in 10% CO2, washed three times with PBS, and stained with a modified Wright's Giemsa stain (Fisher). The phagocytic index was calculated by multiplying the percentage of MΦ that have phagocytosed one or more cells by the average number of engulfed cells per MΦ (17, 19). A minimum of 200 MΦ were counted blindly. Each condition was tested in duplicate using at least four mice per experiment and repeated 3–10 times as indicated.
Assay of in Vivo Phagocytosis
Peritoneal cells were collected by lavage at the indicated time points, and phagocytosis was determined in a blinded fashion as above by visual inspection of cytospun slides stained with either a modified Wright's Giemsa stain or TUNEL staining (below). Where in vivo engulfment of adoptively transferred exudate neutrophils was assessed, exudate neutrophils were prepared as above, and uptake was assessed by visual inspection and blinded scoring of cytospun slides. In some experiments, uptake of PSBE-labeled exudate neutrophils by recipient macrophages, defined as CD45.1+/Ly6G−/F4/80+, was determined by flow cytometry. Time course experiments demonstrated that ingestions of adoptively transferred neutrophils were detectable by 1 h of dwell time in recipient mice but that engulfment was highly reproducible following a 4-h dwell time in vivo (data not shown). Four hours was chosen for further experiments.
Peritoneal Cell Surface Staining and Flow Cytometry
Peritoneal cells were suspended in PBS supplemented with 3% FBS at 1 × 107 cells/ml. 0.5 × 106 cells (50 μl) were incubated with Fc-block for 1 h on ice. Fifty μl of 2× antibody solution (anti-F4/80, anti-CD115, anti-CD206 (or isotype), and anti-Ly6G) in PBS plus 3% FBS was added to cells for a final volume of 100 μl and incubated in the dark on ice for 1 h. Cells were washed twice with PBS plus 3% FBS and analyzed by flow cytometry. Neutrophils were defined as Ly6G+/F4/80−/CD115− cells. CD206 expression was determined on monocytes/macrophages defined as Ly6G−/F4/80+/CD115+. CD206 mean fluorescence intensity was determined by dividing the geometric mean fluorescence of CD206 staining by the geometric mean fluorescence of its own isotype control staining and was expressed as fold over isotype control.
Cytokine Measurement
IL-6 and KC were measured by ELISA from cell-free lavage supernatants according to the manufacturer's instructions.
TUNEL Staining
Colorimetric TUNEL staining was performed on cytospun slides according to the manufacturer's instructions.
Statistical analyses and p value calculations were conducted using ANOVA (JMP Statistical Program (SAS Institute, Cary, NC)). The Dunnett's and Tukey-Kramer tests were used for single and multiple comparisons, respectively.
RESULTS
Lyso-PS Generated by Viable Exudate Neutrophils Drives Clearance by Macrophages in a G2A-dependent Process
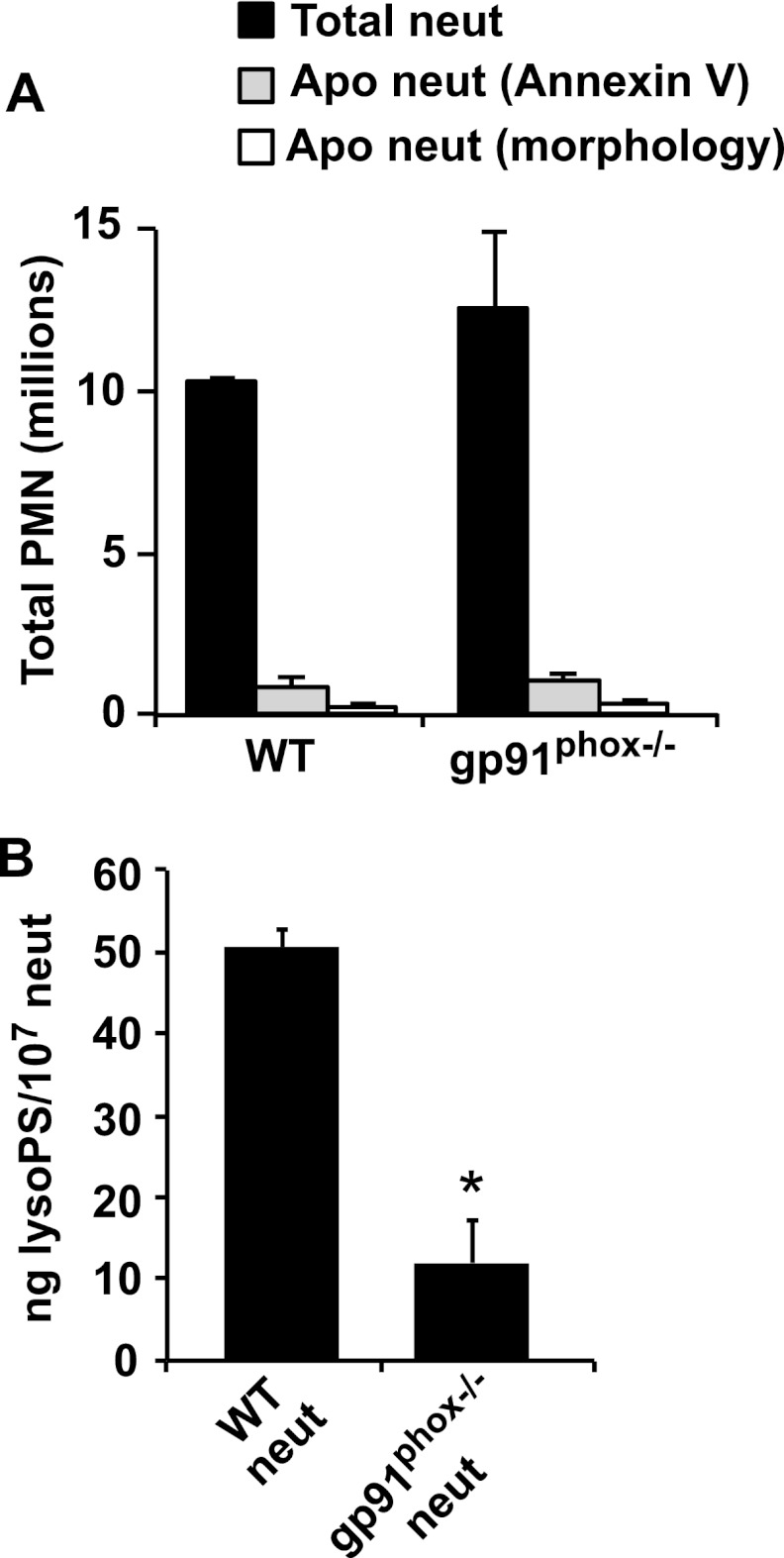
Previous investigation has shown that blood neutrophils activated in vitro can be recognized and removed by macrophages and that lyso-PS can play a role in this process. Accordingly, studies were initiated to explore the concept that lyso-PS generated in recruited neutrophils as a consequence of NADPH oxidase activation leads to an increase in “palatability” for uptake of these viable cells by macrophages. Neutrophils isolated from an acute inflammatory reaction were first characterized for their lyso-PS content as well as their viability in preparation for assessing their ability to be cleared in vitro or when re-instilled into an inflammatory reaction in vivo. Because neutrophils lacking the NADPH oxidase are deficient in lyso-PS generation, these were compared with wild type cells in this analysis (16). C57BL/6 and gp91phox−/− mice were injected with zymosan, and cells were lavaged from inflamed peritonea 6–10 h later. Cell differentials showed that 89 ± 2% (mean ± S.E.) of the inflammatory cells were intact neutrophils and that recovery was equivalent during this time frame for both genotypes (Fig. 1A). Only 6% of exudate neutrophils from either genotype contained visible zymosan. Evidence of apoptosis was rare as follows: 2.9% for wild type and 3.1% for gp91phox−/− as determined by morphology, and 6% for wild type and 8% for gp91phox−/− as determined by annexin V staining (Fig. 1A). Analysis for the presence of lyso-PS by LC/MS/MS showed (16) that wild type exudate neutrophils had five times more lyso-PS (lyso-PShigh) than gp91phox−/− (lyso-PSlow) exudate neutrophils with ∼90% of the 18:0 and 10% of the 18:1 species. As both are thought to signal via G2A (20, 21), their summated concentration in the wild type neutrophils was 3.1 μm based on an average cellular volume of 330 fl per neutrophil (Fig. 1B) (22). Lysophosphatidylcholine levels did not differ between wild type and gp91phox−/− neutrophils (data not shown).
FIGURE 1.
Characterization of exudate neutrophils (neut) harvested from WT and gp91phox−/− mice 6–10 h after intraperitoneal zymosan injection. A, total numbers of exudate and apoptotic neutrophils harvested from the two genotypes. Apoptotic neutrophils were detected by annexin V staining or nuclear morphology on cytospins. B, lipids from exudate neutrophils collected from WT or gp91phox−/− mice were extracted, and lyso-PS was quantified by LC/MS/MS. n = 8–10; *, p < 0.001. Data represent mean ± S.E.
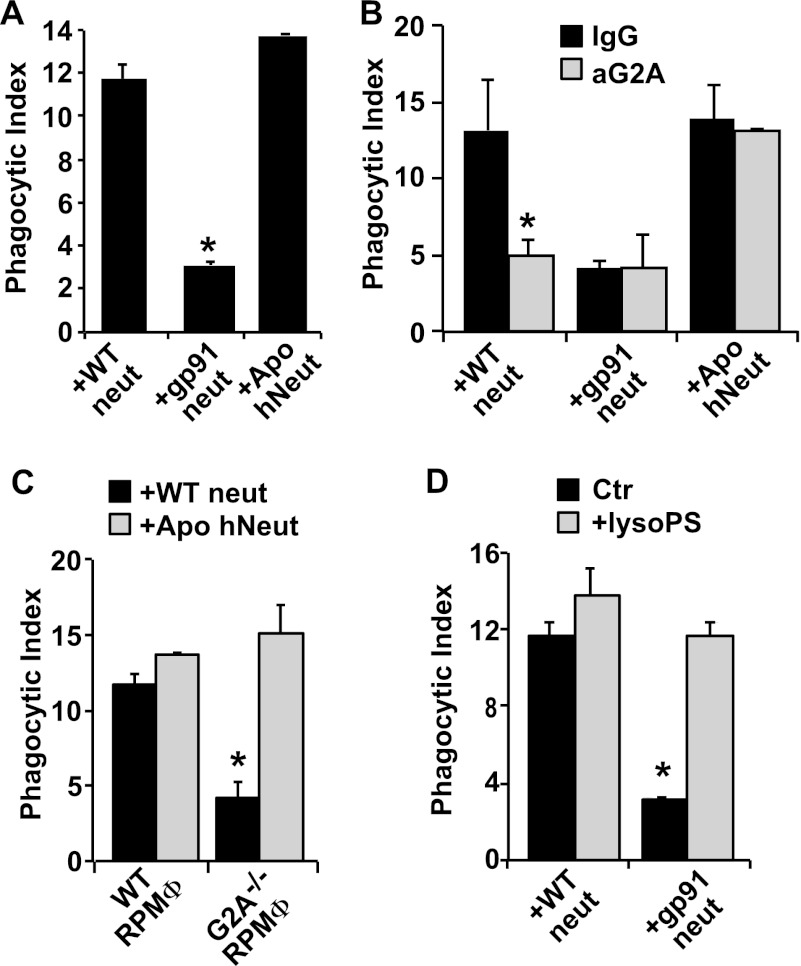
To address the hypothesis that lyso-PS includes a significant clearance signal on emigrated neutrophils, the harvested exudate neutrophils were first co-cultured with resident peritoneal macrophages. After 1 h, cultures were washed and macrophages assessed for phagocytosis of neutrophils. Although ingestions of exudate lyso-PShigh wild type neutrophils were readily visible, engulfments of exudate lyso-PSlow gp91phox−/− neutrophils were significantly fewer (Fig. 2A). As a reference comparison, uptake of apoptotic neutrophils was determined. Human neutrophils isolated from peripheral blood and induced to undergo apoptosis by UV irradiation (∼60–80% positive by annexin V staining and less than 5% propidium iodide positive) were also co-cultured with the macrophages. Quantitatively, lyso-PShigh (and nonapoptotic) wild type exudate neutrophils were only slightly less “palatable” than apoptotic human neutrophils.
FIGURE 2.
Lyso-PS drives engulfment of viable exudate neutrophils (neut) by macrophages in vitro. A, exudate neutrophils from WT or gp91phox−/− mice, or human neutrophils induced to undergo apoptosis by UV irradiation, were co-cultured with RPMΦ, and the phagocytic index was determined after 1 h. n = 8; *, p < 0.05 compared with WT neutrophils. B, RPMΦ were treated with 10 μg/ml blocking antibodies to G2A (or IgG isotype control) prior to co-culture with neutrophils. n = 6; *, p < 0.05 compared with WT neutrophils + isotype control. C, RPMΦ from either WT or G2A−/− mice were co-cultured with neutrophils. n = 4; *, p < 0.05 compared with WT neutrophils co-cultured with WT RPMΦ. D, lyso-PS liposomes were added as indicated to co-cultures. n = 6; *, p < 0.05 compared with WT neutrophil control in the absence of lyso-PS liposomes. Data represent mean ± S.E.
Next, signaling for the apparent palatability of exudate neutrophils was investigated. Previous studies had demonstrated a role for lyso-PS signaling via the G2A receptor on macrophages (16, 17), and thus, blocking antibodies directed against G2A and macrophages from mice genetically deficient in G2A were employed. As shown in Fig. 2B, treatment of peritoneal macrophages with anti-G2A, but not isotype antibodies, significantly blocked the ingestion of lyso-PShigh exudate neutrophils to levels seen for gp91phox−/− neutrophils and had no effect on the minimal uptake seen with the gp91phox−/− cells. UV-irradiated apoptotic human neutrophils do not make lyso-PS (see under “Experimental Procedures”) and have been shown not to signal via G2A (16, 17). As expected, anti-G2A antibody did not reduce their removal. Likewise, when presented to peritoneal macrophages from G2A−/− mice, engulfment of lyso-PShigh exudate neutrophils, but not the apoptotic cells, was markedly reduced (Fig. 2C).
We have previously shown that lyso-PS is not released from the activated neutrophil and have suggested that it is presented on its surface (16). Accordingly, gp91phox−/− exudate neutrophils were presented with lyso-PS-containing liposomes (30 mol %) before testing their palatability to macrophages (16, 17). As shown in Fig. 2D, lyso-PS liposomes fully restored the palatability of gp91phox−/− exudate neutrophils to levels of wild type exudate neutrophils. Similar addition of exogenous lyso-PS to wild type neutrophils did not further enhance their clearance suggesting that signaling by endogenous lyso-PS in/on these exudate neutrophils was already maximal.
Engulfment of apoptotic cells (efferocytosis) has been shown to be a process akin to macropinocytosis and dependent on the Na/H+ antiporter (23, 24). To determine whether lyso-PS-mediated engulfment occurred by a similar process, engulfment assays in the presence of amiloride to block the antiporter were conducted. Amiloride suppressed the uptake of both viable exudate neutrophils as well as apoptotic neutrophils but had no effect on macrophage phagocytosis of killed opsonized Candida albicans (data not shown). Thus, engulfment driven by lyso-PS appears to converge with efferocytosis driven by other signals on apoptotic cells (25). Together, these data demonstrate the following: (i) viable exudate neutrophils are rendered palatable to macrophages likely via a macropinocytotic mechanism, and (ii) this palatability is driven by NADPH oxidase-derived lyso-PS on the neutrophil signaling to G2A on the macrophage.
Lyso-PS Drives “Early” Neutrophil Clearance in Vivo
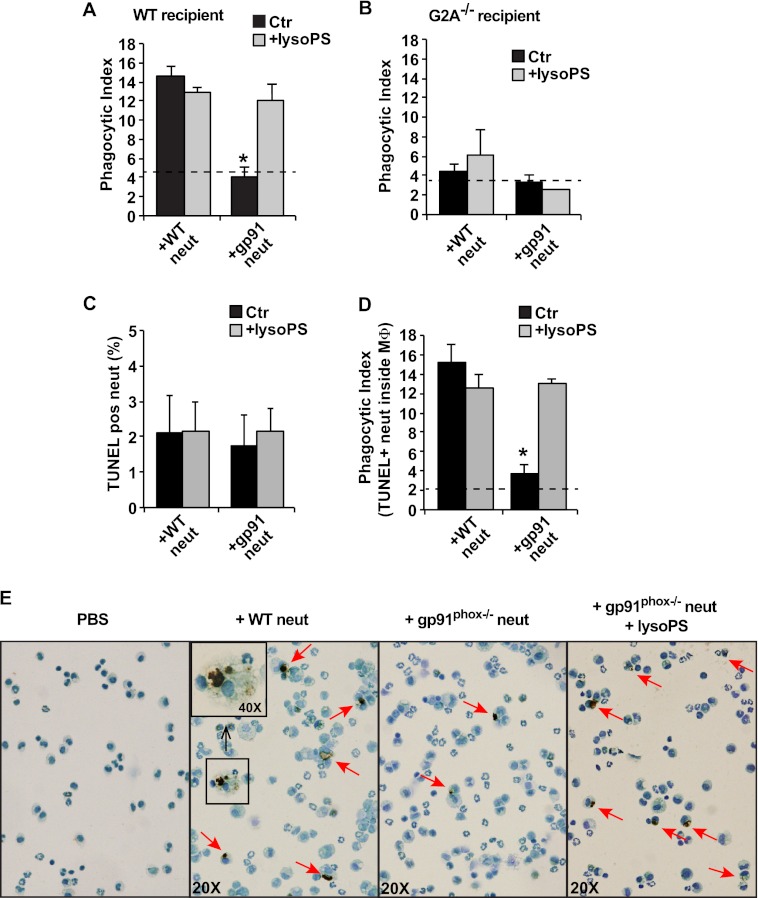
To examine the palatability of emigrated neutrophils in vivo, lyso-PShigh or lyso-PSlow exudate neutrophils from wild type or gp91phox−/− mice, respectively, were harvested, washed, pre-mixed with lyso-PS liposomes (see under “Experimental Procedures”) or not, and instilled into inflamed peritonea of C57BL/6 or G2A−/− mice 5 days after peritonitis induction with zymosan. Five days was chosen because endogenous neutrophils are largely cleared from inflamed peritonea in both recipient genotypes at this time. Four hours after instillation, peritoneal cells were lavaged and analyzed. The numbers of recovered macrophages from mice receiving exudate neutrophils did not differ in comparison with mice injected with only PBS. Ingestions of neutrophils were investigated on cytospins. As shown in Fig. 3A, lyso-PShigh exudate neutrophils from wild type mice were ingested at a significantly greater frequency than exudate neutrophils from gp91phox−/− mice. As in the in vitro experiments, pre-mixing of gp91phox−/− neutrophils with lyso-PS restored their in vivo palatability to that of wild type lyso-PShigh neutrophils. Conversely, few exudate neutrophils of either genotype were engulfed following instillation into peritonea of G2A−/− mice (Fig. 3B). Furthermore, as in the in vitro experiments, pre-mixing of neutrophils with exogenous lyso-PS liposomes did not enhance the clearance of either wild type or gp91phox−/− exudate neutrophils in the inflamed peritonea of G2A−/− mice.
FIGURE 3.
Lyso-PS drives engulfment of viable exudate neutrophils (neut) by macrophages in vivo. Exudate neutrophils (5 × 106) from WT and gp91phox−/− mice, with or without lyso-PS liposome premixing (see “Experimental Procedures”), were adoptively transferred into the peritonea of WT or G2A−/−-recipient mice on day 5 following induction of peritonitis. After 4 h, peritoneal cells were collected by lavage, and the phagocytic index was determined by visual inspection of cytospins. A and B, n = 5; *, p < 0.05 compared with WT neutrophil control. C–E, lavaged cells were TUNEL-stained and analyzed for free TUNEL+ neutrophils (C) and phagocytic index of MΦ containing TUNEL+ material in phagosomes (D). n = 5; *, p < 0.05 compared with WT neutrophil control. E, representative photographs of cytospins from C and D. Red arrows indicate TUNEL+ material in phagosomes. The dotted line in A, B, and D represents the background phagocytic index from control mice that did not receive adoptive transfer of exudate neutrophils. Data represent mean ± S.E.
Because of the 4-h dwell time of the instilled neutrophils in the peritonea for these experiments, cytospins of the harvested cells from recipient mice were stained to identify whether the cells had become apoptotic. TUNEL-positive free neutrophils were rarely seen regardless of donor genotype and with or without pre-mixing with lyso-PS liposomes (Fig. 3C). In contrast, quantification of TUNEL-positive neutrophils inside the macrophages replicated the ingestion data generated by visual inspection. Thus, TUNEL-positive phagosomes were seen with significantly greater frequency following the transfer of lyso-PShigh versus lyso-PSlow neutrophils (Fig. 3, D and E). Importantly, when gp91phox−/− neutrophils were pre-mixed with lyso-PS liposomes, TUNEL-positive ingestions equaled that for lyso-PShigh wild type neutrophils. These data support the hypothesis that lyso-PS is a major “eat me” signal for clearance of viable emigrated neutrophils in vivo and suggest that neutrophil apoptosis as detectable by TUNEL staining occurs within the phagosome after uptake (see under “Discussion”) (26, 27).
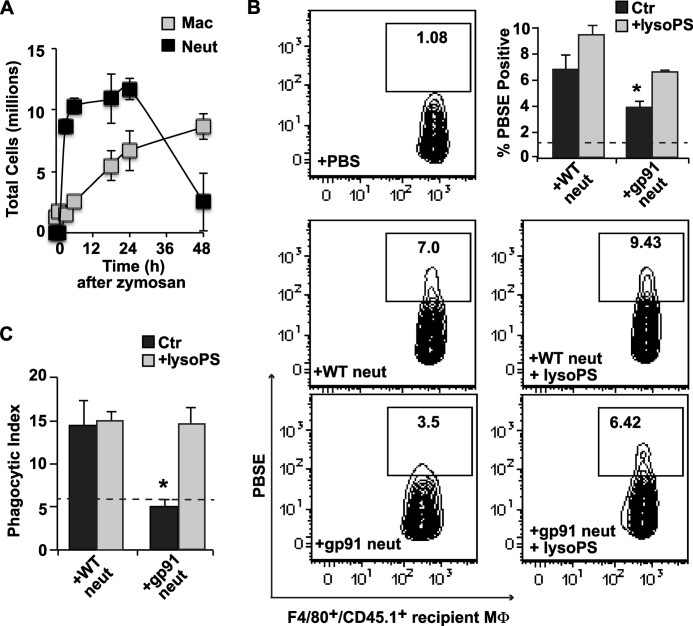
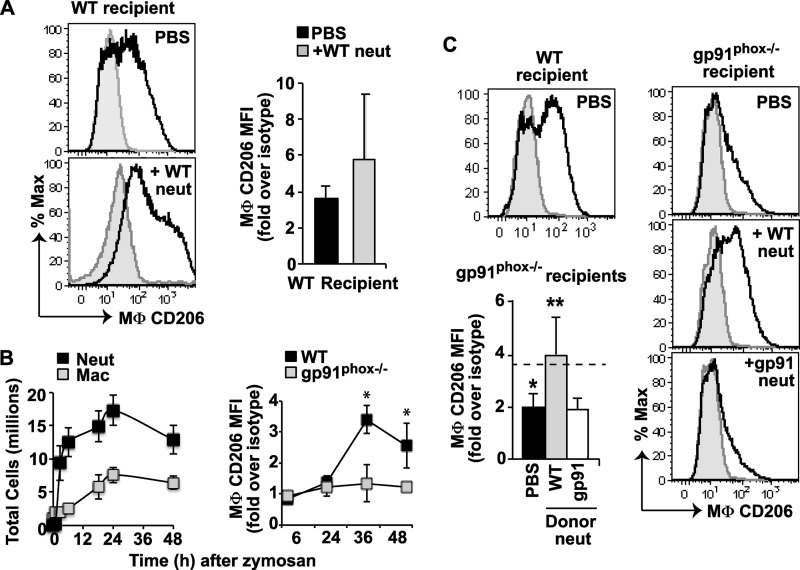
The clearance of instilled exudate neutrophils during earlier stages of the inflammatory process was also investigated. For these experiments, wild type CD45.1 mice were used as recipients at 18 h post zymosan, near the peak of endogenous neutrophil accumulation in the peritoneum (Fig. 4A). Wild type or gp91phox−/− CD45.2 neutrophils were labeled with PBSE and adoptively transferred, and their ingestion by CD45.1+/F480+/Ly6G− recipient macrophages was assessed following 4 h of dwell time in the peritonea. As shown in Fig. 4B, PBSE+ recipient macrophages were twice as frequent when lyso-PShigh wild type exudate neutrophils were instilled in comparison with lyso-PSlow, gp91phox−/− exudate neutrophils, i.e. 7% versus 3.5% of recipient macrophages, respectively. Pre-mixing the neutrophils with lyso-PS prior to transfer normalized the engulfment of gp91phox−/− neutrophils to that of wild type and slightly enhanced that of wild type neutrophils. These same differences in palatability detected by flow cytometry, both at base line and following pre-mixing with lyso-PS, were also confirmed on blinded scoring of cytospins by visual inspection (Fig. 4C).
FIGURE 4.
Lyso-PS drives engulfment of viable exudate neutrophils (neut) by macrophages early in inflammation. A, time course of total neutrophils and macrophages (Mac) after induction of zymosan peritonitis in WT mice. B, 6-h exudate neutrophils (5 × 106) from CD45.2 WT and gp91phox−/− mice were harvested, PBSE-labeled, pre-mixed with lyso-PS liposomes or not, and adoptively transferred into recipient CD45.1 wild type mice at 18 h into zymosan-induced peritonitis. After 4 h, peritoneal cells were collected by lavage, and MΦ of recipient mice defined as F4/80+/CD45.1+/Ly6G− were analyzed for ingested PBSE+ exudate neutrophils. Shown are representative density plots from three independent experiments demonstrating recipient macrophages positive for PBSE following adoptive transfer of WT neutrophils pre-mixed or not with lyso-PS liposomes (middle row), and gp91phox−/− neutrophils pre-mixed or not with lyso-PS liposomes (bottom row). Upper right, bar graph shows summated data of PSBE+ recipient macrophages under each condition. Ctr, control. C, phagocytic indices for these same harvests were determined by visual inspection of cytospins. Dashed lines in B and C represent the background phagocytosis in control wild type mice that received PBS alone (no neutrophils). n = 3; *, p < 0.05 compared with WT neutrophil control. Data represent mean ± S.E.
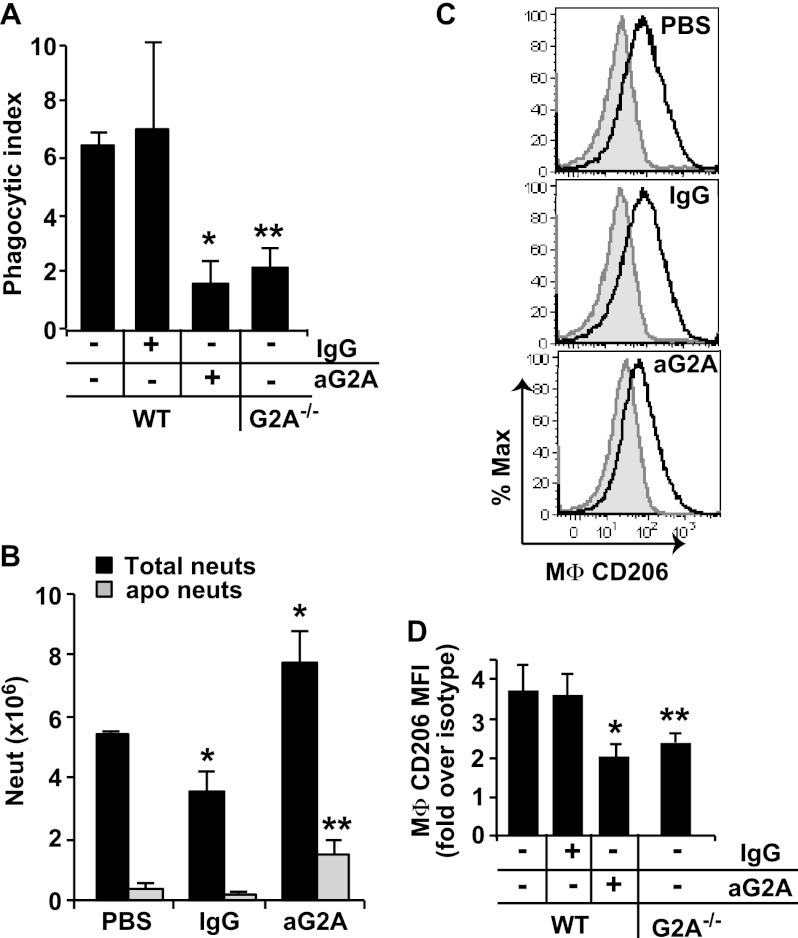
Given that G2A is expressed on both resident and recruited macrophages (16), we asked whether blockade of endogenous lyso-PS signaling would diminish neutrophil removal and delay resolution of neutrophilia in the inflammatory reaction. Blocking antibody to G2A (or isotype control antibody) was administered to wild type mice 18 h after induction of peritonitis with zymosan, and neutrophil clearance was investigated 30 h later. As predicted, neutrophil ingestions by macrophages were significantly decreased following anti-G2A treatment (Fig. 5A). This reduction in clearance was associated with increased recovery of neutrophils in lavage, both viable and morphologically apoptotic, following the G2A blockade (Fig. 5B).
FIGURE 5.
Endogenous lyso-PS signaling via G2A promotes the development of CD206high efferocytic macrophages and resolution of neutrophilia. WT mice were injected intraperitoneally with 100 μg of blocking antibody to G2A (or IgG isotype control or PBS) at 18 h after induction of zymosan peritonitis. Thirty hours later, peritoneal cells were collected by lavage and analyzed. For comparison, cells from G2A−/− mice at 48 h into zymosan-induced peritonitis were also analyzed. A, phagocytic indices were determined by visual inspection of cytospins. n = 6–8; *, p < 0.05 compared with WT control in the absence of antibody blockade; **, p < 0.05 compared with WT control. B, total neutrophils (neuts) and total apoptotic neutrophils (as determined by morphology from cytospins) were quantified. n = 5; *, p = 0.03 compared with WT PBS control; **, p < 0.02 compared with WT PBS control. C, CD206 expression on F4/80+/Ly6G− MΦ was determined by staining with anti-CD206 (solid line) or CD206 isotype control antibody (shaded gray) and analyzed by flow cytometry (shown are representative histograms from six independent experiments). D, geometric means of CD206 expression was determined and expressed as fold over CD206 isotype control staining and summated for WT and G2A−/− peritoneal MΦ. n = 6; *, p < 0.02 compared with WT control; **, p = 0.01 compared with WT control. Data represent mean ± S.E.
Effects of Lyso-PS/G2A Signaling on Macrophage Programming for Enhanced Efferocytosis
Efferocytic capability has been generally associated with “alternative activation” or programming of macrophages to a “resolving” state. Acquisition of CD206 expression on macrophages appears to track with efferocytic capability during the resolution of zymosan-induced peritonitis (28, 29). Accordingly, we asked whether antibody blockade of lyso-PS signaling resulted in diminished expression of CD206 on macrophages. As shown in Fig. 5, C and D, treatment of wild type mice with anti-G2A (but not isotype antibodies) reduced the subsequent expression of CD206 on macrophages. As further proof of concept, inflammatory macrophages from G2A−/− mice were also investigated for efferocytosis and CD206 expression at the same time point during zymosan-induced peritonitis. Diminished efferocytosis (Fig. 5A) and CD206 expression (Fig. 5D) were demonstrated for G2A−/− macrophages relative to wild type, although inflammation in this genotype is somewhat delayed (17) making head-to-head comparisons inexact (see under “Discussion”). Of note, CD36 and 15-lipoxygenase, additional markers associated with alternative activation and implicated in apoptotic cell engulfment (30, 31), were also evaluated, but neither protein was altered in this system (data not shown).
Given these findings, associating blockade of lyso-PS/G2A signaling with diminished macrophage programming for efferocytic capability, we next sought to determine whether adoptively transferred lyso-PShigh exudate neutrophils could drive the acquisition of the resolving macrophage marker, CD206, on the macrophages. Adoptive transfer of lyso-PShigh neutrophils into wild type mice 12 h after initiation of the peritonitis was found to variably enhance expression of CD206 assessed 24 h later (Fig. 6A). We suspect that in these wild type mice the presence of signaling by the endogenous lyso-PShigh neutrophils and relatively high levels of CD206 expression on the macrophages may have reduced detection of an enhancement. Therefore, to further test for effects of lyso-PS signaling on macrophage programming, we turned to lyso-PS-deficient gp91phox−/− mice as recipients of lyso-PShigh exudate neutrophils. As the murine model of CGD, these mice demonstrate delayed expression of CD206 on inflammatory macrophages, deficient efferocytosis, and exaggerated inflammation during zymosan-induced peritonitis (Fig. 6B; compare time course to Fig. 4A) (28). Adoptive transfer of lyso-PShigh neutrophils into these mice at 12 h significantly increased the subsequent expression of CD206 on macrophages 24 h later, resulting in levels comparable with that of wild type macrophages (Fig. 6C). This enhancement was not seen following transfer of lyso-PSlow gp91phox−/− neutrophils.
FIGURE 6.
Adoptive transfer of lyso-PShigh exudate neutrophils (neut) enhances macrophage CD206high expression during peritonitis. A, lyso-PShigh (WT) exudate neutrophils (1 × 107) (or PBS) were adoptively transferred into the peritonea of WT recipient mice at 12 h into zymosan-induced peritonitis. Peritoneal cells were collected by lavage 24 h later, and CD206 expression on F4/80+/Ly6G- MΦ was determined by flow cytometry and expressed as in Fig. 5. Left, histograms from a single experiment. Staining is shown with anti-CD206 (solid line) or CD206 isotype control antibody (shaded gray). Right, summated expression data. n = 8. B, left, time course of total neutrophils and macrophages (Mac) following induction of zymosan peritonitis in gp91phox−/− mice. Right, time course of CD206 expression on macrophages in WT or gp91phox−/− mice following induction of zymosan peritonitis. n = 8–10; *, p < 0.001 compared with gp91phox−/−. C, representative histograms from WT PBS control or gp91phox−/− recipients. Lower left, summated CD206 expression data are shown for gp91phox−/− recipient MΦ. Dashed line represents CD206 expression on MΦ from PBS- treated WT mice. n = 8; *, p < 0.0001 compared with WT PBS control; **, p < 0.0002 compared with CD206 expression on PBS treated gp91phox−/− mice. Data represent mean ± S.E.
Lyso-PS-driven Macrophage Reprogramming Restores “Normal” Resolution of Inflammation in the Murine Model of CGD
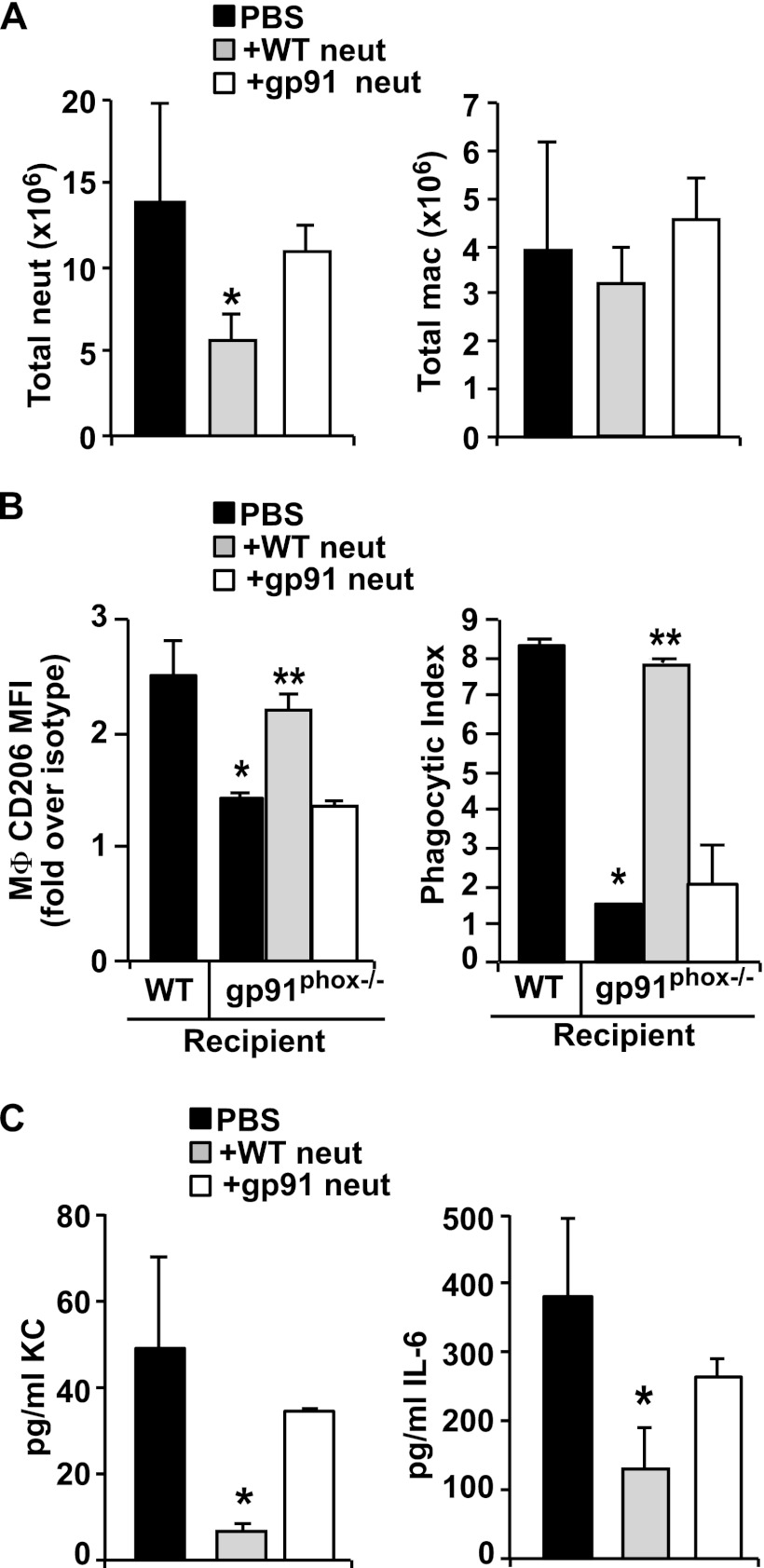
Neutrophilia (Fig. 6B) and the levels of pro-inflammatory cytokines are both prolonged in the CGD gp91phox−/− mice (28, 32, 33). We therefore hypothesized that transfer of lyso-PS expressing viable neutrophils would not only lead to their efficient intrinsic removal (Figs. 3 and 4) and the reprogramming of macrophages (Fig. 6), but would also increase efferocytosis and decrease production of proinflammatory chemokines and cytokines. Adoptive transfer of CD45.1 lyso-PShigh neutrophils into CD45.2 gp91phox−/− recipients allowed us to track donor versus recipient neutrophil accumulation and disappearance over time. At 36 h after transfer, only 3% of harvested peritoneal neutrophils were CD45.1 donor neutrophils, whereas 97% were CD45.2 endogenous neutrophils. Notably, there was a significant reduction in the total numbers of neutrophils in peritoneal harvests from gp91phox−/− recipients that were adoptively transferred with lyso-PShigh neutrophils compared with those receiving PBS or lyso-PSlow (gp91phox−/−) neutrophils (Fig. 7A). Macrophage numbers were unaltered regardless of the adoptively transferred neutrophil genotype (Fig. 7A), although again, macrophage CD206 expression was enhanced following transfer of lyso-PShigh neutrophils (Fig. 7B). Accompanying the enhanced CD206 expression, efferocytic ingestions were also significantly increased in macrophages from gp91phox−/− mice given lyso-PShigh neutrophils and were comparable with levels seen in wild type mice. PBS or adoptively transferred lyso-PSlow neutrophils had no effect. Given the reduction in accumulated neutrophils, we also assessed the levels of mediators associated with neutrophil recruitment. As shown (Fig. 7C), decreased KC and IL-6 levels were found in peritoneal lavage of gp91phox−/− recipients receiving lyso-PShigh exudate neutrophils relative to levels seen in mice receiving either PBS or lyso-PSlow neutrophils (see under “Discussion”).
FIGURE 7.
In addition to driving macrophage programming, lyso-PShigh exudate neutrophils (neut) reduce subsequent neutrophilia and pro-inflammatory mediator levels in gp91phox−/− recipients. gp91phox−/− recipient mice were adoptively transferred with either lyso-PShigh or lyso-PSlow neutrophil (1 × 107) at 12 h into zymosan-induced peritonitis, and 36 h later peritoneal lavage was analyzed. A, total neutrophil and MΦ counts. n = 3–6; *, p < 0.05 compared with PBS control. B, left, geometric mean of CD206 expression on recipient gp91phox−/− MΦ expressed as fold over their respective isotype controls. n = 3–6; *, p < 0.05 compared with WT PBS control; **, p < 0.01 compared with gp91phox−/− PBS control. Right, phagocytic index determined by visual inspection of cytospins. n = 3–6; *, p < 0.001 compared with WT PBS control; **, p < 0.001 compared with gp91phox−/− PBS control. C, levels of KC (left) and IL-6 (right) were measured in cell-free lavage supernatants from gp91phox−/− mice. n = 3–6; *, p < 0.05 compared with PBS control. Data represent mean ± S.E.
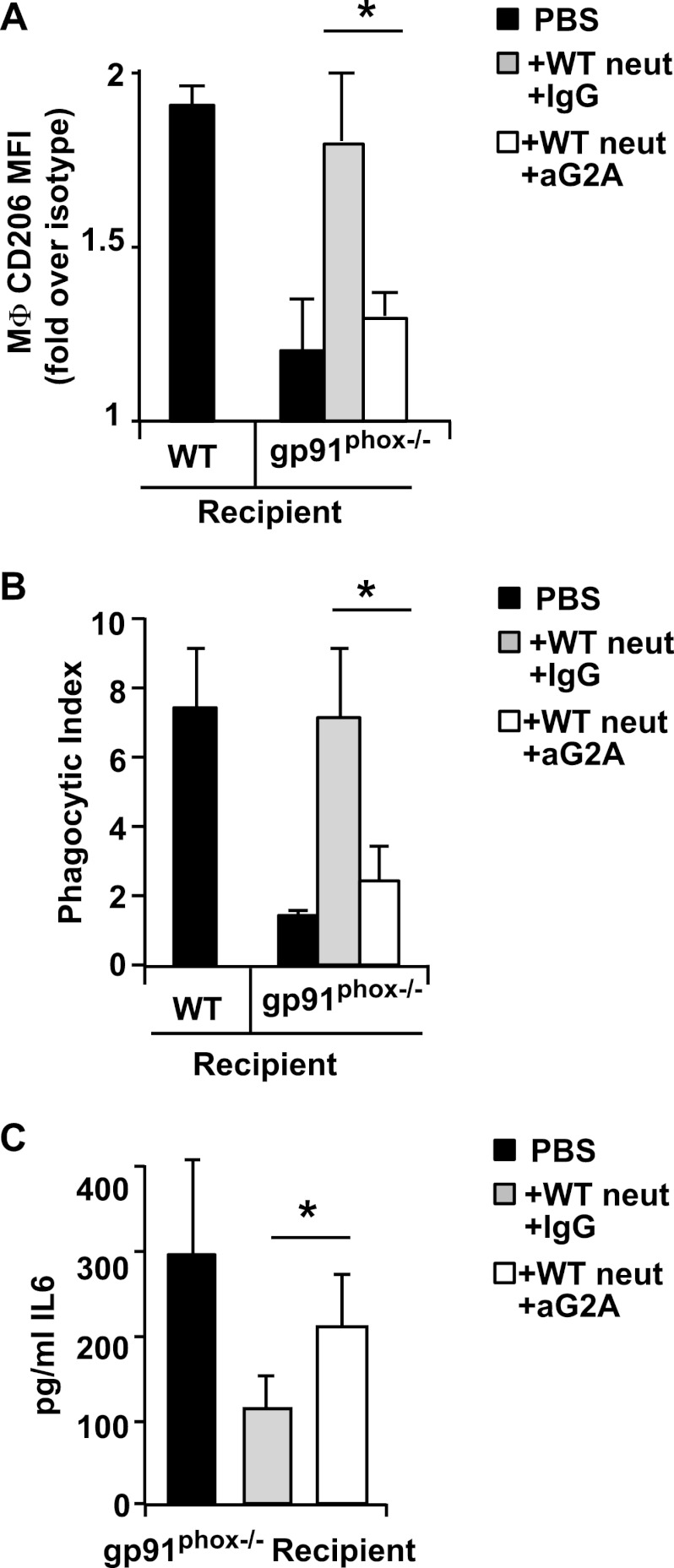
The role of lyso-PS signaling via G2A for these events was verified by antibody blockade. Treatment of the mice with antibody to G2A (but not isotype) 2 h prior to the adoptive transfer resulted in reversal of the heightened CD206 expression (Fig. 8A) and efferocytosis (Fig. 8B) induced by the lyso-PShigh neutrophils. The resulting levels following G2A blockade were comparable with those of mice receiving lyso-PSlow neutrophils. Additionally, the reductions in mediator levels evident in gp91phox−/− mice adoptively transferred with lyso-PShigh neutrophils were also reversed by G2A blockade (Fig. 8C). Taken together, the results demonstrate that provision of lyso-PShigh neutrophils enhanced gp91phox−/− macrophage programming to a resolving state associated with improved efferocytosis and reduced neutrophilia.
FIGURE 8.
Signaling to G2A by lyso-PShigh exudate neutrophils (neut) is required for enhanced efferocytic macrophage programming and reductions in pro-inflammatory mediator levels in gp91phox−/− recipients. gp91phox−/−-recipient mice were injected intraperitoneally with 100 μg of blocking antibody to G2A (or IgG isotype control) 18 h after induction of zymosan-induced peritonitis. WT exudate neutrophils were adoptively transferred 2 h after antibodies, and peritoneal cells were collected by lavage 24 h later and analyzed. A, CD206 expression on MΦ was determined, and geometric means represented as fold over isotype control is shown. n = 5; *, p < 0.005. B, phagocytic indices were determined by visual inspection of cytospins. n = 5; *, p < 0.02. C, IL-6 was determined in cell-free lavage supernatants from gp91phox−/−-recipient mice. n = 5; *, p < 0.005. Data represent mean ± S.E.
DISCUSSION
Our results support the hypothesis that neutrophils themselves govern their own early clearance in acute inflammation and that lyso-PS is the major signal determining their palatability for removal by macrophages. Using antibodies to block G2A signaling and macrophages from G2A−/− mice, a requirement for macrophage G2A was clearly demonstrated (Figs. 2, 5, and 8). Of note, it is thought that lyso-PS (as well as its other related lipid activators, e.g. lyso-PC) signals indirectly through this receptor rather than as a direct ligand (20, 34, 35). We have previously shown that lyso-PS signaling downstream of G2A activates calcium-dependent cytosolic phospholipase A2 and COX for the production of PGE2. PGE2, in turn, signals to adenylyl cyclase for production of cAMP and the activation of PKA and ultimately Rac (17). The activation of Rac is required for macropinocytosis, the clearance of apoptotic cells and of activated, viable neutrophils, and there appears to be considerable convergence between these pathways (Fig. 2) (4, 17, 25). Precedence for the ingestion of viable cells by macrophages is found in other inflammatory contexts (36, 37). As such, lyso-PS-driven clearance likely explains the earlier findings of Lagasse and Weissman (38) in which neutrophils rendered resistant to apoptosis by the overexpression of Bcl-2 were cleared from inflamed peritonea with the same kinetics as unaltered neutrophils. Furthermore, and as shown here, ingested viable cells have been well documented to undergo subsequent apoptosis within the macrophage phagosome (26, 27).
In addition to its rapid signaling for neutrophil clearance, lyso-PS signaling from the activated neutrophil to the macrophage has longer term downstream consequences. Macrophages exposed to lyso-PShigh neutrophils demonstrate a G2A-mediated shift in programming to a CD206high resolving functional capacity associated with enhanced efferocytosis and diminished production of pro-inflammatory cytokines, including those involved in neutrophil recruitment itself. We note that this lyso-PS-driven programming shift was most readily demonstrated in gp91phox−/− mice where the altered macrophage programming state is delayed, efferocytosis is diminished, and the production of KC, IL-6, and IL-1β is prolonged (28, 33, 39–41). By contrast, these resolution events occur quite rapidly in wild type mice, e.g. KC levels recede within 6 h (28), and neutrophil engulfment is readily evident by 18 h (Fig. 4). We hypothesize that endogenous lyso-PS signaling contributes significantly to these events in normal resolution of acute inflammation. Accordingly, blockade of G2A signaling in wild type mice reduced macrophage expression of the CD206 marker, diminished cell removal, and increased the numbers of viable and uningested apoptotic neutrophils (Fig. 5). Unfortunately, attempts to examine even earlier blockades of endogenous lyso-PS signaling from neutrophil immigrants with anti-G2A antibody were confounded by delayed recruitment of both neutrophils and monocyte/macrophages to the peritoneum (data not shown). G2A is present on most hematopoietic cells, including neutrophils, resident, and recruited macrophages as well as on endothelium, and the G2A receptor plays multiple roles in inflammation as is borne out in the knock-out mice (35, 42, 43).
Future investigation will use the gp91phox−/− model to determine the signaling pathway from G2A to the macrophage reprogramming. The changes noted, including CD206 expression, are downstream targets of peroxisome proliferator-activated receptor γ activation, and we have previously shown that peroxisome proliferator-activated receptor γ expression and activation are delayed in these gp91phox−/− mice (28). Other candidates are also possible. PGE2, cAMP, and PKA (44–46) are all lyso-PS signaling intermediaries (17) and have been shown to lead to the development of resolving macrophages. Thus, there are several likely intermediaries of the lyso-PS/G2A signaling pathway.
Notably, in an attempt to drive both lyso-PS-mediated neutrophil clearance and altered macrophage programming, lyso-PS liposomes alone were instilled into inflamed peritonea at various time points following zymosan-induced peritonitis (data not shown). These did not reproduce the findings of adoptively transferred lyso-PShigh neutrophils. Given that we do not know the pharmacokinetics of lyso-PS in vivo, our liposome concentrations or timing may have been inadequate. However, we think it is likely that the presentation of lyso-PS on the neutrophil surface itself is critical. We have previously shown that endogenous lyso-PS produced by neutrophils is always cell-associated, raising the possibility that lyso-PS is locally concentrated on the cell surface and/or co-presented to macrophages with other, as yet, unidentified signals (47, 48). In support of this, earlier studies showed that lyso-PS liposomes added with apoptotic cells, e.g. Jurkat cells, enhanced their G2A-mediated engulfment, although lyso-PS addition to viable cells did not, suggesting that a second signal was needed (16). Furthermore, lyso-PS liposomes significantly enhanced the uptake of apoptotic cell mimics, carboxylated beads presenting a PS head group-like surface, perhaps indicating the need for co-signaling via a macrophage receptor for the PS head group (16, 17). An intriguing possibility is that autocrine signaling by lyso-PS via G2A on the recruited neutrophil (20) may up-regulate other eat me signals on these cells. Thus, although we have shown a requirement for lyso-PS/G2A signaling in the removal of activated neutrophils, we have not proven that it acts alone in this setting.
There is growing recognition that oxidants play significant anti-inflammatory roles in inflammation (12, 14, 49), although the exact mechanisms have not been elucidated. We have previously shown that lyso-PS was the predominant modified PS species identified in activated neutrophils, and although roles for several phospholipases in its production were ruled out, an absolute requirement of an active NADPH oxidase was demonstrated (16, 17). Here, we have shown that lyso-PS plays a critical role in the control and resolution of neutrophilia. Conversely, suppression of the neutrophil NADPH oxidase, and certainly its absence as in CGD, likely contributes to poor neutrophil removal and exacerbation of inflammation (28, 33, 41). From our own study and the investigations of others, which show that tying the loss of the NADPH oxidase to exaggerated inflammation in CGD, two hypotheses regarding the defects in efferocytosis have emerged as follows: (i) oxidase-deficient neutrophils undergo delayed apoptosis with deficient eat me signaling, i.e. do not develop their normal palatability (16, 39, 41, 50), and (ii) CGD inflammatory macrophages do not become appropriately programmed for efferocytosis or to turn off inflammatory mediator production (28, 40, 41). Noteworthy are the implications of the data here that link both hypotheses to deficient lyso-PS signaling from the activated neutrophil. Hence, restoration of lyso-PS on the CGD neutrophil may restore these anti-inflammatory pathways in this disorder. Indeed, the development of chronic inflammation in both human and murine CGD, and autoimmunity in CGD patients and both the gp91phox−/− and G2A−/− murine models (13, 35, 51, 52), suggests that loss of lyso-PS signaling on neutrophils may have additional consequences. Similarly, defects in the programming toward resolving macrophages have been identified in a number of inflammatory disorders and linked to deficient efferocytosis, chronic inflammation, and autoimmunity, e.g. in chronic obstructive pulmonary disease, severe asthma, and systemic lupus erythematosus (7, 53, 54). The data here suggest that proper exploitation of lyso-PS signaling may be a tractable means of therapeutic intervention in such disorders.
Acknowledgments
We appreciate the technical assistance of Lindsay Guthrie and help from Brenda Sebern in the preparation of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants AI058228 (to D. L. B.), HL34303 (to D. L. B., C. C. L., P. M. H., and R. C. M.), and HL81151 (to P. M. H.). This work was also supported by the Chronic Granulomatous Disorder Society (to D. L. B.), the Catherine Kramer Foundation in Pediatric Medicine (to D. L. B.), and the Eugene F. and Easton M. Crawford Charitable Lead Unitrust (to R. F. F.-B.).
- PGE2
- prostaglandin E2
- lyso-PS
- lysophosphatidylserine
- MΦ
- macrophage
- RPMΦ
- resident peritoneal macrophage
- gp91
- gp91phox −/−
- CGD
- chronic granulomatous disease
- KC
- keratinocyte chemoattractant.
REFERENCES
- 1. Bratton D. L., Henson P. M. (2011) Neutrophil clearance. When the party is over, clean-up begins. Trends Immunol. 32, 350–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fadok V. A., Bratton D. L., Guthrie L., Henson P. M. (2001) Differential effects of apoptotic versus lysed cells on macrophage production of cytokines. Role of proteases. J. Immunol. 166, 6847–6854 [DOI] [PubMed] [Google Scholar]
- 3. Serhan C. N., Brain S. D., Buckley C. D., Gilroy D. W., Haslett C., O'Neill L. A., Perretti M., Rossi A. G., Wallace J. L. (2007) Resolution of inflammation. State of the art, definitions and terms. FASEB J. 21, 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. deCathelineau A. M., Henson P. M. (2003) The final step in programmed cell death. Phagocytes carry apoptotic cells to the grave. Essays Biochem. 39, 105–117 [DOI] [PubMed] [Google Scholar]
- 5. Freire-de-Lima C. G., Xiao Y. Q., Gardai S. J., Bratton D. L., Schiemann W. P., Henson P. M. (2006) Apoptotic cells, through transforming growth factor-β, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J. Biol. Chem. 281, 38376–38384 [DOI] [PubMed] [Google Scholar]
- 6. Zhang Y., Kim H. J., Yamamoto S., Kang X., Ma X. (2010) Regulation of interleukin-10 gene expression in macrophages engulfing apoptotic cells. J. Interferon Cytokine Res. 30, 113–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elliott M. R., Ravichandran K. S. (2010) Clearance of apoptotic cells. Implications in health and disease. J. Cell Biol. 189, 1059–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Erwig L. P., Henson P. M. (2008) Clearance of apoptotic cells by phagocytes. Cell Death Differ. 15, 243–250 [DOI] [PubMed] [Google Scholar]
- 9. Mevorach D., Mascarenhas J. O., Gershov D., Elkon K. B. (1998) Complement-dependent clearance of apoptotic cells by human macrophages. J. Exp. Med. 188, 2313–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taylor P. R., Carugati A., Fadok V. A., Cook H. T., Andrews M., Carroll M. C., Savill J. S., Henson P. M., Botto M., Walport M. J. (2000) A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J. Exp. Med. 192, 359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cho H. Y., Gladwell W., Wang X., Chorley B., Bell D., Reddy S. P., Kleeberger S. R. (2010) Nrf2-regulated PPARγ expression is critical to protection against acute lung injury in mice. Am. J. Respir Crit. Care Med. 182, 170–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lopes F., Coelho F. M., Costa V. V., Vieira É. L., Sousa L. P., Silva T. A., Vieira L. Q., Teixeira M. M., Pinho V. (2011) Resolution of neutrophilic inflammation by H2O2 in antigen-induced arthritis. Arthritis Rheum 63, 2651–2660 [DOI] [PubMed] [Google Scholar]
- 13. Holland S. M. (2010) Chronic granulomatous disease. Clin. Rev. Allergy Immunol. 38, 3–10 [DOI] [PubMed] [Google Scholar]
- 14. Gallin J. I., Buescher E. S. (1983) Abnormal regulation of inflammatory skin responses in male patients with chronic granulomatous disease. Inflammation 7, 227–232 [DOI] [PubMed] [Google Scholar]
- 15. Pollock J. D., Williams D. A., Gifford M. A., Li L. L., Du X., Fisherman J., Orkin S. H., Doerschuk C. M., Dinauer M. C. (1995) Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 9, 202–209 [DOI] [PubMed] [Google Scholar]
- 16. Frasch S. C., Berry K. Z., Fernandez-Boyanapalli R., Jin H. S., Leslie C., Henson P. M., Murphy R. C., Bratton D. L. (2008) NADPH oxidase-dependent generation of lysophosphatidylserine enhances clearance of activated and dying neutrophils via G2A. J. Biol. Chem. 283, 33736–33749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frasch S. C., Fernandez-Boyanapalli R. F., Berry K. Z., Leslie C. C., Bonventre J. V., Murphy R. C., Henson P. M., Bratton D. L. (2011) Signaling via macrophage G2A enhances efferocytosis of dying neutrophils by augmentation of Rac activity. J. Biol. Chem. 286, 12108–12122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr., Henson P. M. (1985) Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am. J. Pathol. 119, 101–110 [PMC free article] [PubMed] [Google Scholar]
- 19. Fadok V. A., Savill J. S., Haslett C., Bratton D. L., Doherty D. E., Campbell P. A., Henson P. M. (1992) Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J. Immunol. 149, 4029–4035 [PubMed] [Google Scholar]
- 20. Frasch S. C., Zemski-Berry K., Murphy R. C., Borregaard N., Henson P. M., Bratton D. L. (2007) Lysophospholipids of different classes mobilize neutrophil secretory vesicles and induce redundant signaling through G2A. J. Immunol. 178, 6540–6548 [DOI] [PubMed] [Google Scholar]
- 21. Frasch S. C., Bratton D. L. (2012) Emerging roles for lysophosphatidylserine in resolution of inflammation. Prog. Lipid Res. 51, 199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nibbering P. H., Zomerdijk T. P., Corsèl-Van Tilburg A. J., Van Furth R. (1990) Mean cell volume of human blood leucocytes and resident and activated murine macrophages. J. Immunol. Methods 129, 143–145 [DOI] [PubMed] [Google Scholar]
- 23. Hoffmann P. R., deCathelineau A. M., Ogden C. A., Leverrier Y., Bratton D. L., Daleke D. L., Ridley A. J., Fadok V. A., Henson P. M. (2001) Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J. Cell Biol. 155, 649–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vandivier R. W., Richens T. R., Horstmann S. A., deCathelineau A. M., Ghosh M., Reynolds S. D., Xiao Y. Q., Riches D. W., Plumb J., Vachon E., Downey G. P., Henson P. M. (2009) Dysfunctional cystic fibrosis transmembrane conductance regulator inhibits phagocytosis of apoptotic cells with proinflammatory consequences. Am. J. Physiol. Lung Cell Mol. Physiol. 297, L677–L686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ogden C. A., deCathelineau A., Hoffmann P. R., Bratton D., Ghebrehiwet B., Fadok V. A., Henson P. M. (2001) C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 194, 781–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McIlroy D., Tanaka M., Sakahira H., Fukuyama H., Suzuki M., Yamamura K., Ohsawa Y., Uchiyama Y., Nagata S. (2000) An auxiliary mode of apoptotic DNA fragmentation provided by phagocytes. Genes Dev. 14, 549–558 [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang B., Hirahashi J., Cullere X., Mayadas T. N. (2003) Elucidation of molecular events leading to neutrophil apoptosis following phagocytosis: cross-talk between caspase 8, reactive oxygen species, and MAPK/ERK activation. J. Biol. Chem. 278, 28443–28454 [DOI] [PubMed] [Google Scholar]
- 28. Fernandez-Boyanapalli R., Frasch S. C., Riches D. W., Vandivier R. W., Henson P. M., Bratton D. L. (2010) PPARγ activation normalizes resolution of acute sterile inflammation in murine chronic granulomatous disease. Blood 116, 4512–4522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martinez F. O., Helming L., Gordon S. (2009) Alternative activation of macrophages. An immunologic functional perspective. Annu. Rev. Immunol. 27, 451–483 [DOI] [PubMed] [Google Scholar]
- 30. Schif-Zuck S., Gross N., Assi S., Rostoker R., Serhan C. N., Ariel A. (2011) Saturated-efferocytosis generates pro-resolving CD11b low macrophages: modulation by resolvins and glucocorticoids. Eur. J. Immunol. 41, 366–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stables M. J., Shah S., Camon E. B., Lovering R. C., Newson J., Bystrom J., Farrow S., Gilroy D. W. (2011) Transcriptomic analyses of murine resolution-phase macrophages. Blood 118, e192–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bylund J., MacDonald K. L., Brown K. L., Mydel P., Collins L. V., Hancock R. E., Speert D. P. (2007) Enhanced inflammatory responses of chronic granulomatous disease leukocytes involve ROS-independent activation of NF-κB. Eur. J. Immunol. 37, 1087–1096 [DOI] [PubMed] [Google Scholar]
- 33. Segal B. H., Han W., Bushey J. J., Joo M., Bhatti Z., Feminella J., Dennis C. G., Vethanayagam R. R., Yull F. E., Capitano M., Wallace P. K., Minderman H., Christman J. W., Sporn M. B., Chan J., Vinh D. C., Holland S. M., Romani L. R., Gaffen S. L., Freeman M. L., Blackwell T. S. (2010) NADPH oxidase limits innate immune responses in the lungs in mice. PLoS ONE 5, e9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang L., Radu C. G., Yang L. V., Bentolila L. A., Riedinger M., Witte O. N. (2005) Lysophosphatidylcholine-induced surface redistribution regulates signaling of the murine G protein-coupled receptor G2A. Mol. Biol. Cell 16, 2234–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kabarowski J. H. (2009) G2A and LPC. Regulatory functions in immunity. Prostaglandins Other Lipid Mediat. 89, 73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brown G. C., Neher J. J. (2012) Eaten alive! Cell death by primary phagocytosis. 'Phagoptosis.' Trends Biochem. Sci. 37, 325–332 [DOI] [PubMed] [Google Scholar]
- 37. Silva M. T. (2011) Macrophage phagocytosis of neutrophils at inflammatory/infectious foci. A cooperative mechanism in the control of infection and infectious inflammation. J. Leukocyte Biol. 89, 675–683 [DOI] [PubMed] [Google Scholar]
- 38. Lagasse E., Weissman I. L. (1994) bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J. Exp. Med. 179, 1047–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brown J. R., Goldblatt D., Buddle J., Morton L., Thrasher A. J. (2003) Diminished production of anti-inflammatory mediators during neutrophil apoptosis and macrophage phagocytosis in chronic granulomatous disease (CGD). J. Leukocyte Biol. 73, 591–599 [DOI] [PubMed] [Google Scholar]
- 40. Fernandez-Boyanapalli R. F., Frasch S. C., McPhillips K., Vandivier R. W., Harry B. L., Riches D. W., Henson P. M., Bratton D. L. (2009) Impaired apoptotic cell clearance in CGD due to altered macrophage programming is reversed by phosphatidylserine-dependent production of IL-4. Blood 113, 2047–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sanmun D., Witasp E., Jitkaew S., Tyurina Y. Y., Kagan V. E., Ahlin A., Palmblad J., Fadeel B. (2009) Involvement of a functional NADPH oxidase in neutrophils and macrophages during programmed cell clearance: implications for chronic granulomatous disease. Am. J. Physiol. Cell Physiol. 297, C621–C631 [DOI] [PubMed] [Google Scholar]
- 42. Bolick D. T., Whetzel A. M., Skaflen M., Deem T. L., Lee J., Hedrick C. C. (2007) Absence of the G protein-coupled receptor G2A in mice promotes monocyte/endothelial interactions in aorta. Circ. Res. 100, 572–580 [DOI] [PubMed] [Google Scholar]
- 43. Parks B. W., Gambill G. P., Lusis A. J., Kabarowski J. H. (2005) Loss of G2A promotes macrophage accumulation in atherosclerotic lesions of low density lipoprotein receptor-deficient mice. J. Lipid Res. 46, 1405–1415 [DOI] [PubMed] [Google Scholar]
- 44. Bystrom J., Evans I., Newson J., Stables M., Toor I., van Rooijen N., Crawford M., Colville-Nash P., Farrow S., Gilroy D. W. (2008) Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood 112, 4117–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Medeiros A. I., Serezani C. H., Lee S. P., Peters-Golden M. (2009) Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. J. Exp. Med. 206, 61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rajakariar R., Newson J., Jackson E. K., Sawmynaden P., Smith A., Rahman F., Yaqoob M. M., Gilroy D. W. (2009) Nonresolving inflammation in gp91phox−/− mice, a model of human chronic granulomatous disease, has lower adenosine and cyclic adenosine 5′-monophosphate. J. Immunol. 182, 3262–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Henson P., Bratton D. (2009) in Phagocyte-Pathogen Interactions: Macrophages and the Host Responses to Infection (Russell D. G., Gordon S., eds) pp. 341–365, American Society for Microbiology, Washington, D. C. [Google Scholar]
- 48. Ravichandran K. S. (2010) Find-me and eat-me signals in apoptotic cell clearance. Progress and conundrums. J. Exp. Med. 207, 1807–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Voltan S., Martines D., Elli M., Brun P., Longo S., Porzionato A., Macchi V., D'Incà R., Scarpa M., Palù G., Sturniolo G. C., Morelli L., Castagliuolo I. (2008) Lactobacillus crispatus M247-derived H2O2 acts as a signal transducing molecule activating peroxisome proliferator activated receptor-gamma in the intestinal mucosa. Gastroenterology 135, 1216–1227 [DOI] [PubMed] [Google Scholar]
- 50. Coxon A., Rieu P., Barkalow F. J., Askari S., Sharpe A. H., von Andrian U. H., Arnaout M. A., Mayadas T. N. (1996) A novel role for the β2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity 5, 653–666 [DOI] [PubMed] [Google Scholar]
- 51. Morgenstern D. E., Gifford M. A., Li L. L., Doerschuk C. M., Dinauer M. C. (1997) Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J. Exp. Med. 185, 207–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sanford A. N., Suriano A. R., Herche D., Dietzmann K., Sullivan K. E. (2006) Abnormal apoptosis in chronic granulomatous disease and autoantibody production characteristic of lupus. Rheumatology 45, 178–181 [DOI] [PubMed] [Google Scholar]
- 53. Mukaro V. R., Hodge S. (2011) Airway clearance of apoptotic cells in COPD. Curr. Drug Targets 12, 460–468 [DOI] [PubMed] [Google Scholar]
- 54. Muñoz L. E., Lauber K., Schiller M., Manfredi A. A., Herrmann M. (2010) The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat. Rev. Rheumatol. 6, 280–289 [DOI] [PubMed] [Google Scholar]