Background: The role of the ubiquitin-proteasome pathway under high glucose conditions is unclear.
Results: AMPKα2 interacts with WWP1, and its expression is down-regulated by the ubiquitin proteasome pathway in high glucose culture conditions in C2C12 cells.
Conclusion: WWP1 down-regulates AMPKα2 expression through direct interaction in high glucose culture conditions of skeletal muscle C2C12 cells.
Significance: The ubiquitin proteasome pathway may involve high glucose-induced AMPKα2 down-regulation.
Keywords: AMP-activated kinase (AMPK), Diabetes, Glucose Metabolism, Metabolic Regulation, Ubiquitination
Abstract
It is known that the activity of AMP-activated protein kinase (AMPKα2) was depressed under high glucose conditions. However, whether protein expression of AMPKα2 is also down-regulated or not remains unclear. In this study, we showed that the expression of AMPKα2 was down-regulated in cells cultured under high glucose conditions. Treatment of proteasome inhibitor, MG132, blocked high glucose-induced AMPKα2 down-regulation. Endogenous AMPKα2 ubiquitination was detected by immunoprecipitation of AMPKα2 followed by immunoblotting detection of ubiquitin. The yeast-two hybrid (YTH) approach identified WWP1, an E3 ubiquitin ligase, as the AMPKα2-interacting protein in skeletal muscle cells. Interaction between AMPKα2 and WWP1 was validated by co-immunoprecipitation. Knockdown of WWP1 blocked high glucose-induced AMPKα2 down-regulation. The overexpression of WWP1 down-regulated AMPKα2. In addition, the expression of WWP1 is increased under high glucose culture conditions in both mRNA and protein levels. The level of AMPKα2 was down-regulated in the quadriceps muscle of diabetic animal model db/db mice. Expression of WWP1 blocked metformin-induced glucose uptake. Taken together, our results demonstrated that WWP1 down-regulated AMPKα2 under high glucose culture conditions via the ubiquitin-proteasome pathway.
Introduction
The metabolic syndrome is a combination of metabolic risk factors, including obesity, insulin resistance, hyperglycemia, and dyslipidemia, and it increases the risk of developing cardiovascular disease and diabetes. Maintaining energy balance depends on the efficiency of tightly regulated mechanisms of energy intake and expenditure. Hyperglycemia is a condition in which an excess amount of glucose circulates in the blood plasma. Glucose homeostasis is maintained by a balance between hepatic glucose production and glucose uptake by peripheral tissues. Activation of AMP-activated protein kinase (AMPK)2 enhances glucose uptake through the translocation of the glucose transporter 4 (GLUT4) to the cell membrane and also through regulation of Glut4 gene expression (1–3). The AMPK activator enhances glucose uptake into muscle by increasing cell surface GLUT4 levels (4–6). AMPK may regulate GLUT4 endocytosis (7, 8) and GLUT4 translocation (9, 10). Thus, AMPK is a well-accepted therapeutic target for the metabolic syndrome and type 2 diabetes (11, 12).
AMPK is an enzyme that plays a role in cellular energy homeostasis. AMPK, a heterotrimeric complex comprised of a catalytic subunit and two regulatory subunits, is activated when cellular energy is depleted (13). Activated AMPK switches on ATP-generating catabolic pathways, including glucose and fatty acid oxidation (14–16), while it simultaneously switches off ATP-consuming anabolic pathways (cholesterol, fatty acid, and triacylglycerol synthesis) (17). Thus, AMPK plays as a counter-regulatory mechanism and restores the AMP:ATP ratio. In addition to its roles in energy homeostasis, AMPK has also been shown to be regulated by ubiquitination (18). This fact suggests that the expression level of AMPK may play an important role in in specific biological conditions; however, the molecular link by which ubiquitination acts in high glucose conditions is obscure. It is reported that elevated glucose down-regulated the activity of AMPK in pancreatic cells (19) and rat skeletal muscle (20).
Protein ubiquitination is a mechanism for targeting proteins for rapid proteasomal degradation (21). Ubiquitin is attached covalently to target proteins in a series of reactions driven by ubiquitin ligase. E1 ligases load ubiquitin onto E2 ligases, which in turn modify target proteins. Substrate specificity is generated by E3 ligases, which serve as adaptors. Recent studies demonstrated that the ubiquitin proteasome system may regulate AMPK activity. The de-ubiquitination enzyme USP9X interacts with NUAK1 (AMPK-related kinase 5) (18), implicating a role of AMPK in ubiquitination. This group identified Cidea (cell death-inducing DFFA-like effector a) as an E3 ubiquitin ligase for AMPK (22). Although the basic regulation of AMPK activity by kinases has been delineated, regulation by post-translational modifications such as ubiquitination remains to be determined.
In this study, we found that AMPKα2 directly interacted with E3 ubiquitin ligase WWP1. We also showed that the expression level of AMPKα2 was down-regulated in C2C12 cells cultured under high glucose conditions, and further demonstrated that the ubiquitination is involved in high glucose-induced AMPKα2 down-regulation. These findings provide novel insights into the manner in which high glucose contributes to insulin resistance through down-regulation of AMPKα2 in skeletal muscle cells.
MATERIALS AND METHODS
Reagents
Anti-AMPKα2 antibody were purchased from Cell Signaling Technology (New England Biolabs, Beverly, MA). Anti-WWP1 antibody was purchased from ProteinTech Group, Inc. (Chicago, IL). Anti-ubiquitin antibody, anti-Flag antibody, anti-β-actin antibody, and AICAR were purchased from Cell Signaling Technology (New England Biolabs). Horseradish peroxidase-conjugated secondary antibodies were obtained from Assay Designs and Stressgen (Ann Arbor, MI). Metformin, MG132, and lactacystin were obtained from Calbiochem (San Diego, CA). l-(-)Glucose, d-(+)glucose, and mannitol were purchased from Sigma.
Cell Culture and Differentiation
Mouse myoblast C2C12 and human embryonic kidney (HEK) 293 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics at 37 °C in an incubator with 5% CO2. Cells were grown in culture medium consisting of 500 μl of DMEM (GIBCOTM, Auckland, NZ) containing 0.584 g/liter of l-glutamate and 4.5 g/liter of glucose, and mixed with 500 ml of F-12 medium containing 0.146 g/liter of l-glutamate, 1.8 g/liter of glucose, 100 μg/ml of gentamicin, 2.5 g/liter of sodium carbonate, and 10% heat-inactivated FBS. L6 myoblasts were maintained in cell monolayers in α-MEM supplemented with 10% FBS, 50 units of penicillin per ml, and 50 μg of streptomycin per ml in a 5% CO2 humidified atmosphere at 37 °C. Two days after the myoblasts achieved confluence, differentiation to myotubes was induced by incubating the cells for 6∼7 days in α-MEM supplemented with 2% FBS, which was changed every 2 days. The monolayer myotubes were finally serum-starved in DMEM for 2 h, then used in the following experiments.
Animal Tissue
Male C57BLKS/J-db/db mice and wild-type C57BL/6J mice were obtained from the Central Lab. Animal Inc. (Seoul, Korea) at age 6 weeks. All mice were maintained under standard light (12 hours light/dark), temperature (22 ± 2 °C), and humidity (40 ± 1 0%) conditions. The mice were anesthetized with dry ice, and quadriceps muscle was prepared. All procedures were approved by the Institutional Animal Care and Utilization Committee for Medical Science of Korea University.
Immunoblot Analysis
Cells were grown on 6-well plates and serum-starved for 24 h prior to treatment with the indicated agents. Following treatment of the cells, the media was aspirated, and the cells were washed twice in ice-cold PBS and lysed in 100 μl of lysis buffer. The samples were then briefly sonicated, heated for 5 min at 95 °C, and centrifuged for 5 min. The supernatants were electrophoresed on SDS-PAGE (8%) gels, and transferred to polyvinylidene difluoride membranes. The blots were incubated overnight at room temperature with primary antibodies and then washed six times in Tris-buffered saline/0.1% Tween 20 prior to 1 h incubation with horseradish peroxidase-conjugated secondary antibodies at room temperature. The blots were then visualized via ECL (Amersham Biosciences, Buckinghamshire, UK). In some cases, the blots were stripped and reprobed using other antibodies.
Immunoprecipitation
The amount of proteins from C212 cells or Flag-WWP1-transfected HEK293 cells was determined by the Bradford method. Cellular protein (100 μg) was mixed with 1 μg of anti-Flag or anti-AMPK antibodies and incubated overnight at 4 °C. Then, 10 μl of protein A-Sepharose (Amersham, Uppsala, Sweden) was added to these samples and incubated for another 3 h at 4 °C. After the incubation, samples were washed three times with wash buffer (25 mm HEPES, 5 mm EDTA, 1% Triton X-100, 50 mm NaF, 150 mm NaCl, 10 mm phenylmethylsulfonyl fluoride, 1 μm leupeptin, 1 μm pepstatin, and 1 μm aprotinin A, pH 7.2). The washed samples were resuspended in SDS sample buffer (125 mm Tris-HCl, pH 6.8, 20% [v/v] glycerol, 4% [w/v] SDS, 100 mm dithiothreitol, and 0.1% [w/v] bromphenol blue), and heated at 100 °C for 5 min prior to electrophoresis.
Silencing AMPKα2 and WWP1
C2C12 cells were seeded in 6-well plates and allowed to grow to 70% confluence for 24 h. Transient transfections were performed with transfection reagent (Lipofectamine 2000; Invitrogen) according to the manufacturer's protocol. Briefly, both AMPKα2 siRNA (NM_001013367; Dharmacon, CO), WWP1 siRNA (Genolution Pharmaceuticals, Inc.) and non-targeted control siRNAs (Non-targeting pool; Dharmacon CO) were designed. 5 μl of siRNA and 5 μl of transfection reagent (Lipofectamine 2000) were each diluted with 95 μl of reduced serum media (Opti-MEM; Invitrogen), then mixed. The mixtures were allowed to incubate for 30 min at room temperature and then were added dropwise to each culture well containing 800 μl of reduced serum media (Opti-MEM; final siRNA concentration, 100 nm). Four hours after transfection, the medium was changed with fresh complete medium. The cells were cultivated for 24 h and lysed, and expression of AMPKα2 protein was assayed with Western blotting.
Immunohistochemistry
Seven micrometer-thick cryosections from db/db and control mouse quadriceps femoris muscle were fixed with 4% freshly depolymerized paraformaldehyde for 15 min at 4 °C. After blocking with normal goat serum, sections were immunostained with antibodies against AMPKα for overnight at room temperature. After extensive washing with phosphate-buffered saline (PBS), sections were incubated with FITC-labeled secondary antibody.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
First-strand cDNA synthesis was performed using 1 μg of total RNA isolated from C2C12 at 55 °C for 20 min using the Thermoscript II one-step RT-PCR Kit (Invitrogen, Paisly, UK). Amplification of cDNA was carried out in the same tube using the Gene Amp System 9700 thermocycler (Applied Biosystems, Warrington, UK). Heating to 94 °C for 5 min inactivated the reverse transcriptase. The following PCR conditions were used: 27 cycles of 30 s at 94 °C, 30 s at 56 °C, and 30 s at 72 °C, followed by 7 min at 72 °C. The number of PCR cycles used was optimized to ensure amplification at the exponential phase. 10-μl samples from each RT-PCR product were removed and analyzed by agarose gel electrophoresis. Bands were stained with ethidium bromide and visualized under ultraviolet light. Band intensity quantification was determined by a gel documentation system (Gene Genius, Syngene, UK). The following primers were used: WWP1-sense (5′-TCC TGG TGA CGG AAG AAA AC-3′) and WWP1-antisense (5′-AAC CAG GTG TCT TTG CCA AC-3′); and AMPKα2-sense (5′-CGC CTC TAG TCC TCC ATC AG-3′) and AMPKα2-antisense (5′-CAG CTG TGC TGG AAT CAA AA-3′); and β-actin-sense (5′-CAG GAG GAG CAA TGA TCT TGA-3′) and β-actin antisense (5′-ACT ACC TCA TGA AGA TCC TCA-3′).
GST Pull-down Assay
GST fusion proteins containing different domains of AMPKα2 were engineered by PCR. The fusion proteins were expressed in Escherichia coli bacteria by incubation with 0.2 mm isopropyl β-d-galactopyranoside for 6 h at 37 °C. The bacteria were lysed by sonication, and the fusion proteins were purified with glutathione-agarose beads with buffer A followed by immunoblotting with anti-WWP1 antibody.
Glucose Uptake
Glucose uptake was measured using the 2-deoxy-d-[3H]glucose. Briefly, L6 cells were serum-starved overnight before each experiment, and glucose uptake was measured on day 7. Medium was replaced in the morning (serum-free medium), and cells were exposed to drugs for 1 h. Cells were washed twice in warm PBS before media and drugs were placed in DMEM devoid of glucose for 20 min. 2-Deoxy-d-[3H]glucose (50 nm) was added for 15 min at 37 °C, and the reactions were terminated by washing twice in ice-cold PBS. Cells were lysed in 10% SDS or 50 mm NaOH, and samples were transferred to scintillation vials with scintillant and allowed to sit at room temperature for 1 h before being counted.
Plasmid Construction of Flag-wwp1
The open reading frames of human WWP1 was cloned into the Pcmv-Tag2C vector (Stratagene) between EcoRI and SalI sites. cDNA from Hela cell line was amplified by PCR (forward primer, 5′-C GGA ATT CGA ATG GCC ACT GCT TCA CCA AG-3′; reverse primer 5′-A CGC GTC GAC TCA TTC TTG TCC AAA TCC CTC TG-3′). Kpn1- and Sac1-digested products were ligated into a linearized pCMV-Tag2C vector. The construct was verified by direct sequencing.
Data Analysis
Data are expressed as the means ± S.E. Image Gauge (version 3.12; Fujifilm, Tokyo, Japan) was used for analysis of band intensity. One-way ANOVA was used followed by a Holm-Sidak multiple-range test for comparison between groups. p values <0.05 were considered statistically significant.
RESULTS
AMPKα2 Down-regulated in C2C12 Cells Cultured under High Glucose Conditions
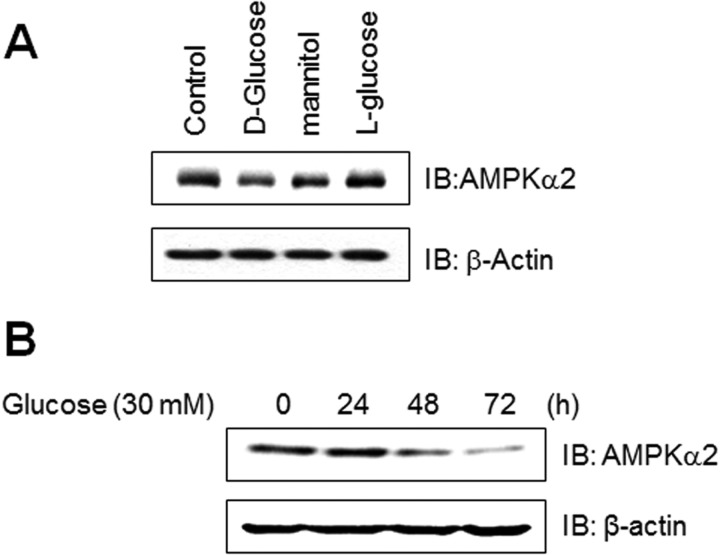
High glucose concentrations are known to have detrimental effects on many cells types. To gain insight into the effect of high glucose conditions on skeletal muscle system, we evaluated the effect of high glucose concentration on the expression of AMPKα2 in skeletal muscle C2C12 cells. The expression of AMPKα2 significantly down regulated in high glucose concentration in cell culture medium. Mannitol is used as the isotonic control. l-Glucose is used as the d-glucose specificity control. The level of AMPKα2 was not affected by these two agents, suggesting that AMPKα2 down-regulation is d-glucose-specific (Fig. 1A). We also assessed the effects of high concentrations of glucose in time dependence. Exposure to 30 mm concentration of glucose for 3 days dramatically down-regulated AMPKα2 expression (Fig. 1B). Together, these results demonstrate that high glucose conditions down-regulates AMPKα2 expression in skeletal muscle cells.
FIGURE 1.
AMPKα2 down-regulated in C2C12 cells cultured under high glucose conditions. A, C2C12 cells were stimulated for the indicated agents, such as d-glucose (30 mm), l-glucose (30 mm), and mannitol (30 mm) for 6 days. The cell lysates (20 μg) were analyzed via Western blotting for anti-AMPKα2 antibody. The anti-β-actin antibody was a protein loading control. B, C2C12 cells were stimulated for the indicated times with 30 mm high glucose. The cell lysates (20 μg) were analyzed via Western blotting for anti-AMPKα2 antibody. The anti-β-actin antibody was a protein loading control.
Proteasome Degradation Pathway Is Involved in High Glucose-induced AMPKα2 Degradation
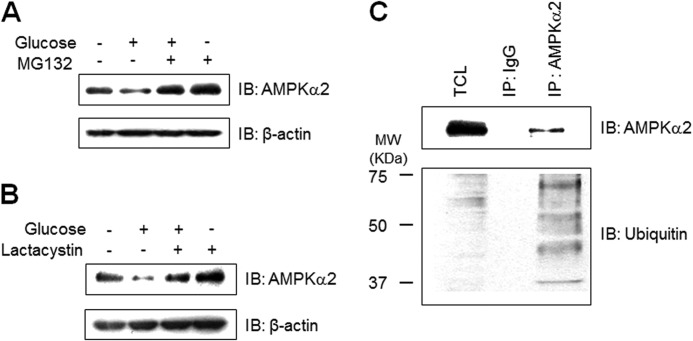
To check the involvement of proteasome degradation in AMPKα2 down-regulation, we analyzed the effect of proteasome inhibition on AMPKα2 expression. MG132, a proteasome inhibitor, blocked high glucose-induced AMPKα2 down-regulation (Fig. 2A). This effect was also observed by another proteasome inhibitor, lactacystin (Fig. 2B), suggesting the involvement of the ubiquitin-proteasome pathway. Next, we tested whether AMPKα2 was ubiquitinated in cells. We immunoprecipitated AMPKα2 from cell lysates, and then analyzed with antibody against ubiquitin. As can be seen, the multi-ubiquitination of AMPKα2 was detected in the immunoprecipitated sample (Fig. 2C). These results demonstrate that proteasome pathway is involved in high glucose-mediated AMPKα2 down-regulation.
FIGURE 2.
Proteasome degradation pathway is involved in high glucose-induced AMPKα2 degradation. A, C2C12 cells were pre-treated 5 μm MG132 for 1 h and stimulated with 30 mm glucose incubation for 48 h. The cell lysates (20 μg) were analyzed via Western blotting for anti-AMPKα2 antibody. The anti-β-actin antibody was a protein loading control. B, C2C12 cells were pre-treated 5 μm lactacystin for 1 h and incubated with 30 mm glucose for 48 h. The cell lysates (20 μg) were analyzed via Western blotting for anti-AMPKα2 antibody. The anti-β-actin antibody was a protein loading control. C, C2C12 cells were immunoprecipitated with anti-AMPKα2 antibody. The immunoprecipitates were analyzed via Western blotting for anti-ubiquitin and AMPKα2 antibodies. TCL, total cell lysates.
AMPKα2 Interacts with WWP1
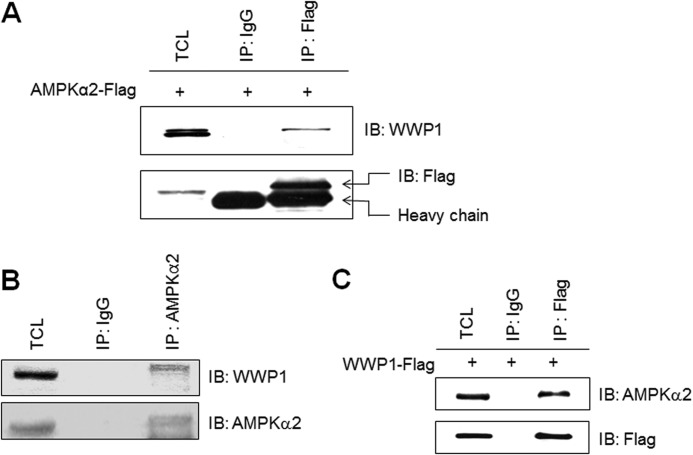
To explain the mechanism of high glucose-mediated AMPKα2 down-regulation, we tried to identify AMPKα2-interacting protein. To identify protein that interacts with the catalytic domain of AMPKα2, we carried out a YTH screen of a skeletal muscle C2C12 cDNA library. In our results, WWP1, a E3 ubiquitin ligase, was identified as an AMPKα2-interacting protein. To determine whether WWP1 interacts with AMPKα2 in mammalian cells, Flag-AMPKα2 was transfected into HEK293 cells. Lysates from transfected cells were immunoprecipitated with antibody against Flag. A band corresponding to WWP1 was detected in the immunoprecipitates, demonstrating interaction (Fig. 3A). Moreover, to assess in vivo interaction, we performed immunoprecipitation of skeletal muscle C2C12 cell protein using an AMPKα2 antibody, followed by Western blotting with an antibody to WWP1. AMPKα2 was co-immunoprecipitated with WWP1 from endogenous C2C12 cells (Fig. 3B). Immunoprecipitation of Flag-WWP1-transfected HEK293 cells using a Flag antibody showed that AMPKα2 was co-immunoprecipitated with WWP1 (Fig. 3C). These results demonstrated that the interaction detected in the YTH also occurs in vivo and in vitro.
FIGURE 3.
AMPKα2 interacts with WWP1. A, HEK293 cells were transiently transfected with Flag-AMPKα2 for 48 h and immunoprecipitated with anti-Flag antibody. The immunoprecipitates were analyzed via Western blotting for anti-WWP1 and Flag antibodies. B, cell lysates (1 mg) of C2C12 cells were immunoprecipitated with anti-AMPKα2 antibody. The immunoprecipitates were analyzed via Western blotting for anti-WWP1 and AMPKα2 antibodies. C, HEK293 cells were transiently transfected with Flag-WWP1 for 48 h and immunoprecipitated with anti-Flag antibody. The immunoprecipitates were analyzed via Western blotting for anti-AMPKα2 and Flag antibodies. TCL, total cell lysates.
Expression Level of AMPKα2 Is Regulated by Expression Status of WWP1
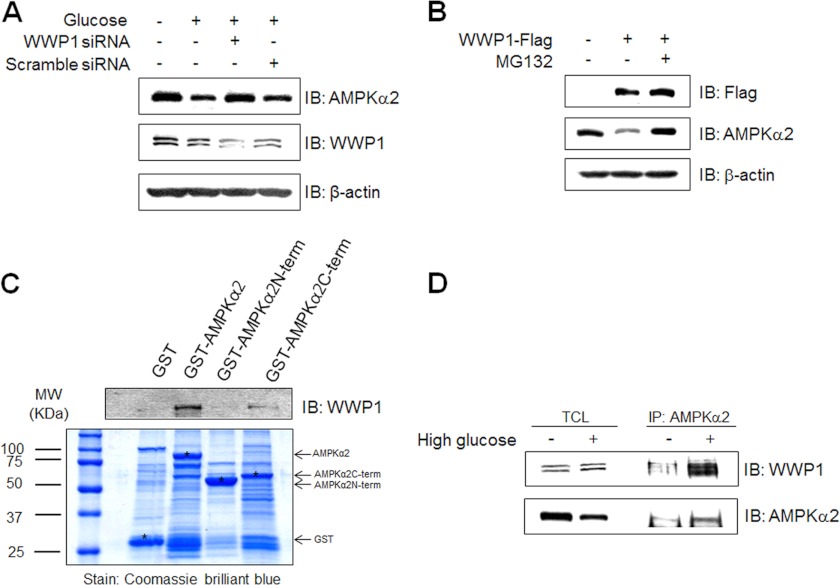
To investigate the relationship between WWP1 and AMPKα2, we first examined the effect of WWP1 knockdown on high glucose-induced AMPKα2 down-regulation. High glucose-induced down-regulation of AMPKα2 was blocked in WWP1 knockdown conditions. However, AMPKα2 down-regulation was apparent in scramble siRNA, implying that WWP1 is important to down-regulate AMPKα2 (Fig. 4A). Interestingly, the anti-WWP1 antibody recognized two protein bands in the C2C12 cell lysates. The intensity of the two bands changed in similar patterns, indicating that WWP1 may be recognized by both protein bands. Next, to further study the function of WWP1, we transiently transfected with Flag-WWP1. The level of AMPKα2 was down-regulated in cells in which WWP1 was overexpressed. This down-regulation was blocked by treatment with proteasome inhibitor (Fig. 4B), suggesting that ubiquitin-proteasome pathway is critical for AMPKα2 down-regulation. To determine the interaction domain, we constructed GST-fused whole AMPKα2, C-terminal AMPKα2, and N-terminal AMPKα2. We incubated GST fusion proteins with cell lysates from C2C12 cells. Targeted AMPKα2 proteins (asterisk) were detected by Coomassie Blue staining. Immunoblotting with anti-WWP1 antibody showed that C-terminal AMPKα2 interacted with WWP1 (Fig. 4C). Interaction between AMPKα2 and WWP1 was enhanced by high glucose incubation (Fig. 4D). Together, these results indicate that WWP1 play an important role in AMPKα2 down-regulation in high glucose culture conditions.
FIGURE 4.
Expression level of AMPKα2 is regulated by expression status of WWP1. A, C2C12 cells were transiently transfected with 50 nm siRNA WWP1 and scramble siRNA for 48 h and then exposed to 30 mm glucose incubation for 48 h. The cell lysates (20 μg) were analyzed via Western blotting for anti-AMPKα2, anti-WWP1 antibodies. The anti-β-actin antibody was a protein loading control. B, C2C12 cells were pre-treated 5 μm MG132 for 1 h and then cells were transiently transfected with Flag-WWP1 for 48 h. The cell lysates (20 μg) were analyzed via Western blotting for anti-AMPKα2, anti-Flag antibodies. The anti-β-actin antibody was a protein loading control. C, C2C12 cells were prepared and incubated with GST fusion proteins of various AMPKα2. Formed protein complexes were isolated by glutathione beads, washed three times with washing buffer, and analyzed by SDS/PAGE and subsequent Coomassie staining. The pull-down sample was also immunoblotted with anti-WWP1 antibody. D, C2C12 cells were exposed to 30 mm glucose incubation for 48 h. The cell lysates (1 mg) of normal and high glucose conditions C2C12 cells were immunoprecipitated with anti-AMPKα2 antibody. The immunoprecipitates were analyzed via Western blotting for anti-WWP1 and AMPKα2 antibodies.
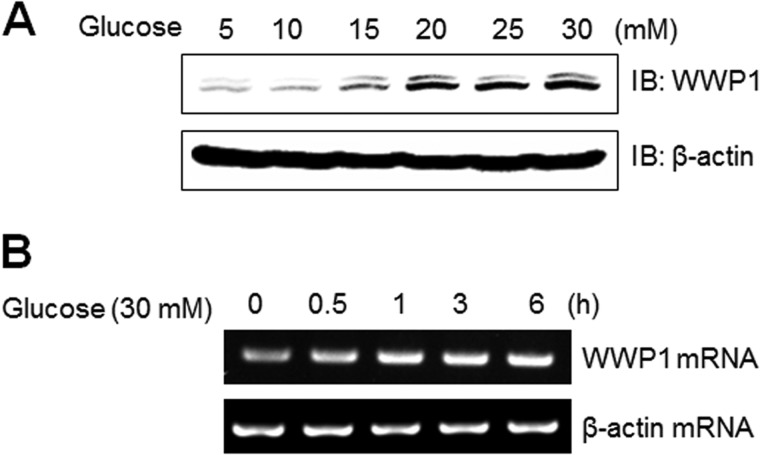
WWP1 Increased in High Glucose Conditions
To gain insight into the role of WWP1 in high glucose culture conditions, we examined the effect of high glucose concentrations on expression of WWP1 both in protein and mRNA levels. When cells were cultured with high concentrations of glucose, Western blot analysis showed that the level of WWP1 protein expression increased in dose-dependently (Fig. 5A). In experiments with RT-PCR, mRNA levels of WWP1 were also increased by incubation of C2C12 cells under a 30 mm concentration glucose condition (Fig. 5B). Together, these results suggest that WWP1 may be regulated by glucose concentration status.
FIGURE 5.
WWP1 increased in high glucose conditions. A, C2C12 cells were stimulated for the indicated doses of glucose for 48 h. The cell lysates (20 μg) were analyzed via Western blotting for anti-WWP1 antibody. The anti-β-actin antibody was a protein loading control. B, total mRNA was prepared for these cells after 30 mm glucose incubation, and RT-PCR was conducted using specific WWP1 primers. The PCR product was then gel-run in 1% agarose, and visualized in UV. β-Actin was employed as a positive control.
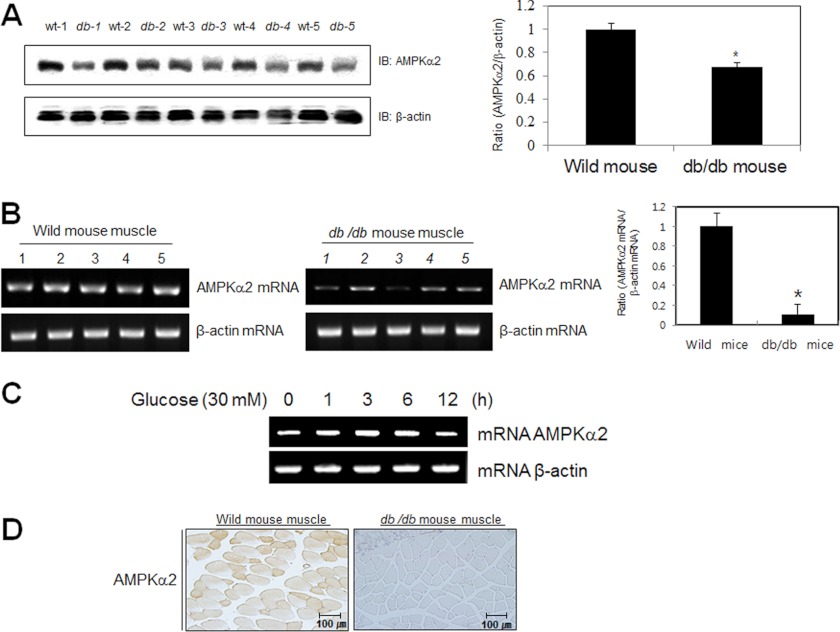
AMPKα2 Decreased in db/db Mouse
To examine the effect of high glucose on AMPKα2 in vivo, we used the type 2 diabetes animal model, db/db mouse. Proteins of the quadriceps from wild type and db/db mice were prepared. Each group had 5 mice. The non-fasting blood glucose levels in db/db mice were significantly higher than those of wild type mice (data not shown). Insulin levels of db/db mice were also increased (data not shown). Hyperinsulinemic-hyperglycemia of db/db mice indicates that these animals show insulin resistance, a primary characteristic of type 2 diabetes. The expression of AMPKα2 was down-regulated in quadriceps muscle of these mice (Fig. 6A). Furthermore, the level of AMPKα2 mRNA was also down-regulated (Fig. 6B). To confirm whether AMPKα2 is regulated at the transcriptional level, we performed RT-PCR experiments in C2C12 cells. The level of AMPKα2 mRNA was slightly down-regulated at 12 h after incubation with high glucose concentration, suggesting that AMPKα2 may be regulated by transcriptional level (Fig. 6C). Immunostaining of db/db mouse and wild type mouse identified AMPKα2 in quadriceps muscle. In hyperglycemic db/db mouse, the level of AMPKα2 was down-regulated compared with euglycemic wild type mouse. (Fig. 6D). The results from the diabetic animal model were correlated with biochemical data. These results indicate that the WWP1 may play a key function in AMPKα2 down-regulation under high glucose conditions.
FIGURE 6.
AMPKα2 down-regulated in db/db mouse. A, Western blot analysis of quadriceps muscles of wild type and db/db mice. The tissue lysates (40 μg) were analyzed via Western blotting for anti-AMPKα2 antibody. The anti-β-actin antibody was a protein loading control. Densitometry analysis (n = 5) of values are means ± S.E. values of the ratios of densities (AMPKα2/β-actin). *, p < 0.05 versus wild type mouse. B, RT-PCR analysis of quadriceps muscles of wild type and db/db mice. Total mRNA was prepared for these mice, and RT-PCR was conducted using specific AMPKα2 primers. The PCR product was then gel-run in 2% agarose, and visualized in UV. β-Actin RNA was employed as a positive control. Densitometry analysis (n = 5) of values are means ± S.E. values of the ratios of densities (AMPKα2 mRNA/β-actin mRNA). *, p < 0.05 versus wild type mouse. C, total mRNA was prepared for these cells after 30 mm glucose incubation, and RT-PCR was conducted using specific AMPKα2 primers. The PCR product was run in 1% agarose gel, and visualized in UV. β-Actin was employed as a positive control. D, histochemistry of the quadriceps muscle of wild type and db/db mice. Each cryosection was stained with anti-AMPKα2 antibody.
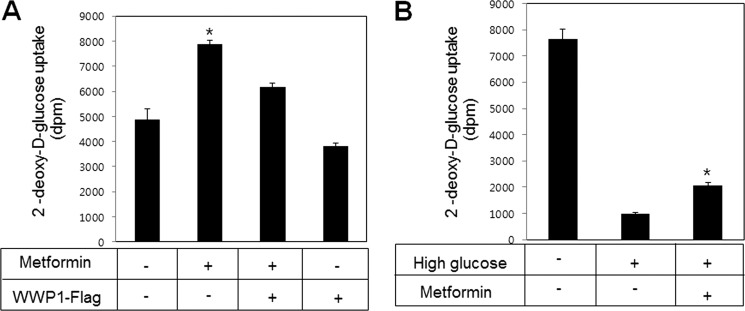
Expression of WWP1 Suppressed the Metformin-induced Glucose Uptake
Finally, to provide physiological relevance of WWP1, we investigated the effect of WWP1 expression on glucose uptake. Metformin-induced glucose uptake was blocked in WWP1 overexpression (Fig. 7A). To gain insight into the role of high glucose-mediated AMPKα2 down-regulation, we examined the effect of metformin on glucose uptake under high glucose culture condition. Attenuation of glucose uptake under high glucose culture conditions was recovered by treatment with metformin, a first choice for the treatment of type 2 diabetes (Fig. 7B). These results indicated that expression of WWP1 is important for metformin-mediated glucose uptake through regulating AMPKα2 expression.
FIGURE 7.
Expression of WWP1 blocked metformin-induced glucose uptake. A, L6 myoblast cells were differentiated for 7 days. Cells were transiently transfected with Flag-WWP1 for 48 h and then treated with metformin for 16 h. Glucose uptake was measured using 2-deoxy-d-[3H]glucose. *, p < 0.05 versus basal. B, L6 myoblast cells were differentiated for 7 days. Cells were incubated to 30 mm glucose for 48 h in the presence of or absence of metformin for 16 h. Glucose uptake was measured using 2-deoxy-d-[3H]glucose. *, p < 0.05 versus high glucose.
DISCUSSION
The principal finding of this study was that WWP1 is involved in high glucose-induced AMPKα2 down-regulation. Specifically, we demonstrated that direct interaction between AMPKα2 and WWP1 is instrumental in AMPKα2 down-regulation.
The anti-diabetic role of AMPK has previously been evaluated in conjunction with its activity. Dysfunction of AMPK activity may lead to metabolic syndrome. There is correlation between the low activation state of AMPK with metabolic disorders associated with insulin resistance and obesity (23, 24). AMPK activity is also suppressed in muscle and liver by sustained hyperglycemia (25). Moreover, many agents that are useful in treating diabetes, including TZDs (26, 27), metformin (28) have been shown to stimulate AMPK activity. The contribution of AMPK activity to the anti-diabetic role has raised questions as to which aspect of the protein expression of AMPK may be relevant to its metabolic role. In the present study, we have established that high glucose culture conditions down-regulated AMPK expression. Additionally, we have demonstrated that WWP1 down-regulated AMPKα2 through direct interaction. The relationship of AMPK with ubiquitination has been suggested. Collectively, our results indicate that AMPK may down-regulate in high glucose culture conditions via the ubiquitination pathway.
The main goal of the present study was to ascertain whether or not AMPKα2 is directly regulated by high glucose, and if so, to determine which mechanisms are involved in this process. Our data have revealed a novel role for WWP1 of AMPKα2 down-regulation in high glucose conditions. Our identification of an AMPKα2-WWP1 interaction in high glucose-treated skeletal muscle cells led us to hypothesize that WWP1 might play a role in the high glucose-mediated down-regulation of AMPKα2. Indeed, this is the first report of a link between AMPKα2 and WWP1 in skeletal muscle cells. It is thus tempting to speculate that WWP1-mediated regulation of AMPKα2 plays a role in diabetes, since AMPKα2 expression has been implicated in insulin resistance. Overall, although the mechanism by which high glucose influences AMPKα2 remains unknown, the findings in this report suggest that high glucose may down-regulate AMPKα2 expression through the WWP1 as part of the process of high glucose-mediated insulin resistance. In the present study, we further demonstrated that high glucose induces expression of WWP1, and overexpression of WWP1 decreased AMPKα2 expression. In addition, wild type WWP1 blocks metformin-mediated GLUT4 translocation. These results demonstrate that WWP1 has an important role in glucose homeostasis through regulating AMPKα2 expression. Regulation of AMPK by ubiquitination has been suggested (29, 30). The AMPK kinases NUAK1 and MARK4 are polyubiquitinated, and expression of NUAK and MARK4 mutants did not interact with the de-ubiquitinating enzyme USP9X de-ubiquitinating enzyme (DUB), thus resulting in AMPK activity (18). This result implies an important role for de-ubiquitination in AMPK activity regulation and further raises the question of what E3 ubiquitin ligases are involved. Because E3 ubiquitin ligases function to recognize specific substrates and to transfer ubiquitin to target protein, the specificity of the ubiquitin process is found in the E3 ubiquitin ligases. In the present study, we found an interaction of AMPKα2 with E3 ubiquitin ligase WWP1. This interaction is presumably required for high glucose-mediated AMPKα2 down-regulation. Combined with the recent report of interaction between proteasome itself and AMPK (31), protein-protein interaction has become a model for ubiquitin-mediated AMPK regulation.
On the basis of our present results, we propose a novel mechanism to explain how the expression of AMPK is down-regulated in high glucose culture conditions. This study revealed that AMPKα2 is down-regulated in hyperglycemic animal db/db mice, while its interaction partner WWP1 is up-regulated. In conclusion, WWP1 appears to be involved in insulin resistance through suppressing AMPKα2 expression. In conclusion, the present study supports the hypothesis that WWP1 may have an important role in hyperglycemic situations, such as diabetes and insulin resistance, through direct interaction with AMPKα2 and down-regulation of its expression.
This study was supported by the National Research Foundation of Korea funded by the Korean government (2010-0011053).
- AMPK
- AMP-activated protein kinase
- AICAR
- 5-aminoimidazole-4-carboxy-amide-1-d-ribofuranoside
- ACC
- acetyl-CoA carboxylase
- ACO
- acyl-CoA oxidase
- GLUT4
- glucose transporter 4
- YTH
- yeast two hybrid
- Cide
- cell death-inducing DFFA-like effector.
REFERENCES
- 1. Holmes B. F., Kurth-Kraczek E. J., Winder W. W. (1999) Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J. Appl. Physiol. 87, 1990–1995 [DOI] [PubMed] [Google Scholar]
- 2. Song H., Guan Y., Zhang L., Li K., Dong C. (2010) SPARC interacts with AMPK and regulates GLUT4 expression. Biochem. Biophys. Res. Commun. 396, 961–966 [DOI] [PubMed] [Google Scholar]
- 3. Nishino Y., Miura T., Miki T., Sakamoto J., Nakamura Y., Ikeda Y., Kobayashi H., Shimamoto K. (2004) Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 up-regulation in the late phase of cardioprotection. Cardiovasc. Res. 61, 610–619 [DOI] [PubMed] [Google Scholar]
- 4. Kramer H. F., Witczak C. A., Fujii N., Jessen N., Taylor E. B., Arnolds D. E., Sakamoto K., Hirshman M. F., Goodyear L. J. (2006) Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes 55, 2067–2076 [DOI] [PubMed] [Google Scholar]
- 5. Buhl E. S., Jessen N., Schmitz O., Pedersen S. B., Pedersen O., Holman G. D., Lund S. (2001) Chronic treatment with 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside increases insulin-stimulated glucose uptake and GLUT4 translocation in rat skeletal muscles in a fiber type-specific manner. Diabetes 50, 12–17 [DOI] [PubMed] [Google Scholar]
- 6. Koistinen H. A., Galuska D., Chibalin A. V., Yang J., Zierath J. R., Holman G. D., Wallberg-Henriksson H. (2003) 5-amino-imidazole carboxamide riboside increases glucose transport and cell-surface GLUT4 content in skeletal muscle from subjects with type 2 diabetes. Diabetes 52, 1066–1072 [DOI] [PubMed] [Google Scholar]
- 7. Karlsson H. K., Chibalin A. V., Koistinen H. A., Yang J., Koumanov F., Wallberg-Henriksson H., Zierath J. R., Holman G. D. (2009) Kinetics of GLUT4 trafficking in rat and human skeletal muscle. Diabetes 58, 847–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang J., Holman G. D. (2005) Insulin and contraction stimulate exocytosis, but increased AMP-activated protein kinase activity resulting from oxidative metabolism stress slows endocytosis of GLUT4 in cardiomyocytes. J. Biol. Chem. 280, 4070–4078 [DOI] [PubMed] [Google Scholar]
- 9. Shi L., Qin N., Hu L., Liu L., Duan H., Niu W. (2011) Tiliroside-derivatives enhance GLUT4 translocation via AMPK in muscle cells. Diabetes Res. Clin. Pract. 92, e41–46 [DOI] [PubMed] [Google Scholar]
- 10. Yamaguchi S., Katahira H., Ozawa S., Nakamichi Y., Tanaka T., Shimoyama T., Takahashi K., Yoshimoto K., Imaizumi M. O., Nagamatsu S., Ishida H. (2005) Activators of AMP-activated protein kinase enhance GLUT4 translocation and its glucose transport activity in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 289, E643–E649 [DOI] [PubMed] [Google Scholar]
- 11. Luo Z., Saha A. K., Xiang X., Ruderman N. B. (2005) AMPK, the metabolic syndrome and cancer. Trends Pharmacol. Sci. 26, 69–76 [DOI] [PubMed] [Google Scholar]
- 12. Zhang B. B., Zhou G., Li C. (2009) AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 9, 407–416 [DOI] [PubMed] [Google Scholar]
- 13. Hardie D. G., Carling D. (1997) The AMP-activated protein kinase–fuel gauge of the mammalian cell? Eur. J. Biochem. 246, 259–273 [DOI] [PubMed] [Google Scholar]
- 14. Makinde A. O., Gamble J., Lopaschuk G. D. (1997) Upregulation of 5′-AMP-activated protein kinase is responsible for the increase in myocardial fatty acid oxidation rates following birth in the newborn rabbit. Circ. Res. 80, 482–489 [DOI] [PubMed] [Google Scholar]
- 15. Ai H., Ihlemann J., Hellsten Y., Lauritzen H. P., Hardie D. G., Galbo H., Ploug T. (2002) Effect of fiber type and nutritional state on AICAR- and contraction-stimulated glucose transport in rat muscle. Am. J. Physiol. Endocrinol. Metab. 282, E1291–1300 [DOI] [PubMed] [Google Scholar]
- 16. Zong H., Ren J. M., Young L. H., Pypaert M., Mu J., Birnbaum M. J., Shulman G. I. (2002) AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. U.S.A. 99, 15983–15987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henin N., Vincent M. F., Gruber H. E., Van den Berghe G. (1995) Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. FASEB J. 9, 541–546 [DOI] [PubMed] [Google Scholar]
- 18. Al-Hakim A. K., Zagorska A., Chapman L., Deak M., Peggie M., Alessi D. R. (2008) Control of AMPK-related kinases by USP9X and atypical Lys(29)/Lys(33)-linked polyubiquitin chains. Biochem. J. 411, 249–260 [DOI] [PubMed] [Google Scholar]
- 19. Joly E., Roduit R., Peyot M. L., Habinowski S. A., Ruderman N. B., Witters L. A., Prentki M. (2009) Glucose represses PPARα gene expression via AMP-activated protein kinase but not via p38 mitogen-activated protein kinase in the pancreatic beta-cell. J. Diabetes 1, 263–272 [DOI] [PubMed] [Google Scholar]
- 20. Saha A. K., Xu X. J., Lawson E., Deoliveira R., Brandon A. E., Kraegen E. W., Ruderman N. B. (2010) Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes 59, 2426–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hershko A. (2005) The ubiquitin system for protein degradation and some of its roles in the control of the cell division cycle. Cell Death Differ. 12, 1191–1197 [DOI] [PubMed] [Google Scholar]
- 22. Qi J., Gong J., Zhao T., Zhao J., Lam P., Ye J., Li J. Z., Wu J., Zhou H. M., Li P. (2008) Downregulation of AMP-activated protein kinase by Cidea-mediated ubiquitination and degradation in brown adipose tissue. EMBO J. 27, 1537–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee W. J., Song K. H., Koh E. H., Won J. C., Kim H. S., Park H. S., Kim M. S., Kim S. W., Lee K. U., Park J. Y. (2005) α-Lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem. Biophys. Res. Commun. 332, 885–891 [DOI] [PubMed] [Google Scholar]
- 24. Martin T. L., Alquier T., Asakura K., Furukawa N., Preitner F., Kahn B. B. (2006) Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J. Biol. Chem. 281, 18933–18941 [DOI] [PubMed] [Google Scholar]
- 25. Assifi M. M., Suchankova G., Constant S., Prentki M., Saha A. K., Ruderman N. B. (2005) AMP-activated protein kinase and coordination of hepatic fatty acid metabolism of starved/carbohydrate-refed rats. Am. J. Physiol. Endocrinol. Metab. 289, E794–800 [DOI] [PubMed] [Google Scholar]
- 26. Fryer L. G., Parbu-Patel A., Carling D. (2002) The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 277, 25226–25232 [DOI] [PubMed] [Google Scholar]
- 27. Saha A. K., Avilucea P. R., Ye J. M., Assifi M. M., Kraegen E. W., Ruderman N. B. (2004) Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem. Biophys. Res. Commun. 314, 580–585 [DOI] [PubMed] [Google Scholar]
- 28. Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., Musi N., Hirshman M. F., Goodyear L. J., Moller D. E. (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thomson D. M., Hansen M. D., Winder W. W. (2008) Regulation of the AMPK-related protein kinases by ubiquitination. Biochem. J. 411, e9–10 [DOI] [PubMed] [Google Scholar]
- 30. Zungu M., Schisler J. C., Essop M. F., McCudden C., Patterson C., Willis M. S. (2011) Regulation of AMPK by the ubiquitin proteasome system. Am. J. Pathol. 178, 4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moreno D., Viana R., Sanz P. (2009) Two-hybrid analysis identifies PSMD11, a non-ATPase subunit of the proteasome, as a novel interaction partner of AMP-activated protein kinase. Int. J. Biochem. Cell Biol. 41, 2431–2439 [DOI] [PubMed] [Google Scholar]