Background: IAP antagonists sensitize toward apoptosis induced by TNF and other TNFR family ligands.
Results: IAP antagonism exerted effects on spontaneous as well as TNF-induced cytokine and chemokine production.
Conclusion: IAPs regulate spontaneous as well as TNF-induced cytokine/chemokine production.
Significance: IAP antagonists modulate cytokine production as well as apoptosis, which could influence their utility as adjuncts to chemotherapy.
Keywords: Apoptosis, Cell Death, Cytokine Induction, Inflammation, Tumor Necrosis Factor (TNF), IAP Antagonists, Inhibitor of Apoptosis Proteins (IAPs), Smac Mimetics
Abstract
Inhibitor of apoptosis proteins (IAPs) play a major role in determining whether cells undergo apoptosis in response to TNF as well as other stimuli. However, TNF is also highly proinflammatory through its ability to trigger the secretion of multiple inflammatory cytokines and chemokines, which is arguably the most important role of TNF in vivo. Indeed, deregulated production of TNF-induced cytokines is a major driver of inflammation in several autoimmune conditions such as rheumatoid arthritis. Here, we show that IAPs are required for the production of multiple TNF-induced proinflammatory mediators. Ablation or antagonism of IAPs potently suppressed TNF- or RIPK1-induced proinflammatory cytokine and chemokine production. Surprisingly, IAP antagonism also led to spontaneous production of chemokines, particularly RANTES, in vitro and in vivo. Thus, IAPs play a major role in influencing the production of multiple inflammatory mediators, arguing that these proteins are important regulators of inflammation in addition to apoptosis. Furthermore, small molecule IAP antagonists can modulate spontaneous as well as TNF-induced inflammatory responses, which may have implications for use of these agents in therapeutic settings.
Introduction
IAPs3 are a diverse family of proteins that have been implicated as regulators of apoptosis, mitosis, and inflammation (1–3). Indeed, it is becoming increasingly apparent that the original designation of this protein family as inhibitors of apoptosis is overly simplistic, and evidence is accumulating to suggest that the IAPs implicated in cell death control, cIAP-1, cIAP-2, and XIAP, also play important roles as regulators of signal transduction events that promote inflammation (4–6). However, to date, the overwhelming majority of studies have focused on the ability of these proteins to modulate apoptosis, particularly in the context of TNF receptor engagement, and few studies have explored the role of the IAPs in the production of proinflammatory mediators. Because TNF is a major driver of inflammation in response to infection as well as in the context of inflammatory diseases (7, 8), the role of IAPs in shaping TNF-dependent inflammatory signaling is an important unresolved question.
Engagement of the TNF receptor with its cognate ligand can result in apoptosis via a caspase-8-dependent pathway that is now relatively well understood (9, 10). TNF-dependent apoptosis proceeds via recruitment of TRADD, FADD, and caspase-8 to the cytoplasmic tails of trimerized TNF receptors, resulting in proximity-induced autoactivation of caspase-8 (9, 11). However, it is frequently overlooked that the majority of cell types fail to undergo apoptosis in response to TNF receptor engagement but can be sensitized through blocking NFκB activation signals (12–15). The identities of the NFκB-induced factor(s) that afford protection from TNF-induced apoptosis have been the subject of debate, with cIAP-1, cIAP-2, A20, and FLIP emerging as candidates. Recent evidence indicates that cIAP-1 and cIAP-2 play particularly influential roles in repressing TNFR-induced cell death signals.
Several groups have independently reported that small molecule IAP antagonists (also called Smac mimetics) can greatly sensitize cells toward TNF-dependent apoptosis (16–19). cIAP-1 and cIAP-2 are required for optimal TNF-induced canonical NFκB activation (20), most likely via their role as ubiquitin ligases for RIPK1, a central participant in TNF-initiated signaling (21, 22). Therefore, antagonism of cIAP-1 and cIAP-2 activities can sensitize toward TNF-induced apoptosis, apparently through suppression of cIAP-1/2-dependent RIPK1 ubiquitination, which is required for canonical NFκB activation (20, 21). Recent studies also suggest that IAPs negatively regulate assembly of a RIPK1-containing complex, “the ripoptosome,” most likely through restraining recruitment of FADD and caspase-8 to RIPK1 (23–25).
As mentioned above, most cell types do not undergo apoptosis directly in response to TNF but require sensitization toward its proapoptotic effects through blocking NFκB activation. Independent of its ability to promote apoptosis, a key aspect of TNF function is its capacity to trigger the production of a battery of proinflammatory cytokines and chemokines, thereby initiating and/or escalating immune responses. Examples of TNF-induced cytokines and chemokines include IL-6, IL-8, GM-CSF, and RANTES, which can instigate and amplify immune responses through triggering the acute phase response, recruitment of neutrophils and basophils to the site of inflammation, and by eliciting increased production of monocytes/macrophages from bone marrow. TNF-induced cytokines have both powerful and diverse effects on the immune system and overproduction of TNF is frequently associated with autoimmune conditions such as rheumatoid arthritis and Crohn disease (7). Given the key role of TNF as a major driver of inflammation in many settings (7, 8), agents that are capable of restraining TNF-induced cytokine production are of considerable therapeutic interest. Because IAP antagonism can dramatically alter sensitivity toward TNF-induced apoptosis, we wondered whether this could also modulate TNF-induced inflammatory cytokine production.
Here, we show that TNF-induced production of multiple proinflammatory cytokines and chemokines can be attenuated through antagonizing IAPs. Knockdown experiments revealed that cIAP-2 and RIPK1 were required for optimal TNF-induced cytokine and chemokine production. Furthermore, a small molecule IAP antagonist also inhibited the production of multiple TNF-induced inflammatory mediators. However, in the absence of TNF stimulation, IAP antagonism alone led to spontaneous production of the chemokines RANTES and IL-8 in certain cell types. These data suggest that strategies aimed at neutralizing IAPs to lower the threshold for apoptosis in cancer chemotherapy may produce outcomes that relate to the ability of such compounds to influence inflammatory cytokine production in addition to cell death. Thus, independent of their role as negative regulators of TNF-induced apoptosis, IAPs positively regulate the production of TNF-induced proinflammatory cytokines and chemokines and therefore act as important regulators of inflammation.
EXPERIMENTAL PROCEDURES
Materials
The following antibodies were used: anti-XIAP, anti-IKBα, and anti-IKKα (BD Biosciences); anti cIAP-1, anti-cIAP-2, and anti-TNFα (R&D Systems); anti-phospho IKKα/β, anti-MEK, anti-phospho MEK, anti-ERK, anti-phospho ERK, anti-p38, anti-phospho-p38, anti-JNK, anti-phospho-JNK, and anti-p100/p52 (Cell Signaling); and anti-RIPK1 and anti-p65 (Santa Cruz Biotechnology). Recombinant TNFα was purchased from Roche Applied Science. BV6 was from Genentech, and Z-VAD-fmk was from Bachem. The following inhibitors were used: UO126, PD98059 (Cell Signaling); BAY117082, ERK peptide, SP600125, SB202190 (Calbiochem); and SB203580 (Invivogen). Unless otherwise indicated, all other reagents were purchased from Sigma.
Cell Culture
HeLa cells were cultured in RPMI medium (Invitrogen), supplemented with 5% fetal calf serum (FCS). HT-29 and HaCaT were cultured in DMEM medium (Invitrogen) supplemented with 10% FCS. Human umbilical vein endothelial cells (PromoCell) were cultured in endothelial cell growth medium with added growth supplement (PromoCell). Cells were cultured at 37 °C in humidified atmosphere with 5% CO2.
Apoptosis Assays
Cells were plated at 2 × 105 cells/well in six-well plates and treated as indicated 24 h later. Apoptosis was enumerated based on cell morphology (cell rounding, detachment from the plate, nuclear condensation, and presence of apoptotic bodies) and by flow cytometry-based annexin V/propidium iodide staining). A minimum of 300 cells was counted in each treatment. Each assay was repeated a minimum of three times.
Measurement of Cytokines and Chemokines
Cells were plated at 2 × 105 cells/well in six-well plates and treated as indicated 24 h later. Cytokines and chemokines were measured from cell culture supernatants using specific paired antibody ELISA kits obtained from R&D Systems (human TNF, human MCP-1, human sICAM-1, human RANTES, human GM-CSF, mouse G-CSF, mouse M-CSF, mouse KC, mouse IL1α); eBiosciences (human IL-6, human IL-8, and mouse MCP-1); Promokine (human CXCL1, mouse IL-6); and Peprotech (mouse RANTES). Each assay was repeated a minimum of three times, and all cytokine assays were carried out using triplicate samples from each culture.
Western Immunoblotting
Cell lysates were prepared using SDS-PAGE loading buffer and were electrophoresed on 8–12% SDS-PAGE gels. Protein expression was examined by Western immunoblotting.
Transfection
To examine the effect of RIPK1 and IAP overexpression, HeLa cells were plated at 2 × 105 cells/well in six-well plates. After 24 h, cells were transfected using GeneJuice (Merck) with the following plasmid constructs: pRK.FLAG.RIPK1, pEBB.HA.cIAP1, pEBB.HA.cIAP2, and pEBB.HA.XIAP.
RNA Interference
To ablate RIPK-1, RIPK3, cIAP-1, cIAP-2, XIAP, p100, and p65 expression in HeLa cells, cells were transfected with 100 nm siRNA using nucleofection (Amaxa) as per the manufacturer's instructions and then seeded at 1 × 105 in six-well plates. After 48 h, cells were treated with TNF, BV6, or transfected with a RIPK1 cDNA. siRNA sequences were as follows: cIAP-1-1, 5′-ggcaaaugcugcggccaaca-3′ (sense); cIAP-1-2, 5′-uucguacauuucucucuuua-3′ (sense); cIAP-2-1, 5′-guucaagccaguuacccuc-3′ (sense); cIAP-2-2 5′-aagugguagggacuugugc-3′ (sense); XIAP-1-1, 5′-gugguaguccuguuucagc-3′ (sense); XIAP-1-2, 5′-gcaguugacaaguguccca-3′ (sense); RIPK-1-1, 5′-ggagcaaacugaauaaugaagagca-3′ (sense); RIPK-1-2, 5′-guacuccgcuuucuguaaa-3′ (sense); RIPK-3, 5′-uaacuugacgcacgacauc-3′ (sense); p100-1, 5′-cagccuaagcagagaggcu-3′ (sense); p100-2, 5′-gaugaagauugagcggccu-3′ (sense); p65-1, 5′-gauugaggagaaacguaaa-3′ (sense); p65-2, 5′-gcccuaucccuuuacguca-3′ (sense).
Animals
C57BL/6 mice were purchased from Harlan. Animal experiments were in accordance with the regulations of the Trinity College Dublin ethics committee and the Irish Department of Health.
RESULTS
Several groups have independently demonstrated that small molecule IAP antagonists greatly sensitize many cell types to TNF-induced apoptosis (16–19). However, because a major role of TNF in vivo is to promote the production of proinflammatory cytokines and chemokines in the context of innate immune responses, we wondered whether IAPs are also capable of influencing TNF-dependent inflammatory responses.
TNF Induces the Production of Multiple Cytokines in the Absence of Apoptosis
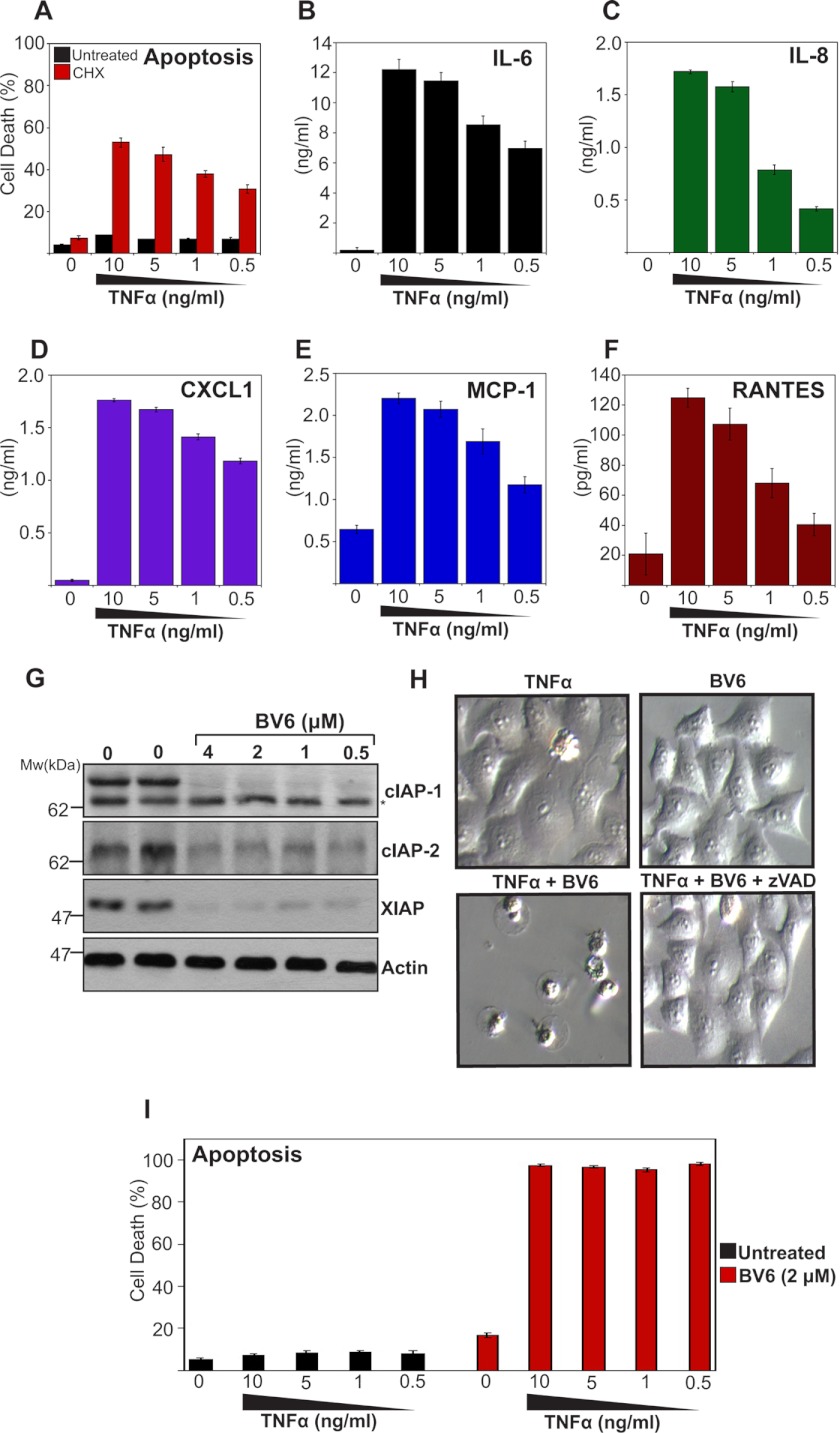
To explore the role of IAPs as regulators of inflammatory cytokine production, we initially used HeLa cells, which are insensitive to the apoptosis-inducing effects of TNF in common with numerous other cell types (Fig. 1A). However, HeLa cells can be sensitized to TNF-induced apoptosis through addition of transcriptional/translational inhibitors that block the actions of TNF-induced NFkB activation (Fig. 1A). Under conditions where TNF failed to induce apoptosis, HeLa cells secreted large amounts of IL-6, IL-8, CXCL1/KC, MCP-1, and RANTES (regulated on activation normal T cell expressed and secreted) in response to TNFR stimulation (Fig. 1, B–F). Thus, independent of its ability to provoke apoptosis, TNF is capable of triggering the production of diverse proinflammatory cytokines and chemokines.
FIGURE 1.
TNF induces a battery of proinflammatory cytokines and chemokines. A, HeLa cells were pretreated or not with cycloheximide (CHX; 2 μm) for 2 h, followed by TNF stimulation at the indicated concentrations. After 24 h, apoptosis was determined by morphological assessment. Data represent triplicate counts of a minimum of 300 cells per treatment. B–F, HeLa cells were stimulated with TNF at the indicated concentrations. After 24 h, cytokine concentrations in the culture supernatants were determined by ELISA. G, HeLa cells were treated with BV6 at the indicated concentrations. After 24 h, levels of endogenous IAPs were analyzed by immunoblotting. * indicates a non-specific band. H, HeLa cells were pretreated for 2 h with BV6 (4 μm) in the presence or absence of Z-VAD-fmk (20 μm), followed by addition of TNF (10 ng/ml). 24 h later, cell cultures were visualized by phase-contrast microscopy. I, percentage apoptosis in cultures from H was determined by morphological assessment as described in A.
TNF-induced Cytokine Production Can Be Inhibited through Antagonism of IAPs
To explore the role of IAPs in TNF-induced cytokine and chemokine production, we used a bivalent small molecule IAP antagonist (BV6) that was previously shown to promote the rapid degradation of cIAP-1 and cIAP-2 (16). Consistent with this, BV6 promoted essentially complete degradation of cIAP-1, cIAP-2, as well as XIAP (Fig. 1G) and greatly sensitized HeLa cells to the apoptosis-inducing effects of TNF (Fig. 1, H and I).
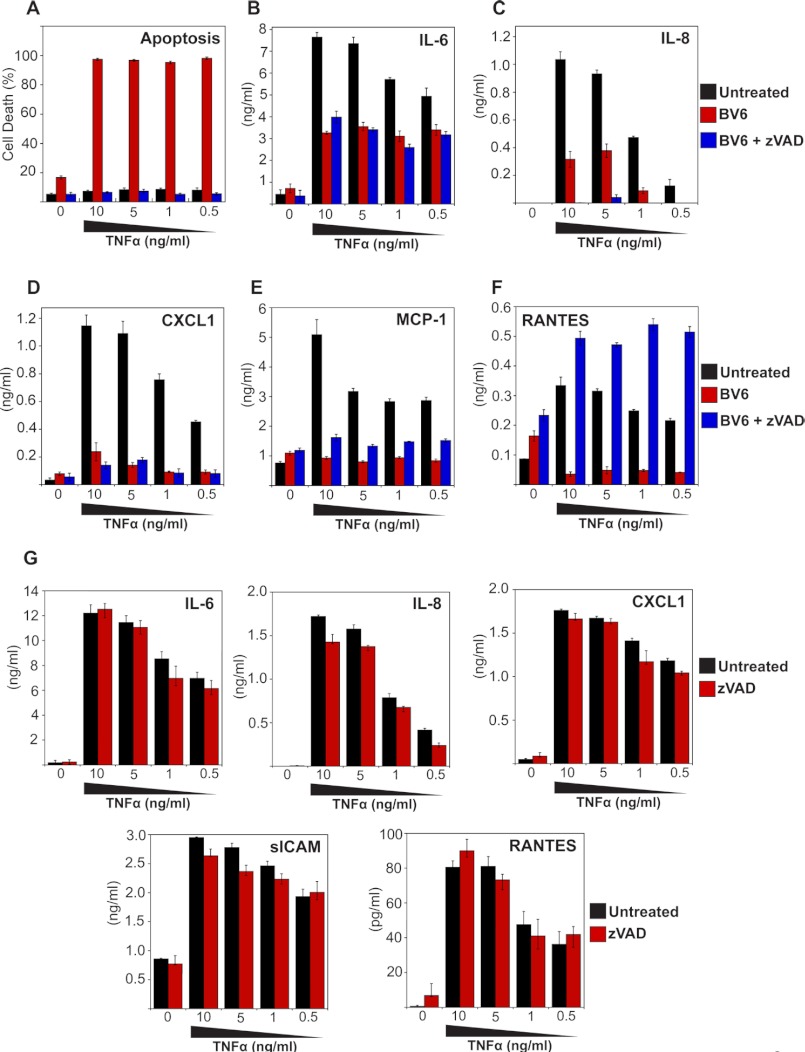
We then explored the impact of IAP antagonism on TNF-induced cytokine production. HeLa cells were stimulated with TNF in the presence or absence of IAP antagonist and, as before, this greatly sensitized toward TNF-induced apoptosis, which was completely suppressed through addition of the polycaspase inhibitor Z-VAD-fmk (Fig. 2A). Importantly, TNF-induced production of IL-6, IL-8, CXCL1, MCP-1, and RANTES were profoundly inhibited through addition of IAP antagonist (Fig. 2, B–F). This strongly suggests that IAPs are required for optimal TNF-induced cytokine production, consistent with previous observations that IAPs are required for canonical NFκB activation upon TNFR ligation (20). Because TNF induced widespread apoptosis in the presence of the IAP antagonist (Fig. 2A), it was possible that TNF-induced cytokine production was impaired merely as a consequence of cell death, leading to a decline in transcription/translation, rather than as a direct consequence of IAP antagonism. However, under conditions where TNF/BV6-initiated cell death was completely suppressed by Z-VAD-fmk (Fig. 2A), TNF-induced cytokine and chemokine production were still inhibited in the presence of BV6 (Fig. 2, B–E). The exception to this was RANTES, the production of which was robustly increased upon blocking cell death with Z-VAD-fmk (Fig. 2F). Indeed, the levels of this chemokine seen in response to TNF/BV6/Z-VAD-fmk treatment exceeded those seen in response to TNF alone (Fig. 2F). Note that Z-VAD-fmk-treatment alone failed to influence TNF-induced cytokine production (Fig. 2G).
FIGURE 2.
TNF-induced cytokine production is attenuated through inhibition of IAPs. A, HeLa cells were pretreated for 2 h with BV6 (4 μm) in the presence or absence of Z-VAD-fmk (20 μm), followed by stimulation with TNF at the indicated concentrations. After 24 h, apoptosis was determined by morphological assessment. Data represent triplicate counts of a minimum of 300 cells per treatment. B–F, cytokine concentrations in the culture supernatants from A were determined by ELISA. G, HeLa cells were pretreated for 2 h with Z-VAD-fmk (20 μm), followed by stimulation with TNF at the indicated concentrations. After 24 h, cytokine concentrations in the culture supernatants were determined by ELISA. Results shown are representative of at least three independent experiments. Error bars represent the mean ± S.E. of triplicate determinations from a representative experiment.
TNF-induced Cytokine Production Can Be Suppressed without Sensitizing toward Apoptosis
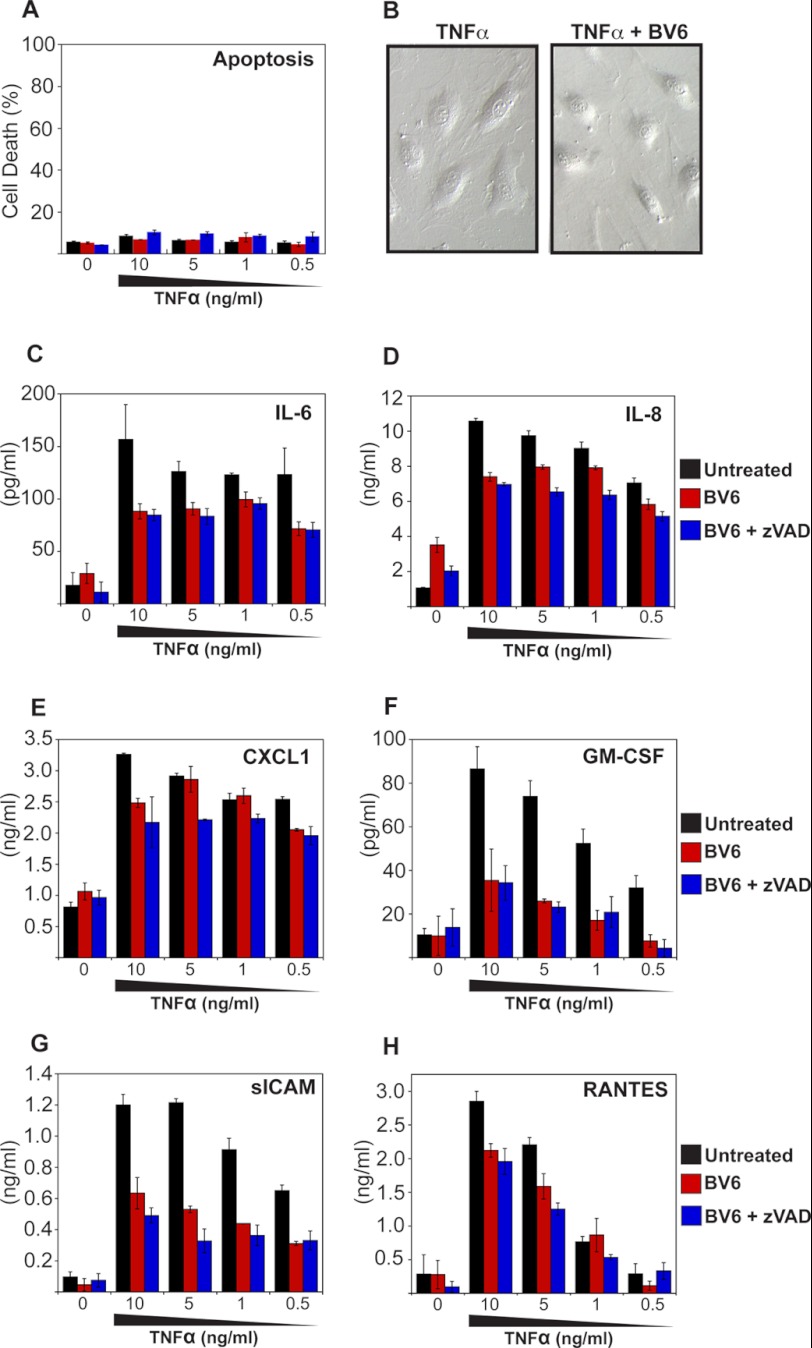
To further separate the effects of IAP antagonism on TNF-induced apoptosis versus TNF-induced cytokine production, we screened a panel of cell types for lack of sensitization to TNF-induced apoptosis in the presence of IAP antagonist (data not shown). These experiments revealed that primary human umbilical vein endothelial cells fail to be sensitized toward the apoptosis-inducing effects of TNF through addition of BV6 (Fig. 3, A and B). This enabled us to resolve whether inhibition of IAPs suppressed TNF-induced cytokine production completely independent of sensitization toward apoptosis. As Fig. 3, C–H, illustrates, BV6 inhibited TNF-induced production of IL-6, IL-8, CXCL1, GM-CSF, sICAM, and RANTES in primary human umbilical vein endothelial cells, despite failing to sensitize these cells toward TNF-induced apoptosis under the same conditions (Fig. 3, A and B). These data again argue that IAPs are required for optimal TNF-induced proinflammatory cytokine and chemokine production and that synthetic IAP antagonists can influence this important aspect of TNF function.
FIGURE 3.
IAP antagonist-mediated suppression of inflammatory cytokines can be uncoupled from sensitization toward apoptosis. A, primary human umbilical vein endothelial cells were pretreated for 2 h with BV6 (4 μm) in the presence or absence of Z-VAD-fmk (20 μm), followed by stimulation with TNF at the indicated concentrations. After 24 h, apoptosis was scored by morphological assessment. B, cell cultures from A, were visualized by phase contrast microscopy. C–H, cytokine concentrations in the culture supernatants from A were determined by ELISA. Results shown are representative of least three independent experiments. Error bars represent the mean ± S.E. of triplicate determinations from a representative experiment.
IAP Antagonism Can Lead to Spontaneous Production of Chemokines
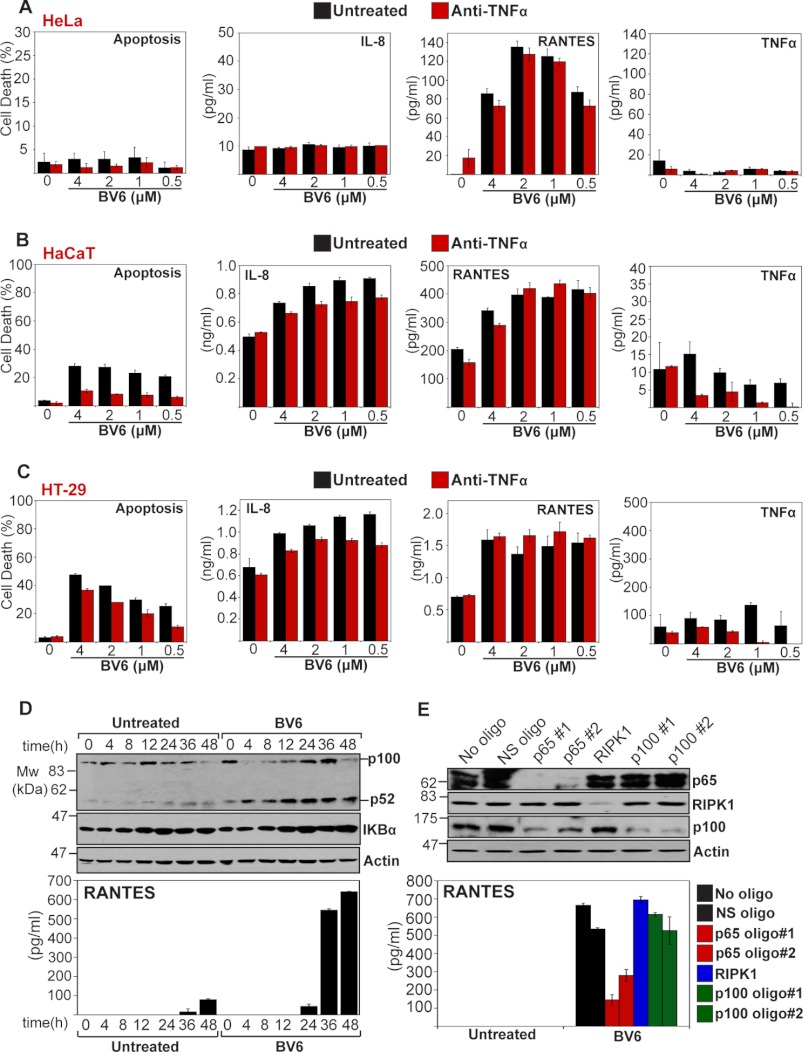
In contrast to the general suppression of TNF-induced cytokine and chemokine production observed in the presence of IAP antagonist, treatment of HeLa cells with BV6 alone led to spontaneous production of RANTES above basal levels (Fig. 2F). Indeed, RANTES production was further enhanced through inhibition of caspase activity in the presence of BV6 (Fig. 2F). To explore this observation further, we assessed the production of several cytokines and chemokines in response to BV6 treatment in HeLa, HaCaT, and HT-29 cell lines (Fig. 4, A–C). These experiments revealed that RANTES, as well as IL-8 to a lesser degree, were consistently induced in the presence of IAP antagonist alone (Fig. 4, A–C), in contrast to the generally suppressive effects seen in response to TNF-induced production of these chemokines (Fig. 2). Moreover, this effect was observed in several independent cell lines (Fig. 4, A–C). IAP antagonist-induced expression of RANTES did not appear to be due to TNF production because this was not attenuated through addition of neutralizing anti-TNF antibodies (Fig. 4, A–C). Thus, interfering with cellular IAPs can produce complex alterations to the pattern of spontaneous as well as TNF-induced production of cytokines and chemokines.
FIGURE 4.
IAP antagonism can lead to spontaneous production of chemokines. A–C, the indicated cell lines were pretreated with neutralizing anti-TNF antibodies (1 μg/ml) for 1 h, followed by addition of BV6 at the indicated concentrations. 24 h later, apoptosis was determined by morphological assessment and cytokine/chemokine concentrations in the culture supernatants were determined by ELISA. D, HeLa cells were seeded at 1 × 106 in 6-cm plates. The following day, cells were treated with BV6 (2 μm). At the indicated time points, cell lysates were analyzed by immunoblotting for levels of endogenous p100/p52 and IKBα, whereas RANTES was measured in the corresponding supernatants by ELISA. E, HeLa cells were transfected either with non-silencing siRNA (NS) or siRNAs targeted against p65, RIPK1, or p100, and 48 h later, cells were treated with BV6 (2 μm). After a further 48 h, cell lysates were analyzed by immunoblotting for the indicated proteins, and RANTES concentrations in the supernatants were determined by ELISA. Results shown are representative of at least three independent experiments. Error bars represent the mean ± S.E. of triplicate determinations from a representative experiment. oligo, oligonucleotide.
IAP antagonists have been shown to promote NIK stabilization as a consequence of cIAP-1 and cIAP-2 degradation, both of which appear to contribute to constitutive degradation of this kinase (16). Therefore, we considered the possibility that BV6-induced chemokine production may be due to the activation of NIK-dependent non-canonical NFκB activation (16). To explore this, we monitored the processing of NFκB2 p100 to p52 during BV6 treatment and confirmed that this indeed occurred in parallel with RANTES production (Fig. 4D), suggesting that non-canonical NFκB activation was responsible for chemokine production as a result of BV6 treatment. However, knockdown of p100 failed to suppress BV6-induced RANTES production, whereas knockdown of RelA/p65 sharply attenuated production of this chemokine under the same conditions (Fig. 4E). This suggests that BV6 induces a complex route to selective NFκB p65 activation, possibly due to the elimination of p100, which as been reported to act as a bona fide inhibitor of a fraction of cytoplasmic RelA/p65 (26).
TNF-dependent Cytokine Production Is Regulated through RIPK1
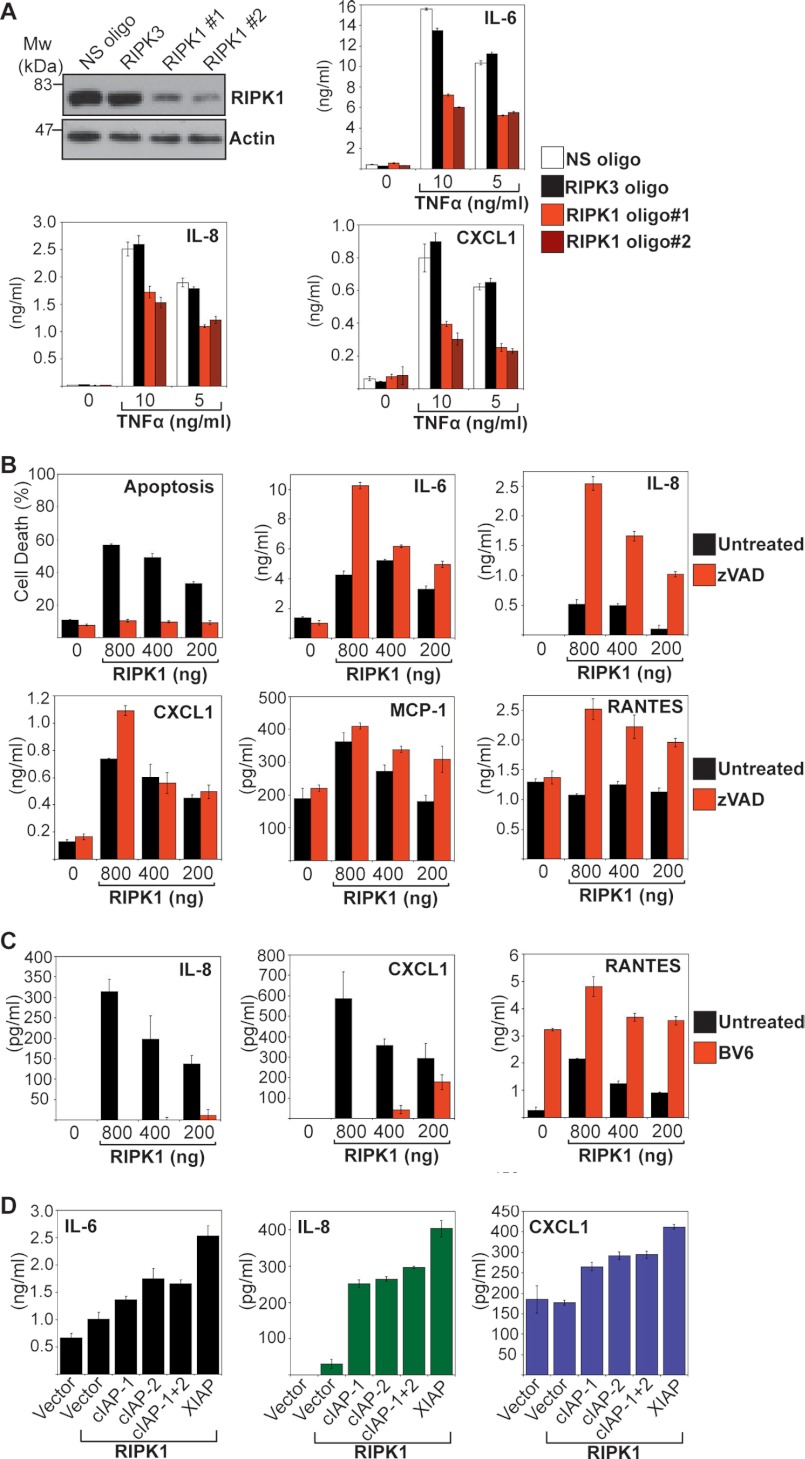
cIAP-1 and cIAP-2 have been implicated as regulators of RIPK1 polyubquitination and recruitment of downstream signaling intermediates in the context of TNFR signaling (21, 27). Because the preceding experiments found that IAP neutralization broadly suppressed TNF-induced cytokine production, this suggested that RIPK1 was important in this context. Thus, we asked whether knockdown of RIPK1 could also inhibit TNF-induced cytokine production. As Fig. 5A shows, silencing of RIPK1 with two different siRNAs greatly attenuated TNF-induced IL-6, IL-8, and CXCL1 production, suggesting that this kinase is required for the proinflammatory effects of TNFR stimulation. Consistent with this, transient overexpression of RIPK-1 also promoted production of IL-6, IL-8, CXCL1, MCP-1, and RANTES from HeLa cells (Fig. 5B). Moreover, inhibition of RIPK1-induced apoptosis using Z-VAD-fmk potentiated production of the latter cytokines, as expected (Fig. 5B). RIPK1-induced production of IL-8 and CXCL1 was robustly inhibited through addition of BV6 (Fig. 5C), although once again, we observed that BV6 enhanced RANTES expression.
FIGURE 5.
RIPK-1 plays a central role in TNF-induced cytokine production. A, HeLa cells were transfected with either non-silencing (NS), RIPK3, or RIPK1-targeted siRNAs then 48 h later, cells were treated with the indicated concentrations of TNF. After a further 24 h, cell lysates were analyzed by immunoblotting for levels of endogenous RIPK1 and cytokine concentrations in the supernatants were determined by ELISA. B, HeLa cells were transfected with empty vector or the indicated concentrations of a cDNA encoding RIPK1 in the presence or absence of Z-VAD-fmk (20 μm). After 24 h, apoptosis was determined by morphological assessment and cytokine concentrations in the culture supernatants were determined by ELISA. C, HeLa cells were transfected with empty vector or the indicated concentrations of a cDNA encoding RIPK1 in the presence or absence of BV6 (2 μm). Z-VAD-fmk (20 μm) was used to suppress RIPK1-induced cell death. After 24 h, cytokine concentrations in the culture supernatants were determined by ELISA. D, HeLa cells were pretreated for 12 h with BV6 (1 μm) then transfected in fresh medium with empty vector, RIPK1 cDNA alone, or RIPK1 cDNA in combination with IAP cDNA as indicated. After 24 h, cytokine concentrations in the culture supernatants were determined by ELISA. Results shown are representative of at least three independent experiments. Error bars represent the mean ± S.E. of triplicate determinations from a representative experiment. oligo, oligonucleotide.
Because RIPK1 activity is required for TNF-induced cytokine production as illustrated above, we next explored whether RIPK1-induced production of IL-6, IL-8, and CXCL1 could be enhanced through co-expression of cIAP-1, cIAP-2, or XIAP. As Fig. 5D illustrates, co-transfection of cIAP1, cIAP-2, or XIAP along with RIPK1 led to enhanced IL-6, IL-8, and CXCL1 production.
Knockdown of cIAP-2 Attenuates RIPK1- and TNF-induced Cytokine Production
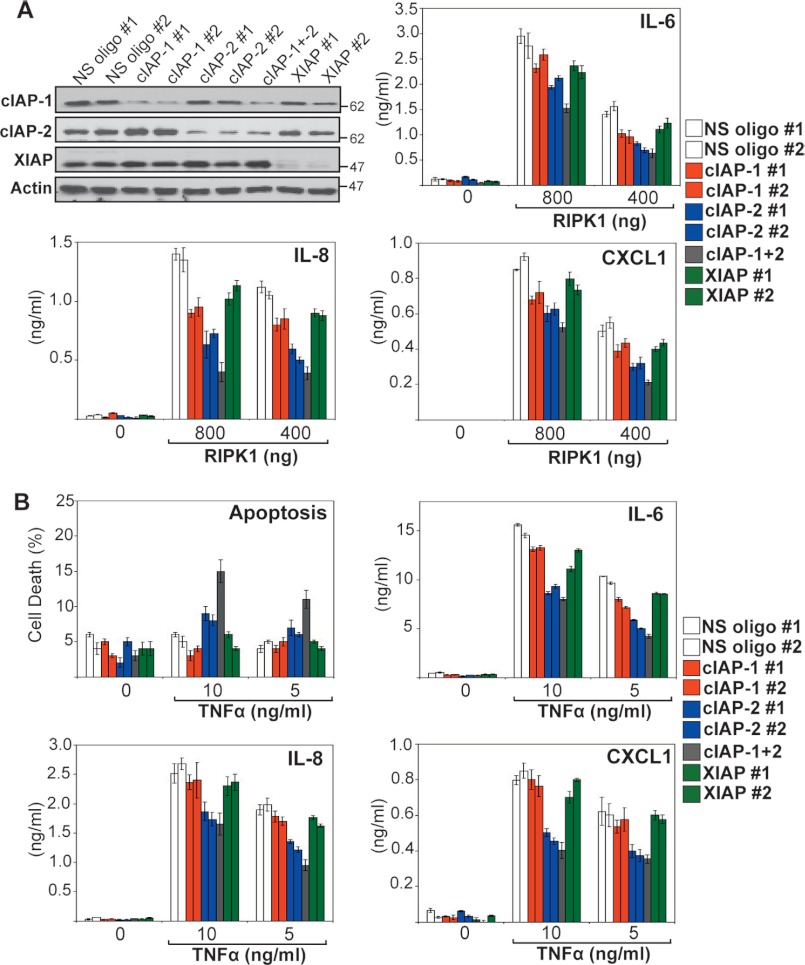
We next explored whether all three IAPs were required for optimal RIPK1-dependent production of cytokines through knocking down endogenous cIAP-1, cIAP-2, and XIAP, followed by transfection of RIPK1 (Fig. 6A). As Fig. 6A illustrates, knockdown of cIAP-2 had the greatest effect on RIPK1-induced cytokine production, with knockdown of both cIAP-1 and cIAP-2 having a greater effect than either alone. By contrast, knockdown of XIAP resulted in only a modest decrease in RIPK1-dependent cytokine production (Fig. 6A). These data suggest that RIPK1-dependent cytokine production requires endogenous cIAP-1 and cIAP-2.
FIGURE 6.
Knockdown of endogenous IAPs attenuates RIPK1- and TNF-induced inflammatory responses. A, HeLa cells were transfected with either non-silencing siRNA (NS) or siRNAs directed against cIAP-1, cIAP-2, or XIAP as indicated. 48 h later, cells were transfected with empty vector or a cDNA encoding RIPK1 in the presence of Z-VAD-fmk (25 μm) for inhibition of RIPK1-mediated cell death. 24 h later, cell lysates were analyzed by immunoblotting for endogenous IAPs, and cytokine concentrations in the culture supernatants were determined by ELISA. B, HeLa cells were transfected with either non-silencing siRNA (NS) or siRNAs directed against cIAP-1, cIAP-2, or XIAP as indicated. 48 h later, cells were treated with the indicated concentrations of TNF. After 24 h, cell death was scored by annexin V/propidium iodide staining, and cytokine concentrations in the resulting supernatants were determined by ELISA. Results shown are representative of at least three independent experiments. Error bars represent the mean ± S.E. of triplicate determinations from a representative experiment.
To confirm the identity of the endogenous IAPs required for optimal TNF-induced cytokine production, we again silenced expression of endogenous XIAP, cIAP-1, or cIAP-2 using specific siRNAs, followed by TNF stimulation. Similar to the effects of using IAP antagonist (BV6), knockdown of cIAP-2 attenuated TNF-induced IL-6, IL-8, and CXCL1 secretion and sensitized toward TNF-induced apoptosis (Fig. 6B). Taken together, these data argue that cIAP-2 in particular is important for optimal TNF-induced cytokine production.
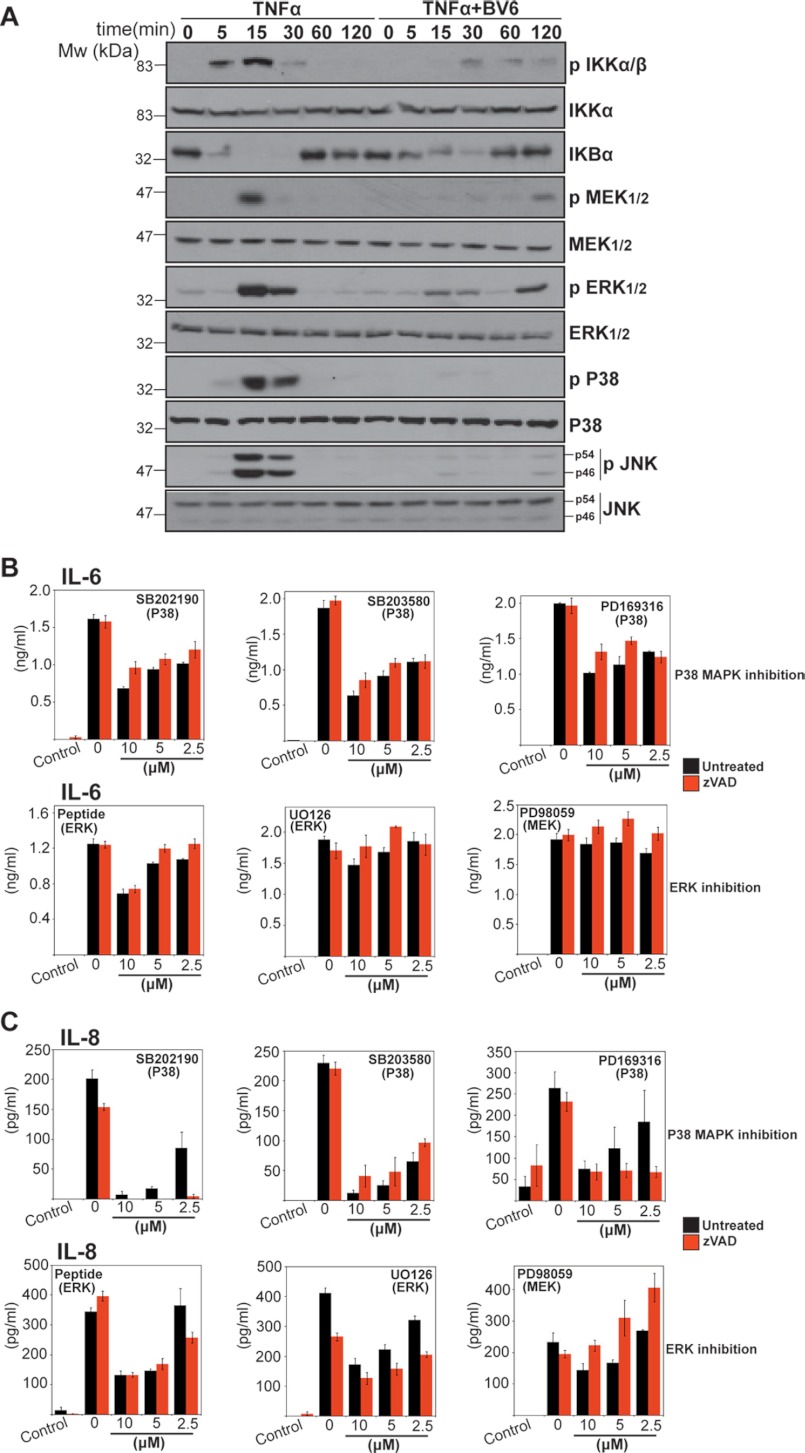
Several TNF-induced Kinases Are Suppressed through IAP Antagonism
TNF promotes the production of proinflammatory cytokines and chemokines through activating several kinases, including IKK kinases, p38MAPK, MEK/ERK, and JNK. Because TNF-induced cytokine production was attenuated through IAP antagonism, this suggested that one or more TNF-induced kinases failed to be activated upon loss of IAPs. To explore this issue, we monitored TNF-induced signal transduction events in the presence and absence of IAP antagonist. As Fig. 7A illustrates, TNF-induced activation of NFκB, MEK/ERK, p38MAPK, and JNK were all greatly attenuated in the presence of BV6. Furthermore, using a panel of kinase inhibitors (Fig. 7, B and C), similar inhibition of TNF-induced IL-6 and IL-8 production was also observed due to inhibition of p38MAPK and of MEK/ERK to a lesser degree (Figs. 7B and 6C). Thus, neutralization of IAPs suppresses TNF-induced production of cytokine and chemokines, most likely as a consequence of interfering with the activation of multiple kinases downstream of TNF receptor engagement.
FIGURE 7.
TNF-induced activation of multiple kinases is inhibited through neutralization of IAPs. A, HeLa cells were treated with TNF (10 ng/ml) in the presence or absence of BV6 (2 μm). Cell lysates were prepared at the indicated time points and analyzed by immunoblotting for the indicated proteins. B and C, HeLa cells were pretreated for 2 h with the indicated concentration of kinase inhibitor in the presence or absence of Z-VAD-fmk (10 μm) and then stimulated with TNF (1 ng/ml). After 24 h, cytokine concentrations in the culture supernatants were determined by ELISA. Results shown are representative of at least three independent experiments. Error bars represent the mean ± S.E. of triplicate determinations from a representative experiment.
IAP Antagonism Can Influence TNF-induced Inflammatory Responses in Vivo
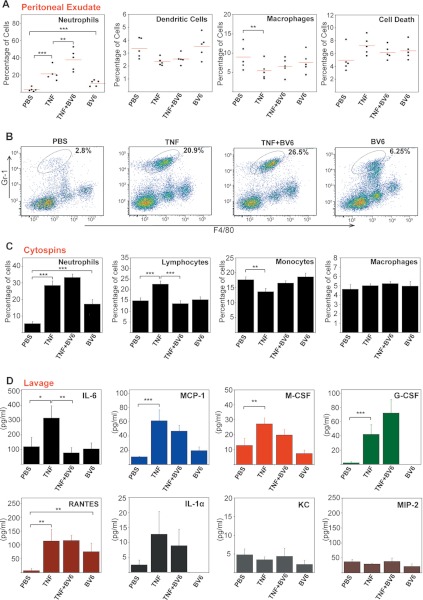
We next explored whether IAP antagonism could influence the pro-inflammatory effects of TNF in vivo by administering recombinant TNF into the peritoneal cavity of wild type mice in the presence and absence of BV6. As expected, TNF-treatment led to a rapid influx of neutrophils into the peritoneum (Fig. 8, A–C), along with elevated levels of IL-6, MCP-1, M-CSF, G-CSF, RANTES, and IL-1α (Fig. 8D). However, TNF-induced recruitment of neutrophils was slightly enhanced in the presence of BV6, and treatment with this IAP antagonist alone also elevated neutrophil counts in the peritoneum (Fig. 8, A–C). The latter observation correlated with elevated production of the granulocytic chemokine, RANTES, in the peritoneal lavage fluid (Fig. 8D), consistent with what we had earlier observed in vitro (Fig. 4). Furthermore, co-administration of BV6 with TNF robustly inhibited TNF-induced IL-6 production (Fig. 8D), with more modest inhibitory effects observed with MCP-1 and M-CSF, whereas production of other cytokines and chemokines was either unaffected or slightly enhanced due to BV6 co-administration (Fig. 8D). Once again, these data demonstrate that interfering with IAP function can influence spontaneous as well as TNF-driven inflammatory responses.
FIGURE 8.
IAP antagonism modulates TNF-induced inflammatory effects in vivo. A, female C57BL/6 mice (five per treatment group) were injected (intraperitoneally, 200 μl) with PBS or BV6 (20 μm). After 2 h, mice were injected again with either PBS or TNF (1 μg per mouse). After a further 10 h, mice were sacrificed, and the peritoneal cavity was washed with 5 ml of PBS. Peritoneal lavage-derived cells were immunostained and analyzed by flow cytometry. Percentage cell death was determined by Aqua positivity. B, peritoneal lavage-derived neutrophils (CD11b+, Gr1 high, F4/80+) from A were quantified by flow cytometry. C, cytospins were made from peritoneally derived cells from A and were stained with hematoxylin/eosin, and the percentage of individual immune cell types was assessed by morphology. D, cytokine/chemokine concentrations in the peritoneal lavage fluid from A were determined by ELISA. Error bars represent the mean ± S.E. *, p < 0.1; **, p < 0.05; ***, p < 0.01 by Student's t test.
Collectively, these data indicate that, in addition to their role as arbiters of cell death, IAPs also contribute to the regulation of spontaneous as well as TNF-induced cytokine and chemokine production, a property that may have significant implications for use of IAP antagonists as therapeutic agents.
DISCUSSION
Here, we have shown that interfering with IAP function, either through knockdown of IAPs or use of a small molecule IAP antagonist (BV6), can influence TNF-induced production of a range of proinflammatory cytokines and chemokines such as IL-6, IL-8, GM-CSF, CXCL1, and RANTES. These effects were independent of the ability of IAP antagonists to sensitize target cells toward apoptosis. However, IAP antagonism also led to spontaneous production of chemokines, such as RANTES, both in vitro as well as in vivo. Furthermore, knockdown of endogenous IAPs also attenuated TNF-induced inflammatory responses.
A number of chronic inflammatory conditions such as rheumatoid arthritis, Crohn disease, and psoriasis are associated with overproduction of TNF (8). Consequently, biologics such as anti-TNF antibodies capable of inhibiting the actions of this cytokine exhibit considerable therapeutic benefit (28, 29). TNF exerts its pathologic effects in such conditions through a cascade effect, which elicits the production of additional cytokines and chemokines from a variety of cell types, thereby provoking further escalation of immune responses. Our observations that small molecule IAP antagonists can attenuate some of the proinflammatory effects of TNF suggest that targeting IAPs may be a useful strategy in inflammatory diseases. However, the observation that IAP antagonism can also lead to spontaneous production of chemokines, such as RANTES and IL-8, from certain cell types, both in vitro as well as in vivo, is a potentially significant complicating factor for the use of such agents. The latter effects may be due to the activation of a fraction of RelA/p65 as a consequence of NIK-dependent p100 processing to p52 (Fig. 4, D–E). IAP antagonists have been shown to promote NIK stabilization as a consequence of cIAP-1 and cIAP-2 degradation, both of which appear to contribute to constitutive degradation of this kinase (16, 30). However, p100 was not required for BV6-induced RANTES production, apparently ruling out a role for non-canonical NFκB here. Surprisingly, RelA/p65 was involved in spontaneous RANTES production upon treatment with BV6 alone (Fig. 4E). This suggests that IAP antagonists can induce a complex route to RelA/p65 activation, possibly due to the elimination of p100, which has been reported to act as a bona fide inhibitor of a fraction of cytoplasmic RelA/p65 (26). Further studies will be required to resolve this issue.
IAP antagonists are also under investigation for their ability to provoke apoptosis in tumor cell types, either as single agents or in combination with other cytotoxic drugs. Where IAP antagonists display single agent efficacy, this has been shown to be due to sensitization of such tumors to a TNF-dependent autocrine loop where cells increase TNF production and become sensitized to this cytokine due to elimination of the IAP-mediated survival pathway (16–19). TNF has also been implicated in promoting tumor initiation and progression via a process dubbed “smoldering inflammation,” which can recruit cells of the innate immune system to the tumor site as a consequence of production of cytokines and chemokines such as IL-6 and IL-8 (31). Innate immune cells such as neutrophils and macrophages are capable of provoking further mutations as a consequence of the production of reactive oxygen and can affect tumor progression through release of additional growth promoting cytokines and chemokines such as IL-6, IL-8, and CXCL1/KC, which can have direct effects on tumor cell proliferation, resistance to apoptosis, and can instigate a wound healing response that can promote local neovascularization. Thus, the use of agents that can suppress the proinflammatory effects of TNF, in addition to sensitizing tumor cells toward apoptosis, can simultaneously achieve two desirable goals at once: lowering the threshold for apoptosis and breaking the inflammatory cycle that can permit tumor progression and metastasis.
RIPK1 is likely to be the key target of IAPs in the TNF pathway to cytokine production. Indeed, we have also shown here that knockdown of RIPK1 greatly attenuated TNF-induced cytokine production. Moreover, overexpression of RIPK1 was sufficient to promote the production of several proinflammatory chemokines (Fig. 5B). In this regard, it is interesting to note that the RIPK1-related kinase, RIPK3, has been implicated in a proinflammatory form of cell death that has been dubbed necroptosis (32, 33). However, studies on RIPK3-dependent necroptosis have assumed that necroptosis induces a proinflammatory state due to cell rupture and the release of endogenous alarmins (34). However, it is equally possible that deregulated RIPK3 activation may also lead to the transcriptional induction of conventional cytokines and chemokines, as we have shown here for RIPK1. Thus, necroptosis may be highly proinflammatory due to enhanced RIPK1/RIPK3-driven inflammatory cytokine production, rather than necrosis-associated alarmin release. Further studies will be required to resolve this issue.
In summary, independent of their role as inhibitors of apoptosis, our findings suggest that IAPs are important regulators of TNF-induced inflammatory cytokine and chemokine production. Thus, IAPs serve as key arbiters of inflammation as well as apoptosis.
This work was supported by Strategic Research Cluster 07/SRC/B1144 and Principal Investigator 08/IN.1/B2031 Grants from Science Foundation Ireland.
- IAP
- inhibitor of apoptosis protein
- TNFR
- tumor necrosis factor receptor
- Z-VAD-fmk
- benzyloxycarbonyl
- fmk
- fluoromethyl ketone.
REFERENCES
- 1. O'Riordan M. X., Bauler L. D., Scott F. L., Duckett C. S. (2008) Inhibitor of apoptosis proteins in eukaryotic evolution and development: a model of thematic conservation. Dev. Cell 15, 497–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gyrd-Hansen M., Meier P. (2010) IAPs: from caspase inhibitors to modulators of NF-κB, inflammation and cancer. Nat. Rev. Cancer 10, 561–574 [DOI] [PubMed] [Google Scholar]
- 3. Silke J., Brink R. (2010) Regulation of TNFRSF and innate immune signalling complexes by TRAFs and cIAPs. Cell Death Differ. 17, 35–45 [DOI] [PubMed] [Google Scholar]
- 4. Bertrand M. J., Doiron K., Labbé K., Korneluk R. G., Barker P. A., Saleh M. (2009) Cellular inhibitors of apoptosis cIAP1 and cIAP2 are required for innate immunity signaling by the pattern recognition receptors NOD1 and NOD2. Immunity 30, 789–801 [DOI] [PubMed] [Google Scholar]
- 5. Vince J. E., Wong W. W., Gentle I., Lawlor K. E., Allam R., O'Reilly L., Mason K., Gross O., Ma S., Guarda G., Anderton H., Castillo R., Häcker G., Silke J., Tschopp J. (2012) Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity 36, 215–227 [DOI] [PubMed] [Google Scholar]
- 6. Damgaard R. B., Nachbur U., Yabal M., Wong W. W., Fiil B. K., Kastirr M., Rieser E., Rickard J. A., Bankovacki A., Peschel C., Ruland J., Bekker-Jensen S., Mailand N., Kaufmann T., Strasser A., Walczak H., Silke J., Jost P. J., Gyrd-Hansen M. (2012) The Ubiquitin Ligase XIAP Recruits LUBAC for NOD2 Signaling in Inflammation and Innate Immunity. Mol. Cell 46, 746–758 [DOI] [PubMed] [Google Scholar]
- 7. Feldmann M. (2008) Many cytokines are very useful therapeutic targets in disease. J. Clin. Invest. 118, 3533–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brennan F. M., McInnes I. B. (2008) Evidence that cytokines play a role in rheumatoid arthritis. J. Clin. Invest. 118, 3537–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ashkenazi A., Dixit V. M. (1998) Death receptors: signaling and modulation. Science 281, 1305–1308 [DOI] [PubMed] [Google Scholar]
- 10. Wang L., Du F., Wang X. (2008) TNF-α induces two distinct caspase-8 activation pathways. Cell 133, 693–703 [DOI] [PubMed] [Google Scholar]
- 11. Van Antwerp D. J., Martin S. J., Verma I. M., Green D. R. (1998) Inhibition of TNF-induced apoptosis by NF-κB. Trends Cell Biol. 8, 107–111 [DOI] [PubMed] [Google Scholar]
- 12. Beg A. A., Sha W. C., Bronson R. T., Ghosh S., Baltimore D. (1995) Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 376, 167–170 [DOI] [PubMed] [Google Scholar]
- 13. Van Antwerp D. J., Martin S. J., Kafri T., Green D. R., Verma I. M. (1996) Suppression of TNF-α-induced apoptosis by NF-κB. Science 274, 787–789 [DOI] [PubMed] [Google Scholar]
- 14. Beg A. A., Baltimore D. (1996) An essential role for NF-κB in preventing TNF-α-induced cell death. Science 274, 782–784 [DOI] [PubMed] [Google Scholar]
- 15. Wang C. Y., Mayo M. W., Baldwin A. S., Jr. (1996) TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science 274, 784–787 [DOI] [PubMed] [Google Scholar]
- 16. Varfolomeev E., Blankenship J. W., Wayson S. M., Fedorova A. V., Kayagaki N., Garg P., Zobel K., Dynek J. N., Elliott L. O., Wallweber H. J., Flygare J. A., Fairbrother W. J., Deshayes K., Dixit V. M., Vucic D. (2007) IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFα-dependent apoptosis. Cell 131, 669–681 [DOI] [PubMed] [Google Scholar]
- 17. Vince J. E., Wong W. W., Khan N., Feltham R., Chau D., Ahmed A. U., Benetatos C. A., Chunduru S. K., Condon S. M., McKinlay M., Brink R., Leverkus M., Tergaonkar V., Schneider P., Callus B. A., Koentgen F., Vaux D. L., Silke J. (2007) IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell 131, 682–693 [DOI] [PubMed] [Google Scholar]
- 18. Gaither A., Porter D., Yao Y., Borawski J., Yang G., Donovan J., Sage D., Slisz J., Tran M., Straub C., Ramsey T., Iourgenko V., Huang A., Chen Y., Schlegel R., Labow M., Fawell S., Sellers W. R., Zawel L. (2007) A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-α signaling. Cancer Res. 67, 11493–11498 [DOI] [PubMed] [Google Scholar]
- 19. Petersen S. L., Wang L., Yalcin-Chin A., Li L., Peyton M., Minna J., Harran P., Wang X. (2007) Autocrine TNFα signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell 12, 445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Varfolomeev E., Goncharov T., Fedorova A. V., Dynek J. N., Zobel K., Deshayes K., Fairbrother W. J., Vucic D. (2008) c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor α (TNFα)-induced NF-κB activation. J. Biol. Chem. 283, 24295–24299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bertrand M. J., Milutinovic S., Dickson K. M., Ho W. C., Boudreault A., Durkin J., Gillard J. W., Jaquith J. B., Morris S. J., Barker P. A. (2008) cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 30, 689–700 [DOI] [PubMed] [Google Scholar]
- 22. Dynek J. N., Goncharov T., Dueber E. C., Fedorova A. V., Izrael-Tomasevic A., Phu L., Helgason E., Fairbrother W. J., Deshayes K., Kirkpatrick D. S., Vucic D. (2010) c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling. EMBO J. 29, 4198–4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tenev T., Bianchi K., Darding M., Broemer M., Langlais C., Wallberg F., Zachariou A., Lopez J., MacFarlane M., Cain K., Meier P. (2011) The ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol. Cell 43, 432–448 [DOI] [PubMed] [Google Scholar]
- 24. Feoktistova M., Geserick P., Kellert B., Dimitrova D. P., Langlais C., Hupe M., Cain K., MacFarlane M., Häcker G., Leverkus M. (2011) cIAPs block ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell 43, 449–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moulin M., Anderton H., Voss A. K., Thomas T., Wong W. W., Bankovacki A., Feltham R., Chau D., Cook W. D., Silke J., Vaux D. L. (2012) IAPs limit activation of RIP kinases by TNF receptor 1 during development. EMBO J. 31, 1679–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Basak S., Kim H., Kearns J. D., Tergaonkar V., O'Dea E., Werner S. L., Benedict C. A., Ware C. F., Ghosh G., Verma I. M., Hoffmann A. (2007) A fourth IkappaB protein within the NF-kappaB signaling module. Cell 128, 369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vucic D., Dixit V. M., Wertz I. E. (2011) Ubiquitylation in apoptosis: a post-translational modification at the edge of life and death. Nat. Rev. Mol. Cell Biol. 12, 439–452 [DOI] [PubMed] [Google Scholar]
- 28. Scheinecker C., Redlich K., Smolen J. S. (2008) Cytokines as therapeutic targets: advances and limitations. Immunity 28, 440–444 [DOI] [PubMed] [Google Scholar]
- 29. Taylor P. C., Feldmann M. (2009) Anti-TNF biologic agents: still the therapy of choice for rheumatoid arthritis. Nat. Rev. Rheumatol. 5, 578–582 [DOI] [PubMed] [Google Scholar]
- 30. Zarnegar B. J., Wang Y., Mahoney D. J., Dempsey P. W., Cheung H. H., He J., Shiba T., Yang X., Yeh W. C., Mak T. W., Korneluk R. G., Cheng G. (2008) Noncanonical NF-κB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat. Immunol. 9, 1371–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mantovani A., Allavena P., Sica A., Balkwill F. (2008) Cancer-related inflammation. Nature 454, 436–444 [DOI] [PubMed] [Google Scholar]
- 32. Zhang D. W., Shao J., Lin J., Zhang N., Lu B. J., Lin S. C., Dong M. Q., Han J. (2009) RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–336 [DOI] [PubMed] [Google Scholar]
- 33. Green D. R., Oberst A., Dillon C. P., Weinlich R., Salvesen G. S. (2011) RIPK-dependent necrosis and its regulation by caspases: A mystery in five acts. Mol. Cell 44, 9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duprez L., Takahashi N., Van Hauwermeiren F., Vandendriessche B., Goossens V., Vanden Berghe T., Declercq W., Libert C., Cauwels A., Vandenabeele P. (2011) RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity 35, 908–918 [DOI] [PubMed] [Google Scholar]