Background: The role of RNA-binding protein HuR in angiogenesis is not known.
Results: In macrophages, HuR and miR-200b antagonistically regulate VEGF-A and angiogenesis.
Conclusion: Interplay of HuR and miRNAs in the macrophage regulates tumor angiogenesis in the mouse and embryonic vascular development in zebrafish.
Significance: HuR modulation of miRNA function highlights the importance of post-transcriptional gene regulatory mechanisms in biology and disease.
Keywords: Angiogenesis, Macrophages, MicroRNA, RNA-binding Protein, Vascular Endothelial Growth Factor (VEGF)
Abstract
HuR, also known as Elavl1, is an RNA-binding protein that regulates embryonic development, progenitor cell survival, and cell stress responses. The role of HuR in angiogenesis is not known. Using a myeloid-specific HuR knock-out mouse model (Elavl1Mø KO), we show that HuR expression in bone marrow-derived macrophages (BMDMs) is needed to maintain the expression of genes enriched in AU-rich elements and U-rich elements in the 3′-UTR. In addition, BMDMs from Elavl1Mø KO mice also showed alterations in expression of several miRNAs. Interestingly, computational analysis suggested that miR-200b, which is up-regulated in Elavl1Mø KO BMDMs, interacts with myeloid mRNAs very close to the HuR binding sites, suggesting competitive regulation of gene expression. One such mRNA encodes vascular endothelial growth factor (VEGF)-A, a major regulator of angiogenesis. Immunoprecipitation of RNA-protein complexes and luciferase reporter assays indicate that HuR antagonizes the suppressive activity of miR-200b, down-regulates miR-200b expression, and promotes VEGF-A expression. Indeed, Vegf-a and other angiogenic regulatory transcripts were down-regulated in Elavl1Mø KO BMDMs. Interestingly, tumor growth, angiogenesis, vascular sprouting, branching, and permeability were significantly attenuated in Elavl1Mø KO mice, suggesting that HuR-regulated myeloid-derived factors modulate tumor angiogenesis in trans. Zebrafish embryos injected with an elavl1 morpholino oligomer or miR-200b mimic showed angiogenesis defects in the subintestinal vein plexus, and elavl1 mRNA rescued the repressive effect of miR-200b. In addition, miR-200b and HuR morpholino oligomer suppressed the activity of a zVEGF 3′-UTR luciferase reporter construct. Together, these studies reveal an evolutionarily conserved post-transcriptional mechanism involving competitive interactions between HuR and miR-200b that controls angiogenesis.
Introduction
Post-transcriptional gene regulation is important in a wide array of biological processes including embryonic development, cell stress responses, and oncogenesis. This process is controlled at least in part by RNA-binding proteins (RBPs)2 and microRNAs (miRNAs) (1, 2). They bind specific regulatory elements on transcripts and control the fate of mRNAs such as processing, stability, and translation. HuR (also known as Elavl1) is an RNA-binding protein that interacts with an AU-rich element (ARE), a regulatory sequence motif located in the 3′-UTR of transcripts (3, 4). Recent findings indicate that HuR association with target mRNA correlates directly with RNA abundance, supporting the role of HuR as an RNA stabilizer (5). mRNA targets that associate with HuR have been identified by several techniques such as RIP-ChIP, PAR-CLIP, and iCLIP (5–9). Consensus HuR binding sequence motifs such as AREs and U-rich elements have been elucidated from such studies. HuR-associated mRNAs regulate numerous cellular processes including inflammation, cell cycle, tumorigenesis, cell survival, and apoptosis.
Recent in vivo studies revealed that Elavl1 knock-out mice are embryonic lethal due to a defect in placentation (10). Our laboratory showed that postnatal deletion of Elavl1 led to rapid lethality due to apoptosis of intestinal and hematopoietic progenitor cells (11). In addition, thymus-specific deletion of Elavl1 resulted in the defective egress of mature thymocytes (12), and overexpression of Elavl1 in murine innate immune cells suppressed inflammatory responses (13). A recent study concluded that HuR suppressed inflammatory processes in myeloid cells and protected mice from colitis-induced intestinal cancer (14). Moreover, cell adhesion-dependent stabilization of mRNAs in macrophages was dependent on HuR (15). These studies suggest that HuR is a key post-transcriptional regulator of numerous biological responses. However, the role of HuR in pathological processes is not well understood.
Angiogenesis, the process by which new blood vessels develop from a pre-existing vascular system, is required for embryonic development, physiological processes such as wound healing and corpus luteum formation, as well as in pathological processes such as tumor progression. Hypoxia, a fundamental angiogenic stimuli, induces key angiogenic cytokines such as vascular endothelial growth factor (VEGF)-A primarily by transcriptional mechanisms. Bone marrow (BM)-derived myeloid cells are also recruited and facilitate tumor growth by producing proangiogenic growth factors, cytokines, and matricellular regulators (16). Generally, tumor vessels are structurally and functionally abnormal, largely as a result of the unbalanced, local overexpression of angiogenic factors such as VEGF-A (17, 18). Tumor vasculature is irregularly organized and contains aberrantly associated mural cells. This abnormality is thought to contribute to the leakiness of vessels and to increase interstitial pressure in the tumor (19, 20). These studies indicate that tight control of VEGF-A expression as well as other angiogenic factors is essential for maintaining normal vascular homeostasis and productive angiogenesis.
Although transcriptional mechanisms are well characterized to regulate the induction of VEGF-A, several post-transcriptional mechanisms are also involved. The Vegf-a 3′-UTR contains several important cis-acting elements, including CA-rich element, IFN-γ-activated inhibitor of translation element (GAIT), and ARE, and is regulated by its RBPs such as AUF, tristetraprolin, heterogeneous nuclear ribonucleoprotein (hnRNP L) and HuR (21–25). ARE-binding proteins such as AUF and tristetraprolin destabilize Vegf-a mRNA in macrophage and tumor cells, respectively (24, 25). Furthermore, hnRNP L and the GAIT complex regulate VEGF-A expression in a mutually exclusive, stimulus-dependent manner through an RNA conformational change known as riboswitch (26). Under hypoxia, hnRNP L overrides GAIT silencing by triggering a VEGF-A mRNA structural change to a translation-permissive RNA conformation, in which the GAIT element is occluded. Finally, HuR stabilizes VEGF-A mRNA under hypoxia (22). Despite these important observations, the molecular details of how HuR regulates VEGF-A expression are not well understood.
Recent studies suggest that RBPs cooperate with miRNA and control gene expression (1, 27–29). Systems-level bioinformatics analysis of 3′-UTRs have revealed that the ARE motif bound by AUF, tristetraprolin, and HuR is overrepresented near miRNA target sites, suggesting that ARE-binding proteins work in concert with miRNA-dependent mechanisms (30). Moreover, transcriptome-wide mapping of HuR binding sites revealed that HuR frequently binds to transcripts near or overlapping with predicted miRNA sites (5). These observations along with others suggest that ARE-binding proteins such as HuR either antagonize or cooperate with miRNA-dependent gene regulation. For example, HuR allows the expression of the CAT-1 mRNA by counteracting miR-122-dependent repression (31) or, in contrast, represses c-myc mRNA by recruiting let-7 (32). The functional interconnections between HuR and miRNAs may provide additional levels of control in regulating gene expression and thus provide biological robustness in gene regulatory mechanisms. Given the profound phenotypic effects of miRNAs, RBP interactions with miRNAs may be very critical for biological and pathological outcomes.
Little is known about how post-transcriptional gene regulatory mechanisms influence angiogenesis in vivo. Here, we demonstrate that HuR antagonism of miR-200b-mediated gene repression of angiogenic regulators such as VEGF-A controls tumor angiogenesis.
EXPERIMENTAL PROCEDURES
Animals (Mice and Zebrafish)
Myeloid specific deletion of Elavl1 in mice was generated by crossing Elavl1 floxed mice (11) with mice expressing Cre recombinase driven by the lysozyme M promoter (33). All studies were performed under protocols approved by the Weill Cornell Medical College Animal Care and Use Committee. For subcutaneous tumor isograft model, Lewis lung carcinomas (LLC) cells (106) were injected subcutaneously into Elavl1f/f and Elavl1Mø KO mice which were determined to be >97% C57BL/6 background. 14 days later, tumors were collected and analyzed further. For Matrigel plug experiments, mice were injected subcutaneously with 0.3 ml of Matrigel supplemented with basic FGF (2.5 μg/ml) and 8 units of heparin. At 6 days, Matrigel plugs were harvested and processed for confocal images. For zebrafish husbandry, wild-type zebrafish and fli1:EGFP lines were obtained from the Zebrafish International Research Center, maintained at 28.5 °C and staged as described by Westerfield (53).
BMDM Culture and ELISA Analysis
Bone marrow cells obtained by the lavage of femur and tibia from Elavl1f/f and Elavl1Mø KO mice were cultured in complete DMEM with 20% of L929 cell culture medium for 7 days (34). 1 × 106 BMDMs in a 6-well plate were seeded and transfected using Lipofectamine 2000 (Invitrogen) without or with 50 nm antagomir for miR-200b (Ambion). Supernatant was collected the next day and measured for VEGF secretion by ELISA (R&D Systems).
RNA Isolation, Mouse Exon Chip Array, and qRT-PCR Analysis
Total RNA was isolated according to the manufacturer's protocol (Clontech), and RNA quality was checked by an Agilent 2100 bioanalyzer (Agilent Technologies). 500 ng was hybridized on the mouse Exon 1.0 ST array (Affymetrix) and scanned at the Weill Cornell Medical College core facility. Scanned chip data were analyzed with AltAnalyze (35) using default parameters. To validate mRNA expression by qRT-PCR, 1 μg of RNA was reverse-transcribed with Superscript III reverse transcriptase (Invitrogen), and cDNAs were amplified with specific primers (primer bank) using SYBR Green master mix (Quanta BioSciences).
miRNA Isolation, miRNA High Throughput Sequencing, qRT-PCR Analysis, and miRNA Target Prediction
Small RNAs were isolated according to the manufacturer's protocol (Clontech). RNAs were ligated to 5′ and 3′ adaptors with RNA ligase and followed by reverse transcription (Illumina Small RNA Sample Preparation kit version 1.5; Illumina). The cDNAs were amplified by PCR for 16 cycles. PCR products were purified on a nondenaturing acrylamide gel with size selected and sequenced for 42 cycles on the Illumina Genome Analyzer II. The resulting sequences were trimmed and mapped to miRBase 627 mouse stem-loop sequences using the Bowtie alignment program (version 0.12.7) allowing up to 1 mismatch. The alignment reads were normalized as proportion of total miRNA reads, and the normalized reads were analyzed for differentially expressed miRNAs using the Fisher's exact test. miRNA was subject to poly(A) tailing reaction and reverse-transcribed with an oligo(dT) adaptor primer (qScript miRNA cDNA synthesis kit; Quanta) and followed by qPCR amplication with the specific miRNA sequence primer and oligo(dT) adaptor sequence primer (PerfeCTa SYBR Green system, Quanta). Expression of specific miRNAs was normalized to the value of U6 snRNA.
Bioinformatic Motif Analysis and Distance between HuR Binding Site and miRNA Seeding Site
ARE and HuR high affinity sequences were obtained from published studies (9, 36). RNA motif scanning and counting in the 3′-UTRs, 5′-UTRs, exons, introns and the transcribed strand only of ENSEMBL transcript models were performed using previously published programs (37). Motif counts were compared between gene sets using two-tailed Wilcoxon tests; overall motif overrepresentation was evaluated using the hypergeometric distribution. High confidence miRNA target predictions were downloaded from the TargetScan website (release 6.2, June 2012). AREs and U-rich sequences in 3′-UTRs and within 50 nucleotides or less of TargetScan predicted seeds were determined. To examine whether AREs and U-rich sequences/miRNA target sites are closer to each other than expected by chance, we uniformly randomly redistributed the same number of miRNA target sites in the same 3′-UTRs and calculated how often AREs and U-rich sequences are within 50 nucleotides or less of random miRNA targets. We repeated this procedure 100 times and reported how frequently the number of AREs and U-rich sequences within 50 nucleotides or less of random miRNA targets surpassed the true observed value, thus generating a HuR binding site proximity p value for each miRNA.
Luciferase miRNA Target Reporter Assay
PCR products of Vegf-a 3′-UTR fragment (1059–1455) generated using the forward primer 5′-GCGCTAGCATCTTCCCTTCCCAAGGA and the reverse primer 5′-GCCTCGAGCAAAGGAATGTGTGGTGG were cloned into the NheI and XhoI sites of the multiple cloning site of pmirGLO vector (Promega), a dual luciferase reporter (firefly and Renilla). Zebrafish Vegfaa 3′-UTR (871–1236) was amplified from 24 h postfertilization (hpf) zebrafish embryos cDNA using two primers with adaptors: SacI, 5′-GAGCTCATTTGTGTTGGGACAACATGAAGAAATTGTG, and XbaI, 5′-TCTAGACAGATTCATGAAACAATAACACG. Fragment was inserted in pmirGLO vector by underlined restriction enzymes. Mutations were introduced in the complementary with the seed region of miR-200b on Vegf-a 3′-UTR (1194–1222). Primers containing mutated target sequences are 5′-GCTTAACCAACCGCGGTGAAAAAAACCCTACTCTTTAAT and 5′-CCGGGTTTTTTTCACCGCGGTTGGTTAATATTTAATTTCAAC. HEK293T cells were co-transfected with 25 ng/ml pLuc vectors containing Vegf-a 3′-UTR or mutant Vegf-a 3′-UTR with 20 nm miR-200b mimics (Ambion) using Lipofectamine-2000 (Invitrogen). After 24 h, the cells were lysed, and luciferase activity was measured using a luminometer. Firefly luciferase values were normalized with Renilla luciferase values.
RNA-binding Protein Immunoprecipitation (RIP) Assay
The binding of HuR or Ago-2 with Vegf-a mRNA was determined by immunoprecipitation followed by qRT-PCR as described (9). BMDMs (5 × 107) isolated from two mice were lysed with polysome lysis buffer supplemented with RNase inhibitors and protease inhibitors. The supernatant was incubated overnight at 4 °C with no antibody, control IgG, Ago-2 monoclonal antibody (Wako), or HuR monoclonal antibody (Clonegene) and then incubated with protein G Dynabeads (25 μl) for 4 h at 4 °C. After washing five times, the pellet was treated with 10 units of DNase I (Promega) in 100 μl of buffer for 10 min at 37 °C and then treated with 5 μg of proteinase K (Roche Applied Science) in 100 μl of buffer for 30 min at 55 °C. The supernatant was collected, and RNA was extracted using acid phenol chloroform (Ambion). Extracted RNA was subjected to qRT-PCR analysis as described above.
Tumor Vessel Leakiness
Mice bearing LLC tumors were anesthetized with kentamine/xylazine and received 0.25 mg of 70-kDa Texas Red-conjugated dextran (Molecular Probes) via tail vein injection. 25 min later, 0.25 mg of 2000-kDa fluorescein isothiocyanate (FITC)-conjugated dextran (Molecular Probes) was injected intravenously. 5 min later, mice were euthanized with CO2. Tumors were collected and postfixed overnight and embedded in OCT frozen blocks. Blocks were cut into approximately 50-mm thick cryosections using the cryomicrotome (Leica). The intravascular FITC-dextran and extravasated Texas Red-dextran in sections were observed by confocal microscopy (Olympus Fluoview).
Flow Cytometry and Tumor-associated Macrophage Isolation
Tumors were minced and digested with 2 mg/ml collagenase A (Roche Applied Science) for 45 min at 37 °C, followed by passing through a 40-μm nylon mesh to collect a single cell suspension. The suspensions were centrifuged and washed twice with PBS. For flow cytometry, cells were stained with myeloid-derived suppressor cells, a CD11b antibody, and a Gr1 antibody markers and counted with LSRII flow cytometer (BD Biosciences). The acquired data were analyzed with FlowJo software (Treestar). For tumor-associated myeloid (TAM) cell isolation, suspended single cells were incubated with anti-F4/80 antibody and processed for the positive selection using the Macs column (Miltenyi Biotec). Collected TAM cells were lysed for RNA purification followed by qRT-PCR analysis or for protein extraction followed by proteome profile angiogenesis array (R&D Systems).
Immunohistochemistry and Immunofluorescence
Tumors were fixed in 4% paraformaldehyde and processed for paraffin or frozen sections. Paraffin sections were stained with CD31 antibody (BD Pharmingen) and counterstained with methyl green (Vector Laboratories). Confocal images of tumor vasculature in frozen sections were obtained by co-staining of rat anti-CD31 antibody (BD Pharmingen) and Cy3-conjugated anti-smooth muscle actin (SMA) antibody (Sigma). Zebrafish embryos were fixed in 4% paraformaldehyde overnight and processed for whole mount immunohistochemistry with anti-HuR antibody (Clonegene), alkaline phosphatase staining (Roche Applied Science), or immunofluorescence staining with anti-GFP antibody (Invitrogen) as described (38). Brightfield images were captured using Zeiss Axioskop2 microscope with an AxioCam digital camera (Zeiss). Immunofluorescence images were taken using FluoView (Olympus) or Zeiss LSM510 upright confocal microscope.
Morpholino Design, Capped mRNA Synthesis, and Microinjection
elavl1 morpholino antisense oligomers (MOs) were synthesized by GeneTools: ATG-MO (translational blocking antisense, 5′-TGTGGTCTTCGTAACCGTTCGACAT-3′) or SPL-MO (splicing antisense, 5′-AGAGCACCTTATGTCACATTACCTT-3′) and dissolved in distilled water. A full-length elavl1 clone (Open Biosystems) was inserted into the EcoRI and XbaI sites of the pCS2 vector. After linearization, the plasmids were used to synthesize capped elavl1 mRNA in vitro using an Ambion mRNA synthesis kit. MOs, miR-200b mimic (Ambion), or mRNA was micro-injected into one-two cell stage embryos (Harvard apparatus).
Alkaline Phosphatase Staining Assay
In situ detection of zebrafish vessels was done using the staining method described (39).
Statistics
Data are expressed as means ± S.E. Statistical significance was determined by two-tailed unpaired t test with Prism software. Other statistical tests such as hypergeometric test were performed using the R statistical software.
RESULTS
Myeloid HuR Regulates the Expression of ARE-bearing mRNAs
To investigate the specific role of HuR in myeloid cells, we crossed Elavl1 floxed mice (Elavl1f/f) with mice expressing Cre recombinase driven by the lysozyme M promoter (LysM-Cre) (33), generating Elavl1Mø KO. Elavl1Mø KO mice appeared normal and reproduced with Mendelian frequency. The efficiency of Elavl1 gene excision and suppression of HuR expression in isolated BMDMs from Elavl1Mø KO mice was highly effective as determined by quantitative RT-PCR and Western blot analysis (Fig. 1, A and B). To examine whether the loss of HuR affects myeloid cell proliferation or differentiation, we performed flow cytometry in bone marrow and blood to phenotype myeloid populations (Fig. 1, C and D). The population of immature (CD11b+Gr1−/low) and mature myeloid cells (CD11b+Gr1high) in bone marrow was similar in both Elavl1f/f and Elavl1Mø KO mice. Similarly, neutrophils (CD11b+Ly6G+F4/80−) as well as monocytes/macrophages (CD11b+Ly6G−F4/80+) in blood showed no discernable differences. These data suggest that HuR is not essential for myeloid cell proliferation, survival, or differentiation in vivo.
FIGURE 1.
Deletion of Elavl1 in myeloid cells results in down-regulation of ARE-bearing mRNAs. A and B, efficiency of Elavl1 deletion in isolated BMDMs as determined by quantitative RT-PCR (A) and Western blotting (B). Data are representative of at least five independent experiments. C and D, flow cytometry analysis. Bone marrow cells isolated from Elavl1f/f and Elavl1Mø KO mice were stained for immature myeloid (CD11b+Gr1−/low) and mature myeloid cells (CD11b+Gr1high). Blood cells were stained for neutrophils (CD11b+Ly6G+F4/80−) and monocytes/macrophages (CD11b+Ly6G−F4/80+). n = 3 mice/group. E, microarray analysis indicating that 575 genes were down-regulated and 234 genes were up-regulated in Elavl1Mø KO BMDMs. -fold change >1.4, n = 4 mice/group. F, heat map of differentially expressed ARE-containing mRNAs. Differentially expressed genes in Elavl1Mø KO BMDMs are color-coded based on the -fold change relative to Elavl1f/f BMDMs (blue, down-regulated genes; red, up-regulated genes). The heat map was produced by open source software, Matrix2png. G, the average number of ARE (AUUUA) in each region (3′-UTRs, 5′-UTRs, exons, and introns) of genes analyzed by microarray. Note that the number of ARE binding sites in 3′-UTR of down-regulated genes in Elavl1Mø KO BMDMs is significantly higher, compared with 3′-UTR of up-regulated or stable genes. H, count number of high affinity of HuR motif (UUUUUUU, UUUAUUU, UUUCUUU, UUUGUUU, UUACUUU, UUAUUUU, UUGUUUU, UUUUGUU, GUUUUUU, UUUUUUC) in each genomic region, such as 3′-UTR, intron, exon, and 5′-UTR genes detected in the microarray as above.
To gain insights into myeloid genes regulated by HuR, we investigated global gene expression profiles in BMDMs isolated from Elavl1f/f mice and Elavl1Mø KO mice using Affymetrix exon-chip microarrays. Among 809 differentially expressed genes in four biological replicates, 71% (575 genes) of differentially expressed genes in Elavl1 KO BMDMs were down-regulated (Fig. 1E). When analyzed in the Ingenuity Pathway Analysis software, cardiovascular system development and function were identified, suggesting a role for HuR in regulating the expression of angiogenic regulatory genes in myeloid cells.
HuR is known as a stabilizer of ARE-bearing mRNAs. Thus, we hypothesized that the loss of HuR in myeloid cells led to destabilization of many transcripts bearing the HuR binding motif in 3′-UTR regions. To identify the relationships between HuR binding sites and mRNA expression, we examined ARE-bearing mRNAs in 809 differentially expressed genes using the ARED search engine (36). 46 genes were identified that contain ARE motifs in their 3′-UTR (Fig. 1F). Interestingly, angiogenic regulatory genes such as, Vegf-a, Jag1 (Jagged 1), Ptgs2 (prostaglandin-endoperoxide synthase 2), Vcam1 (vascular cell adhesion molecule 1), and Nos2 (nitric-oxide synthase 2), which contain ARE motifs, were down-regulated in Elavl1 KO BMDMs, suggesting that HuR stabilizes ARE-containing angiogenic regulatory RNAs in macrophages.
We further examined the enrichment of ARE and HuR high-affinity binding motifs (U-rich sequences (7) in the 3′-UTRs, introns, exons, and 5′-UTRs of all transcripts that changed expression in the microarray studies (Fig. 1G). The average number of AREs in 3′-UTRs of down-regulated genes was significantly higher than that in 3′-UTRs of stable or up-regulated genes (p < 1e-9, Wilcoxon test). Accordingly, 3′-UTRs of down-regulated genes in Elavl1 KO BMDMs are more likely to have higher copies of ARE than 3′-UTRs of stable or up-regulated genes (p < 1e-11, hypergeometric test), indicating significant stabilization of 3′-UTR ARE-containing transcripts by HuR. Similar findings were observed even when the density of ARE sites was considered (data not shown). Further, U-rich sequences that bind HuR with high affinity (7) (Fig. 1H) were also associated with down-regulated transcripts. However, we did not find significant differences of ARE or U-rich sequence enrichment in 5′-UTRs, exons, or introns, indicating that HuR activity is exerted mostly through the regulatory elements present in the 3′-UTRs. Together, these studies suggest that myeloid HuR stabilizes a large number of transcripts including angiogenic regulatory genes bearing ARE or U-rich sequences in their 3′-UTRs.
HuR Regulates the Expression of miRNAs in BMDMs
We next characterized the miRNA expression prolife in WT and Elavl1Mø KO BMDM by high throughput RNA sequencing. Two biological replicates of WT and Elavl1Mø KO BMDM miRNA preparations were sequenced, resulting in >32 million total reads that perfectly aligned to 627 mouse miRNA loci described in miRBase. We further analyzed miRNA species that were most abundant (top 45% in both WT and KO). Of 282 miRNA species, 10 miRNAs (miR-126-3p, 143-3p, 196a-5p, 100, 199a-3p, 126-5p, 20b, 200b, 199b, 199a-5p) were up-regulated, and two miRNAs (miR-1249, 3108) were down-regulated (Fig. 2A). Up-regulation of miR-126-3p, 143-3p, 196a-5p, 199a-3p, 200b, 199a-5p and down-regulation of miR-1249, 3108 were further tested and confirmed by qRT-PCR analysis (Fig. 2B). These data suggest that lack of HuR in BMDMs results in alteration in abundance of certain miRNAs, which may have profound effects on gene expression in the myeloid cells and may influence some of their phenotypes.
FIGURE 2.
Regulation of the BMDM miRNome by HuR. A, high throughput sequencing analysis of miRNAs. Top 10 up-regulated miRNAs are listed in the order of abundance. -fold change >1.7, p < 0.1, n = 2 mice/group. B, up-regulated miRNAs (miR-126-3p, 143-3p, 196a-5p,199a-5p, 200b, 199a-3p) and down-regulated miRNAs (miR-1249, 3108) confirmed by qRT-PCR. Data represent mean ± S.E. (error bars); n = 8 mice (miR-126-3p, 143-3p, 199a-3p), n = 6 mice (miR-200b, 199a-5p,196a-5p, 1249), n = 4 mice (miR-3108)/group. **, p < 0.005; *, p < 0.05. C, number of miRNA targets predicted by TargetScan, number of predicted miRNA targets with an ARE/U-rich site within 50 nucleotides of miRNA seed site, and number of targets with adjacent miRNA and ARE/U-rich binding sites in GO:0001944 vascular development. Note that miR-200b target sites and ARE/U-rich elements are overall closer to each other than expected by chance (p < 1e-2), and 12 of these genes are involved in vascular development.
We next explored the hypothesis that miRNA suppression of gene expression via the RNA-induced silencing complex may be influenced by HuR interaction with mRNAs targeted by these miRNAs. We therefore used bioinformatic tools to search for targets of HuR-regulated miRNAs. Using TargetScan, miRNA targets were identified for five of the up-regulated miRNAs we validated by qRT-PCR (the other three up-regulated miRNAs did not have any predicted high confidence targets in the June 2012 TargetScan release (6.2)) (Fig. 2C). Of the five miRNAs, only miR-200b showed significant presence of ARE or U-rich HuR binding sites in close proximity (<50 nucleotides) from the miRNA seed sequence in the 3′-UTR of transcripts. For example, 257 mRNAs showed the presence of adjacent ARE and miR-200b binding sites whereas 276 mRNAs contained adjacent U-rich and miR-200b binding sites (Fig. 2C). We further observed that 12 of these mRNAs (Vegfa, Cited2, Mkl2, Efnb2, Gjc1, Pten, Pkd1, Reck, Srf, Qk, Vezf1, and Zfpm2) are known to be involved in vascular development. These bioinformatic analyses suggest that interaction between miR-200b and HuR on mRNAs regulates the expression of angiogenic regulatory genes.
Myeloid HuR and miR-200b Regulate VEGF-A Expression in a Competitive Manner
Vegf-a, one of the strongly down-regulated genes in Elavl1Mø KO BMDMs and a key angiogenic growth factor, was chosen for further analysis. Significantly attenuated expression of Vegf-a mRNA and secretion of VEGF-A protein were observed in Elavl1Mø KO BMDMs (Fig. 3A). To examine the direct interaction of HuR to Vegf-a mRNA in myeloid cells, RAW264.7 macrophage lysates were immunoprecipitated with anti-HuR antibody followed by qRT-PCR for Vegf-a mRNA (Fig. 3B). Vegf-a mRNA was immunoprecipitated by the HuR antibody in a dose-dependent manner, suggesting direct interaction between HuR and Vegf-a mRNA.
FIGURE 3.
HuR and miR-200b regulate Vegf-a expression in a competitive manner. A, reduced expression of VEGF-A in Elavl1Mø KO BMDMs. Expression of Vegf-a transcript was determined by qRT-PCR (left) and VEGF-A secretion in the supernatant of cultured BMDMs analyzed by ELISA (right). Data are shown as -fold change relative to Elavl1f/f BMDMs. Data reflect mean ± S.E. (error bars) Left; n = 5 mice/group; p = 0.008. Right, n = 7 mice/group; p = 0.001. VEGF secretion varied between 20 and 100 pg/ml between experiments. B, HuR binding to Vegf-a mRNA in Raw264.7 cells in a dose-dependent manner. Cell lysates were immunoprecipitated with anti-HuR antibody (0.1, 0.5, and 2.5 μg) or control IgG antibody (0.1, 0.5, and 2.5 μg) followed by qRT-PCR to measure the level of Vegf-a mRNA. Data are presented as relative level of Vegf-a mRNA normalized to Hprt mRNA. *, p = 0.026. C, schematic of murine Vegf-a 3′-UTR elements. HSR, hypoxia stability region; inset, region used to generate a reporter construct. The black box in the inset is expanded to show the sequence containing HuR binding site and seed region of miR-200b. Note that the U-rich motif on Vegf-a 3′-UTR is located in the proximity of the seed region of miR-200b. The predicted binding site was generated using Diana microT 4.0 (52). D, sequence conservation of Vegf-a 3′-UTR among human, mouse, and rat. The murine Vegf-a 3′-UTR (1059–1455) which was used to generate a luciferase reporter includes an U-rich element (underlined in red) and the seed region of miR-200b (underlined in blue). Note the fragment (hg18/chr6:43,861,550–43,861,596) which was identified as a HuR target by PAR-CLIP analysis (7) is conserved. Lower panel shows the complete conservation of miR-200b sequences among human, mouse, rat, and zebrafish. E, miR-200b inhibits the expression of a reporter bearing Vegf-a 3′-UTR. Schematic of reporter plasmids; pLuc, pLuc-Vegf-a (3′) reporter bearing the fragment of Vegf-a 3′-UTR (1059–1455), and pLuc-Vegf-a (3′mut) reporter bearing six mutant nucleotides in the seed region of miR-200b is shown. Each plasmid (25 ng/ml) was transfected into HEK293T cells with miR-200b (5 or 20 nm) or control miRNA, and 24 h later, luciferase activity was measured. Firefly luciferase values were divided by Renilla luciferase values to normalize variation in transfection efficiency. Data are presented as -fold change compared with control miRNA treatment. Data are mean ± S.E. -Fold change was averaged from four independent experiments. **, p < 0.01 (control miRNA versus 5 nm or 20 nm miR-200b). F, repressive activity of miR-200b enhanced in the Elavl1-silenced HEK293T cells. siHuR or control siRNA (10, 50 nm) was transfected 1 day prior to transfection of luciferase reporter with miR-200b (20 nm). Data represent mean ± S.E. of three independent experiments. *, p < 0.05 (control siRNA versus 50 nm siElavl1). Knockdown efficiency of Elavl1 with siRNA was confirmed by Western blot analysis in E. G, Vegf-a mRNA was significantly enriched in Ago-2-bound complex in Elavl1Mø KO BMDMs. Lysates from Elavl1f/f or Elavl1Mø KO BMDMs were immunoprecipitated with anti-Ago2 antibody (0.1, 1 μg) or control IgG antibody (0.1, 1 μg) and subjected to qRT-PCR to quantify Vegf-a mRNA. Data are presented as the relative level of Vegf-a mRNA normalized to Hprt mRNA. ***, p < 0.0001 (Ago-2, 1 μg of Elavl1f/f versus Ago-2, 1 μg Elavl1Mø KO). H, supernatants of BMDMs treated with miR-200b antagomir (50 nm) were analyzed with VEGF-A ELISA. Data are shown as -fold change relative to Elavl1f/f BMDMs with control treatment averaged from four independent experiments. **, p = 0.019 (control Elavl1f/f versus Elavl1Mø KO).
We examined the murine Vegf-a 3′-UTR for HuR binding sites (U-rich 7-mer or AU-rich sites) and potential miRNA binding sites using four different algorithms, namely, miRanda, TargetScan, PicTar, and Diana search engines. Interestingly, a region in the Vegf-a 3′-UTR sequence (1194–1222) contains a putative HuR binding site (U-rich 7-mer) in proximity to a highly conserved miR-200b seed (predicted as an miR-200n target site) by all four programs (Fig. 3C). This region is conserved between mouse and human VEGF-A (hg18: chr6: 43,861,550–43,861,596) and was identified as a HuR interaction site in HEK293 cells by ELAVL1 PAR-CLIP analysis (Fig. 3D) (7). We therefore tested whether HuR and miR-200b compete for the same regulatory element in the Vegf-a 3′-UTR.
To test whether the Vegf-a regulatory element (1194–1222) containing the U-rich 7-mer and the miR-200b seed is functionally important for expression, we generated a reporter construct bearing Vegf-a 3′-UTR (1059–1455) or a mutant reporter in which the region of complementary with miR-200b seed sequence was mutated (Fig. 3E). Transfection of miR-200b mimic reduced the luciferase activity in HEK293T cells expressing Vegf-a 3′-UTR reporter but not the mutant or the empty vector (Fig. 3E). Interestingly, in HuR knockdown 293T cells, miR-200b further reduced the luciferase activity of a reporter bearing Vegf-a 3′-UTR, suggesting that the absence of HuR may potentiate the suppression of Vegf-a mRNA by miR-200b (Fig. 3F). These data suggest that the (1194–1222) regulatory element of the Vegf-a 3′-UTR is subject to negative regulation by miR-200b and positive regulation by HuR.
To further examine whether the miR-200b-RISC complex interacts with the Vegf-a 3′-UTR and whether this interaction is competed by HuR binding, we immunoprecipitated Elavl1f/f and Elavl1Mø KO BMDMs lysates with the anti-Ago-2 antibody (an essential component of RISC) and examined the level of Vegf-a mRNA by qRT-PCR analysis (Fig. 3G). Expression of Ago-2 polypeptide was similar between Elavl1f/f and Elavl1Mø KO BMDMs (data not shown). Higher levels of Vegf-a mRNA were associated with the Ago-2 complex in Elavl1Mø KO BMDMs compared with that in Elavl1f/f BMDMs, indicating that HuR blocks the ability of RISC complex to engage Vegf-a transcript and suppress VEGF-A expression.
Furthermore, inhibition of miR-200b activity by a specific antagomir in Elavl1Mø KO BMDMs partially rescued the repression of VEGF-A, whereas treatment of Elavl1f/f BMDMs (which have high HuR expression) with the miR-200b antagomir did not affect VEGF-A expression (Fig. 3H). Together, these data strongly suggest that Vegf-a mRNA in macrophages is subject to post-transcriptional regulation by miR-200b and HuR in a competitive manner.
Myeloid HuR Regulates Tumor Growth and Angiogenesis
We next investigated the role of myeloid HuR in tumor growth and angiogenesis. We implanted LLC cells subcutaneously and studied tumor growth and the formation of intratumoral vessels. Tumor growth in Elavl1Mø KO mice was significantly attenuated compared with Elavl1f/f mice (Fig. 4A). Tumor cell apoptosis by TUNEL staining was significantly increased in tumors of Elavl1Mø KO mice whereas tumor cell proliferation was not altered (Fig. 4, B and C), indicating that the loss of myeloid HuR resulted in tumor cell death and reduced tumor mass.
FIGURE 4.

Deletion of Elavl1 in myeloid cells results in reduced LLC tumor growth. A, LLC tumors injected subcutaneously into Elavl1f/f mice and Elavl1Mø KO mice. Tumor weight in Elavl1f/f mice was 0.623 ± 0.045 (mean ± S.E., n = 49) and in Elavl1Mø KO mice was 0.324 ± 0.028 (n = 55). p < 0.0001. B, quantitative analysis of TUNEL-positive cells in LLC tumors. Magnification, ×20; WT = 6.73 ± 3.36 versus KO = 48.80 ± 8.96, p = 0.0001, n = 15 images from three mice/group. Scale bars, 50 μm. C, quantitative analysis of percentage of Ki-67 positive cells in LLC tumors. WT = 56.99 ± 0.86 versus KO = 57.36 ± 1.03, p = 0.0001, n = 16 images from four mice/group. Magnification: 20×; scale bars = 50 μm. D, immunohistochemical analysis with a macrophage-specific F4/80 antibody in LLC tumors from Elavl1f/f mice and Elavl1Mø KO mice. n = 6 tumors/group. Scale bars, 50 μm. E, flow cytometry analysis of myeloid-derived suppressor cells (CD11b+Gr1+) in dissociated cells from LLC tumors from Elavl1f/f mice and Elavl1Mø KO mice. n = 4 mice/group.
Tumor growth is known to be supported by the BMDM cells recruited to the tumor microenvironment (40). To examine whether the loss of myeloid HuR perturbs recruitment of myeloid cells to the tumor, we immunostained tumors for F4/80+ (macrophages) or CD11b+Gr1+ (myeloid-derived suppressor cells) (Fig. 4, D and E). The population of myeloid-derived suppressor cells (CD11b+Gr1+) by flow cytometry and F4/80+ macrophages by immunostaining in tumors of Elavl1f/f mice and Elavl1Mø KO mice did not show significant differences. This suggests that myeloid HuR function is not required for recruitment/migration or egress of myeloid cells into the tumor microenvironment.
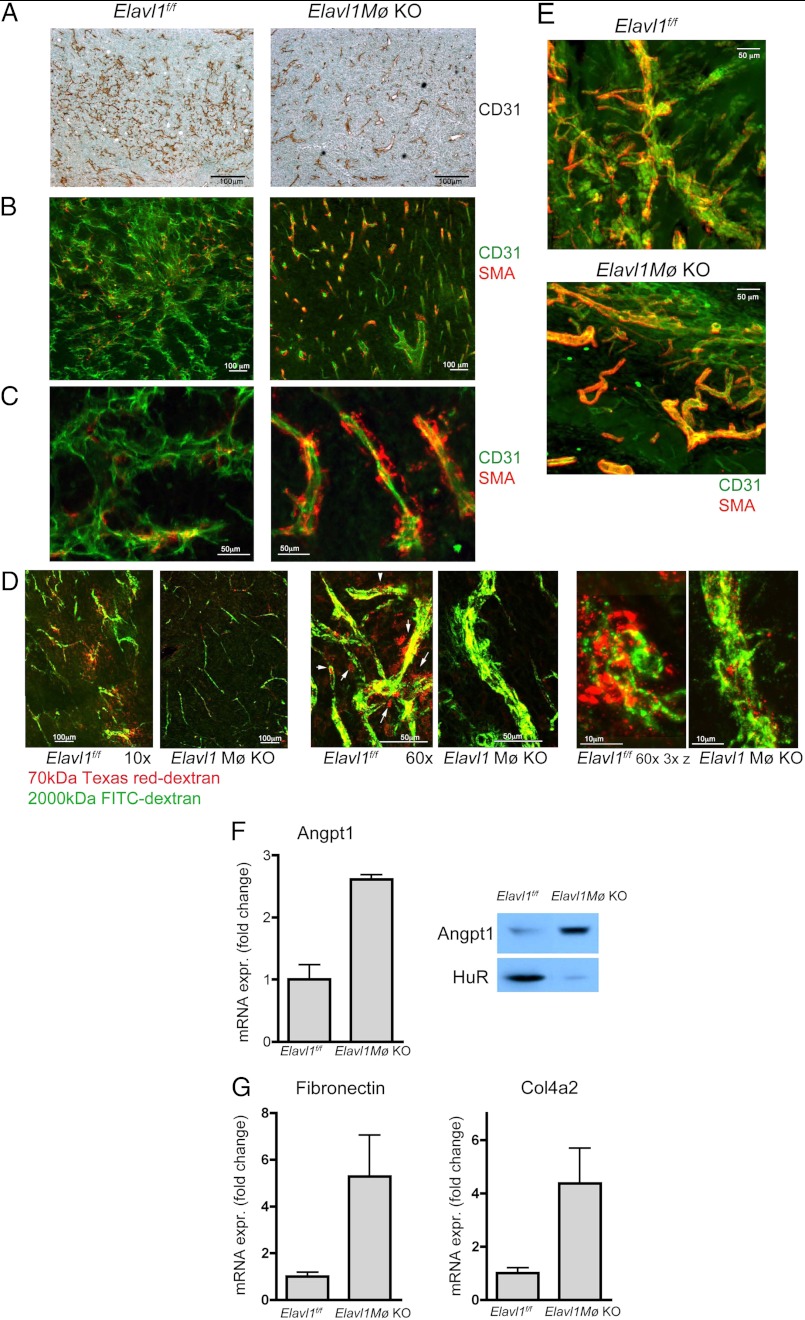
CD31 immunohistochemistry staining of tumor sections indicates that Elavl1Mø KO mice had reduced vascular density, sprouting and branching, and contained vessels with dilated lumens compared with the WT counterparts (Fig. 5A). To examine the pericyte coverage of tumor vessels, tumor tissue sections were immunostained for CD31 and α-SMA. As shown in Fig. 5, B and C, intratumoral vessels in the Elavl1Mø KO mice contained more SMA+ mural cells. We also examined vascular perfusion and leakiness by injecting fluorescent dextrans (70-kDa Texas Red-conjugated and 2000-kDa FITC-conjugated) intravenously and imaging the tumor sections by confocal fluorescence microscopy. Tumor vessels in Elavl1f/f mice were highly permeable because a significant amount of 70-kDa dextran was found in extravascular regions (Fig. 5D). In sharp contrast, most 70-kDa dextran remained in the intravascular regions in tumors from Elavl1Mø KO mice. The 2000-kDa FITC-dextran decorated the outline of the intratumoral vessels. These data indicate that tumor vascular permeability in Elavl1Mø KO mice was markedly decreased, suggesting the phenotypic alteration of tumor vasculature in Elavl1Mø KO mice. Mature and stable vessels with better pericyte coverage and reduced branching were also observed in growth factor-induced angiogenesis in Matrigel plugs implanted subcutaneously in Elavl1Mø KO mice (Fig. 5E). In addition, the increased synthesis of vascular maturation markers such as angiopoietin 1, fibronectin, and type IV collagen a2 were observed in Elavl1 KO BMDMs (Fig. 5, F and G). Together, the findings strongly suggest that myeloid HuR is critically important for tumor angiogenesis, vascular phenotype alterations, and tumor growth.
FIGURE 5.
Deletion of Elavl1 in myeloid cells results in normalized vasculature. A, representative images of CD31 immunostaining of tumors. n = 3 mice/group. B and C, confocal fluorescence microscopy images of endothelial cells (CD31, green) and pericytes (smooth muscle actin, red) in LLC tumors. n = 5 mice/group. D, tumor vascular permeability. Fluorescent microscopy images of FITC-dextran (2000 kDa) and Texas Red-dextran (70 kDa) angiography on LLC isografts are shown. Arrows indicate extravasated Texas Red-dextran, indicating vascular leakage. Elavl1f/f mice, n = 8; Elavl1Mø KO mice, n = 6). E, representative images of CD31 and SMA co-immunostaining of neovessels in growth factor impregnated-Matrigel plugs. n = 3 mice/group. F, up-regulated expression of angiopoietin 1 in Elavl1Mø KO BMDMs as determined by qRT-PCR and Western blotting. G, increased mRNA expression of fibronectin and type IV collagen α2. Data represent mean ± S.E. (error bars). n = 4 mice/group.
HuR and miR-200b Regulate Angiogeneis in the Subintestinal Vein Plexus in Zebrafish
To investigate whether interplay between HuR and miR-200b regulates developmental angiogenesis, we utilized the zebrafish model. The zebrafish genome encodes a HuR homologue (elavl1) that is highly conserved (85% identity between fish and mouse) (41). In addition, miR-200b is completely invariant between fish and mammals. Because mammalian HuR antibody detects the zebrafish homolog specifically, we used whole mount immunostaining to determine the embryonic expression of pattern of Hur. elavl1 was highly and ubiquitously expressed in zebrafish embryos at 24 and 72 hpf (Fig. 6A). We determined that two different MOs targeting elavl1 (ATG-MO, translational blocking MO; SPL-MO, splice blocker MO) both suppressed HuR protein synthesis in a dose-dependent manner at 24 hpf, thus validating specificity for knockdown (Fig. 6A).
FIGURE 6.

HuR and miR-200b regulate SIV plexus angiogenesis in zebrafish. A, dose-dependent knockdown of elavl1 by translational antisense morpholino (ATG-MO) or splicing antisense morpholino (SPL-MO). Protein lysates extracted from 24 hpf embryos injected with indicated MOs were assessed for HuR knockdown by Western blotting. Lateral views of whole mount immunostained embryos at 24 and 72 hpf indicate the ubiquitous expression of HuR. B, schematic of zebrafish vegfaa 3′-UTR element which contains adjacent HuR binding site and miR-200b seed sequence. The predicted binding site was generated using TargetScan. C, miR-200b suppression of luciferase reporter fused to zebrafish vegfaa 3′-UTR in transfected HEK293T cells. ***, p < 0.0001; n = 4. Data are presented as -fold change compared with control miRNA treatment. D, ability of miR-200b to suppress the activity of the luciferase reporter fused to zebrafish vegfaa 3′-UTR in transfected HEK293T cells is enhanced when HuR is down-regulated by siRNA. **, p = 0.007 control siRNA versus 10 or 50 nm siElavl1; n = 2. Data are presented as -fold change compared with control miRNA treatment. E, knockdown of HuR or overexpression of miR-200b in fli1:EGFP embryos resulting in reduced SIV basket size and branching vessels. Confocal images (left) and alkaline phosphatase-stained images (right) show SIVs in fli1:EGFP embryos injected with a control MO, elavl1MO (2 ng/embryo), miR-200b mimics (57 pg/embryo), or co-injected with synthesized mRNA of elavl1 (200pg/embryo) and miR-200b mimics (57pg/embryo) at 72 hpf. F, HuR antagonizing repressive effect of miR-200b on SIV formation. Confocal images (left) and alkaline phosphatase-stained images (right) show SIVs in 72 hpf fli1:EGFP embryos injected with a suboptimal dose of elavl1MO (1 ng/embryo) or miR-200b mimics (14 pg/embryo), or co-injected with elavl1MO (1 ng/embryo) and miR-200b mimics (14 pg/embryo). The results are combined from at least two independent experiments, and the number of embryos in each group under “Results.”
In zebrafish, the vegfaa 3′-UTR contains HuR- and miR-200b binding sites that are only 9 bases apart (Fig. 6B). To test whether elav1 MO and miR-200b regulate vegfaa expression, we cloned the vegfaa 3′-UTR (from nucleotides 871–1236) downstream of the luciferase ORF. As shown in Fig. 6C, miR-200b suppressed expression from the vegfaa 3′-UTR reporter in a dose-dependent manner in HEK293 cells; moreover, siRNA against HuR further suppressed reporter expression.
To examine the role of HuR in angiogenesis, we knocked down elavl1 expression in tg(fli1:EGFP) vascular reporter embryos (Fig. 6E, left). Injection of embryos with elavl1MO (2 ng/embryo) resulted in a severe impairment in subintestinal vein (SIV) vascular development at 72 hpf, although other vessels including intersegmental vessels were not discernibly affected. The miR-200b mimic (57 pg/embryo) yielded similar defects in SIV vascular development, suggesting an anti-angiogenic role of miR-200b in SIV plexus development. Alkaline phosphatase-stained images (Fig. 6E, right) further confirmed impaired vascular development in the SIV plexus in 94% of embryos injected with elavl1MO (68/72) and 88% of embryos injected with miR-200b mimics (53/60). Importantly, when embryos injected with the anti-angiogenic dose of miR-200b (57pg) were co-injected with elavl1 mRNA (200 pg/embryo) 71% of the embryos (40/56) displayed normal SIV plexus development, suggesting that HuR relieved miR-200b mediated anti-angiogenesis in the SIV plexus. Furthermore, at a suboptimal dose of elavl1MO (1 ng) or miR-200b mimic (14 pg), the embryos develop with little or no vascular defects detected at 72 hpf (57/63, and 54/58 embryos, respectively; Fig. 6F). However, when these same doses of elavl1MO and miR-200b mimic were simultaneously administered, 61% of the embryos (38/62) exhibited a severe defect in SIV plexus angiogenesis, again consistent with the two factors competitively regulating common angiogenic targets during embryogenesis.
DISCUSSION
A major finding of this work is that HuR plays a critical role regulating myeloid cell gene expression at the post-transcriptional level. HuR is not essential for myeloid cell survival, proliferation, differentiation, or trafficking. This is in sharp contrast to the essential role of HuR in hematopoietic progenitor cell survival (11). Thus, once hematopoietic progenitors are committed to the myeloid lineage, the HuR function switches from an essential prosurvival role to that of myeloid phenotype switch.
Using the myeloid-specific Elavl1 (Hur) knock-out model, we analyzed the transcriptome of primary bone marrow-derived macrophages. Significant down-regulation of ARE- and U rich-motif containing transcripts was observed. These data strongly suggest that an important function of HuR is to stabilize transcripts in macrophages. mRNAs bearing ARE sequences, which function as instability elements in 3′-UTR, constitute approximately 8% of the transcribed genome (36). These encode mostly immediate early genes, inflammatory cytokines, and growth factors, suggesting a role for post-transcriptional regulation in processes such as inflammation and oncogenesis. Our analysis of primary macrophages clearly showed the positive correlation of the number of putative HuR binding sites (AREs and U-rich sequences) in 3′-UTR/transcript and degree of HuR-dependent RNA stabilization, a finding that was echoed in other systems (5).
Second, our data show that miRNA expression profiles in BMDM were altered by lack of HuR expression. In particular, we found that several miRNAs (miR-126-3p, 143-3p, 196a-5p, 199a-5p, 200b, and 199a-3p) were up-regulated, and two (miR-3108, 1249) were down-regulated in BMDMs that lack HuR. Multiple mechanisms could account for such changes in miRNA expression in the absence of HuR. For example, RBPs are shown to regulate the biogenesis of miRNAs. A recent study found that HuR interaction with H19 primary transcripts in the nucleus suppresses miR-675 generation from the first exon of H19 lincRNA (54). The KH-type splicing regulatory protein (KSRP), a key mediator of mRNA decay by interacting with ARE-containing mRNAs, promotes the biogenesis of a subset of miRNAs (42). Some miRNA precursors reside in introns, and their expression often correlates with the transcription of the host gene or can be also regulated by RBPs during the precursor processing. miR-7 resides in an intron of HNRNPK, and the biogenesis of mature miR-7 is derepressed in siRNA-mediated knockdown of HuR in HeLa cells (6). Like miR-7, the miR-200b precursors reside in the first intron of Ttll10-001 gene, and HuR binding to its intron and the flanking exons may directly influence the efficiency of miRNA generation. Alternatively, HuR-regulated genes could be involved in up-regulation of miRNA gene transcription or stability. Nevertheless, because a single miRNA can have profound phenotypic effects, we explored the possibility that HuR interaction with miRNA function is involved in post-transcriptional gene regulatory circuits.
Many genes involved in the angiogenic response were altered in the Elavl1Mø KO mice, suggesting a functional role in blood vessel development. For example, Vegf-a, Jag1, Vcam1, and Nos2 were down-regulated. However, HuR in the macrophages is unlikely to play a role in developmental angiogenesis because Elavl1Mø KO mice did not show developmental or blood vessel defects. Thus, we hypothesized that macrophage HuR plays a role in the postnatal process regulated by macrophages such as tumor angiogenesis. In particular, we focused on Vegf-a, which is a major angiogenic factor important in tumor angiogenesis.
Although the Vegf-a gene is strongly induced by hypoxia and cytokines at the level of transcription, it is also regulated at the post-transcriptional level by RNA-binding proteins and miRNAs such as AUF, hnRNP L, miR-15b, 16, and 20a/b (24, 26, 43). HuR is known to bind directly to Vegf-a mRNA (Fig. 3B and Ref. 7). Our data show that in the absence of HuR, basal expression of Vegf-a mRNA and polypeptide was significantly suppressed. We also describe a novel site on the Vegf-a 3′-UTR where HuR and the miR-200b-RISC complex competitively regulate VEGF-A. HuR and miR-200b binding sites overlap, and the ability of miR-200b to suppress Vegf-a expression was antagonized by HuR. Importantly, more Ago-2-containing RISC complex was associated with Vegf-a mRNA in the absence of HuR. Functionally, miR-200b suppressed Vegf-a 3′-UTR luciferase reporter expression, and an antagomir of miR-200b restored VEGF expression in macrophages that lack HuR. These data strongly suggest that competitive interaction between HuR and miR-200b at the 3′-UTR of Vegf-a constitutes an RNA regulon that is important for the expression of VEGF-A.
Transcriptome-wide mapping analysis of the spatial relationship between HuR binding sites and Ago binding sites suggested that the functional antagonism of HuR is most likely due to competition for physical access at proximal sites (5). Dnd1, another RBP, also relieves miRNA activity by binding its target 3′-UTR at a location that overlaps with miRNA binding sites and blocking miRNA association with its target sites (44). hnRNP L, another RBP, also competes with miR-297/299 for the CA-rich element in the Vegf-a 3′-UTR (26). Thus, the antagonism of RBP in proximal miRNA-mediated repression may be a general mechanism in post-transcriptional gene regulation. A recent study demonstrates that HuR leads to the dissociation of miRNA-RISC complex from target mRNAs irrespective of the distance between miRNA-RISC sites and HuR binding site, suggesting that the HuR effect is unlikely due to steric hindrance of miRNA-RISC (45).
Interestingly, we found that post-transcriptional gene regulation by myeloid HuR is critical in tumor angiogenesis in mice. Subcutaneous LLC implants in myeloid Elavl1 KO mice showed reduced vascular density, vessel branching, vascular leakage, and attenuated tumor growth, suggesting that HuR promotes the proangiogenic phenotype in macrophages that enhance tumor growth. TAMs play a critical role in regulating the malignant transition by turning on the angiogenic switch in a mouse model of breast cancer (46). We did not observe any difference in the number of TAMs between the WT and myeloid Elavl1 KO mice despite the angiogenic defect in tumors in myeloid Elavl1 KO mice. This suggests that myeloid HuR is not required for trafficking and/or retention of these cells into the tumor microenvironment. VEGF-A is an angiogenic factor that is regulated exquisitely at multiple levels. Previous studies have shown that HuR regulates VEGF-A (22, 47), suggesting that VEGF-A is a critical downstream factor regulated by HuR that impacts tumor angiogenesis.
Our data indicate that HuR also antagonizes the anti-angiogenic effect of miR-200b in zebrafish embryos. Recent studies demonstrated the anti-angiogenic role of miR-200b by targeting Ets-1, GATA2, VEGFR2, and VEGF (48–50). RBPs such as DAZL and Dead end (Dnd1), which are expressed in zebrafish germ cells, were shown to relieve miRNA-mediated repression (44, 51). These studies suggest that the combinatorial action of RBPs and miRNAs in post-transcriptional gene regulation is widespread and evolutionarily conserved. The developmental angiogenic defects we noted in zebrafish elav1 morphants were not global, but restricted (at least most obviously) to a specific vascular plexus. Thus, we cannot rule out that subtle angiogenic defects may also occur in the murine HuR knock-out mouse. Alternatively, the mouse may better compensate for loss of HuR during embryogenesis.
In conclusion, HuR promotes angiogenesis by limiting the anti-angiogenic effect of miR-200b. It suppresses miR-200b expression and antagonizes its suppressive action on VEGF-A mRNA. Complex post-transcriptional control of VEGF-A expression may be necessary to achieve precise spatial and temporal control of this critical angiogenic factor. We speculate that the complex interplay between RBPs and miRNAs allow specificity, precision, and robustness of post-transcriptional gene regulation.
Acknowledgment
We thank Professor Thomas Tuschl, Rockefeller University, New York, for advice on miRNA expression experiments.
This work is supported, in whole or in part, by National Institutes of Health Grants HL49094 (to T. H.) and HL56182 (to T. E.).
- RBP
- RNA-binding protein
- ARE
- AU-rich element
- BM
- bone marrow
- BMDM
- bone marrow-derived macrophage
- Elavl1Mø KO
- myeloid-specific HuR knock-out mouse model
- GAIT
- IFN-γ-activated inhibitor of translation element
- hnRNP
- heterogeneous nuclear ribonucleoprotein
- hpf
- hours postfertilization
- LLC
- Lewis lung carcinoma
- miRNA
- microRNA
- MO
- morpholino antisense oligomer
- qPCR
- quantitative PCR
- RIP
- RNA-binding protein immunoprecipitation
- SIV
- subintestinal vein
- SMA
- smooth muscle actin
- TAM
- tumor-associated myeloid.
REFERENCES
- 1. Keene J. D. (2007) RNA regulons: coordination of post-transcriptional events. Nat. Rev. Genet. 8, 533–543 [DOI] [PubMed] [Google Scholar]
- 2. Moore M. J. (2005) From birth to death: the complex lives of eukaryotic mRNAs. Science 309, 1514–1518 [DOI] [PubMed] [Google Scholar]
- 3. Shaw G., Kamen R. (1986) A conserved AU sequence from the 3′-untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell 46, 659–667 [DOI] [PubMed] [Google Scholar]
- 4. Brennan C. M., Steitz J. A. (2001) HuR and mRNA stability. Cell. Mol. Life Sci. 58, 266–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mukherjee N., Corcoran D. L., Nusbaum J. D., Reid D. W., Georgiev S., Hafner M., Ascano M., Jr., Tuschl T., Ohler U., Keene J. D. (2011) Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol. Cell 43, 327–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lebedeva S., Jens M., Theil K., Schwanhäusser B., Selbach M., Landthaler M., Rajewsky N. (2011) Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol. Cell 43, 340–352 [DOI] [PubMed] [Google Scholar]
- 7. Kishore S., Jaskiewicz L., Burger L., Hausser J., Khorshid M., Zavolan M. (2011) A quantitative analysis of CLIP methods for identifying binding sites of RNA-binding proteins. Nat. Methods 8, 559–564 [DOI] [PubMed] [Google Scholar]
- 8. Uren P. J., Burns S. C., Ruan J., Singh K. K., Smith A. D., Penalva L. O. (2011) Genomic analyses of the RNA-binding protein Hu antigen R (HuR) identify a complex network of target genes and novel characteristics of its binding sites. J. Biol. Chem. 286, 37063–37066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. López de Silanes I., Zhan M., Lal A., Yang X., Gorospe M. (2004) Identification of a target RNA motif for RNA-binding protein HuR. Proc. Natl. Acad. Sci. U.S.A. 101, 2987–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katsanou V., Milatos S., Yiakouvaki A., Sgantzis N., Kotsoni A., Alexiou M., Harokopos V., Aidinis V., Hemberger M., Kontoyiannis D. L. (2009) The RNA-binding protein Elavl1/HuR is essential for placental branching morphogenesis and embryonic development. Mol. Cell. Biol. 29, 2762–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghosh M., Aguila H. L., Michaud J., Ai Y., Wu M. T., Hemmes A., Ristimaki A., Guo C., Furneaux H., Hla T. (2009) Essential role of the RNA-binding protein HuR in progenitor cell survival in mice. J. Clin. Invest. 119, 3530–3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Papadaki O., Milatos S., Grammenoudi S., Mukherjee N., Keene J. D., Kontoyiannis D. L. (2009) Control of thymic T cell maturation, deletion and egress by the RNA-binding protein HuR. J. Immunol. 182, 6779–6788 [DOI] [PubMed] [Google Scholar]
- 13. Katsanou V., Papadaki O., Milatos S., Blackshear P. J., Anderson P., Kollias G., Kontoyiannis D. L. (2005) HuR as a negative posttranscriptional modulator in inflammation. Mol. Cell 19, 777–789 [DOI] [PubMed] [Google Scholar]
- 14. Yiakouvaki A., Dimitriou M., Karakasiliotis I., Eftychi C., Theocharis S., Kontoyiannis D. L. (2012) Myeloid cell expression of the RNA-binding protein HuR protects mice from pathologic inflammation and colorectal carcinogenesis. J. Clin. Invest. 122, 48–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang J., Modi Y., Yarovinsky T., Yu J., Collinge M., Kyriakides T., Zhu Y., Sessa W. C., Pardi R., Bender J. R. (2012) Macrophage β2 integrin-mediated, HuR-dependent stabilization of angiogenic factor-encoding mRNAs in inflammatory angiogenesis. Am. J. Pathol. 180, 1751–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Du R., Lu K. V., Petritsch C., Liu P., Ganss R., Passegué E., Song H., Vandenberg S., Johnson R. S., Werb Z., Bergers G. (2008) HIF1α induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 13, 206–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dvorak H. F. (2002) Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 20, 4368–4380 [DOI] [PubMed] [Google Scholar]
- 18. Jain R. K. (2003) Molecular regulation of vessel maturation. Nat. Med. 9, 685–693 [DOI] [PubMed] [Google Scholar]
- 19. Ferrara N., Carver-Moore K., Chen H., Dowd M., Lu L., O'Shea K. S., Powell-Braxton L., Hillan K. J., Moore M. W. (1996) Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380, 439–442 [DOI] [PubMed] [Google Scholar]
- 20. Oosthuyse B., Moons L., Storkebaum E., Beck H., Nuyens D., Brusselmans K., Van Dorpe J., Hellings P., Gorselink M., Heymans S., Theilmeier G., Dewerchin M., Laudenbach V., Vermylen P., Raat H., Acker T., Vleminckx V., Van Den Bosch L., Cashman N., Fujisawa H., Drost M. R., Sciot R., Bruyninckx F., Hicklin D. J., Ince C., Gressens P., Lupu F., Plate K. H., Robberecht W., Herbert J. M., Collen D., Carmeliet P. (2001) Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat. Genet. 28, 131–138 [DOI] [PubMed] [Google Scholar]
- 21. Ray P. S., Jia J., Yao P., Majumder M., Hatzoglou M., Fox P. L. (2009) A stress-responsive RNA switch regulates VEGF-A expression. Nature 457, 915–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levy N. S., Chung S., Furneaux H., Levy A. P. (1998) Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J. Biol. Chem. 273, 6417–6423 [DOI] [PubMed] [Google Scholar]
- 23. Claffey K. P., Shih S. C., Mullen A., Dziennis S., Cusick J. L., Abrams K. R., Lee S. W., Detmar M. (1998) Identification of a human VPF/VEGF 3′-untranslated region mediating hypoxia-induced mRNA stability. Mol. Biol. Cell 9, 469–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fellows A., Griffin M. E., Petrella B. L., Zhong L., Parvin-Nejad F. P., Fava R., Morganelli P., Robey R. B., Nichols R. C. (2012) AUF1/hnRNP D represses expression of VEGF in macrophages. Mol. Biol. Cell 23, 1414–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Essafi-Benkhadir K., Onesto C., Stebe E., Moroni C., Pagès G. (2007) Tristetraprolin inhibits Ras-dependent tumor vascularization by inducing vascular endothelial growth factor mRNA degradation. Mol. Biol. Cell 18, 4648–4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jafarifar F., Yao P., Eswarappa S. M., Fox P. L. (2011) Repression of VEGFA by CA-rich element-binding microRNAs is modulated by hnRNP L. EMBO J. 30, 1324–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang S. H., Hla T. (2011) Gene regulation by RNA-binding proteins and microRNAs in angiogenesis. Trends Mol. Med. 17, 650–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Filipowicz W., Bhattacharyya S. N., Sonenberg N. (2008) Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 9, 102–114 [DOI] [PubMed] [Google Scholar]
- 29. Chan C. S., Elemento O., Tavazoie S. (2005) Revealing posttranscriptional regulatory elements through network-level conservation. PLoS Comput. Biol. 1, e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jacobsen A., Wen J., Marks D. S., Krogh A. (2010) Signatures of RNA-binding proteins globally coupled to effective microRNA target sites. Genome Res. 20, 1010–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bhattacharyya S. N., Habermacher R., Martine U., Closs E. I., Filipowicz W. (2006) Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell 125, 1111–1124 [DOI] [PubMed] [Google Scholar]
- 32. Kim H. H., Kuwano Y., Srikantan S., Lee E. K., Martindale J. L., Gorospe M. (2009) HuR recruits let-7/RISC to repress c-Myc expression. Genes Dev. 23, 1743–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clausen B. E., Burkhardt C., Reith W., Renkawitz R., Förster I. (1999) Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 [DOI] [PubMed] [Google Scholar]
- 34. Weischenfeldt J., Porse B. (2008) Bone marrow-derived macrophages (BMM): isolation and application. Cold Spring Harb. Protoc. 2008, pdb.prot5080 [DOI] [PubMed] [Google Scholar]
- 35. Salomonis N., Schlieve C. R., Pereira L., Wahlquist C., Colas A., Zambon A. C., Vranizan K., Spindler M. J., Pico A. R., Cline M. S., Clark T. A., Williams A., Blume J. E., Samal E., Mercola M., Merrill B. J., Conklin B. R. (2010) Alternative splicing regulates mouse embryonic stem cell pluripotency and differentiation. Proc. Natl. Acad. Sci. U.S.A. 107, 10514–10519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bakheet T., Williams B. R., Khabar K. S. (2006) ARED 3.0: the large and diverse AU-rich transcriptome. Nucleic Acids Res. 34, D111–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Elemento O., Slonim N., Tavazoie S. (2007) A universal framework for regulatory element discovery across all genomes and data types. Mol. Cell 28, 337–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zygmunt T., Gay C. M., Blondelle J., Singh M. K., Flaherty K. M., Means P. C., Herwig L., Krudewig A., Belting H. G., Affolter M., Epstein J. A., Torres-Vázquez J. (2011) Semaphorin-PlexinD1 signaling limits angiogenic potential via the VEGF decoy receptor sFlt1. Dev. Cell 21, 301–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Avraham-Davidi I., Ely Y., Pham V. N., Castranova D., Grunspan M., Malkinson G., Gibbs-Bar L., Mayseless O., Allmog G., Lo B., Warren C. M., Chen T. T., Ungos J., Kidd K., Shaw K., Rogachev I., Wan W., Murphy P. M., Farber S. A., Carmel L., Shelness G. S., Iruela-Arispe M. L., Weinstein B. M., Yaniv K. (2012) ApoB-containing lipoproteins regulate angiogenesis by modulating expression of VEGF receptor 1. Nat. Med. 18, 967–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qian B. Z., Pollard J. W. (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Good P. J. (1995) A conserved family of elav-like genes in vertebrates. Proc. Natl. Acad. Sci. U.S.A. 92, 4557–4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Trabucchi M., Briata P., Garcia-Mayoral M., Haase A. D., Filipowicz W., Ramos A., Gherzi R., Rosenfeld M. G. (2009) The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature 459, 1010–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hua Z., Lv Q., Ye W., Wong C. K., Cai G., Gu D., Ji Y., Zhao C., Wang J., Yang B. B., Zhang Y. (2006) miRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One 1, e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kedde M., Strasser M. J., Boldajipour B., Oude Vrielink J. A., Slanchev K., le Sage C., Nagel R., Voorhoeve P. M., van Duijse J., Ørom U. A., Lund A. H., Perrakis A., Raz E., Agami R. (2007) RNA-binding protein Dnd1 inhibits microRNA access to target mRNA. Cell 131, 1273–1286 [DOI] [PubMed] [Google Scholar]
- 45. Kundu P., Fabian M. R., Sonenberg N., Bhattacharyya S. N., Filipowicz W. (2012) HuR protein attenuates miRNA-mediated repression by promoting miRISC dissociation from the target RNA. Nucleic Acids Res. 40, 5088–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lin E. Y., Li J. F., Gnatovskiy L., Deng Y., Zhu L., Grzesik D. A., Qian H., Xue X. N., Pollard J. W. (2006) Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 66, 11238–11246 [DOI] [PubMed] [Google Scholar]
- 47. Galbán S., Kuwano Y., Pullmann R., Jr., Martindale J. L., Kim H. H., Lal A., Abdelmohsen K., Yang X., Dang Y., Liu J. O., Lewis S. M., Holcik M., Gorospe M. (2008) RNA-binding proteins HuR and PTB promote the translation of hypoxia-inducible factor 1α. Mol. Cell. Biol. 28, 93–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McArthur K., Feng B., Wu Y., Chen S., Chakrabarti S. (2011) MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes 60, 1314–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chan Y. C., Roy S., Khanna S., Sen C. K. (2012) Down-regulation of endothelial microRNA-200b supports cutaneous wound angiogenesis by desilencing GATA binding protein 2 and vascular endothelial growth factor receptor 2. Arterioscler. Thromb. Vasc. Biol. 32, 1372–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chan Y. C., Khanna S., Roy S., Sen C. K. (2011) miR-200b targets Ets-1 and is down-regulated by hypoxia to induce angiogenic response of endothelial cells. J. Biol. Chem. 286, 2047–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Takeda Y., Mishima Y., Fujiwara T., Sakamoto H., Inoue K. (2009) DAZL relieves miRNA-mediated repression of germ-line mRNAs by controlling poly(A) tail length in zebrafish. PLoS One 4, e7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maragkakis M., Vergoulis T., Alexiou P., Reczko M., Plomaritou K., Gousis M., Kourtis K., Koziris N., Dalamagas T., Hatzigeorgiou A. G. (2011) DIANA-microT Web server upgrade supports Fly and Worm miRNA target prediction and bibliographic miRNA to disease association. Nucleic Acids Res. 39, W145–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Westerfield M. (1994) in The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio), 2.1 Ed., Institute of Neuroscience, University of Oregon, Eugene, OR [Google Scholar]
- 54. Keniry A., Oxley D., Monnier P., Kyba M., Dandolo L., Smits G., Reik W. (2012) The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and lgf1r. Nat. Cell Biol. 14, 659–665 [DOI] [PMC free article] [PubMed] [Google Scholar]