Background: BH3-only proteins play a key role in the activation of Bax and Bak during apoptosis.
Results: Interactions among Bcl-2 proteins have been detected in living cells.
Conclusion: Interactions observed in living cells fit to “mixed models.”
Significance: This study in living cells demonstrates direct activation of Bax and Bak by BH3-only proteins.
Keywords: Apoptosis, Bax, Bcl-2 Family, Cell Death, Mitochondrial Apoptosis, BH3-only, Bax/Bak Activation, Bimolecular Fluorescence Complementation (BiFC)
Abstract
The key event in the mitochondrial pathway of apoptosis is the activation of Bax and Bak by BH3-only proteins through a molecular mechanism that is still a matter of debate. Here we studied interactions among anti- and proapoptotic proteins of the Bcl-2 family in living cells by using bimolecular fluorescence complementation analysis. Our results indicate that the antiapoptotic proteins Mcl-1 and Bcl-xL bind preferably to the BH3-only proteins Bim, PUMA, and Noxa but can also bind to Bak and Bax. We also found a direct interaction between Bim, PUMA, or Noxa with either Bax or Bak during apoptosis induction. In HeLa cells, interaction of Bim with Bax occurs in cytosol, and then Bim-Bax complexes translocate to mitochondria. Complexes of either PUMA or Noxa with Bax or Bak were always detected at mitochondria. Overexpression of Bcl-xL or Mcl-1 delayed Bim/Bax translocation to mitochondria. These results reveal the ability of main BH3-only proteins to directly activate Bax and Bak in living cells and suggest that a complex network of interactions regulate the function of Bcl-2 family members during apoptosis.
Introduction
Bcl-2 proteins are key players of apoptosis induction, regulating mitochondrial permeabilization and release of proteins that participate in the execution phase of apoptosis. This family comprises anti- and proapoptotic members that share at least one of the four Bcl-2 homology (BH)2 domains BH1 to BH4 (1). Proapoptotic members are divided into two subsets: multidomain Bax-like proteins such as Bax, Bak, and Bok and BH3-only proteins. Multidomain proteins Bax and Bak are the “effectors” for mitochondrial permeabilization, and simultaneous lack of both proteins renders cells resistant to most apoptotic inducers (2, 3). Both proteins are usually present in healthy cells in an inactive state, although Bax can be transcriptionally up-regulated in response to some death stimuli (4). Induction of apoptosis occurs through the BH3-only protein-induced activation of Bax and Bak, but there is a significant controversy about the precise mechanism underlying this process. Two different models were proposed initially (5). According to the indirect (displacement) model, Bax and Bak remain blocked by antiapoptotic members of the family, which are displaced by BH3-only proteins upon apoptosis initiation. Among BH3-only proteins, Bim, Bid, and PUMA would be more efficient because of their ability to bind all the antiapoptotic members of the family. The direct model classifies BH3-only proteins in two groups: sensitizers and activators. In this model, Bim, Bid, and PUMA have been proposed to be direct activators of Bax and Bak, although two recent reports have suggested that Noxa could also act as an activator (6, 7). Other BH3-only proteins, such as Bad and Bik, would act as sensitizers, freeing activators from antiapoptotic proteins of the family. Evidence in support of the direct model comes from experiments with BH3-derived peptides. Bim, Bid, or PUMA peptides can bind Bax and Bak and induce cytochrome c release from isolated mitochondria, whereas BH3 peptides derived from other BH3-only proteins lack this ability (8). However, a demonstration of a direct interaction of full-length, putative BH3-only activator proteins and Bax/Bak in intact cells remains elusive, fueling controversy about this subject.
In this work we studied interactions among members of the Bcl-2 family during apoptosis in living cells using the bimolecular fluorescence complementation (BiFC) technique (9). For BiFC experiments, each protein to be studied is fused to a half of a fluorescent protein. Interaction between the two proteins analyzed induces complementation of the fluorescent protein fragments, and the fluorescent signal can be monitored. BiFC has been used to study protein interactions in mammalian cells (10, 11), including oligomerization of caspase 2 (12). Also, BiFC has been used recently to study Bax homodimerization and translocation to mitochondria (13). BiFC, contrary to other fluorescence-based methods, captures transient interactions (14). By using BiFC, we found that Mcl-1 and Bcl-xL can associate to both BH3-only and multidomain proapoptotic proteins. We also demonstrate that Bim, PUMA, and Noxa can bind to Bax and Bak. Complexes of Bim with Bax are detected in cytosol and translocate to mitochondria prior to cell death. We also found differences in the relative participation of the H1α and BH3 domains of Bax and Bak in the interaction with BH3-only proteins.
EXPERIMENTAL PROCEDURES
Construction of the pBiFC and pBabe Vectors
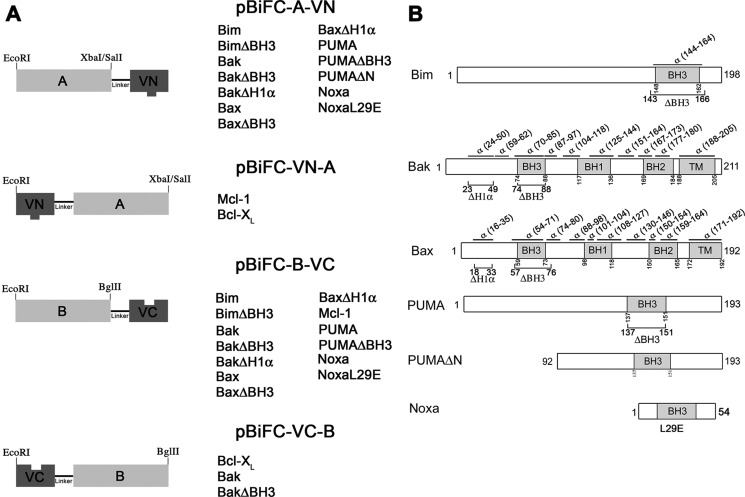
The coding sequences for human Mcl-1, Bcl-xL, Bim, Bak, Bax, PUMA, and Noxa were subcloned by standard PCR strategies into BiFC plasmids containing Venus fragments (FLAG/VN173 or HA/VC155; VN, VC: N/C-terminal fragment of the Venus protein) to generate the constructs depicted in Fig. 1A. Constructs with the Venus moiety at the N or C terminus were tested for Mcl-1, Bcl-xL, Bak, and Bax. Appropriate restriction sites (EcoRI/XbaI or EcoRI/SalI for pBiFC-VN173 and EcoRI/BglII for pBiFC-VC155) were incorporated. Deletions of either the BH3 domain or the first α helix of some Bcl-2 family proteins (Fig. 1B) were generated by PCR overlap (15). A variant of human PUMA-α lacking the first 92 amino acids (PUMAΔN) and a single-substitution mutant of Noxa (NoxaL29E) were also subcloned into pBiFC-VN173 using the same methods described above. We confirmed correct localization and functionality of Mcl-1, Bcl-xL, Bak, and Bax fusion proteins. For instance, we verified that Bax-EYFP is able to translocate to mitochondria and induce apoptosis and shows the same distribution within the cell that the endogenous protein (data not shown).
FIGURE 1.
Schematic representation of gene constructs generated for BiFC experiments. A, unique restriction sites in the Multicloning site of pBiFC vectors were used for cloning the proteins of interest (EcoRI, XbaI, SalI, and BglII) fused to corresponding fragment of Venus fluorescent protein. B, secondary structure of proteins and mutations generated for the study of interactions by BiFC technique.
The cDNA of Mcl-1 or Bcl-xL were subcloned in the retroviral vector pBabe (a kind gift from Reinhard Wallich, University of Heidelberg) between the EcoRI/SalI and EcoRI/NotI sites, respectively. DNA coding for the fusion proteins Bax-EYFP and EYFP-Bax were generated by PCR overlap. Purified fragments were also subcloned into a pBabe vector using the EcoRI and SalI restriction sites. The nucleotide sequence corresponding to GSRSIAT was introduced between the cDNAs of the two proteins to act as a linker. The accuracy of all generated plasmids was verified by gene sequencing.
Cell Lines
Human HeLa cervix adenocarcinoma cells were from the ATCC. Cells stably overexpressing either Mcl-1 or Bcl-xLwere generated by retroviral infection using the pBABE vector. Clones exhibiting a high expression of the proteins relative to the controls infected with the empty vector were obtained by limiting dilution and expanded after puromycin or fluorescence selection. Cells were routinely cultured at 37 °C in DMEM supplemented with 10% FCS, l-glutamine, and penicillin/streptomycin.
Transient Transfection Assays
For BiFC assays, cells grown to at least 50% confluence were transfected with the appropriate amount of each vector using Lipofectamine 2000 (Invitrogen) according to the instructions of the manufacturer. A ratio of 1 mg DNA:3 ml Lipofectamine was used for all experiments. Cotransfection with equal amounts of the vector pAL2-mRFP, containing mRFP cDNA, was performed to assess transfection efficiency and relative fluorescence intensities. The amount of each vector was adjusted so that fusion proteins were expressed at levels similar to that of endogenous proteins (data not shown) to avoid random complementation of Venus protein fragments. Addition of 50 μm Z-VAD-fmk (Bachem) was necessary to inhibit caspases and maintain cell integrity when cells were transfected with two proapoptotic proteins. Transfected cells were cultured at 37 °C for 24 h, and BiFC complex formation was analyzed by time lapse fluorescence microscopy and flow cytometry. Apoptosis was also evaluated when necessary by measuring phosphatidylserine exposure by flow cytometry as described below.
Western Blot Analysis
To determine the expression level of the fusion proteins, SDS-PAGE and Western blot analyses of cytoplasmic cell lysates were performed. Proteins were immunodetected by using appropriate primary and peroxidase-labeled secondary antibodies (Sigma) and visualized with Immobilon Western chemiluminescent HRP substrate (Millipore). Specific antibodies against the following proteins were used: Bim (Calbiochem), Bak (BD Biosciences), Bax (BD Biosciences), PUMA (Cell Signaling Technology, Inc.), Mcl-1 (Santa Cruz Biotechnology), and Noxa (Abcam). Protein loading control was achieved by membrane reprobing with an anti-α-tubulin antibody (Sigma).
Confocal Microscopy
For confocal microscopy, cells were grown and transfected on coverslips, washed with PBS, fixed in 4% paraformaldehyde at room temperature for 20 min, washed, and mounted in Fluoromount G (Southern Biotechnology). When necessary, unfixed cells were stained with 50 nm MitoTracker Red (CMXRos, Invitrogen) and/or Hoechst 33342 (1 μg/ml, Invitrogen) to analyze mitochondria and nuclear morphology, respectively. Images were collected using a Leica SP2 AOBS confocal scanning microscope in sequential mode with a ×63 oil immersion lens, a line average of 16, and a format of 1024 × 1024 pixels. The confocal pinhole was 1 Airy unit. Nuclei stained with Hoechst were visualized with an Olympus FV10i confocal scanning microscope. The images were collected in sequential mode with a ×60 oil immersion lens, a line average of 8, and a format of 1024 × 1024 pixels. The confocal pinhole was 1 Airy unit. Images were exported without image manipulation from the Leica confocal software or FV10-ASW 2.0 viewer software into Adobe Photoshop CS2 v12 to generate the figures.
Time Lapse Microscopy
Cells were plated on μ-Slide 8-well tissue culture plates (Ibidi) and transfected with the vectors indicated in each experiment. Optimal culture conditions (37 °C, 5% CO2) were maintained using an environmental control chamber. Cells were monitored for 72 h with a HCX PL S-APO 40.0 × 0.75 DRY objective in a Leica AF6000 LX system. Images were acquired with a CCD camera (C9100-02, Hamamatsu) at 7-min intervals using LAS AF software (Leica) to process images. Snapshots of selected times were collected using LAS AF software (Leica).
Flow Cytometry
Venus (BiFC) and mRFP signals in cells were quantified in a FACSCalibur flow cytometer using 488 nm and 635 nm excitation lasers, respectively. A gating analysis on the basis of mRFP fluorescence was performed to exclude non-transfected cells. The mean fluorescence intensities of the BiFC complexes were normalized to the mean fluorescence intensity of mRFP. At least 10,000 cells were analyzed in each experiment. Cell death was analyzed by determining phosphatidylserine exposure. Cells were incubated at room temperature in 100 ml of annexin-binding buffer (140 mm NaCl, 2.5 mm CaCl2, 10 mm Hepes/NaOH (pH 7.4)) containing 2 μl of annexin V-phycoerythrin (PE) (Immunostep) for 15 min. Cells were diluted to 1 ml with annexin-binding buffer prior to flow cytometry analysis. Mitochondrial membrane potential (ΔΨm) analyses were performed by incubating cells with 60 nm tetramethylrhodamine ethyl ester (Invitrogen) at 37 °C for 15 min and performing a flow cytometry analysis.
RESULTS
Antiapoptotic Proteins Bind Both to BH3-only and Multidomain Proapoptotic Proteins
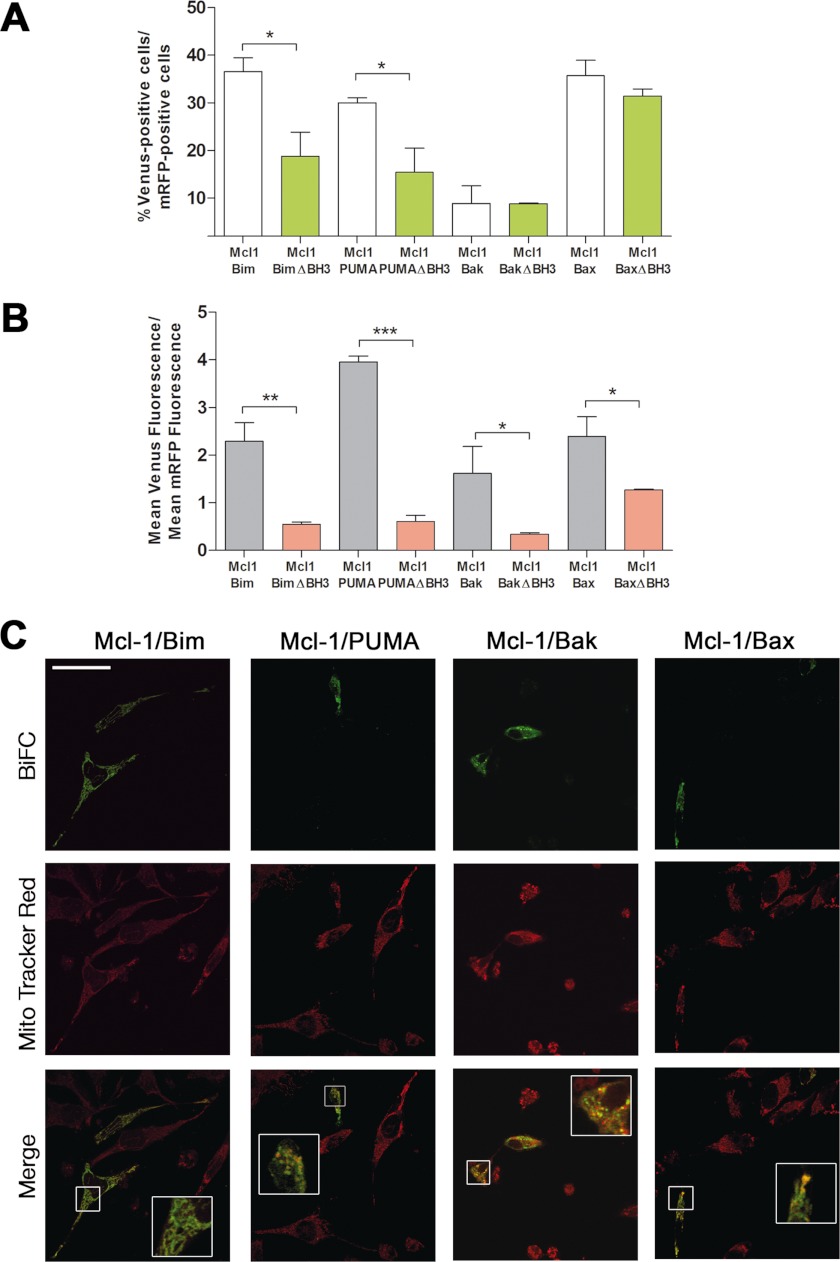
One of the main discrepancies between the “direct” and the “displacement” models is the way antiapoptotic proteins block the activation of multidomain proapoptotic proteins. We first explored interactions of either Mcl-1 or Bcl-xL with proapoptotic members of the Bcl-2 family in HeLa cells by using BiFC. Cells were cotransfected with two vectors, one encoding Mcl-1 or Bcl-xL fused to the N- or C-terminal fragment of Venus (VN-Mcl-1, VN-Bcl-xL, VC-Bcl-xL) and the other encoding Bim, PUMA, Bax, or Bak fused to the N- or C-terminal fragment of Venus (Fig. 1). Complementation of the two Venus halves revealed that Mcl-1 interacts with the BH3-only proteins Bim and PUMA, as indicated by a significant percentage of Venus fluorescent cells and high Venus/mRFP fluorescence ratios (Figs. 2, A and B). Deletion of the BH3 domain of Bim (16) or PUMA (17, 18) has been reported to abolish their proapoptotic effect, and we observed that ablation of this domain significantly reduced their binding to Mcl-1 (Fig. 2, A and B). Mcl-1 complexes with Bim and PUMA showed mainly a punctuate distribution and colocalized with MitoTracker staining (Fig. 2C). Similar results were obtained when cells were transfected with vectors encoding Mcl1-VC/PUMA-VN fusions (data not shown). We also observed interaction between Mcl1-VC/Bim-VN fusions with a similar percentage of Venus-positive cells and Venus/mRFP ratio than the one measured with VN-Mcl1/Bim-VC in Fig. 2, A and B. However, the Mcl1-VC/Bim-VN pair complexes showed a cytosolic distribution (data not shown), suggesting that formation of the complexes affected translocation to mitochondria when the moiety is located at the C terminus of Mcl-1.
FIGURE 2.
Interactions of Mcl-1 with proapoptotic proteins of the Bcl-2 family. A and B, HeLa cells were cotransfected with vectors expressing the fusion protein VN-Mcl-1 and Bim, PUMA, Bax, or Bak fused to the other half of Venus (VC). Deletion mutants lacking the BH3 domain (ΔBH3) were transfected as indicated. A vector encoding mRFP was also included in the transfection. After transfection, cells were cultured for 24 h, trypsinized, and Venus and mRFP fluorescence was analyzed by flow cytometry. Results are mean ± S.D. of seven independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001. C, HeLa cells were seeded on coverslips and transfected as indicated in A and B. 24 h after transfection, cells were stained with the mitochondrial probe MitoTracker Red (50 nm), fixed, and analyzed by confocal microscopy as indicated under “Experimental Procedures.” Scale bar = 50 μm. The insets show the enlarged x2.5 boxed regions.
As indicated previously, the displacement model postulates that Bax and Bak are kept in check by antiapoptotic members of the Bcl-2 family, such as Mcl-1. In our system, association of Mcl-1 to Bak could be appreciated in a small portion of cells (Fig. 2, A and B), and binding to Bax was detected in around 50% of transfected cells. In both cases, relative fluorescence levels were reduced when BH3 domains were deleted (Fig. 2B), indicating that this domain is involved in the binding of Bak or Bax to Mcl-1, as other authors have reported previously (19–21). Deletion mutants were expressed at the same level as full-length proteins (data not shown), ruling out the possibility that the decrease in BiFC fluorescence was due to reduced expression of the constructs. In the case of Bax, deletion of the BH3 produced a reduction in Venus intensity but did not completely abrogated association to Mcl-1, suggesting that other domains could also be involved. Mcl-1-Bax and Mcl-1-Bak complexes showed discrete mitochondrial localization, as determined by confocal microscopy in MitoTracker stained cells (Fig. 2C).
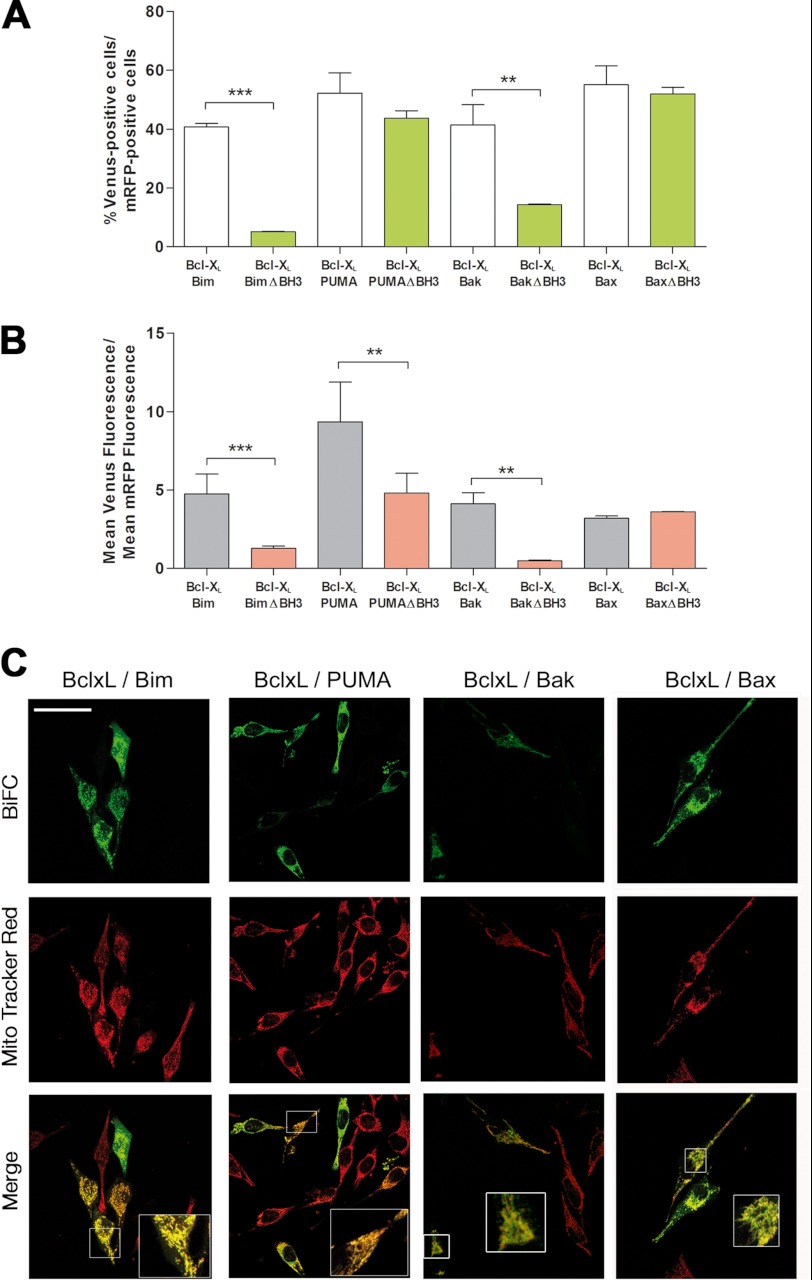
Bcl-xL associated to BH3-only proteins in a high percentage of cells, and interactions with Bax and Bak were also observed (Fig. 3A). Deletion of the BH3 domains of Bim, PUMA, or Bak reduced their interaction with Bcl-xL (Fig. 3B), although, in the case of PUMA, the percentage of fluorescent cells remained high (A). These results could indicate that PUMA can interact with Bcl-xL through different domains but that in the absence of the BH3 domain, the association probably does not allow for an efficient complementation of the Venus halves, yielding a lower fluorescence intensity. Confocal microscopy revealed that Bcl-xL complexes with Bim, PUMA, Bax, or Bak showed a mitochondrial localization (Fig. 3C).
FIGURE 3.
Interactions of Bcl-xLwith proapoptotic proteins of the Bcl-2 family. A and B, HeLa cells were cotransfected with vectors expressing the fusion protein VC- Bcl-xL and Bim, PUMA, Bax, or Bak fused to the other half of Venus (VN). Deletion mutants lacking the BH3 domain (ΔBH3) were transfected as indicated. A vector encoding mRFP was also included in the transfection. After transfection, cells were cultured for 24 h, trypsinized, and then Venus and mRFP fluorescence was analyzed by flow cytometry. Results are mean ± S.D. of seven independent experiments. * p < 0.05; **, p < 0.01; ***, p < 0.001. C, HeLa cells were seeded on coverslips and transfected as indicated in A and B. 24 h after transfection cells were stained with the mitochondrial probe MitoTracker Red (50 nm), fixed, and analyzed by confocal microscopy as indicated under “Experimental Procedures.” Scale bar = 50 μm. The insets show the enlarged (x2.5) boxed regions.
On the whole, these results indicate that in healthy cells, Mcl-1 and Bcl-xL restrain BH3-only proteins but can also block Bax and Bak, especially the former. In this way, our results agree with previous reports showing a high affinity of Bim and PUMA for Mcl-1 (22). The low percentage of cells positive for Mcl-1/Bak association in BiFC experiments is in accordance with previous findings. Coimmunoprecipitation of Mcl-1 with Bak has been reported by several groups (23–25) but not by others (3), and in some cases these interactions depended on the detergent used to prepare cell lysates (26). In Bim/Bid Double Knock-Out (DKO) mouse embryonic fibroblasts, only 10% of total Mcl-1 was found to coimmunoprecipitate with Bak (6). Our current results in living cells indicate that both Mcl-1 and Bcl-xL may interact with Bax. Previous immunoprecipitation approaches have not revealed interaction between Mcl-1 and Bax (26), although earlier yeast two-hybrid studies suggested this possibility (27).
Bim/Bax Complexes Form in Cytosol and Translocate to Mitochondria during Apoptosis
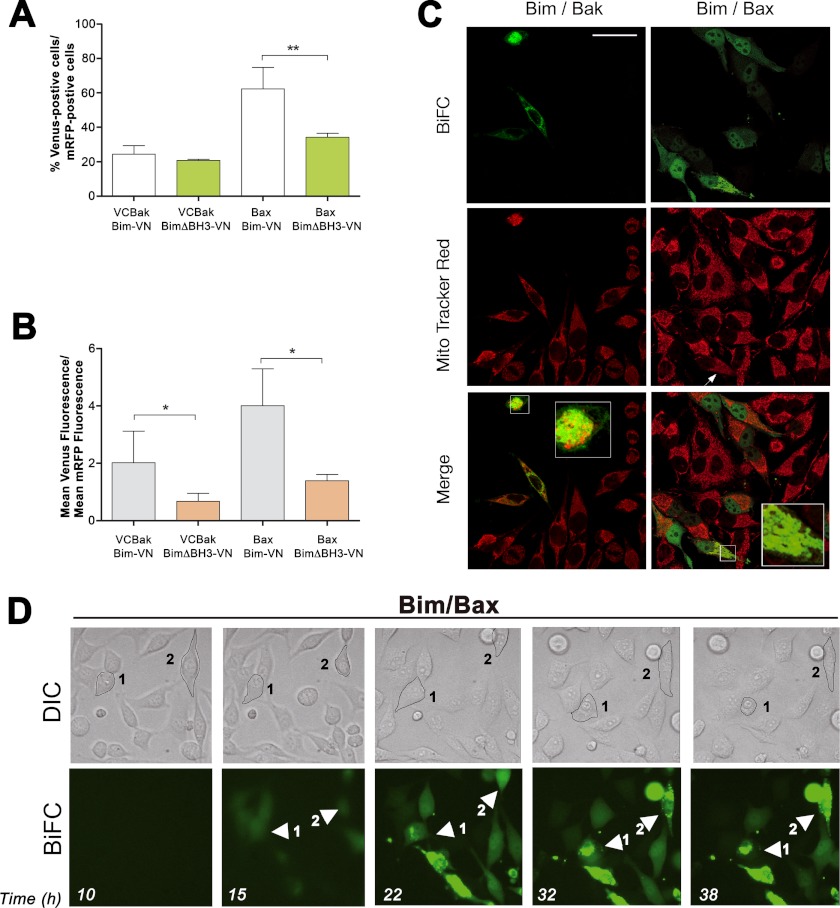
The direct model of activation relies on putative interactions between “activator” BH3-only proteins and Bax/Bak effectors. However, solid evidence for these interactions in living cells is lacking. Some authors have proposed that activator BH3-only proteins could activate Bax and Bak through transient, difficult-to-detect interactions. The BiFC technique could thus be adequate to capture these probably transient associations because complementation of the two halves of the fluorescent protein is an irreversible process. Simultaneous overexpression of either Bim-VN or PUMA-VN and Bax-VC or VC-Bak induced apoptosis, especially evident in the case of PUMA. For this reason, cells were pretreated with the pan-caspase inhibitor Z-VAD-fmk to delay apoptosis and preserve cell integrity, thus facilitating the visualization of protein-protein interactions. Bim-Bak complexes were detected in around 20% of transfected cells (Fig. 4A). Deletion of the BH3 domain of Bim disrupted its association to Bak, as reflected by the significant decrease in the Venus/mRFP intensity ratio (Fig. 4B). Distribution of the Bim/Bak pairs suggested mitochondrial localization (Fig. 4C). Cotransfection with a vector encoding a mitochondria-targeted mRFP confirmed that Bim-Bak complexes were located at mitochondria (data not shown). Importantly, Bim/Bak Venus-positive cells displayed a reduced mitochondrial transmembrane potential, as indicated by the faint MitoTracker staining (Fig. 4C, arrows).
FIGURE 4.
Visualizing direct interactions of Bim with Bak and Bax. A and B, HeLa cells were cotransfected with vectors encoding the fusion protein Bim-VN and Bax or Bak fused to the other half of Venus (VC). Deletion mutants lacking the BH3 domain (ΔBH3) were transfected as indicated. A vector encoding mRFP was also included in the transfection. After transfection, cells were cultured for 24 h, trypsinized, and Venus and mRFP fluorescence was analyzed by flow cytometry. Results are mean ± S.D. of six to ten independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001. C, HeLa cells were seeded on coverslips and transfected as indicated in A and B. 24 h after transfection cells were stained with 50 nm MitoTracker Red, fixed, and analyzed by confocal microscopy as indicated under “Experimental Procedures.” Scale bar = 50 μm. The insets show the enlarged (x2.5) boxed regions. D, time lapse microscopy reveals translocation of Bim-Bax complexes from the cytosol to mitochondria. Cells were plated on μ-Slide 8-well tissue culture plates and transfected with the vectors containing the Bim-VN and Bax-VC constructs. Cells were monitored for 72 h in a Leica AF6000 LX system as indicated under “Experimental Procedures.” Images were acquired with a CCD camera (C9100-02, Hamamatsu) at 7-min intervals, and LAS AF software (Leica) was used to process the images. Also shown is a representative time lapse sequence from supplemental movies Mov1BimBax and Mov2BimBax showing phase-contrast images (upper panel) and Venus fluorescence (lower panel). DIC, differential interference contrast.
Strikingly, around 60% of mRFP-positive cells exhibited Venus fluorescence after cotransfection with the Bim/Bax BiFC pair (Fig. 4A). A high Venus/mRFP fluorescence ratio also indicated a strong association between Bim and Bax (Fig. 4B). Deletion of the BH3 domain of Bim significantly reduced this interaction (Fig. 4, A and B). Interestingly, Bim/Bax association was found in the cytosol of healthy cells (Fig. 4C), suggesting that Bim binds to Bax before translocation to mitochondria. Bax-Bim complexes were also found partially in nuclei, a fact that can be explained by the ability of small cytosolic proteins to diffuse through nuclear pores (28). Both endogenous Bax and the EYFP-Bax were found in cytosol and nuclei (data not shown), as described previously (29). In apoptotic cells, however, Venus fluorescence showed a punctated distribution (Fig. 4C) congruent with mitochondrial distribution. Cells containing mitochondrial Bim-Bax complexes presented collapsed ΔΨm (Fig. 4C, arrows). Time lapse microscopy confirmed that Bim associates with Bax in cytosol and then Bim-Bax complexes translocate to mitochondria, preceding cell shrinking and other morphological features of apoptosis (Fig. 4D and supplemental moviesMov1BimBax and Mov2BimBax). Similarly, Yivgi-Ohana et al. (13) have reported that Bax and Bid associate in cytosol and translocate together to mitochondria.
PUMA Can Bind Directly to Bak and Bax in Intact Cells
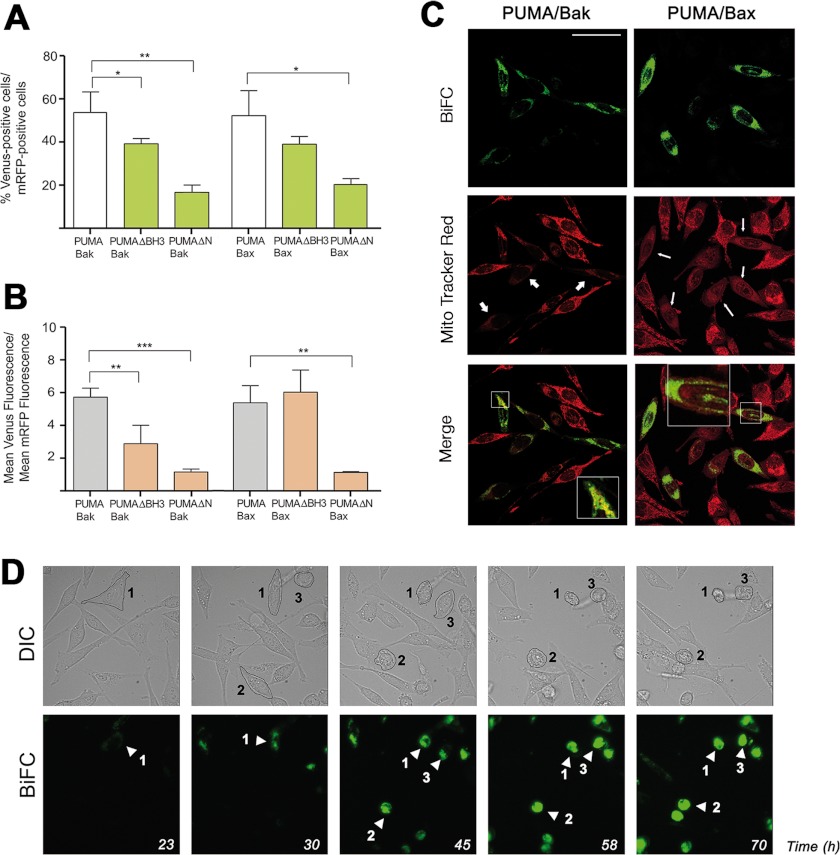
PUMA is a potent apoptosis inducer, and its expression is usually induced by p53-dependent and -independent death stimuli (17, 30). Overexpression of PUMA has been reported to induce apoptosis (31). On the basis of biochemical studies (32) and on triple genetic ablation of Bim, Bid, and PUMA (33), the latter has been proposed to be an activator of Bax and Bak. To explore this hypothesis in living cells, we tested whether PUMA could interact with Bax and Bak in BiFC experiments. As found previously, transfection with PUMA and the Bax/Bak vector induced high mortality, demonstrating the functionality of fusion proteins. We found that a high percentage of cells were positive for Venus fluorescence after transfection with PUMA-VN and VC-Bak or PUMA-VN and Bax-VC (Fig. 5A), indicating that PUMA directly interacts with each of these proteins. Strikingly, the interaction with Bax was also detected when using a BH3-truncated form of PUMA (Fig. 5, A and B). Because PUMAΔBH3 has lost its proapoptotic activity (data not shown and Refs. 17, 18), these results could indicate that this binding of PUMA to Bax is not sufficient to induce its activation. A possible explanation for this observation would be that PUMA could establish another interaction with Bax independent of its BH3 domain, but only the interaction via its BH3 is productive to induce the conformational activation of Bax. A PUMA mutant lacking the N-terminal domain did not interact with Bax and Bak (Fig. 5, A and B). Thus, this latter domain could be involved in the binding, but not in the activation, of PUMA to Bax and Bak. Another possibility would be that the correct binding of PUMA to antiapoptotic proteins, which involves an intact BH3 domain (Figs. 2 and 3), could be critical for its proapoptotic activity, in agreement with previous studies (34). Both the PUMA-Bak and PUMA-Bax complexes showed a punctated distribution, suggesting mitochondrial localization, and cells containing these pairs displayed a lower MitoTracker staining (Fig. 5C, arrows), indicating loss of ΔΨm, an event frequently associated with cell death. Bax and PUMA pairs appeared shortly before the initiation of cell death (Fig. 5D and supplemental movies Mov3 PUMABax and Mov4 PUMABax), and they were not detected in cytosol, contrary to the observations with Bim/Bax pairs (Fig. 4). These results are congruent with the reported mitochondrial localization of PUMA (34).
FIGURE 5.
Visualizing direct interactions of PUMA with Bak and Bax. A and B, HeLa cells were cotransfected with vectors expressing the fusion protein PUMA-VN and Bax or Bak fused to the other half of Venus (VC). Deletion mutants lacking the BH3 domain (ΔBH3) or amino acids 1–94 (ΔN) were transfected as indicated. A vector encoding mRFP was also included in the transfection. After transfection, cells were cultured for 24 h, trypsinized, and Venus and mRFP fluorescence was analyzed by flow cytometry. Results are mean ± S.D. of five to ten independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001. C, HeLa cells were seeded on coverslips and transfected as indicated in A and B. 24 h after transfection cells were stained with 50 nm MitoTracker Red, fixed, and analyzed by confocal microscopy as indicated under “Experimental Procedures.” Scale bar = 50 μm. The insets show the enlarged (x2.5) boxed regions. D, time lapse microscopy reveals the interaction of PUMA and Bax in mitochondria prior to cell death induction. Cells were plated on μ-Slide 8-well tissue culture plates and transfected with the vectors containing the PUMA-VN and Bax-VC constructs. Cells were monitored for 72 h in a Leica AF6000 LX system, as indicated under “Experimental Procedures.” Images were acquired with a CCD camera (C9100-02, Hamamatsu) at 7-min intervals, and LAS AF software (Leica) was used to process the images. Also shown is a representative time lapse sequence from supplemental movies Mov3PUMABax and Mov4PUMABax showing phase-contrast images (upper panel) and Venus fluorescence (lower panel). DIC, differential interference contrast.
Different Role of BH3 and H1α Domains of Bax and Bak in the Binding of BH3-only Proteins
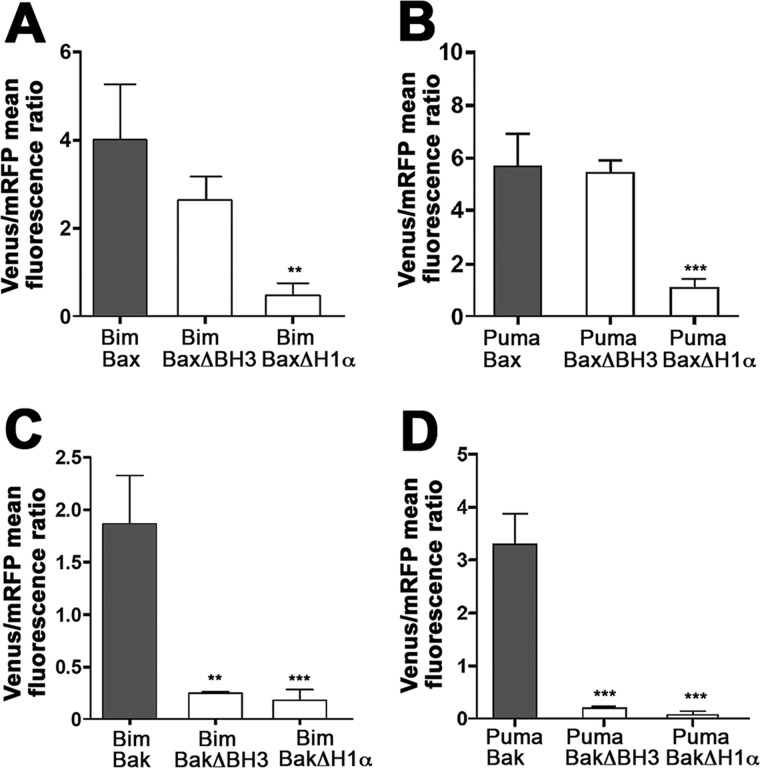
The α 1 helix (H1α) of Bax has been suggested to be the binding site for BH3-only proteins (35–37). Thus, we have explored the involvement of H1α and BH3 domains of Bax and Bak in the corresponding binding to Bim and PUMA. We transfected cells with forms of Bax and Bak lacking the H1α helix or the BH3 domains, and expression of these truncated proteins were confirmed by Western blot analysis (data not shown). Our present results indicate that the H1α of Bax is essential for Bim-Bax and PUMA-Bax interactions (Fig. 6) in living cells, according to previous reports on the basis of biochemical data (35–37). Interestingly, the BH3 domain of Bax did not seem to be significantly involved in interactions with BH3-only proteins (Fig. 6, A and B). In contrast, both the H1α and BH3 domains of Bak were involved in its association with Bim and PUMA (Fig. 6, C and D). The same differences were observed when the percentage of the corresponding BiFC-positive cells was analyzed (data not shown). Taken together, these results indicate that significant structural differences exist in the interaction of BH3-only proteins with either Bax or Bak, highlighting functional differences between these two proapoptotic proteins.
FIGURE 6.
Implication of BH3 and H1α domains of Bak and Bax in their interaction with Bim and PUMA. HeLa cells were transfected with vectors expressing the fusion proteins Bax-VC/Bim-VN (A), Bax-VC/PUMA-VN (B), Bak-VC/Bim-VN (C), or Bak-VC/PUMA-VN (D). Deletion mutants of Bax and Bak (lacking either the BH3 domain, ΔBH3, or the first α helix, ΔH1α) were transfected as indicated (white bars). A vector encoding mRFP was also included in the transfection. After transfection, cells were cultured for 24 h, trypsinized, and then Venus and mRFP fluorescence was analyzed by flow cytometry. Results are mean ± S.D. of four to seven independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Noxa Can Bind to Antiapoptotic and Proapoptotic Proteins
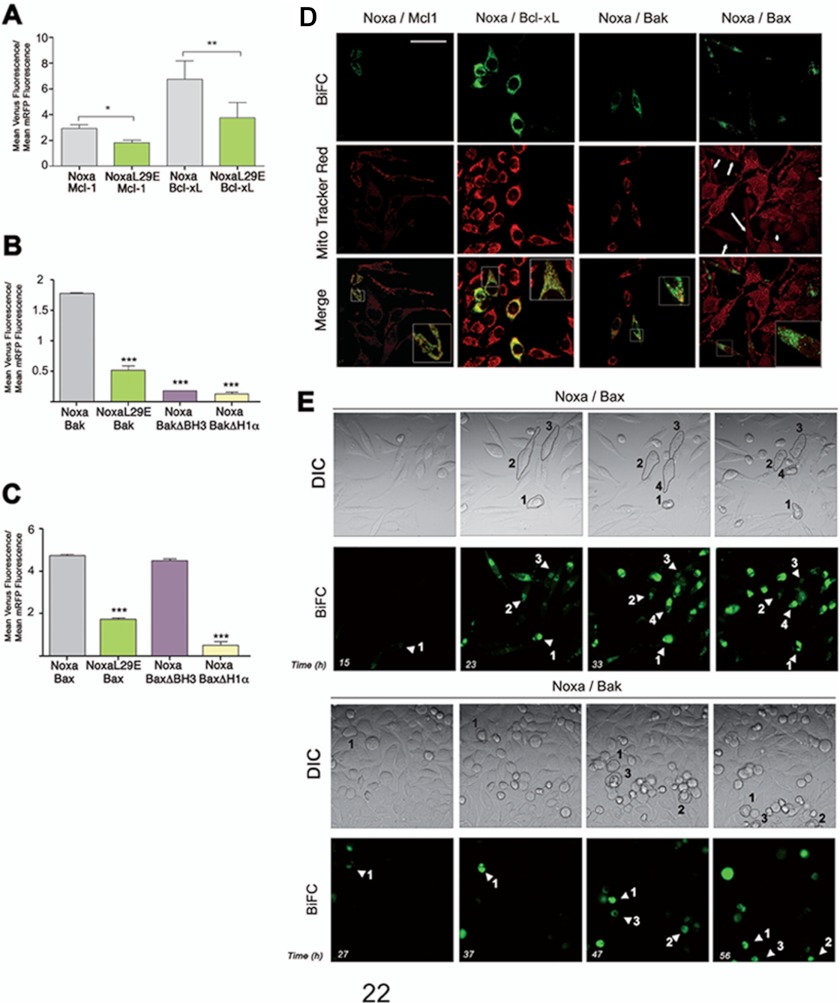
Similar to PUMA, Noxa is transcriptionally induced in response to certain apoptotic stimuli (38), and its overexpression induces cell death (39). Two recent reports have proposed that the BH3-only protein Noxa could also act as a direct activator of Bax and Bak (6, 7). We have analyzed the relevance of this finding in living cells by using the BiFC technique. As shown in Fig. 6, Noxa formed complexes with Mcl-1 and Bcl-xL (Fig. 7A), as expected, but also with Bak (B) and Bax (C). Mutation of Leu-29 to Glu abolished its binding to other proteins of the family (Fig. 7), confirming the implication of the BH3 domain in these interactions (7, 40). The H1α of Bax, but not its BH3 domain, was necessary for Noxa-Bax association (Fig. 7C). However, both regions were required for Bak to interact with Noxa (Fig. 7B), as observed for Bim and PUMA (Fig. 6, A and B). The Noxa-Mcl-1 and Noxa-Bcl-xL pairs exhibited a punctate pattern in the cytoplasm and partial mitochondrial localization (Fig. 7D). Noxa associated with multidomain proteins Bak and Bax seemed to localize exclusively at mitochondria (Fig. 7D). Cells positive for Venus fluorescence with the pair Noxa-Bak or Noxa-Bax presented reduced mitochondrial transmembrane potential (Fig. 7D, arrows) and time lapse microscopy showed that association of Noxa to Bax (supplemental movies Mov5NoxaBax and Mov6NoxaBax) and Bak (supplemental moviesMov7NoxaBak and Mov8NoxaBak) preceded cell death (Fig. 7, E and F).
FIGURE 7.
Interactions of Noxa with antiapoptotic proteins Mcl-1 and direct interaction with proapoptotic proteins Bak and Bax. A, B, and C, HeLa cells were cotransfected with vectors expressing the fusion protein Noxa-VN and Mcl-1, Bcl-xL, Bax, or Bak fused to the other half of Venus (VC). Deletion mutants lacking the BH3 domain (ΔBH3) or the single-substitution mutant (NoxaL29E) were transfected as indicated. A vector encoding mRFP was also included in the transfection. After transfection, cells were cultured for 24 h, trypsinized, and then Venus and mRFP fluorescence were analyzed by flow cytometry. Results are mean ± S.D. of four independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001. D, HeLa cells were seeded on coverslips and transfected as indicated in A, B, and C. 24 h after transfection, cells were stained with 50 nm MitoTracker Red, fixed, and analyzed by confocal microscopy as indicated under “Experimental Procedures.” Scale bar = 50 μm. The insets show the enlarged boxed regions. E and F, time lapse microscopy reveals the formation of the Noxa-Bax (E) and Noxa-Bak complexes (F) before cell death induction. Cells were plated on μ-Slide 8-well tissue culture plates and transfected with the vectors containing the Noxa-VN and Bax-VC/Bak-VC constructs. Cells were monitored for 72 h in a Leica AF6000 LX system, as indicated under “Experimental Procedures.” Images were acquired with a CCD camera (C9100-02, Hamamatsu) at 7-min intervals, and LAS AF software (Leica) was used to process the images. Also shown is a representative time lapse sequence from supplemental movies Mov5NoxaBax, Mov6NoxaBax, Mov7NoxaBak, and Mov8NoxaBak showing Venus fluorescence (lower panel) and phase-contrast images (upper panel). DIC, differential interference contrast.
Bcl-xL or Mcl-1 Overexpression Delays Interaction of BH3-only Proteins with Bax and Bak
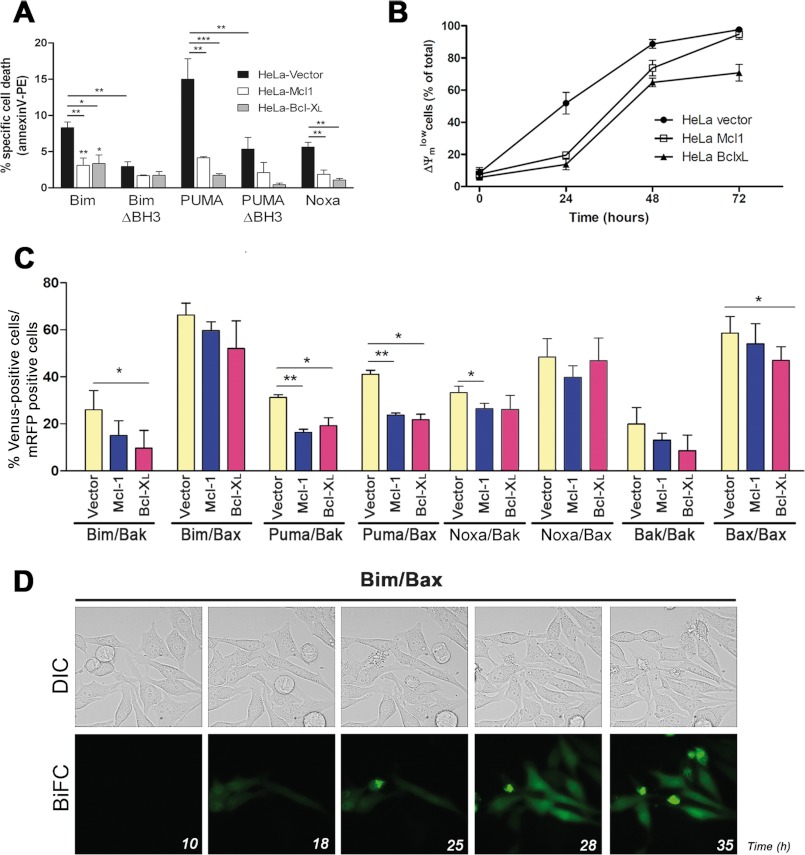
Interactions between BH3-only proteins and multidomain proteins were also analyzed in HeLa cells overexpressing either Bcl-xL or Mcl-1 to determine whether these antiapoptotic proteins affected the association of BH3-only proteins to Bax and Bak. We verified that both Bcl-xL and Mcl-1 proteins reduced cell death induced by transfection of Bim or PUMA-containing vectors (Fig. 8A). Overexpression of Bcl-xL or Mcl-1 also delayed the collapse of mitochondrial potential associated to etoposide-induced apoptosis (Fig. 8B). Fluorescence complementation due to interactions of either Bim or Noxa with Bak and of PUMA with either Bax or Bak was reduced in cells overexpressing Bcl-xL or Mcl-1 (Fig. 8C). Bim/Bax fluorescence also decreased, although the difference was not statistically significant. In all cases, interactions were not completely eliminated, suggesting that overexpression of antiapoptotic proteins at this level offered only a partial protection, delaying activation of Bax and Bak. These data were in agreement with the effect of Bcl-xL and Mcl-1 in etoposide-induced apoptosis (Fig. 8B). Also, the greater protection offered by Bcl-xL was in accord with its greater effect in disrupting the interactions of Bim or PUMA with Bax or Bak (Fig. 8C). Time lapse microscopy also demonstrated that overexpression of these antiapoptotic proteins delayed binding of BH3 proteins to Bax or Bak (Fig. 8D and supplemental moviesMov9Bcl-x LBimBax and Mov10Bcl-x LBimBax). As shown in Fig. 8D, the number of cells displaying a punctated Venus fluorescence pattern (mitochondrial Bim/Bax complexes) and apoptotic morphology was lower in cells overexpressing Bcl-xL than in control cells at equivalent times after transfection (Fig. 4D). These latter results agree with the fact that Bcl-xL or Mcl-1 protected cells from apoptosis for the first 24 h (Fig. 8B).
FIGURE 8.
Effect of Bcl-xL or Mcl-1 overexpression on the interactions of BH3-only proteins Bim and PUMA with Bak/Bax. A, HeLa-Vector, HeLa-Bcl-xL, or HeLa-Mcl-1 stable cells lines were transfected with vectors encoding different proapoptotic proteins or corresponding deletion mutants. A phosphatidylserine exposure analysis was performed by annexin V-PE staining and flow cytometry 24 h after transfection. Results are mean ± S.D. of three to four independent experiments. B, time course analysis of the percentage of cells with low mitochondrial membrane potential (ΔΨmlow) after treatment with 100 μm etoposide confirms a delay in cell death when cells overexpress antiapoptotic proteins. C, HeLa cells overexpressing antiapoptotic proteins Bcl-xL or Mcl-1 or containing the empty vector (HeLa-Vector) were cotransfected with the vector pairs indicated together with a vector encoding mRFP. D, time lapse microscopy reveals that overexpression of Bcl-xL delays translocation to the mitochondria of Bim-Bax pairs. HeLa- Bcl-xL cells were plated on μ-Slide 8-well tissue culture plates and transfected with the vectors containing the Bim-VN and Bax-VC constructs. Cells were monitored for 72 h in a Leica AF6000 LX system, as indicated under “Experimental Procedures.” Images were acquired with a CCD camera (C9100-02, Hamamatsu) at 7-min intervals. LAS AF software (Leica) was used to process the images. Also shown is a representative time lapse sequence from supplemental movies Mov9BclXlBimBax and Mov10BclXlBimBax showing phase-contrast images (upper panel) and Venus fluorescence (lower panel).
DISCUSSION
In the intrinsic pathway of apoptosis, death signals converge on Bax and Bak proteins to induce their activation, leading to mitochondrial permeabilization and release of proteins that execute cell death. Activation of Bax and Bak is carried out by BH3-only proteins, but the precise mechanism is still a matter of controversy. Two models have been proposed and extensively studied in recent years: the indirect (displacement) model and the direct model (41). Both models are mainly on the basis of the different binding preferences displayed among members of the Bcl-2 family. In this work, we have visualized interactions among proteins of the Bcl-2 family in living HeLa cells by using the BiFC technique. All main results were confirmed in HCT116 cells (data not shown). First, our results indicate that Mcl-1 and Bcl-xL bind to BH3-only proteins, although a fraction of these proteins also interacts with Bax or Bak. Yet, these results are compatible with the possibility that a certain amount of antiapoptotic proteins may keep in check activated Bak and Bax. This hypothesis would also agree with “mixed” models, such as the “embedded together” model (42) or the “unified” model (43). Other authors have also proposed that an improved description of how Bcl-2 proteins work should share features of both direct and indirect models (5, 8, 44). Anyway, mixed models also imply the direct activation of Bax and Bak by BH3-only proteins. Available data supporting the direct activation of Bax and Bak mainly rely on the use of BH3-peptides or recombinant proteins on cell-free systems, namely liposomes (6) or isolated mitochondria (8). Also, a recent genetic study has shown that the simultaneous absence of Bid, Bim, and PUMA proteins causes developmental abnormalities in mice, related to defective apoptosis, as well as resistance to apoptosis ex vivo induced by different stimuli such as dexamethasone, etoposide, and cytokine withdrawal (33). According to this study, the phenotype of triple knockout mice supports the role of Bid, Bim, and PUMA as direct activators in the intrinsic cell death pathway. Another genetic study has proved that the function of BH3-only proteins depends not only on their ability to displace antiapoptotic members of the Bcl-2 family but also on their capacity to bind Bax (44). Mice expressing mutant forms of Bim in which the BH3 domain was replaced by that of Bad, PUMA, or Noxa, accumulate white blood cells and splenocytes, indicating a reduced proapoptotic activity of mutant forms of Bim (44). These authors demonstrate that Bim can co-immunoprecipitate with Bax and this association, in addition to Bim engagement of prosurvival proteins, would mediate its proapoptotic activity. However, data supporting a direct interaction between putative activators and multidomain proteins in living cells are scarce. To our knowledge, this is the first report showing a direct interaction of the full-length BH3-only proteins with Bax and Bak in living mammalian cells. Two recent reports have studied interactions between antiapoptotic proteins of the Bcl-2 family and BH3-only proteins in living cells by using FLIM FRET (45) or redistribution assays (46), but an association of these latter to Bax or Bak was not addressed. Aranovich et al. (45) found that interactions between proteins of the Bcl-2 family in living cells were similar to previous in vitro observations, but, interestingly, they observed differences in the case of Bim, highlighting the need to study these interactions in their cellular context. BiFC analysis has also allowed us to determine the subcellular localization of complexes and to visualize the translocation of Bim/Bax pairs from the cytosol to mitochondria. In the absence of death signals, Bim is found in cytosol, associated to the LC8 subunit of the motor protein dynein in several cell types (16). During apoptosis, Bim translocates to mitochondria (16), where it exerts its proapoptotic function. Our present results show that Bim and Bax first associate in cytosol, and then Bim/Bax complexes translocate to mitochondria. Current models propose that activator BH3 proteins that normally reside in the cytosol first translocate to the mitochondrial membrane and then bind to Bax (41). It is conceivable that current biochemical techniques do not allow detection of the Bim-Bax complexes that rapidly translocate to mitochondria, as demonstrated by time lapse microscopy. Bim is blocked in healthy cells through binding to Mcl-1, but some apoptotic stimuli, e.g. glucocorticoids (47, 48), increase Bim levels exceeding the buffering capacity of Mcl-1. This frees Bim that can bind and activate Bax and Bak. It has also been proposed that PUMA may be a direct activator of Bax and Bak, mainly on the basis of data from cell-free experiments (36). Our present results confirm that PUMA can bind to multidomain proteins in intact cells. However, a PUMA mutant lacking the BH3 domain was still able to associate with Bax, although it was devoid of apoptotic activity (17, 18). In contrast, the BH3-deletion mutant of PUMA did not bind to Mcl-1 and only weakly to Bcl-xL. These results could indicate that PUMA can act both as a weak, direct activator and as a sensitizer, this latter mechanism being more relevant for its apoptotic function. Alternatively, wild-type PUMA might efficiently interact during apoptosis with multidomain proapoptotic and antiapoptotic proteins via its BH3 domain. PUMAΔBH3 mutant could still interact with Bax via another different region, but this interaction would be unproductive to apoptosis. In support of this latter explanation, it has been demonstrated that PUMA efficiently interacts via its BH3 domain with H1α in Bax (36). On the other hand, although Noxa has long been considered as a sensitizer, recent reports have shown that it can bind in vitro to Bax and Bak (6). We have visualized such interactions in living cells, supporting the role of Noxa as an activator of both Bax and Bak.
Using mutant fusion proteins, we also studied the participation of different domains of Bax and Bak in their interaction with anti- and proapoptotic proteins. Although Bax and Bak share a high sequence homology and function as “effectors” for mitochondrial outer membrane permeabilization, they still present some differences in their mechanisms of action. Our studies herein corroborate these differences at protein interaction level. Both proteins exhibited a similar partner profile, but the domains implicated in protein-protein interactions were different. Interactions between Bax and BH3-only proteins depended on the presence of H1α, according to the proposed role of this domain as a “receptor” for activator BH3-only proteins (35–37). Antiapoptotic proteins could also bind to Bax through a region including this H1α, thus preventing interaction of Bax with activator BH3-only proteins (49). The fact that overexpression of Bcl-xL or Mcl-1 reduced these interactions supports this hypothesis. Importantly, our results unveil functional differences between Bax and Bak. Contrary to the observed with Bax, both the H1α and the BH3 domains of Bak were essential for the binding of activator BH3 proteins, suggesting that the Bak-BH3-only and Bax-BH3-only complexes are structurally different. Mcl-1 and Bcl-xL would prevent Bak oligomerization by keeping in check “activated” Bak BH3 domains, but inhibition of Bax by antiapoptotic protein could affect different domains, such as the H1α involved in activation by BH3-only proteins.
In conclusion, with the help of the minimally invasive BiFC technique, we were able to visualize interactions between different Bcl-2 family proteins in living cells. The analysis of these interactions indicates a more prominent role than believed for most BH3-only proteins in the activation of Bax and Bak. The results also suggest that a complex network of interactions regulates the biological function of Bcl-2 family proteins, according to recently proposed models that include both positive and negative regulation of Bax and Bak during apoptosis.
Acknowledgments
We thank María Royo (Microscopy and Image Service, Instituto de Investigación Sanitaria de Aragón) for excellent technical assistance and help with the live cell fluorescence microscopy system. We also thank Karen Vousden (Beatson Institute, Glasgow, United Kingdom), Cristina Muñoz-Pinedo (IDIBELL, Barcelona, Spain), and José Alberto Carrodeguás (Instituto Universitario de Investigación en Biocomputación y Física de Sistemas Complejos, BIFI, University of Zaragoza) for plasmids.
This study was supported by Ministerio de Ciencia e Innovación Grants SAF2007-60748, SAF2010-14920, and Red Temática de Investigación Cooperativa en Cáncer-Instituto de Salud Carlos III Grant RD06/0020, and Gobierno de Aragón/Fondo Social Europeo Grant B16.

This article contains supplemental movies 1–10.
- BH
- Bcl-2 homology
- BiFC
- bimolecular fluorescence complementation
- VN
- Venus protein N-terminal fragment
- VC
- Venus protein C-terminal fragment
- mRFP
- monomeric Red Fluorescent Protein
- PE
- phycoerythrin
- TMRE
- tetramethylrhodamine
- Z-VAD-fmk
- Z- Val-Ala-DL-Asp-fluoromethylketone.
REFERENCES
- 1. Youle R. J., Strasser A. (2008) The BCL-2 protein family. Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9, 47–59 [DOI] [PubMed] [Google Scholar]
- 2. Wei M. C., Zong W.-X., Cheng E. H., Lindsten T., Panoutsakopoulou V., Ross A. J., Roth K. A., MacGregor G. R., Thompson C. B., Korsmeyer S. J. (2001) Proapoptotic BAX and BAK. A requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. López-Royuela N., Pérez-Galán P., Galán-Malo P., Yuste V. J., Anel A., Susín S. A., Naval J., Marzo I. (2010) Different contribution of BH3-only proteins and caspases to doxorubicin-induced apoptosis in p53-deficient leukemia cells. Biochem. Pharmacol. 79, 1746–1758 [DOI] [PubMed] [Google Scholar]
- 4. Buckbinder L., Talbott R., Velasco-Miguel S., Takenaka I., Faha B., Seizinger B. R., Kley N. (1995) Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature 377, 646–649 [DOI] [PubMed] [Google Scholar]
- 5. Villunger A., Labi V., Bouillet P., Adams J., Strasser A. (2011) Can the analysis of BH3-only protein knockout mice clarify the issue of “direct versus indirect” activation of Bax and Bak[quest]. Cell Death Differ. 18, 1545–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Du H., Wolf J., Schafer B., Moldoveanu T., Chipuk J. E., Kuwana T. (2011) BH3 domains other than Bim and Bid can directly activate Bax/Bak. J. Biol. Chem. 286, 491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dai H., Smith A., Meng X. W., Schneider P. A., Pang Y. P., Kaufmann S. H. (2011) Transient binding of an activator BH3 domain to the Bak BH3-binding groove initiates Bak oligomerization. J. Cell Biol. 194, 39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuwana T., Bouchier-Hayes L., Chipuk J. E., Bonzon C., Sullivan B. A., Green D. R., Newmeyer D. D. (2005) BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Molecular Cell 17, 525–535 [DOI] [PubMed] [Google Scholar]
- 9. Kerppola T. K. (2008) Bimolecular fluorescence complementation (BiFC) analysis as a probe of protein interactions in living cells. Annu. Rev. Biophys. 37, 465–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee Y., Lee J., Kwon I., Nakajima Y., Ohmiya Y., Son G. H., Lee K. H., Kim K. (2010) Coactivation of the CLOCK-BMAL1 complex by CBP mediates resetting of the circadian clock. J. Cell Sci. 123, 3547–3557 [DOI] [PubMed] [Google Scholar]
- 11. Wang R., Li Q., Helfer C. M., Jiao J., You J. (2012) Bromodomain protein Brd4 Associated with acetylated chromatin is important for maintenance of higher-order chromatin structure. J. Biol. Chem. 287, 10738–10752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bouchier-Hayes L., Oberst A., McStay G. P., Connell S., Tait S. W., Dillon C. P., Flanagan J. M., Beere H. M., Green D. R. (2009) Characterization of cytoplasmic caspase-2 activation by induced proximity. Mol. Cell 35, 830–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yivgi-Ohana N., Eifer M., Addadi Y., Neeman M., Gross A. (2011) Utilizing mitochondrial events as biomarkers for imaging apoptosis. Cell Death Dis. 2, e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kerppola T. K. (2006) Visualization of molecular interactions by fluorescence complementation. Nat. Rev. Mol. Cell Biol. 7, 449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee J., Lee H. J., Shin M. K., Ryu W. S. (2004) Versatile PCR-mediated insertion or deletion mutagenesis. BioTechniques 36, 398–400 [DOI] [PubMed] [Google Scholar]
- 16. Puthalakath H., Huang D. C., O'Reilly L. A., King S. M., Strasser A. (1999) The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell 3, 287–296 [DOI] [PubMed] [Google Scholar]
- 17. Nakano K., Vousden K. H. (2001) PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 7, 683–694 [DOI] [PubMed] [Google Scholar]
- 18. Yu J., Zhang L., Hwang P. M., Kinzler K. W., Vogelstein B. (2001) PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell 7, 673–682 [DOI] [PubMed] [Google Scholar]
- 19. Czabotar P. E., Lee E. F., van Delft M. F., Day C. L., Smith B. J., Huang D. C., Fairlie W. D., Hinds M. G., Colman P. M. (2007) Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc. Natl. Acad. Sci. U.S.A. 104, 6217–6222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kazi A., Sun J., Doi K., Sung S. S., Takahashi Y., Yin H., Rodriguez J. M., Becerril J., Berndt N., Hamilton A. D., Wang H. G., Sebti S. M. (2011) The BH3 α-helical mimic BH3-M6 disrupts Bcl-X(L), Bcl-2, and MCL-1 protein-protein interactions with Bax, Bak, Bad, or Bim and induces apoptosis in a Bax- and Bim-dependent manner. J. Biol. Chem. 286, 9382–9392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ku B., Liang C., Jung J. U., Oh B. H. (2011) Evidence that inhibition of BAX activation by BCL-2 involves its tight and preferential interaction with the BH3 domain of BAX. Cell Res. 21, 627–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen L., Willis S. N., Wei A., Smith B. J., Fletcher J. I., Hinds M. G., Colman P. M., Day C. L., Adams J. M., Huang D. C. (2005) Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 17, 393–403 [DOI] [PubMed] [Google Scholar]
- 23. Cuconati A., Mukherjee C., Perez D., White E. (2003) DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev. 17, 2922–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leu J. I., Dumont P., Hafey M., Murphy M. E., George D. L. (2004) Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat. Cell Biol. 6, 443–450 [DOI] [PubMed] [Google Scholar]
- 25. Willis S. N., Chen L., Dewson G., Wei A., Naik E., Fletcher J. I., Adams J. M., Huang D. C. (2005) Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 19, 1294–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Germain M., Milburn J., Duronio V. (2008) MCL-1 inhibits BAX in the absence of MCL-1/BAX Interaction. J. Biol. Chem. 283, 6384–6392 [DOI] [PubMed] [Google Scholar]
- 27. Sedlak T. W., Oltvai Z. N., Yang E., Wang K., Boise L. H., Thompson C. B., Korsmeyer S. J. (1995) Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc. Natl. Acad. Sci. U.S.A. 92, 7834–7838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang R., Brattain M. G. (2007) The maximal size of protein to diffuse through the nuclear pore is larger than 60 kDa. FEBS Lett. 581, 3164–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoetelmans R., van Slooten H. J., Keijzer R., Erkeland S., van de Velde C. J., Dierendonck J. H. (2000) Bcl-2 and Bax proteins are present in interphase nuclei of mammalian cells. Cell Death Differ. 7, 384–392 [DOI] [PubMed] [Google Scholar]
- 30. Villunger A., Michalak E. M., Coultas L., Müllauer F., Böck G., Ausserlechner M. J., Adams J. M., Strasser A. (2003) p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 302, 1036–1038 [DOI] [PubMed] [Google Scholar]
- 31. Jabbour A. M., Heraud J. E., Daunt C. P., Kaufmann T., Sandow J., O'Reilly L. A., Callus B. A., Lopez A., Strasser A., Vaux D. L., Ekert P. G. (2009) Puma indirectly activates Bax to cause apoptosis in the absence of Bid or Bim. Cell Death Differ. 16, 555–563 [DOI] [PubMed] [Google Scholar]
- 32. Gallenne T., Gautier F., Oliver L., Hervouet E., Noël B., Hickman J. A., Geneste O., Cartron P. F., Vallette F. M., Manon S., Juin P. (2009) Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members. J. Cell Biol. 185, 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ren D., Tu H. C., Kim H., Wang G. X., Bean G. R., Takeuchi O., Jeffers J. R., Zambetti G. P., Hsieh J. J., Cheng E. H. (2010) BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science 330, 1390–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yee K. S., Vousden K. H. (2008) Contribution of membrane localization to the apoptotic activity of PUMA. Apoptosis 13, 87–95 [DOI] [PubMed] [Google Scholar]
- 35. Gavathiotis E., Suzuki M., Davis M. L., Pitter K., Bird G. H., Katz S. G., Tu H. C., Kim H., Cheng E. H., Tjandra N., Walensky L. D. (2008) BAX activation is initiated at a novel interaction site. Nature 455, 1076–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cartron P. F., Gallenne T., Bougras G., Gautier F., Manero F., Vusio P., Meflah K., Vallette F. M., Juin P. (2004) The first α helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol. Cell 16, 807–818 [DOI] [PubMed] [Google Scholar]
- 37. Gavathiotis E., Reyna D. E., Davis M. L., Bird G. H., Walensky L. D. (2010) BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol. Cell 40, 481–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Puthalakath H., Strasser A. (2002) Keeping killers on a tight leash. Transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Diff. 9, 505–512 [DOI] [PubMed] [Google Scholar]
- 39. Oda E., Ohki R., Murasawa H., Nemoto J., Shibue T., Yamashita T., Tokino T., Taniguchi T., Tanaka N. (2000) Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288, 1053–1058 [DOI] [PubMed] [Google Scholar]
- 40. Pang Y. P., Dai H., Smith A., Meng X. W., Schneider P. A., Kaufmann S. H. (2012) Bak conformational changes induced by ligand binding. Insight into BH3 domain binding and Bak homo-oligomerization. Sci. Rep. 2, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shamas-Din A., Brahmbhatt H., Leber B., Andrews D. W. (2011) BH3-only proteins. Orchestrators of apoptosis. Biochim. Biophys. Acta 1813, 508–520 [DOI] [PubMed] [Google Scholar]
- 42. Leber B., Lin J., Andrews D. W. (2007) Embedded together. The life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis 12, 897–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Llambi F., Moldoveanu T., Tait S. W., Bouchier-Hayes L., Temirov J., McCormick L. L., Dillon C. P., Green D. R. (2011) A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol. Cell 44, 517–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mérino D., Giam M., Hughes P. D., Siggs O. M., Heger K., O'Reilly L. A., Adams J. M., Strasser A., Lee E. F., Fairlie W. D., Bouillet P. (2009) The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J. Cell Biol. 186, 355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aranovich A., Liu Q., Collins T., Geng F., Dixit S., Leber B., Andrews D. W. (2012) Differences in the mechanisms of proapoptotic BH3 proteins binding to Bcl-XL and Bcl-2 quantified in live MCF-7 cells. Mol. Cell 45, 754–763 [DOI] [PubMed] [Google Scholar]
- 46. Wong C., Anderson D. J., Lee E. F., Fairlie W. D., Ludlam M. J. (2012) Direct visualization of Bcl-2 family protein interactions using live cell fluorescent protein redistribution assays. Cell Death Dis. 3, e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Erlacher M., Michalak E. M., Kelly P. N., Labi V., Niederegger H., Coultas L., Adams J. M., Strasser A., Villunger A. (2005) BH3-only proteins Puma and Bim are rate-limiting for γ-radiation- and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood 106, 4131–4138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. López-Royuela N., Balsas P., Galán-Malo P., Anel A., Marzo I., Naval J. (2010) Bim is the key mediator of glucocorticoid-induced apoptosis and of its potentiation by rapamycin in human myeloma cells. Biochim. Biophys. Acta 1803, 311–322 [DOI] [PubMed] [Google Scholar]
- 49. Ding J., Zhang Z., Roberts G. J., Falcone M., Miao Y., Shao Y., Zhang X. C., Andrews D. W., Lin J. (2010) Bcl-2 and Bax interact via the BH1–3 groove-BH3 motif interface and a novel interface involving the BH4 motif. J. Biol. Chem. 285, 28749–28763 [DOI] [PMC free article] [PubMed] [Google Scholar]