Background: Mitochondrial glutathione transport has not been extensively studied within the CNS.
Results: Cerebellar neurons and astrocytes use distinct mechanisms of mitochondrial glutathione transport.
Conclusion: Inhibition of a single mitochondrial glutathione transporter renders neurons more susceptible to oxidative and nitrosative stress.
Significance: Mitochondrial glutathione transport is essential to protect neurons from oxidative and nitrosative stress conditions common to neurodegenerative diseases.
Keywords: Glutathione; Mitochondria; Mitochondrial Transport; Nitrosative Stress; Oxidative Stress; 2-Oxoglutarate Carrier (OGC, Slc25a11); Dicarboxylate Carrier (DIC, Slc25a10); Mitochondrial Glutathione Transport; Mitochondrial Oxidative Stress
Abstract
Mitochondrial oxidative stress significantly contributes to the underlying pathology of several devastating neurodegenerative disorders. Mitochondria are highly sensitive to the damaging effects of reactive oxygen and nitrogen species; therefore, these organelles are equipped with a number of free radical scavenging systems. In particular, the mitochondrial glutathione (GSH) pool is a critical antioxidant reserve that is derived entirely from the larger cytosolic pool via facilitated transport. The mechanism of mitochondrial GSH transport has not been extensively studied in the brain. However, the dicarboxylate (DIC) and 2-oxoglutarate (OGC) carriers localized to the inner mitochondrial membrane have been established as GSH transporters in liver and kidney. Here, we investigated the role of these carriers in protecting neurons from oxidative and nitrosative stress. Immunoblot analysis of DIC and OGC in primary cultures of rat cerebellar granule neurons (CGNs) and cerebellar astrocytes showed differential expression of these carriers, with CGNs expressing only DIC and astrocytes expressing both DIC and OGC. Consistent with these findings, butylmalonate specifically reduced mitochondrial GSH in CGNs, whereas both butylmalonate and phenylsuccinate diminished mitochondrial GSH in astrocytes. Moreover, preincubation with butylmalonate but not phenylsuccinate significantly enhanced susceptibility of CGNs to oxidative and nitrosative stressors. This increased vulnerability was largely prevented by incubation with cell-permeable GSH monoethylester but not malate. Finally, knockdown of DIC with adenoviral siRNA also rendered CGNs more susceptible to oxidative stress. These findings demonstrate that maintenance of the mitochondrial GSH pool via sustained mitochondrial GSH transport is essential to protect neurons from oxidative and nitrosative stress.
Introduction
Mitochondrial oxidative stress and mitochondrial dysfunction are convergence points in the underlying pathologies of several devastating neurodegenerative disorders (1). For instance, previous studies have provided significant evidence for increased mitochondrial oxidative damage and organelle dysfunction in Parkinson disease (PD)2 and amyotrophic lateral sclerosis (ALS) (2–4). Overall, mitochondrial dysfunction leads to an increase in reactive oxygen species (ROS), which damage mitochondrial DNA, lipids, and proteins (1). Moreover, ROS-induced alterations in mitochondrial membrane potential and permeability can trigger a positive feedback mechanism known as ROS-induced ROS release, which has the potential to propagate oxidative stress signals from mitochondria to mitochondria, thus exacerbating cellular injury (5). These findings indicate that mitochondrial free radical scavenging systems, such as the essential antioxidant glutathione (GSH), are critical to protect neuronal cells from mitochondrial oxidative stress.
GSH exists as a discrete mitochondrial pool and a much larger cytosolic store. The mitochondrial GSH pool is considered to be an indispensable antioxidant reservoir. For example, selective depletion of the mitochondrial GSH pool within cerebellar granule neurons (CGNs) induced opening of the mitochondrial permeability transition pore, increased ROS production, and caused significant cell death, whereas depletion of the cytosolic GSH pool did not elicit these deleterious effects (6). In a similar manner, specific depletion of mitochondrial GSH in astrocytes rendered these glial cells more vulnerable to both oxidative and nitrosative stress (7). Based on these previous studies, mitochondrial GSH appears to be a critical antioxidant that supports both glial and neuronal cell viability.
Several factors indicate that GSH transport into mitochondria occurs via a facilitated process. These factors include a lack of the enzymes necessary for GSH synthesis within mitochondria, the negative potential of the inner mitochondrial membrane, the net negative charge of GSH at physiological pH, and the approximately equal concentrations of GSH in the cytosolic and mitochondrial compartments (8, 9). Previous studies have shown that two inner mitochondrial membrane anion transporters, the dicarboxylate carrier (DIC, Slc25a10) and the 2-oxoglutarate carrier (OGC, Slc25a11), are capable of transporting GSH into renal and hepatic mitochondria (9–11). Mechanisms of mitochondrial GSH transport in the brain have only recently been investigated. Using isolated mitochondria from whole brain, one study determined that mitochondrial GSH transport may depend on the function of the tricarboxylate carrier (12). A second study showed that cortical neurons and astrocytes express both DIC and OGC. However, in isolated mitochondria from whole cortical tissue, only the activity of DIC appeared to influence mitochondrial GSH content (13). To date, the mechanism of mitochondrial GSH transport utilized by neurons specifically has not been examined. Furthermore, the effects of inhibiting either DIC- or OGC-dependent mitochondrial GSH transport on neuronal susceptibility to oxidative or nitrosative stress are unknown. Here, we demonstrate a key role for DIC-dependent mitochondrial GSH transport in the maintenance of the mitochondrial GSH pool and protection of CGNs from oxidative and nitrosative stress.
EXPERIMENTAL PROCEDURES
Materials
Butylmalonic acid, phenylsuccinic acid, primary antibody against β-tubulin, poly-l-lysine, GSH monoethylester, l-malate, and Hoechst stain were purchased from Sigma-Aldrich. HA14-1 was acquired from Alexis Biochemicals (Enzo Life Sciences, Plymouth Meeting, PA). The GSH assay kit was purchased from Oxford Biomedical (Rochester Hills, MI). Anti-MAP2 was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The mitochondrial/cytosolic fractionation kit was from Biovision (Mountain View, CA). Antibody against Cox-IV was obtained from Cell Signaling (Beverly, MA). Primary rat astrocyte cultures were purchased from ScienCell (Carlsbad, CA). Anti-GFAP, anti-GAPDH, anti-DIC (Slc25a10), and anti-OGC (Slc25a11) antibodies were purchased from Abcam (Cambridge, MA). Sodium nitroprusside (SNP) was received from Calbiochem. Horseradish peroxidase-conjugated secondary antibodies and reagents for enhanced chemiluminescence detection were purchased from Amersham Biosciences. The ViraBind® adenovirus miniprep kit and QuickTiterTM adenovirus titer immunoassay kit were purchased from Cell Biolabs (San Diego, CA). Adenoviral siRNA1 and siRNA2 against DIC and the scrambled adenoviral control were constructed as described previously (14). siRNA1 targets DIC from bp 119GCU UCG AAU GAC UGG AAU G, and siRNA2 targets DIC from bp 354GCA GAU UUG GUC AAU GUC A, whereas the scrambled siRNA sequence (GAG ACC CTA TCC GTG ATT A) has no known gene homology (14).
CGN Cell Culture
CGNs were isolated and cultured from P7 Sprague-Dawley rat pups based on the methods described previously (15). CGNs were maintained in basal modified Eagle's medium, containing 10% heat inactivated fetal bovine serum, 25 mm potassium chloride, 2 mm l-glutamine and 100 units/ml/100 μg/ml penicillin/streptomycin at 37 °C in 10% CO2.
Primary Rat Cerebellar Astrocyte Cell Culture
Primary rat cerebellar astrocyte cultures were grown on poly-l-lysine-coated flasks in basal modified Eagle's medium containing 10% heat-inactivated fetal bovine serum, 2 mm l-glutamine, and 100 units/ml/100 μg/ml penicillin/streptomycin. Cells were plated on poly-l-lysine-coated 6-well plates and grown to ∼80% confluence at 37 °C in 10% CO2, at which time cells were utilized for the described experiments.
Immunofluorescence Staining
CGNs and/or astrocytes were treated as described under “Results” and fixed with 4% paraformaldehyde. Cells stained for β-tubulin were blocked and permeabilized in 5% BSA, 0.2% Triton X-100 in PBS for 1 h. Cells were then inbucated with primary antibody overnight at 4 °C, at a dilution of 1:500 in 2% BSA, 0.2% Triton X-100 in PBS. Cells were then washed five times in PBS and incubated in secondary antibody for 1 h at room temperature at a dilution of 1:250 in 2% BSA, 0.2% Triton X-100 in PBS. Cells were then washed five times and placed in anti-quench solution. For cells with only nuclei stained, cells were fixed as above and incubated with Hoechst dye (8 μg/ml) in PBS for 1 h at room temperature. Cells were then washed five times with PBS and placed in anti-quench. Images were captured using a Zeiss Axiovert-zoom inverted epifluorescence microscope.
Mitochondrial/Cytosolic Fractionation
Cells were treated as indicated under “Results” or in the figure legends, after which the medium was aspirated, and cells were washed once in ice-cold PBS, pH 7.4. 200 μl of cytosolic buffer (provided in the kit, diluted 1:5 in double-distilled H2O, with added protease inhibitor mixture and 1 mm DTT as per the manufacturer's recommendations) was added to the cells and allowed to incubate on ice for 20 min. Cells were scraped and harvested and then homogenized with 40 passes of a Dounce homogenizer. Samples were spun down at 720 relative centrifugal force for 10 min at 4 °C. The supernatant from each sample was transferred to a new tube labeled “mitochondrial fraction” and spun at 10,000 relative centrifugal force for 30 min at 4 °C. The supernatant was then transferred to a new tube labeled “cytosolic fraction,” and the pellet in the mitochondrial fraction tube was resuspended in 150 μl of mitochondrial buffer (provided in the kit, with added protease inhibitor mixture and 1 mm DTT as per the manufacturer's recommendations). Samples were measured immediately for GSH content as described below.
GSH Assay
GSH was measured using an assay kit (DTNB) from Oxford Biomedical Research, following the manufacturer's protocol. All GSH measurements were normalized to protein concentration. GSH concentrations were determined using a GSH standard in the DTNB assay and reported as nmol of GSH/mg of protein. The percentage of mitochondrial GSH versus and percentage of cytosolic GSH was calculated using the slope of the kinetic assay and the appropriate dilution factor.
Cell Lysis
CGNs were infected with the indicated adenoviral construct (shown under “Results”) as described below, after which CGNs were washed in 1× PBS over ice and incubated with 1% Triton X-100 in Wahl buffer (20 mm HEPES, pH 7.4, 50 mm NaCl, 1 mm EGTA, 5 mm β-glycerophosphate, 30 mm sodium pyrophosphate, 100 μm sodium orthovanadate, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) for 10 min. Cells were harvested by scraping and spun down at 10,000 rpm for 2 min, and the cell supernatant was removed.
Immunoblot Analysis
Following mitochondrial/cytosolic fractionation or cell lysis, a BCA protein assay (Pierce) was used to determine protein concentration. Equal concentrations of protein were resolved by SDS-PAGE and immunoblotted as described previously (15).
Purification and Titering of Adenoviral Constructs
Adenoviral constructs were amplified in a HEK 293 AD cell line by adding 10 μl of the respective adenovirus to 4 ml of medium for 1.5 h, after which 16 ml of medium was added to the flask, and cells were incubated at 5% CO2 and 37 °C for 48 h or until the beginning signs of cytotoxicity were observed. Cells were harvested, and the adenoviral constructs were purified as per the manufacturer's recommendations in the ViraBind® adenovirus miniprep kit. Adenoviral constructs were titered as per the manufacturer's recommendations in the QuickTiterTM adenovirus titer immunoassay kit.
Adenoviral Knockdown of DIC
CGNs were infected with adenoviral constructs at a multiplicity of infection of 100 for 48 h, after which experiments were completed.
Statistical Analysis
One-way analysis of variance tests followed by post hoc Tukey's tests were performed to determine statistical significance between data sets. A p value of <0.05 was considered statistically significant. A minimum of three independent experiments were completed for each figure.
RESULTS
Differential Expression of the Mitochondrial GSH Transporters, OGC and DIC, within CGNs and Cerebellar Astrocytes
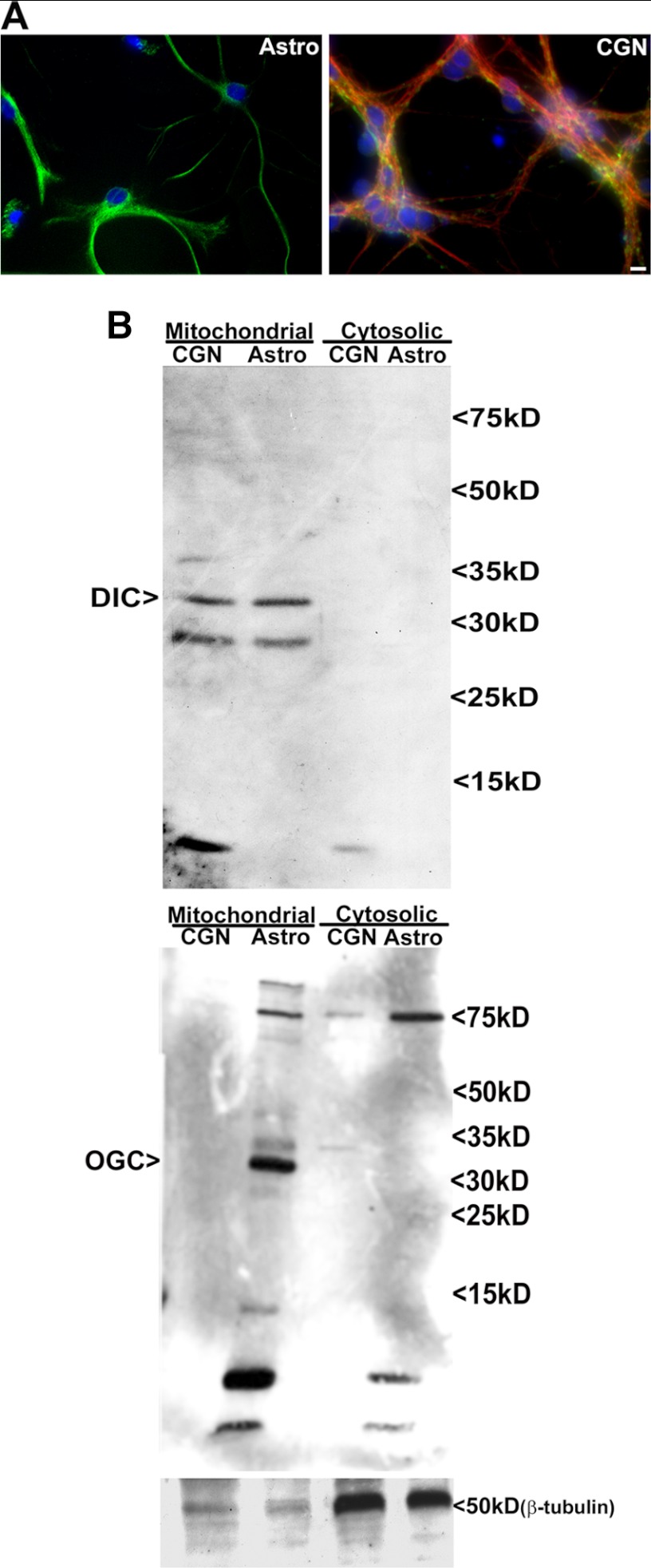
To determine the specific cellular expression of the previously identified mitochondrial GSH transporters within the cerebellum, we employed primary rat CGN and primary rat cerebellar astrocyte cultures (Fig. 1A). We first completed immunoblots against DIC and OGC on mitochondrial fractions of these primary cultures. Here, we show that CGNs express DIC but no detectable OGC. In contrast, cerebellar astrocytes express both DIC and OGC (Fig. 1B).
FIGURE 1.
Differential expression of the mitochondrial GSH transporters, OGC and DIC, within CGNs and cerebellar astrocytes. A, primary rat CGN cultures were fixed and stained for β-tubulin (red), MAP2 (green), and Hoechst (blue). Primary rat astrocyte cultures were fixed and stained for GFAP (green) and Hoechst (blue). B, CGN and cerebellar astrocyte cultures were subfractionated into mitochondrial and cytoplasmic fractions using differential centrifugation as described under “Experimental Procedures.” Protein lysates from the mitochondrial and cytosolic fractions were resolved using SDS-PAGE and immunoblotted for DIC and OGC. Immunoblotting for β-tubulin was used as a measure of fraction purity.
Inhibition of DIC but Not OGC Specifically Reduces the Mitochondrial GSH Pool in CGNs
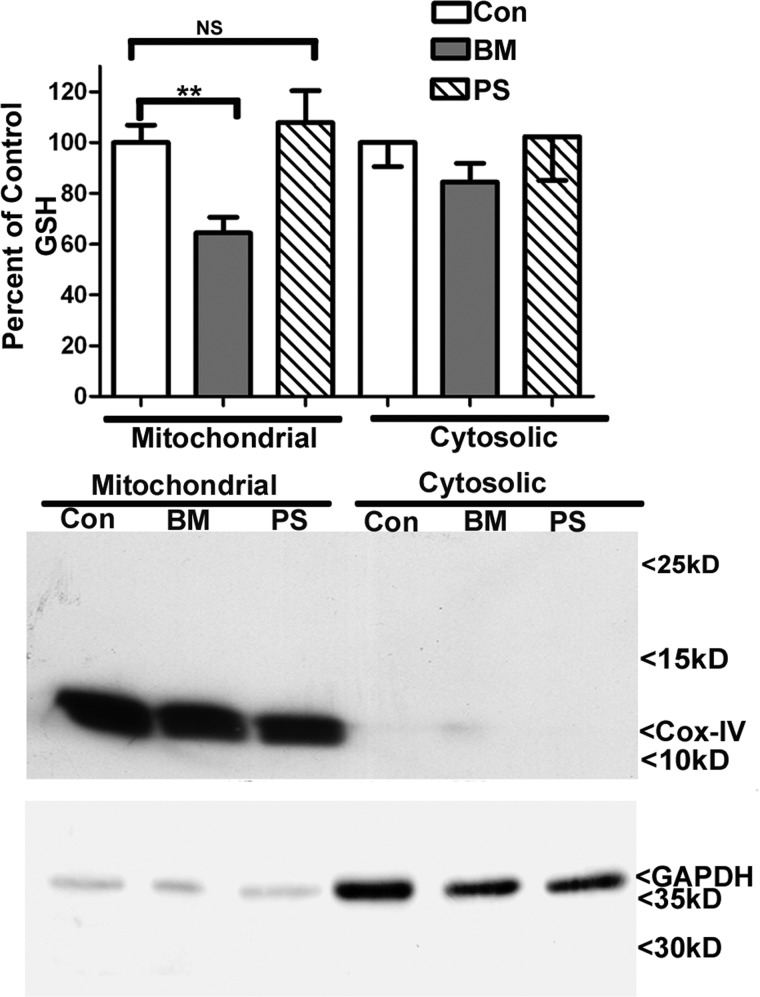
We next examined the effects of butylmalonate and phenylsuccinate, DIC and OGC inhibitors, respectively, on mitochondrial GSH levels within CGNs. Dose responses were initially completed in order to establish a maximal non-cytotoxic level of each of these inhibitors (data not shown). Accordingly, CGNs were treated with 5 mm butylmalonate or 5 mm phenylsuccinate overnight. After subfractionation of CGNs into mitochondrial and cytoplasmic fractions, GSH was quantified using a DTNB colorimetric assay. CGNs showed a significant reduction of the mitochondrial GSH pool when treated with butylmalonate, but they displayed no decrement in the cytosolic pool (Fig. 2). Interestingly, phenylsuccinate had no discernible effect on either the mitochondrial or cytosolic pools of GSH within CGNs (Fig. 2). These results are consistent with the immunoblot analysis, which demonstrated that the CGNs expressed DIC but not OGC (Fig. 1B). Immunoblot analysis of the subcellular fractions for cytochrome c oxidase IV and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) verified the purity of the mitochondrial and cytosolic fractions, respectively (Fig. 2). Furthermore, mitochondrial GSH comprised 14.3 ± 2.7% of total cellular GSH at a concentration of 6.9 ± 1.10 nmol/mg, consistent with previous studies (mean ± S.E., n = 6) (16–19).
FIGURE 2.
Inhibition of DIC but not OGC specifically reduces the mitochondrial GSH pool in CGNs. CGN cultures were treated with vehicle (DMSO; Con), 5 mm butylmalonate (BM), or 5 mm phenylsuccinate (PS) overnight. Cells were then subfractionated into mitochondrial and cytoplasmic fractions using differential centrifugation. Mitochondrial and cytosolic fractions were measured for total GSH content using a DTNB assay. Data are represented as a percentage of control GSH. **, p < 0.01 compared with control; NS, not significant; n = 4 experiments with each treatment performed in duplicate. Protein lysates were resolved using SDS-PAGE and immunoblotted for cytochrome c oxidase IV (Cox-IV) and GAPDH to indicate pure fractionations. Error bars, S.E.
Inhibition of DIC or OGC Function Specifically Decreases the Mitochondrial GSH Pool in Cerebellar Astrocytes
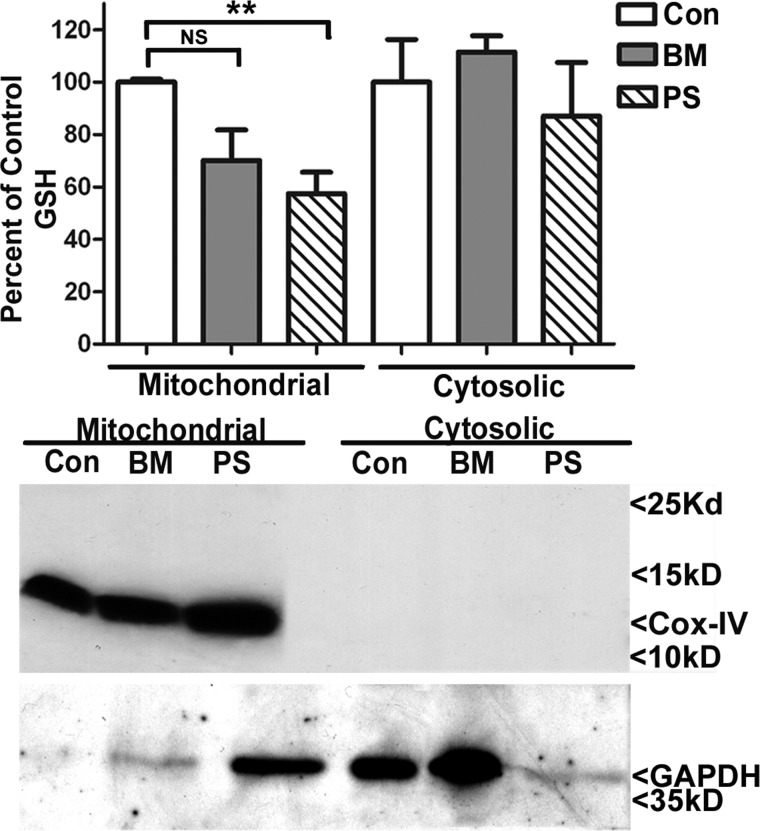
In order to resolve the mitochondrial GSH transporters utilized in cerebellar astrocytes, these cultures were incubated overnight with 5 mm butylmalonate or 5 mm phenylsuccinate, after which the cerebellar astrocytes were subfractionated, and GSH was quantified as described above. Cerebellar astrocytes displayed a significant level of mitochondrial GSH depletion with phenylsuccinate but no effect of this inhibitor on the cytosolic pool of GSH (Fig. 3). Although the results for butylmalonate did not reach statistical significance, the trend toward mitochondrial GSH depletion with this inhibitor is in agreement with the previous immunoblot analysis showing that these astrocytes express both DIC and OGC (Fig. 1B). Furthermore, mitochondrial GSH comprised 13.4 ± 3.9% of total cellular GSH at a concentration of 2.0 ± 0.78 nmol/mg, consistent with previous studies (mean ± S.E., n = 4) (17–19). Cytochrome c oxidase IV blots showed consistent mitochondrial fractions. Although immunoblotting indicated that the mitochondrial phenylsuccinate sample contains GAPDH, this finding alone does not definitively demonstrate cytosolic contamination of this fraction. For instance, a previous study showed that GAPDH can localize to the mitochondria under conditions of mitochondrial permeability transition pore activation (20). Given the significant depletion of mitochondrial GSH observed with phenylsuccinate, this mitochondrial perturbation may be occurring in astrocytes. Second, if the phenylsuccinate mitochondrial samples did indeed contain significant cytosolic contamination, then we would expect to see a marked increase in mitochondrial GSH compared with controls because the cytosolic GSH pool is much larger than the mitochondrial GSH pool. Instead, we observe the opposite effect, where phenylsuccinate significantly reduces the mitochondrial GSH pool in cerebellar astrocytes (Fig. 3).
FIGURE 3.
Inhibition of DIC or OGC function specifically decreases the mitochondrial GSH pool in cerebellar astrocytes. Cerebellar astrocyte cultures were treated with vehicle (DMSO; Con), 5 mm butylmalonate (BM), or 5 mm phenylsuccinate (PS) overnight. Cells were then subfractionated into mitochondrial and cytoplasmic fractions using differential centrifugation. Mitochondrial and cytosolic fractions were measured for total GSH content using a DTNB assay. Data are represented as a percentage of control GSH. **, p < 0.01 compared with control; NS, not significant; n = 3 experiments with each treatment performed in duplicate. Protein lysates were resolved using SDS-PAGE and immunoblotted for cytochrome c oxidase IV (Cox-IV) and GAPDH to indicate pure fractions. Error bars, S.E.
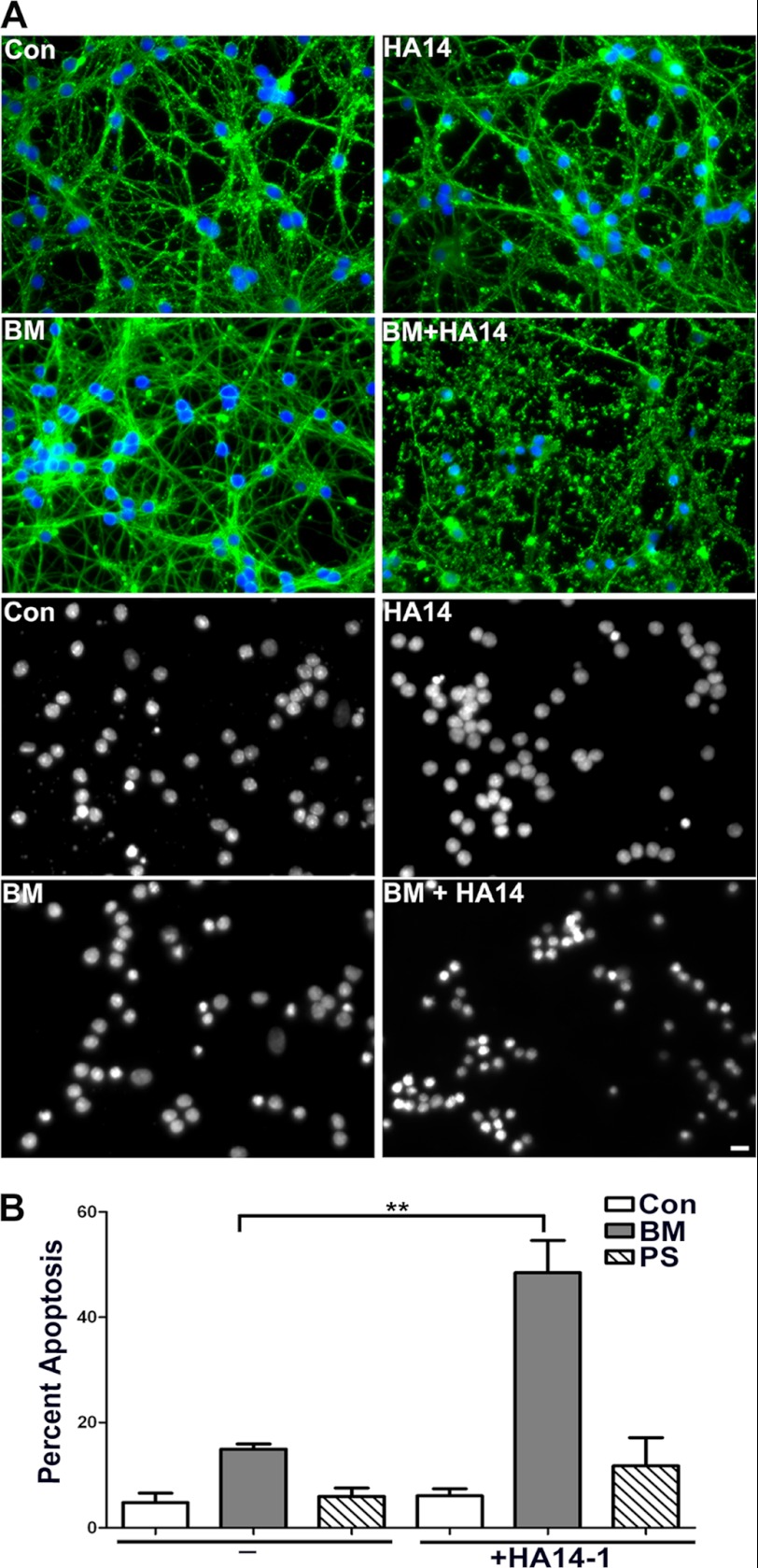
Inhibition of DIC-dependent Mitochondrial GSH Transport Renders CGNs More Susceptible to Oxidative Stress Induced by HA14-1
The unique dependence of CGNs on DIC-dependent transport provided a valuable model for examining the effects of specific inhibition of mitochondrial GSH transport on neuronal sensitivity to oxidative stress. To this end, CGNs were preincubated overnight with either 5 mm phenylsuccinate or 5 mm butylmalonate. Incubation with these doses of inhibitors are not cytotoxic on their own (Fig. 4, A (BM panels) and B (quantification)). Next, CGNs were treated for an additional 4 h with 12.5 μm HA14-1, alone or in combination with butylmalonate or phenylsuccinate. We have previously shown that HA14-1 causes GSH-sensitive intrinsic apoptosis and mitochondrial oxidative stress, through the inhibition of Bcl-2 in CGNs (15). Therefore, we utilized this Bcl-2 homology-3 domain (BH3) mimetic, HA14-1, as a model of oxidative stress. Incubation with 12.5 μm HA14-1 alone for 4 h had no significant effect on CGN survival (Fig. 4, A (HA14 panels) and B (quantification)). However, inhibition of mitochondrial GSH transport through the DIC using butylmalonate rendered the CGNs significantly more sensitive to this oxidative stressor (Fig. 4, A (BM + HA14 panels) and B (quantification)). In contrast, preincubation with the OGC inhibitor, phenylsuccinate, did not significantly impact the susceptibility of CGNs to oxidative stress induced by HA14-1, again consistent with our data showing that CGNs do not express OGC (Fig. 4B, quantification).
FIGURE 4.
Inhibition of DIC-dependent mitochondrial GSH transport renders CGNs more susceptible to oxidative stress induced by HA14-1. A, CGN cultures were treated with vehicle (Con) or 5 mm butylmalonate (BM) overnight. The following day, cells were either left untreated (BM or Con) or treated with 12.5 μm HA14-1 (HA14 or BM + HA14) for 4 h. After treatment, cells were fixed, and β-tubulin (FITC; green) and nuclei (Hoechst; blue) were stained (top four panels). The bottom panels show decolorized nuclei from separate fields. Scale bar, 10 μm. B, cells were treated as in A, with the addition of a 5 mm phenylsuccinate pretreatment and HA14-1 (PS + HA14) or no treatment (PS) the following day. Cells were fixed, and nuclei were stained with Hoechst. Apoptosis was quantified by determining the percentage of cells with condensed and/or fragmented nuclei. **, p < 0.01 versus butylmalonate alone; n = 4 experiments with each treatment performed in duplicate. Error bars, S.E.
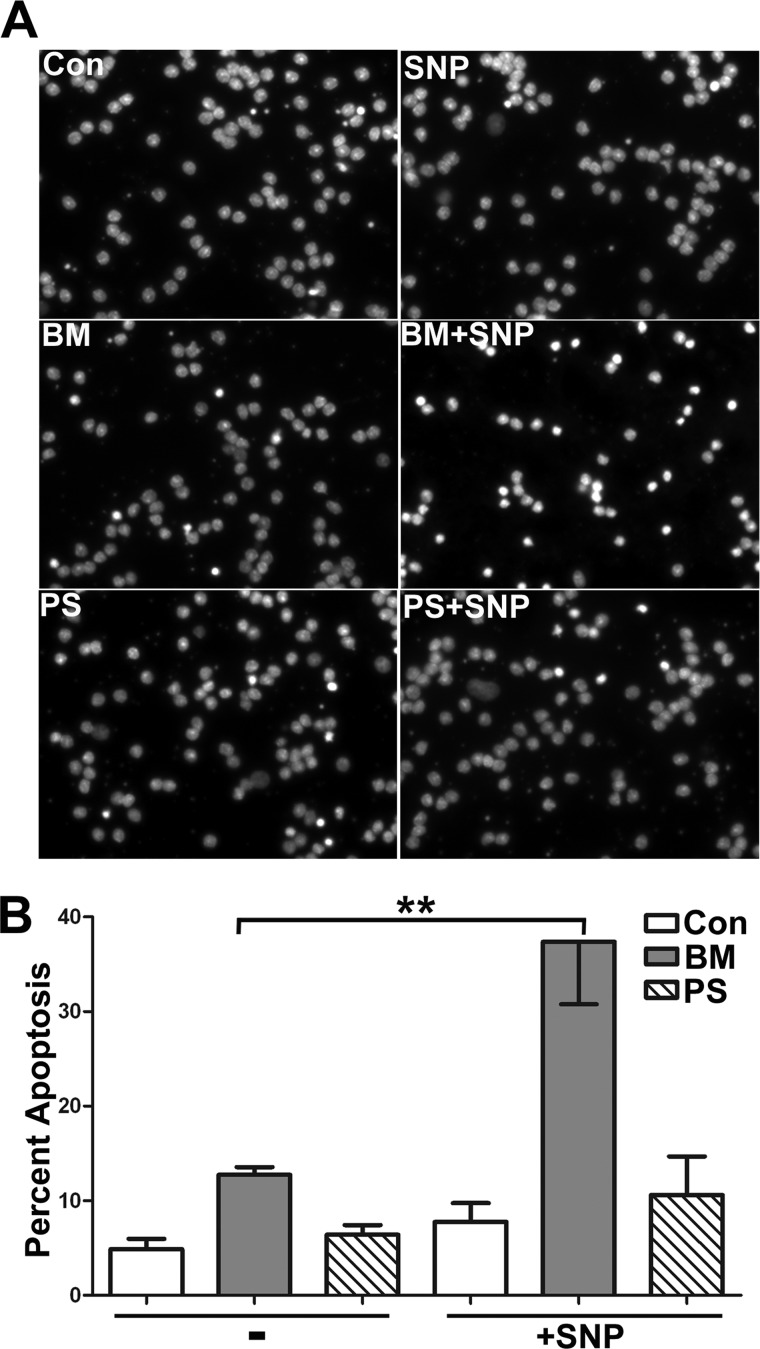
Inhibition of DIC-dependent Mitochondrial GSH Transport Renders CGNs More Susceptible to Nitrosative Stress Induced by the Nitric Oxide Donor, SNP
We next examined the effects of selective mitochondrial GSH depletion, through the inhibition of mitochondrial GSH transport, on the vulnerability of CGNs to nitrosative stress. As described above, CGNs were preincubated overnight with either 5 mm phenylsuccinate or 5 mm butylmalonate. Incubations with these doses of inhibitors were not cytotoxic to CGNs on their own (Fig. 5A, BM panel and PS panel). Next, CGNs were treated for an additional 6 h with the nitric oxide donor SNP, at a concentration (25 μm) that was also not cytotoxic on its own (Fig. 5A, SNP panel). Some CGNs were also treated with SNP in combination with butylmalonate or phenylsuccinate. Consistent with the results described above for HA14-1, preincubation with butylmalonate but not phenylsuccinate rendered CGNs more susceptible to nitrosative stress-induced apoptosis (Fig. 5A, compare BM + SNP and PS + SNP panels). Quantification of these data showed a statistically significant increase in CGN apoptosis induced by SNP in cells preincubated with butylmalonate versus cells incubated with butylamalonate alone (Fig. 5B).
FIGURE 5.
Inhibition of DIC-dependent mitochondrial GSH transport renders CGNs more susceptible to nitrosative stress induced by the nitric oxide donor, SNP. A, CGN cultures were treated with vehicle (Con), 5 mm phenylsuccinate (PS), or 5 mm butylmalonate (BM) overnight. The following day cells were either left untreated (Con, PS, or BM) or treated with 25 μm SNP (SNP, PS + SNP, or BM + SNP) for 6 h. Cells were then fixed and stained with Hoechst dye. Scale bar, 10 μm. B, cells were treated as in A, and apoptosis was quantified by determining the percentage of condensed and/or fragmented nuclei. **, p < 0.01 versus butylmalonate alone; n = 4 experiments with each treatment performed in duplicate. Error bars, S.E.
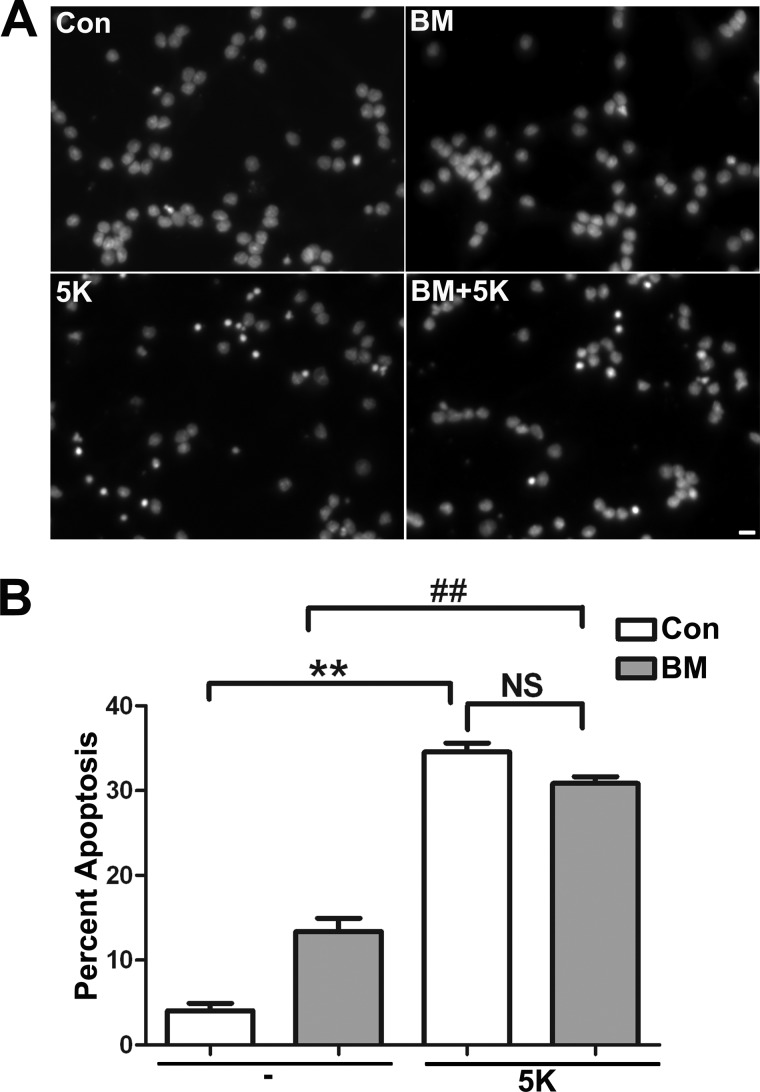
Inhibition of DIC-dependent Mitochondrial GSH Transport Does Not Render CGNs More Susceptible to Apoptosis Induced by Deprivation of Depolarizing Potassium
Previous studies have shown that removing depolarizing potassium (5K) induces CGN apoptosis and that this insult is insensitive to the addition of GSH-MEE (21, 22). Therefore, to determine if incubation of CGNs with the DIC inhibitor, butylmalonate, nonspecifically enhances the sensitivity of CGNs to any toxic insult, we examined the effects of 5K within this system. Incubation with 5K alone for 10 h had a significant deleterious effect on CGN survival (Fig. 6, A (5K panel) and B (quantification)). However, inhibition of mitochondrial GSH transport through the DIC, using butylmalonate, did not render the CGNs more sensitive to 5K (Fig. 6, A (BM + 5K panel) and B (quantification)). Therefore, inhibition of GSH transport through the DIC does not indiscriminately render CGNs more susceptible to GSH-insensitive insults.
FIGURE 6.
Inhibition of DIC-dependent mitochondrial GSH transport does not render CGNs more susceptible to apoptosis induced by deprivation of depolarizing potassium. A, CGN cultures were treated with vehicle (Con) or 5 mm butylmalonate (BM) overnight. The following day, medium was either changed to 25 mm potassium minus serum (Con or BM) or changed to 5 mm potassium minus serum (5K or BM + 5K). Cells were then fixed, and nuclei (Hoechst; blue) were stained. Panels show decolorized nuclei from representative fields. Scale bar, 10 μm. B, cells were treated as in A, after which cells were fixed, and nuclei were stained with Hoechst. Apoptosis was quantified by determining the percentage of cells with condensed and/or fragmented nuclei. ##, p < 0.01 versus butylmalonate alone; **, p < 0.01 versus control; NS, not significant; n = 3 experiments with each treatment performed in duplicate. Error bars, S.E.
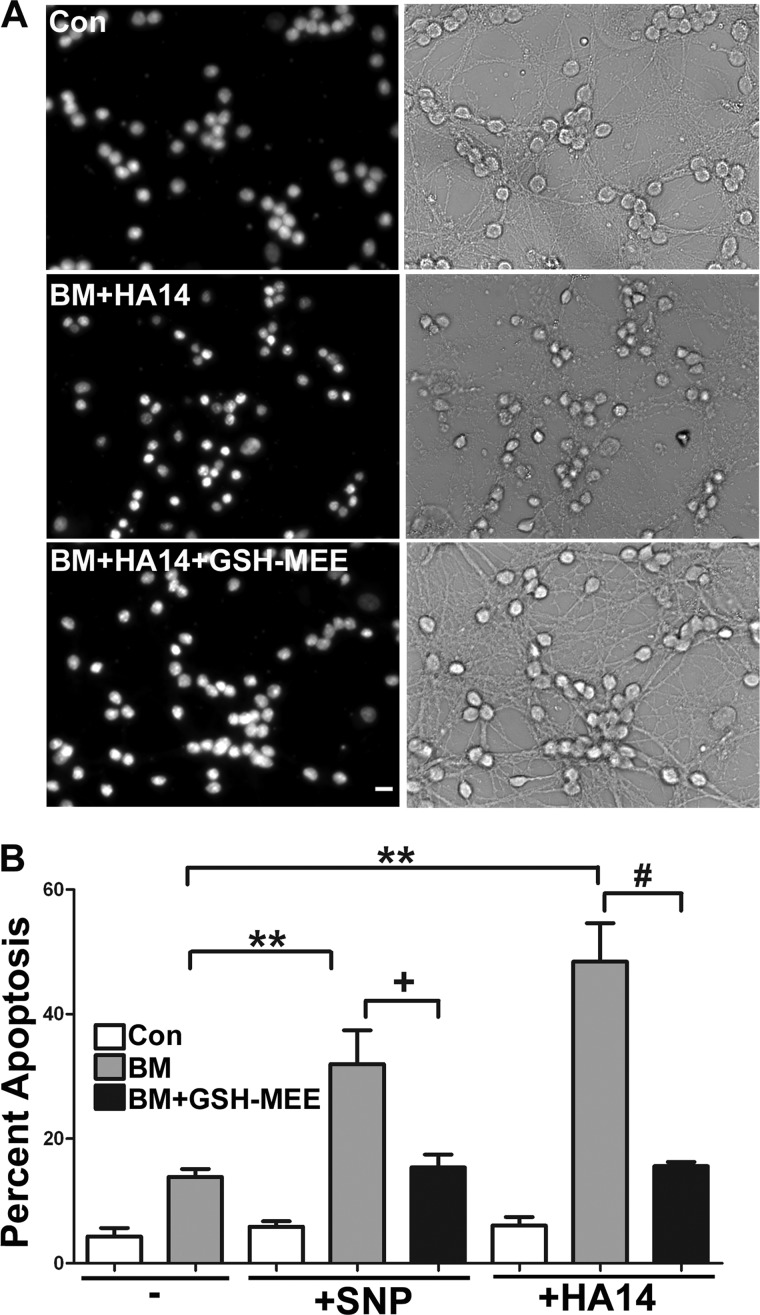
A Cell-permeable Form of GSH Protects CGNs from the Sensitizing Effects Induced by Inhibition of DIC-dependent Mitochondrial GSH Transport
Next, we used a cell-permeable form of GSH, GSH monoethylester, to determine if the sensitization of CGNs to oxidative and nitrosative stress induced by the inhibition of DIC-dependent mitochondrial GSH transport was indeed due to the observed reduction in mitochondrial GSH (see Fig. 2). CGNs were preincubated with 5 mm butylmalonate overnight, after which they were treated with either 12.5 μm HA14-1 (for 4 h) or 25 μm SNP (for 6 h) in the absence or presence of 2 mm GSH monoethylester. Again, incubation with butylmalonate, SNP, and HA14-1 individually did not induce significant levels of apoptosis compared with control conditions (Fig. 7B). However, as shown previously, preincubation with butylmalonate induced significant susceptibility to both oxidative and nitrosative stress caused by these insults (Fig. 7, A (BM +HA14 panels) and B (quantification)). Moreover, the cell-permeable form of GSH significantly protected CGNs from the sensitizing effects induced by inhibition of DIC-dependent mitochondrial GSH transport (Fig. 7A, BM + HA14 + GSH-MEE panels). Quantification of these data showed statistically significant protection upon the addition of GSH monoethylester, when compared with the sensitizing conditions of pretreatment with butylmalonate and the subsequent addition of HA14-1 or SNP (Fig. 7B).
FIGURE 7.
A cell-permeable form of GSH protects CGNs from the sensitizing effects induced by inhibition of DIC-dependent mitochondrial GSH transport. A, CGN cultures were treated with vehicle (Con) or 5 mm butylmalonate (BM) overnight. The following day, cells were either left untreated (Con or BM) or treated with 12.5 μm HA14-1 in the presence or absence of 2 mm GSH monoethylester (GSHMEE) for 4 h, after which cells were fixed, and nuclei (Hoechst; blue) were stained. The left panels show decolorized nuclei from representative fields. The right panels show corresponding bright field images. Scale bar, 10 μm. B, cells were treated as in A; in addition, some cells were incubated with 25 μm SNP in the presence or absence of 2 mm GSH monoethylester. Cells were fixed, and nuclei were stained with Hoechst. Apoptosis was quantified by determining the percentage of cells with condensed and/or fragmented nuclei. **, p < 0.01 versus butylmalonate alone; +, p < 0.05 versus butylmalonate + SNP; ##, p < 0.01 versus butylmalonate + HA14-1; n = 3 experiments with each treatment performed in duplicate. Error bars, S.E.
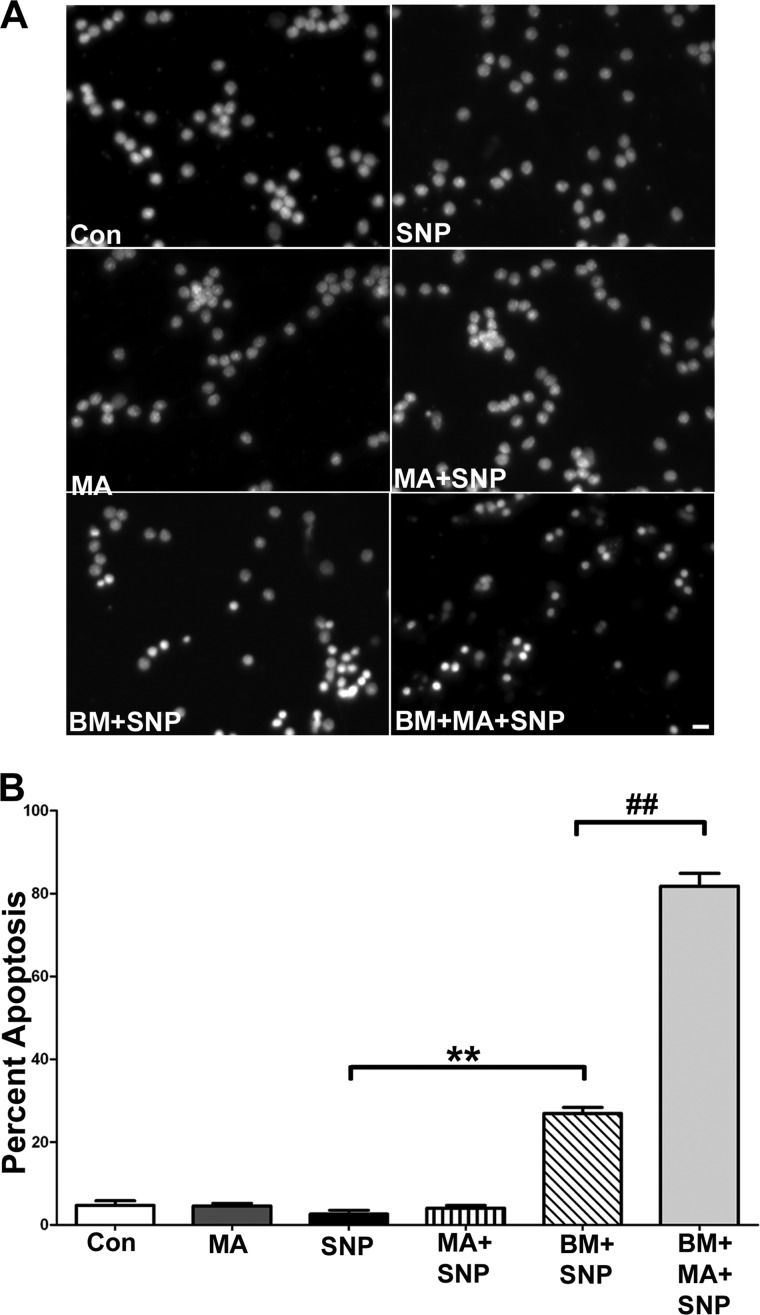
The Addition of Malate Fails to Protect CGNs from the Sensitizing Effects Induced by Inhibition of DIC-dependent Mitochondrial GSH Transport
Mitochondrial anion transporters work in concert with one another in order to transport critical molecules into and out of the mitochondria for energy production. Previous studies have shown that the DIC has the ability to transport malate bidirectionally in exchange for phosphate (23). However, the tricarboxylate carrier transports malate into the mitochondria in exchange for citrate. Therefore, the DIC and the tricarboxylate carrier work in tandem, where DIC transports malate out of the mitochondria and the tricarboxylate carrier transports malate into the mitochondria (24). These transport processes are critical for cell survival because citrate is a crucial component of fatty acid synthesis within the cytosol, whereas malate is required for the citric acid cycle in the mitochondrial matrix. Therefore, an important control experiment when inhibiting DIC-dependent transport function is to examine if malate is capable of protecting CGNs from the sensitizing effects of butylmalonate. This experiment is necessary to demonstrate that the sensitizing effects induced by inhibiting DIC-dependent transport function are not due to disruption of the malate transport capacity of the DIC. Here, we preincubated CGNs with 5 mm butylmalonate in the absence or presence of 5 mm malate. Following these preincubations, CGNs were then treated for 6 h with 25 μm SNP. In the presence of malate, preincubation with butylmalonate and subsequent SNP treatment induced an even greater amount of CGN apoptosis as compared with butylmalonate in the presence of SNP alone (Fig. 8A, compare BM + SNP panel with BM + MA + SNP panel). However, preincubation with malate alone followed by SNP treatment did not increase apoptosis beyond control levels (Fig. 8A, MA + SNP panel). Quantification of these data showed a statistically significant increase in vulnerability to nitrosative stress after CGNs were preincubated with both malate and butylmalonate compared with butylmalonate preincubation alone (Fig. 8B).
FIGURE 8.
The addition of malate fails to protect CGNs from the sensitizing effects induced by inhibition of DIC-dependent mitochondrial GSH transport. A, CGN cultures were treated with vehicle (Con), 5 mm butylmalonate (BM), or 5 mm malate (MA) overnight. The following day, cells were either left untreated or were treated with 25 μm SNP (SNP, BM + SNP, MA + SNP, or BM + MA + SNP) for 6 h, after which cells were fixed, and nuclei (Hoechst; blue) were stained. Scale bar, 10 μm. B, cells were treated as in A. Apoptosis was quantified by determining the percentage of cells with condensed and/or fragmented nuclei. **, p < 0.01 versus SNP alone; ##, p < 0.01 versus butylmalonate + SNP; n = 5 experiments with each treatment performed in duplicate. Error bars, S.E.
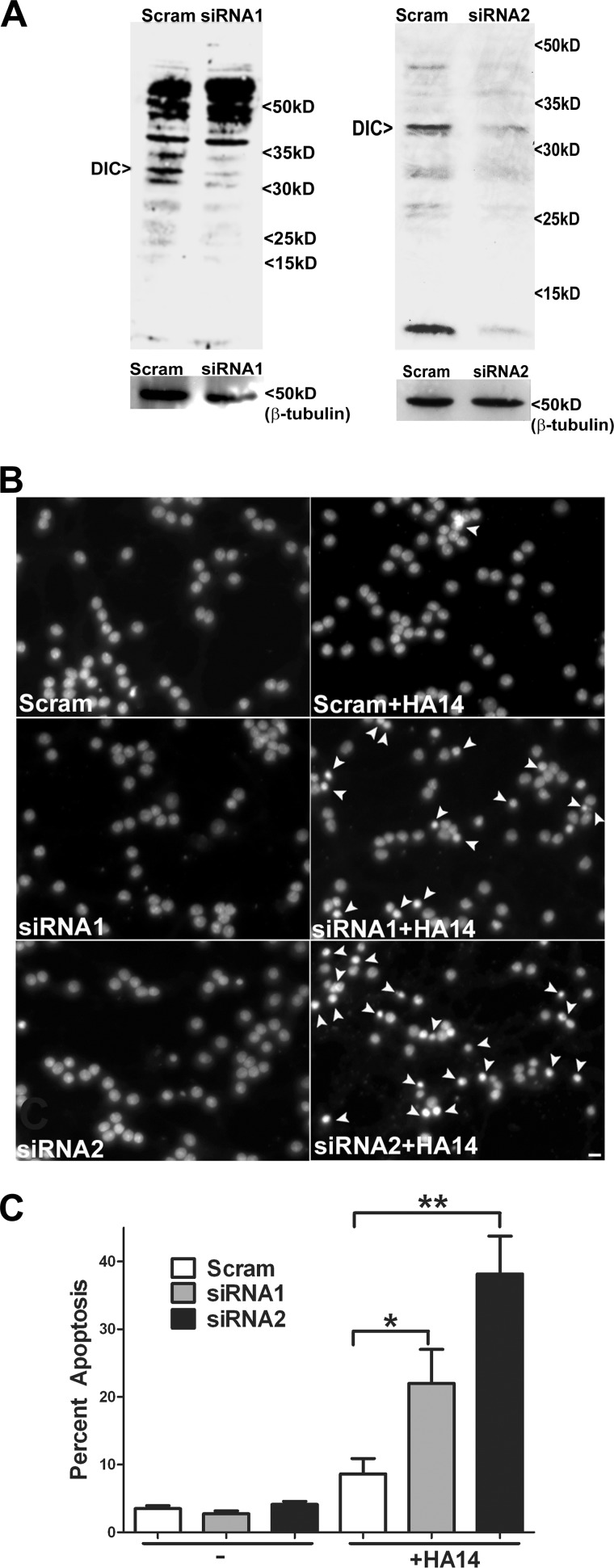
Adenoviral siRNA-mediated Knockdown of DIC Renders CGNs More Susceptible to Oxidative Stress
Finally, we utilized two distinct adenoviral constructs encoding siRNA against rat DIC and a scrambled control construct to examine their effects on neuronal vulnerability to oxidative stress. After 48 h of infection with the adenoviral constructs, CGNs were treated with 12.5 μm HA14-1 for 4 h. In addition, a parallel group of cells from each adenoviral infection was lysed and analyzed for DIC protein levels. As expected, the siRNA DIC adenoviral constructs consistently induced a significant knockdown of DIC expression compared with CGNs infected with the scrambled control (Fig. 9A, siRNA1, 33.6 ± 16%, n = 3; siRNA2, 48 ± 11%, n = 9; mean ± S.E., reduction in DIC protein level normalized to β-tubulin). Neither of the adenoviral constructs caused any significant apoptosis on their own (Fig. 9B, Scram, siRNA1, and siRNA2 panels). However, CGNs infected with either the DIC siRNA1 or DIC siRNA2 adenovirus and subsequently treated with HA14-1 showed a significant increase in apoptosis compared with CGNs infected with the scrambled control virus and then incubated with HA14-1 (Fig. 9B, compare Scram + HA14 with siRNA1 + HA14 and siRNA2 + HA14 panels). Quantification of these data showed that knockdown of DIC with either of two distinct adenoviral siRNA constructs significantly sensitized CGNs to oxidative stress-induced apoptosis when compared with infection with the scrambled control construct (Fig. 9C).
FIGURE 9.
Adenoviral siRNA-mediated knockdown of DIC renders CGNs more susceptible to oxidative stress. A, CGNs were infected for 48 h with a scrambled (Scram) or two distinct DIC-specific siRNA (siRNA1 or siRNA2) adenoviruses. Cells were lysed; proteins were resolved by SDS-PAGE and immunoblotted for DIC (upper blots) and β-tubulin (lower blots). B, CGNs were infected as described in A, after which cells were then either left untreated (Scram, siRNA1, or siRNA2) or treated with 12.5 μm HA14-1 (Scram + HA14, siRNA1 + HA14, or siRNA2 + HA14) for 4 h. Cells were fixed, and nuclei were stained with Hoechst. The panels show representative fields of decolorized nuclei. Arrowheads indicate apoptotic nuclei. Scale bar, 10 μm. C, cells were infected/treated as in B, and apoptosis was quantified as the percentage of cells with condensed and/or fragmented nuclei. *, p < 0.05 versus scrambled + HA14; **, p < 0.01 versus scrambled + HA14; n = 3 experiments with each treatment performed in triplicate. Error bars, S.E.
DISCUSSION
Given that previous studies have demonstrated enhanced sensitivity of both neurons and astrocytes to specific depletion of mitochondrial GSH, it is perhaps not surprising that inhibition of mitochondrial GSH transport would produce similar effects (6, 7). However, it is striking that the discrete inhibition of a single inner membrane transport protein, DIC, is sufficient to significantly sensitize neurons to both oxidative and nitrosative stress. We chose to use CGNs as a model for inhibition of mitochondrial GSH transport based on their apparent dependence on DIC-mediated GSH transport. Interestingly, a mere ∼32% reduction in mitochondrial GSH through specific inhibition of DIC leads to a dramatic increase in the susceptibility of CGNs to both oxidative and nitrosative stress-induced apoptosis. We used both chemical inhibition of DIC (butylmalonate) and molecular knockdown of DIC (adenoviral siRNA) in order to show that discrete inhibition of this single transporter could render CGNs more susceptible to apoptosis. As discussed previously, the mitochondrial GSH pool is an indispensable reservoir of this critical antioxidant. Several studies have shown that specific depletion of mitochondrial GSH versus depletion of the cytosolic GSH pool leads to neuronal degeneration as well as increased vulnerability of astrocytes to both oxidative and nitrosative stress (6, 7). Furthermore, depletion of mitochondrial GSH may occur upstream of mitochondrial dysfunction and oxidative stress, suggesting that reductions in mitochondrial GSH may act as a trigger for these pathological mechanisms (25). Based on our data, it becomes an interesting possibility that dysfunction of one or more mitochondrial GSH transport proteins could lead to the enhanced susceptibility of neurons in the CNS to oxidative and nitrosative stress. This may be particularly relevant within chronic neurodegenerative diseases (e.g. PD or ALS), which have been hypothesized to occur via multiple “hits” involving gene-environment interactions as underlying pathological mechanisms (26). To date, a role for DIC or OGC dysfunction in neurodegeneration has not been examined.
In the context of neurodegenerative diseases, the devastating triad of GSH depletion, mitochondrial dysfunction, and oxidative stress has been implicated in the underlying pathology of a large number of disorders, such as Alzheimer disease, PD, and ALS (1, 2, 27–30). Specifically, GSH has been shown to be depleted within the substantia nigra of PD patients (31). Moreover, depletion of nigral GSH was detected in a mouse model of PD before the onset of complex I deficiency and neurodegeneration (32). Similarly, in a familial mouse model of ALS, the G93A mutant hSOD1 mouse, GSH depletion in the spinal cord was positively correlated with disease onset and progression (33). Forced reduction of GSH levels in the G93A mutant hSOD1 mouse model, through the knockdown of γ-glutamyl cysteine ligase modifier subunit (the rate-limiting enzyme in GSH synthesis) led to a decrease in life span, increased oxidative stress, and accelerated motor dysfunction (34). Furthermore, in sporadic ALS patients, erythrocyte GSH levels were found to be diminished, whereas lipid peroxidation levels were significantly increased compared with healthy age-matched controls (35). Despite this abundant and compelling evidence for a key role of GSH depletion within neurodegenerative diseases, neither mitochondrial GSH nor mitochondrial GSH transport mechanisms explicitly have been studied within models of these diseases.
To further support the role of DIC-dependent mitochondrial GSH transport as a protective mechanism in neurons, while excluding the contribution of interference with the malate transport function of DIC, we examined whether malate would protect CGNs from the sensitizing effects of DIC inhibition to nitrosative stress. Our results show that the addition of malate in the presence of the DIC inhibitor butylmalonate caused CGNs to become more susceptible to nitrosative stress than in the presence of butylmalonate alone. This result can be attributed to the competitive inhibition produced by butylmalonate and malate together on DIC-dependent mitochondrial GSH transport. Butylmalonate inhibits DIC through competitive inhibition by binding to the transport site, and because it is an analog, it does not become transported or metabolized (9, 36). Therefore, the addition of malate with butylmalonate at the same concentration would allow malate to outcompete butylmalonate for transport. However, by adding both of these chemicals together, GSH would have to compete with both malate and butylmalonate, therefore causing an even more significant reduction in mitochondrial GSH transport and a greater vulnerability to nitrosative stress. In contrast to malate addition, the sensitizing effects induced by DIC inhibition with butylmalonate could be rescued by adding back GSH as a cell-permeable esterified form. Therefore, because malate was unable to protect CGNs from the enhanced susceptibility induced by DIC inhibition, whereas adding back GSH did prevent these effects, we are able to rule out deficits in fatty acid synthesis and the citric acid cycle as the mechanism by which CGNs become vulnerable to apoptosis, leaving mitochondrial GSH transport as the most probable mechanism. Depletion of mitochondrial GSH and increased susceptibility to oxidative and nitrosative stress did not occur in CGNs exposed to phenylsuccinate, ruling out the possibility that chemical analogs of commonly transported anions are capable of non-specifically inducing neuronal susceptibility to these insults. Furthermore, butylmalonate did not render the CGNs more vulnerable to a GSH-insensitive insult, 5K-induced apoptosis. The specificity of the sensitizing effects of butylmalonate is also supported through the use of two distinct adenoviral siRNAs to knock down DIC, which similarly induced increased susceptibility to oxidative stress compared with a scrambled control virus.
Previous studies have shown that whole brain mitochondria utilize the tricarboxylate carrier (Slc25a1) as a mitochondrial GSH transport protein, whereas mitochondria isolated specifically from cerebral cortex, which express both DIC and OGC, use primarily DIC as a mitochondrial GSH transport protein (12, 13). Our study shows that CGNs are dependent on DIC, whereas cerebellar astrocytes use both DIC and OGC as mitochondrial GSH transport proteins. CGNs appear to be unique because they are insensitive to phenylsuccinate; however, based on the relatively small decrease in mitochondrial GSH (∼32%) observed with butylmalonate, it cannot be ruled out that a second inner membrane transporter, such as the tricarboxylate carrier, is also being used to transport GSH into CGN mitochondria. Overall, more studies are necessary to determine the identity of a second transport protein that contributes significantly to mitochondrial GSH transport within CGNs.
Several studies have shown that mitochondrial oxidative stress plays a key role within neurodegenerative diseases, such as PD and ALS. In one such study, a partial knockdown of the mitochondrial specific manganese-superoxide dismutase (SOD2) led to a significant decrease in survival and a greater decline in motor function within the G93A mutant hSOD1 mouse model (37). In addition, overexpression of SOD2 in a differentiated human neuroblastoma cell line protected from cell death induced by the overexpression of several different mutant SOD1 proteins (38). These studies indicate the importance of maintaining the mitochondrial redox status for neuronal viability. Mitochondrial SOD2 and mitochondrial GSH (via the activity of GSH peroxidase) work in concert to abolish the free radicals generated by leakage from the electron transport chain, and these antioxidants are equally important in maintaining the redox balance within mitochondria. Therefore, the prior mentioned studies focused on SOD2, in combination with our data, indicate that the antioxidant repertoire of mitochondria is critical in protecting neurons from pathological mechanisms common to many neurodegenerative diseases.
In addition to defining the specific transport proteins involved in mitochondrial GSH transport in the CNS, the regulation of mitochondrial GSH transport is also an area that is not well understood. We have previously shown that Bcl-2 plays a central role in maintaining the mitochondrial GSH pool in CGNs and is also capable of directly binding GSH (15). Furthermore, Bcl-2 is an interacting partner of OGC, and co-overexpression of these proteins leads to a significant increase in mitochondrial GSH (16). Finally, BH3-only proteins (e.g. Bim) and BH3 mimetics antagonize the Bcl-2/GSH interaction and inhibit GSH transport into isolated rat brain mitochondria (15, 16). Therefore, Bcl-2 appears to play a crucial role in regulating mitochondrial GSH transport. This point becomes particularly relevant when considering that Bcl-2 function is often compromised within neurodegenerative diseases. For instance, Bcl-2 expression has been shown to be significantly decreased within the spinal cord of ALS patients (39). In the G93A mutant hSOD1 mouse model of familial ALS, the mutant SOD1 protein was found to interact with Bcl-2 and form dysfunctional aggregates with Bcl-2 at mitochondria (40). This interaction between G93A mutant SOD1 and Bcl-2 was also shown to induce a toxic conformational change in Bcl-2, which prevents it from performing its anti-apoptotic function (41). Therefore, it would be of interest to determine if these deficits in Bcl-2 protein expression or its interaction with G93A mutant SOD1 lead to consequential decreases in mitochondrial GSH transport in the context of specific neurodegenerative diseases like ALS.
In summary, we have shown that by modulating mitochondrial GSH transport through the specific inhibition of a discrete inner membrane transporter, DIC, neurons become more vulnerable to apoptosis induced by oxidative or nitrosative stress. This study provides novel insights into the importance of mitochondrial GSH transport in maintaining neuronal survival and suggests that understanding the mechanisms involved in the regulation of mitochondrial GSH transport in neurons may reveal novel therapeutic targets for neurodegenerative disorders.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 NS062766 (to D. A. L.). This work was also supported by a Merit Review grant from the Department of Veterans Affairs (to D. A. L.).
- PD
- Parkinson disease
- ALS
- amyotrophic lateral sclerosis
- BH3
- Bcl-2 homology-3 domain
- DIC
- dicarboxylate carrier (Slc25a10)
- DTNB
- 5′-dithiobis(2-nitrobenzoic acid)
- HA14-1
- ethyl [2-amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)]-4H-chromene-3-arboxylate, 2-amino-6-bromo-α-cyano-3-(ethoxycarbonyl)-4H-1-benzopyran-4-acetic acid ethyl ester
- OGC
- 2-oxoglutarate carrier (Slc25a11)
- ROS
- reactive oxygen species
- SNP
- sodium nitroprusside
- SOD1
- copper-zinc superoxide dismutase
- SOD2
- manganese superoxide dismutase
- 5K
- 5 mm potassium
- CGN
- cerebellar granule neuron.
REFERENCES
- 1. Lin M. T., Beal M. F. (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795 [DOI] [PubMed] [Google Scholar]
- 2. Murata T., Ohtsuka C., Terayama Y. (2008) Increased mitochondrial oxidative damage and oxidative DNA damage contributes to the neurodegenerative process in sporadic amyotrophic lateral sclerosis. Free Radic. Res. 42, 221–225 [DOI] [PubMed] [Google Scholar]
- 3. Arthur C. R., Morton S. L., Dunham L. D., Keeney P. M., Bennett J. P., Jr. (2009) Parkinson's disease brain mitochondria have impaired respirasome assembly, age-related increases in distribution of oxidative damage to mtDNA and no differences in heteroplasmic mtDNA mutation abundance. Mol. Neurodegener. 4, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Isobe C., Abe T., Terayama Y. (2010) Levels of reduced and oxidized coenzyme Q-10 and 8-hydroxy-2′-deoxyguanosine in the cerebrospinal fluid of patients with living Parkinson's disease demonstrate that mitochondrial oxidative damage and/or oxidative DNA damage contributes to the neurodegenerative process. Neurosci. Lett. 469, 159–163 [DOI] [PubMed] [Google Scholar]
- 5. Zorov D. B., Juhaszova M., Sollott S. J. (2006) Mitochondrial ROS-induced ROS release. An update and review. Biochim. Biophys. Acta 1757, 509–517 [DOI] [PubMed] [Google Scholar]
- 6. Wüllner U., Seyfried J., Groscurth P., Beinroth S., Winter S., Gleichmann M., Heneka M., Löschmann P., Schulz J. B., Weller M., Klockgether T. (1999) Glutathione depletion and neuronal cell death. The role of reactive oxygen intermediates and mitochondrial function. Brain Res. 826, 53–62 [DOI] [PubMed] [Google Scholar]
- 7. Muyderman H., Wadey A. L., Nilsson M., Sims N. R. (2007) Mitochondrial glutathione protects against cell death induced by oxidative and nitrative stress in astrocytes. J. Neurochem. 102, 1369–1382 [DOI] [PubMed] [Google Scholar]
- 8. Griffith O. W., Meister A. (1985) Origin and turnover of mitochondrial glutathione. Proc. Natl. Acad. Sci. U.S.A. 82, 4668–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lash L. H. (2006) Mitochondrial glutathione transport. Physiological, pathological and toxicological implications. Chem. Biol. Interact. 163, 54–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Z., Lash L. H. (1998) Evidence for mitochondrial uptake of glutathione by dicarboxylate and 2-oxoglutarate carriers. J. Pharmacol. Exp. Ther. 285, 608–618 [PubMed] [Google Scholar]
- 11. Chen Z., Putt D. A., Lash L. H. (2000) Enrichment and functional reconstitution of glutathione transport activity from rabbit kidney mitochondria. Further evidence for the role of the dicarboxylate and 2-oxoglutarate carriers in mitochondrial glutathione transport. Arch. Biochem. Biophys. 373, 193–202 [DOI] [PubMed] [Google Scholar]
- 12. Wadey A. L., Muyderman H., Kwek P. T., Sims N. R. (2009) Mitochondrial glutathione uptake. Characterization in isolated brain mitochondria and astrocytes in culture. J. Neurochem. 109, 101–108 [DOI] [PubMed] [Google Scholar]
- 13. Kamga C. K., Zhang S. X., Wang Y. (2010) Dicarboxylate carrier-mediated glutathione transport is essential for reactive oxygen species homeostasis and normal respiration in rat brain mitochondria. Am. J. Physiol. Cell Physiol. 299, C497–C505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huypens P., Pillai R., Sheinin T., Schaefer S., Huang M., Odegaard M. L., Ronnebaum S. M., Wettig S. D., Joseph J. W. (2011) The dicarboxylate carrier plays a role in mitochondrial malate transport and in the regulation of glucose-stimulated insulin secretion from rat pancreatic beta cells. Diabetologia 54, 135–145 [DOI] [PubMed] [Google Scholar]
- 15. Zimmermann A. K., Loucks F. A., Schroeder E. K., Bouchard R. J., Tyler K. L., Linseman D. A. (2007) Glutathione binding to the Bcl-2 homology-3 domain groove. A molecular basis for Bcl-2 antioxidant function at mitochondria. J. Biol. Chem. 282, 29296–29304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilkins H. M., Marquardt K., Lash L. H., Linseman D. A. (2012) Bcl-2 is a novel interacting partner for the 2-oxoglutarate carrier and a key regulator of mitochondrial glutathione. Free Radic. Biol. Med. 52, 410–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ravindranath V., Shivakumar B. R., Anandatheerthavarada H. K. (1989) Low glutathione levels in brain regions of aged rats. Neurosci. Lett. 101, 187–190 [DOI] [PubMed] [Google Scholar]
- 18. Sagara J. I., Miura K., Bannai S. (1993) Maintenance of neuronal glutathione by glial cells. J. Neurochem. 61, 1672–1676 [DOI] [PubMed] [Google Scholar]
- 19. Huang J., Philbert M. A. (1995) Distribution of glutathione and glutathione-related enzyme systems in mitochondria and cytosol of cultured cerebellar astrocytes and granule cells. Brain Res. 680, 16–22 [DOI] [PubMed] [Google Scholar]
- 20. Tarze A., Deniaud A., Le Bras M., Maillier E., Molle D., Larochette N., Zamzami N., Jan G., Kroemer G., Brenner C. (2007) GAPDH, a novel regulator of the pro-apoptotic mitochondrial membrane permeabilization. Oncogene 26, 2606–2620 [DOI] [PubMed] [Google Scholar]
- 21. D'Mello S. R., Galli C., Ciotti T., Calissano P. (1993) Induction of apoptosis in cerebellar granule neurons by low potassium. Inhibition of death by insulin-like growth factor I and cAMP. Proc. Natl. Acad. Sci. U.S.A. 90, 10989–10993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zimmermann A. K., Loucks F. A., Le S. S., Butts B. D., Florez-McClure M. L., Bouchard R. J., Heidenreich K. A., Linseman D. A. (2005) Distinct mechanisms of neuronal apoptosis are triggered by antagonism of Bcl-2/Bcl-xL versus induction of BH3-only protein Bim. J. Neurochem. 94, 22–36 [DOI] [PubMed] [Google Scholar]
- 23. Mizuarai S., Miki S., Araki H., Takahashi K., Kotani H. (2005) Identification of dicarboxylate carrier Slc25a10 as malate transporter in de novo fatty acid synthesis. J. Biol. Chem. 280, 32434–32441 [DOI] [PubMed] [Google Scholar]
- 24. Kajimoto K., Terada H., Baba Y., Shinohara Y. (2005) Essential role of citrate export from mitochondria at early differentiation stage of 3T3-L1 cells for their effective differentiation into fat cells, as revealed by studies using specific inhibitors of mitochondrial di- and tricarboxylate carriers. Mol. Genet. Metab. 85, 46–53 [DOI] [PubMed] [Google Scholar]
- 25. Merad-Boudia M., Nicole A., Santiard-Baron D., Saillé C., Ceballos-Picot I. (1998) Mitochondrial impairment as an early event in the process of apoptosis induced by glutathione depletion in neuronal cells. Relevance to Parkinson's disease. Biochem. Pharmacol. 56, 645–655 [DOI] [PubMed] [Google Scholar]
- 26. Sulzer D. (2007) Multiple hit ypotheses for dopamine neuron loss in Parkinson's disease. Trends Neurosci. 30, 244–250 [DOI] [PubMed] [Google Scholar]
- 27. Castellani R., Hirai K., Aliev G., Drew K.L., Nunomura A., Takeda A., Cash A. D., Obrenovich M. E., Perry G., Smith M. A. (2002) Role of mitochondrial dysfunction in Alzheimer's disease. J. Neurosci. Res. 70, 357–360 [DOI] [PubMed] [Google Scholar]
- 28. Mattiazzi M., D'Aurelio M., Gajewski C. D., Martushova K., Kiaei M., Beal M. F., Manfredi G. (2002) Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J. Biol. Chem. 277, 29626–29633 [DOI] [PubMed] [Google Scholar]
- 29. Eckert A., Keil U., Marques C. A., Bonert A., Frey C., Schüssel K., Müller W. E. (2003) Mitochondrial dysfunction, apoptotic cell death, and Alzheimer's disease. Biochem. Pharmacol. 66, 1627–1634 [DOI] [PubMed] [Google Scholar]
- 30. Henchcliffe C., Beal M. F. (2008) Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat. Clin. Pract. Neurol. 4, 600–609 [DOI] [PubMed] [Google Scholar]
- 31. Perry T. L., Yong V. W. (1986) Idiopathic Parkinson's disease, progressive supranuclear palsy and glutathione metabolism in the substantia nigra of patients. Neurosci. Lett. 67, 269–274 [DOI] [PubMed] [Google Scholar]
- 32. Chinta S. J., Kumar M. J., Hsu M., Rajagopalan S., Kaur D., Rane A., Nicholls D. G., Choi J., Andersen J. K. (2007) Inducible alterations of glutathione levels in adult dopaminergic midbrain neurons result in nigrostriatal degeneration. J. Neurosci. 27, 13997–14006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chi L., Ke Y., Luo C., Gozal D., Liu R. (2007) Depletion of reduced glutathione enhances motor neuron degeneration in vitro and in vivo. Neuroscience 144, 991–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vargas M. R., Johnson D. A., Johnson J. A. (2011) Decreased glutathione accelerates neurological deficit and mitochondrial pathology in familial ALS-linked hSOD1(G93A) mice model. Neurobiol. Dis. 43, 543–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Babu G. N., Kumar A., Chandra R., Puri S. K., Singh R. L., Kalita J., Misra U. K. (2008) Oxidant-antioxidant imbalance in the erythrocytes of sporadic amyotrophic lateral sclerosis patients correlates with the progression of disease. Neurochem. Int. 52, 1284–1289 [DOI] [PubMed] [Google Scholar]
- 36. Palmieri F., Prezioso G., Quagliariello E., Klingenberg M. (1971) Kinetic study of the dicarboxylate carrier in rat liver mitochondria. Eur. J. Biochem. 22, 66–74 [DOI] [PubMed] [Google Scholar]
- 37. Andreassen O. A., Ferrante R. J., Klivenyi P., Klein A. M., Shinobu L. A., Epstein C. J., Beal M. F. (2000) Partial deficiency of manganese superoxide dismutase exacerbates a transgenic mouse model of amyotrophic lateral sclerosis. Ann. Neurol. 47, 447–455 [PubMed] [Google Scholar]
- 38. Flanagan S. W., Anderson R. D., Ross M. A., Oberley L. W. (2002) Overexpression of manganese superoxide dismutase attenuates neuronal death in human cells expressing mutant (G37R) Cu/Zn-superoxide dismutase. J. Neurochem. 81, 170–177 [DOI] [PubMed] [Google Scholar]
- 39. Mu X., He J., Anderson D. W., Trojanowski J. Q., Springer J. E. (1996) Altered expression of bcl-2 and bax mRNA in amyotrophic lateral sclerosis spinal cord motor neurons. Ann. Neurol. 40, 379–386 [DOI] [PubMed] [Google Scholar]
- 40. Pasinelli P., Belford M. E., Lennon N., Bacskai B. J., Hyman B. T., Trotti D., Brown R. H., Jr. (2004) Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron 43, 19–30 [DOI] [PubMed] [Google Scholar]
- 41. Pedrini S., Sau D., Guareschi S., Bogush M., Brown R. H., Jr., Naniche N., Kia A., Trotti D., Pasinelli P. (2010) ALS-linked mutant SOD1 damages mitochondria by promoting conformational changes in Bcl-2. Hum. Mol. Genet. 19, 2974–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]