Synopsis
The autonomic nervous system helps regulate glucose homeostasis by acting on pancreatic islets of Langerhans. Despite decades of research on the innervation of the pancreatic islet, the exact mechanisms used by the autonomic nervous input to influence islet cell biology have not been elucidated. Major limitations include the lack of adequate anatomical and physiological techniques. Here we discuss how these major barriers can be overcome to study the role of the autonomic innervation of the pancreatic islet in glucose metabolism. We describe recent advances in microscopy and novel approaches to study the effects of nervous input that may help clarify how autonomic axons regulate islet biology.
Introduction
A well functioning endocrine pancreas, the islets of Langerhans, is crucial for survival. The islets of Langerhans secrete hormones that maintain constant levels of plasma glucose. Dysfunction or death of the insulin-secreting beta cells leads to potentially life-threatening fluctuations in plasma glucose levels and is a major cause of diabetes mellitus, a devastating disease affecting millions worldwide. Already in the 19th century, Claude Bernard proposed that the nervous system is implicated in the regulation of plasma glucose 1 and since their discovery Langerhans recognized a rich innervation of the islets 2. Many studies have linked the autonomic nervous system to metabolic control and islet function 3–8. However, the importance of the autonomic innervation of the islets in the maintenance of glucose homeostasis remains controversial for two main reasons: a) there is a lack of detailed anatomical studies of islet innervation, in particular of human islets, and b) it has been difficult to discern the effects autonomic fibers have locally on islet cell physiology from the confounding effects the autonomic input has simultaneously on many other organs. In this review, we will first give a brief overview of the literature on islet innervation, trying to illustrate the difficulties inherent to this research. We will then focus on approaches that are currently used to circumvent major limitations of studying the structural and functional innervation of the islet.
Several components of the autonomic nervous system have been identified in islets of Langerhans
Based on numerous studies, the consensus is that the endocrine pancreas is richly innervated by the autonomic nervous system 3–8. Parasympathetic and sympathetic nerves travel to the islet through the neurovascular stalk. Within the islets, the nerves follow the blood vessels and terminate within the pericapillary space, within the capillary basement membrane, or closely apposed to the endocrine cells 9. These fibers do not form classical synapses with endocrine cells but rather have release sites near these islet cells. It has been proposed that neurotransmitters are released into the interstitial space to affect a group of adjacent islet cells.
The distribution and density of the different types of nerve fibers have been examined in several species. A dense sympathetic innervation, as identified by the presence of catecholamine-synthesizing enzymes, has been reported in rodents, dogs, and cats 10–13. Studies based on the cholinesterase technique have revealed the parasympathetic innervation in the cat, rat, and rabbit 14–18. Fibers containing neuropeptides have also been reported, but it is unclear whether these represent unique fiber populations or whether these peptides are localized in the autonomic fibers 19. In addition, there is a network of sensory fibers containing the neuropeptides calcitonin gene-related peptide (GGRP) and substance P. Thus far, the innervation of human islets has been merely mentioned in a few studies 20–22.
Although the work cited above provides convincing evidence that autonomic fibers innervate the islets, the precise organization of islet innervation is mostly unknown. Only a few markers have been used, and because these markers have not been combined in immunofluorescence studies, it is unclear how the different fiber systems relate to each other. For instance, it is not known whether the patterns of the parasympathetic and sympathetic innervations are complementary or if they overlap in specific regions of the islet. There have been few attempts to identify peptides as co-transmitters in cholinergic and adrenergic fibers. Furthermore, relying on the cholinesterase technique may be misleading because esterases do not map exclusively to parasympathetic nerves. Other important prototypical cholinergic markers for the parasympathetic system (e.g. ChAT, vAChT) have barely been examined. There are no systematic analyses on how and where the axons terminate within the islets, and what particular cells they innervate. It is further obvious that our current view of islet innervation is based mainly on rodent studies, and that, given their unique cytoarchitecture, the situation in human islets may be very different 23–25.
Several neurotransmitter receptors are expressed on the plasma membrane of islet cells
Parasympathetic axons and sympathetic axons release acetylcholine and noradrenaline that act on cholinergic and adrenergic receptors, respectively. Activation of cholinergic muscarinic receptors stimulates insulin and glucagon secretion 26–28. Release of Ca2+ from intracellular stores in response to muscarinic receptor activation is the major cause for the insulinotropic action, but the signaling underlying the enhanced glucagon secretion needs to be established. Activation of alpha2-adrenergic receptors inhibits glucose-induced insulin secretion via hyperpolarization of the beta cell. Activation of beta2-adrenergic receptors stimulates insulin and glucagon secretion. Thus, the actions of a neurotransmitter vary according to the activated receptor type. To add to this complexity, neuropeptides, which are also released from autonomic axons, including VIP, NPY, and, in some species, galanin, also have effects on islet cells 5, 29. It is important to note that most of the in vitro studies have been conducted with rodent islets. Because the effects of neurotransmitter on islet endocrine cells may vary considerably between species, it will be important to establish the neurotransmitter receptor profiles in human islet cells, in particular because these could represent potential therapeutic targets.
Physiological studies on the effects of the autonomic nervous system on islet function
The overall effect of parasympathetic stimulation is an increase in insulin secretion. Several studies support the view that acetylcholine is released to stimulate insulin secretion. Exogenous treatment with acetylcholine or other muscarinic agonists stimulates insulin secretion in vivo. This effect can be blocked with the muscarinic antagonist atropine 16, 17. In vivo studies in the dog and the baboon reported that stimulation of the vagus nerve increases insulin secretion 30–32, but this stimulation also increases the secretion of other islet hormones such as glucagon, somatostatin, and pancreatic polypeptide 16, 33, 34. The net effect of sympathetic nerve stimulation seems to be a lowering in of plasma insulin concentration. Exogenous treatment with catecholamines and electrical activation of sympathetic nerves inhibits insulin secretion in vivo 35–37. Alpha-adrenergic receptor blockade counteracts this inhibition of insulin secretion 36, 38, suggesting noradrenaline as the mediator.
Several other direct and indirect mechanisms, however, could contribute to the effects of noradrenaline on insulin secretion: noradrenaline may activate beta-adrenergic receptors on beta cells and adrenergic receptors on alpha cells. This makes it difficult to interpret the results. Moreover, this effect cannot be attributed solely to the release of noradrenaline from nerve fibers close to beta cells because other tissues innervated by the activated nerves are also stimulated. The sympathetic nervous system further exerts profound effects on the secretion of the other islet hormones. Splanchnic nerve stimulation and noradrenaline application increases glucagon secretion and decreases somatostatin secretion 34, 36–40. To our knowledge, similar studies have not been performed in human beings.
It is important to note that autonomic innervation of the endocrine pancreas modulates function of the islet. It does not determine it. The islet can work without innervation (see below), but it needs nervous input to adapt the hormonal secretory output to changes in the internal and external environments. Thus, the autonomic input plays a role in adjusting glucose homeostasis in response to food intake or stress 5, 41. In this sense, it is similar to autonomic input to the heart, where the parasympathetic and sympathetic inputs adjust the force and pace of the basic heartbeat in response to behavioral challenges. Moreover, the autonomic nervous input may not only be important for acutely modulating hormone secretion. As experiments in mice lacking neurotransmitter receptors have shown, it is also likely that the nervous input plays a trophic role in maintaining a healthy population of beta cells 28.
Sensory axons and the link to diabetes
In addition to the efferent fibers of the autonomic nervous system, the islets are also innervated by a network of sensory fibers. These fibers leave the pancreas along the sympathetic fibers within the splanchnic nerves and transmit information about noxious stimuli. Fibers containing the sensory neuropeptides GGRP and substance P have been observed in the endocrine pancreas. Vanilloid receptors are localized in sensory fibers and generally report pain information. Whether these fibers are involved in the regulation of islet hormone secretion remains to be determined. Intriguingly, recent papers suggest that eliminating the pancreatic sensory innervation dramatically affects autoimmune diabetes in NOD mice 42, 43 and contributes to defective insulin secretion in the Zucker diabetic rat, an animal model for type 2 diabetes 44. These studies raise the possibility that signals derived from the sensory component of the autonomic nervous system can alter insulin secretion and islet inflammation, thus indirectly affecting the development of autoimmunity and type 2 diabetes. It is unclear, however, to which extent human islets are innervated by sensory fibers. Finding out which receptors are expressed on these fibers is crucial to be able to propose that a similar mechanism provides a link between the nervous system and the natural history of diabetes in human beings.
Establishing the role of autonomic innervation of the islet in glucose homeostasis is challenging
To establish that a tissue is innervated investigators can a) show that neurotransmitters are present within the efferent autonomic axon; b) show that the neurotransmitter is released in response to stimulation of the efferent axon; and c) show specific receptors for the neurotransmitter are present on the postsynaptic cell. However, performing these studies in the pancreas is technically challenging. Many physiological events under parasympathetic and sympathetic control can indirectly interfere with insulin or glucagon secretion. It has been very difficult to discern the direct effects of autonomic terminals in the islet from the confounding effects of the autonomic nervous system elsewhere (e.g. incretin secretion, activation of the adrenal medulla). Selective stimulation of islet innervation is difficult. For instance, to achieve a specific activation of the pancreas, investigators have to use electrical activation of the mixed autonomic nerves along a pancreatic artery with a concomitant blockade of the joint preganglionic cholinergic nerves 36, 37. Furthermore, if not applied locally, exogenous application of neurotransmitters can influence multiple organs and tissues and, as a result, the effects are the sum of a multitude of activities. An additional limitation is that the responses of islet cells to nerve stimulation can only be measured indirectly in the systemic circulation (e.g. hormone plasma levels). It is not possible to record receptor activation directly in the postsynaptic cells.
Consequently, although several studies suggest the involvement of islet innervation, the importance of the autonomic nervous system in regulating islet hormone secretion is unclear. The list of neurotransmitters that may modulate islet function is long and confusing because of species differences and uncertainty about the physiological relevance of the effects observed with in vivo models. In human subjects, for instance, decreased glucose tolerance after vagotomy has been reported 45, 46, but patients who have undergone pancreas transplantation (and thus may have denervated islets) remain euglycemic without therapy 47–50. For similar reasons, doubts exist about whether sympathetic nerve fibers exert a major influence on the basal and postprandial insulin secretory responses. The contribution of autonomic signals to the glucagon secretory response in vivo is also unclear. Autonomic activation most likely plays a major role in dogs 51 and in monkeys 52, but combined adrenergic and muscarinic cholinergic blockade has no effect on the glucagon response to hypoglycemia in humans 53, 54. Furthermore, this glucagon response is not reduced in adrenalectomized, spinal-cord-sectioned, or vagotomized humans 55–59.
We agree with other investigators 5, 40 in that the lack of experimental tools has prevented critical demonstrations of the effects of autonomic innervation. A successful new approach has been to generate mutant mice lacking a particular neurotransmitter receptor in a particular islet cell. Thus, studies of knockout mice lacking the M3 muscarinic receptor in beta cells showed that M3 receptors play an important role in promoting insulin secretion and maintaining glucose homeostasis28. This strategy eliminates potential confounding factors caused by the widespread distribution of autonomic neurotransmitter receptors. This approach, however, is too cumbersome to allow a larger screening of the role of the many putative receptors on islet cells and it certainly cannot be used to examine the physiological role of islet cell receptors in humans. Furthermore, these receptors are not used exclusively to mediate neural input and could be responding to humoral and paracrine signals.
New approaches to study the anatomical aspects of islet innervation
A major hurdle to studying innervation patterns is related to the structure of the neuronal axon. Histological examination of axons is difficult because they are elongated, meandering, and thin structures. Although an axon can be longer than a meter, it is generally less than 5 μm thick. Axons are bundled in nerves, but when they reach their target tissues they branch extensively and run in tortuous pathways through the tissue parenchyma, often along blood vessels, to establish contacts with effector cells. On their way to their final target, axons may contact many different types of cells and traverse tissues with a variety of functions. Autonomic axons, in particular, do not form specialized terminal endings but secrete their neurotransmitter content at axonal varicosities in close vicinity of target cells. The lack of a well-defined terminal structure makes it difficult to determine whether or not a particular cell type is innervated by the autonomic nervous system.
In view of these difficulties, how can we study the innervation of the pancreatic islet? To visualize axons, investigators can use axonal tracing techniques in animal models60. With retrograde and anterograde axonal tracing the exact origin of the innervation as well as the terminal innervation patterns can be determined in detail. These experiments cannot be performed in human beings. Instead, investigators most commonly detect molecules that serve as axonal markers. These can be targeted with specific antibodies using immunohistochemical techniques. The markers include both structural (i.e. neurofilaments, myelin, acetylated actin) and functional molecules such as neurotransmitters and proteins involved in synthesis, packaging, secretion, or degradation of neurotransmitters (i.e. biosynthetic enzymes, vesicular transporters). Labeled axons are then visualized using bright field or fluorescence microscopy (Figure 1). In most studies, data are presented as micrographs showing labeled axonal shafts and varicosities within the tissue of interest. Because these images are generally taken from relatively thin sections (5–15 μm), only fragments of the axons or dispersed varicosities are visualized. Often, these cannot be discerned as neuronal elements or even be distinguished from staining artifacts. In addition, the results are qualitative because only few images can be shown in a paper. As a consequence, many results in the literature can be considered anecdotal.
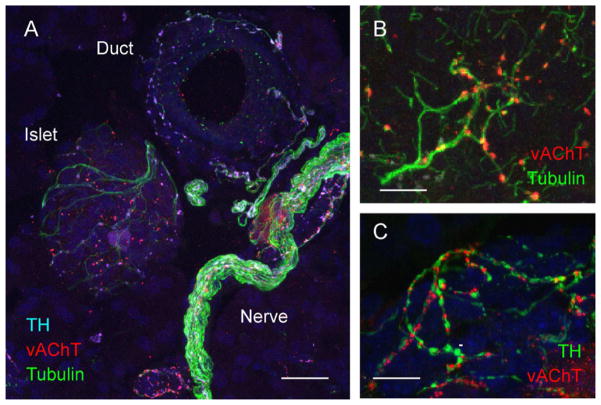
Figure 1.
Visualization of autonomic axons in the pancreas. (A) Maximal projection of a Z-stack of 40 confocal images (Z step = 0.5 μm) of a mouse pancreatic section showing axons labeled for acetylated tubulin (green), vesicular acetylcholine transporter (vAChT, red), and tyrosine hydroxylase (TH, light blue). Notice that acetylated tubulin is present in the entire axon whereas vAChT and TH are more restricted to axon varicosities. TH and vAChT label noradrenergic, sympathetic and cholinergic, parasympathetic axons, respectively. (B) At higher magnification tubulin-stained axons are beaded with vAChT-positive varicosities. (C) Sympathetic and parasympathetic axons run together in the same nerve tracts as shown by the close association between TH-labeled and vAChT labeled axons. Scale bars = 20 μm (A) and 10 μm (B, C).
To be able to understand the innervation pattern of an organ it is crucial to visualize large portions of the axonal plexus penetrating the tissue. This can be accomplished by using the immunostaining approaches mentioned above on thicker histological sections (40–60 μm). Labeled axons are then imaged with confocal microscopy to produce Z-stacks of confocal images. These are used to render the axonal plexus in three dimensions. In these three-dimensional reconstructions, axon trajectories and branching can be followed, and the terminal fields of axons become visible as agglomerations of varicosities. When the cells of the innervated tissue are labeled with cell specific markers, it is possible to determine which cell populations are within the terminal field of an axon, which gives a first hint of which cells are targeted by the nervous input. Most studies of islet innervation, however, have not attempted to identify the cellular targets of innervation. As a consequence, in many published images, axons seem lost in the pancreatic parenchyma in search for a target.
How can we determine which cells are targets of autonomic axons in the islet? This is not straightforward because autonomic axons do not show the same terminal specializations typical of axonal endings in the central nervous system or at neuromuscular endplates. To help us address this issue we can make two assumptions: a) varicosities are the sites at which neurotransmitters are released, and cells closely apposed to these varicosities can be considered targets 61 and b) because of the limits of optical resolution, close apposition between axonal varicosities and target cells (< 200 nm) can be revealed as an overlap in the axonal and the cellular labeling. This not only defines autonomic innervation conceptually but also provides a strategy to visualize the contacts established with effector cells. Using this approach we recently determined the cellular targets for parasympathetic and sympathetic axons in mouse and human islets 62. We dissected out in detail the preferential axonal targets in the islet and, based on these anatomical data, proposed that the autonomic nervous system uses different mechanisms to regulate islet function in mouse and human islets.
It is very likely that the anatomical observations have a functional correlate, but to be accurate and predictive this extrapolation requires in-depth structural studies and should include quantitative results. Few studies, however, have provided numbers on islet innervation mostly because the technology to measure axonal volumes in the tissue or contacts with target cells was not available until recently. With modern software applications, it is now possible to determine the volume of labeled structures within a defined tissue volume. The fraction of axonal volume to tissue volume gives an estimate of innervation densities that can be used to examine differences between tissues, species, or experimental conditions. For instance, the innervation density in the endocrine pancreas can be compared to that in the exocrine pancreas, which reveals a much stronger innervation of the islet in the mouse pancreas 62. Similarly, the volume of the overlap between the axonal staining and target cell staining can be used to determine if a cell population is innervated. Using this approach, we found that vascular cells, but not endocrine cells, are the major targets of sympathetic innervation in the human islet 62.
These examples show that it is feasible to get quantitative estimates of innervation patterns, without which studies are only subjective or anecdotal. There is a series of technical difficulties and critical checkpoints, however, to be considered when visualizing pancreatic innervation, in particular in human specimens:
Well-preserved human tissue specimens are not always available because pancreatic tissue is sensitive to post-mortem damage. Specimens come from cadaveric donors and the endocrine tissue is surrounded by acinar tissue loaded with digestive enzymes. Many specimens may be needed to get satisfactory results.
Effective and fast fixation of the pancreatic tissue and right choice of the fixative agent is needed to preserve the marker epitopes on target molecules, which will help detect axonal markers. For instance, long fixation in formalin, the default fixative agent used in most pathological laboratories, may impair antigenicity. It is important to perform controls to establish that antigenicity is not lost and that antibodies are recognizing the target proteins. The exocrine pancreas surrounding the islets can be inspected for the presence of labeled axons in major nerves or running along major blood vessels (Figure 2). In principle, staining of these axons should be conserved across species.
Inclusion or embedding of the tissue and the process of de-embedding (i.e., deparaffinization) may impair antigenicity. We recommend sectioning the fixed, frozen, Optimal Cutting Temperature (OCT)-embedded pancreas on a cryostat microtome.
Generally, tissues are cut into sections with cellular or subcellular thickness (5–15 um), which is not suitable for visualization of thin and elongated structural elements. In these thin sections it is difficult to distinguish between transected fibers, varicosities, and staining artifacts. Thicker sections (40–60 μm) are preferable.
Staining procedures, including the best use of detergents and optimized incubation times, are crucial to allow optimal penetration of antibodies. Antigen retrieval or amplification of the immunostaining staining may be necessary to visualize axonal markers.
Choice of commercial antibodies recognizing axon-specific markers is pivotal (Table 1). Using several antibodies for each axonal marker may be needed.
State of the art confocal microscope to acquire images with the optimal resolution (60X objective lenses with high numerical aperture) is required to image thin axons and small varicosities. Z-stacks of confocal images have to be acquired at the optimal z resolution to allow three-dimensional rendering of images.
Cellular imaging software (e.g. Volocity 3D Image Analysis Software, PerkinElmer) is necessary to reconstruct axonal tress and reveal clusters of varicosities in three dimensions (Figure 3). Software is also needed to measure the volumes of labeled structures and to detect cell contacts as defined by staining overlap.
Once volumes of labeled structures are measured, the axonal density per tissue volume can be calculated. The density of contacts of axons with specific types of cells can also be calculated as the fraction of the volume of the staining overlap to the volume of the staining of the cell population of interest. It is important to select adequate detection parameters to filter out background noise and staining artifacts (e.g. by carefully selecting detection thresholds). A limitation of this approach is that axon shafts close to target cells may also be included in the analyses. To avoid this, it is preferable to use axon markers that are concentrated in varicosities (e.g. vesicular neurotransmitter transporters).
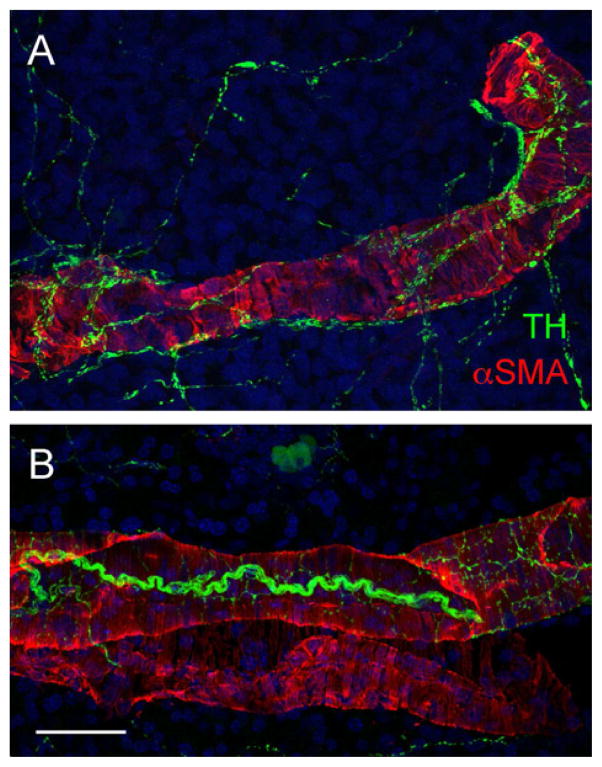
Figure 2.
Control immunostaining in the pancreas. Maximal projection of a Z-stack of 40 confocal images (Z step = 0.5 μm) of a human (A) and a mouse (B) pancreatic section showing vascular smooth muscle cells of a large artery labeled for alpha smooth muscle actin (aSMA, red) and the sympathetic nerves running along the vessel labeled for TH (green). Notice that the staining patterns are similar in both species. Scale bar = 20 μm.
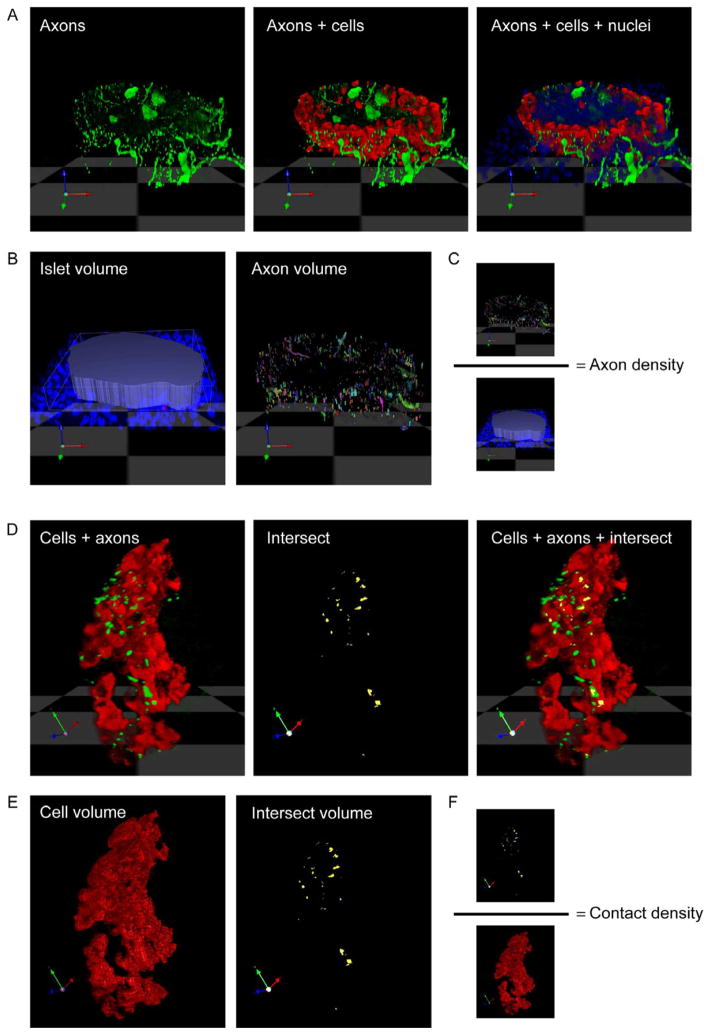
Figure 3.
Representation of axon density and contact density quantification. (A) Three dimensional rendering of a z-stack of confocal images (n = 40) of a mouse pancreatic section stained for TH (green), glucagon (red), and DAPI (blue). (B) Islet volume (in voxels) is determined by outlining the islet using DAPI staining. The volume of axonal staining is determined within this islet volume. (C) The axon (innervation) density is calculated as the quotient axon volume/ islet volume. (D) TH-labeled axons can be seen in close proximity to glucagon-labeled alpha cells. Shown is a higher magnification and rotated image of the islet in A. The proximity of axons to target cells can be detected using an algorithm in Volocity software (“intersect). Intersect between axons and cells is shown in yellow. Notice that few axonal varicosities contact target cells (yellow and green overlap in the merged image on the right). (E) Cellular (cell) volume is determined based on cell immunostaining, intersect volume is determined as the close proximity or overlap of axon and cell immunostaining. (F) The contact density is calculated as the quotient intersect volume/ cellular volume.
Physiological approaches to study innervation
In vivo investigation of the influence of innervation on islet function has been mostly limited to studies in which the effects of systemic intervention (i.e. stimulation of the vagus nerve, exogenous application of neurotransmitters) are measured with systemic metabolic readouts (i.e. blood insulin levels). Under these circumstances, the stimulation is not specific to the islet, which raises a series of issues: 1) the parasympathetic and sympathetic branches of the autonomic nervous system control many physiological events in the body. Application of agonists or antagonists and stimulation or transection of the autonomic nerves can indirectly affect islet hormone secretion; 2) autonomic axons release a panoply of neurotransmitters and neuropeptides, which makes it difficult to interpret the effects of autonomic input on islet hormone secretion in vivo. For instance, vagal stimulation can release at least five neurotransmitters (acetylcholine, VIP, PACAP, GRP, and nitric oxide), the relative contribution of which differs between organs and species; 3) several intestinal hormones, particularly glucose-dependent insulin-releasing peptide (GIP) and glucagon-like peptide-1 (GLP-1), potently increase insulin secretion. GIP - and GLP-1-secreting cells express neurotransmitter receptors, and both the stimulation of autonomic nerves and agonists stimulate their release; and 4) stimulation of autonomic input can change regional blood flow, which may impact islet hormone secretion into the bloodstream. The fact is that selective stimulation of pancreas innervation is difficult 36, 37, and specific stimulation of axons contacting cells within the islet may never be achieved in vivo, in particular in the human pancreas. For these reasons, results need to be interpreted with caution.
Even if it were possible to selectively stimulate islet innervation, we would only be able to measure the effects on islet cells indirectly, namely by using changes in hormone blood concentration as a readout. Hormone levels in the blood, however, are the end product of a chain of events occurring in the islet as well as downstream after interactions with target tissues. The autonomic nervous input may influence directly the secretion of islet hormones, which may act as paracrine signals regulating the secretion of other islet hormones. Alternatively, sympathetic input to islet blood vessels may change regional blood flow and increase hormone release into the circulation. The current readouts do not allow discerning the proximal mechanisms used by the local innervation to regulate islet function. A common approach to detect parasympathetic activation of islet function is to measure the concentration of pancreatic polypeptide (PP) in the circulation because PP is released only in response to vagal activation 8. Although it is an important tool for measuring vagal activity in the pancreas, a caveat here is that PP blood levels may not reflect parasympathetic input to most islets because endocrine cells producing and releasing PP are concentrated in a distinct region of the human pancreas in islets almost devoid of beta and alpha cells 63–65.
New, creative approaches are needed to overcome the limitations of studying the functional role of islet innervation. Emerging efforts include in vivo studies in human beings using pharmacological agents that act selectively on neurotransmitter release from autonomic axons. Thus, endogenous release of noradrenaline can be specifically stimulated from sympathetic axons by infusing the indirect sympathomimetic agent tyramine 66. This produces only modest effects on potentially confounding circulating noradrenaline levels. Using this approach, an effect of sympathetic innervation on insulin secretion was finally demonstrated in human beings 66. Whether this effect was mediated by axons acting directly on beta cells or by other mechanisms in the islet could not be shown. Unfortunately, monitoring islet activity in the organism is very challenging, and non- or minimally-invasive technologies to monitor islet cell function with sufficient spatial resolution have not been developed.
In an attempt to study beta cell function in a relatively intact environment, organotypic pancreatic slices containing islets are used for electrophysiological recordings 67–69. This preparation preserves intra-islet cellular communication and islet architecture in their native state, including the distal portion of the autonomic innervation. In principle, it should be feasible to stimulate local axons electrically while recording cellular responses in the islet, which allows characterizing the local mechanisms by which axons influence endocrine cell function. This approach has not been extended to the human pancreas, but it should be possible to produce living pancreatic slices from human pancreatic biopsies. Needless to say, in this preparation the pancreas is isolated from the organism and therefore these ex vivo experiments cannot determine the effects the islet’s nervous input has on glucose homeostasis.
It may turn out that studying the influence of innervation on islet function in vivo in the pancreas of human beings is not feasible. Recent efforts to image beta cells in vivo include magnetic resonance imaging and position emission tomography 70, 71. Such methods likely will improve quantifying transplanted islet mass but have low spatial resolution and do not allow functional monitoring of islets. An alternative strategy is to make human islets accessible for imaging by transplanting them into an animal model. Islets have been imaged after transplantation under the kidney capsule, where it has been possible to visualize the revascularization process 72, 73. However, the instability and inaccessibility of this transplantation site makes this approach very challenging, in particular for functional or longitudinal studies.
To dissociate the neural effects from hormonal and other confounding effects and to record islet function locally and systemically after manipulation of the neural input, investigators could use an experimental platform in which islets are transplanted into the anterior chamber of the eye for functional monitoring 74–76. In the early 1870s, van Dooremaal made the extraordinary observation that tumor cells injected into the anterior chamber of the eye formed progressively growing tumors 77. Since then, the anterior chamber of the eye has been widely used as a model system to study the biology of several tissues. Ovaries have been transplanted into the anterior eye chamber to study the physiology of ovulation 78, 79 and a wide variety of peripheral and central nervous tissues have been shown to proliferate and mature histologically after intraocular transplantation 80, 81. This site has also been used extensively to study the survival and growth of pancreatic tissue grafts 82, 83. Human xenografts survive in the anterior chamber of the eye of athymic nude rodents, where they become strongly and appropriately innervated 81, 84–86.
In this transplantation site biological phenomena can be monitored repeatedly without invasive procedures because the cornea is transparent. The grafts are easily vascularized and innervated because of the rich blood and nerve supply of the iris that forms the bed of the anterior chamber of the eye. The iris contains noradrenergic and cholinergic nerve fibers that control pupillary diameter. These fibers have been shown to innervate the intraocular grafts appropriately and can be specifically activated by changing the illumination 80, 87 or by topical drug application. Therefore, the autonomic input to the grafted tissue can be modulated non-invasively and locally. Taking advantage of these features can provide an experimental platform that allows local, specific manipulation of the neural input to the islets while imaging islet cell function in real-time in response to neural activation. To measure the effects of this manipulation on glucose homeostasis, systemic readouts such as detection of glucose and hormone plasma levels during glucose and insulin tolerance tests can be used. Local, cellular readouts include imaging of cytoplasmic [Ca2+], electrophysiological recordings, or measurements of blood flow.
A potential limitation is that human tissue will not be innervated to the same degree as mouse tissue because of species incompatibilities. However, these species incompatibilities may not be problematic because human tissues have been successfully transplanted into the mouse eye, where they become strongly and appropriately innervated 81, 85, 86. It is further important to clarify that the intraocular islet grafts are not under the control of the autonomous nervous system as they are in the pancreas. Thus, it is not possible to study the influences of feeding behavior and stress on nervous input to the islet. That islets are disconnected from this circuit and still work appropriately, however, can be advantageous. Cutting islet grafts from their natural nervous connections and replacing these with an artificial innervation may indeed be the only way to distinguish the direct effects of parasympathetic and sympathetic innervation from all other confounding factors that can affect islet function and survival. Autonomic axons in islet grafts in the eye can be activated selectively and non-invasively, enabling acute and chronic intervention while the physiological effects of islet innervation are monitored in a living organism.
Concluding remarks
There is a gap in our knowledge about how the nervous system controls human glucose homeostasis, and there are few comprehensive morphological studies of the innervation of the human islet of Langerhans. Malfunction of the islet of Langerhans is a hallmark of diabetes, a condition affecting millions. Despite many years of investigation, a clear picture of human islet microanatomy is emerging slowly. Still missing is a consolidated structural model that puts together the different subsets of human endocrine cells with vascular, immune, and neural elements. To understand the biology of the islet will also require establishing functional links between these different structures in vivo. A cohesive picture of the microanatomy of the islet of Langerhans will help us identify the sequential events leading to the failure of this micro-organ during diabetes.
Supplementary Material
KEY POINTS.
Malfunction of the islet of Langerhans is a hallmark of diabetes, a condition affecting millions.
The islets of Langerhans secrete hormones that maintain constant levels of plasma glucose. Dysfunction or death of the insulin-secreting beta cells leads to potentially life-threatening fluctuations in plasma glucose levels and is a major cause of diabetes mellitus, a devastating disease affecting millions worldwide.
Acknowledgments
Funding sources: Diabetes Research Institute Foundation, NIH grants NIH grants R56DK084321 and R01DK084321
Footnotes
Conflict of interest: Nil
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bernard C. Leçons De Physiologie Expérimentale Appliquée À La Médecine. Paris: Bailliere; 1855. [Google Scholar]
- 2.Langerhans P, Morrison H. Contributions to the microscopic anatomy of the pancreas. The Johns Hopkins press; Baltimore: 1937. [Google Scholar]
- 3.Woods S, Porte DJ. Neural control of the endocrine pancreas. Physiol Rev. 1974;54:596–619. doi: 10.1152/physrev.1974.54.3.596. [DOI] [PubMed] [Google Scholar]
- 4.Satin L, Kinard T. Neurotransmitters and their receptors in the islets of Langerhans of the pancreas: what messages do acetylcholine, glutamate, and GABA transmit? Endocrine. 1998;8:213–223. doi: 10.1385/ENDO:8:3:213. [DOI] [PubMed] [Google Scholar]
- 5.Ahrén B. Autonomic regulation of islet hormone secretion-- implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- 6.Gilon P, Henquin J. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr Rev. 2001;22:565–604. doi: 10.1210/edrv.22.5.0440. [DOI] [PubMed] [Google Scholar]
- 7.Gerald J, Taborsky J. Handbook of Physiology. Oxford University Press, American Physiological Society; United States of America: 2001. [Google Scholar]
- 8.Teff KL. How neural mediation of anticipatory and compensatory insulin release helps us tolerate food. Physiol Behav. 2011;103:44–50. doi: 10.1016/j.physbeh.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonner-Weir S. Islets of Langerhans:Morphology and postnatal growth. Lippincott Williams & Wilkins; Boston, MA: 2005. [Google Scholar]
- 10.Cegrell L. Catecholamines in pancreas of human fetuses. Acta Physiol Scand. 1968:14–16. [Google Scholar]
- 11.Ahrén B, Ericson L, Lundquist I, Lorén I, Sundler F. Adrenergic innervation of pancreatic islets and modulation of insulin secretion by the sympatho-adrenal system. Cell Tissue Res. 1981;216:15–30. doi: 10.1007/BF00234541. [DOI] [PubMed] [Google Scholar]
- 12.Ahrén B, Bötcher G, Kowalyk S, Dunning BE, Sundler F, Taborsky GJ. Galanin is co-localized with noradrenaline and neuropeptide Y in dog pancreas and celiac ganglion. Cell Tissue Res. 1990;261:49–58. doi: 10.1007/BF00329437. [DOI] [PubMed] [Google Scholar]
- 13.Stach W, Radke R. Innervation of islands of Langerhans. Light and electron microscopic studies of the pancreas in laboratory animals. Endokrinologie. 1982;79:210–220. [PubMed] [Google Scholar]
- 14.COUPLAND R. The innervation of pan creas of the rat, cat and rabbit as revealed by the cholinesterase technique. 92:143–149. [PMC free article] [PubMed] [Google Scholar]
- 15.Esterhuizen A, Spriggs T, Lever J. Nature of islet-cell innervation in the cat pancreas. Diabetes. 1968;17:33–36. doi: 10.2337/diab.17.1.33. [DOI] [PubMed] [Google Scholar]
- 16.Ahrén B, Taborsky GJ, Porte D. Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia. 1986;29:827–836. doi: 10.1007/BF00870137. [DOI] [PubMed] [Google Scholar]
- 17.Brunicardi F, Shavelle D, Andersen D. Neural regulation of the endocrine pancreas. Int J Pancreatol. 1995;18:177–195. doi: 10.1007/BF02784941. [DOI] [PubMed] [Google Scholar]
- 18.Love J, Szebeni K. Morphology and histochemistry of the rabbit pancreatic innervation. Pancreas. 1999;18:53–64. doi: 10.1097/00006676-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Ahrén B, Böttcher G, Kowalyk S, Dunning B, Sundler F, Taborsky GJ. Galanin is co-localized with noradrenaline and neuropeptide Y in dog pancreas and celiac ganglion. Cell Tissue Res. 1990;261:49–58. doi: 10.1007/BF00329437. [DOI] [PubMed] [Google Scholar]
- 20.Ahrén B, Berggren PO, Rorsman P, Ostenson CG, Efendic S. Neuropeptides in the regulation of islet hormone secretion--localization, effects and mode of action. Adv Exp Med Biol. 1991;291:129–142. doi: 10.1007/978-1-4684-5931-9_11. [DOI] [PubMed] [Google Scholar]
- 21.Shimosegawa T, Asakura T, Kashimura J, Yoshida K, Meguro T, Koizumi M, Mochizuki T, Yanaihara N, Toyota T. Neurons containing gastrin releasing peptide-like immunoreactivity in the human pancreas. Pancreas. 1993;8:403–412. doi: 10.1097/00006676-199307000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Ding WG, Kimura H, Fujimura M, Fujimiya M. Neuropeptide Y and peptide YY immunoreactivities in the pancreas of various vertebrates. Peptides. 1997;18:1523–1529. doi: 10.1016/s0196-9781(97)00237-4. [DOI] [PubMed] [Google Scholar]
- 23.Brissova M, Fowler M, Nicholson W, Chu A, Hirshberg B, Harlan D, Powers A. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 24.Cabrera O, Berman D, Kenyon N, Ricordi C, Berggrern P, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosco D, Armanet M, Morel P, Niclauss N, Sgroi A, Muller YD, Giovannoni L, Parnaud G, Berney T. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes. 2010;59:1202–1210. doi: 10.2337/db09-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verspohl EJ, Tacke R, Mutschler E, Lambrecht G. Muscarinic receptor subtypes in rat pancreatic islets: binding and functional studies. Eur J Pharmacol. 1990;178:303–311. doi: 10.1016/0014-2999(90)90109-j. [DOI] [PubMed] [Google Scholar]
- 27.Havel P, Akpan J, Curry D, Stern J, Gingerich R, Ahren B. Autonomic control of pancreatic polypeptide and glucagon secretion during neuroglucopenia and hypoglycemia in mice. Am J Physiol. 1993;265:R246–254. doi: 10.1152/ajpregu.1993.265.1.R246. [DOI] [PubMed] [Google Scholar]
- 28.Gautam D, Han S, Hamdan F, Jeon J, Li B, Li J, Cui Y, Mears D, Lu H, Deng C, Heard T, Wess J. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006;3:449–461. doi: 10.1016/j.cmet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Dunning B, Taborsky GJ. Galanin--sympathetic neurotransmitter in endocrine pancreas? Diabetes. 1988;37:1157–1162. doi: 10.2337/diab.37.9.1157. [DOI] [PubMed] [Google Scholar]
- 30.Daniel PM, Henderson JR. The effect of vagal stimulation on plasma insulin and glucose levels in the baboon. J Physiol. 1967;192:317–327. doi: 10.1113/jphysiol.1967.sp008302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frohman LA, Ezdinli EZ, Javid R. Effect of vagotomy and vagal stimulation on insulin secretion. Diabetes. 1967;16:443–448. doi: 10.2337/diab.16.7.443. [DOI] [PubMed] [Google Scholar]
- 32.Kaneto A, Kosaka K, Nakao K. Effects of stimulation of the vagus nerve on insulin secretion. Endocrinology. 1967;80:530–536. doi: 10.1210/endo-80-3-530. [DOI] [PubMed] [Google Scholar]
- 33.Alejandro R, Feldman E, Bloom A, Kenyon N. Effects of cyclosporin on insulin and C-peptide secretion in healthy beagles. Diabetes. 1989;38:698–703. doi: 10.2337/diab.38.6.698. [DOI] [PubMed] [Google Scholar]
- 34.Holst JJ, Grønholt R, Schaffalitzky de Muckadell OB, Fahrenkrug J. Nervous control of pancreatic endocrine secretion in pigs. I. Insulin and glucagon responses to electrical stimulation of the vagus nerves. Acta Physiol Scand. 1981;111:1–7. doi: 10.1111/j.1748-1716.1981.tb06697.x. [DOI] [PubMed] [Google Scholar]
- 35.Porte D, Williams RH. Inhibition of insulin release by norepinephrine in man. Science. 1966;152:1248–1250. doi: 10.1126/science.152.3726.1248. [DOI] [PubMed] [Google Scholar]
- 36.Bloom SR, Edwards AV. Characteristics of the neuroendocrine responses to stimulation of the splanchnic nerves in bursts in the conscious calf. J Physiol. 1984;346:533–545. doi: 10.1113/jphysiol.1984.sp015039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahrén B, Veith RC, Taborsky GJ. Sympathetic nerve stimulation versus pancreatic norepinephrine infusion in the dog: 1). Effects on basal release of insulin and glucagon. Endocrinology. 1987;121:323–331. doi: 10.1210/endo-121-1-323. [DOI] [PubMed] [Google Scholar]
- 38.Kurose T, Seino Y, Nishi S, Tsuji K, Taminato T, Tsuda K, Imura H. Mechanism of sympathetic neural regulation of insulin, somatostatin, and glucagon secretion. Am J Physiol. 1990;258:E220–227. doi: 10.1152/ajpendo.1990.258.1.E220. [DOI] [PubMed] [Google Scholar]
- 39.Holst JJ, Jensen SL, Knuhtsen S, Nielsen OV. Autonomic nervous control of pancreatic somatostatin secretion. Am J Physiol. 1983;245:E542–548. doi: 10.1152/ajpendo.1983.245.6.E542. [DOI] [PubMed] [Google Scholar]
- 40.Dunning BE, Taborsky GJ. Galanin--sympathetic neurotransmitter in endocrine pancreas? Diabetes. 1988;37:1157–1162. doi: 10.2337/diab.37.9.1157. [DOI] [PubMed] [Google Scholar]
- 41.Havel PJ, Taborsky GJ. The contribution of the autonomic nervous system to changes of glucagon and insulin secretion during hypoglycemic stress. Endocr Rev. 1989;10:332–350. doi: 10.1210/edrv-10-3-332. [DOI] [PubMed] [Google Scholar]
- 42.Winer S, Tsui H, Lau A, Song A, Li X, Cheung RK, Sampson A, Afifiyan F, Elford A, Jackowski G, Becker DJ, Santamaria P, Ohashi P, Dosch HM. Autoimmune islet destruction in spontaneous type 1 diabetes is not beta-cell exclusive. Nat Med. 2003;9:198–205. doi: 10.1038/nm818. [DOI] [PubMed] [Google Scholar]
- 43.Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, Tsui H, Tang L, Tsai S, Santamaria P, Driver JP, Serreze D, Salter MW, Dosch HM. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 44.Gram D, Ahrén B, Nagy I, Olsen U, Brand C, Sundler F, Tabanera R, Svendsen O, Carr R, Santha P, Wierup N, Hansen A. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur J Neurosci. 2007;25:213–223. doi: 10.1111/j.1460-9568.2006.05261.x. [DOI] [PubMed] [Google Scholar]
- 45.Linquette M, Fourlinnie JC, Lagache G. [Study of blood sugar and insulin after vagotomy and pyloroplasty in man] Ann Endocrinol (Paris) 1969;30:96–102. [PubMed] [Google Scholar]
- 46.Hakanson R, Liedberg G, Lundquist I. Effect of vagal denervation on insulin release after oral and intravenous glucose. Experientia. 1971;27:460–461. doi: 10.1007/BF02137312. [DOI] [PubMed] [Google Scholar]
- 47.Pozza G, Bosi E, Secchi A, Piatti P, Touraine J, Gelet A, Pontiroli A, Dubernard J, Traeger J. Metabolic control of type I (insulin dependent) diabetes after pancreas transplantation. Br Med J (Clin Res Ed) 1985;291:510–513. doi: 10.1136/bmj.291.6494.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diem P, Redmon J, Abid M, Moran A, Sutherland D, Halter J, Robertson R. Glucagon, catecholamine and pancreatic polypeptide secretion in type I diabetic recipients of pancreas allografts. J Clin Invest. 1990;86:2008–2013. doi: 10.1172/JCI114936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blackman J, Polonsky K, Jaspan J, Sturis J, Van Cauter E, Thistlethwaite J. Insulin secretory profiles and C-peptide clearance kinetics at 6 months and 2 years after kidney-pancreas transplantation. Diabetes. 1992;41:1346–1354. doi: 10.2337/diab.41.10.1346. [DOI] [PubMed] [Google Scholar]
- 50.Madsbad S, Christiansen E, Tibell A, Tydén G, Rasmussen K, Burcharth F. Beta-cell dysfunction following successful segmental pancreas transplantation. Danish-Swedish Study Group of Metabolic Effect of Pancreas Transplantation. Transplant Proc. 1994;26:469–470. [PubMed] [Google Scholar]
- 51.Havel P, Taborsky G. The autonomic nervous system and insulin secretion. London: 1994. [DOI] [PubMed] [Google Scholar]
- 52.Havel PJ, Valverde C. Autonomic mediation of glucagon secretion during insulin-induced hypoglycemia in rhesus monkeys. Diabetes. 1996;45:960–966. doi: 10.2337/diab.45.7.960. [DOI] [PubMed] [Google Scholar]
- 53.Hilsted J, Frandsen H, Holst JJ, Christensen NJ, Nielsen SL. Plasma glucagon and glucose recovery after hypoglycemia: the effect of total autonomic blockade. Acta Endocrinol (Copenh) 1991;125:466–469. doi: 10.1530/acta.0.1250466. [DOI] [PubMed] [Google Scholar]
- 54.Towler DA, Havlin CE, Craft S, Cryer P. Mechanism of awareness of hypoglycemia. Perception of neurogenic (predominantly cholinergic) rather than neuroglycopenic symptoms. Diabetes. 1993;42:1791–1798. doi: 10.2337/diab.42.12.1791. [DOI] [PubMed] [Google Scholar]
- 55.Ensinck JW, Walter RM, Palmer JP, Brodows RG, Campbell RG. Glucagon responses to hypoglycemia in adrenalectomized man. Metabolism. 1976;25:227–232. doi: 10.1016/0026-0495(76)90053-6. [DOI] [PubMed] [Google Scholar]
- 56.Brodows RG, Campbell RG, Al-Aziz AJ. Lack of central autonomic regulation of substrate during early fasting in man. Metabolism. 1976;25:803–807. doi: 10.1016/0026-0495(76)90150-5. [DOI] [PubMed] [Google Scholar]
- 57.Palmer JP, Henry DP, Benson JW, Johnson DG, Ensinck JW. Glucagon response to hypoglycemia in sympathectomized man. J Clin Invest. 1976;57:522–525. doi: 10.1172/JCI108305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmer JP, Werner PL, Hollander P, Ensinck JW. Evaluation of the control of glucagon secretion by the parasympathetic nervous system in man. Metabolism. 1979;28:549–552. doi: 10.1016/0026-0495(79)90196-3. [DOI] [PubMed] [Google Scholar]
- 59.Corral R, Frier B. Acute hypoglycemia in man: neural control of pancreatic islet cell function. Metabolism. 1981;30:160–164. doi: 10.1016/0026-0495(81)90166-9. [DOI] [PubMed] [Google Scholar]
- 60.Caicedo A, Herbert H. Topography of descending projections from the inferior colliculus to auditory brainstem nuclei in the rat. J Comp Neurol. 1993;328:377–392. doi: 10.1002/cne.903280305. [DOI] [PubMed] [Google Scholar]
- 61.Burnstock G. Autonomic neurotransmission: 60 years since sir Henry Dale. Annu Rev Pharmacol Toxicol. 2009;49:1–30. doi: 10.1146/annurev.pharmtox.052808.102215. [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, Caicedo A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011;14:45–54. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahier J, Goebbels R, Henquin J. Cellular composition of the human diabetic pancreas. Diabetologia. 1983;24:366–371. doi: 10.1007/BF00251826. [DOI] [PubMed] [Google Scholar]
- 64.Orci L, Malaisse-Lagae F, Baetens D, Perrelet A. Pancreaticpolypeptide- rich regions in human pancreas. Lancet. 1978;2:1200–1201. doi: 10.1016/s0140-6736(78)92181-5. [DOI] [PubMed] [Google Scholar]
- 65.Baetens D, Malaisse-Lagae F, Perrelet A, Orci L. Endocrine pancreas: three-dimensional reconstruction shows two types of islets of langerhans. Science. 1979;206:1323–1325. doi: 10.1126/science.390711. [DOI] [PubMed] [Google Scholar]
- 66.Gilliam LK, Palmer JP, Taborsky GJ. Tyramine-mediated activation of sympathetic nerves inhibits insulin secretion in humans. J Clin Endocrinol Metab. 2007;92:4035–4038. doi: 10.1210/jc.2007-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Speier S, Rupnik M. A novel approach to in situ characterization of pancreatic beta-cells. Pflugers Arch. 2003;446:553–558. doi: 10.1007/s00424-003-1097-9. [DOI] [PubMed] [Google Scholar]
- 68.Meneghel-Rozzo T, Rozzo A, Poppi L, Rupnik M. In vivo and in vitro development of mouse pancreatic beta-cells in organotypic slices. Cell Tissue Res. 2004;316:295–303. doi: 10.1007/s00441-004-0886-6. [DOI] [PubMed] [Google Scholar]
- 69.Huang YC, Rupnik M, Gaisano HY. Unperturbed islet α-cell function examined in mouse pancreas tissue slices. J Physiol. 2011;589:395–408. doi: 10.1113/jphysiol.2010.200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arifin DR, Bulte JW. Imaging of pancreatic islet cells. Diabetes Metab Res Rev. 2011;27:761–766. doi: 10.1002/dmrr.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Malaisse WJ, Maedler K. Imaging of the β-cells of the islets of Langerhans. Diabetes Res Clin Pract. 2012 doi: 10.1016/j.diabres.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 72.Bertera S, Geng X, Tawadrous Z, Bottino R, Balamurugan AN, Rudert WA, Drain P, Watkins SC, Trucco M. Body window-enabled in vivo multicolor imaging of transplanted mouse islets expressing an insulin-Timer fusion protein. Biotechniques. 2003;35:718–722. doi: 10.2144/03354st01. [DOI] [PubMed] [Google Scholar]
- 73.Nyqvist D, Köhler M, Wahlstedt H, Berggren PO. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes. 2005;54:2287–2293. doi: 10.2337/diabetes.54.8.2287. [DOI] [PubMed] [Google Scholar]
- 74.Speier S, Nyqvist D, Kohler M, Caicedo A, Leibiger I, Berggren P. Noninvasive high-resolution in vivo imaging of cell biology in the anterior chamber of the mouse eye. Nature Protocols. 2008;3:1278–1286. doi: 10.1038/nprot.2008.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Speier S, Nyqvist D, Cabrera O, Yu J, Molano R, Pileggi A, Moede T, Kohler M, Wilbertz J, Leibiger B, Ricordi C, Leibiger I, Caicedo A, Berggren P. Noninvasive in vivo imaging of pancreatic islet cell biology. Nature Medicine. 2008;14:574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perez VL, Caicedo A, Berman DM, Arrieta E, Abdulreda MH, Rodriguez-Diaz R, Pileggi A, Hernandez E, Dubovy SR, Parel JM, Ricordi C, Kenyon NM, Kenyon NS, Berggren PO. The anterior chamber of the eye as a clinical transplantation site for the treatment of diabetes: a study in a baboon model of diabetes. Diabetologia. 2011;54:1121–1126. doi: 10.1007/s00125-011-2091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dooremaal V. Die Entwicklung der in fremden Grund versetzten lebenden Geweba Albrecht Von Graefes. Arch Ophthalmol. 1873;19:358–373. [Google Scholar]
- 78.Goodman L. Observations on transplanted immature ovaries in the eye of the adult male and female rats. Anat Rec. 1934;59:223–251. [Google Scholar]
- 79.FALCK B. Site of production of oestrogen in rat ovary as studied in micro-transplants. Acta Physiol Scand Suppl. 1959;47:1–101. doi: 10.1111/j.1748-1716.1960.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 80.Taylor D, Seiger A, Freedman R, Olson L, Hoffer B. Electrophysiological analysis reinnervation of transplants in the anterior chamber of the eye by the autonomic ground plexus of the iris. Proc Natl Acad Sci U S A. 1978;75:1009–1012. doi: 10.1073/pnas.75.2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bickford-Wimer P, Granholm AC, Bygdeman M, Hoffer B, Olson L, Seiger A, Strömberg I. Human fetal cerebellar and cortical tissue transplanted to the anterior eye chamber of athymic rats: electrophysiological and structural studies. Proc Natl Acad Sci U S A. 1987;84:5957–5961. doi: 10.1073/pnas.84.16.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adeghate E, Donáth T. Morphological findings in long-term pancreatic tissue transplants in the anterior eye chamber of rats. Pancreas. 1990;5:298–305. doi: 10.1097/00006676-199005000-00009. [DOI] [PubMed] [Google Scholar]
- 83.Adeghate E, Donáth T. Transplantation of tissue grafts into the anterior eye chamber: a method to study intrinsic neurons. Brain Res Brain Res Protoc. 2000;6:33–39. doi: 10.1016/s1385-299x(00)00034-9. [DOI] [PubMed] [Google Scholar]
- 84.Olson L, Strömberg I, Bygdeman M, Granholm AC, Hoffer B, Freedman R, Seiger A. Human fetal tissues grafted to rodent hosts: structural and functional observations of brain, adrenal and heart tissues in oculo. Exp Brain Res. 1987;67:163–178. doi: 10.1007/BF00269464. [DOI] [PubMed] [Google Scholar]
- 85.Granholm AC, Eriksdotter-Nilsson M, Strömberg I, Stieg P, Seiger A, Bygdeman M, Geffard M, Oertel W, Dahl D, Olson L. Morphological and electrophysiological studies of human hippocampal transplants in the anterior eye chamber of athymic nude rats. Exp Neurol. 1989;104:162–171. doi: 10.1016/s0014-4886(89)80010-x. [DOI] [PubMed] [Google Scholar]
- 86.Granholm AC, Gerhardt GA, Bygdeman M, Strömberg I. Human fetal xenografts of brainstem tissue containing locus coeruleus neurons: functional and structural studies of intraocular grafts in athymic nude rats. Exp Neurol. 1992;118:7–17. doi: 10.1016/0014-4886(92)90017-k. [DOI] [PubMed] [Google Scholar]
- 87.Tucker DC, Gist R. Sympathetic innervation alters growth and intrinsic heart rate of fetal rat atria maturing in oculo. Circ Res. 1986;59:534–544. doi: 10.1161/01.res.59.5.534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.