Abstract
18F-FDG PET is a new noninvasive tool for inflammation functional imaging. Low spatial resolution is now compensated by coregistration with CT or MRI. New mechanistic insights have emerged from animal and histology to explain the obtained signals by hypoxia, macrophage infiltration, and differentiation. Mixed results have been found in biomarkers studies. Interesting data have come recently linking plaque anatomy and function in carotids and in aortic aneurysms as well as inflammation and events. In coronary arteries, plaque assessment is still hampered by myocardium uptake but developments are being made. 18-FDG PET has been able to monitor inflammation before and after several therapies in animals and humans but to date the lack of standardization and the absence of prospective event-driven studies prevent this promising technique to be used in clinical practice.
Keywords: Noninvasive imaging, Inflammation, 18f-fluorodesoxyglucose, Positron emission tomography, Macrophages, Atherosclerotic plaque
Introduction
Conventional clinical imaging by angiography gives information about site and severity of disease but not the more important plaque composition. Noninvasive assessment of atherosclerotic plaques is needed to better predict events in the at-risk but asymptomatic, provide information regarding the underlying vascular biology, and track the effect of novel interventions, which will rely on interference with key identified pathways. Molecular imaging with positron emission tomography (PET) is sensitive enough to detect inflammation within atherosclerotic plaques. The tracer 18F-fluorodeoxyglucose (18F-FDG) is taken up by macrophages and has proven to be a robust noninvasive surrogate of plaque composition and activity. This review will outline the recent evidence and other emerging applications for FDG PET in atherosclerosis imaging.
18F-FDG Pet Technique
The metabolic 18F-FDG tracer is a glucose analogue, which preferentially accumulates in macrophages. In clinical and preclinical studies of atherosclerosis, areas of high macrophage density correlate with enhanced FDG uptake in vessels with plaque. However the precise mechanisms leading to the uptake remain uncertain as other cells such as neutrophils, endothelial cells, lymphocytes, or smooth muscular cells (SMC) also seem to contribute to the uptake. A recent in vitro study revealed that 18F-FDG PET appears to visualize the early stage of foam cell formation in vulnerable plaques [1•]. The uptake was decreased to the control level after the cells had differentiated completely to foam cells. Another in vitro study reported that hypoxia is a potent and sufficient stimulus for increased glucose uptake in macrophages and foam cells, suggesting that hypoxia rather than pro-inflammatory stimuli could augment FDG uptake in plaques [2•].
Techniques and method of image analysis of FDG PET for atherosclerosis are not standardized. One of the major technical parameter conditioning the quality of the contrast obtained is FDG circulation time. It must be long enough to allow for FDG uptake in areas of interest and for background FDG levels to be reduced to generate a favorable target-to-background signal and must be as short as possible to allow a better workflow for imaging departments and make the 18F-FDG PET examinations more acceptable to the patient. While in oncology a 1 hour time is commonly accepted, in vascular imaging this time varies from 60 to 180 minutes according to prior studies. Dynamic studies revealed that18-FDG uptake occurs over a longer time course in arteries than in tumors, so a longer circulation time of at least 90 minutes is recommended. This recommendation was confirmed by a recent study conducted on patients with symptomatic carotid atherosclerotic plaques using a combined PET/CT system at 1, 2, and 3 hours after injection which showed an increase in quantitative parameters of FDG uptake, whereas those of blood pools were decreased.
An exact estimation of tracer uptake in plaque is crucial for PET imaging especially for studies assessing the activity of plaque before and after pharmacologic intervention. Different methods have been proposed to quantify FDG uptake in atherosclerosis: vessel wall-to-blood ratio, whole vessel-to-blood ratio, differential uptake ratio, blood pool ratio, and standardized uptake value (SUV). The SUV, which is the decay-corrected tissue concentration of FDG (in kBq/g), corrected for injected FDG dose and lean body mass, is a widely accepted method. The target-to-background ratio (TBR) is a blood corrected uptake quantification and may be obtained by dividing the SUVmax of the lesion by an averaged SUV of the blood-pool. Reproducibility of both SUV and TBR has already been established. Derlin et al recently reported that additional correction by division with SUV blood-pool might lead to an over-correction and concluded that there is no reason to prefer TBR over SUV max for the quantification of tracer uptake [3].
The exact uptake estimation is also hampered by the limited spatial resolution of the PET (about ~4.5–6 mm), while atherosclerotic plaques size is usually about the same. Therefore, quantification of tracer uptake in those structures can be significantly affected by partial volume error and by surrounding tissue activity. In the carotid area, lymph nodes, salivary glands, and pharyngeal tissues sometimes show high uptake, potentially affecting the carotid artery count.
This low spatial resolution is now compensated by co-registration with other imaging modality to localize 18F-FDG uptake to the underlying anatomy. So far, PET+CT and combined PET/CT have been the most used for several reasons: ease of coregistration of PET and CT images, CT attenuation correction, faster scan times, and the wide availability of combined scanners in cancer-imaging programs. Combined CT or MR systems avoid coregistration issues and combined PET/MR systems are becoming more and more available. Such systems provide the possibility of MR tissue characterization (high resolution of soft tissue) without CT related irradiation. The PET/MR combination is an excellent morphologic and functional imaging modality for atherosclerotic plaque studies.
Animal Studies
In rabbit models of progressive atherosclerosis, after balloon aortic injury and high-fat, atherogenic diets, 18F-FDG uptake has been shown to be higher in diseased regions as compared with healthy arterial wall and can be reduced with reversion to a normal diet [4]. In another study [5], rabbits were fed with high fat diet for 5 months after aortic endothelia damage then changed for normal diet plus atorvastatin for 4 months. Rabbits with induced atherosclerosis showed significantly higher 18F-FDG uptake than the control group and after 4 months of atorvastatin treatment and diet modification, SUVs decreased significantly. However, no marked difference was found in TBR, the number of macrophages, the number of SMC and the cap-to-core ratio between groups. However, TBR correlated significantly with macrophage number.
Several other treatments were evaluated on rabbits. Pioglitazone, a peroxisome proliferator-activated receptor-γ agonist with potent anti-inflammatory properties was administrated in rabbits with induced atheroma by a combination of hyperlipidemic diet and balloon endothelial denudations [6•]. Animals underwent 18-FDG PET/CT and dynamic contrast enhanced (DCE)-MRI at baseline, 1 month, and 3 months after treatment initiation. SUV increased in the control group but remained stable in the treatment group. Immunohistologic examination of the aortas demonstrated a significant decrease in macrophage in the pioglitazone group with respect to control animals. Positive correlations between SUV and macrophage density and between neovessels as detected by DCE-MRI were detected.
The other tested drug is ezetimibe, a lipid lowering therapy that inhibits intestinal transport of cholesterol. Ezetimibe was administrated to atherosclerotic rabbits that were pharmacologically triggered for plaque disruption. Serum and aortic wall cholesterol, plaque area, and thrombosis area were significantly lower in the treated group vs control with also less inflammation detected by PET and RAM 11 macrophage staining [7].
Aortic Aneurysm, Looking Beyond Structure
Arterial wall inflammation is present in aortic aneurysm and is thought to be one of the main features leading to rupture. Experimental aortic aneurysms can be induced in apolipoprotein E−/− mice via systemic administration of angiotensin II. Nahrendorf et al [8] showed that SUVs were increased in the aneurysms. Therefore, PET/CT could be used to track and monitor inflammation within aortic aneurysms during their different stages and detect if inflammation within the arterial wall could predict growth and/or rupture. The main problem of this attitude is that experimental aneurysms are induced by mechanisms, which may not be transposable in humans. Therefore, no definitive answer on the physiopathology should be drawn from those studies.
New Approach to Image Coronary Arteries Inflammation
The main limitation to 18F-FDG PET imaging of the coronary arteries is myocardial uptake. In a recent pilot study of coronary imaging in mice [9], verapamil administration decreased myocardial 18-FDG uptake by 31 % in the group administered with 1 mg/kg and 37 % in those that received a 20 mg/kg dose. This type of intervention with a drug commonly used in humans may facilitate coronary atherosclerotic plaque inflammation imaging by 18F-FDG PET scans.
Some controversial data were however published contradicting the potential direct link between inflammation as seen in Pet/CT and plaque activity. Falk et al [10] demonstrated with micro PET that spontaneously developed advanced atherosclerotic lesions in mice aorta were, paradoxically, associated with reduced FDG uptake, and accelerated carotid atherosclerosis also failed to increase FDG-uptake.
FDG and Biomarkers
A wide variety of biomarkers are available to measure inflammation in the blood. It is well known that atherosclerosis is associated with systemic inflammation, but there is, to date, no strong evidence to tell which is the best biomarker that is the most predictive of future cardiovascular events.
There are mixed results when comparisons are made between C-reactive protein (CRP) and 18F-FDG uptake. A first study found that patients in the highest quartile of CRP had the greatest degree of vascular 18-FDG uptake, regardless of their LDL-cholesterol levels [11]. Interestingly, other emerging inflammatory markers such as lipoprotein-associated phospholipase A(2) or monocyte chemo attractant protein-1 were not coherently (NB: consistently?) associated with TBR values. In another study on carotids in patients undergoing endarterectomy, gene expression markers of vulnerability GLUT-1, CD68, cathepsin K, and HK2 were found to predict the degree of arterial FDG accumulation [12]. Finally, hsCRP levels and traditional cardiovascular risk factors in 142 non-diabetic subjects without history of cardiovascular disease were compared with carotid intimamedia thickness (CIMT), brachial-ankle pulse wave velocity (baPWV), and vascular inflammation represented as TBR using 18-FDG PET/CT [13]. In both low- and intermediate-Framingham risk score (FRS) categories, mean TBR values in subjects with higher hsCRP levels were significantly increased compared with those with lower hsCRP levels. However, baPWV and CIMT values did not significantly differ according to hsCRP levels in the same FRS categories. Mean TBR levels positively correlated with FRS, body mass index (BMI), whereas negatively correlated with HDL-cholesterol. Besides, hsCRP, LDL-cholesterol, BMI, and insulin resistance were independently associated with mean TBR values.
Recently, Myers et al [14•] showed in patients with symptomatic peripheral artery disease no significant correlation between CD68 level (as a measure of macrophage content in the atherectomy section) and FDG uptake in the peripheral arteries. The authors claimed that differences in lesion extraction technique, lesion size, the degree of inflammation, and imaging coregistration techniques may have been responsible for this lack of results.
Adipokines and Inflammation
Obese patients, and especially those with increased visceral fat, display inflammation independent of atherosclerosis. The adipose tissue itself hosts inflammatory reactions and synthesizes several pro-(resistin) or anti-inflammatory (adiponectin) hormones.
Those adipokines were studied in obese and controls subjects [15•] and compared with CIMT and TBR measured using 18-FDG PET. The mean TBR values were significantly higher in the obese subjects, although their mean CIMT levels were the same. Mean TBR values were negatively correlated to adiponectin levels and positively with resistin levels. TBR values were also independently associated with BMI and hsCRP.
In another study involving biomarkers, a marker of adipotoxicity, the adipocyte fatty acid-binding protein (AFBP) was measured in 87 men without previously diagnosed cardiovascular disease or diabetes [16]. Circulating A-FABP levels had positive and independent correlation with maximum TBR values. The maximum TBR levels exhibited an additive linear increment according to the rise in tertiles of the A-FABP levels in subjects with and without metabolic syndrome.
New Biomarkers and Perspectives
Because they are rather easy to measure, often cheap and sometimes widely available, biomarkers have been a subject of research in atherosclerosis and new markers are being discovered on a regular basis. For example, pigment epithelium-derived factor (PEDF), the serum level of which has been independently associated with vascular inflammation in PET and CIMT [17]. Metalloproteases (MMP) are enzymes known to be present in fibrous caps. MMP-9 in particular has been found in high quantity in vulnerable plaques and linked with fibrous cap disruption caused by the MMP-9 collagen lysis. Pedersen et al [18] found in human atherosclerotic carotid artery plaques that MMP-9 gene expression correlated with (18)FDG uptake.
Biomarkers represent a very exciting research topic but the negative results by Myers et al should recommend us to be cautious with pre clinical results. Moreover, clinical decisions driven by their measurements are still not standardized, as well as the timing of their reassessment, even with hsCRP, the most studied one. Finally, the biomarker may reflect the state of inflammation in the blood and may not necessarily reflect the inflammation in the vessel wall (as measured by FDG-PET).
Carotid Studies
Anatomy and Function
Carotid plaque screening with ultrasound is frequently done in cardiology, neurology, and diabetology. What to do with the discovered plaques is however sometimes more controversial.
Several features of the plaque itself such as its morphology or echolucency can be used to refine difficult clinical decisions. Two studies combining carotid 18F-FDG PET/CT and ultrasound have shown that carotid arterial walls TBR for echolucent plaque were significantly greater than TBR for calcified plaques, and than TBR of normal walls [19, 20].
Another study compared plaque 18F-FDG uptake, plaque anatomy in CT, staining for CD68 (macrophage density), and vascular endothelial growth factor in 21 patients undergoing endarterectomy [21].
The mean surgically excised carotid plaque 18F-FDG metabolism was superior to the contralateral one. Plaque uptake was positively correlated to CD68 staining and vascular endothelial growth factor and inversely correlated to calcium on CT. Among carotid plaque imaging modalities, MRI has emerged as one of the most powerful tools to assess carotid plaque composition. In a recent study [22•], the relationship between inflammation in PET and several plaque compositional features (HRM) of carotid plaques associated with high-risk of clinical event was studied in patients with PET and MRI coregistration.
Carotid inflammation increased with the number of HRM observed supporting the concept that plaque inflammation and composition are closely linked. Diabetics and patients with impaired glucose tolerance display greater carotid FDG uptake but other uptake determinants are not well known. In 82 patients with coronary artery disease, Bucerius et al [23] showed increased carotid inflammation defined by a TBR >1.8 in 67 % of the patients. They also demonstrated that the maximum TBR and SUV were associated with BMI>30 kg/m2, male gender, age >65 years, smoking, hypertension, and with the numbers of components of the metabolic syndrome.
Carotid Inflammation and Stroke
The natural history of plaque is very different in the carotid than in the coronary arteries. If they both display inflammation, the same forces and risk factors do not drive their growth. Plus, if the overwhelming majority of acute coronary syndromes are due to local plaque disruption, only half of TIA and stroke are caused by atherosclerosis. Therefore, one of the main challenges in patients with acute cerebrovascular event and atherosclerosis is to know whether their carotid plaques are responsible for their accident and if surgery should be performed to avoid recurrence.
There is circumstantial evidence from retrospective series of cancer patients undergoing PET that correlated subsequent stroke with foci of FDG uptake in the carotid arteries or aortic arch in a case–control study [24]. Stroke recurrence and responsibility of carotid plaque in the ipsilateral stroke has been linked to microembolic signals (MES) detected by transcranial Doppler. In a sample of recently symptomatic patients with carotid stenosis, an association was found between in vivo measures of plaque inflammation detected by PET (and co registered with MRI) and the presence of MES [25].
After an ischemic stroke with an ipsilateral stenosis over 70 %, PET/CT was able to detect significant differences between ipsilateral and contralateral asymptomatic plaques, thus reinforcing the fact that inflammation is active in acute setting [26]. Also, Masteling et al [27] showed, using micro PET, that FDG uptake and macrophage infiltration were different among carotid endarterectomy pieces excised from patients with different symptoms. Maximum FDG uptake was significantly higher in patients after a stroke compared with those with transient ischemic attack or amaurosis fugax patients, or compared with asymptomatic patients.
A recent study included 60 consecutive patients with a recent stroke, transient ischemic attack, or retinal embolism and ipsilateral carotid stenosis (≥50 %) [28••]. Twenty-two percent had stroke recurrence within 90 days and FDG uptake in ipsilateral carotid plaque was greater in patients with early recurrent stroke. These preliminary results reinforce the need for prospective evaluation of FDG-PET in event-driven studies to evaluate the role of this imaging technology as a clinically useful tool.
The BioImage Study [29], part of the High Risk Plaque Initiative, aims to identify imaging markers of risk for cardiovascular events over 3 years of follow-up. The patients recruited to this study will be imaged with a combination of MRI to identify carotid and aortic plaques, CT for arterial calcification and stenosis and 18FDG-PET/CT to measure carotid and aortic inflammation. The predictive ability of advanced imaging tests such as these will be compared with FRS alone.
Coronary Studies
A difficult but not impossible challenge [30], to improve poor resolution of PET, coronary images can be coregistered with coronary CT images. While resolving the problem, it severely increases the radiation exposure for the patient, hindering the possibility or repetition of those exams and posing the difficult issue of stochastic effects of radiation with a possible rise in cancer incidence.
18-FDG PET imaging of coronary atherosclerosis would be valuable. However it has many limitations as previously mentioned [31].
In 2010, Saam et al [32] measured FDG uptake in the coronary arteries of subjects with cancer. Evaluation of coronary artery 18-FDG uptake was only possible in half of the close to 300 patients imaged; the remainder had myocardial 18-FDG uptake that swamped any signal from the coronary plaque. In those interpretable images, there was a correlation between the number of cardiovascular risk factors and the quantity of calcified plaques present in the coronary arteries. Rogers et al [33] more recently used dietary manipulation in patients treated by percutaneous coronary intervention for stable angina and acute coronary syndromes (ACS). CT coronary angiography (CTA) images were registered to the PET/CT images. They demonstrated higher FDG uptake in the stented segments in acute coronary syndrome, as well as in the relatively fixed left main coronary artery, and the ascending aorta, consistent with a multifocal inflammatory response in unstable atherosclerosis. This very interesting study clearly demonstrates the role of inflammation in the mechanisms underlying ACS. It also shows the feasibility of coronary PET/CT imaging using 18F-FDG tracers. This however requires a very strict one day fat-allowed, carbohydrate-restricted diet. With this specific diet, low myocardial FDG uptake was obtained in up to 67 % of patients in opposition of 69 % of high myocardial uptake in control patients [34]. The high level of radiation exposure from the CTA is of concern. Coronary 18F-FDG PET/CT imaging may have an important role to play along with other molecular imaging modalities in the next few years when technical obstacles will be passed [35].
Myocardium Studies in Coronary Artery Disease
Ischemic cardiomyopathy is heterogeneous and complex, varying from predominantly hibernating muscle that will benefit most from revascularization to irreversible scarring, which will not. PET imaging is well validated to assess myocardial perfusion and metabolism after myocardial infarction (MI) [36]. It has also been associated with outcome in a post hoc analysis of the PET and Recovery Following Revascularization-2 study where post-MI revascularization improved outcome in patients with a myocardial perfusion-metabolism mismatch of over 7 % and did not in those with <7 % [37]. Yet, as mentioned above, what is seen through increase SUVs is controversial.
Recently, inflammation in mice within MI was studied with 18-FDG PET, MRI, and histology [38]. Infarcts showed high 18-FDG uptake on day 5 after MI, in good correlation to cellularity and with considerable monocyte recruitment. This study also showed interesting data on the non-ischemic myocardium with increase of monocytes, adhesion molecules, chemokines, and matrix metalloproteinase activity. Cell recruitment was slower in the infarct zone where it was maximal at day 10. FDG signal approached control values at day 14.
This very interesting pilot study shows the possibility for noninvasive imaging to approach mechanisms at stake at a molecular level with the synergy of PET and MRI. Since 18F decays with a half-life of 110 minutes, serial noninvasive spatiotemporal imaging of inflammation also appears feasible.
Aortic Studies
In the aorta, the main focus is abdominal aneurysms. Aneurysms are disease of the media and adventice with disruption of elastin fibres and inflammation. Aneurysms evolve over time brutally or progressively with very few strong predictors identified besides smoking. Aneurysms rupture challenge vital prognostic of patients and if their size is closely linked to their rupture risk, this acute event is not yet totally predictable.
Atherosclerosis, Calcification, and Aging
A recent retrospective study on serial 18F-FDG-PET/CTs of 100 cancer patients assessed the prevalence and the evolution of 18F-FDG aortic uptake [39]. It confirmed that atherosclerotic plaques are very common as increased aortic uptake was seen in 70 % of the patients on the initial scan and changed on the second scan to 55 %. 18-FDG vascular uptake correlated with vascular calcifications. Calcifications were stable over time, but 18-FDG uptake changed in more than half of the patients, supporting the postulate that inflammation is not a stable process in atheroma. Both calcification and 18-FDG uptake correlated with age. No correlation was seen between 18F-FDG uptake and CT determined calcification stability.
Aortic Aneurysms
Current guidelines for stable patients recommend surgical or endovascular intervention based largely on aortic dimensions, although it is well known that rupture can also occur in small aneurysms. It has been shown more recently that abdominal aortic FDG uptake is associated with higher wall stress [40] and correlates with aneurysm instability, symptoms and with macrophage infiltration.
In patients presenting with acute aortic syndrome who underwent acute FDG-PET imaging, there was a trend to less progression of disease in those with lower degrees of FDG [41] uptake. A study by Kato et al [42] in subjects with acute and chronic dissection suggested worse outcomes in those with the highest aortic FDG uptake at the maximal dilatation site. Whilst these are small studies conducted on potentially unstable populations, they suggest a possible role in risk stratification and planning of intervention in abdominal aortic aneurysm and type B aortic dissection.
One of the major challenges is to predict aneurysm growth. Preliminary findings from an observational longitudinal pilot study [43] suggest that there is an inverse trend between 18-FDG uptake and future expansion. Aortic aneurysms with lower metabolic activity may therefore be more likely to expand over a 12-month follow up.
Several studies studied aneurysms of pre-surgical size to assess their inflammation pattern. Interestingly, Palombo et al [44] showed that aneurysms which ranged from 4.8–5.4 cm had lower metabolic activity than the normal adjacent aorta wall or than the control patients aortas. A more recent study showed direct relationship between cell density and FDG uptake in asymptomatic aortic aneurysm also close to surgical threshold [45•]. In this work, PET/CT was performed on 12 candidates for aortic abdominal aneurisms surgical repair and in controls. Autoradiographic images were coregistered with immunohistochemical pictures of cell density and type. No visible uptake in abdominal aorta occurred in any patient or control subject. Immunohistochemistry documented a severe loss of wall structure, with low numbers of cells. Tracer retention directly correlated with overall cell density. This recent study was therefore able to relate the previously reported low prevalence of inflammation on PETCT with the loss of tissue structure and the marked decrease in cell density.
Taken together these studies on aortic aneurysms confirm the prominent role of inflammation in aneurisms growth, especially at early stages. Further validation is required in larger event-driven studies to assess the right place of inflammation imaging by PET.
Therapy Monitoring Studies Using 18 FDG Pet
As in the rabbits study [6•], 4-month treatment with pioglitazone attenuated atherosclerotic plaque inflammation in patients with impaired glucose tolerance or diabetes in comparison of glimepiride independently of glucose lowering [46].
Statins have demonstrated potential anti-inflammatory effects in vitro, unrelated to the LDL-cholesterol decrease. This was also shown in a randomized prospective study where 6 months of treatment with atorvastatin 20 mg was associated with a significant reduction in TBR in the ascending aorta and femoral artery of adults with dyslipidemia with stable angina pectoris [47].
The recently published phase 2b, double-blind, multicenter DAL-plaque study [48••] in high risk patients studied the effect on arterial wall after a 24-month treatment by dalcetrapib, a CETP-inhibitor raising HDL-cholesterol. The FDG-PET/CT measure of carotid artery showed a 7 % reduction in most-diseased-segment TBR in the dalcetrapib group compared with the placebo group at 6 months (Fig. 1).
Fig. 1.
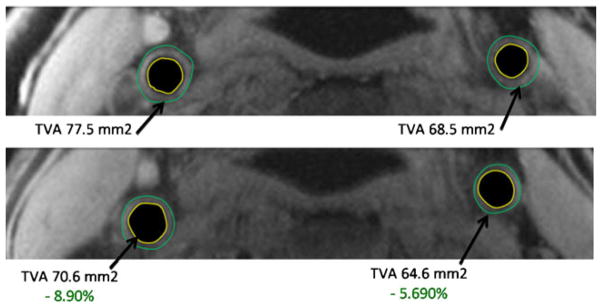
Sample MRI images from a patient at baseline top) and treated with dalcetrapib 600 mg for 24 months (bottom) showing regression in total vessel area (top panel). The MRI metrics used as endpoints are shown in the bottom panel. LCC = left carotid artery. RCC = right carotid artery. Wall outer boundary is denoted in green. Wall inner boundary is denoted in yellow. Total vessel area is lumen area + wall area. Normalized wall index is wall area divided by total vessel area and represents a ratio with no units
Conclusion
During the last years, more evidence has been gathered to reinforce the huge potential represented by inflammation imaging with 18FDG PET in preclinical field and in research.
More insight on what the received signals really stand for has come, especially from the vast amount of multimodal imaging studies that combined PET with CT or MRI (Table 1).
Table 1.
Important recent findings in 18FDG-PET atherosclerosis imaging
| Field of research | Important recent findings summary |
|---|---|
| Technique | FDG uptake is driven by inflammation but also hypoxia |
| Animals |
|
| Biomarkers |
|
| Carotid plaques |
|
| Stroke |
|
| Coronary arteries |
|
| Myocardium |
|
| Aortic aneurysms |
|
| Therapy monitoring in humans | PET able to track FDG uptake decrease in humans under atorvastatin, pioglitazone and HDL raising compound dalcetrapib |
Noninvasive arterial inflammation imaging has opened new fields of interest like the association of atherosclerosis with COPD, HIV, or periodontal disease [49]. It also been validated in inflammatory arteritis such as Erdheim Chester disease or Takasu arteritis [50].
Whilst the majority of work used FDG as a PET tracer, more specific tracers have emerged:
(11)C-acetate for imaging [3] of fatty acid synthesis in the atherosclerotic vessel wall
18F-sodium fluoride, which was successfully used in aortic valve stenosis and in coronary arteries
The translocator protein/peripheral benzodiazepine receptor (TSPO) ligands PK11195 and DAA1106, which bind to macrophage-rich regions in human carotid plaque in vitro but with less convincing results in vivo
In the future, ongoing hardware developments, such as the introduction of combined PET and MRI scanners, should help to harness the most attractive aspects of both modalities to have a more profound look into the biology of structures as small as the atherosclerotic plaques.
Ultimately, the lack of event-driven studies to date, the heaviness of the equipment, and the radiation exposure greatly hamper the widespread use of this technique in clinical practice, and is an issue that must be addressed.
Acknowledgments
This work was supported in part by NIH/NHLBI R01 HL071021, R01 HL078667; NIH/NBIB R01 EB009638; NIH/NHLBI Program of Excellence in Nanotechnology (PEN) Award, Contract #HHSN268201000045C; and NIH/NCRR CTSA UL1RR029887 (Imaging Core), and by funds from the Mount Sinai Cardiovascular Institute and the Department of Radiology. David Rosenbaum’s work was partially supported by the Fédération Française de Cardiologie, Paris, France.
Footnotes
Disclosure No potential conflicts of interest relevant to this article was reported.
Contributor Information
David Rosenbaum, Email: david.rosenbaum@psl.aphp.fr, Unité de Prévention Cardiovasculaire, Pole Cardiologie, métabolisme, Groupe Hospitalier Pitié-Salpêtrière, Assistance Publique-Hôpitaux de Paris, 83, Boulevard de l’Hôpital, 75651 Paris Cedex 13, France. UMR S939, Université Pierre et Marie Curie, Paris 6, Paris, France.
Antoine Millon, Translational and Molecular Imaging Institute, Mount Sinai School of Medicine, Mount Sinai Hospital, One Gustave L. Levy Place, New York, NY 10528, USA.
Zahi A. Fayad, Email: Zahi.Fayad@mssm.edu, Translational and Molecular Imaging Institute, Mount Sinai School of Medicine, Mount Sinai Hospital, One Gustave L. Levy Place, New York, NY 10528, USA
References
Papers of particular interest, published recently have been highlighted as:
• Of importance
•• Of major importance
- 1•.Ogawa M, Nakamura S, Saito Y, Kosugi M, Magata Y. What can be seen by 18F-FDG PET in atherosclerosis imaging? The effect of foam cell formation on 18F-FDG uptake to macrophages in vitro. Society of Nuclear Medicine official publication. J Nucl Med. 2012;53:55–8. doi: 10.2967/jnumed.111.092866. In this study, authors look at the very cause of FDG uptake and show the different role of macrophages and foam cells. [DOI] [PubMed] [Google Scholar]
- 2•.Folco EJ, Sheikine Y, Rocha VZ, et al. Hypoxia but not inflammation augments glucose uptake in human macrophages. Implications for imaging atherosclerosis with 18fluorine-labeled 2-deoxy-D-glucose positron emission tomography. J Am Coll Cardiol. 2011;58:603–14. doi: 10.1016/j.jacc.2011.03.044. Inflammation is not the only trigger for FDG uptake, in this study, various stimuli are overlooked and hypoxia seems to be the most important one. [DOI] [PubMed] [Google Scholar]
- 3.Derlin T, Habermann CR, Lengyel Z, et al. Feasibility of 11C-acetate PET/CT for imaging of fatty acid synthesis in the atherosclerotic vessel wall. J Nucl Med. 2011;52:1848–54. doi: 10.2967/jnumed.111.095869. [DOI] [PubMed] [Google Scholar]
- 4.Worthley SG, Zhang ZY, Machac J, et al. In vivo noninvasive serial monitoring of FDG-PET progression and regression in a rabbit model of atherosclerosis. Int J Cardiovasc Imaging. 2009;25:251–7. doi: 10.1007/s10554-008-9377-2. [DOI] [PubMed] [Google Scholar]
- 5.Zhao QM, Feng TT, Zhao X, et al. Imaging of atherosclerotic aorta of rabbit model by detection of plaque inflammation with fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography. Chin Med J. 2011;124:911–7. [PubMed] [Google Scholar]
- 6•.Vucic E, Dickson SD, Calcagno C, et al. Pioglitazone modulates vascular inflammation in atherosclerotic rabbits noninvasive assessment with FDG-PET-CT and dynamic contrast-enhanced MR imaging. JACC Cardiovasc Imaging. 2011;4:1100–9. doi: 10.1016/j.jcmg.2011.04.020. One of the first studies to demonstrate a decrease in inflammation not mediated by statin, a proof of concept study but with a drug that has been withdrawn from the market. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel R, Janoudi A, Vedre A, et al. Plaque rupture and thrombosis are reduced by lowering cholesterol levels and crystallization with ezetimibe and are correlated with fluorodeoxyglucose positron emission tomography. Arterioscler Thromb Vasc Biol. 2011;31:2007–14. doi: 10.1161/ATVBAHA.111.226167. [DOI] [PubMed] [Google Scholar]
- 8.Nahrendorf M, Keliher E, Marinelli B, et al. Detection of macrophages in aortic aneurysms by nanoparticle positron emission tomography-computed tomography. Arterioscler Thromb Vasc Biol. 2011;31:750–7. doi: 10.1161/ATVBAHA.110.221499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaeta C, Fernandez Y, Pavia J, et al. Reduced myocardial 18F-FDG uptake after calcium channel blocker administration. Initial observation for a potential new method to improve plaque detection. Eur J Nucl Med Mol Imaging. 2011;38:2018–24. doi: 10.1007/s00259-011-1873-2. [DOI] [PubMed] [Google Scholar]
- 10.Laurberg JM, Olsen AK, Hansen SB, et al. Imaging of vulnerable atherosclerotic plaques with FDG-microPET: no FDG accumulation. Atherosclerosis. 2007;192(2):275–82. doi: 10.1016/j.atherosclerosis.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Yoo HJ, Kim S, Park MS, et al. Vascular inflammation stratified by C-reactive protein and low-density lipoprotein cholesterol levels: analysis with 18F-FDG PET. J Nucl Med. 2011;52:10–7. doi: 10.2967/jnumed.110.080838. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen SF, Graebe M, Fisker Hag AM, Hojgaard L, Sillesen H, Kjaer A. Gene expression and 18FDG uptake in atherosclerotic carotid plaques. Nucl Med Commun. 2010;31:423–9. doi: 10.1097/MNM.0b013e32833767e0. [DOI] [PubMed] [Google Scholar]
- 13.Yang SJ, Kim S, Choi HY, et al. High-sensitivity C-reactive protein in the low- and intermediate-Framingham risk score groups: analysis with (18)F-fluorodeoxyglucose positron emission tomography. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 14•.Myers KS, Rudd JH, Hailman EP, et al. Correlation between arterial FDG uptake and biomarkers in peripheral artery disease. JACC Cardiovasc Imaging. 2012;5:38–45. doi: 10.1016/j.jcmg.2011.08.019. A negative study important as it reminds us to be careful with pre clinical studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Choi HY, Kim S, Yang SJ, et al. Association of adiponectin, resistin, and vascular inflammation: analysis with 18F-fluorodeoxyglucose positron emission tomography. Arterioscler Thromb Vasc Biol. 2011;31:944–9. doi: 10.1161/ATVBAHA.110.220673. Obesity is one of the next challenges of western societies and relations between visceral fat and cardiovascular disease are of the greatest interest for the future. [DOI] [PubMed] [Google Scholar]
- 16.Yoo HJ, Kim S, Park MS, et al. Serum adipocyte fatty acid-binding protein is associated independently with vascular inflammation: analysis with (18)F-fluorodeoxyglucose positron emission tomography. J Clin Endocrinol Metab. 2011;96:E488–92. doi: 10.1210/jc.2010-1473. [DOI] [PubMed] [Google Scholar]
- 17.Tahara N, Yamagishi S, Tahara A, et al. Serum level of pigment epithelium-derived factor is a marker of atherosclerosis in humans. Atherosclerosis. 2011;219:311–5. doi: 10.1016/j.atherosclerosis.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen SF, Graebe M, Hag AM, Hoejgaard L, Sillesen H, Kjaer A. Microvessel density but not neoangiogenesis is associated with (18)F-FDG uptake in human atherosclerotic carotid plaques. Mol Imaging Biol. 2012;14(3):384–92. doi: 10.1007/s11307-011-0507-1. [DOI] [PubMed] [Google Scholar]
- 19.Choi YS, Youn HJ, Chung WB, et al. Uptake of F-18 FDG and ultrasound analysis of carotid plaque. J Nucl Cardiol. 2011;18:267–72. doi: 10.1007/s12350-011-9338-3. [DOI] [PubMed] [Google Scholar]
- 20.Graebe M, Pedersen SF, Hojgaard L, Kjaer A, Sillesen H. 18FDG PET and ultrasound echolucency in carotid artery plaques. JACC Cardiovasc Imaging. 2010;3:289–95. doi: 10.1016/j.jcmg.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Menezes LJ, Kotze CW, Agu O, et al. Investigating vulnerable atheroma using combined (18)F-FDG PET/CT angiography of carotid plaque with immunohistochemical validation. J Nucl Med. 2011;52:1698–703. doi: 10.2967/jnumed.111.093724. [DOI] [PubMed] [Google Scholar]
- 22•.Figueroa AL, Subramanian SS, Cury RC, et al. Distribution of inflammation within carotid atherosclerotic plaques with high-risk morphological features: a comparison between positron emission tomography activity, plaque morphology, and histopathology. Circ Cardiovasc Imaging. 2012;5:69–77. doi: 10.1161/CIRCIMAGING.110.959478. Important results coming from this study combining anatomic and molecular imaging. [DOI] [PubMed] [Google Scholar]
- 23.Bucerius J, Duivenvoorden R, Mani V, et al. Prevalence and risk factors of carotid vessel wall inflammation in coronary artery disease patients: FDG-PET and CT imaging study. JACC Cardiovasc Imaging. 2011;4:1195–205. doi: 10.1016/j.jcmg.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grandpierre S, Desandes E, Meneroux B, et al. Arterial foci of F-18 fluorodeoxyglucose are associated with an enhanced risk of subsequent ischemic stroke in cancer patients: a case-control pilot study. Clin Nucl Med. 2011;36:85–90. doi: 10.1097/RLU.0b013e318203bb42. [DOI] [PubMed] [Google Scholar]
- 25.Moustafa RR, Izquierdo-Garcia D, Fryer TD, et al. Carotid plaque inflammation is associated with cerebral microembolism in patients with recent transient ischemic attack or stroke: a pilot study. Circ Cardiovasc Imaging. 2010;3:536–41. doi: 10.1161/CIRCIMAGING.110.938225. [DOI] [PubMed] [Google Scholar]
- 26.Kwee RM, Truijman MT, Mess WH, et al. Potential of integrated [18F] fluorodeoxyglucose positron-emission tomography/CT in identifying vulnerable carotid plaques. AJNR Am J Neuroradiol. 2011;32:950–4. doi: 10.3174/ajnr.A2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masteling MG, Zeebregts CJ, Tio RA, et al. High-resolution imaging of human atherosclerotic carotid plaques with micro 18F-FDG PET scanning exploring plaque vulnerability. J Nucl Cardiol. 2011;18:1066–75. doi: 10.1007/s12350-011-9460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Marnane M, Merwick A, Sheehan OC, et al. Carotid plaque inflammation on (18) F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Ann Neurol. 2012;71(5):709–18. doi: 10.1002/ana.23553. Very important study, demonstrating for the first time, in a well-conducted way, the prognostic importance of high mean and max carotid SUV (2.1 and 2.8, respectively) [DOI] [PubMed] [Google Scholar]
- 29.Muntendam P, McCall C, Sanz J, Falk E, Fuster V. High-risk plaque I. The BioImage study: novel approaches to risk assessment in the primary prevention of atherosclerotic cardiovascular disease–study design and objectives. Am Heart J. 2010;160:49–57e1. doi: 10.1016/j.ahj.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 30.Camici PG, Rimoldi OE, Gaemperli O, Libby P. Noninvasive anatomic and functional imaging of vascular inflammation and unstable plaque. Eur Heart J. 2012;33(11):1309–17. doi: 10.1093/eurheartj/ehs067. [DOI] [PubMed] [Google Scholar]
- 31.Rogers IS, Tawakol A. Imaging of coronary inflammation with FDG-PET: feasibility and clinical hurdles. Curr Cardiol Rep. 2011;13:138–44. doi: 10.1007/s11886-011-0168-3. [DOI] [PubMed] [Google Scholar]
- 32.Saam T, Rominger A, Wolpers S, et al. Association of inflammation of the left anterior descending coronary artery with cardiovascular risk factors, plaque burden and pericardial fat volume: a PET/CT study. Eur J Nucl Med Mol Imaging. 2010;37:1203–12. doi: 10.1007/s00259-010-1432-2. [DOI] [PubMed] [Google Scholar]
- 33.Rogers IS, Nasir K, Figueroa AL, et al. Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovasc Imaging. 2010;3:388–97. doi: 10.1016/j.jcmg.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Balink H, Hut E, Pol T, Flokstra FJ, Roef M. Suppression of 18F-FDG myocardial uptake using a fat-allowed, carbohydrate-restricted diet. J Nucl Med Technol. 2011;39:185–9. doi: 10.2967/jnmt.110.076489. [DOI] [PubMed] [Google Scholar]
- 35.Hucker WJ, Jaffer FA. F-FDG PET imaging of atherosclerosis–a new approach to detect inflamed, high-risk coronary plaques? Curr Cardiovasc Imaging Rep. 2011;4:1–3. doi: 10.1007/s12410-010-9054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuster A, Morton G, Chiribiri A, Perera D, Vanoverschelde JL, Nagel E. Imaging in the management of ischemic cardiomyopathy: special focus on magnetic resonance. J Am Coll Cardiol. 2012;59:359–70. doi: 10.1016/j.jacc.2011.08.076. [DOI] [PubMed] [Google Scholar]
- 37.D’Egidio G, Nichol G, Williams KA, et al. Increasing benefit from revascularization is associated with increasing amounts of myocardial hibernation: a substudy of the PARR-2 trial. JACC Cardiovasc Imaging. 2009;2:1060–8. doi: 10.1016/j.jcmg.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Lee WW, Marinelli B, van der Laan AM, et al. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol. 2012;59:153–63. doi: 10.1016/j.jacc.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meirelles GS, Gonen M, Strauss HW. 18F-FDG uptake and calcifications in the thoracic aorta on positron emission tomography/computed tomography examinations: frequency and stability on serial scans. J Thorac Imaging. 2011;26:54–62. doi: 10.1097/RTI.0b013e3181d9c9f9. [DOI] [PubMed] [Google Scholar]
- 40.Xu XY, Borghi A, Nchimi A, et al. High levels of 18F-FDG uptake in aortic aneurysm wall are associated with high wall stress. Eur J Vasc Endovasc Surg. 2010;39:295–301. doi: 10.1016/j.ejvs.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Reeps C, Pelisek J, Bundschuh RA, et al. Imaging of acute and chronic aortic dissection by 18F-FDG PET/CT. J Nucl Med. 2010;51:686–91. doi: 10.2967/jnumed.109.072298. [DOI] [PubMed] [Google Scholar]
- 42.Kato K, Nishio A, Kato N, Usami H, Fujim aki T, Murohara T. Uptake of 18F-FDG in acute aortic dissection: a determinant of unfavorable outcome. J Nucl Med. 2010;51:674–81. doi: 10.2967/jnumed.109.065227. [DOI] [PubMed] [Google Scholar]
- 43.Kotze CW, Groves AM, Menezes LJ, et al. What is the relationship between (1)F-FDG aortic aneurysm uptake on PET/CT and future growth rate? Eur J Nucl Med Mol Imaging. 2011;38:1493–9. doi: 10.1007/s00259-011-1799-8. [DOI] [PubMed] [Google Scholar]
- 44.Palombo D, Lucertini G, Pane B, Spinella G. Screening for abdominal aortic aneurysm. Questions and results. Acta Chirurgica Belgica. 2011;111:7–11. doi: 10.1080/00015458.2011.11680695. [DOI] [PubMed] [Google Scholar]
- 45•.Marini C, Morbelli S, Armonino R, et al. Direct relationship between cell density and FDG uptake in asymptomatic aortic aneurysm close to surgical threshold: an in vivo and in vitro study. Eur J Nucl Med Mol Imaging. 2012;39:91–101. doi: 10.1007/s00259-011-1955-1. This study highlights the link between anatomy, cellularity, and inflammation, going against the false idea that a large aneurysm would have very high FDG uptake. [DOI] [PubMed] [Google Scholar]
- 46.Mizoguchi M, Tahara N, Tahara A, et al. Pioglitazone attenuates atherosclerotic plaque inflammation in patients with impaired glucose tolerance or diabetes a prospective, randomized, comparator-controlled study using serial FDG PET/CT imaging study of carotid artery and ascending aorta. JACC Cardiovasc Imaging. 2011;4:1110–8. doi: 10.1016/j.jcmg.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Ishii H, Nishio M, Takahashi H, et al. Comparison of atorvastatin 5 and 20 mg/d for reducing F-18 fluorodeoxyglucose uptake in atherosclerotic plaques on positron emission tomography/computed tomography: a randomized, investigator-blinded, open-label, 6-month study in Japanese adults scheduled for percutaneous coronary intervention. Clin Therapeut. 2010;32:2337–47. doi: 10.1016/j.clinthera.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 48••.Fayad ZA, Mani V, Woodward M, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel noninvasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378:1547–59. doi: 10.1016/S0140-6736(11)61383-4. Important study at several levels: 1) for using non invasive imaging as an endpoints; 2) for using a novel HDL raising agent; 3) for giving hope to patients in secondary prevention even with not completely positive results. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fifer KM, Qadir S, Subramanian S, et al. Positron emission tomography measurement of periodontal (18)f-fluorodeoxyglucose uptake is associated with histologically determined carotid plaque inflammation. J Am Coll Cardiol. 2011;57:971–6. doi: 10.1016/j.jacc.2010.09.056. [DOI] [PubMed] [Google Scholar]
- 50.Tezuka D, Haraguchi G, Ishihara T, et al. Role of FDG PET-CT in Takayasu Arteritis: sensitive detection of recurrences. JACC Cardiovasc Imaging. 2012;5:422–9. doi: 10.1016/j.jcmg.2012.01.013. [DOI] [PubMed] [Google Scholar]


