Introduction
The actin cytoskeleton is a fundamental regulator of cellular morphology, including the morphology of neurons. Actin is present in several neuronal compartments, and is highly enriched in dendritic spines, the sites of most excitatory synapses in the central nervous system (CNS). Structurally, spines consist of a head region that is connected to the dendrite shaft through a neck region (Hering and Sheng 2001). The spine head contains the post-synaptic density (PSD), the most prominent spine microdomain, which contains a dense concentration of glutamatergic receptors responsible for excitatory transmission (Newpher and Ehlers 2009). Outside of the PSD are additional spine compartments, including the perisynaptic domain, which is roughly 100-200nm from the edge of the PSD (Newpher and Ehlers 2009). Spines exist in an array of morphologies, and actin rearrangements are primarily responsible for regulating the morphology of spine heads as well as for regulating de novo spine formation. Over the past decade it has become clear that spine structure and function are tightly associated such that spines with large heads have a higher content of functional AMPA receptors than small spines (Matsuzaki et al. 2001), and the morphogenesis of small spines into large spines is correlated with the potentiation of AMPARs (Matsuzaki et al. 2004). Spine formation increases after learning (Yang et al. 2009), and alterations in spine numbers and morphology are characteristic features of many neuropsychiatric disorders (Fiala et al. 2002; Penzes et al. 2011a).
Here we discuss the importance of actin in regulating dendritic spine morphogenesis. The regulation of actin in spines is complex, and as we discuss, it is best conceptualized as a hierarchical network in which molecules function in discrete levels as defined by their “molecular distance” to the actin cytoskeleton. At the highest level in this network (i.e., most distant from actin) are synaptic surface receptors such as glutamatergic NMDA and AMPA receptors, receptor tyrosine kinases such as ephrin receptors, and non-kinase adhesion receptors such as N-cadherin, among others (Penzes et al. 2008). Here we focus on the molecules that act downstream of synaptic surface receptors to regulate actin in spines. Guanine exchange factors (GEFs) relay signals from synaptic receptors to the actin cytoskeleton, but typically require several intermediary proteins to do so. Notably, GEFs activate small GTPases, which in turn, directly regulate the activity of numerous molecules broadly termed “small GTPase effectors”. These effector molecules stimulate or repress the activity of actin binding proteins, which, as their name implies, bind to actin and directly affect actin polymerization.
The functional relevance of having numerous molecular steps between synaptic receptor activity on the one hand, and actin regulation on the other, was the subject of a recent review (Penzes et al. 2008). Here we delineate the direct role for actin in spine morphogenesis, and then deconstruct the signal transduction pathways known to mediate actin polymerization, focusing on GEF-small GTPase interactions, the relationships between small GTPases and their effectors, and the mechanisms by which actin binding proteins regulate actin dynamics. In each instance we focus on the known biochemical mechanisms that govern these interactions and highlight notable exceptions to the hierarchical framework described. We then discuss the evidence linking actin-regulatory networks to the pathogenesis of neuropsychiatric disorders. Finally, after deconstructing these actin-regulatory pathways, we take a holistic approach to help direct future studies that will illuminate how these numerous pathways are regulated both spatially and temporally in spines. As informative guides, figures 1 and 2 illustrate the interactions between molecules for two of the networks discussed in most detail in this review.
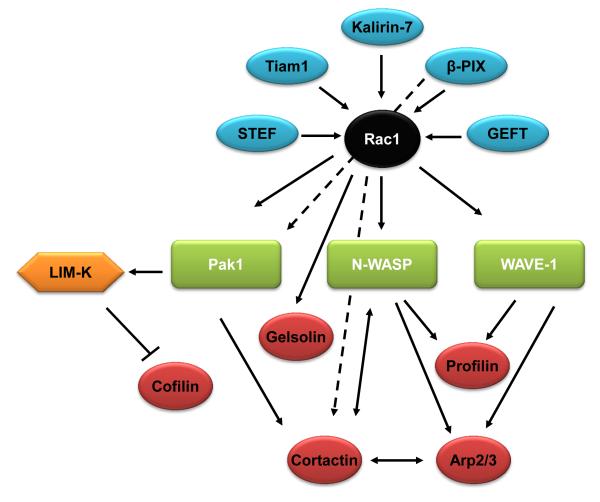
Figure 1. The Rac1-actin regulatory network.
The upstream and downstream regulators of the Rac1 network are illustrated. Rac1 GEFs are shown in blue, and direct Rac1 effectors are in green. Actin-binding proteins are shown in red. LIM-K is neither a direct Rac1 effector nor an actin-binding protein in this network. Solid arrows indicate pathways that follow the typical GEF-small GTPase-small GTPase effector-actin binding protein hierarchy, while dashed arrows indicate pathways that violate this hierarchy.
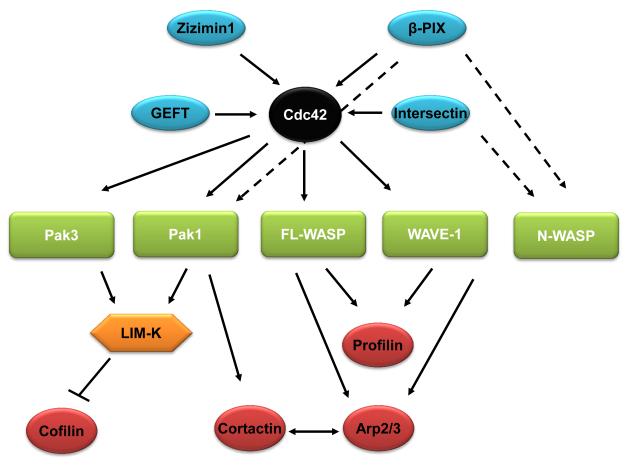
Figure 2. The cdc42-actin regulatory network.
The upstream and downstream regulators of cdc42 network are illustrated. Cdc42 GEFs are shown in blue, and direct cdc42 effectors are in green. Actin-binding proteins are shown in red. LIM-K is neither a direct Rac1 effector nor an actin-binding protein in this network. Solid arrows indicate pathways that follow the typical GEF-small GTPase-small GTPase effector-actin binding protein hierarchy, while dashed arrows indicate pathways that violate this hierarchy.
Dendritic spines are rich in actin
The overall stability of molecules within the PSD depends on their association, even if indirect, with F-actin. Spines are rich in F-actin, and the necessity of F-actin for the synaptic localization of proteins has been assessed by treating cells with pharmacological inhibitors of F-actin formation. Inhibition of de novo F-actin formation with Latrunculin A, which binds to and sequesters monomeric actin thereby preventing new F-actin formation, reduces the number of spines containing the GluR1 AMPA receptor (AMPAR) subunit as well as the number of synaptic clusters expressing the NMDA receptor (NMDAR) subunit, NR1 (Allison et al. 1998). Moreover, glutamate-mediated AMPAR internalization can be blocked by treatment with jasplakinolide, a stabilizer of actin filaments (Zhou et al. 2001), and jasplakinolide prevents spine loss resulting from intense NMDAR activation (Halpain et al. 1998). That surface receptor internalization is facilitated by actin instability is suggestive of different actin pools in spines (Cingolani and Goda 2008), as receptor internalization would require the activity of actin-based motor proteins such as myosin VI (Osterweil et al. 2005), which presumably function normally in the presence of latrunculin A.
As scaffolding molecules help link surface rectors to the actin cytoskeleton in spines, it is not surprising that several scaffolding molecules in the PSD, such as PSD-Zip45 and shank, are also destabilized by F-actin depolymerization (Usui et al. 2003). The importance of actin in stabilizing scaffolding molecules in the PSD is underscored by recent findings indicating that scaffold molecules can directly interact with actin binding proteins, and these actin binding proteins possess the capability to simultaneously bind a scaffold and interact with F-actin (Qualmann et al. 2004). Unlike most scaffolding proteins examined, PSD-95 levels are fairly stable upon F-actin inhibition suggesting that it acts as a core protein for the PSD (Kuriu et al., 2006).
In addition to promoting the stability of PSD proteins, actin is a principal regulator of spine morphology. Within spines, actin exists as either monomeric units (G-actin) or in multiple units of filamentous actin (F-actin) comprised of multiple associated G-actin units. Actin growth is polarized with one end exhibiting more rapid growth than the other. The rapidly growing end is termed the plus or barbed end and the opposite end termed the negative or pointed end (Cingolani and Goda 2008; Dillon and Goda 2005). In F-actin, individual G-actin subunits are bound by covalent interactions that allow for rapid assembly and disassembly (Dillon and Goda 2005). ATP-actin monomers are added to F-actin plus ends, and ADP-actin monomers are removed from F-actin negative ends, at F-actin steady state (Bindschadler et al. 2004). In spines, actin filaments turnover rapidly and show a treadmilling activity that extends from the tip to the base of the spine. Large spines have a greater velocity of actin flow than small spines, which is theorized to contribute to a greater expansive force on the spine head (Honkura et al. 2008). NMDAR activation shifts the G-actin to F-actin equilibrium toward F-actin with associated spine enlargement. Conversely, prolonged low-frequency stimulation causes actin depolymerization resulting in spine shrinkage and even spine elimination (Okamoto et al. 2004).
Recent studies have found that spines contain subdomains of actin, and that while the PSD contains a low concentration of actin molecules, perisynaptic regions have an abundance of polymerized actin foci (Frost et al. 2010). Spine enlargement, however, is not simply the result of an increase in the polymerization of existing actin filaments, as the formation of a novel pool of actin in spines occurs following the activation of synaptic glutamate receptors. This actin pool, termed the enlargement pool, is concentrated in the spine head and is critical for spine enlargement (Honkura et al. 2008). The formation of this enlargement pool of actin occurs concomitantly with increased spine membrane reshaping, suggesting it is responsible for alterations in spine head morphology. Failure to confine the enlargement pool of actin will result in shrinkage of the spine head, indicating that the aggregation of new filaments is necessary for long-term spine head enlargement (Honkura et al. 2008).
The exact mechanisms that govern the formation of new spines are incompletely understood, yet actin rearrangements are pivotal for de novo spine formation. Studies examining the mechanisms of spine formation have investigated the transitional states between filopodia and mature spines as filopodia are believed to be spine precursors (Ziv and Smith 1996). The interaction between the neuron-specific actin binding protein drebrin A and PSD-95 could be critical in the transition from filopodia to spine formation (Sekino et al. 2007; Takahashi et al. 2003), as drebrin A promotes the clustering of F-actin and PSD-95 in incipient spines (Takahashi et al. 2003). PSD-95 clusters are much scarcer in filopodia relative to spines, and PSD-95 clustering might thus signify the transition to spine formation (Okabe et al. 2001). Other studies have suggested that the dominance of actin binding proteins in filopodia and mature spines might differ such that filopodia rely on actin binding proteins that promote the formation of unbranched actin filaments, while spines rely on those that promote actin filament branching (Hotulainen et al. 2009).
Small GTPases: critical regulators of actin dynamics in spines
We now turn our attention to understanding the biochemical interactions that regulate actin in spines. As discussed in the introduction, this regulation can be conceptualized as a hierarchical network in which molecules function in discrete levels defined by their molecular distance to actin. In this network, small GTPases are intermediary between surface receptor activity and actin rearrangement, and we thus focus our attention on this class of molecules first. We then discuss the upstream regulators of small GTPases in spines, GEFs, and then discuss how GEF-small GTPase pathways regulate actin dynamics through small GTPase effectors and actin-binding proteins.
Members of the Rho subfamily of Ras-like small GTPases are critical regulators of the actin cytoskeleton in numerous cell types, including in neurons (Luo 2002). Ras-like small GTPases regulate the development and morphology of dendrites and spines. Small GTPases act as “binary switches” that exist in either an inactive or active form, and are inactive in their GDP-bound state and active when bound to GTP. Small GTPase activity is regulated through several mechanisms. First, guanine nucleotide exchange factors (GEFs) facilitate the exchange of GDP for GTP on small GTPases thereby activating them. Conversely, GTPase activating proteins (GAPs) stimulate the intrinsic GTPase activity of small GTPases resulting in the conversion to an inactive state. Finally, guanine nucleotide dissociation inhibitors (GDIs) can regulate the activity of some small GTPases by directly binding to them and regulating their cellular localization and proximity to upstream regulators (Heasman and Ridley 2008; Schmidt and Hall 2002).
The Rho subfamily of small GTPases includes the members Rac1, cdc42, and RhoA. A variety of evidence indicates that Rac1 promotes spine formation, while RhoA inhibits spine maintenance (Nakayama and Luo 2000; Tashiro et al. 2000). Specifically, the overexpression of a dominant negative form of Rac1 drastically decreases the number of spines and synapses in cultured hippocampal slices and dissociated neurons (Nakayama et al. 2000; Penzes et al. 2003), while constitutively active RhoA promotes spine elimination in hippocampal neurons (Tashiro et al. 2000). Similar to Rac1, cdc42 promotes spine morphogenesis as dominant negative cdc42 and cdc42 RNAi inhibit spine formation (Irie and Yamaguchi 2002; Wegner et al. 2008). Recent evidence indicates that the precise regulatory balance of small GTPase activity is critical to spine morphogenesis. Notably, although Rac1 activity typically increases forebrain spine formation, excessive and prolonged Rac1 activity can in some instances inhibit spine morphogenesis (Hayashi-Takagi et al. 2010).
Ras and Rap are related GTPases in the Ras subfamily that produce opposing effects on dendritic spines. Ras is rapidly activated following single spine glutamate uncaging, and once activated, Ras can diffuse from activated spines to neighboring, unactivated spines. The spread of activated Ras might directly contribute to the facilitation of plasticity across syapses as Ras activity allows for sustained increases in spine volume following subthreshold synaptic stimulation (Harvey et al. 2008). Moreover, Ras has been shown to stabilize synapses and traffic AMPA receptors into spines in a phosphoinositide 3-kinase (PI3K)–Akt dependent manor (Zhu et al. 2002). Consistent with Ras activity in driving spine plasticity, mice expressing constitutively active Ras show an increase in pyramidal neuron spine density in both apical and basal dendrites (Arendt et al. 2004). Inactivators of Rap, on the other hand, increase spine head size (McAvoy et al. 2009; Pak et al. 2001), and mice overexpressing a particular Rap isoform, Rap2, show a reduction in forebrain spine density as well as increased long-term depression Ryu et al. 2008).
GEFs: linking surface receptors to small GTPases
Through catalyzing the exchange of GDP for GTP, guanine exchange factors (GEFs) serve to activate small GTPases. The activity of GEFs is regulated by several means: through direct protein-protein interactions, through the regulation of GEF subcellular localization, and several GEFs have intramolecular inhibitory interactions that function as intrinsic activity regulators. The Dbl homology domain (DH) present in most GEFs is necessary and sufficient for catalyzing GDP to GTP exchanges (Schmidt and Hall 2002).
RhoA is associated with spine shrinkage and destabilization, thus, GEFs that activate this GTPase have analogous effects on dendritic spine morphology. For example, the recently identified GEF-H1 activates RhoA, and its knockdown increases forebrain spine density (Kang et al. 2009), while its overexpression decreases spine area (Ryan et al. 2005). Moreover, treatment of neurons with the AMPAR antagonist NBQX decreases forebrain spine density and increases RhoA activity through GEF-H1 (Kang et al. 2009). δ-Catenin, a member of the p120-Catenin subfamily of armadillo proteins, increases spine density by inactivating the RhoA GEF P190RhoGEF (Kim et al. 2008).
Several GEFs for Rap1 have been identified, including MR-GEF, PDZGEF1, and nRap GEP (Ohtsuka et al. 1999; Rebhun et al. 2000). The loss of PDZGEF1 increases spine density in hippocampal neurons (Lee et al. 2011). Of note, the RapGEF Epac2 is expressed in forebrain spines and is activated by cAMP. Epac2 activity leads to reduced spine AMPA receptor content, depressed excitatory transmission, as well as spine destabilization as demonstrated by live imaging studies. Conversely, inhibition of Epac2 leads to spine enlargement and stabilization (Woolfrey et al. 2009).
Among the GEFs for Ras are RasGRF1 and RasGRF2. As expected from the loss of Ras activity, RasGRF1 knockdown reduces spine density in hippocampal neurons (Lee et al. 2011). A recent study revealed an intriguing link between Ras and Rap signaling in neurons. The polo-like kinase 2 (Plk2/SNK) is a serine/threonine kinase whose activity increases Rap activity while simultaneously inhibiting Ras activity. This effect on inhibiting Ras activity occurs through Plk2’s ability to phosphorylate and degrade RasGRF1 and via its role in stimulating the activity of the RasGAP SynGAP. The effect of Plk2 in increasing Rap activity occurs is due to its ability to stimulate PDZGEF1 activity and inhibit the activity of SPAR, a Rap GAP. Plk2 overexpression decrease spine density and size, while its knockdown increases spine size and density (Lee et al. 2011).
There are several well characterized Rac1 GEFs, including Tiam1, Betapix (βPix), STEF, and kalirin. Through the activation of Rac1, RacGEFs increase spine morphogenesis in forebrain neurons. Tiam1 knockdown reduces dendritic spine density, and Tiam1 regulates spine morphogenesis downstream of NMDAR-mediated CaMKII activity and EphB (Tolias et al. 2005; Tolias et al. 2007). Reduction of βPix levels reduces spine density in forebrain neurons, and βPix is critical for CaMK kinase-CaMK1-mediated spine morphogenesis (Saneyoshi et al. 2008). The RacGEF STEF (Tiam2) is a critical regulator of dendritic growth in young neurons (Takemoto-Kimura et al. 2007), but its role in spine morphogenesis remains unknown.
Kalirin expression is mainly restricted to the central nervous system where it is highly expressed in the hippocampus and cortex, and within neurons, kalirin is highly expressed in dendrites and dendritic spines. Kalirin is a prominent regulator of dendritic growth and facilitates spine morphogenesis in both young and mature neurons (Penzes and Jones 2008). The KALRN gene gives rise to four major protein products: kalirin-5, kalirin-7, kalirin-9, and kalirin-12. The kalirin-7, kalirin-9, and kalirin-12 isoforms are derived from the same promoter and are generated through alternate splicing. Kalirin-5 on the other hand uses a unique promoter. Kalirin-5 and kalirin-7 each contain a single catalytic domain, while kalirin-9 and kalirin-12 contain an additional catalytic domain not present in the other isoforms. Kalirin-7 is the most abundantly expressed isoform in the adult forebrain (Johnson et al. 2000; Rabiner et al. 2005).
A variety of evidence indicates that the first kalirin GEF domain shared by all major isoforms is a specific activator of the small GTPase Rac1, while the second GEF domain present only in the kalirin-9 and kalirin-12 isoforms is a specific activator of the small GTPase RhoA (Penzes et al. 2001a). The first GEF domain (DHPH1) binds to Rac1 but does not bind to either RhoA or cdc42. The second GEF domain (DHPH2) binds to both Rac1 and to RhoA. Studies in heterologous cells have found that DHPH1 activates Rac1 but does not affect the activity of cdc42. Although DHPH2 binds to both RhoA and to Rac1, this domain activates RhoA without affecting the activity of Rac1 (Penzes et al. 2001a). In neurons, the DHPH1 domain of kalirin-7 promotes spine morphogenesis (Penzes et al. 2001b). Kalirin is critical in facilitating spine structural plasticity and spine AMPAR content following NMDAR-mediated CamKII activation (Xie et al. 2007). Cdk5 also regulates spine morphogenesis through kalirin-7 (Xin et al. 2008), and mice lacking kalirin expression show reduced forebrain spine density (Cahill et al. 2009; Ma et al. 2008).
The GEF intersectin is among the most well characterized GEFs for cdc42 in neurons. The overexpression of an intersectin mutant lacking its GEF domain results in a reduction in spine area and increases the preponderance of long, immature spines (Irie and Yamaguchi 2002; Thomas et al. 2009). Unlike most RacGEFs, βPix also acts as a GEF for cdc42, indicating that it likely affects spine morphogenesis through multiple small GTPase targets (Manser et al. 1998). Zizimin1 is a cdc42 GEF and is unique in that lacks a DH domain characteristic of most GEFs. Zizimin1’s GEF activity comes from a unique domain termed the CDM-zizimin homology domain 2 (CZH2) located at the C-terminus that binds cdc42 (Meller et al. 2002). Zizimin1 exists in an autoinhibited state attributable to direct binding of its N-terminus to the CZH2 domain. This interaction impedes binding to cdc42, and it is currently unclear what mechanisms enable intersectin activation (Meller et al. 2008). In hippocampal neurons, the overexpression of Zizimin1 promotes dendritic growth, while its knockdown suppress the growth of dendrites. The effects of zizimin1 on dendritic spines remains to be determined (Kuramoto et al. 2009).
A recently identified GEF, GEFT, strongly activates Rac1, cdc42, and to a lesser degree, RhoA (Guo et al. 2003). GEFT is highly expressed in the brain, and its overexpression increases spine size and the preponderance of mature spines (Bryan et al. 2004). It is not clear which GTPase regulated by GEFT is principally responsible for its effects on spines. Of note is that Rac1, but not cdc42 or RhoA, mediates the effect of GEFT of neurite growth (Bryan et al. 2004). Whether Rac1 is also primary responsible for GEFT’s effects on spine morphogenesis requires future attention.
In addition to the catalytic DH domain present in most GEFs, several GEFs share additional subunits which affect their ability to activate their small GTPase targets. Several GEFs contain SH3 domains. SH3 domains bind to proline-rich sequences (Mayer 2001), and the role for SH3 domains in several GEFs has been studied. Notably, Rac1 was recently shown to directly interact with the SH3 domain of βPIX (ten Klooster et al. 2006). This interaction is mediated by the proline-rich C-terminus of Rac1, and does not require Rac1 to be in its activated form. The association with βPIX is sufficient to drive Rac1 to the plasma membrane (ten Klooster et al. 2006); thus, the SH3-Rac1 interaction could help drive the membrane targeting of Rac1 in neurons where it is strategically positioned for activation by the GEF DH domain. In some instances SH3 domains of GEFs seem to inhibit small GTPase activity. The fifth SH3 domain of intersectin, for example, intramolecularly interacts with the catalytic DH domain, which would be expected to interfere with nucleotide exchange (Ahmad and Lim 2010).
Several RacGEFs contain PDZ-interacting motifs, including kalirin-5, kalirin-7, Tiam1, and STEF. This motif allows these GEFs to directly interact with a variety of PDZ domain-containing proteins, including synaptic scaffolding proteins such as PSD-95 in the case of kalirin-7 (Penzes et al. 2001b), and receptor adhesion proteins such as Syndecan1 in the case of Tiam1 (Shepherd et al. 2010). Evidence suggests that the PDZ-interacting motif of RacGEFs is important for their targeting to spines, and for some RacGEFs, this region is necessary for spine promoting effects (Penzes et al. 2001b). In the case of kalirin-7 the interaction with PSD-95 inhibits its ability to activate Rac1 (Penzes et al. 2001b). This suggests that disassociation from PSD-95 might be a critical initial step in fostering kalirin-7 activity. Consistent with this, global elevations in synaptic activity drastically reduces the interaction of kalirin-7 with PSD-95 in the cortex (Hayashi-Takagi et al. 2010).
A recent study revealed that under certain signaling conditions small GTPases can activate GEFs through a non-canonical pathway. In the presence of nerve growth factor (NGF) Ras binds to Tiam1 thereby allowing Tiam1 to bind to and activate Rac1 (Shirazi Fard et al. 2010). Other studies support the idea that crosstalk between small GTPases occurs. Cdc42 can increase Rac1 activation, Rac1 can cause RhoA activation, and RhoA, in turn, can reduce Rac1 activity (Li et al. 2002; Nobes and Hall 1995).
Downstream effectors of small GTPases and actin-binding proteins: an intimate link to actin
Among the most well characterized downstream target of Rac1 and cdc42 in neurons is the p21-activated kinase (Pak) (Manser et al. 1994). The group 1 Pak family is comprised of Pak1, Pak2, and Pak3. Group 1 Pak members, and in particular Pak1, have an established role in promoting spine morphogenesis (Nikolic 2008). Rac1 activity has been shown to increase the activity of Pak1, and the ability to activate Pak1 is also seen with cdc42 (Knaus et al. 1998). Small GTPases activate Pak1 by relieving its intramolecular autoinhibition (Tu and Wigler 1999).
Rac1 and cdc42 show differential propensity to activating certain group 1 Pak members. Notably, b-galactosidase two-hybrid assays have shown that Rac1 shows minimal interaction with Pak3 when compared to that of cdc42 (about 2% relative to cdc42), and overexpression experiments have shown that cdc42 shows a 10 fold higher affinity for binding Pak3 relative to Rac1. These findings extend to the level of GEFs as the Rac1 GEF Tiam1 is unable to activate Pak3, which is in stark contrast to the surge in Pak3 activity caused by the cdc42 GEF intersectin (Kreis et al. 2007). Overall, it seems that cdc42 is a broader activator of group 1 Paks than Rac1.
Rac1 and cdc42 facilitate the interaction of Pak1 with LIM-kinase, thereby causing LIM-kinase activation (Edwards et al. 1999). LIM-kinase activity, in turn, favors actin polymerization by the phosphorylation and consequent inactivation of cofilin, a negative regulator of F-actin polymerization (Arber et al. 1998; Yang et al. 1998). Cofilin has a high affinity for GDP-actin but not for GTP-actin, and it binds to GDP-actin solely at the F-actin pointed end; cofilin causes a more than twenty fold increase in the rate of GDP-actin dissociation from F-actin pointed ends (Carlier et al. 1997). Indeed, the overexpression of constitutively active cofilin reduces spine head area and spine head to length ratios, indicative of immature spine phenotypes (Shi et al. 2009). Surprisingly, small GTPases that promote spine morphogenesis in some instances paradoxically dephosphorylate cofilin thereby increasing its activity: Ras acts through the MAPkinase/Erkkinase and phosphatidylinositol-3-kinase (PI3K) pathway to dephosphorylate cofilin in fibroblasts (Nebl et al. 2004). Whether this occurs in neurons remains unclear, but nevertheless indicates that the relationship between small GTPases and cofilin with regard to actin regulation is likely more complicated than previously believed.
RacGEFs can in some instances interact with Pak kinases independent of small GTPases. For example, βPIX interacts with Pak1 via its SH3 domain (ten Klooster et al. 2006). As Rac1 also binds to the SH3 domain of βPIX, cellular studies have found that Pak1 and Rac1 competitively bind to βPIX. The exact implications for the binding of Pak1 to βPIX with regard to actin remodeling is not completely clear. Of note, phospho-active Pak1 cannot bind to βPIX (Mott et al. 2005), indicating that βPIX selectively binds inactive Pak1, and upon activation by small GTPases, Pak1 disassociates from βPIX (ten Klooster et al. 2006).
Rac1 and cdc42 can also regulate the actin cytoskeleton through the direct activation of the Wiskott-Aldrich syndrome protein (WASP) family. The WASP protein family includes full-length WASP and neural-WASP (N-WASP). WASP and N-WASP have a GBD/CRIB motif that binds small GTPases, and the N-terminal Wh1 domain of the WASP family binds to F-actin (Egile et al. 1999) and to the WASP-interacting protein (WIP) (Ramesh et al. 1997). WIP can facilitate actin polymerization as well as protect WASP from degradation (Anton et al. 2007; Ramesh et al. 1997). In resting state, WASP and N-WASP are autoinhibited as a consequence of an intramolecular interaction between the GBD domain and the C-terminus. This occludes its interaction with actin regulatory molecules, and small GTPase binding effectively relieves this autoinhibition (Kim et al. 2000; Rohatgi et al. 2000).
N-WASP is highly expressed in the brain (Miki et al. 1996), is present in adult rodent synaptsomes (Irie and Yamaguchi, 2002), and is enriched in forebrain excitatory synapses (Wegner et al. 2008). N-WASP expression is present at birth in both the hippocampus and cortex and levels increase at around 2-weeks of age in rodents, coinciding with the period of elevated spine formation. N-WASP levels then remain elevated throughout adulthood (Tsuchiya et al. 2006). Actin polymerization assays have shown that Rac1 preferentially activates N-WASP over full-length WASP, while cdc42 preferentially activates full-length WASP over N-WASP. The differential affinity of Rac1 and cdc42 for N-WASP and WASP, respectively, is partially due to the differences in the GTPase-binding CRIB domains of the WASP members (Tomasevic et al. 2007). That Rac1 activates N-WASP is significant given that N-WASP knockdown has been shown to reduce spine density as well as PSD-95 clusters, while the overexpression of N-WASP increases spine density (Wegner et al. 2008).
In some instances GEFs can directly interact with downstream regulators of actin-binding proteins irrespective of small GTPase activation as shown by the ability of intersectin-1 to directly interact with N-WASP in vitro and in vivo (Hussain et al. 2001). This interaction stimulates intersectin-1’s ability to activate cdc42. These findings indicate that although cdc42 does not seem to directly activate N-WASP, cdc42 might nevertheless be capable of driving N-WASP activity via interactions with GEFs. It is interesting that the SH3 domain of intersectin-1 binds N-WASP (Hussain et al. 2001). Whether the SH3 domains of other GEFs can also directly interact with small GTPase effectors such as N-WASP has not been reported.
As another exception to the linear GEF-small GTPase-GTPase effector-actin binding protein pathway, the WH1 domain of N-WASP directly interacts with the proline-rich domain of a particular β-PIX isoform, β-PIX-b (Park et al. 2011). This interaction stimulates actin dynamics in non-neuronal cells, and in neurons it promotes spine formation. As cdc42 activity is also critical for β-PIX-b’s effects on spines, this suggests that multiple B-PIX-b domains are responsible for regulating spines, and might do so in a cooperative manner. As different β-PIX-b domains bind to N-WASP and cdc42, it is possible that β-PIX-b strategically brings activated cdc42 in close proximity to N-WASP thereby fostering cdc42-mediated N-WASP activation (Park et al. 2011).
Rac1 can also affect actin polymerization through the regulation of WAVE-1. WAVE proteins share a similar structure to WASP proteins, but lack a GTPase binding CRIB domain (Miki et al. 1998). Rac1 immunoprecipitates with WAVE, and WAVE functions downstream of Rac1 to control actin clustering (Miki et al. 1998). WAVE1 exists in a constitutively active form and its activity is trans-inhibited by a protein complex. Rac1 acts not to directly activate WAVE1 per se, but rather to relieve WAVE1 inhibition by directly binding to and dissembling proteins in the WAVE1 inhibitory complex (Eden et al. 2002). WAVE1 is expressed in the spines of mature forebrain neurons, and is important for spine maintenance (Soderling et al. 2007) and spine maturation (Kim et al. 2006). Mice with reduced WAVE1 expression show striking deficits in spatial memory (Soderling et al. 2003), consistent with a role for actin regulators in cognitive processes.
A primary downstream target of N-WASP and WAVE1 is the arp2/3 complex (Takenawa and Suetsugu 2007). Arp2/3 is found in the majority of spine heads, and arp2/3 activity favors spine maturation as reducing its activity increases filopodia formation and decreases the number of both thin and mushroom spines. However, loss of arp2/3 does not affect filopodial growth (Hotulainen et al. 2009). Arp2/3’s role seems to lie in promoting the branching of actin filaments and might thus be crucial for spine head enlargement (Hotulainen et al. 2009; Korobova and Svitkina 2010; Mullins et al. 1998). Knockdown of Arp3 reduces forebrain spine density (Wegner et al. 2008).
WASP and WAVE contain a VCA region (verprolin homology, cofilin homology domain, acidic region) at their C-terminus where the V region binds to actin monomers while the CA region binds to arp2/3 (Miki and Takenawa 2003). The VCA region of WASP and WAVE is essential for actin polymerization, and the overexpression of a N-WASP mutant lacking the VCA region causes spine loss, akin to a dominant-negative protein (Wegner et al. 2008). The interaction of WASP or WAVE with arp2/3 helps form an actin trimer necessary to drive actin polymerization: the V domain of WASP/WAVE bind an actin monomer and arp2 and apr3 each bind an actin monomer (Kurisu and Takenawa 2009). Arp2/3 can then promote actin filament growth in the rapidly growing plus direction (Pollard and Beltzner 2002).
The N-terminus of cortactin directly binds to the arp2/3 complex, and although cortactin by itself is not sufficient to drive actin polymerization, cortactin expedites arp2/3-mediated actin polymerization. Unlike WASP and WAVE which simultaneously interact with actin monomers and arp2/3 to drive polymerization, cortactin stimulates arp2/3 polymerization by interacting with F-actin (Uruno et al. 2001). Although both cortactin and N-WASP bind arp2/3, they interact with partially non-overlapping arp2/3 domains which allow these molecules to form a ternary complex (Weaver et al. 2002). This could explain the ability of N-WASP and cortactin to synergistically promote actin branching (Weaver et al. 2001). Cortactin is enriched in spines, and its knockdown reduces spine density, while cortactin overexpression increases spine length (Hering and Sheng 2003). Both Rac1 and Pak phosphorylate cortactin (Head et al. 2003; Webb et al. 2006). Pak-mediated cortactin phosphorylation can attenuate cortactin’s interaction with F-actin (Webb et al. 2006), while a separate study found that Pak’s phosphorylation of cortactin increases its association with N-WASP (Grassart et al. 2010). The implications of Pak’s regulation of cortactin on dendritic spines has not been reported.
Another potential means by which Rac1 affects actin is by regulating gelsolin. Gelsolin severs actin filaments, is important for calcium-mediated actin depolymerization in neurons (Furukawa et al. 1997), and also binds and caps the barbed ends of actin filaments (Harris and Weeds 1984). Other studies using knockout neurons suggest that gelsolin can stabilize actin (Star et al. 2002). Rac1 negatively regulates the interaction of gelsolin with actin in non-neuronal cells (Arcaro 1998). Whether Rac1 has similar effects in neurons remains unknown. Further, it seems that Rac1 activity in and of itself is not sufficient to regulate gelsolin-actin interactions suggesting that a downstream Rac1 effector is critical. Pak does not alter gelsolin’s interaction with actin (Arcaro 1998), and the exact Rac1 effector responsible for gelsolin regulation has not been delineated.
The actin binding protein profilin is another key regulator of actin polymerization. Profilin can promote the rapid assembly of actin filaments by binding to ADP-actin. This interaction greatly increases the rate of ADP to ATP exchange on actin monomers thereby replenishing the pool of ATP-actin. ATP-actin can then be added to an elongating actin filament (Witke 2004). WAVE directly interacts with profilin, and profilin is critical for WAVE-induced clustering of actin filaments (Miki et al. 1998). N-WASP also interacts with and regulates actin polymerization through profilin (Suetsugu et al. 1998), and this interaction might be fostered by the profilin interacting motif present in the WIP protein (Ramesh et al. 1997). Spines containing profilin show greater PSD lengths than those lacking profilin (Lamprecht et al. 2006). Synaptic activity increases profilin levels in spine heads, and interfering with profilin targeting to spines causes spine destabilization (Ackermann and Matus 2003).
RhoA’s effects on actin are thought to be partially mediated by mammalian diaphanous (mDia), a member of the diaphanous-related forming family of actinnucleating proteins (Schmandke and Strittmatter 2007). RhoA binds to, and stimulates the function of, mDia. mDia binds to the plus end of actin filaments thereby preventing the binding of proteins that terminate actin polymerization (Ridley 2006). Additional RhoA effectors in neurons are the Rho-associated kinases (ROCKs) (Nakayama et al. 2000; Schmandke and Strittmatter 2007). ROCK1 (p160ROCK) can activate LIM-kinase, and evidence suggests that ROCK1 negatively impacts the activity of Rac1 and cdc42 (Hirose et al. 1998). The latter effect may contribute to the negative role of RhoA activity on spine morphogenesis, and indeed ROCK inhibitors (e.g, Y27632) increase spine density (Kang et al. 2009).
Actin regulatory signal transduction pathways are implicated in neurological disorders
As changes in spine density and morphology are characteristic of many neuropsychiatric disorders (Penzes et al. 2011a), much evidence links components of the GEF-small GTPase-GTPase effector-actin binding protein pathway to disorders characterized by spine loss. Starting with GEFs, KALRN transcript expression is reduced in the forebrain of schizophrenia and Alzheimer’s disease (AD) patients (Hill et al. 2006; Narayan et al. 2008; Youn et al. 2007), and several missense mutations in the KALRN gene have been identified in schizophrenia patients (Kushima et al.). Mice lacking kalirin show several schizophrenia-relevant phenotypes, including deficits in sensory-motor gating (Cahill et al. 2009). Kalirin-7 also interacts with the protein product of multiple well-characterized schizophrenia susceptibility genes (Cahill et al. 2011; Hayashi-Takagi et al. 2010).
Besides kalirin, several other GEFs have been implicated in neuropsychiatric disorders. STEF has a role in processing the amyloid precursor protein (Zaldua et al. 2007), the dysregulation of which is thought to contribute to synaptic degeneration in AD (Esteban 2004; Isacson et al. 2002), and a mutation in a member of the PIX RacGEF family invariably leads to mental retardation in male members of a Dutch pedigree (Kutsche et al. 2000). Coding mutations in the EPAC2 gene have been associated with autism (Bacchelli et al. 2003), and these mutations affect Rap activity and spine morphogenesis (Penzes et al. 2011b; Woolfrey et al. 2009). Moreover, increased protein expression of Epac2 has been detected in the prefrontal cortex and hippocampus of depressed suicide victims (Dwivedi et al. 2006). The expression of intersectin transcript is increased in patients with Alzheimer’s disease and Down’s syndrome, and proteins levels are elevated in the frontal cortex in Down’s syndrome as well (Hunter et al. 2011; Pucharcos et al. 1999; Wilmot et al. 2008). Moreover mice overexpressing intersectin1 show Down’s syndrome relevant phenotypes, particularly reduced activity (Hunter et al. 2011).
Small GTPases and their effectors have also been implicated in numerous neurological disorders. Cdc42 transcript expression is reduced in the prefrontal cortex of schizophrenia patients (Hill et al. 2006). Rap1 protein levels are reduced in the frontal cortex of major depressive disorder and schizophrenia, while levels of B-raf, a downsteam Rap1 target, are reduced in bipolar disorder and schizophrenia (Yuan et al. 2010). Similarly, depressed suicide victims show reduced levels of Rap1 protein and active Rap1 levels in the prefrontal cortex and hippocampus (Dwivedi et al. 2006). Costello syndrome is rare disorder characterized by mental retardation, seemingly abnormal brain growth, along with a host of other symptoms including craniofacial features (Gripp et al. 2010; Tidyman and Rauen 2008). Mutations in the HRAS gene are associated with Costello syndrome, some of which lead to greatly heightened Ras activity (Aoki et al. 2005; Tidyman and Rauen 2008). In the AD brain, insoluble deposits of the protein amyloid beta (Abeta) are evident. Abeta activates RhoA, and the inactivation of RhoA seems to protect dendrites from the deleterious effects of Abeta (Chacon et al. 2011).
Mutations in Pak3 have been associated with mental retardation (Allen et al. 1998; Bienvenu et al. 2000), and numerous studies implicate the Rac1-Pak pathway in animal models of Fragile X syndrome. Studies have shown that the FMRP protein, whose function is disrupted in Fragile X syndrome, is a negative regulator of Rac1, and evidence suggests that the loss of FMRP can cause spine loss by Rac1 overactivation (Bongmba et al. 2011). Similarly, Pak inhibition can rescue the spine loss of FMR1 knockout mice (Hayashi et al. 2007). In contrast, a recent report found that Rac1 and Pak activation in response to theta burst stimulation in the hippocampus is deficient in FMR1 KO mice, indicating that a loss of Rac1-Pak activity could also be relevant to Fragile X animal models (Chen et al. 2010). Dysregulation of Pak has also been implicated in Alzheimer’s disease as a reduction in Pak protein expression and activity occurs in the forebrain of AD patients, and the rescue of Pak loss in a cellular AD model prevents the loss of postsynaptic proteins required for actin clustering (Zhao et al. 2006).
Finally, emerging evidence has linked several actin binding proteins to neuropsychiatric disorders as well. Fibrillar Abeta increases LIM-kinase activity with a consequent increase in the level of phospho-inactive cofilin. Phospho-LIM-kinase levels are upregulated in the AD brain, specifically in areas rich in Abeta pathology, and this increase Is theorized to contribute to the increased incidence of dystrophic neurites in AD (Heredia et al. 2006). The protein expression of arp2/3 is reduced in the fetal human cerebral cortex in Down’s syndrome, and its loss could contribute to the altered arrangement of the cytoskeleton is the disease (Weitzdoerfer et al. 2002). Finally, gelsolin transcript levels are reduced in the dorsolateral prefrontal cortex of schizophrenia patients (Hakak et al. 2001), and a recent study supports potential associations of single nucleotide polymorphisms in the gene encoding gelsolin, GSN, with schizophrenia (Xi et al. 2004).
Conclusions and future directions
Given the role for spine morphogenesis in learning and memory, and the links of aberrant spine regulation in contributing to numerous neuropsychiatric disorders (Penzes et al. 2011a), understanding the precise mechanisms governing the regulation of actin in spines remains an important avenue of research. As discussed, there are numerous signaling cascades that link surface receptors in spines to the actin cytoskeleton, and crosstalk between pathways in likely the norm rather than the exception. When deconstructing the signaling pathways that regulate actin, it would seem that a large degree of functional redundancy occurs, making it difficult to determine how a precise regulation of actin can occur. For example, the activation of different RacGEFs would seem to result in the activation of nearly identical downstream signal transduction pathways. How then is signaling specificity achieved?
First off, matters are more complex than simple linear pathways would suggest, as it is unlikely that the activation of a particular molecule in this network invariably leads to the activation of its full complement of identified downstream targets. Consistent with this, evidence indicates that GEFs can prime their small GTPase targets to activate specific effectors and not others (Zhou et al. 1998). This could be attributable to the spatial regulation of GEFs in different neuronal compartments or even in different spine regions such that their activated small GTPase counterparts have access to only a limited number of their known effectors. Also, non-overlapping regions of an individual small GTPase can interact with different downstream effectors (Xu et al. 1994). It is thus possible that different GEFs can cause different domains of a particular small GTPase to become available for interaction with effectors (Zhou et al. 1998). Also, because GEFs can in some instances directly interact with downsteam targets of small GTPases (Hussain et al. 2001), this could couple small GTPases to specific pathways under certain conditions.
The signaling specificity between GEFs and their targets leads to the greater issue of delineating which signal transduction pathways are the dominant regulators of the actin rearrangements that underlie spine morphogenesis. Evidence indicates that the expression profile of individual GEFs is tightly regulated during development, and in some instances in a brain-region specific manner (Penzes et al. 2008). The comparative stoichiometrics of different GEFs in spines has not been thoroughly examined, but would help determine if some GEFs have prominent roles during specific developmental windows and in particular brain regions. The preferential activation of particular pathways in the hierarchical network described is likely largely determined by the activation of specific spine surface receptors. Indeed, different GEFs interact with different receptor complexes (Penzes et al. 2008), and because the expression of these receptors is also likely regulated both temporally and in a brain-region specific manner, this would be predicted to alter the activity of corresponding pathways in the network.
Finally, the spatial localization and regulation of the molecules in this network merit future attention. This is especially important considering that actin pools required for long-term spine stabilization, as seen in spines with large heads, are located outside of the PSD (Honkura et al. 2008), and in fact the PSD largely lacks polymerized actin (Frost et al. 2010). This indicates that signals originating from spine surface receptors must spatially propagate to actin within spines. This would necessitate that molecules critical for long-term spine stability be strategically localized outside of the PSD and in the vicinity of stable actin pools. Indeed, electron microscopy (EM) studies have shown that molecules such as Pak1 and Pak3 are particularly enriched perisynaptically (Ong et al. 2002), and arp2 as well as cortactin are enriched away from the PSD in the spine “core” region (Racz and Weinberg 2004; Racz and Weinberg 2008). Although molecules such as GEFs are enriched in the PSD, it would be interesting to determine if GEFs can shuttle between synaptic and perisynaptic regions (Figure 3). If so, does the subspine localization of these molecules differ depending on spine morphology? Also, if GEFs show a prominent localization perisynaptically, what molecules stabilize them away from the PSD? Examining these issues in the future would illuminate some of the most fundamental aspects of actin regulation as it relates to spine morphogenesis.
Figure 3. The hypothetical regulation of GEFs subspine localization.
On the left is a spine with a small head. In blue is the postsynaptic density (PSD) where numerous scaffolding proteins are enriched. In spines actin is scarce in the PSD and is enriched in the spine core. GEFs bind to scaffolding proteins in the PSD, but this presents a fundamental spatial problem. How do GEFs bound to the PSD interact with the actin cytoskeleton located a considerable distance outside of the PSD? We propose that in spines with large heads as shown on the right, GEFs might be localized outside of the PSD and in the vicinity of the actin cytoskeleton. This localization of GEFs perisynaptically would be a critical initiator of spine head enlargement.
Acknowledgements
This work was supported by grant R01MH071316 (to P.P.), and training grant 1F31AG031621-01A2 to M.E.C.
References
- Ackermann M, Matus A. Activity-induced targeting of profilin and stabilization of dendritic spine morphology. Nat Neurosci. 2003;6(11):1194–200. doi: 10.1038/nn1135. [DOI] [PubMed] [Google Scholar]
- Ahmad KF, Lim WA. The minimal autoinhibited unit of the guanine nucleotide exchange factor intersectin. PLoS One. 2010;5(6):e11291. doi: 10.1371/journal.pone.0011291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen KM, Gleeson JG, Bagrodia S, Partington MW, MacMillan JC, Cerione RA, Mulley JC, Walsh CA. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat Genet. 1998;20(1):25–30. doi: 10.1038/1675. [DOI] [PubMed] [Google Scholar]
- Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci. 1998;18(7):2423–36. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton IM, Jones GE, Wandosell F, Geha R, Ramesh N. WASP-interacting protein (WIP): working in polymerisation and much more. Trends Cell Biol. 2007;17(11):555–62. doi: 10.1016/j.tcb.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Niihori T, Kawame H, Kurosawa K, Ohashi H, Tanaka Y, Filocamo M, Kato K, Suzuki Y, Kure S. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37(10):1038–40. doi: 10.1038/ng1641. others. [DOI] [PubMed] [Google Scholar]
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393(6687):805–9. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- Arcaro A. The small GTP-binding protein Rac promotes the dissociation of gelsolin from actin filaments in neutrophils. J Biol Chem. 1998;273(2):805–13. doi: 10.1074/jbc.273.2.805. [DOI] [PubMed] [Google Scholar]
- Arendt T, Gartner U, Seeger G, Barmashenko G, Palm K, Mittmann T, Yan L, Hummeke M, Behrbohm J, Bruckner MK. Neuronal activation of Ras regulates synaptic connectivity. Eur J Neurosci. 2004;19(11):2953–66. doi: 10.1111/j.0953-816X.2004.03409.x. others. [DOI] [PubMed] [Google Scholar]
- Bacchelli E, Blasi F, Biondolillo M, Lamb JA, Bonora E, Barnby G, Parr J, Beyer KS, Klauck SM, Poustka A. Screening of nine candidate genes for autism on chromosome 2q reveals rare nonsynonymous variants in the cAMP-GEFII gene. Mol Psychiatry. 2003;8(11):916–924. doi: 10.1038/sj.mp.4001340. others. [DOI] [PubMed] [Google Scholar]
- Bienvenu T, des Portes V, McDonell N, Carrie A, Zemni R, Couvert P, Ropers HH, Moraine C, van Bokhoven H, Fryns JP. Missense mutation in PAK3, R67C, causes X-linked nonspecific mental retardation. Am J Med Genet. 2000;93(4):294–8. doi: 10.1002/1096-8628(20000814)93:4<294::aid-ajmg8>3.0.co;2-f. others. [DOI] [PubMed] [Google Scholar]
- Bindschadler M, Osborn EA, Dewey CF, Jr., McGrath JL. A mechanistic model of the actin cycle. Biophys J. 2004;86(5):2720–39. doi: 10.1016/S0006-3495(04)74326-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongmba OY, Martinez LA, Elhardt ME, Butler K, Tejada-Simon MV. Modulation of dendritic spines and synaptic function by Rac1: A possible link to Fragile X syndrome pathology. Brain Res. 2011;1399:79–95. doi: 10.1016/j.brainres.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan B, Kumar V, Stafford LJ, Cai Y, Wu G, Liu M. GEFT, a Rho family guanine nucleotide exchange factor, regulates neurite outgrowth and dendritic spine formation. J Biol Chem. 2004;279(44):45824–32. doi: 10.1074/jbc.M406216200. [DOI] [PubMed] [Google Scholar]
- Cahill ME, Jones KA, Rafalovich I, Xie Z, Barros CS, Muller U, Penzes P. Control of interneuron dendritic growth through NRG1/erbB4-mediated kalirin-7 disinhibition. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill ME, Xie Z, Day M, Barbolina MV, Miller CA, Weiss C, Radulovic J, Sweatt JD, Disterhoft JF, Surmeier DJ. Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes. Proc Natl Acad Sci U S A. 2009;106(31):13058–63. doi: 10.1073/pnas.0904636106. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier MF, Laurent V, Santolini J, Melki R, Didry D, Xia GX, Hong Y, Chua NH, Pantaloni D. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol. 1997;136(6):1307–22. doi: 10.1083/jcb.136.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon PJ, Garcia-Mejias R, Rodriguez-Tebar A. Inhibition of RhoA GTPase and the subsequent activation of PTP1B protects cultured hippocampal neurons against amyloid beta toxicity. Mol Neurodegener. 2011;6(1):14. doi: 10.1186/1750-1326-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LY, Rex CS, Babayan AH, Kramar EA, Lynch G, Gall CM, Lauterborn JC. Physiological activation of synaptic Rac>PAK (p-21 activated kinase) signaling is defective in a mouse model of fragile X syndrome. J Neurosci. 2010;30(33):10977–84. doi: 10.1523/JNEUROSCI.1077-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9(5):344–56. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- Dillon C, Goda Y. The actin cytoskeleton: integrating form and function at the synapse. Annu Rev Neurosci. 2005;28:25–55. doi: 10.1146/annurev.neuro.28.061604.135757. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Mondal AC, Rizavi HS, Faludi G, Palkovits M, Sarosi A, Conley RR, Pandey GN. Differential and brain region-specific regulation of Rap-1 and Epac in depressed suicide victims. Arch Gen Psychiatry. 2006;63(6):639–48. doi: 10.1001/archpsyc.63.6.639. [DOI] [PubMed] [Google Scholar]
- Eden S, Rohatgi R, Podtelejnikov AV, Mann M, Kirschner MW. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature. 2002;418(6899):790–3. doi: 10.1038/nature00859. [DOI] [PubMed] [Google Scholar]
- Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1(5):253–9. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- Egile C, Loisel TP, Laurent V, Li R, Pantaloni D, Sansonetti PJ, Carlier MF. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J Cell Biol. 1999;146(6):1319–32. doi: 10.1083/jcb.146.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban JA. Living with the enemy: a physiological role for the beta-amyloid peptide. Trends Neurosci. 2004;27(1):1–3. doi: 10.1016/j.tins.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39(1):29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Frost NA, Shroff H, Kong H, Betzig E, Blanpied TA. Single-molecule discrimination of discrete perisynaptic and distributed sites of actin filament assembly within dendritic spines. Neuron. 2010;67(1):86–99. doi: 10.1016/j.neuron.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K, Fu W, Li Y, Witke W, Kwiatkowski DJ, Mattson MP. The actin-severing protein gelsolin modulates calcium channel and NMDA receptor activities and vulnerability to excitotoxicity in hippocampal neurons. J Neurosci. 1997;17(21):8178–86. doi: 10.1523/JNEUROSCI.17-21-08178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassart A, Meas-Yedid V, Dufour A, Olivo-Marin JC, Dautry-Varsat A, Sauvonnet N. Pak1 phosphorylation enhances cortactin-N-WASP interaction in clathrin-caveolin-independent endocytosis. Traffic. 2010;11(8):1079–91. doi: 10.1111/j.1600-0854.2010.01075.x. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Hopkins E, Doyle D, Dobyns WB. High incidence of progressive postnatal cerebellar enlargement in Costello syndrome: brain overgrowth associated with HRAS mutations as the likely cause of structural brain and spinal cord abnormalities. Am J Med Genet A. 2010;152A(5):1161–8. doi: 10.1002/ajmg.a.33391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Stafford LJ, Bryan B, Xia C, Ma W, Wu X, Liu D, Songyang Z, Liu M. A Rac/Cdc42-specific exchange factor, GEFT, induces cell proliferation, transformation, and migration. J Biol Chem. 2003;278(15):13207–15. doi: 10.1074/jbc.M208896200. [DOI] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98(8):4746–51. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci. 1998;18(23):9835–44. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HE, Weeds AG. Plasma gelsolin caps and severs actin filaments. FEBS Lett. 1984;177(2):184–8. doi: 10.1016/0014-5793(84)81280-6. [DOI] [PubMed] [Google Scholar]
- Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321(5885):136–40. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, Makino Y, Seshadri AJ, Ishizuka K, Srivastava DP. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010 doi: 10.1038/nn.2487. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi ML, Rao BS, Seo JS, Choi HS, Dolan BM, Choi SY, Chattarji S, Tonegawa S. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci U S A. 2007;104(27):11489–94. doi: 10.1073/pnas.0705003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head JA, Jiang D, Li M, Zorn LJ, Schaefer EM, Parsons JT, Weed SA. Cortactin tyrosine phosphorylation requires Rac1 activity and association with the cortical actin cytoskeleton. Mol Biol Cell. 2003;14(8):3216–29. doi: 10.1091/mbc.E02-11-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9(9):690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Heredia L, Helguera P, de Olmos S, Kedikian G, Sola Vigo F, LaFerla F, Staufenbiel M, de Olmos J, Busciglio J, Caceres A. Phosphorylation of actin-depolymerizing factor/cofilin by LIM-kinase mediates amyloid beta-induced degeneration: a potential mechanism of neuronal dystrophy in Alzheimer’s disease. J Neurosci. 2006;26(24):6533–42. doi: 10.1523/JNEUROSCI.5567-05.2006. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci. 2001;2(12):880–8. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- Hering H, Sheng M. Activity-dependent redistribution and essential role of cortactin in dendritic spine morphogenesis. J Neurosci. 2003;23(37):11759–69. doi: 10.1523/JNEUROSCI.23-37-11759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11(6):557–66. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- Hirose M, Ishizaki T, Watanabe N, Uehata M, Kranenburg O, Moolenaar WH, Matsumura F, Maekawa M, Bito H, Narumiya S. Molecular dissection of the Rho-associated protein kinase (p160ROCK)-regulated neurite remodeling in neuroblastoma N1E-115 cells. J Cell Biol. 1998;141(7):1625–36. doi: 10.1083/jcb.141.7.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkura N, Matsuzaki M, Noguchi J, Ellis-Davies GC, Kasai H. The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron. 2008;57(5):719–29. doi: 10.1016/j.neuron.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Hotulainen P, Llano O, Smirnov S, Tanhuanpaa K, Faix J, Rivera C, Lappalainen P. Defining mechanisms of actin polymerization and depolymerization during dendritic spine morphogenesis. J Cell Biol. 2009;185(2):323–39. doi: 10.1083/jcb.200809046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter MP, Nelson M, Kurzer M, Wang X, Kryscio RJ, Head E, Pinna G, O’Bryan JP. Intersectin 1 contributes to phenotypes in vivo: implications for Down’s syndrome. Neuroreport. 2011;22(15):767–72. doi: 10.1097/WNR.0b013e32834ae348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain NK, Jenna S, Glogauer M, Quinn CC, Wasiak S, Guipponi M, Antonarakis SE, Kay BK, Stossel TP, Lamarche-Vane N. Endocytic protein intersectin-l regulates actin assembly via Cdc42 and N-WASP. Nat Cell Biol. 2001;3(10):927–32. doi: 10.1038/ncb1001-927. others. [DOI] [PubMed] [Google Scholar]
- Irie F, Yamaguchi Y. EphB receptors regulate dendritic spine development via intersectin, Cdc42 and N-WASP. Nat Neurosci. 2002;5(11):1117–8. doi: 10.1038/nn964. [DOI] [PubMed] [Google Scholar]
- Isacson O, Seo H, Lin L, Albeck D, Granholm AC. Alzheimer’s disease and Down’s syndrome: roles of APP, trophic factors and ACh. Trends Neurosci. 2002;25(2):79–84. doi: 10.1016/s0166-2236(02)02037-4. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Penzes P, Eipper BA, Mains RE. Isoforms of kalirin, a neuronal Dbl family member, generated through use of different 5′- and 3′-ends along with an internal translational initiation site. J Biol Chem. 2000;275(25):19324–33. doi: 10.1074/jbc.M000676200. [DOI] [PubMed] [Google Scholar]
- Kang MG, Guo Y, Huganir RL. AMPA receptor and GEF-H1/Lfc complex regulates dendritic spine development through RhoA signaling cascade. Proc Natl Acad Sci U S A. 2009;106(9):3549–54. doi: 10.1073/pnas.0812861106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature. 2000;404(6774):151–8. doi: 10.1038/35004513. [DOI] [PubMed] [Google Scholar]
- Kim H, Han JR, Park J, Oh M, James SE, Chang S, Lu Q, Lee KY, Ki H, Song WJ. Delta-catenin-induced dendritic morphogenesis. An essential role of p190RhoGEF interaction through Akt1-mediated phosphorylation. J Biol Chem. 2008;283(2):977–87. doi: 10.1074/jbc.M707158200. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Sung JY, Ceglia I, Lee KW, Ahn JH, Halford JM, Kim AM, Kwak SP, Park JB, Ho Ryu S. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature. 2006;442(7104):814–7. doi: 10.1038/nature04976. others. [DOI] [PubMed] [Google Scholar]
- Knaus UG, Wang Y, Reilly AM, Warnock D, Jackson JH. Structural requirements for PAK activation by Rac GTPases. J Biol Chem. 1998;273(34):21512–8. doi: 10.1074/jbc.273.34.21512. [DOI] [PubMed] [Google Scholar]
- Korobova F, Svitkina T. Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol Biol Cell. 2010;21(1):165–76. doi: 10.1091/mbc.E09-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis P, Thevenot E, Rousseau V, Boda B, Muller D, Barnier JV. The p21-activated kinase 3 implicated in mental retardation regulates spine morphogenesis through a Cdc42-dependent pathway. J Biol Chem. 2007;282(29):21497–506. doi: 10.1074/jbc.M703298200. [DOI] [PubMed] [Google Scholar]
- Kuramoto K, Negishi M, Katoh H. Regulation of dendrite growth by the Cdc42 activator Zizimin1/Dock9 in hippocampal neurons. J Neurosci Res. 2009;87(8):1794–805. doi: 10.1002/jnr.21997. [DOI] [PubMed] [Google Scholar]
- Kurisu S, Takenawa T. The WASP and WAVE family proteins. Genome Biol. 2009;10(6):226. doi: 10.1186/gb-2009-10-6-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushima I, Nakamura Y, Aleksic B, Ikeda M, Ito Y, Shiino T, Okochi T, Fukuo Y, Ujike H, Suzuki M. Resequencing and Association Analysis of the KALRN and EPHB1 Genes And Their Contribution to Schizophrenia Susceptibility. Schizophr Bull. doi: 10.1093/schbul/sbq118. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsche K, Yntema H, Brandt A, Jantke I, Nothwang HG, Orth U, Boavida MG, David D, Chelly J, Fryns JP. Mutations in ARHGEF6, encoding a guanine nucleotide exchange factor for Rho GTPases, in patients with X-linked mental retardation. Nat Genet. 2000;26(2):247–50. doi: 10.1038/80002. others. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, Farb CR, Rodrigues SM, LeDoux JE. Fear conditioning drives profilin into amygdala dendritic spines. Nat Neurosci. 2006;9(4):481–3. doi: 10.1038/nn1672. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Lee Y, Rozeboom A, Lee JY, Udagawa N, Hoe HS, Pak DT. Requirement for Plk2 in orchestrated ras and rap signaling, homeostatic structural plasticity, and memory. Neuron. 2011;69(5):957–73. doi: 10.1016/j.neuron.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Aizenman CD, Cline HT. Regulation of rho GTPases by crosstalk and neuronal activity in vivo. Neuron. 2002;33(5):741–50. doi: 10.1016/s0896-6273(02)00621-9. [DOI] [PubMed] [Google Scholar]
- Luo L. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu Rev Cell Dev Biol. 2002;18:601–35. doi: 10.1146/annurev.cellbio.18.031802.150501. [DOI] [PubMed] [Google Scholar]
- Ma XM, Kiraly DD, Gaier ED, Wang Y, Kim EJ, Levine ES, Eipper BA, Mains RE. Kalirin-7 is required for synaptic structure and function. J Neurosci. 2008;28(47):12368–82. doi: 10.1523/JNEUROSCI.4269-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367(6458):40–6. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell. 1998;1(2):183–92. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4(11):1086–92. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429(6993):761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer BJ. SH3 domains: complexity in moderation. J Cell Sci. 2001;114(Pt 7):1253–63. doi: 10.1242/jcs.114.7.1253. [DOI] [PubMed] [Google Scholar]
- McAvoy T, Zhou MM, Greengard P, Nairn AC. Phosphorylation of Rap1GAP, a striatally enriched protein, by protein kinase A controls Rap1 activity and dendritic spine morphology. Proc Natl Acad Sci U S A. 2009;106(9):3531–6. doi: 10.1073/pnas.0813263106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller N, Irani-Tehrani M, Kiosses WB, Del Pozo MA, Schwartz MA. Zizimin1, a novel Cdc42 activator, reveals a new GEF domain for Rho proteins. Nat Cell Biol. 2002;4(9):639–47. doi: 10.1038/ncb835. [DOI] [PubMed] [Google Scholar]
- Meller N, Westbrook MJ, Shannon JD, Guda C, Schwartz MA. Function of the N-terminus of zizimin1: autoinhibition and membrane targeting. Biochem J. 2008;409(2):525–33. doi: 10.1042/BJ20071263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki H, Miura K, Takenawa T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 1996;15(19):5326–35. [PMC free article] [PubMed] [Google Scholar]
- Miki H, Suetsugu S, Takenawa T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 1998;17(23):6932–41. doi: 10.1093/emboj/17.23.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki H, Takenawa T. Regulation of actin dynamics by WASP family proteins. J Biochem. 2003;134(3):309–13. doi: 10.1093/jb/mvg146. [DOI] [PubMed] [Google Scholar]
- Mott HR, Nietlispach D, Evetts KA, Owen D. Structural analysis of the SH3 domain of beta-PIX and its interaction with alpha-p21 activated kinase (PAK) Biochemistry. 2005;44(33):10977–83. doi: 10.1021/bi050374a. [DOI] [PubMed] [Google Scholar]
- Mullins RD, Heuser JA, Pollard TD. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc Natl Acad Sci U S A. 1998;95(11):6181–6. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20(14):5329–38. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama AY, Luo L. Intracellular signaling pathways that regulate dendritic spine morphogenesis. Hippocampus. 2000;10(5):582–6. doi: 10.1002/1098-1063(2000)10:5<582::AID-HIPO8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Narayan S, Tang B, Head SR, Gilmartin TJ, Sutcliffe JG, Dean B, Thomas EA. Molecular profiles of schizophrenia in the CNS at different stages of illness. Brain Res. 2008 doi: 10.1016/j.brainres.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebl G, Fischer S, Penzel R, Samstag Y. Dephosphorylation of cofilin is regulated through Ras and requires the combined activities of the Ras-effectors MEK and PI3K. Cell Signal. 2004;16(2):235–43. doi: 10.1016/s0898-6568(03)00133-5. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Spine microdomains for postsynaptic signaling and plasticity. Trends Cell Biol. 2009;19(5):218–27. doi: 10.1016/j.tcb.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Nikolic M. The Pak1 kinase: an important regulator of neuronal morphology and function in the developing forebrain. Mol Neurobiol. 2008;37(2-3):187–202. doi: 10.1007/s12035-008-8032-1. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81(1):53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Hata Y, Ide N, Yasuda T, Inoue E, Inoue T, Mizoguchi A, Takai Y. nRap GEP: a novel neural GDP/GTP exchange protein for rap1 small G protein that interacts with synaptic scaffolding molecule (S-SCAM) Biochem Biophys Res Commun. 1999;265(1):38–44. doi: 10.1006/bbrc.1999.1619. [DOI] [PubMed] [Google Scholar]
- Okabe S, Miwa A, Okado H. Spine formation and correlated assembly of presynaptic and postsynaptic molecules. J Neurosci. 2001;21(16):6105–14. doi: 10.1523/JNEUROSCI.21-16-06105.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7(10):1104–12. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- Ong WY, Wang XS, Manser E. Differential distribution of alpha and beta isoforms of p21-activated kinase in the monkey cerebral neocortex and hippocampus. Exp Brain Res. 2002;144(2):189–99. doi: 10.1007/s00221-002-1016-x. [DOI] [PubMed] [Google Scholar]
- Osterweil E, Wells DG, Mooseker MS. A role for myosin VI in postsynaptic structure and glutamate receptor endocytosis. J Cell Biol. 2005;168(2):329–38. doi: 10.1083/jcb.200410091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak DT, Yang S, Rudolph-Correia S, Kim E, Sheng M. Regulation of dendritic spine morphology by SPAR, a PSD-95-associated RapGAP. Neuron. 2001;31(2):289–303. doi: 10.1016/s0896-6273(01)00355-5. [DOI] [PubMed] [Google Scholar]
- Park J, Kim Y, Park ZY, Park D, Chang S. Neuronal specific betaPix-b stimulates actin-dependent processes via the interaction between its PRD and WH1 domain of N-WASP. J Cell Physiol. 2011 doi: 10.1002/jcp.22863. [DOI] [PubMed] [Google Scholar]
- Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, Huganir RL. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37(2):263–74. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, Srivastava DP. Convergent CaMK and RacGEF signals control dendritic structure and function. Trends Cell Biol. 2008;18(9):405–13. doi: 10.1016/j.tcb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011a;14(3):285–93. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Kambampati V, Mains RE, Eipper BA. Distinct roles for the two Rho GDP/GTP exchange factor domains of kalirin in regulation of neurite growth and neuronal morphology. J Neurosci. 2001a;21(21):8426–34. doi: 10.1523/JNEUROSCI.21-21-08426.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Sattler R, Zhang X, Huganir RL, Kambampati V, Mains RE, Eipper BA. The neuronal Rho-GEF Kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron. 2001b;29(1):229–42. doi: 10.1016/s0896-6273(01)00193-3. [DOI] [PubMed] [Google Scholar]
- Penzes P, Jones KA. Dendritic spine dynamics--a key role for kalirin-7. Trends Neurosci. 2008;31(8):419–27. doi: 10.1016/j.tins.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Woolfrey KM, Srivastava DP. Epac2-mediated dendritic spine remodeling: implications for disease. Mol Cell Neurosci. 2011b;46(2):368–80. doi: 10.1016/j.mcn.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD, Beltzner CC. Structure and function of the Arp2/3 complex. Curr Opin Struct Biol. 2002;12(6):768–74. doi: 10.1016/s0959-440x(02)00396-2. [DOI] [PubMed] [Google Scholar]
- Pucharcos C, Fuentes JJ, Casas C, de la Luna S, Alcantara S, Arbones ML, Soriano E, Estivill X, Pritchard M. Alu-splice cloning of human Intersectin (ITSN), a putative multivalent binding protein expressed in proliferating and differentiating neurons and overexpressed in Down syndrome. Eur J Hum Genet. 1999;7(6):704–12. doi: 10.1038/sj.ejhg.5200356. [DOI] [PubMed] [Google Scholar]
- Qualmann B, Boeckers TM, Jeromin M, Gundelfinger ED, Kessels MM. Linkage of the actin cytoskeleton to the postsynaptic density via direct interactions of Abp1 with the ProSAP/Shank family. J Neurosci. 2004;24(10):2481–95. doi: 10.1523/JNEUROSCI.5479-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiner CA, Mains RE, Eipper BA. Kalirin: a dual Rho guanine nucleotide exchange factor that is so much more than the sum of its many parts. Neuroscientist. 2005;11(2):148–60. doi: 10.1177/1073858404271250. [DOI] [PubMed] [Google Scholar]
- Racz B, Weinberg RJ. The subcellular organization of cortactin in hippocampus. J Neurosci. 2004;24(46):10310–7. doi: 10.1523/JNEUROSCI.2080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz B, Weinberg RJ. Organization of the Arp2/3 complex in hippocampal spines. J Neurosci. 2008;28(22):5654–9. doi: 10.1523/JNEUROSCI.0756-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh N, Anton IM, Hartwig JH, Geha RS. WIP, a protein associated with wiskott-aldrich syndrome protein, induces actin polymerization and redistribution in lymphoid cells. Proc Natl Acad Sci U S A. 1997;94(26):14671–6. doi: 10.1073/pnas.94.26.14671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebhun JF, Castro AF, Quilliam LA. Identification of guanine nucleotide exchange factors (GEFs) for the Rap1 GTPase. Regulation of MR-GEF by M-Ras-GTP interaction. J Biol Chem. 2000;275(45):34901–8. doi: 10.1074/jbc.M005327200. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16(10):522–9. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Ho HY, Kirschner MW. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J Cell Biol. 2000;150(6):1299–310. doi: 10.1083/jcb.150.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan XP, Alldritt J, Svenningsson P, Allen PB, Wu GY, Nairn AC, Greengard P. The Rho-specific GEF Lfc interacts with neurabin and spinophilin to regulate dendritic spine morphology. Neuron. 2005;47(1):85–100. doi: 10.1016/j.neuron.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Saneyoshi T, Wayman G, Fortin D, Davare M, Hoshi N, Nozaki N, Natsume T, Soderling TR. Activity-dependent synaptogenesis: regulation by a CaM-kinase kinase/CaM-kinase I/betaPIX signaling complex. Neuron. 2008;57(1):94–107. doi: 10.1016/j.neuron.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmandke A, Strittmatter SM. ROCK and Rho: biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist. 2007;13(5):454–69. doi: 10.1177/1073858407303611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16(13):1587–609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- Sekino Y, Kojima N, Shirao T. Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem Int. 2007;51(2-4):92–104. doi: 10.1016/j.neuint.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Shepherd TR, Klaus SM, Liu X, Ramaswamy S, DeMali KA, Fuentes EJ. The Tiam1 PDZ domain couples to Syndecan1 and promotes cell-matrix adhesion. J Mol Biol. 2010;398(5):730–46. doi: 10.1016/j.jmb.2010.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Pontrello CG, DeFea KA, Reichardt LF, Ethell IM. Focal adhesion kinase acts downstream of EphB receptors to maintain mature dendritic spines by regulating cofilin activity. J Neurosci. 2009;29(25):8129–42. doi: 10.1523/JNEUROSCI.4681-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi Fard S, Kele J, Vilar M, Paratcha G, Ledda F. Tiam1 as a signaling mediator of nerve growth factor-dependent neurite outgrowth. PLoS One. 2010;5(3):e9647. doi: 10.1371/journal.pone.0009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling SH, Guire ES, Kaech S, White J, Zhang F, Schutz K, Langeberg LK, Banker G, Raber J, Scott JD. A WAVE-1 and WRP signaling complex regulates spine density, synaptic plasticity, and memory. J Neurosci. 2007;27(2):355–65. doi: 10.1523/JNEUROSCI.3209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling SH, Langeberg LK, Soderling JA, Davee SM, Simerly R, Raber J, Scott JD. Loss of WAVE-1 causes sensorimotor retardation and reduced learning and memory in mice. Proc Natl Acad Sci U S A. 2003;100(4):1723–8. doi: 10.1073/pnas.0438033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Star EN, Kwiatkowski DJ, Murthy VN. Rapid turnover of actin in dendritic spines and its regulation by activity. Nat Neurosci. 2002;5(3):239–46. doi: 10.1038/nn811. [DOI] [PubMed] [Google Scholar]
- Suetsugu S, Miki H, Takenawa T. The essential role of profilin in the assembly of actin for microspike formation. EMBO J. 1998;17(22):6516–26. doi: 10.1093/emboj/17.22.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Sekino Y, Tanaka S, Mizui T, Kishi S, Shirao T. Drebrin-dependent actin clustering in dendritic filopodia governs synaptic targeting of postsynaptic density-95 and dendritic spine morphogenesis. J Neurosci. 2003;23(16):6586–95. doi: 10.1523/JNEUROSCI.23-16-06586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto-Kimura S, Ageta-Ishihara N, Nonaka M, Adachi-Morishima A, Mano T, Okamura M, Fujii H, Fuse T, Hoshino M, Suzuki S. Regulation of dendritogenesis via a lipid-raft-associated Ca2+/calmodulin-dependent protein kinase CLICK-III/CaMKIgamma. Neuron. 2007;54(5):755–70. doi: 10.1016/j.neuron.2007.05.021. others. [DOI] [PubMed] [Google Scholar]
- Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8(1):37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Minden A, Yuste R. Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex. 2000;10(10):927–38. doi: 10.1093/cercor/10.10.927. [DOI] [PubMed] [Google Scholar]
- ten Klooster JP, Jaffer ZM, Chernoff J, Hordijk PL. Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J Cell Biol. 2006;172(5):759–69. doi: 10.1083/jcb.200509096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Ritter B, Verbich D, Sanson C, Bourbonniere L, McKinney RA, McPherson PS. Intersectin regulates dendritic spine development and somatodendritic endocytosis but not synaptic vesicle recycling in hippocampal neurons. J Biol Chem. 2009;284(18):12410–9. doi: 10.1074/jbc.M809746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidyman WE, Rauen KA. Noonan, Costello and cardio-facio-cutaneous syndromes: dysregulation of the Ras-MAPK pathway. Expert Rev Mol Med. 2008;10:e37. doi: 10.1017/S1462399408000902. [DOI] [PubMed] [Google Scholar]
- Tolias KF, Bikoff JB, Burette A, Paradis S, Harrar D, Tavazoie S, Weinberg RJ, Greenberg ME. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45(4):525–38. doi: 10.1016/j.neuron.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Tolias KF, Bikoff JB, Kane CG, Tolias CS, Hu L, Greenberg ME. The Rac1 guanine nucleotide exchange factor Tiam1 mediates EphB receptor-dependent dendritic spine development. Proc Natl Acad Sci U S A. 2007;104(17):7265–70. doi: 10.1073/pnas.0702044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasevic N, Jia Z, Russell A, Fujii T, Hartman JJ, Clancy S, Wang M, Beraud C, Wood KW, Sakowicz R. Differential regulation of WASP and N-WASP by Cdc42, Rac1, Nck, and PI(4,5)P2. Biochemistry. 2007;46(11):3494–502. doi: 10.1021/bi062152y. [DOI] [PubMed] [Google Scholar]
- Tsuchiya D, Kitamura Y, Takata K, Sugisaki T, Taniguchi T, Uemura K, Miki H, Takenawa T, Shimohama S. Developmental expression of neural Wiskott-Aldrich syndrome protein (N-WASP) and WASP family verprolin-homologous protein (WAVE)-related proteins in postnatal rat cerebral cortex and hippocampus. Neurosci Res. 2006;56(4):459–69. doi: 10.1016/j.neures.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Tu H, Wigler M. Genetic evidence for Pak1 autoinhibition and its release by Cdc42. Mol Cell Biol. 1999;19(1):602–11. doi: 10.1128/mcb.19.1.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uruno T, Liu J, Zhang P, Fan Y, Egile C, Li R, Mueller SC, Zhan X. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat Cell Biol. 2001;3(3):259–66. doi: 10.1038/35060051. [DOI] [PubMed] [Google Scholar]
- Usui S, Konno D, Hori K, Maruoka H, Okabe S, Fujikado T, Tano Y, Sobue K. Synaptic targeting of PSD-Zip45 (Homer 1c) and its involvement in the synaptic accumulation of F-actin. J Biol Chem. 2003;278(12):10619–28. doi: 10.1074/jbc.M210802200. [DOI] [PubMed] [Google Scholar]
- Weaver AM, Heuser JE, Karginov AV, Lee WL, Parsons JT, Cooper JA. Interaction of cortactin and N-WASp with Arp2/3 complex. Curr Biol. 2002;12(15):1270–8. doi: 10.1016/s0960-9822(02)01035-7. [DOI] [PubMed] [Google Scholar]
- Weaver AM, Karginov AV, Kinley AW, Weed SA, Li Y, Parsons JT, Cooper JA. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr Biol. 2001;11(5):370–4. doi: 10.1016/s0960-9822(01)00098-7. [DOI] [PubMed] [Google Scholar]
- Webb BA, Zhou S, Eves R, Shen L, Jia L, Mak AS. Phosphorylation of cortactin by p21-activated kinase. Arch Biochem Biophys. 2006;456(2):183–93. doi: 10.1016/j.abb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Wegner AM, Nebhan CA, Hu L, Majumdar D, Meier KM, Weaver AM, Webb DJ. N-wasp and the arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. J Biol Chem. 2008;283(23):15912–20. doi: 10.1074/jbc.M801555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzdoerfer R, Fountoulakis M, Lubec G. Reduction of actin-related protein complex 2/3 in fetal Down syndrome brain. Biochem Biophys Res Commun. 2002;293(2):836–41. doi: 10.1016/S0006-291X(02)00291-7. [DOI] [PubMed] [Google Scholar]
- Wilmot B, McWeeney SK, Nixon RR, Montine TJ, Laut J, Harrington CA, Kaye JA, Kramer PL. Translational gene mapping of cognitive decline. Neurobiol Aging. 2008;29(4):524–41. doi: 10.1016/j.neurobiolaging.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witke W. The role of profilin complexes in cell motility and other cellular processes. Trends Cell Biol. 2004;14(8):461–9. doi: 10.1016/j.tcb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Woolfrey KM, Srivastava DP, Photowala H, Yamashita M, Barbolina MV, Cahill ME, Xie Z, Jones KA, Quilliam LA, Prakriya M. Epac2 induces synapse remodeling and depression and its disease-associated forms alter spines. Nat Neurosci. 2009;12(10):1275–84. doi: 10.1038/nn.2386. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZR, Qin W, Yang YF, He G, Gao SH, Ren MS, Peng YW, Zhang Z, He L. Transmission disequilibrium analysis of the GSN gene in a cohort of family trios with schizophrenia. Neurosci Lett. 2004;372(3):200–3. doi: 10.1016/j.neulet.2004.09.041. [DOI] [PubMed] [Google Scholar]
- Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeier DJ, Penzes P. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56(4):640–56. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X, Wang Y, Ma XM, Rompolas P, Keutmann HT, Mains RE, Eipper BA. Regulation of Kalirin by Cdk5. J Cell Sci. 2008;121(Pt 15):2601–11. doi: 10.1242/jcs.016089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Barry DC, Settleman J, Schwartz MA, Bokoch GM. Differing structural requirements for GTPase-activating protein responsiveness and NADPH oxidase activation by Rac. J Biol Chem. 1994;269(38):23569–74. [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462(7275):920–4. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393(6687):809–12. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- Youn H, Jeoung M, Koo Y, Ji H, Markesbery WR, Ji I, Ji TH. Kalirin is under-expressed in Alzheimer’s disease hippocampus. J Alzheimers Dis. 2007;11(3):385–97. doi: 10.3233/jad-2007-11314. [DOI] [PubMed] [Google Scholar]
- Yuan P, Zhou R, Wang Y, Li X, Li J, Chen G, Guitart X, Manji HK. Altered levels of extracellular signal-regulated kinase signaling proteins in postmortem frontal cortex of individuals with mood disorders and schizophrenia. J Affect Disord. 2010;124(1-2):164–9. doi: 10.1016/j.jad.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaldua N, Gastineau M, Hoshino M, Lezoualc’h F, Zugaza JL. Epac signaling pathway involves STEF, a guanine nucleotide exchange factor for Rac, to regulate APP processing. FEBS Lett. 2007;581(30):5814–8. doi: 10.1016/j.febslet.2007.11.053. [DOI] [PubMed] [Google Scholar]
- Zhao L, Ma QL, Calon F, Harris-White ME, Yang F, Lim GP, Morihara T, Ubeda OJ, Ambegaokar S, Hansen JE. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9(2):234–42. doi: 10.1038/nn1630. others. [DOI] [PubMed] [Google Scholar]
- Zhou K, Wang Y, Gorski JL, Nomura N, Collard J, Bokoch GM. Guanine nucleotide exchange factors regulate specificity of downstream signaling from Rac and Cdc42. J Biol Chem. 1998;273(27):16782–6. doi: 10.1074/jbc.273.27.16782. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Xiao M, Nicoll RA. Contribution of cytoskeleton to the internalization of AMPA receptors. Proc Natl Acad Sci U S A. 2001;98(3):1261–6. doi: 10.1073/pnas.031573798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17(1):91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]