Abstract
The discovery of new catalysts that can generate complex organic compounds via enantioselective transformations is central to advances in the life sciences;i for this reason, many chemists try to discover catalysts that can be used to produce chiral molecules with a strong preference for one mirror image isomer.ii The ideal catalyst should be devoid of precious elementsiii and should bring reactions to completion in a few hours using operationally simple procedures. In this manuscript, we introduce a set of small organic molecules that can catalyze reactions of unsaturated organoboron reagents with imines and carbonyls; the products of the reactions are enantiomerically pure amines and alcohols, which can be used to synthesize more complex, biologically active molecules. A distinguishing feature of this new catalyst class is the presence of a 'key' proton embedded within their structure. The catalyst is derived from the abundant amino acid valine and was prepared in large quantities in four steps using inexpensive reagents. Reactions are scalable, do not demand stringent conditions, and can be performed with as little as 0.25 mol % catalyst in less than six hours at room temperature to generate products in >85% yield and ≥97:3 enantiomeric ratio. The efficiency, selectivity and operational simplicity of the transformations and the range of boron-based reagents render this advance vital to future progress in chemistry, biology and medicine.
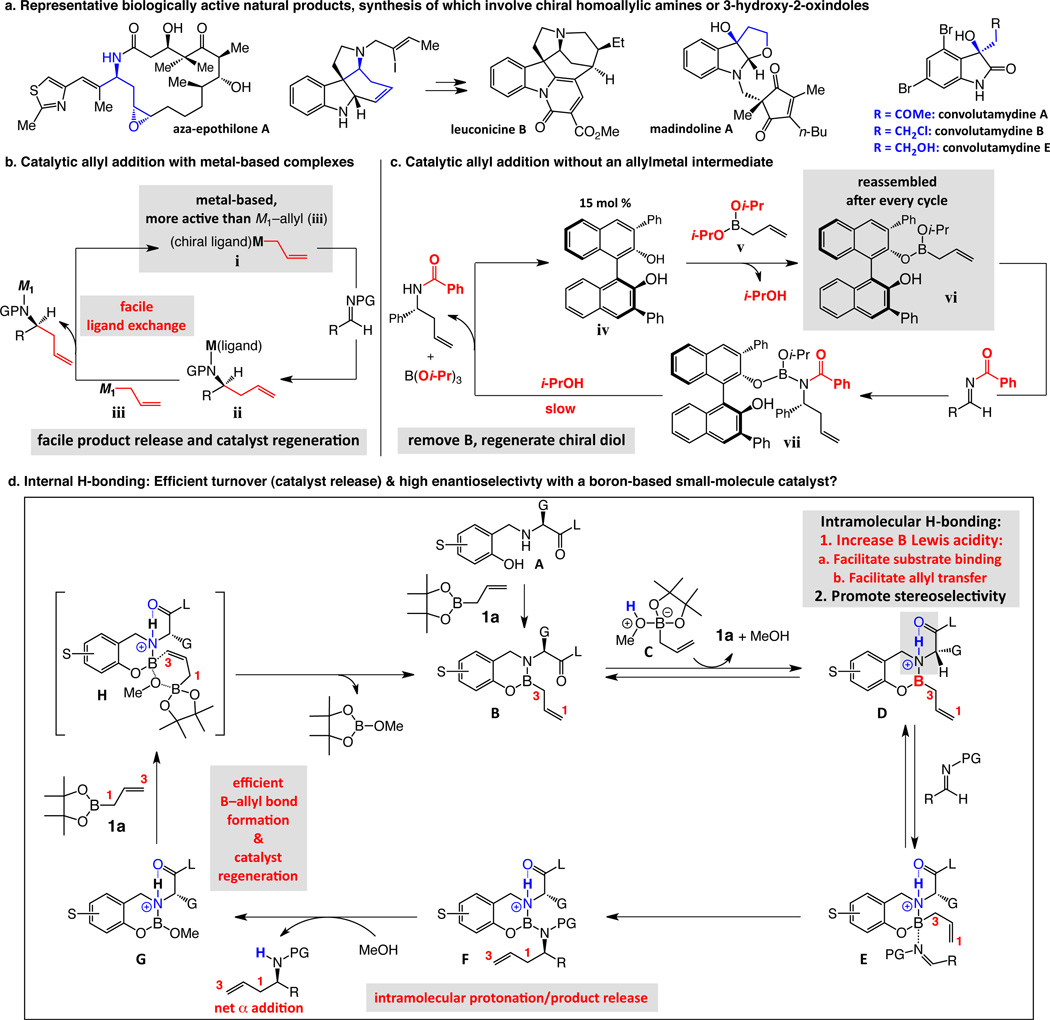
Many biologically active molecules contain nitrogen-substituted carbon stereogenic centers. Routes for efficient preparation of enantiomerically enriched homoallylic amines are therefore of considerable consequenceiv. Anti-cancer agents aza-epothilones A–Dv (see Fig. 1a), leuconicines A–Bvi, natural products that can reverse multi-drug resistance, and immunosuppressant FR235222vii are among entities the synthesis of which involves homoallylic amines. Enantioselective addition of an allyl group to an aldimine has thus been the subject of substantial scrutinyiv. Catalytic protocols have been introduced for preparing homoallylic amines and derivatives with high enantioselectivity; nevertheless, all lack several of the abovementioned attributes. Some demand the intermediacy of allylindiumsviii, prepared in situ from allyl halides and the costly metalix,x; others entail the use of a rare elementxi. Moreover, the following drawbacks are encountered frequently: difficult-to-access or expensive ligandsxii, high catalyst loadings (e.g., ≥10 mol %)viii–x,xii–xiii, long reaction times (e.g., >8 hours)viii–xi,xiii–xiv,xv,xvi exceedingly low temperatures (e.g., −50 °C or lower)xv,xvii, narrow substrate rangeix,xv,xvi,xviii, and the need for allyltinxi or moisture-sensitive reagentsxiii.
Figure 1. The significance of homoallylic amines and alcohols; three approaches to their catalytic enantioselective synthesis.
a, Biologically active natural products synthesized via homoallylic amines and alcohols. b, With a metal-containing catalyst, high rates are achieved through facile ligand exchange. c, In a metal-free system, the catalyst must be reassembled prior to each cycle. d, A catalyst might be designed with an internal H-bond promoting fast reaction rates and high enantioselectivities. Catalytic cycles deliver net α addition (C1–B→C1–C) resulting from two γ-selective processes (G→B and D→F). Facile catalyst regeneration may occur through allylation of G via H. PG = protecting group.
Readily obtainable catalysts for efficient, sustainable and practical enantioselective additions to ketones are equally sought after. Isatins are carbonyl-containing entities that can be converted to enantiomerically enriched 3-hydroxy-2-oxindoles found within alkaloids of substantial biological consequencexix. Examples are proteasome inhibitors TMC-95A–D with ample potential in the treatment of cancer and immune disordersxx, and interleukin 6 inhibitor and anti-osteoporosis agent madindoline A (Fig. 1a); proper configuration of the tertiary hydroxyl unit is needed for high activityxxi. A few reports concentrate on catalytic enantioselective allyl additions to isatins; limitations including the need for toxic tin-based reagents,xxii scarce metal salts,xxiii and moderate selectivitiesxxii tarnish these notable advances.
Deliberations regarding catalyst design, alongside consideration of the mechanistic attributes of different extant approaches to catalytic enantioselective allyl additions, led us to opt for metal-free catalysts; several factors led to such a conclusion. Most allylmetal reagents are sensitive to oxygen and moisture;xxiv their use entails vigilantly controlled conditions. Furthermore, transformations with π-allylmetal complexes are usually either not diastereoselectiveix,xvii or one possible isomer remains inaccessiblexiii,xxiii regardless of whether the E or Z allylic reagent is employed. While strategies involving stoichiometric quantities of enantiomerically pure substrates offer stereoselective alternatives, the transformations suffer from similar limitations (see the Supplementary Information for bibliography).
There was one metal-free catalytic method for enantioselective allyl addition to iminesxiii; reactions, however, proceed less readily and demand higher catalyst amounts and longer reaction times than when allylmetal species are involved (Fig. 1c); additionally, moisture-sensitive allylboron derivatives are required (cf. v, Fig. 1c), and similar to transformations with crotylmetal reagents, only one product diastereomer can be preparedxiii. Reactions with metal-containing systems are likely more efficient because of swift ligand exchange leading to fast catalyst regeneration (Fig. 1b): the swap between a homoallyl metal-amide (ii) and an allyl reagent (iii) to re-form the active complex i can occur rapidly. In contrast, allylboron vi needs to be re-assembled after each cycle (Fig. 1c): the enantiomerically enriched vii must first be converted to diol iv by protolytic removal of the boron and product moieties; the diol then reacts with allylboron v to regenerate vi. Mechanistic studies indicate that it is indeed the regeneration of the diol iv and the chiral catalyst that hampers reaction ratexxv. Thus, to ensure re-formation of vi, a more reactive but moisture sensitive allylboron (v) was prepared and used.
The above analysis led us to conclude that a pathway must be conceived such that the catalyst is reproduced rapidly but without a sensitive allylboron and the benefit of the favorable kinetics available to metal-containing intermediates. Accordingly, we drafted the blueprint outlined in Fig. 1d. Aminophenol (A) offered an attractive possibility; such molecules are structurally modular and synthesized by dependable manipulations; chiral allylboron B would be generated by reaction with the relatively robust (pinacolato)allylboron 1a. At this juncture, several challenges become evident: (1) The boron center, bearing a comparatively electron-donating amine ligand, would have to be rendered sufficiently Lewis acidic to bind readily with the substrate. (2) The stereogenic center resides at a conformationally mobile arm of the catalyst; high enantioselectivity would demand strong differentiation between the diastereotopic faces of the coordinated imine. (3) A mechanism for quick catalyst regeneration would have to be identified. We envisioned that a solution could involve an internal H-bond, bridging the catalyst’s amide carbonyl and boron-bound nitrogen (Fig. 1d). Such electrostatic attraction would elevate the boron center Lewis acidity to facilitate substrate binding (→E) and C–C bond formation (→F) and rigidify the catalyst•substrate complex E, engendering high enantioselectivity. Turnover could be facilitated by acceleration of product release through intramolecular protonation in F, generating the desired product and chiral allylboron intermediate G, which can then react to regenerate the catalytically active B through a structure such as H (Fig. 1). As will be detailed below, the facility with which H can be accessed is central to the high turnover rates achieved and has stereochemical consequences that are among the hallmarks of the present system. We further noted a significant implication of the projected mechanistic scenario: once the boron-based catalyst (B) is generated (i.e., after the first cycle), subsequent cycles would deliver net α addition of an allyl unit (C1–B→C1–C) resulting from two γ-selective processes (i.e., G→B and D→F; Fig. 1d).
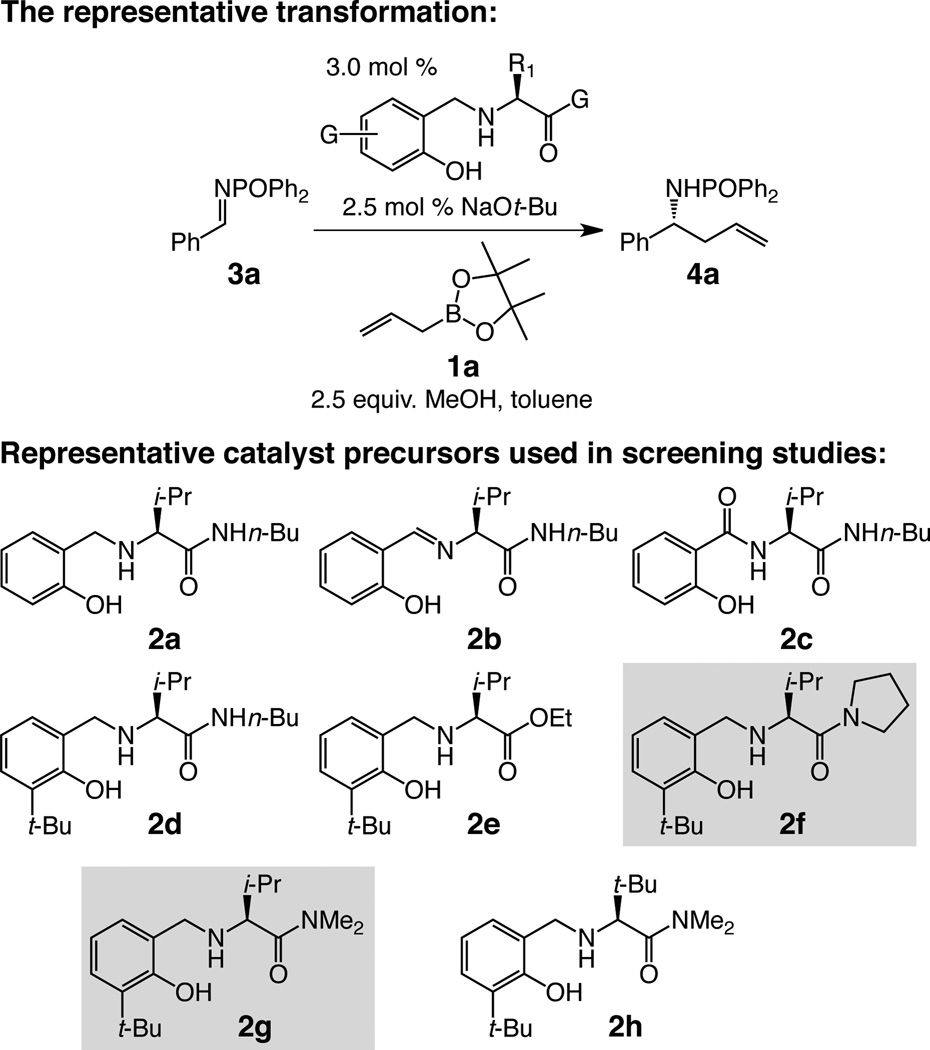
We first probed the ability of aminophenols 2a–2h (Table 1) to serve as catalyst precursors for reactions involving commercially available (pinacolato)allylboron 1a and N-phosphinoylimine 3a. The choice of the N-activating group, despite its ostensible non-optimal atom economy, was for several reasons. The derived imines, aryl- or alkyl-containing, can be prepared efficiently; such entities are relatively robust and generate products that are easy to purify due to their strong tendency to be crystalline (chromatography avoided). There are inexpensive and efficient mildly acidic methods for removal of the phosphorous-based protecting group and generation of the parent amines.xxiv,xxvi Such protocols tolerate many commonly used functional groups and do not require strongly reductive conditions (e.g., diisobutylaluminum hydridexiii required in Fig. 1c), or costly metal salts (e.g., SmI2xviii,xxvii) and/or alkyllithium reagentsx.
Table 1.
Examination of various amino alcohols
| Entry no. | Amino Alcohol; Mol % |
Time (h); T (°C) |
Conv. (%)§ | e.r. † |
|---|---|---|---|---|
| 1 | 2a; 3.0 | 4.0; 22 | 71 | 74.5:2.5 |
| 2 | 2b; 3.0 | 4.0; 22 | <2 | ND |
| 3 | 2c; 3.0 | 4.0; 22 | <10 | ND |
| 4 | 2d; 3.0 | 4.0; 22 | >98 | 91:9 |
| 5 | 2e; 3.0 | 4.0; 22 | 47 | 80:20 |
| 6 | 2f; 3.0 | 4.0; 22 | >98 | 96:4 |
| 7 | 2g; 3.0 | 4.0; 22 | >98 | 96.5:3.5 |
| 8 | 2h; 3.0 | 4.0; 22 | 97 | 98:2 |
When imine 3a and allylboron 1a are subjected to 3.0 mol % amino alcohol 2a (Fig. 2, Table 1, entry 1), 2.5 mol % NaOt-Bu and 2.5 equivalents of MeOH, 71% conversion to homoallylamide 4a is observed in four hours [74.5:25.5 enantiomeric ratio (e.r.)]. With Schiff base 2b or amide 2c (entries 2–3), there is minimal transformation. Placement of a sizeable t-butyl unit adjacent to the phenol group in 2d (entry 4), incorporated with the idea to discourage dimerization of two or more amino alcohols in solution, led to improved efficiency (>98% conv.); the superior enantioselectivity (91:9 e.r.) reflects a substantially more facile process initiated by the chiral catalyst, since control experiments indicate that allyl addition proceeds with reasonable efficiency in its absence (70% conv., 75 min, 22 °C). Lower e.r. and diminished reactivity is furnished by less Lewis basic ethyl ester 2e (Table 1, entry 5). With dialkylamides 2f and 2g (entries 6–7) additions proceed to completion readily, affording the desired amide in approximately 96:4 e.r. Reaction with 2h (entry 8) is more selective (98:2 e.r.) but requires the exorbitantly expensive tert-Leu residue. Lastly, similar efficiency and enantioselectivity is attained when organic amines are used as base [e.g., 1,8-diazabicycloundec-7-ene (dbu)].
Figure 2. Examination of chiral amino alcohols as candidates for catalyst precursors.
The lack of activity shown by catalysts derived from 2b and 2c is consistent with the mechanistic scenario outlined in Fig. 1d, as the requisite chiral allylboron species cannot be generated. Also consistent is the low activity and enantioselectivity by ester-containing 2e, underscoring the pivotal role of the catalyst’s Lewis basic C-terminus in establishing an H-bond.
Reactions were carried out in toluene under an atmosphere of nitrogen gas; ND = not determined.
§ Conversion to the desired product as measured by analysis of 400 MHz 1H NMR spectra of unpurified mixtures versus an internal standard of 9-methylanthracene; the variance of values is estimated to be <±2%.
† Enantiomeric ratios were determined by HPLC analysis; the variance of values is estimated to be <±2%. See the Supplementary Information for details.
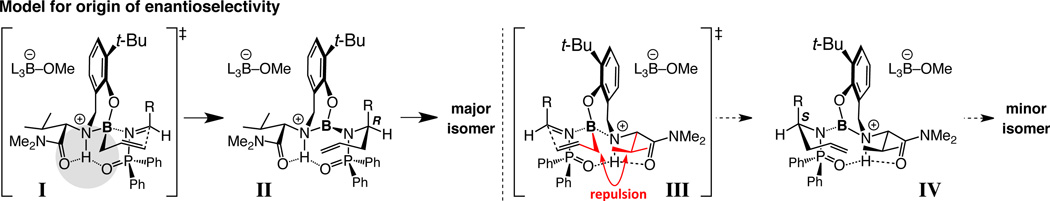
A wide array of imines undergoes allyl additions with 3.0 mol % of amino alcohol 2g and 1.5 equivalents of allylboron 1a within six hours at ambient temperature (Fig. 3, Tables 2–3). Homoallylamides, including those that bear heterocyclic moieties, such as a furyl or a pyridyl unit (entries 11–12, Table 2), are isolated often in >85% yield and ≥97:3 e.r. As the syntheses of 4m and 4n illustrate, use of 2-substituted allylboron reagents results in equally efficient and enantioselective processes. The method can be extended to additions with alkenyl- and alkyl-substituted aldimines (Table 3).
Figure 3. Efficient and enantioselective catalytic allyl additions to aldimines.
Aryl-, alkenyl-, alkynyl- and alkylimines can be used to generate homoallylic amides with high efficiency and enantioselectivity (Tables 2–3). Mechanistic models account for the observed enantioselectivity and involve H-bonding interactions that bring the reaction components together, promote high enantiotopic face differentiation by enforcing an organized transition structure, and facilitate bond formation by minimizing electron–electron repulsion caused by the converging heteroatoms; this model is supported by the X-ray crystal structures of 2g and its HCl salt, which contain a proton-bridge connecting the amine and carbonyl units (see the Supplementary Information).
Reactions were carried out in toluene under an atmosphere of nitrogen gas.
§Conversion to the desired product as measured by analysis of 400 MHz 1H NMR spectra of unpurified mixtures versus an internal standard of 9-methylanthracene; the variance of values is estimated to be <±2%.
§§Yield of isolated product after purification; the variance of values is estimated to be ±2%.
†Enantiomeric ratios were determined by HPLC analysis; the variance of values is estimated to be <±2%. See the Supplementary Information for details.
Table 2.
Catalytic enantioselective allyl additions to aryl-substituted imines
 | ||||
|---|---|---|---|---|
| Entry no. | Ar | Time (h) | Conv. (%);§ Yield (%)§§ |
e.r.† |
| 1 | Ph; 3a | 4.0 | >98; 95 | 96.5:3.5 |
| 2 | o-FC6H4; 3b | 4.0 | >98; 91 | 98:2 |
| 3 | o-BrC6H4; 3c | 4.0 | >98; 86 | 97.5:2.5 |
| 4 | o-MeC6H4; 3d | 6.0 | >98; 91 | 93.5:6.5 |
| 5 | m-BrC6H4; 3e | 4.0 | >98; 95 | 98:2 |
| 6 | p-BrC6H4; 3f | 6.0 | >98; 91 | 97:3 |
| 7 | p-CF3C6H4; 3g | 4.0 | >98; 93 | 98:2 |
| 8 | p-MeO2CC6H4; 3h | 4.0 | >98; 92 | 98:2 |
| 9 | p-MeOC6H4; 3i | 4.0 | >98; 98 | 96.5:3.5 |
| 10 | p-(n-Bu)2C6H4; 3j | 4.0 | 95; 93 | 92:8 |
| 11 | 2-furyl; 3k | 6.0 | >98; 93 | 98:2 |
| 12 | 3-pyridyl; 3l | 4.0 | 90; 75 | 98:2 |
Table 3.
Catalytic enantioselective allyl additions to alkenyl-, alkynyl- and alkyl-substituted imines
 | ||||
|---|---|---|---|---|
| Entry no. | G | Mol % 2g; Mol % NaOt-Bu |
Conv. (%);§ Yield (%)§§ |
e.r.† |
| 1 | 3.0; 2.5 | >98; 84 | >99:1 | |
| 2 |  |
3.0; 2.5 | >98; 95 | >99:1 |
| 3 | 3.0; 2.5 | >98; 98 | >99:1 | |
| 4 | 3.0; 2.5 | >98; 96 | 98:2 | |
| 5 | 2.5; 2.5 | >98; 96 | 98:2 | |
| 6 | 3.0; 2.5 | >98; 95 | 88:12 | |
| 7 | 6.0; 5.0 | 66; 50 | >99:1 | |
| 8 | 6.0; 5.0 | 70; 51 | >99:1 | |
| 9 | 6.0; 8.5 | 90; 71 | 97.5:2.5 | |
Stereochemical models, supported by computational studies (Supplementary Information), are presented in Fig. 3. Association of the N-phosphinoylimine with the boron center and allyl addition takes place as depicted (I→II); the allyl and the i-Pr groups of the catalyst in III bring about steric repulsion. The proposed scenario assigns an additional role to H-bonding: A three-pronged association involving the catalyst’s amine and amide carbonyl and the phosphinoyl unit is established; reactions with N-aryl imines, which lack an approprite H-bond acceptor, engender minimal enantioselectivity. Other observations support the hypothesis regarding the internal H-bond (Fig. 1d). Kinetic studies point to the C–C bond forming step as rate determining, with imines bearing electron-donating groups reacting at a slower pace (Supplementary Information). There is <2% conversion without MeOH. Furthermore, treatment of a solution of 2g with one equivalent of NaOt-Bu results in rapid and complete phenol deprotonation. The addition of five equivalents of MeOH does not lead to major changes; when two equivalents of allylboron 1a are introduced, allowing for Lewis acid activation of the alcohol additive (Fig. 1d), the phenol is regenerated immediately (>98%). The above observations support the notion that, overall, the mixture is a buffered acidic solution.
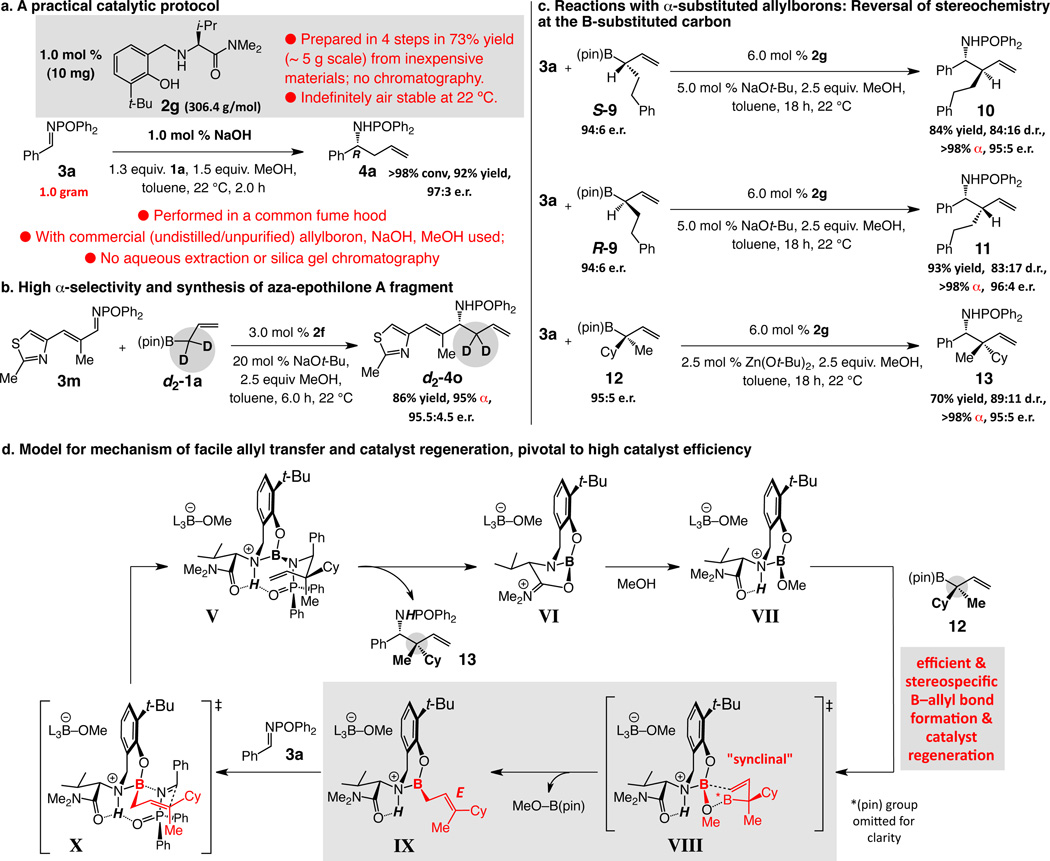
Several key features of the catalytic system are outlined in Fig. 4. The chiral amino alcohol has the low molecular weight of 306.4 g/mole; it is prepared on multi-gram scale by an uncomplicated four-step sequence involving valine, inexpensively available as either enantiomer, and other cheap materials. Purification of 2g, indefinitely stable to air and moisture, entails routine filtration without the need for costly chromatography procedures. Enantioselective additions are scaleable, as the case in Fig. 4a illustrates; reaction work-up is no more than solvent evaporation. Analytically pure homoallylamide is obtained by trituration; distillation or silica gel chromatography is, again, not needed. Such a simple and cost effective product isolation procedure (no need for expensive chromatography solvents) is largely due to the diphenylphosphinoyl unit, more than compensating for its perceived lack of atom economy.
Figure 4. Practical, scaleable and highly α-selective catalytic enantioselective allyl additions to imines.
a, Amino alcohol 2g is prepared in multi-gram quantities inexpensively by simple procedures; additions are easily performed on gram scale. b, Deuterium-labeling experiments support the preference for high α selectivity. c, Various attributes of the chiral catalyst allow access to homoallylamides with an additional tertiary or quaternary carbon stereogenic center with high α- diastereo- and enantioselectivity. d, The stereochemical outcome with substituted allylboron reagents support the proposed mechanism and shed light on the efficient and stereoselective allyl transfer phase of the catalytic cycle (catalyst regeneration/product release). H-bonding in VII stimulates enhanced Lewis acidity at the chiral catalyst’s boron center, favoring donation by the π bond of the organoboron reagent 12 (cf. VIII), facilitating stereoselective generation of IX.
Conversions and diastereomeric ratios were measured by analysis of 400 MHz 1H NMR spectra of unpurified mixtures; the variance of values estimated to be <±2%. Yields correspond to isolated and purified products (±2%). Enantiomeric ratios were determined by HPLC analysis (±2%). See the Supplementary Information for experimental details and spectroscopic analyses.
Congruent with the pathway in Fig. 1d, and confirmed by the reaction with d2-1a (Fig. 4b), the overall transformation takes place with net α selectivity (d2-4o; 95% α)xxvii. Homoallylic amide 4o can be used in enantioselective synthesis of anti-cancer agents aza-epothilones (Fig. 1a).v The ability to convert the C–B of an allylboron entity to a C–C bond, while generating a N-substituted stereogenic center, has critical implications vis-à-vis its utility in stereoselective synthesis. With allylboron S-9 or its enantiomer R-9, accessed in 94:6 e.r. by a Cu-catalyzed protocolxxviii, homoallylamides 10 and 11 are obtained in 84% and 93% yield, 84:16 and 83:17 diastereomeric ratio (d.r.), respectively, and 95:5 e.r. (for the major diastereomer); reaction with allylboron 12xxviii, bearing a quaternary carbon stereogenic center (95:5 e.r.), delivers 13 in 70% yield (pure diastereomer), 89:11 d.r. and 95:5 e.r. (major isomer). Alternative diastereomeric products can be synthesized through the use of the other enantiomer of a chiral allylboron (10 vs. 11, Fig. 4c). There is complete α selectivity in all instances. The route charted in Fig. 1d implies that a net γ-selective addition should result from the initial catalytic cycle (i.e., boron-based catalyst B first generated by ligand exchange); that none of the homoallylamine from overall γ-addition is detected suggests that the catalyst is derived from a minute fraction of the amino alcohol, or B is initially formed by a pathway to be elucidated. Reaction with sterically demanding 12, for reasons that remain to be determined, proceeds more readily when performed with Zn(Ot-Bu)2.
The reversal in the stereochemical identities in the reactions shown in Fig. 4c, ascertained through X-ray crystallography, supports the suggested general mechanism and the pivotal allyl exchange step leading to rapid catalyst regeneration. As initially put forth in Fig. 1d, stereoselective formation of 13 begins with product release by intramolecular proton transfer (Fig. 4d), leading to the formation of VI, wherein the boron center is stabilized by chelation with the Lewis basic amide group. Subsequent reaction with MeOH yields VII. Stereoselective generation of chiral allylboron species IX can proceed via VIII, involving a synclinal (cyclic) transition stateiv; otherwise, the corresponding Z isomer of IX or a mixture of the two, would be formed and the reverse diastereoselectivity or little stereochemical preference would be observed. Selective formation of 13 would take place through transition complex X.
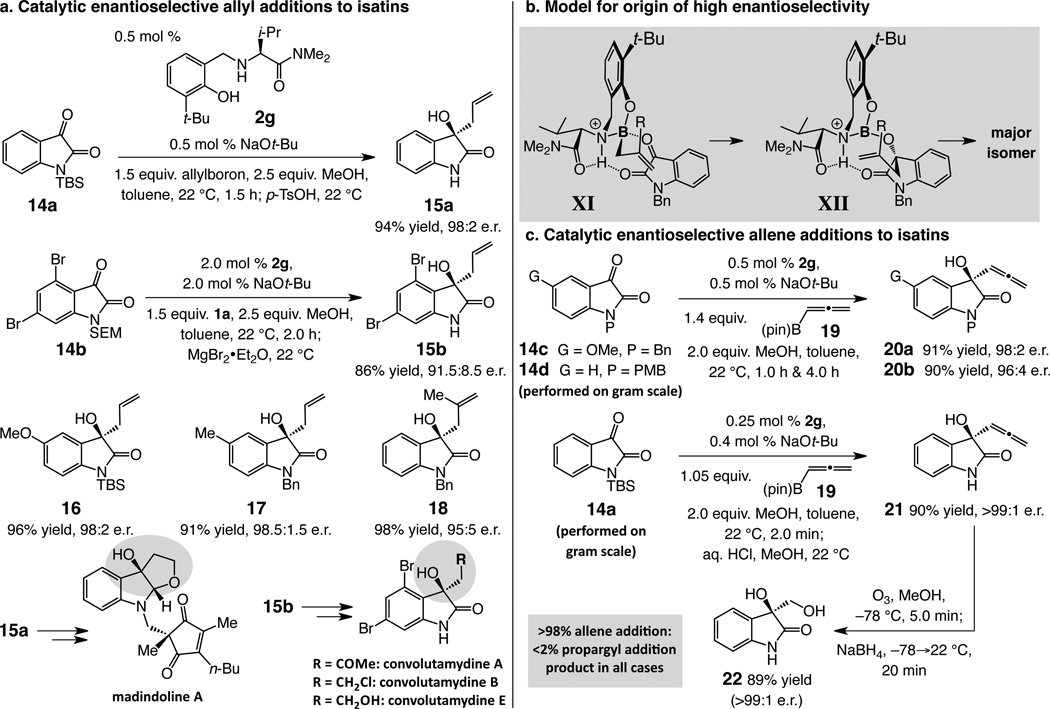
The catalytic strategy can be applied to carbonyl-containing substrates, entities that do not readily lend themselves to chiral auxiliary approaches. The catalyst derived from 2g promotes efficient enantioselective reactions with isatins, potential precursors to tertiary alcohols used in drug development.xix With 0.5–2.0 mol % 2g and 1.5 equivalents of the allylboron reagent, addition to N-protected isatins is complete at 22 °C within two hours (Fig. 5a); homoallylic alcohols are obtained in 84–98% yield and 91.5:8.5–98.5:1.5 e.r. As the syntheses of 15a–b exemplify, enantioselective allyl addition/amide deprotection can be carried out in a single vessel easily and with exceptional efficiency. Homoallyl carbinol 15a is applicable to the synthesis of madindoline Axxix and 15b is a potential intermediate en route to different convolutamydines (Fig. 3a)xxx. A stereochemical model similar to that offered for additions to imines applies (XI and XII, Fig. 5b). Allyl addition to acetophenone under the same conditions proceeds with high efficiency (3.0 mol % 2g, >98% conv. in 4.0 h) but in 70:30 e.r., consistent with the proposed mechanistic model.
Figure 5. Catalytic enantioselective additions to isatins and reactions with an allenylboron reagent.
a, Enantioselective allyl additions to isatins afford homoallylic alcohols. b, A stereochemical model proposed to account for the enantioselectivities. c, Broad applicability is illustrated by enantioselective allene additions to isatins, performed with commercially available organoboron reagent 19.
All reactions were carried out in toluene under an atmosphere of nitrogen gas. Conversions measured by analysis of 400 MHz 1H NMR spectra of unpurified mixtures; the variance of values estimated to be <±2%. Yields correspond to isolated and purified products (±2%). Enantiomeric ratios were determined by HPLC analysis (±2%). See the Supplementary Information for details. TBS = t-butyldimethylsilyl; Bn = benzyl; PMB = p-methoxybenzyl; SEM = 2-(trimethylsilyl)ethoxymethyl.
Another readily accessible organoboron reagent may be utilized in the present set of catalytic transformations: in the presence of 0.5 mol % 2g, reaction of benzyl amide 14c or p-methoxybenzyl amide 14d with commercially available (pinacolato)allenylboron 19 is complete within four hours at ambient temperature, affording allenyl carbinols 20a and 20b in 98:2 and 96:4 e.r. and 91% and 90% yield, respectively (Fig. 5c). Similar to the reaction with 14d, addition to silylamide 14a can be performed on gram scale in a standard fume hood with 0.25 mol % 2g and 1.05 equivalent of 19; C–C bond formation is complete within two minutes and the silyl group is removed through mild acidic workup to afford 21, which can be isolated in high purity without chromatography, in 90% overall yield and >99:1 e.r. The enantioselective synthesis of α-hydroxy alcohol 22 further demonstrates utility; the enantiomerically pure diol, not easily accessed by an alternative protocol, can serve as precursor to various derivatives. All allene additions proceed with complete α selectivity (<2% of propargyl products detected).
The ease of accessing the present class of amino alcohol-derived catalysts, the importance of amines and alcohols to the preparation of biologically active molecules, as well as the simplicity, economy and selectivity with which the catalytic transformations proceed, foreshadow a lasting impact on future efforts in catalyst development and chemical synthesis. Development of other efficient and enantioselective C–C bond forming reactions promoted by the present catalyst class is in progress.
METHODS SUMMARY
Preparation of catalyst solution
Aminophenol 2g (15.0 mg, 0.049 mmol) is weighed out into a 4 ml vial to which is added 263 µl of a solution of sodium hydroxide (1.95 mg, 0.049 mmol) in reagent grade methanol [a 111 mg NaOH pellet (Fisher) is dissolved in 15 ml solvent]. After removal of solvent, 0.5 ml of technical grade anhydrous toluene is added and concentrated in vacuo to remove residual methanol and water. The resulting white solid is dried at 0.5 Torr for 30 min and the vial sealed with a cap containing a teflon septum. Toluene (1.0 ml) is added to yield a suspension.
Gram-scale procedure for allyl addition
A round bottom flask (50 ml, not flame dried, equipped with a magnetic stirring bar) is charged with imine 3a (1.0 g, 3.3 mmol) and subjected to 0.5 Torr for 30 min, purged with dry nitrogen and sealed with a rubber septum. Toluene (30 ml) is added followed by allylboronic acid pinacol ester 1a (800 µl, 4.26 mmol, 1.3 equiv.) from a septum-sealed bottle (Frontier Scientific, used as received) and methanol (200 µl, 4.92 mmol) from a septum-sealed bottle (Acros, 99.9% ExtraDry, used as received). A suspension of the catalyst containing aminophenol 2g (10.1 mg, 0.033 mmol) and sodium hydroxide (1.31 mg, 0.033 mmol, 0.01 equiv.) in 0.67 ml toluene is added through a syringe to the mixture. After two hours, the solvent is evaporated and the residue is taken up in 30 ml technical grade hexanes. The suspension is subjected to sonication for two minutes, filtered and washed four times with 3 ml hexanes. The product is dried at 0.5 Torr and obtained in 92% yield (1.04 g, 3.01 mmol, e.r. = 97.5:2.5). Elemental analysis for C22H22NOP: Calcd: C, 76.06; H, 6.38; N, 4.03. Found: C, 75.77; H, 6.43; N 3.98.
Supplementary Material
Acknowledgements
This research was supported by the United States National Institutes of Health, Institute of General Medical Sciences (Grant GM-57212). S. T. was a Swiss National Science Foundation Postdoctoral Fellow; E. M. V. was an AstraZeneca Graduate Fellow. We thank B. Li for assistance in securing X-ray structures, S. J. Meek, S. J. Malcolmson and K. L. Tan for helpful discussions, Boston College for providing access to computational facilities and Frontier Scientific, Inc. for gifts of various organoboron reagents.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions D. L. S. and T. P. were involved in the discovery, design and development of the catalysts; D. L. S., S. T. and T. P. worked on applications to enantioselective additions to imines; D. L. S. and E. M. V. developed the enantioselective allyl and allene additions to isatins, respectively; D. L. S., S. T., T. P. and F. H. carried out mechanistic and computational studies. This work is part of a collaborative program between M. L. S. and A. H. H. involving the development of amino acid-derived chiral catalysts. A. H. H. conceived, designed and directed the investigations and wrote the manuscript with revisions provided by D. L. S. and E. M. V.
The authors declare no competing financial interests.
References
- i.Lovering F, Bikker J, Humblet C. Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009;52:6752–6756. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- ii.Ojima I, editor. Catalytic Asymmetric Synthesis. Hoboke, New Jersey: Wiley; 2010. [Google Scholar]
- iii.Nakamura E, Sato K. Managing the scarcity of chemical elements. Nature Mat. 2011;10:158–161. doi: 10.1038/nmat2969. [DOI] [PubMed] [Google Scholar]
- iv.Yus M, González-Gómez JC, Foubelo F. Catalytic enantioselective allylation of carbonyl compounds and imines. Chem. Rev. 2011;111:7774–7854. doi: 10.1021/cr1004474. [DOI] [PubMed] [Google Scholar]
- v.Borzilleri RM, et al. A novel application of a Pd(0)-catalyzed nucleophilic substitution reaction to the regio- and stereoselective synthesis of lactam analogues of the epothilone natural products. J. Am. Chem. Soc. 2000;122:8890–8897. [Google Scholar]
- vi.Sirasani G, Andrade RB. Total synthesis of (−)-leuconicine A and B. Org. Lett. 2011;13:4736–4737. doi: 10.1021/ol202056w. [DOI] [PubMed] [Google Scholar]
- vii.Xie W, Zhou B, Pei D, Ma D. Total synthesis of cyclic tetrapeptide FR235222, a potent immunosuppressant that inhibits mammalian histone deacetylases. Org. Lett. 2005;7:2775–2777. doi: 10.1021/ol050991r. [DOI] [PubMed] [Google Scholar]
- viii.Kim SJ, Jang DO. Indium-mediated catalytic enantioselective allylation of N-benzoylhydrazones using a protonated chiral amine. J. Am. Chem. Soc. 2010;132:12168–12169. doi: 10.1021/ja1035336. [DOI] [PubMed] [Google Scholar]
- ix.Tan KL, Jacobsen EN. Indium-mediated asymmetric allylation of acylhydrazones using a chiral urea catalyst. Angew. Chem. Int. Edn. 2007;46:1315–1317. doi: 10.1002/anie.200603354. [DOI] [PubMed] [Google Scholar]
- x.Kargbo R, Takahashi Y, Bhor S, Cook GR, Lloyd-Jones GC, Shepperson IR. Readily accessible, modular, and tunable BINOL 3,3’-perfluoroalkylsulfones: Highly efficient catalysts for enantioselective In-mediated imine allylation. J. Am. Chem. Soc. 2007;129:3846–3847. doi: 10.1021/ja070742t. [DOI] [PubMed] [Google Scholar]
- xi.Aydin J, Kumar K-s, Sayah MJ, Wallner OA, Szabó KJ. Synthesis and catalytic application of chiral 1,1’-bi-2-naphthol and biphenanthrol-based pincer complexes: Selective allylation of sulfonimines with allyl stannane and allyl trifluoroborate. J. Org. Chem. 2007;72:4689–4697. doi: 10.1021/jo070288b. [DOI] [PubMed] [Google Scholar]
- xii.Wada R, Shibuguchi T, Makino S, Oisaki K, Kanai M, Shibasaki M. Catalytic enantioselective allylation of ketoimines. J. Am. Chem. Soc. 2006;128:7687–7691. doi: 10.1021/ja061510h. [DOI] [PubMed] [Google Scholar]
- xiii.Lou S, Moquist PN, Schaus SE. Asymmetric allylboration of acyl imines catalyzed by chiral diols. J. Am. Chem. Soc. 2007;129:15398–15404. doi: 10.1021/ja075204v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xiv.Chakrabarti A, Konishi H, Yamaguchi M, Schneider U, Kobayashi S. Indium(I)-catalyzed asymmetric allylation, crotylation, and α-chloroallylation of hydrazones with rare constitutional and high configurational selectivities. Angew. Chem. Int. Edn. 2010;49:1838–1841. doi: 10.1002/anie.200906308. [DOI] [PubMed] [Google Scholar]
- xv.Naodovic M, Wadamoto M, Yamamoto H. Enantioselective Ag-catalyzed allylation of aldimines. Eur. J. Org. Chem. 2009;2009:5129–5131. doi: 10.1002/ejoc.200900669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xvi.Ferraris D, et al. Catalytic, enantioselective alkylation of α-imino esters: The synthesis of nonnatural α-amino acid derivatives. J. Am. Chem. Soc. 2002;124:67–77. doi: 10.1021/ja016838j. [DOI] [PubMed] [Google Scholar]
- xvii.Vieira EM, Snapper ML, Hoveyda AH. Enantioselective synthesis of homoallylic amines through reactions of (pinacolato)allylborons with aryl-, heteroaryl-, alkyl-, or alkene-substituted aldimines catalyzed by chiral C1-symmetric NHC–Cu complexes. J. Am Chem. Soc. 2011;133:3332–3335. doi: 10.1021/ja200311n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xviii.Hamada T, Manabe K, Kobayashi S. Catalytic asymmetric allylation of hydrazono esters in aqueous media by using ZnF2–chiral diamine. Angew. Chem. Int. Edn. 2003;42:3927–3930. doi: 10.1002/anie.200351778. [DOI] [PubMed] [Google Scholar]
- xix.Peddibhotla S. 3-Substituted-3-hydroxy-2-oxindole, an emerging new scaffold for drug discovery with potential anti-cancer and other biological activities. Curr. Bioact. Compd. 2009;5:20–38. [Google Scholar]
- xx.Coste A, Couty F, Evano G. TMC-95A–D and analogues: Chemistry and biology. Comp. Rend. Chemie. 2008;11:1544–1573. [Google Scholar]
- xxi.Yamamoto D, Sunazuka T, Hirose T, Kojima N, Kaji E, Omura S. Design, synthesis, and biological activities of madindoline analogues. Bioorg. Med. Chem. Lett. 2006;16:2807–2811. doi: 10.1016/j.bmcl.2006.01.107. [DOI] [PubMed] [Google Scholar]
- xxii.Hanhan NV, Sahin AH, Chang TW, Fettinger JC, Franz AK. Catalytic asymmetric synthesis of 3-hydroxy-2-oxindoles. Angew. Chem. Int. Edn. 2010;49:744–747. doi: 10.1002/anie.200904393. [DOI] [PubMed] [Google Scholar]
- xxiii.Itoh J, Han SB, Krische MJ. Enantioselective allylation, crotylation, and reverse prenylation of substituted isatins: Iridium-catalyzed C–C bond-forming transfer hydrogenation. Angew. Chem. Int. Edn. 2009;48:6313–6316. doi: 10.1002/anie.200902328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxiv.Vieira EM, Haeffner F, Snapper ML, Hoveyda AH. A robust, efficient and highly enantioselective method for synthesis of homopropargyl amines. Angew. Chem. Int. Edn. 2012;51:6618–6621. doi: 10.1002/anie.201202694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxv.Barnett DS, Moquist PN, Schaus SE. The mechanism and an improved asymmetric allylboration of ketones catalyzed by chiral biphenols. Angew. Chem. Int. Edn. 2009;48:8679–8682. doi: 10.1002/anie.200904715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxvi.Weinreb SM, Orr RK. N-Phosphinoylimines: An emerging class of reactive intermediates for stereoselective organic synthesis. Synthesis. 2005;8:1205–1227. [Google Scholar]
- xxvii.Fujita M, Nagano T, Schneider U, Hamada T, Kobayashi S. Zn-catalyzed asymmetric allylation for the synthesis of optically active allylglycine derivatives. Regio- and stereoselective formal α-addition of allylboronates to hydrazono esters. J. Am. Chem. Soc. 2008;130:2914–2915. doi: 10.1021/ja710627x. [DOI] [PubMed] [Google Scholar]
- xxviii.Guzman-Martinez A, Hoveyda AH. Enantioselective synthesis of allylboronates bearing a tertiary or quaternary B-substituted stereogenic carbon by NHC–Cu-catalyzed substitution reactions. J. Am Chem. Soc. 2010;132:10634–10637. doi: 10.1021/ja104254d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxix.Itoh T, Ishikawa H, Hayashi Y. Asymmetric aldol reaction of acetaldehyde and isatin derivatives for the total syntheses of ent-convolutamydine E and CPC-1 and a half fragment of madindoline A and B. Org. Lett. 2009;11:3854–3857. doi: 10.1021/ol901432a. [DOI] [PubMed] [Google Scholar]
- xxx.Cravotto G, et al. Convolutamydine A: the first authenticated absolute configuration and enantioselective synthesis. Tetrahedron Asymm. 2006;17:3070–3074. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.