Abstract
We investigated the existence of a temporal association between age at initiation of cannabis use and age at onset of psychotic illness in 997 participants from the 2010 Survey of High Impact Psychosis (SHIP) in Australia. We tested for group differences in age at onset of psychotic illness and in the duration of premorbid exposure to cannabis (DPEC). Analyses were repeated in subgroups of participants with a schizophrenia-spectrum disorder (SSD), a diagnosis of lifetime cannabis dependence (LCD), and a comorbid SSD/LCD diagnosis. The association between age at initiation of cannabis use and age at onset of psychotic illness was linear and significant, F(11, 984) = 13.77, P < .001, even after adjusting for confounders. The effect of age at initiation of cannabis use on DPEC was not significant (mean duration of 7.8 years), and this effect was similar in participants with a SSD, LCD, and comorbid SSD/ LCD diagnosis although a shift toward shorter premorbid exposure to cannabis was noted in the SSD/LCD subgroup (mean duration of 7.19 years for SSD/LCD). A temporal direct relationship between age at initiation of cannabis use and age at onset of psychotic illness was detected with a premorbid exposure to cannabis trend of 7–8 years, modifiable by higher severity of premorbid cannabis use and a diagnosis of SSD. Cannabis may exert a cumulative toxic effect on individuals on the pathway to developing psychosis, the manifestation of which is delayed for approximately 7–8 years, regardless of age at which cannabis use was initiated.
Key words: cannabis, psychosis, schizophrenia, age at onset, causality, DIP, SHIP
Introduction
There is ongoing debate regarding the association between cannabis use and age at onset of psychosis (AOP). A recent meta-analysis by Large et al. (2011)1 reported an earlier mean AOP in samples with cannabis use and made a strong argument for causality, although increased use of cannabis by those approaching the onset of psychosis, ie, “self-medication” was considered a reasonable interpretation of the association. If cannabis use brings forward the AOP, then one may anticipate that a temporal relationship between age at initiation of cannabis use (AIC) and AOP might be observed after adjustment for confounder effects. However, few studies have specifically addressed this question within sufficiently large samples of participants with psychosis.
Several small studies have demonstrated that AIC is significantly associated with AOP.2–5 In 123 consecutive referrals with first-episode psychosis to an early intervention service, Barnett et al. (2007)2 reported that AIC, cocaine, ecstasy, and amphetamine use was significantly associated with age at first-psychotic symptoms. In a sample of 99 participants with a first-psychotic episode, Leeson et al. (2012)3 reported a linear relationship between AIC and age at onset of psychotic symptoms. The average duration of premorbid exposure to cannabis (DPEC) was reported as 6 years and 33 days. In a sample of 57 participants with a nonaffective psychotic disorder who gave a history of heavy cannabis use, Galvez-Buccollini et al. (2012)4 also reported a significant association between AIC and AOP after adjusting for cofounding variables; the DPEC was reported as 7±4.3 years. In a sample of 80 young participants with early onset schizophrenia-spectrum disorders (mean AOP was 16.6 years), Estrada et al. (2011)5 reported that AIC correlated significantly with AOP. Taken together, these small studies suggest a temporal relationship between AIC and AOP in nonaffective psychotic disorders.
Our goal was to further investigate the association between AIC, AOP, and DPEC in a large Australian sample of participants with psychotic disorders.
Methods
Participants
Information on participants was obtained from the 2010 Survey of High Impact Psychosis (SHIP), which recruited people with psychosis from across 7 sites in 5 states of Australia in 2010. The survey used a 2-phase representative sampling design covering an estimated resident population aged 18−64 years of 1.5 million people, or approximately 10% of the Australian population in that age range.6,7 Of the 7955 people in contact with mental health services who screened positive for psychosis, 1825 were randomly selected and engaged in the interview and assessment process, and all participants provided written informed consent. For full details of the methodology, see Morgan et al. (2012).6
Measures of Diagnoses and Substance Use
Participants were assessed using the Diagnostic Interview for Psychosis (DIP), a standardized semistructured interview for psychosis.8 National training workshops were conducted for all interviewers, in addition to onsite training, with weekly intersite teleconferences throughout the survey. Interrater reliability was assessed in the course of the field interviews and the level of agreement achieved among interviewers was good (averaged pairwise agreement of 0.94 for ICD-10 diagnoses). Diagnostic classification of cases was made using the OPCRIT diagnostic computer algorithm9 to score the DIP responses; this aticle uses diagnoses based on ICD-10 criteria. The DIP also enables the interviewer to assess family history for schizophrenia and other psychiatric disorders. In addition, the DIP includes items on current and past substance use for alcohol, tobacco, cannabis, amphetamines, lysergic acid diethylamide (LSD), cocaine, ecstasy, and heroin, yielding age of first use, frequency of use in the 12 months prior to psychiatric symptoms first appearing, frequency of substance use in the 12 months prior to interview, and a lifetime diagnosis of substance dependence. For the purposes of this study, 997 participants who reported use of cannabis prior to onset of psychosis (daily/almost daily use, use 1–2 days/week, use 2–4 times/month, or use less frequently than once per month) were considered for further analysis.
Definition of AOP
AOP was determined after interviewing the participant and reviewing the hospital file if consent was given. AOP was recorded to the nearest year and defined as the earliest age at which medical advice was sought for psychiatric reasons, or age at which any psychiatric symptom diagnostic of psychotic or major affective illnesses began to cause subjective distress or impair functioning. If there were no clear symptoms described, then age at first hospital admission was recorded.
Results
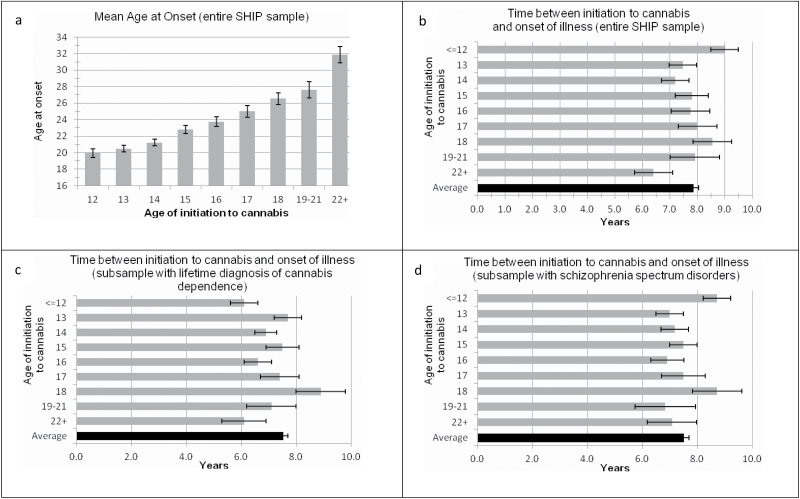
Univariate General Linear Models (GLM) framework was used to test for group differences in AOP among patients clustered in AIC groups and for possible sex differences. AIC were clustered into 9 groups (ranging from ≤12 to 21+). The effect of AIC on AOP was significant, F(8,978) = 25.37, P < .001, adjusted R 2 = 0.19 (see figure 1a), whereas the effect of sex was not, F(1,977) = 2.02, P = .16. The effect of AIC on AOP remained significant after family history of schizophrenia or other psychiatric disorders was used as covariate, F(11, 984) = 13.77, P < .001, R 2 = 0.20. Age at interview, SSD, and lifetime cannabis dependence (LCD) were not considered as covariates because they shared large proportion of variance with both dependent and independent variables.10 However, a potential confounding influence of SSD and LCD was indirectly examined by analyzing separately these subsets of the entire SHIP sample. Using the Curve Estimation procedure (to examine which regression model best fit to the data), we tested 3 models (linear, cubic, and quadratic) and found that a linear model of association provided a better fit to the data, F(1,994) = 224.32, P < .001.
Fig. 1.
Figure showing mean (with standard errors) age at onset of psychosis according to age at initiation of cannabis use and time gap between initiation to cannabis and onset of psychosis.
Based on the observed linear association between AIC and AOP, univariate GLM was also used to test for group differences in DPEC. Furthermore, we examined potential trends in our data. For the entire sample the effect of AIC on DPEC was not significant, F(8,988) = 1.28, P = .25, adjusted R 2 = 0.002, for trend F(8,988) = 0.944, P = .33. Mean DPEC for the entire SHIP sample was 7.85 (SD = 6.2) years (figure 1b).
In order to further ascertain whether greater presumed severity of premorbid use of cannabis, diagnosis of schizophrenia-spectrum disorder (SSD), or a combination may alter mean DPEC and DPEC among AIC groups, we repeated these analyses for 3 overlapping subsets. In patients who had a LCD, the effect of AIC on DPEC was just significant, F(8,781) = 1.99, P = .044, adjusted R 2 = 0.01, but there was no significant trend observed, F(8,781) = 2.60, P = .11; the mean DPEC was 7.51 (SD = 5.6) years (figure 1c). In patients who had an ICD-10 SSD (schizophrenia, schizoaffective disorder, and delusional disorder), the effect of AIC on DPEC was not significant, F(8,725) = 1.18, P = .31, adjusted R 2 = 0.002, and no significant trend was observed, F(8,725) = 0.75, P = .39; mean DPEC was 7.50 (SD=5.9) years (figure 1d). Finally, in patients who had SSD and comorbid LCD, the effect of AIC on DPEC was just significant, F(8, 581) = 2.01, P = .043, R 2 < 0.014, but there was no significant trend observed, F(8, 581) = 2.01, P = .15; the mean DPEC was 7.19 (SD = 5.4) years.
Discussion
These findings, derived retrospectively from a large population of representatively ascertained participants with psychosis in Australia,6,7 extend previous reports on the association between cannabis and onset of psychosis by demonstrating that AIC is directly and linearly associated with AOP and that an average delay of 7–8 years (mean 7.85, SD = 6.2) is observed from the first exposure to cannabis to the onset of psychotic disorders.
Our study can be seen as complementary to previous work in the general population that has established a temporal association between early cannabis use and risk for psychotic disorders11,12 and with McGrath et al. (2010)13 who demonstrated in a prospective birth cohort that significant risk for nonaffective psychosis increases after 6 years of exposure to cannabis. We also confirm previously reported linear association between AIC and AOP and offer validation to previous work reporting similar mean DPEC (approximately 6–8 years).2–4 Notwithstanding some of the inherent limitations to our study imposed by acquiring retrospective information from the DIP (ie, possible recall bias, lack of data on the premorbid pattern of use of other illicit substances until the year prior to onset of psychosis) and the limitation of excluding from analysis illicit substances other than cannabis that may further modulate AOP, this is the largest study examining the effects of AIC use in psychotic disorders.
Much research has focused on adolescence as a particularly vulnerable period of brain maturation for those exposed to cannabis. This neurodevelopmental “window of vulnerability” is supported by findings that demonstrate that early cannabis exposure is a risk factor for psychosis-related outcomes in young adults.11,13–16 Despite the fact that we also demonstrate a linear trend between early AIC and AOP, our findings do not support a neurodevelopmental “window of vulnerability” hypothesis because those participants who first used cannabis at 12 years of age, for instance, had on average a similar temporal trajectory to illness to those who were first exposed to cannabis at 19 years of age. As noted by Moore et al. (2007),17 arguments for why earlier use of cannabis might have more harmful effects are intuitively compelling, but no robust evidence supports this view. The increased risk of psychosis in people using cannabis from a younger age observed in the Dunedin cohort11 could indicate, for instance, a greater cumulative exposure to cannabis rather than a sensitive period of exposure.
We can only speculate on the reasons behind the apparent 7–8-year consistent trend of cannabis exposure in people who develop psychosis. It could be argued that both AIC and AOP tend to cluster independently of each other in particular periods in the life-span (AIC in adolescence, AOP in early adulthood); therefore, collinearity resulting in a relatively constant DPEC might be expected even if no causal link between cannabis and psychosis existed. However, this is unlikely to explain why mean DPEC is relatively constant across a wide range of AIC groups extending from 12 to over 21 years of age. Furthermore, if the observed temporal association between AIC and AOP was entirely unrelated to causal effects of cannabis exposure on psychosis onset, we would not observe a shift toward a shorter mean DPEC (7.19 years) in participants with SSD and comorbid LCD.
Our findings would be consistent with the notion that cannabis exposure exerts a cumulative toxic effect, particularly in people on the pathway to developing SSD, the manifestation of which is delayed for approximately 7–8 years, regardless of the AIC. While the mechanisms by which cannabis may exert such delayed effects are unclear, several authors have suggested a mechanism involving sensitization of the mesolimbic dopaminergic system, triggered by repeated stimulation with cannabis, to which susceptible individuals may be especially vulnerable,14,18,19 possibly due to a heightened, genetically determined sensitivity to the psychotomimetic effects of cannabis.20
Funding
Australian Government Department of Health and Ageing.
Acknowledgments
This publication is based on data collected in the framework of the 2010 Australian National Survey of High Impact Psychosis. The members of the Survey of High Impact Psychosis Study Group are: V. Morgan (National Project Director), A. Jablensky (Chief Scientific Advisor), A. Waterreus (National Project Coordinator), R. Bush, V. Carr, D. Castle, M. Cohen, C. Galletly, C. Harvey, B. Hocking, A. Mackinnon, P. McGorry, J. McGrath, A. Neil, S. Saw, H. Stain. Ethics approvals for the study were obtained from relevant institutional human research ethics committees. The study was funded by the Australian Government Department of Health and Ageing. This report acknowledges, with thanks, the hundreds of mental health professionals who participated in the preparation and conduct of the survey and the many Australians with psychotic disorders who gave their time and whose responses form the basis of this publication. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry. 2011; 68: 555–561 [DOI] [PubMed] [Google Scholar]
- 2. Barnett JH, Werners U, Secher SM, et al. Substance use in a population-based clinic sample of people with first-episode psychosis. Br J Psychiatry. 2007; 190: 515–520 [DOI] [PubMed] [Google Scholar]
- 3. Leeson VC, Harrison I, Ron MA, Barnes TR, Joyce EM. The effect of cannabis use and cognitive reserve on age at onset and psychosis outcomes in first-episode schizophrenia. Schizophr Bull. 2012; 38: 873–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Galvez-Buccollini JA, Proal AC, Tomaselli V, et al. Association between age at onset of psychosis and age at onset of cannabis use in non-affective psychosis. Schizophr Res. 2012; 139: 157–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Estrada G, Fatjó-Vilas M, Muñoz MJ, et al. Cannabis use and age at onset of psychosis: further evidence of interaction with COMT Val158Met polymorphism. Acta Psychiatr Scand. 2011; 123: 485–492 [DOI] [PubMed] [Google Scholar]
- 6. Morgan VA, Waterreus A, Jablensky A, et al. People living with psychotic illness in 2010: the second Australian national survey of psychosis. Aust N Z J Psychiatry. 2012; 46: 735–752 [DOI] [PubMed] [Google Scholar]
- 7. Moore E, Mancuso SG, Slade T, Galletly C, Castle DJ. The impact of alcohol and illicit drugs on people with psychosis: the second Australian national survey of psychosis. Aust N Z J Psychiatry. 2012; 46: 864–878 [DOI] [PubMed] [Google Scholar]
- 8. Castle DJ, Jablensky A, McGrath JJ, et al. The diagnostic interview for psychoses (DIP): development, reliability and applications. Psychol Med. 2006; 36: 69–80 [DOI] [PubMed] [Google Scholar]
- 9. McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry. 1991; 48: 764–770 [DOI] [PubMed] [Google Scholar]
- 10. Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001; 110: 40–48 [DOI] [PubMed] [Google Scholar]
- 11. Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002; 325: 1212–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ. 2002; 325: 1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McGrath J, Welham J, Scott J, et al. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry. 2010; 67: 440–447 [DOI] [PubMed] [Google Scholar]
- 14. Stefanis NC, Delespaul P, Henquet C, Bakoula C, Stefanis CN, Van Os J. Early adolescent cannabis exposure and positive and negative dimensions of psychosis. Addiction. 2004; 99: 1333–1341 [DOI] [PubMed] [Google Scholar]
- 15. Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003; 33: 15–21 [DOI] [PubMed] [Google Scholar]
- 16. Schubart CD, van Gastel WA, Breetvelt EJ, et al. Cannabis use at a young age is associated with psychotic experiences Psychol Med. 2010; 7: 1–10 [DOI] [PubMed] [Google Scholar]
- 17. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007; 370: 319–328 [DOI] [PubMed] [Google Scholar]
- 18. Kuepper R, van Os J, Lieb R, Wittchen HU, Höfler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ. 2011; 342: d738 doi:10.1136/bmj.d738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010; 468: 203–212 [DOI] [PubMed] [Google Scholar]
- 20. Investigators Group Evidence that familial liability for psychosis is expressed as differential sensitivity to cannabis: an analysis of patient-sibling and sibling-control pairs. Arch Gen Psychiatr. 2011; 68: 138–147 [DOI] [PubMed] [Google Scholar]