Abstract
Central dopaminergic hyperactivity has been one of the main hypotheses of the pathophysiology of schizophrenia since the 1970s. Excess dopamine (DA) neurotransmission in the striatum is hypothesized to alter the processing of information and result in psychotic symptoms in schizophrenia. Single photon emission computerized tomography (SPECT) provides in vivo indices of DA neurotransmission. Our study aimed to compare dopamine transporter (DAT) availability between drug-naive patients with schizophrenia and controls using SPECT. DAT availability through [99mTc]-TRODAT-1 SPECT was compared between 47 drug-naive patients with recent-onset schizophrenia and 112 healthy controls. We also conducted a random-effects meta-analysis of the available literature synthesizing the results of 6 comparable published articles as well as our current data. The mean specific striatal binding showed a statistical trend for a reduction among the patients compared with controls (estimated difference = 0.071; 95% CI −0.01, 0.15; P = .08). There was an effect of gender, whereby females had a higher ratio of specific striatal binding than males. Age was negatively correlated with the ratio of specific striatal binding, both in patients and controls. The meta-analysis provided a pooled standardized effect size (Cohen’s d) of −0.07 (95% CI −0.31, 0.18; P = .60) for the patient vs control comparison in TRODAT binding, with no evidence of heterogeneity between studies or publication bias. Our findings suggest that striatal DAT levels are not altered in the early stages of schizophrenia before medication is introduced. We identified gender differences and aging effects that could have significance for future studies.
Keywords: drug-naive schizophrenia, dopamine, dopamine transporter, TRODAT, single photon emission tomography, meta-analysis
Introduction
Central dopaminergic hyperactivity has been one of the main hypotheses of the pathophysiology of schizophrenia since the 1970s.1,2 Although it has undergone a number of revisions, in its current form, excess dopamine (DA) neurotransmission in the striatum is hypothesized to alter the processing of information and result in psychotic symptoms.2,3 Neurochemical imaging techniques such as single photon emission computerized tomography (SPECT) and positron emission tomography (PET) provide in vivo indices of the stages of DA neurotransmission, including its presynaptic synthesis, DA release into the synapse, and the levels of DA receptors and dopamine transporters (DATs).2
Elevated DA synthesis capacity has been consistently reported in 6-fluoro-(18F)-L-3,4-dihydroxyphenylalanine ([18F]-DOPA) and L-[β-11C]-3,4-dihydroxyphenylalanine ([11C]-DOPA) PET studies in schizophrenia, including in the first episode,3,4 and has been shown to predate the onset of schizophrenia in individuals with prodromal psychotic symptoms.5 Synaptic DA can be studied using challenge approaches that stimulate DA release or deplete synaptic DA levels. These approaches are based on the competition between DA and radioligands such as raclopride and [123I] iodobenzamide for binding to DA receptors,6 although recent evidence indicates the process is more complex than suggested by a simple competition model.7 Studies using challenge approaches have found evidence of increased radiotracer displacement in patients with schizophrenia compared with controls, indicating greater DA release,8 and increased synaptic DA levels.9,10 DA neurotransmission in the striatum is largely terminated by the reuptake of DA into the presynaptic DA nerve terminals by DATs. Thus, alterations in DAT availability could also alter DA neurotransmission and contribute to the pathoetiology of schizophrenia. DAT availability has been investigated in schizophrenia with evidence of a decrease in some studies11,12 but no difference or an increase in others.13–16 A recent review found that there was significant between-study heterogeneity, which may be due to the small sample sizes and the inclusion of patients at different stages and on different treatments. Howes OD et al (in preparation) There is therefore a need for a large study of drug-naive first-episode patients to determine whether there are DAT abnormalities associated with the onset of the illness.
Our study focused on the role of DA presynaptic regulation. We compared DAT availability using [99mTc]-TRODAT-1 SPECT between 47 drug-naive patients with recent-onset schizophrenia and 112 healthy controls in the largest study to date. We then conducted an updated meta-analysis of studies that used SPECT imaging, including our new data, to compare DAT availability in antipsychotic-naive patients with schizophrenia with that in controls to determine if there is evidence of altered DAT availability in drug-naive patients with schizophrenia.
Methods
Sample
All study participants were living in Tainan City, the fifth largest in Taiwan with a population of 1,873,579. A total of 47 drug-naive first-episode patients with schizophrenia were recruited at the psychiatric outpatient clinic of the National Cheng Kung University Hospital. One hundred and twelve healthy community residents of Tainan City were also recruited through research advertisements. They were recruited to enable the study of factors underlying normal variation in DAT activity and as controls for a number of planned psychiatric and neurological research studies. These controls were interviewed by senior psychiatrists who had been practicing for more than 10 years, using the Chinese version of the Mini International Neuropsychiatric Interview,17 to ensure that the subjects were free of any Axis I or Axis II psychiatric disorders. These subjects were physically healthy and without any history of alcohol or other substance abuse or dependence. Any subject who had been taking psychotropic medications, including antipsychotics or antidepressants, were excluded. Brain magnetic resonance images (MRIs) and routine blood tests done in the controls were normal. All the participants recruited in our previous study,14 11 patients and 12 controls, were also included in the present local study. The mean duration of illness was 15.8 months (SD = 33.5, median = 5.4, interquartile range = 16.5).
Before any procedure was performed, written informed consent was obtained from each of the participants after a complete explanation of the study. The Ethical Committee for Human Research at the National Cheng Kung University Hospital approved the study protocol. Inclusion criteria for all participants were as follows: (1) patients should fulfill Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for schizophrenia; (2) age between 18 and 60; (3) no physical illness and with stable vital signs and no evidence of substance abuse/dependence as assessed during the clinical interview with the research psychiatrist at the time of enrollment; (4) never received any antipsychotic or antidepressant treatment and were free of any psychotropic medication at the time of testing. These patients had never had such psychotropic medications prescribed, and our clinic was their first contact with psychiatric services. Exclusion criteria for all participants were as follows: (1) other comorbid psychiatric illnesses, substance abuse/dependence, or neurological illnesses; (2) mental retardation; (3) all female participants of childbearing age had to take an acceptable form of contraceptive throughout the duration of the study in order to be included and all female participants underwent an instant urine pregnancy test prior to starting the experiment; (4) all patients who were deemed at risk of acute suicide/self-harm were excluded from the study for their safety.
Assessment Battery
Before receiving any treatment, the patients underwent all the baseline assessments within 7 days of entering the study. The healthy controls received the same assessments.
[99mTc]-TRODAT-1 SPECT and MRI.
Each subject received a bolus intravenous injection of 740 MBq (20 mCi) of [99mTc]-TRODAT-1 (Institute of Nuclear Energy Research, Lungtan, Taiwan) in a quiet environment approximately 10 minutes after the intravenous line was set up. The brain SPECT images were acquired 4 hours after the injection. To avoid tilt and misalignment of the participant’s head in the image scanner, we carefully positioned participants and monitored them during scanning and used a head holder to further reduce movement artifacts. Before image acquisition, the participant was informed of the necessity to avoid head movement. Sinograms were reviewed blind to diagnosis to determine whether postacquisition correction for head movements was needed. Movement correction was conducted by using the motion correction software ICON (Siemens, version 8.5 KB21).
We used a triple-headed rotating gamma camera (Multispect 3; Siemens, Hoffman Estates, IL, USA) with ultra high-resolution fan-beam collimators, which yields an image resolution of approximately 8.5 mm for the full-width half maximum (FWHM). The SPECT images were acquired over a circular 360° rotation, with 120 steps, at a rate of 50 seconds per step, in a 128 × 128 × 16 matrix. The images were then reconstructed using Butterworth and Ramp filters18 (cutoff frequency = 0.3 Nyquist, power factor = 8), with attenuation according to Chang’s method.19 The reconstructed transverse images were realigned parallel to the canthomeatal line. The slice thickness of each transverse image was 2.89 mm. For semiquantitative analyses, 6 consecutive transverse slices on which the highest striatum uptake was best visualized were combined to obtain a 17.34-mm-thick slice. Then regions of interest (ROIs) were placed over the striatum and the occipital cortex. The ROIs were drawn directly on the SPECT images by an experienced nuclear medicine physician who was blind to the participants' clinical data. The participants' MRIs (SIGNA CV-I, 1.5 T; GE, USA), obtained within 2 weeks after SPECT examination, were used as a visual reference to determine the ROIs. The sizes of all ROIs were at least twice that of the FWHM. The specific striatal [99mTc]-TRODAT-1 binding (which represents striatal DAT availability) was calculated as the mean count in the striatal ROI divided by the mean count in the occipital region (St/Oc).20
Psychopathology Ratings.
On the day of recruitment, standardized psychopathology ratings using Clinical Global Impression Severity of Illness (CGI-S),21 Global Assessment of Functioning (GAF; range 0–100 from poorest to optimal functioning),22 and the Structured Clinical Interview for Positive And Negative Symptoms Scale (SCI-PANSS; range 30–210 from least to most symptomatic)23 were collected for all patients.
Statistical Analyses
Local Study.
The main aim of our study was to assess the differences between patients and controls in the specific striatal [99mTc]-TRODAT-1 binding, considering both the left and right striatum measures. Mixed modeling was used to compare [99mTc]-TRODAT-1 binding between the 2 clinical groups, and to allow for possible group differences between left and right sites, we tested if a group by laterality interaction was evident. On the basis of previous literature, we considered that age, sex, and tobacco smoking are potential confounders and therefore included these as covariates in the analysis.24–26 The models included subject-varying intercepts to acknowledge correlation between the 2 repeated measures per participant.
The second aim of this study was to evaluate the effects of aging, sex, and tobacco smoking on the specific striatal [99mTc]-TRODAT-1 binding, and we present the regression coefficients obtained from the model described above. Our third and final objective was to use the data to assess if any group effect varies with age or gender. Thus, we expanded the above model to test an interaction of group by age or group by gender, respectively.
The association between the specific striatal [99mTc]-TRODAT-1 binding and psychopathology ratings in patients was analyzed by using Spearman's rho correlations.
Demographic differences between patients and controls were examined with Chi-square tests for categorical variables or with Student t tests for continuous variables. For the latter, Levene's test was used to assess the assumption of equality of variances. Diagnostic plots as well as one-sample Kolmogorov-Smirnov tests were used to test for normality. Statistical significance was established at P < .05. SPSS version16 (SPSS Inc., Chicago, IL, USA) was used for all analyses.
Meta-Analysis
We conducted a random-effects meta-analysis combining the previous published literature as well as the local study presented here. We searched the Institute for Scientific Information Web of Knowledge, Scopus, and PubMed (U.S. National Library of Medicine, NLM) using the following 2 sets of key words: (1) “drug-naive, schizophrenia, dopamine transporter” and (2) “drug naïve, schizophrenia, TRODAT, dopamine transporter.” The search covered between 1960 and the end of 2010 and yielded a total of 40 articles. Of these, we excluded 3 reviews, 4 conference abstracts, 2 animal studies, 8 basic/genetic studies, 8 articles of other psychiatric illnesses, 2 articles on medicated patients with schizophrenia, and 7 articles on ligands different from TRODAT. Thus, the meta-analysis finally included 6 articles published between 2003 and 2010 that focused on drug-naive schizophrenia patients using [99mTc]-TRODAT-1.
Our local data were included as the seventh study. The standardized mean difference between controls and patients was computed for each primary study as Cohen’s d.27 The meta-analysis, tests for effect moderation, and publication bias were conducted using, respectively, the metan, metareg, and metabias routines available in STATA 10 (Stata Corporation, College Station, TX, USA).
Results
Local Study
The sample included 47 drug-naive patients with a DSM-IV diagnosis of schizophrenia as well as 112 healthy controls.
The demographic characteristics of the patient and control groups are summarized in table 1. Patients and controls had a similar gender and tobacco smoking status distribution. However, compared with the controls, the patients were significantly younger (t = −4.19, df = 117.7; P < .001), less likely to be married (χ2 = 11.47; P = .001), and had fewer years of education (t = −2.62, df = 154; P = .01). The ratio of specific striatal binding in both patients and controls was normally distributed (P > .2 and diagnostic plots). The group by laterality interaction was not significant and was therefore dropped from our model (F = 2.02, df = 1, 157; P = .16). After controlling for age, sex, and tobacco smoking, the mean specific striatal binding showed a trend for a difference between patients and controls, where patients had a reduction in their TRODAT binding (estimated difference between controls and patients = 0.071; 95% CI −0.01, 0.15; F = 3.19, df = 1, 154; P = .08). These results are summarized in table 2.
Table 1.
Demographic Characteristics of the Participants
| Schizophrenia (n = 47) | Normal Control (n = 112) | Statistical Test | |||
| Mean (SD) | Mean (SD) | t/χ2 | df | P | |
| Age (years), range | 27.2 (8.7), 17.0–52.7 | 34.3 (12.0), 18.9–58.8 | −4.19 | 117.7 | <0.001 |
| Sex (male/female) | 26/21 | 59/53 | 0.09 | 1 | 0.761 |
| Smoking status (yes/no) | 5/42 | 19/93 | 1.03 | 1 | 0.300 |
| Marital status (M/S) | 5/42 | 42/70 | 11.47 | 1 | 0.001 |
| Years of education, range | 12.6 (3.2), 5–21 | 14.2 (3.5), 0–22 | −2.62 | 154 | 0.010 |
Note: M includes married and living with the partner; S includes single, divorced, and married but separated.
Table 2.
The DAT Availability (St/Oc) by Group and by Sex
| n | Total | Right | Left | ||||
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | ||
| Group | |||||||
| Schizophrenia | 47 | 2.20 (0.25) | 1.75–2.81 | 2.20 (0.26) | 1.80–2.90 | 2.19 (0.24) | 1.70–2.73 |
| Normal control | 112 | 2.20 (0.24) | 1.57–2.71 | 2.20 (0.24) | 1.57–2.71 | 2.20 (0.24) | 1.58–2.71 |
| Sex | |||||||
| Male | 85 | 2.17 (0.23) | 1.73–2.66 | 2.17 (0.24) | 1.73–2.71 | 2.17 (0.23) | 1.67–2.67 |
| Female | 74 | 2.23 (0.25) | 1.57–2.81 | 2.23 (0.25) | 1.57–2.90 | 2.24 (0.25) | 1.58–2.73 |
Note: DAT, dopamine transporter.
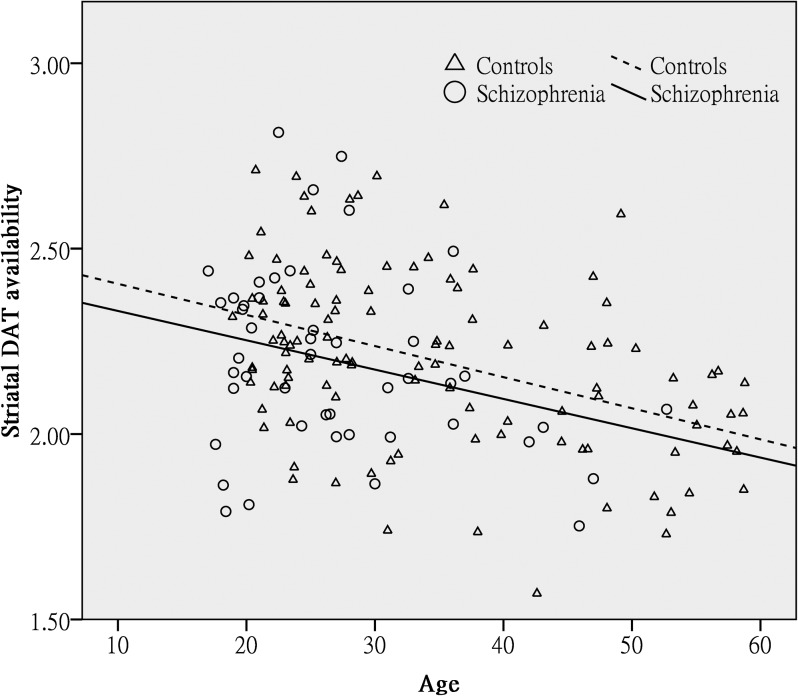
In the same model, there was a significant effect of gender, whereby females had a higher ratio of the specific striatal binding than males (estimated difference between males and females = −0.08; 95% CI −0.15, −0.002; F = 4.11, df = 1, 154; P = .04). Tobacco smoking did not have a significant influence on TRODAT binding (estimated difference between smokers and nonsmokers = −0.06; 95% CI −0.17, 0.04; F = 1.39, df = 1, 154; P = .24). Finally, there was a highly significant effect of age, whereby TRODAT binding declined with advancing age (estimated change per decade of age = −0.1; 95% CI −1.2, −0.6; F = 31.87, df = 1, 154; P < .001). Of note, there was no significant interaction between age and group (F = 0.37, df = 1, 153; P = .55) or between group and sex (F = 0.20, df = 1, 153; P = .66), indicating that the age decline in TRODAT was similar in patients and controls as well as in both genders. These findings are summarized in table 2 and figure 1.
Fig. 1.
The relation between age and striatal DAT availability in patients with schizophrenia and controls. As described in the main analyses, having adjusted by the main effects of group, sex, and tobacco smoking, there was a significant decline of TRODAT binding with advancing age but no significant interaction between the age and group. This graph shows regression lines describing the relationship between TRODAT binding and age within patients and control samples separately. The almost parallel lines illustrate the similar rates of decline in the 2 groups. DAT, dopamine transporter.
Of the 47 patients included, 43 completed the GAF, 42 the PANSS, and 39 the CGI scales. No significant correlations were found between the specific striatal binding and psychopathological rating scores (table 3).
Table 3.
Spearman's Rho Correlations of Striatal DAT Availability and Psychopathology in the Patient Group
| PANSS | CGI-S | GAF | ||||
| Positive | Negative | General Psychopathology | Sum | |||
| n | 42 | 43 | 43 | 42 | 39 | 43 |
| Mean (SD) | 22.0 (6.2) | 20.1 (7.1) | 37.2 (11.4) | 79.3 (21.6) | 4.7 (1.0) | 41.1 (15.0) |
| Range | 7–34 | 8–42 | 16–62 | 32–138 | 2–6 | 5–75 |
| Striatal DAT availability | ||||||
| ρ | 0.25 | 0.05 | 0.17 | 0.15 | 0.17 | 0.18 |
| P | 0.11 | 0.74 | 0.27 | 0.35 | 0.32 | 0.25 |
Note: DAT, dopamine transporter; PANSS, Positive and Negative Syndrome Scale; CGI-S, Clinical Global Impression Severity of Illness; GAF, Global Assessment of Functioning.
Systematic Review.
To date, 6 SPECT studies with [99mTc]-TRODAT-1 have been published (table 4).13–15,28–30 Schmitt et al15 found no significant difference in [99mTc]-TRODAT-1 binding to the striatal DAT between 20 drug-naive patients and 12 normal controls, even though the controls seemed to have a larger mean value of TRODAT binding ratio than did patients in their another study.28 Similarly, Hsaio et al13 reported no significant average differences in TRODAT uptake ratio between 12 drug-naive patients with schizophrenia and 12 controls; however, the patients showed a lack of right-left asymmetry in striatal uptake of TRODAT. Finally, our previous study with 11 patients and 12 controls14 and the study of Chou et al30 with 7 patients and 11 controls also failed to identify any deficits among drug-naive patients. The largest study in our review,29 with 28 patients and 12 normal controls, was again with no significant differences of DAT availability between patients and controls. However, the authors highlighted that in a subsample of 18 patients with a positive syndrome as defined by Kay,23 the severity of hallucinations was inversely correlated with DAT availability. This finding differs from the rest of the studies we reviewed that showed no evidence of correlation between symptoms and DAT availability.13–15,28
Table 4.
Summary of All [99mTc]-TRODAT-1 Relevant Published Articles
| Authors | Year | Journal | Patients | Controls | Psychopathological rating | DAT study only | DAT and DA receptor study | Summary | |||||||
| n | Male/female | Age | n | Matched | Male/female | Age | |||||||||
| Mean | SD | Mean | SD | ||||||||||||
| Chou et al | 2010 | Clinical Psychopharmacology and Neuroscience | 7 | — | 22.4 | 5.3 | 11 | Age | — | 25.2 | 4.5 | Nil | Yes | No difference in striatal DAT availability between patients and controls | |
| Schmitt et al | 2008 | Schizophrenia Research | 20 | 18/2 | 29.3 | 6.5 | 12 | Age and sex | 9/3 | 30.5 | 8 | CGI, GAF, and PANSS | Yes | No difference in striatal DAT availability between patients and controls | |
| Schmitt et al | 2006 | European Archives of Psychiatry and Clinical Neuroscience | 28 | 23/5 | 30.7 | 8.9 | 12 | Age | 10/2 | 31.7 | 8.4 | CGI, GAF, and PANSS | Yes | No difference in striatal DAT availability between patients and controls | |
| Schmitt et al | 2005 | Journal of Psychopharmacology | 10 | 6/4 | 34.9 | 12.1 | 10 | Age and sex | — | 37.8 | 10.8 | BPRS, PANSS, and SANS | Yes | No difference in striatal DAT availability between patients and controls | |
| Yang et al | 2004 | American Journal of Psychiatry | 11 | 6/5 | 26.3 | 10.2 | 12 | — | 9/3 | 33.3 | 12.9 | PANSS | Yes | No difference in striatal DAT availability between patients and controls | |
| Hsiao et al | 2003 | Schizophrenia Research | 12 | 2/10 | 25.9 | 7.7 | 12 | Age and sex | — | 29.8 | 8.6 | SCAN and PANSS | Yes | No difference in striatal DAT availability between patients and controls | |
Note: DAT, dopamine transporter; DA, dopamine; CGI, Clinical Global Impression; GAF, Global Assessment of Functioning; PANSS, Positive and Negative Syndrome Scale; BPRS, Brief Psychiatric Rating Scale; SANS, Schedule for Assessment of Negative Symptoms; SCAN, Standardized Clinical Assessment for Neuropsychiatry.
Meta-Analysis
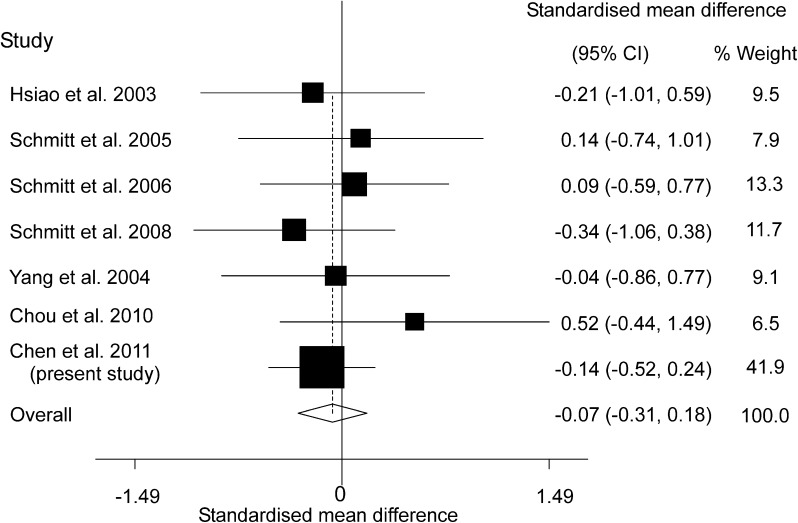
We conducted a random-effects meta-analysis of the available literature including 6 previously published articles as well as our current data. To avoid including them twice, our current data here excluded 11 patients and 12 controls recruited in our previous study14 for the meta-analysis. The average ROI value of TRODAT binding over left and right hemispheres was used for studies where this information was available. The combined sample included a total of 124 drug-naive patients with schizophrenia and 169 healthy controls. The pooled standardized effect size computed as a Cohen’s d 27 was −0.07 (95% CI −0.31, 0.18; P = .60) indicating that patients and controls do not differ significantly. There was no significant between-study heterogeneity in effect sizes (coefficient = −0.07; P = .61). Figure 2 provides a forest plot for the meta-analysis. Furthermore, there was no evidence of publication bias (Egger’s test coefficient = 0.90; P = .28).
Fig. 2.
Meta-analysis of TRODAT literature and local study focusing on drug-naive patients.
Discussion
Our findings show that the specific striatal binding ratio of drug-naive patients with schizophrenia was not significantly different from that of normal controls, indicating that both groups had similar DAT availability. Our study is the largest to date, including over twice the sample of drug-naive/drug-free patients of the next largest study of DAT in schizophrenia.31 Another strength of our study is that all the subjects were drug naive and at a uniform and early stage of their illness. Our findings thus extend previous work in a larger and clinically homogenous sample. They are consistent with all the previous SPECT studies of schizophrenia using [99mTc]-TRODAT-1 that were included in our meta-analysis.13–15,28–30
The meta-analysis including our data found there was no evidence for a difference in striatal [99mTc]-TRODAT-1 binding in drug-naive patients with schizophrenia compared with controls. Considering the range of effect sizes included in the 95% CI, the standardized effect size is unlikely to exceed 0.31, which is conventionally considered to be a small effect.27 With this effect size, as many as 188 drug-naive patients and an equal number of controls would need to be tested to have 85% power to detect a difference (two-group T test with a 5% two-sided significance level). The overall conclusion of our work is that drug-naive patients in the early stages of schizophrenia have normal [99mTc]-TRODAT-1 binding and that any impairments, if present, would be of a small magnitude. Given the normal variation in binding, any potential deficits in drug-naive patients are unlikely to be clinically significant. Although [99mTc]-TRODAT-1 binds to serotonin transporters, blocking studies show that the specific binding in the striatum is almost entirely due to DATs32; hence, our findings reflect that DAT availability is unaltered in drug-naive patients with schizophrenia.
However, it remains conceivable that there is a transient alteration in DAT at or before the onset of the disorder that may have been missed by all of the above studies including our own because our patients probably suffered from psychotic symptoms for months or even years prior to their SPECT scan.
Methodological Considerations
We need to consider the possible influence of tobacco smoking because a decrease in DAT availability in the striatum of smokers has been described in previous studies.26 A study of nonsmokers only would not be representative of most clinical populations, and we included participants regardless of their smoking habit. Our patient and control groups were not significantly different in smoking status; hence, smoking is unlikely to have confounded our results. Nevertheless, all our analyses were adjusted for tobacco smoking in this sample.
One potential limitation of our study is that the ROIs were manually drawn directly on the SPECT image. While there is the potential for observer bias using this approach, we avoided this by ensuring that the ROIs were delineated by an experienced nuclear medicine physician independent of the clinical assessment and blind to the subject’s clinical group. We delineated the ROI on the SPECT image rather than on the MRI because this does not require the ROI to be transformed from MRI to SPECT space. However, studies have found similar results using both approaches33,34 indicating that the choice of approach is unlikely to have influenced our findings.
Another potential limitation is that patients recruited in our study for SPECT imaging had to be relatively cooperative in order to complete the procedure. This is an issue for most imaging studies and may potentially affect the generalizability of our findings to uncooperative patients.
Interpretation of Our Findings
The presynaptic DAT plays a key role in regulating the DA content in the synaptic cleft by transporting it back into DA terminals, effectively modulating the concentration of DA available for postsynaptic receptor binding.35 There are 2 main interpretations of our findings: on the one hand, if DA was able to displace [99mTc]-TRODAT-1 from DAT, our failure to find a difference in specific binding could be due to elevated synaptic DA levels in schizophrenia masking an increase in DAT levels.10 However, while the definitive studies have not yet been conducted, the available evidence indicates that [99mTc]-TRODAT-1 binding is not sensitive to variations in DA levels seen in vivo.36 A more likely interpretation is thus that DAT levels are unaltered in schizophrenia. This is important because it indicates that there is no compensatory increase in DAT levels in response to the increased release and synaptic levels of DA previously reported in schizophrenia.8,10 This interpretation is consistent with evidence that fluctuations in brain DA levels do not alter the abundance of the DAT.37 Our findings might thus suggest pharmacological augmentation of DAT as a potential novel treatment strategy for schizophrenia.
We identified a gender effect in the combined sample, whereby females had a higher specific striatal radiotracer binding ratio than males, which is consistent with a previous study.24 The effect size for gender in our study is 0.25 (Cohen’s d) and although this is a small effect, it might be of relevance. It would seem to be in line with the later age of onset and lower incidence of schizophrenia in females.38 The lower DAT availability in males could contribute to their earlier age of onset because, putatively, they would have less capacity to buffer excess striatal DA release.
Our finding that there is a negative relationship between age and DAT availability is consistent with a previous study showing that the specific uptake of [99mTc]-TRODAT-1 radiotracer decreases with advancing age in healthy individuals.25 Because the density of striatal DA D2/D3 receptors also declines with age in healthy individuals,39 our data show the consistency of this age effect on both pre- and postsynaptic DAergic function.
The PANSS scores for positive and negative symptoms of schizophrenia and general psychopathology did not correlate with the [99mTc]-TRODAT-1 binding ratio. This is again consistent with previous research13,14,28 and with the absence of alterations in [99mTc]-TRODAT-1 availability in our patients as a group.
Conclusions
Our findings, in the largest first-episode drug-naive and healthy sample studied to date, suggest that striatal DAT availability is not altered in the early stages of schizophrenia before medication is introduced. Instead, we identified gender differences and confirmed aging effects that could have clinical significance and may be taken into account in future patient studies.
Funding
National Science Council of Taiwan (NSC 91-2314-B-006-074, NSC 92-2314-B-006-111, NSC 93-2314-B-006-107, NSC 95-2314-B-006-115-MY2, and NSC 99-2314-B-006-019-MY3); Atomic Energy Council of Taiwan (NSC 91-NU-7-006-002 and NSC 92-NU-7-006-004); NIHR Biomedical Research Centre for Mental Health at the South London and Maudsley National Health Service Foundation Trust and Institute of Psychiatry, Kings College London. E.B. holds a Medical Research Council new investigator award.
Acknowledgments
The authors would like to thank Ms Ching Lin Chu, Ms Tsai Hua Chang, and Mr Chien Ting Lin for their administrative support. The funding institutions of this study had no further role in the study design, the collection, analysis and interpretation of data, the writing of this article, or the decision to submit for publication. The authors report no financial relationships with commercial interests.
References
- 1.Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- 2.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyon GJ, Abi-Dargham A, Moore H, Lieberman JA, Javitch JA, Sulzer D. Presynaptic regulation of dopamine transmission in schizophrenia. Schizophr Bull. 2011;37:108–117. doi: 10.1093/schbul/sbp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howes OD, Egerton A, Allan V, McGuire P, Stokes P, Kapur S. Mechanisms underlying psychosis and antipsychotic treatment response in schizophrenia: insights from PET and SPECT imaging. Curr Pharm Des. 2009;15:2550–2559. doi: 10.2174/138161209788957528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howes OD, Montgomery AJ, Asselin M-C, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 6.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Guo N, Guo W, Kralikova M, et al. Impact of D2 receptor internalization on binding affinity of neuroimaging radiotracers. Neuropsychopharmacology. 2010;35:806–817. doi: 10.1038/npp.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 9.Kegeles LS, Abi-Dargham A, Frankle WG, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 10.Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laakso A, Bergman J, Haaparanta M, et al. Decreased striatal dopamine transporter binding in vivo in chronic schizophrenia. Schizophr Res. 2001;52:115–120. doi: 10.1016/s0920-9964(00)00095-5. [DOI] [PubMed] [Google Scholar]
- 12.Mateos JJ, Lomena F, Parellada E, et al. Lower striatal dopamine transporter binding in neuroleptic-naive schizophrenic patients is not related to antipsychotic treatment but it suggests an illness trait. Psychopharmacology (Berl) 2007;191:805–811. doi: 10.1007/s00213-006-0570-5. [DOI] [PubMed] [Google Scholar]
- 13.Hsiao MC, Lin KJ, Liu CY, Tzen KY, Yen TC. Dopamine transporter change in drug-naive schizophrenia: an imaging study with 99mTc-TRODAT-1. Schizophr Res. 2003;65:39–46. doi: 10.1016/s0920-9964(03)00006-9. [DOI] [PubMed] [Google Scholar]
- 14.Yang YK, Yu L, Yeh TL, Chiu NT, Chen PS, Lee IH. Associated alterations of striatal dopamine D2/D3 receptor and transporter binding in drug-naive patients with schizophrenia: a dual-isotope SPECT study. Am J Psychiatry. 2004;161:1496–1498. doi: 10.1176/appi.ajp.161.8.1496. [DOI] [PubMed] [Google Scholar]
- 15.Schmitt GJ, la Fougere C, Dresel S, et al. Dual-isotope SPECT imaging of striatal dopamine: first episode, drug naive schizophrenic patients. Schizophr Res. 2008;101:133–141. doi: 10.1016/j.schres.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Sjoholm H, Bratlid T, Sundsfjord J. 123I-beta-CIT SPECT demonstrates increased presynaptic dopamine transporter binding sites in basal ganglia in vivo in schizophrenia. Psychopharmacology (Berl) 2004;173:27–31. doi: 10.1007/s00213-003-1700-y. [DOI] [PubMed] [Google Scholar]
- 17.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 18.Friston KJ, Frith CD, Liddle PF, Dolan RJ, Lammertsma AA, Frackowiak RS. The relationship between global and local changes in PET scans. J Cereb Blood Flow Metab. 1990;10:458–466. doi: 10.1038/jcbfm.1990.88. [DOI] [PubMed] [Google Scholar]
- 19.Chang LT. A method for attenuation correction in radionuclide computed tomography. IEEE Trans Nucl Sci. 1978;25:638–642. [Google Scholar]
- 20.Hwang WJ, Yao WJ, Wey SP, Ting G. Reproducibility of 99mTc-TRODAT-1 SPECT measurement of dopamine transporters in Parkinson's disease. J Nucl Med. 2004;45:207–213. [PubMed] [Google Scholar]
- 21.Guy W. ECDEU Assessment for Psychopharmacology. Revised ed. Washington, DC: US Department of Health, Education, and Welfare. Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. [Google Scholar]
- 22.Hopper K, Wanderling J. Revisiting the developed versus developing country distinction in course and outcome in schizophrenia: results from ISoS, the WHO collaborative followup project. International Study of Schizophrenia. Schizophr Bull. 2000;26:835–846. doi: 10.1093/oxfordjournals.schbul.a033498. [DOI] [PubMed] [Google Scholar]
- 23.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 24.Kuikka JT, Tiihonen J, Karhu J, Bergstrom KA, Rasanen P. Fractal analysis of striatal dopamine re-uptake sites. Eur J Nucl Med. 1997;24:1085–1090. doi: 10.1007/BF01254238. [DOI] [PubMed] [Google Scholar]
- 25.Mozley PD, Acton PD, Barraclough ED, et al. Effects of age on dopamine transporters in healthy humans. J Nucl Med. 1999;40:1812–1817. [PubMed] [Google Scholar]
- 26.Newberg A, Lerman C, Wintering N, Ploessl K, Mozley PD. Dopamine transporter binding in smokers and nonsmokers. Clin Nucl Med. 2007;32:452–455. doi: 10.1097/01.rlu.0000262980.98342.dd. [DOI] [PubMed] [Google Scholar]
- 27.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1988. [Google Scholar]
- 28.Schmitt GJ, Meisenzahl EM, Frodl T, et al. The striatal dopamine transporter in first-episode, drug-naive schizophrenic patients: evaluation by the new SPECT-ligand[99mTc]TRODAT-1. J Psychopharmacol. 2005;19:488–493. doi: 10.1177/0269881105056530. [DOI] [PubMed] [Google Scholar]
- 29.Schmitt GJ, Frodl T, Dresel S, et al. Striatal dopamine transporter availability is associated with the productive psychotic state in first episode, drug-naive schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 2006;256:115–121. doi: 10.1007/s00406-005-0618-2. [DOI] [PubMed] [Google Scholar]
- 30.Chou Y-H, Huang W-S, Hsu J-W, Lee S-M, Yang A-S, Yang K-C. Simultaneous measurement of dopamine transporters and D2/D3 receptors in drug naïve schizophrenic patients. Clin Psychopharmacol Neurosci. 2010;8:97–104. [Google Scholar]
- 31.Laruelle M, Abi-Dargham A, van Dyck C, et al. Dopamine and serotonin transporters in patients with schizophrenia: an imaging study with [(123)I]beta-CIT. Biol Psychiatry. 2000;47:371–379. doi: 10.1016/s0006-3223(99)00257-7. [DOI] [PubMed] [Google Scholar]
- 32.Dresel SH, Kung MP, Huang X, et al. In vivo imaging of serotonin transporters with [99mTc]TRODAT-1 in nonhuman primates. Eur J Nucl Med. 1999;26:342–347. doi: 10.1007/s002590050396. [DOI] [PubMed] [Google Scholar]
- 33.Inoue M, Katsumi Y, Hayashi T, et al. Sensory stimulation accelerates dopamine release in the basal ganglia. Brain Res. 2004;1026:179–184. doi: 10.1016/j.brainres.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 34.Wang GJ, Volkow ND, Levy AV, et al. MR-PET image coregistration for quantitation of striatal dopamine D2 receptors. J Comput Assist Tomogr. 1996;20:423–428. doi: 10.1097/00004728-199605000-00020. [DOI] [PubMed] [Google Scholar]
- 35.Bannon MJ, Michelhaugh SK, Wang J, Sacchetti P. The human dopamine transporter gene: gene organization, transcriptional regulation, and potential involvement in neuropsychiatric disorders. Eur Neuropsychopharmacol. 2001;11:449–455. doi: 10.1016/s0924-977x(01)00122-5. [DOI] [PubMed] [Google Scholar]
- 36.Fernagut PO, Li Q, Dovero S, et al. Dopamine transporter binding is unaffected by L-DOPA administration in normal and MPTP-treated monkeys. PLoS One. 2010;5:e14053. doi: 10.1371/journal.pone.0014053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moody CA, Granneman JG, Bannon MJ. Dopamine transporter binding in rat striatum and nucleus accumbens is unaltered following chronic changes in dopamine levels. Neurosci Lett. 1996;217:55–57. doi: 10.1016/0304-3940(96)13048-2. [DOI] [PubMed] [Google Scholar]
- 38.Faraone SV, Chen WJ, Goldstein JM, Tsuang MT. Gender differences in age at onset of schizophrenia. Br J Psychiatry. 1994;164:625–629. doi: 10.1192/bjp.164.5.625. [DOI] [PubMed] [Google Scholar]
- 39.Chen PS, Yang YK, Lee YS, et al. Correlation between different memory systems and striatal dopamine D2/D3 receptor density: a single photon emission computed tomography study. Psychol Med. 2005;35:197–204. doi: 10.1017/s0033291704003101. [DOI] [PubMed] [Google Scholar]