Abstract
Low serum folate levels previously have been associated with negative symptom risk in schizophrenia, as has the hypofunctional 677C>T variant of the MTHFR gene. This study examined whether other missense polymorphisms in folate-regulating enzymes, in concert with MTHFR, influence negative symptoms in schizophrenia, and whether total risk allele load interacts with serum folate status to further stratify negative symptom risk. Medicated outpatients with schizophrenia (n = 219), all of European origin and some included in a previous report, were rated with the Positive and Negative Syndrome Scale. A subset of 82 patients also underwent nonfasting serum folate testing. Patients were genotyped for the MTHFR 677C>T (rs1801133), MTHFR 1298A>C (rs1801131), MTR 2756A>G (rs1805087), MTRR 203A>G (rs1801394), FOLH1 484T>C (rs202676), RFC 80A>G (rs1051266), and COMT 675G>A (rs4680) polymorphisms. All genotypes were entered into a linear regression model to determine significant predictors of negative symptoms, and risk scores were calculated based on total risk allele dose. Four variants, MTHFR 677T, MTR 2756A, FOLH1 484C, and COMT 675A, emerged as significant independent predictors of negative symptom severity, accounting for significantly greater variance in negative symptoms than MTHFR 677C>T alone. Total allele dose across the 4 variants predicted negative symptom severity only among patients with low folate levels. These findings indicate that multiple genetic variants within the folate metabolic pathway contribute to negative symptoms of schizophrenia. A relationship between folate level and negative symptom severity among patients with greater genetic vulnerability is biologically plausible and suggests the utility of folate supplementation in these patients.
Keywords: gene-environment interaction, methylation, epigenetics, genetic risk score
Introduction
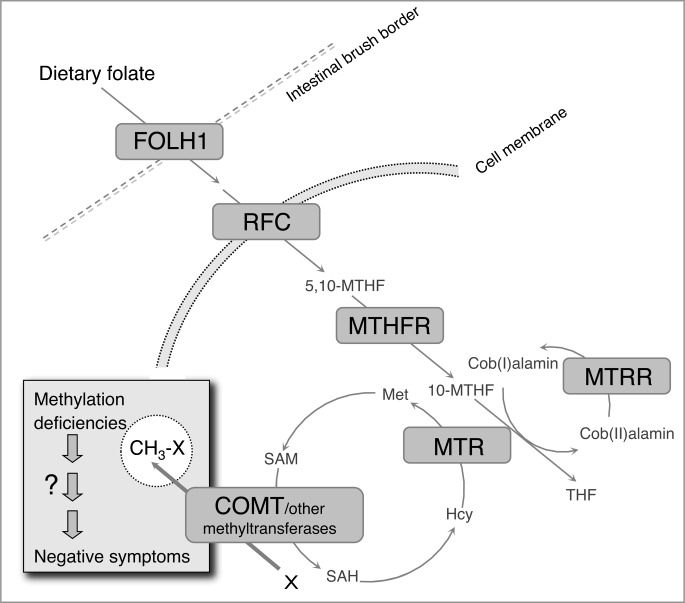
Folate supplies the substrate for intracellular methylation reactions that are essential to normal brain development and function. Methylation governs such vital processes as DNA synthesis and repair, gene expression, neurotransmitter synthesis and degradation, and homocysteine metabolism.1 The availability of 1-carbon moieties for methylation reactions is regulated both by dietary folate intake and by cellular machinery mediating folate absorption through the gut, translocation of folate into cells, and conversion of precursors to methyl donors such as S-adenosylmethionine (SAM) (figure 1).2
Fig. 1.
The folate metabolic pathway. THF: tetrahydrofolate; Met: methionine; SAM: S-adenosylmethionine; SAH: S-adenosyl homocysteine; Hcy: homocysteine.
Altered folate metabolism has been implicated in several neuropsychiatric disorders, including schizophrenia. Reduced maternal folate intake3 and increased maternal homocysteine blood level4 during neurodevelopment have been associated with substantial increases in schizophrenia risk. Low blood levels of folate have been observed in several cohorts of schizophrenia patients,5–7 and vitamin supplementation regimens that include folate8 and methylfolate9 have been associated with symptomatic improvement. Moreover, common functional genetic variants in 2 genes regulating folate metabolism, methylenetetrahydrofolate reductase (MTHFR)10 and methionine synthase (MTR),11 have been associated with increased schizophrenia risk. MTHFR in particular has emerged as a strong candidate gene, with the low-functioning 677T allele significantly augmenting schizophrenia risk across 20 case-control studies,10 although not reaching the threshold of genome-wide significance.
One specific aspect of schizophrenia, negative symptoms, exhibits especially strong ties to folate metabolism and possibly to folate-related genes. Negative symptoms, which include apathy, impoverished speech, flattened affect, and social withdrawal, contribute greatly to functional disability in schizophrenia and are not substantially improved by antipsychotic medications.12–14 Previous work has demonstrated a significant inverse correlation between serum folate level and severity of negative symptoms in schizophrenia.7 MTHFR 677C>T genotype contributes to this relationship: patients who carry at least 1 copy of the 677T allele, which causes a 35% reduction in MTHFR activity,15 demonstrate greater negative symptom severity; among patients homozygous for the hypofunctional 677T allele, those who also have low serum folate are at especially high risk for negative symptoms.16
In turn, MTHFR genotype may also influence response of negative symptoms to folate supplementation in schizophrenia. In a recent small (n = 32) randomized, double-blind placebo-controlled study,17 only patients who carried at least 1 copy of the T allele (C/T + T/T, or T allele carriers) exhibited a decline in negative symptom severity after 12 weeks of daily treatment with 2 mg folic acid (P = .01), although the magnitude of the decline did not differ compared with T allele carriers who received placebo (P = .06). In T allele carriers (P = .003), but not C/C patients (P = .37), the change in serum folate concentration was inversely correlated with the change in negative symptom scores.
Here, we examined whether other common missense genetic variants in the folate metabolic pathway contribute to negative symptoms in schizophrenia and specifically to the relationship between negative symptom severity and serum folate level. We first used linear regression to determine if polymorphisms in 4 other genes that regulate folate metabolism—folate hydrolase (FOLH1, also referred to as glutamate carboxypeptidase II, GCP-II), reduced folate carrier (RFC), MTR, and methionine synthase reductase (MTRR)—contributed to the severity of negative symptoms, in addition to the known effects of MTHFR. The regression model also included the catechol-O-methltransferase (COMT) 675G>A (158Val>Met) polymorphism, which has previously been shown to interact with MTHFR 677C>T to influence executive dysfunction18 and related prefrontal impairment19 in schizophrenia, as well as homocysteine levels in elderly individuals.20 Next, using only those variants that were independent predictors of negative symptom severity, we determined a risk score for each subject, which reflected the total number of risk alleles present within each individual. We then determined whether risk score correlated with negative symptom severity in patients with low vs high serum folate levels, hypothesizing that high folate would protect against the cumulative detrimental effects of the identified risk alleles.
Methods
The present study comprised an augmented version of a cohort we have previously described.16 Study procedures were approved by the Partners HealthCare and Massachusetts Department of Mental Health institutional review boards, and all participants provided written informed consent.
Participants and Symptom Ratings
We included 219 medicated chronic schizophrenia outpatients (mean age 48 ± 14 y, 70% male) of European descent from an urban community mental health center clinic. The diagnosis of schizophrenia was confirmed by a consensus diagnostic conference based on results from a clinical diagnostic interview, chart review, and review of clinical history with treating physicians. Patients were administered the Positive and Negative Syndrome Scale (PANSS)21 to assess symptom severity by trained raters who were blind to genotype and serum folate level.
Genotype
DNA was obtained from blood samples and genotyped for 6 single nucleotide polymorphisms (SNPs) across 5 genes that regulate folate metabolism: FOLH1, RFC, MTHFR, MTR, and MTRR (table 1). Specific variants were selected on the basis of (1) common occurrence in the general population (minor allele frequency >0.2), (2) coding for nonsynonymous mutations in amino acid sequences, and (3) previous support in the literature for association with schizophrenia and/or measurable effects on folate or homocysteine metabolism. Patients were also genotyped for the COMT 675G>A polymorphism. No additional genetic variants were studied. Genotyping was conducted using the MassArray platform (Sequenom). All genotypes demonstrated Hardy Weinberg equilibrium (table 1). The 2 MTHFR SNPs were in linkage disequilibrium (R 2 = .19, D′ = 1).
Table 1.
Missense Variants in the Folate Metabolic Pathway
| Gene | Location | SNP | AA Change | MAF | HWE |
| FOLH1 | 11p11.2 | rs202676 (484T>C) | 75Tyr>His | 0.21 (C) | P = .41 |
| RFC | 21q22.3 | rs1051266 (80A>G) | 27His>Arg | 0.46 (A) | P = .59 |
| MTHFR | 1p36.3 | rs1801131 (1298A>C) | 429Glu>Ala | 0.32 (C) | P = .88 |
| rs1801133 (677C>T) | 222Ala>Val | 0.37 (T) | P = .67 | ||
| MTR | 1q43 | rs1805087 (2756A>G) | 919Asp>Gly | 0.19 (G) | P = 1.00 |
| MTRR | 5p15.31 | rs1801394 (203A>G) | 22Ile>Met | 0.50 (G) | P = .42 |
| COMT | 22q11.21 | rs4680 (675G>A) | 158Val>Met | 0.49 (A) | P = .89 |
Note: SNP, single nucleotide polymorphism; AA, amino acid; MAF, minor allele frequency; HWE, Hardy Weinberg equilibrium.
Serum Folate
Nonfasting serum folate levels, obtained on the day of PANSS ratings, were available for a subset of 82 patients. Folate concentrations were determined using cloned enzyme donor immunoassay kits (Bio-Rad) according to the manufacturer’s instructions. The group of patients for whom folate levels were available did not differ from the remainder of the subject pool by age or PANSS scores (all P’s ≥ .1), although female subjects comprised a smaller proportion of the folate group (15%) than the nonfolate group (31%) (χ2 = 7.09, P = .01). Subjects in the folate group were further divided into high folate (≥12.0 ng/ml) and low folate (<12.0 ng/ml) subjects based on a median split, following the same method as Roffman et al.16 Anticonvulsant use, which can influence serum folate level, did not differ between the folate and nonfolate groups (χ2 = 1.76, P = .19) or between the low and high folate groups (χ2 = 0.67, P = .59).
Statistical Analysis
One multiple linear regression model was used to determine independent effects of each of the 7 SNPs on the PANSS negative symptom subscale. For each SNP, genotype was entered as 0, 1, or 2, depending on the number of minor alleles; the regression model determined whether allele load significantly (2 tailed P < .05) predicted negative symptom severity. All SNP variables were entered simultaneously into the model. An identical analysis was attempted for the PANSS positive symptom subscale.
An allelic risk score was then calculated for each subject based on the total number of alleles (0, 1, or 2 per SNP) across all of the SNPs that had been identified as independently predicting negative symptoms. This information was then entered into a multiple linear regression model for PANSS negative symptom scores, including allelic risk score, folate group (ie, high vs low folate), and risk score × folate group interaction.
Results
SNPs Predictive of Negative Symptoms
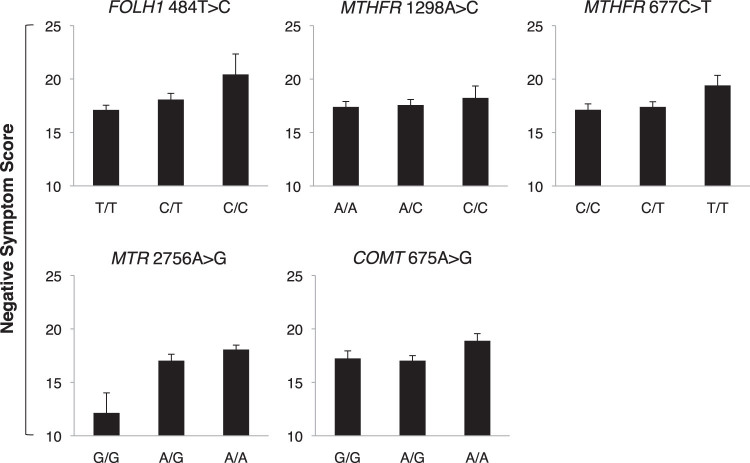
Regression analyses are reported in table 2 and figure 2. For negative symptoms, as previously reported in a smaller version of the same cohort (n = 135 Caucasian participants),16 MTHFR 677T allele load was significantly associated with negative symptom severity. In addition, FOLH1 484C, MTR 2756A, and COMT 675A allele load each predicted negative symptom scores. MTHFR 1298C allele load was associated with negative symptom scores at trend level. The model accounted for 10.2% of the variance in negative symptoms (R 2). In contrast, none of the polymorphisms studied were significantly associated with positive symptom scores (all P’s > .10). Inclusion of anticonvulsant use as an additional factor in the regression model did not change the significance level of any result.
Table 2.
Multiple Linear Regression Examining Genotype Effects on Symptom Profiles
| Gene | SNP | PANSS Negative Symptoms | PANSS Positive Symptoms | ||||||
| β | t | P | R 2 change | β | t | P | R 2 change | ||
| FOLH1 | rs202676 (484T>C) | .139 (C) | 2.11 | .036 | .019 | .097 | 1.43 | .155 | .000 |
| RFC | rs1051266 (80A>G) | .000 | 0.01 | .995 | .000 | .058 | 0.85 | .395 | .003 |
| MTHFR | rs1801131 (1298A>C) | .145 (C) | 1.87 | .063 | .015 | .113 | 1.40 | .162 | .009 |
| rs1801133 (677C>T) | .204 (T) | 2.65 | .009 | .030 | .028 | 0.35 | .727 | .000 | |
| MTR | rs1805087 (2756A>G) | .197 (A) | 3.00 | .003 | .038 | .067 | 0.98 | .328 | .004 |
| MTRR | rs1801394 (203A>G) | .014 | 0.22 | .828 | .000 | .009 | 0.13 | .897 | .000 |
| COMT | rs4680 (675G>A) | .139 (A) | 2.12 | .035 | .019 | .019 | 0.27 | .784 | .000 |
Note: SNP: single nucleotide polymorphism; PANSS: Positive and Negative Syndrome Scale. Overall model statistics: for negative symptoms, R 2 = .102, F 7,218 = 3.41, P = .002; for positive symptoms, R 2 = .032, F 7,218 = 0.98, P = .446. For significant or trend-level SNPs, the risk allele is given in parentheses next to the beta statistic.
Fig. 2.
Genetic variants in the folate metabolic pathway that significantly predicted negative symptom severity. Error bars indicate SE.
Table 3.
Number of Subjects in Risk Allele Load Groups
| Risk Allele Load | 4 SNP Model | 5 SNP Model | ||||
| All Subjects | Low Folate | High Folate | All Subjects | Low Folate | High Folate | |
| 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 1 | 4 | 1 | 0 | 0 | 0 | 0 |
| 2 | 29 | 2 | 9 | 14 | 1 | 4 |
| 3 | 45 | 7 | 5 | 32 | 1 | 6 |
| 4 | 75 | 15 | 15 | 63 | 8 | 15 |
| 5 | 53 | 11 | 12 | 68 | 17 | 9 |
| 6 | 10 | 5 | 0 | 36 | 13 | 7 |
| 7 | 2 | 0 | 0 | 6 | 1 | 0 |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 219 | 41 | 41 | 219 | 41 | 41 |
Note: SNP: single nucleotide polymorphism.
Cumulative Effects of Risk SNPs
To illustrate more directly the cumulative effects of the identified risk SNPs on negative symptoms, subjects were assigned to groups based on the total number of risk alleles (table 3). Given the trend-level significance of MTHFR 1298C as a predictor of negative symptoms, this allelic risk load analysis was conducted twice, first using a 4 SNP score (incorporating MTHFR 677C>T, FOLH1 484C>T, MTR 2756A>G, and COMT 675G>A and resulting in a total score that ranged from 0 to 8) and then a 5 SNP score (which also incorporated MTHFR 1298A>C, resulting in a total score that ranged from 0 to 10). The 4 and 5 SNP scores effectively summarize the genetic risk associated with the individual genotypes because there was insignificant change in goodness of fit when reducing from the 4 or 5 separate predictors to the single risk scores (P = .62 and .79, respectively). Both the 4 SNP (F 3,213 = 5.63, P = .001) and 5 SNP (F 4,213 = 5.15, P = .001) linear regression models fit the negative symptom data significantly better than MTHFR 677C>T alone.
Because MTHFR effects on negative symptoms had already been described in a subset of 135 participants,16 we also determined whether the 4 and 5 SNP scores derived in the present study predicted negative symptoms separately in that group (old cohort), as well as in the 84 subjects not previously reported (new cohort). These relationships were significant in both the old cohort (4 SNP: R = .25, P = .004; 5 SNP: R = .31, P = .0003) and the new cohort (4 SNP: R = .38, P = .003; 5 SNP: R = .28, P = .01).
To determine whether allelic risk load was correlated with specific negative symptoms, we conducted an exploratory analysis of each of the 7 individual items within the PANSS negative symptom subscale. After Bonferroni correction for multiple comparisons (P = .05/7 = .007), several items correlated significantly with allelic risk load. For the 4 SNP model, these included blunted affect (R = .26, P = .0001), poor rapport (R = .22, P = .001), and lack of spontaneity (R = .192, P = .004); for the 5 SNP model, they included blunted affect (R = .28, P = .00002) and emotional withdrawal (R = .26, P = .00008).
Interaction With Folate
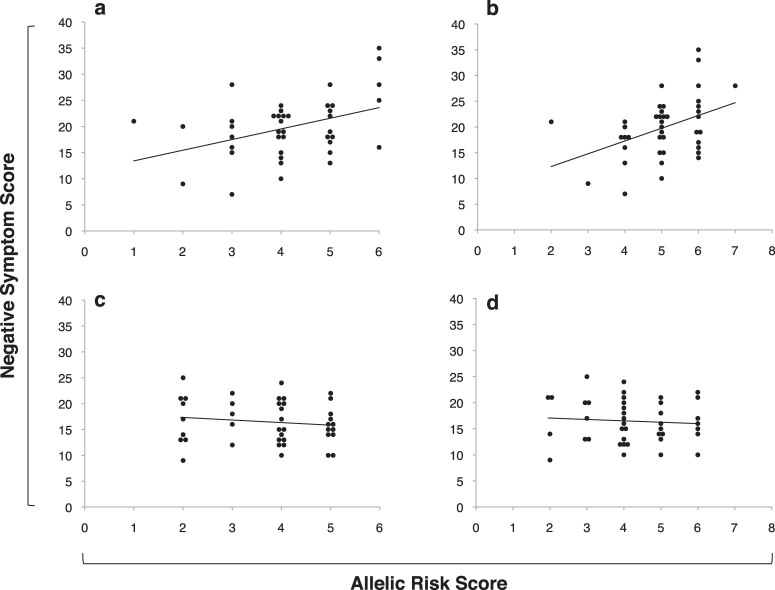
Among subjects with serum folate data, linear regression indicated a significant interaction between allelic risk score and folate group, using either the 4 SNP score (overall model P = .0004, R 2 = .21; interaction β = −1.16, P = .009) or the 5 SNP score (overall model P = .0004, R 2 = .21; interaction β=−1.29, P = .008). Post hoc Pearson’s correlations were determined to examine the relationship between allelic risk scores and negative symptoms in the low and high folate groups. For the low folate group, both the 4 SNP score (R = .41, P = .008, see figure 3a) and 5 SNP score (R = .42, P = .007, see figure 3b) predicted negative symptom severity. These correlations remained significant when allelic risk score groups with fewer than 5 subjects were removed from the analysis (4 SNP score: R = .44, P = .006; 5 SNP score: R = .38, P = .017). Conversely, for the high folate group, neither the 4 SNP score (R = −.14, P = .40, see figure 3c) nor the 5 SNP score (R = −.08, P = .63, see figure 3d) was associated with negative symptom severity.
Fig. 3.
Among individuals with low serum folate, there was a significant relationship between negative symptoms and allelic risk score, using either 4 single nucleotide polymorphism (SNP) score (a) or 5 SNP score (b) models. Conversely, among high folate individuals, there was no significant relationship between negative symptoms and allelic risk score, using either 4 SNP score (c) or 5 SNP score (d) models. Data points represent individual participants and are jittered to avoid overlap.
Discussion
Dietary folate supplies the primary substrate for enzymes in the folate metabolic pathway, which in turn provides 1-carbon moieties for DNA methylation, homocysteine metabolism and other vital transmethylation reactions. Functional polymorphisms in the folate pathway influence the efficiency of downstream methylation events, and in the presence of reduced substrate, low-functioning genetic variants can become rate limiting.22 For example, previous work with the MTHFR 677C>T variant indicated that among individuals homozygous for the fully functional C allele, genomic DNA methylation, and homocysteine metabolism were not dependent on serum folate level; however, for individuals homozygous for the hypofunctional 677T variant, DNA methylation and homocysteine metabolism were strongly dependent on serum folate concentration.23 We reported an analogous pattern with respect to negative symptom severity in schizophrenia, where folate levels influenced negative symptoms in T/T but not C/C patients.16 By the same token, among T/T individuals, higher serum folate levels conferred DNA methylation patterns,23 and negative symptom scores16 that did not differ substantially from C/C subjects, suggesting that T allele-related MTHFR dysfunction is surmountable in the presence of increased dietary folate.
The present findings suggest that genetic variation throughout the folate metabolic pathway—not limited just to MTHFR—contributes to negative symptom severity in schizophrenia. Missense variants in 3 additional genes, FOLH1, MTR, and COMT were also independently associated with negative symptom scores. Moreover, we found evidence of a cumulative effect of MTHFR, FOLH1, MTR, and COMT, where patients who carried a higher number of risk variants across these 4 genes exhibited more severe negative symptoms but only in the presence of low serum folate levels.
FOLH1 (also called GCP-II) is a glutamate carboxypeptidase that is anchored to the intestinal brush border, where it converts dietary polyglutamylated folates into monoglutamyl folates that can be transported into the body. The 484T>C variant is located in exon 2 of the structural transmembrane region. In a study of the Hordaland homocysteine cohort, Halsted and colleagues24 reported elevated homocysteine among individuals with the C/T genotype, although there was no significant genotype effect on serum folate. Here, the 484C variant was associated with more severe negative symptoms. Of note, FOLH1 is also expressed in the brain where it is known as NAALADase and cleaves N-acetylaspartylglutamate (NAAG) into N-acetylaspartate (NAA) and glutamate.25 NAA is a marker of neuronal integrity for which hippocampal and prefrontal levels are consistently reduced in magnetic resonance spectroscopy studies of schizophrenia,26,27 while glutamatergic dysfunction in schizophrenia is well established.28 FOLH1 therefore could represent an important target in schizophrenia pathophysiology through its effects on numerous implicated pathways.
The 2756A variant of MTR has also been associated with elevated homocysteine levels compared with 2756G carriers in numerous studies (reviewed in ref. 22). Given that MTR remethylates homocysteine to methionine, homocysteine elevations in 2756A carriers suggest that this version of MTR confers reduced activity. Kempisty and colleagues11 found that the MTR 2756G allele predicted increased risk of schizophrenia and bipolar disorder. In this study, however, it was the 2756A allele that appeared detrimental with respect to negative symptoms. MTR 2756A>G is thus similar to MTHFR 677C>T, in that the allelic variant associated with reduced availability of 1-carbon moieties is the same one that predicts greater negative symptom severity.
The present results extend our previous MTHFR analyses to a larger cohort, confirming detrimental effects of the 677T variant on negative symptoms. Here, we also found a trend-level effect of MTHFR 1298A>C polymorphism, which is also hypofunctional but not to the same degree as 677C>T.29 Both the 677T and 1298C alleles have been associated with significant increases in schizophrenia risk in a recent large meta-analysis using the SZGene database10; however, as of March 2011, only the 677T allele remained significant in SZGene (N = 20 studies comprising 4362 patients and 5840 controls; OR 1.15; 95% CI 1.04–1.26). The 2 MTFHR polymorphisms are in linkage disequilibrium and hence are not statistically independent with regard to association analyses; however, because each variant induces its own well-characterized functional effect on the level of MTHFR activity, it is reasonable to consider them separately when tallying risk allele load.
The COMT 675G>A variant, which has been consistently implicated in prefrontal function in brain imaging studies30,31 but not in schizophrenia risk,10 was included in the regression analysis due to its previously reported interactive effects with MTHFR 677C>T on executive dysfunction18 and related prefrontal impairment19 in schizophrenia and on homocysteine metabolism.20 Here, we observed a detrimental effect of the low-activity COMT 675A (158Met) allele on negative symptom severity, in contrast to our previous work which implicated the high activity 675G (158Val) allele in executive dysfunction. It is equally noteworthy that a study examining cumulative effects of COMT 675G>A and MTHFR 677C>T on schizophrenia risk in a Dutch cohort32 implicated the same combination of the low-activity alleles (675A and 677T) as in the present report, although this finding failed to replicate in a Korean cohort.33 The molecular interactions of COMT with MTHFR and other genes in the folate pathway are likely complex, and different combinations of MTHFR and COMT genotypes have been found to exacerbate hyperhomocysteinemia,20 preeclampsia risk,34 and low putamen volumes in geriatric depression.35 While the nature of these interactions may vary by context, the results of the present study may be more straightforward to interpret, given that they consistently associate detrimental effects with low-functioning variants across the folate metabolic pathway.
Although common genetic variants may contribute approximately one-third of the total genetic liability in schizophrenia,36 effects of individual variants are small, and many variants that show consistent replication in candidate gene studies are still not strong enough to reach genome-wide significance. Understanding how variants of small effect combine to exert clinically meaningful influences on schizophrenia phenotypes will be critical in deciphering the genetic architecture of the disorder. Increasingly, genome wide association studies and other high-throughput genetic investigations are relying on metabolic pathway analyses in order to pool risk variants into biologically meaningful contexts.37,38 In this study, we have used an analogous—albeit “bottom-up” rather than “top-down”—approach to explore how genetic variants across a single implicated metabolic pathway contribute to negative symptom risk in schizophrenia. Our results suggest that patients who possess a greater number of hypofunctional variants in the folate pathway are at particularly high risk for negative symptoms, perhaps reflecting a cumulative effect of these variants on downstream methylation reactions. Using a similar approach, other investigators have identified pooled effects of folate-related genes on cancer risk.39,40 The approach of canvassing genetic variants in implicated biological pathways to generate cumulative risk scores holds promise in resolving the so-called “missing heritability” in schizophrenia and other complex genetic disorders in psychiatry41–just as in the present study, where the net effects of folate-related variants outweighed the influence of MTHFR 677C>T alone on negative symptom severity.
Our previous finding that the MTHFR 677T allele was protective against positive symptoms was not supported in this larger cohort, which is not surprising given the lack of association between folate level and positive symptoms.7 The reasons for a selective effect of folate-related genes on negative symptoms remain unknown and merit further examination. It also remains possible that other genes that regulate methylation could contribute to heterogeneity of positive symptoms in schizophrenia. Of note, several older studies (reviewed by Grayson et al42) found that administration of methionine, a methyl donor, to patients with schizophrenia induced a worsening of positive symptoms in some patients and had no effect on others. Larger studies that are powered to interrogate broader systems of methylation-related genes in schizophrenia may help clarify how methylation status influences positive vs negative symptoms.
Several limitations in the present study should be acknowledged. First, the size of the cohort is relatively small, especially for the folate analysis, and as with all genetic association studies, findings should be viewed as preliminary until replicated. At the same time, the study was strongly hypothesis driven, and the results are consistent with a considerable literature implicating abnormal folate metabolism in schizophrenia. Second, only a limited number of variants in the folate-related genes of interest were studied, reflecting the small cohort size. However, each variant conferred a change in the amino acid sequence in the associated protein, and in most cases, functional correlates and/or associations with schizophrenia risk had previously been established. Third, subjects varied with regard to psychotropic medication use, a potential concern given the known effects of anticonvulsant medications on folate metabolism. The lack of a difference in anticonvulsant use between high and low folate subjects, and the lack of an anticonvulsant effect on the relationship between genotype and negative symptoms diminishes this concern.
With these limitations in mind, the present findings support the role of heritable variation in folate metabolism in negative symptoms of schizophrenia. They suggest that even among patients who carry multiple risk alleles, negative symptoms can be ameliorated in the presence of elevated serum folate levels. Additional work is needed to examine the utility of folate supplementation for negative symptoms in schizophrenia, especially among patients who may be predisposed to risk due to genetic variation in their folate metabolic profile.
Funding
Harvard-Massachusetts Institute of Technology Division of Health Science and Technology Clinical Investigator Training Program, Howard Hughes Medical Institute Early Career Physician Scientist Award, and Charles King Trust/Campbell and Hall/Alden Trust (to J.L.R.); National Institute of Mental Health (MH079799 to J.W.S. and MH0060450, MH070831, MH002025 to D.C.G.) and a NARSAD Independent Investigator Award to D.C.G.
Acknowledgments
Disclosures: Drs D.C.G. and J.L.R. have submitted a US patent application concerning the effects of MTHFR, MTR, and FOLH1 on response to folate supplementation in schizophrenia. Dr D.C.G. reports having served as a consultant or advisor to: Xytis, Forest Labs, Pfizer, Indevus Pharmaceuticals, H. Lundbeck, Schering-Plough, Eli Lilly, Takeda, Biovail, Solvay, Hoffman-La Roche, Cypress, and Dainippon Sumitomo. He received grant support from PamLab, Pfizer, Janssen, Novartis, and GlaxoSmithKline. Dr J.L.R. has received fellowship support from the Harvard-MIT Division of Health Sciences Clinical Investigator Training Program, which receives funding through unrestricted educational grants from Merck and Pfizer. No other authors report biomedical financial interests or potential conflicts of interest. Presented in part at the American College of Neuropsychopharmacology 2009 Annual Meeting.
References
- 1.Frankenburg FR. The role of one-carbon metabolism in schizophrenia and depression. Harv Rev Psychiatry. 2007;15:146–160. doi: 10.1080/10673220701551136. [DOI] [PubMed] [Google Scholar]
- 2.Greene ND, Stanier P, Copp AJ. Genetics of human neural tube defects. Hum Mol Genet. 2009;18:R113–R129. doi: 10.1093/hmg/ddp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.St Clair D, Xu M, Wang P, et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959-1961. JAMA. 2005;294:557–562. doi: 10.1001/jama.294.5.557. [DOI] [PubMed] [Google Scholar]
- 4.Brown AS, Bottiglieri T, Schaefer CA, et al. Elevated prenatal homocysteine levels as a risk factor for schizophrenia. Arch Gen Psychiatry. 2007;64:31–39. doi: 10.1001/archpsyc.64.1.31. [DOI] [PubMed] [Google Scholar]
- 5.Herran A, Garcia-Unzueta MT, Amado JA, Lopez-Cordovilla JJ, Diez-Manrique JF, Vazquez-Barquero JL. Folate levels in psychiatric outpatients. Psychiatry Clin Neurosci. 1999;53:531–533. doi: 10.1046/j.1440-1819.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- 6.Muntjewerff JW, van der Put N, Eskes T, et al. Homocysteine metabolism and B-vitamins in schizophrenic patients: low plasma folate as a possible independent risk factor for schizophrenia. Psychiatry Res. 2003;121:1–9. doi: 10.1016/s0165-1781(03)00200-2. [DOI] [PubMed] [Google Scholar]
- 7.Goff DC, Bottiglieri T, Arning E, et al. Folate, homocysteine, and negative symptoms in schizophrenia. Am J Psychiatry. 2004;161:1705–1708. doi: 10.1176/appi.ajp.161.9.1705. [DOI] [PubMed] [Google Scholar]
- 8.Levine J, Stahl Z, Sela BA, et al. Homocysteine-reducing strategies improve symptoms in chronic schizophrenic patients with hyperhomocysteinemia. Biol Psychiatry. 2006;60:265–269. doi: 10.1016/j.biopsych.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Godfrey PS, Toone BK, Carney MW, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336:392–395. doi: 10.1016/0140-6736(90)91942-4. [DOI] [PubMed] [Google Scholar]
- 10.Allen NC, Bagade S, McQueen MB, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 11.Kempisty B, Sikora J, Lianeri M, et al. MTHFD 1958G>A and MTR 2756A>G polymorphisms are associated with bipolar disorder and schizophrenia. Psychiatr Genet. 2007;17:177–181. doi: 10.1097/YPG.0b013e328029826f. [DOI] [PubMed] [Google Scholar]
- 12.Goff DC, Heckers S, Freudenreich O. Schizophrenia. Med Clin North Am. 2001;85:663–689. doi: 10.1016/s0025-7125(05)70335-7. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 14.Mohamed S, Rosenheck R, Swartz M, Stroup S, Lieberman JA, Keefe RS. Relationship of cognition and psychopathology to functional impairment in schizophrenia. Am J Psychiatry. 2008;165:978–987. doi: 10.1176/appi.ajp.2008.07111713. [DOI] [PubMed] [Google Scholar]
- 15.Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 16.Roffman JL, Weiss AP, Purcell S, et al. Contribution of methylenetetrahyrdofolate reductase (MTHFR) polymorphisms to negative symptoms in schizophrenia. Biol Psychiatry. 2008;63:42–48. doi: 10.1016/j.biopsych.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Hill M, Shannahan K, Jasinski S, et al. Folate supplementation in schizophrenia: a possible role for MTHFR genotype. Schizophr Res. 2011;127:41–45. doi: 10.1016/j.schres.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Roffman JL, Weiss AP, Deckersbach T, et al. Interactive effects of COMT Val108/158Met and MTHFR C677T on executive function in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2008;147:990–995. doi: 10.1002/ajmg.b.30684. [DOI] [PubMed] [Google Scholar]
- 19.Roffman JL, Gollub RL, Calhoun VD, et al. MTHFR 677C --> T genotype disrupts prefrontal function in schizophrenia through an interaction with COMT 158Val --> Met. Proc Natl Acad Sci U S A. 2008;105:17573–17578. doi: 10.1073/pnas.0803727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tunbridge EM, Harrison PJ, Warden DR, Johnston C, Refsum H, Smith AD. Polymorphisms in the catechol-O-methyltransferase (COMT) gene influence plasma total homocysteine levels. Am J Med Genet B Neuropsychiatr Genet. 2008;147:996–999. doi: 10.1002/ajmg.b.30700. [DOI] [PubMed] [Google Scholar]
- 21.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 22.Sharp L, Little J. Polymorphisms in genes involved in folate metabolism and colorectal neoplasia: a HuGE review. Am J Epidemiol. 2004;159:423–443. doi: 10.1093/aje/kwh066. [DOI] [PubMed] [Google Scholar]
- 23.Friso S, Choi SW, Girelli D, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci U S A. 2002;99:5606–5611. doi: 10.1073/pnas.062066299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halsted CH, Wong DH, Peerson JM, et al. Relations of glutamate carboxypeptidase II (GCPII) polymorphisms to folate and homocysteine concentrations and to scores of cognition, anxiety, and depression in a homogeneous Norwegian population: the Hordaland Homocysteine study. Am J Clin Nutr. 2007;86:514–521. doi: 10.1093/ajcn/86.2.514. [DOI] [PubMed] [Google Scholar]
- 25.Bacich DJ, Pinto JT, Tong WP, Heston WD. Cloning, expression, genomic localization, and enzymatic activities of the mouse homolog of prostate-specific membrane antigen/NAALADase/folate hydrolase. Mamm Genome. 2001;12:117–123. doi: 10.1007/s003350010240. [DOI] [PubMed] [Google Scholar]
- 26.Bertolino A, Nawroz S, Mattay VS, et al. Regionally specific pattern of neurochemical pathology in schizophrenia as assessed by multislice proton magnetic resonance spectroscopic imaging. Am J Psychiatry. 1996;153:1554–1563. doi: 10.1176/ajp.153.12.1554. [DOI] [PubMed] [Google Scholar]
- 27.Marenco S, Bertolino A, Weinberger DR. In vivo NMR measures of NAA and the neurobiology of schizophrenia. Adv Exp Med Biol. 2006;576:227–240. doi: 10.1007/0-387-30172-0_16. discussion 361–223. [DOI] [PubMed] [Google Scholar]
- 28.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lievers KJ, Boers GH, Verhoef P, et al. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med. 2001;79:522–528. doi: 10.1007/s001090100253. [DOI] [PubMed] [Google Scholar]
- 30.Roffman JL, Weiss AP, Goff DC, Rauch SL, Weinberger DR. Neuroimaging-genetic paradigms: a new approach to investigate the pathophysiology and treatment of cognitive deficits in schizophrenia. Harv Rev Psychiatry. 2006;14:78–91. doi: 10.1080/10673220600642945. [DOI] [PubMed] [Google Scholar]
- 31.Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 32.Muntjewerff JW, Gellekink H, den Heijer M, et al. Polymorphisms in catechol-O-methyltransferase and methylenetetrahydrofolate reductase in relation to the risk of schizophrenia. Eur Neuropsychopharmacol. 2007;18:99–106. doi: 10.1016/j.euroneuro.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Kang HJ, Choe BM, Kim SH, et al. No association between functional polymorphisms in COMT and MTHFR and schizophrenia risk in korean population. Epidemiol Health. 2010;32:e2010011. doi: 10.4178/epih/e2010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill LD, York TP, Kusanovic JP, et al. Epistasis between COMT and MTHFR in maternal-fetal dyads increases risk for preeclampsia. PLoS One. 2011;6:e16681. doi: 10.1371/journal.pone.0016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan CC, McQuoid DR, Taylor WD, Payne ME, Ashley-Koch A, Steffens DC. Association analysis of the COMT/MTHFR genes and geriatric depression: an MRI study of the putamen. Int J Geriatr Psychiatry. 2009;24:847–855. doi: 10.1002/gps.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mill J, Tang T, Kaminsky Z, et al. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet. 2008;82:696–711. doi: 10.1016/j.ajhg.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Dushlaine C, Kenny E, Heron E, et al. Molecular pathways involved in neuronal cell adhesion and membrane scaffolding contribute to schizophrenia and bipolar disorder susceptibility. Mol Psychiatry. 2011;16:286–292. doi: 10.1038/mp.2010.7. [DOI] [PubMed] [Google Scholar]
- 39.Rouissi K, Ouerhani S, Oliveira E, et al. Polymorphisms in one-carbon metabolism pathway genes and risk for bladder cancer in a Tunisian population. Cancer Genet Cytogenet. 2009;195:43–53. doi: 10.1016/j.cancergencyto.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Wang M, Zhu H, Fu G, Zhang Z, Lu Q, Wang S. Polymorphisms of methylenetetrahydrofolate reductase and methionine synthase genes and bladder cancer risk: a case-control study with meta-analysis. Clin Exp Med. 2009;9:9–19. doi: 10.1007/s10238-008-0013-1. [DOI] [PubMed] [Google Scholar]
- 41.Maher B. Personal genomes: the case of the missing heritability. Nature. 2008;456:18–21. doi: 10.1038/456018a. [DOI] [PubMed] [Google Scholar]
- 42.Grayson DR, Chen Y, Dong E, Kundakovic M, Guidotti A. From trans-methylation to cytosine methylation: evolution of the methylation hypothesis of schizophrenia. Epigenetics. 2009;4:144–149. doi: 10.4161/epi.4.3.8534. [DOI] [PubMed] [Google Scholar]