Abstract
Striatal dysfunction is thought to be a fundamental element in schizophrenia. Striatal dopamine dysfunction impacts on reward processing and learning and is present even at rest. Here, we addressed the question whether and how spontaneous neuronal activity in the striatum is altered in schizophrenia. We therefore assessed intrinsic striatal activity and its relation with disorder states and symptom dimensions in patients with schizophrenia. We performed resting-state functional (rs-fMRI) and structural magnetic resonance imaging as well as psychometric assessment in 21 schizophrenic patients during psychosis. On average 9 months later, we acquired follow-up data during psychotic remission and with comparable levels of antipsychotic medication. Twenty-one age- and sex-matched healthy controls were included in the study. Independent component analysis of fMRI data yielded spatial maps and time-courses of coherent ongoing blood-oxygen-level-dependent signal fluctuations, which were used for group comparisons and correlation analyses with scores of the positive and negative syndrome scale. During psychosis, coherent intrinsic activity of the striatum was increased in the dorsal part and correlated with positive symptoms such as delusion and hallucination. In psychotic remission of the same patients, activity of the ventral striatum was increased and correlated with negative symptoms such as emotional withdrawal and blunted affect. Results were controlled for volumetric and medication effects. These data provide first evidence that in schizophrenia intrinsic activity is changed in the striatum and corresponds to disorder states and symptom dimensions.
Keywords: schizophrenia, psychosis, intrinsic brain activity, resting-state fMRI, striatum
Introduction
Striatal dysfunction is thought to be a fundamental element in schizophrenia.1 Especially, dopamine transmission in the striatum is increased during prodromal and psychotic states.2,3 Such elevated dopamine levels correlate with positive disease symptoms and antidopaminergic drugs reduce these symptoms in most patients.4
It is still unclear, how striatal dopamine relates to brain activity in humans, but animal experiments revealed that increased dopamine modulates spontaneous activity in the striatum.5,6 Furthermore, theoretical accounts suggest that changed spontaneous brain activity contributes to psychotic symptoms by worsening the signal-to-noise ratio (SNR) of evoked and intrinsic activity.7,8 In particular, noisy striatal signals are assumed to contribute to aberrant salience processing and disrupted reinforcement learning that both underlie positive symptoms in schizophrenia such as delusions.9–11
These data suggest that an investigation of intrinsic brain activity in humans might help to better understand the pathophysiology of schizophrenia. Few studies reported on global connectivity changes in the intrinsic functional architecture of schizophrenic patients.12 Others have concentrated on cortical networks of intrinsic activity and found distributed changes in frontal, temporal, and parietal cortices.13–16 However, in vivo evidence for specifically altered intrinsic activity in the resting-state signal of the striatum is missing.
In order to test for aberrant intrinsic striatal activity, we measured ongoing hemodynamic signal fluctuations with fMRI during a 10-minutes rest period (rs-fMRI) in patients with schizophrenia and healthy controls (HCs). Patients were assessed during psychosis and during psychotic remission. We decomposed the rs-fMRI data with independent component analysis (ICA) into spatially independent maps of intrinsically coupled brain regions with associated blood-oxygen-level-dependent (BOLD) signal fluctuations.17 From these spatial maps, we selected a previously described basal ganglia resting-state network (BGN) including the striatum.18 Additionally, we selected any further intrinsic cortical network with striatal contributions. The ICA approach allows the simultaneous exploration of intrinsic connectivity changes of the striatum within several networks.19 We controlled our analyses of intrinsic functional connectivity for medication effects and structural changes.
We addressed the following questions: (1) is intrinsic striatal activity changed in schizophrenia; (2) are potential changes modulated by psychosis, which is characterized by hyperdopaminergia1; and (3) are changes related to symptom dimensions of schizophrenia.
Methods
Participants and Task
Twenty-one patients and 21 HCs participated in the study (table 1). All participants provided informed consent in accordance with the Human Research Committee guidelines of the Klinikum Rechts der Isar, Technische Universität München. Patients were recruited from the Department of Psychiatry, Klinikum Rechts der Isar, TU München, controls by word of mouth advertising. Participants’ examination included medical history, psychiatric interview, psychometric assessment, and blood tests for patients (all performed by D.S. and M.S.). Psychiatric diagnoses were based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV).20 The Structured Clinical Interview for DSM-IV (SCID-I German version) was used to assess the presence of psychiatric diagnoses. The severity of clinical symptoms was measured with the positive and negative syndrome scale (PANSS)21 on the day of scanning. D.S. and M.S. have been professionally trained for SCID and PANSS-based interviews with interrater reliability for diagnoses and scores of more than 95%. The global level of social, occupational, and psychological functioning was measured with the Global Assessment of Functioning Scale.22
Table 1.
Demographic and Clinical Characteristics
| HC (n = 21) | SP (n = 21) | SPR (n = 13) | SP (n = 21) vs HC (n = 21)a | SPR (n = 13) vs HC (n = 13)a | SP (n = 13) vs SPR (n = 13)b | ||||
| Measure | Mean (SD) | Mean (SD) | Mean (SD) | T Score | P Value | T Score | P Value | T Score | P Value |
| Age | 33.57 (13.6) | 34.05 (12.27) | 33.69 (10.53) | −0.121 | 0.904 | −0.330 | 0.745 | ||
| Sex (male/female) | 10/11 | 10/11 | 9/4 | ||||||
| PANSS | |||||||||
| Total | 30.14 (0.65) | 80.76 (20.77) | 52.75 (13.93) | 8.96 | .000* | 3.240 | .004* | 6.466 | .000* |
| Positive | 7.05 (0.22) | 19.4 (6.09) | 11.92 (3.63) | 9.091 | .000* | 4.801 | .000* | 3.212 | .008* |
| Negative | 7 10 (0.44) | 21.14 (8.20) | 13.58 (5.63) | 7.84 | .000* | 4.102 | .000* | 3.345 | .007* |
| General | 16.05 (0.22) | 39.81 (11.06) | 27.25 (8.30) | 9.846 | .000* | 4.858 | .000* | 4.473 | .001* |
| GAF | 99.76 (1.09) | 39.62 (11.68) | 59.25 (14.44) | −23.492 | .000* | −10.046 | .000* | −3.627 | .004* |
| CPZ | 388.61 (384.67) | 206.95 (189.67) | 1.281 | .227 | |||||
Note: HC, healthy control group; SP, group of patients with schizophrenia during psychosis; SPR, group of patients with schizophrenia during psychotic remission; PANSS, Positive and Negative Syndrome Scale; GAF, Global Assessment of Functioning Scale; CPZ, chlorpromazine equivalent dose.
Two-sample t test.
Paired t test.
*Significant for P < .05 corrected for multiple comparisons.
All patients were diagnosed with schizophrenia. Further inclusion criteria were an age between 18 and 60 years, current psychotic symptoms for the fMRI-session during psychosis (SP) and remission of psychotic symptoms (indicated by significantly decreased PANSS scores) for the fMRI-session during psychotic remission (SPR). Patients were inpatient during SP and ambulatory during SPR. On average about 9 months after psychosis (t mean = 285.84 days, SD = 167.66), 13 of 21 patients approved a second investigation during remission. The 8 remaining patients that also were diagnosed as remitted from psychosis by external psychiatrists could not be convinced of an additional reexamination and rescanning. Importantly, the subsample of patients that was reexamined did not differ from the initial patient group with respect to demographical or medication parameters (table 1) but had significantly reduced PANSS scores.
Patients were free of any current or past neurological or internal systemic disorder, current depressive or manic episode, substance abuse (except nicotine), and cerebral pathology in MRI. Three of 21 patients during psychosis and 4 of 13 patients during psychotic remission were free of antipsychotic medication. All other patients received mono- or dual therapy with atypical antipsychotic medication, including Amilsupride (2 cases during SP/1 case during SPR), Olanzapine (11/1), Clozapine (4/3), Quetiapine (2/3), Ziprasidone (1/0), Risperidone (5/2), Aripiprazole (2/1), and Paliperidone (3/1) (see online supplementary table S1 for individual medication protocols and dosage; see also table 1 for mean chlorpromazine (CPZ) equivalent dose23). Importantly, CPZ did not differ significantly between SP and SPR. All controls were free of any current or past psychiatric, neurological or systemic disorder, or psychotropic medication.
All participants underwent 10 minutes of rs-fMRI with the instruction to keep their eyes closed and not to fall asleep. We verified that subjects stayed awake by interrogating via intercom immediately after the rs-fMRI scan. Before and after scanning, a medical examination of patients validated their stable condition and investigated whether they had feelings of odd situations during the scanning. No patient dropped out during the first scanning session. HCs were scanned only once to define the range of normal BGN coherence; thus, it was not possible to directly test for schizophrenia-specific changes between first and second scan. However, several independent rs-fMRI studies in HCs confirmed the consistency of rs-fMRI networks across days and months.24,25
FMRI Data Acquisition and Analysis
MRI was performed on a 3 T whole-body MR scanner (Achieva; Philips, Netherland) using an 8-channel phased-array head coil. For coregistration and volumetric analysis, T1-weighted anatomical data were obtained from each subject by using a magnetization-prepared rapid acquisition gradient echo sequence (echo time [TE] = 4 ms, time of repetition [TR] = 9 ms, inversion time [TI] = 100 ms, flip angle = 5°, field of view [FoV] = 240 × 240 mm2, matrix = 240 × 240, 170 slices, voxel size = 1 × 1 × 1 mm3). FMRI data were collected using a gradient echo planar imaging sequence (TE = 35 ms, TR = 2000 ms, flip angle = 82°, FoV = 220 × 220 mm2, matrix = 80 × 80, 32 slices, slice thickness = 4 mm, and 0 mm interslice gap; 10 minutes of scanning result in 300 volumes).
For each participant, the first 3 functional scans of each fMRI-session were discarded due to magnetization effects. Statistical parametric mapping 8 (SPM8) (Wellcome Department of Cognitive Neurology, London) was used for motion correction, spatial normalization into the stereotactic space of the Montreal Neurological Institute and spatial smoothing with an 8 × 8 × 8 mm Gaussian kernel. None of the participants had to be excluded due to excessive head motion (linear shift <3 mm across run and on a frame-to-frame basis, rotation <1.5°). We also verified nonsignificant differences in the SNR ratio of the fMRI data between healthy subjects (mean: 47.27, SD: 10.79) and patient group (mean: 46.21, SD: 11.6) with P = .76.
Preprocessed data were decomposed into 40 spatial independent components within a group ICA framework,17 which is based on the infomax algorithm and implemented in the GIFT-software (http://icatb.sourceforge.net). Dimensionality estimation was performed by using the minimum description length criteria and resulted in 40 components representing the mean of all individual estimates. Before volumes were entered into ICA analysis, voxel-wise z-transformation on time course data yijk(t) was applied by subtracting the mean and dividing by the SD σijk (, t time, i,j,k directions in space). The sensitivity of the multivariate ICA algorithm for correlation of variance between voxels, ie, functional connectivity, was thereby rendered independent of the original BOLD signal magnitude across subjects. Data were concatenated and reduced by 2-step principal component analysis, followed by independent component estimation with the infomax algorithm. We subsequently ran 40 ICA (ICASSO) to ensure stability of the estimated components. This results in a set of average group components, which are then back reconstructed into single-subject space. We then applied a multiple spatial regression with a mask containing caudate nucleus and putamen to the 40 independent components to automatically select those including the striatum (figure 1, supplementary figure S1 and tables S2 and S3). The mask was generated with the WFU-Pickatlas (http://www.fmri.wfubmc.edu/). Before we entered the individual’s spatial maps into second-level statistics, we reintegrated the initially calculated scaling factor σijk into the data by voxel-wise multiplication in order to preserve each individual’s profile of variance magnitude while leaving the normalized time course component unchanged.26
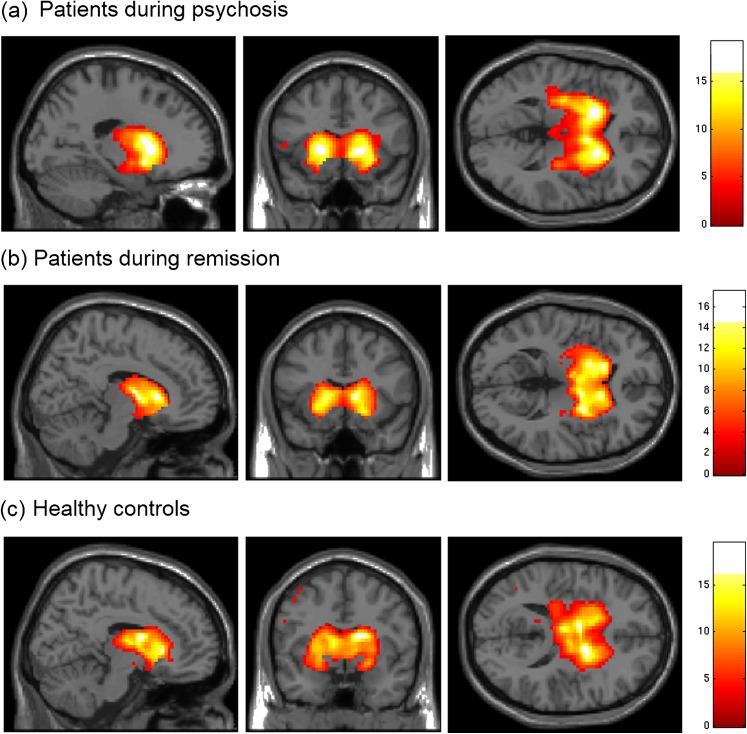
Fig. 1.
Spatial maps of coherent intrinsic activity within the basal ganglia network (BGN). After independent component analysis of resting-state fMRI data, spatial maps of single-subject ICs representing the BGN were entered into voxel-wise one-sample t tests across individuals of each group and thresholded at P < .05, corrected for false discovery rate. Statistical parametric maps representing brain areas with significantly covarying activity were superimposed on a single-subject high-resolution T1 image (color scale representing z values from 0 to 16). The BGN includes the striatum, pallidum, and thalamus. (a) Patients during psychosis, (b) patients during psychotic remission, and (c) healthy controls.
To statistically evaluate spatial maps of selected independent components (ICs), we calculated voxel-wise one-sample t tests on participants’ reconstructed spatial maps for each group and session, using SPM8 (P < .05, false discovery rate [FDR], figure 1). To analyze group effects, participants’ spatial maps were entered into two-sample t tests with striatal volumes as covariate of no interest when comparing patients with HC (P < .05 FDR-corrected, figure 2, supplementary table S4). To evaluate the temporal aspect of selected ICs, we investigated the frequency distribution of each IC’s time course. The power spectral density of each participant’s time course for each session was calculated and then averaged across frequencies ranging from 0.01 to 0.1 Hz. Differences across groups were assessed by using two-sample t tests (supplementary figure S2).
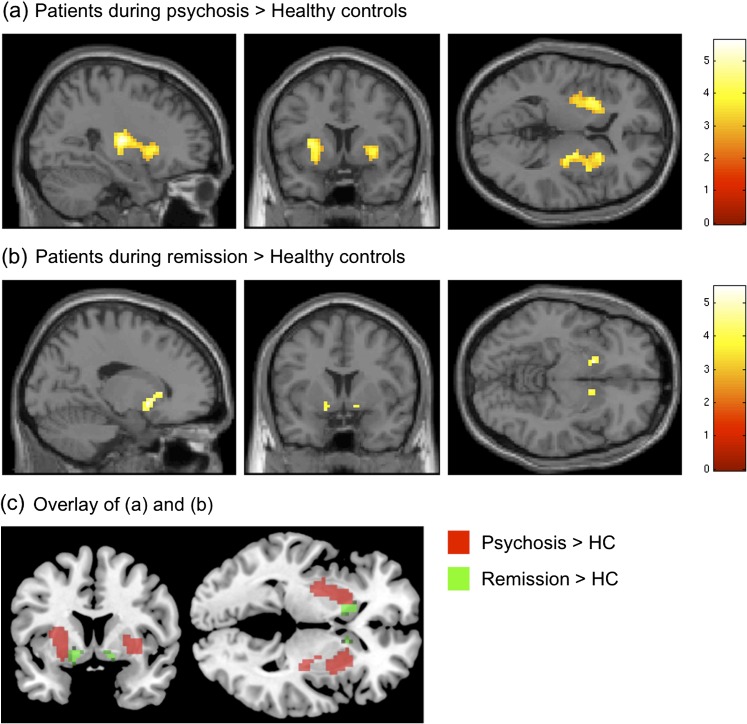
Fig. 2.
Increasingly synchronized intrinsic activity in distinct locations of the striatum depends on disorder state. Statistical parametric maps (SPMs) of brain areas with significantly increased covarying activity in patients. (a) Two-sample t test between patients during psychosis and healthy controls (HCs), Montreal Neurological Institute (MNI)-coordinates [x,y,z] of the SPM: [27,12,4]; (b) Two-sample t test between patients during psychotic remission and HCs, MNI-coordinates: [17,5,−8]; (c) Overlay of (a) and (b) reveals spatially distinct subregions within the striatum, MNI-planes (y,z): [−7,−1]. Striatal volume was entered as covariate of no interest. All t tests were thresholded at P < .05 and corrected for false discovery rate. SPMs were superimposed on a single-subject high-resolution T1 image (color scale representing z values from 0 to 6).
The relation between striatal activity and symptom dimensions was studied within a region of interest (ROI)–based approach. ROI-restricted z scores (derived from subjects’ BGN-ICs) were partially correlated with PANSS scores in patients with striatal volume and antipsychotic medication CPZ as regressors of no interest (partial correlation, P < .05; figure 3, table 1). Striatal ROIs were created by using the MARSBAR-toolbox (Release 0.42, http://marsbar.sourceforge.net/). Centers of spheric ROIs with 6 mm radius were derived from the study of Martinez and colleagues,27 including left and right associative striatum (±24, 12, 0) and left and right limbic striatum (±12, 9, −9). For patients’ BGN-IC of each session, z scores were extracted for each ROI and averaged across each bilateral ROI-pair.
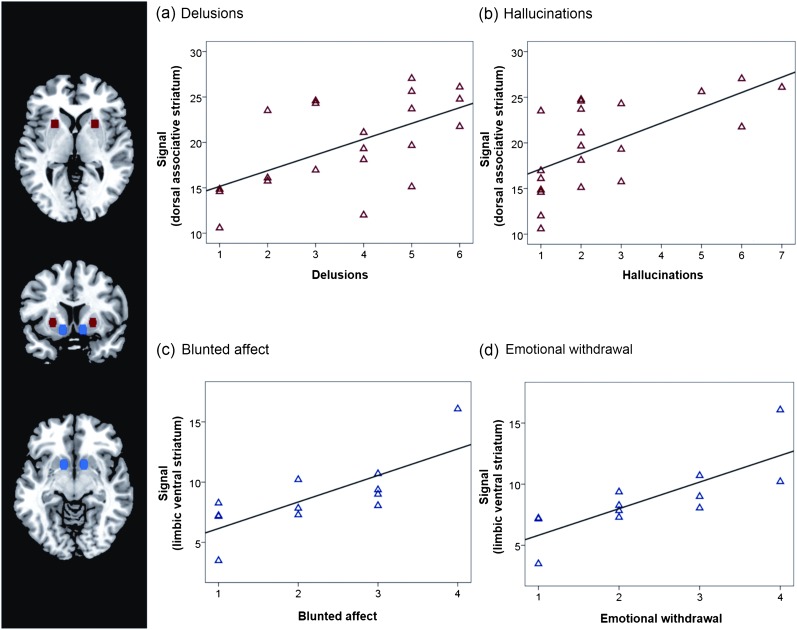
Fig. 3.
Positive and negative symptoms of schizophrenia are related to intrinsic activity in dorsal and ventral parts of the striatum. In patients, the positive syndrome scale of positive and negative syndrome scale (PANSS) correlated with intrinsic activity in the dorsal striatum, while the negative syndrome scale was related to activity in the ventral striatum. In this figure, PANSS subscores responsible for the overall syndrome-brain relationship are plotted. From the positive syndrome scale, only (a) delusions and (b) hallucinations were significantly correlated with coherent activity in the dorsal striatum (red region of interest [ROI], Montreal Neurological Institute (MNI)-coordinates: ±24,12,027) during psychosis (P < .05). The negative scores (c) blunted affect and (d) emotional withdrawal were significantly correlated with activity in the ventral striatum (blue ROI, MNI: ±12,9,−927) during psychotic remission (P < .05). ROI-signals were calculated as averaged z scores across left/right ROI from single-subject ICs of the basal ganglia network (figure 1). Partial correlations were corrected for striatal volume and antipsychotic medication (CPZ).
Voxel-Based Morphometry Analysis
In order to control any functional connectivity results for potentially structural alterations, we calculated an averaged volumetric score for the striatum and included that as a covariate of no interest. For data preprocessing and analysis, the voxel-based morphometry 8 (VBM8) toolbox (http://dbm.neuro.uni-jena.de/vbm.html) was used. Images were corrected for bias-field inhomogeneity, registered using linear (12-parameter affine) and nonlinear transformations, and tissue classified into gray matter (GM), white matter, and cerebrospinal fluid within the same generative model.28 The resulting GM images were modulated to account for volume changes resulting from the normalization process. Here, we only considered nonlinear volume changes so that further analyses did not have to account for differences in head size. Finally, images were smoothed with a Gaussian kernel of 8 mm (FWHM). We then calculated an averaged score for bilateral ventral and dorsal striatum and tested that for group differences. Additionally, we included this averaged VBM score as covariate of no interest in the above-described functional analysis of group differences across spatial IC maps.
Results
The Basal Ganglia Network Includes the Ventral and Dorsal Striatum
For each subject, ICA of fMRI data resulted in spatial maps, displayed in z scores, and associated time course values. Together, these measures represent the relative degree to which the component contributes to the overall BOLD signal at a given time point. Automated component selection by spatially regressing a striatum mask with all spatial maps revealed a subcortical basal ganglia network (BGN) in each individual (supplementary table S2). This BGN was spatially consistent across groups and sessions, matched previous results,18 and included the striatum (nucleus caudatus, putamen), globus pallidus, and thalamus (figure 1, one-sample t test, P < .05, correction for false discovery rate). Additionally, 3 further ICs with cortical centers of mass had minor striatal contribution (supplementary figure S1 and tables S2 and S3).
Spatial Maps of Coherent Intrinsic Brain Activity Reflect Psychosis and Psychotic Remission in Distinct Parts of the Striatum
Compared with controls, psychotic patients showed increased activity in the associative and sensorimotor striatum of the BGN (figure 2 and supplementary table S4, two-sample t test, P < .05 FDR, corrected for striatal volume). During psychotic remission, the same patients showed stronger activity in bilateral limbic striatum (figure 2 and supplementary table S4, two-sample t test, P < .05 FDR, corrected for striatal volume) when compared with HC. The 3 cortical ICs with striatal involvement did not differ across groups and sessions (P < .001 uncorrected).
Spectral Power of Time Courses of Synchronous Activity Was Changed During Psychotic Remission
As a complementary measure to the spatial information provided by component maps, we estimated the spectral power of BGN-time courses, averaged for frequencies from 0.01 to 0.1 Hz, and compared them across groups and sessions (supplementary figure S2; two-sample t tests for comparisons with controls, paired t test for comparisons across sessions). Patients in psychotic remission had significantly reduced power compared with controls (P = .02). Patients during psychosis had a trend for reduced power compared with controls (P = .08). Again, the other ICs with striatal involvement did not differ across groups and sessions for temporal aspects of striatal activity.
Intrinsic Striatal Activity Predicted Positive and Negative Symptoms
To study the relation between increased striatal activity and symptom dimensions, we correlated ROI-restricted z scores with total PANSS scores in patients. The coordinates of ROIs for functional subdivisions of the striatum (limbic/ventral and associative/dorsal) were independently derived from a previous brain imaging study.27 During psychosis, we found that synchronous activity of the dorsal striatum correlated with total positive (r = .53, P < .05) but not with negative (r = .02) symptoms (partial correlation, corrected for striatal volume and medication (CPZ), Bonferroni corrected for 4 tests); during psychotic remission, the correlation between activity in the ventral striatum and total negative symptoms demonstrated a trend to significance (r = .35, P = .09 corrected), while there was no relation to positive symptoms (r = −.07). Post hoc analyses of correlations with PANSS subscores revealed that the severity of delusions and hallucinations during psychosis positively correlated with activity increases within the associative striatum (figure 3, supplementary table S5, P < .05). During psychotic remission, the severity of blunted affect and emotional withdrawal positively correlated with activity increases within the limbic striatum (figure 3, supplementary table S5, P < .05).
Changes of Striatal Coactivity Are Not Explained by Medication or Striatal Volume Changes
Finally, we investigated whether medication or structural changes in the striatum influence our result of coactivity changes. For each subject, we summarized his/her medication in terms of chlorpromazine equivalents (CPZ) and tested these for differences across disease states and for any correlation with striatal connectivity measures. We found no difference between psychosis and psychotic remission for the patient group (paired t test, P < .05, see table 1). Furthermore, the voxel-wise correlation analysis—restricted to the striatum—between z scores reflecting coactivity and CPZs across patients during psychosis and psychotic remission respectively revealed no significant correspondence (P < .05, FDR-corrected). Also, the analysis of volumetric data revealed no significant group differences for the striatum between healthy subjects and patients (P = .57).
Discussion
This study revealed changed intrinsic striatal activity in patients with schizophrenia. Changes were modulated by psychosis and related to various symptom dimensions in spatially distinct regions: Coherent intrinsic activity in the dorsal striatum was increased during psychosis and predictive for delusion and hallucination; the ventral striatum, however, showed increased activity during psychotic remission and predicted blunted affect and emotional withdrawal in the same patients. These findings extend our knowledge about striatal dysfunction in schizophrenia and suggest a link between intrinsic activity, symptom dimensions, and possibly striatal dopamine dysfunction.
The Link Between Changed Intrinsic Activity in the Striatum and Symptom Dimensions in Schizophrenia
The striatum is integrated into multiple cortico—basal ganglia—cortical loops. The ventral part projects into limbic, the associative part into associative, and the sensorimotor part into sensorimotor cortices.29,30 Activity across these corticostriatal loops is coordinated particularly at the striatal level.30 In order to retrieve distinct intrinsic networks with striatal contribution, we decomposed the rs-fMRI data with ICA and subsequently analyzed several components. Voxel-wise z values in a component’s spatial map reflect the strength of functional connectivity to all other parts of this particular network.17,19 While we found no disease-related differences of functional connectivity in the cortical networks covering parts of the basal ganglia, the BGN itself revealed strong changes of coherent intrinsic activity within the striatum.
Dorsal Striatum Activity Correlates With Positive Symptoms
We found that coherent intrinsic activity in the dorsal striatum was increased during psychosis but not during psychotic remission. Moreover, activity in the associative part correlated with positive symptoms and particularly with delusion and hallucination. With respect to regional specificity and behavioral relevance, our result is well in line with previous findings focusing on dopaminergic dysfunctions.2,3 For example, Howes and colleagues2 found elevated dopamine function of the associative striatum to be linked with positive signs in patients with prodromal schizophrenia. The consistency between our resting-state fMRI study and previous dopamine-related resting-state positron emission tomography studies in terms of brain-behavior relations suggest a link between intrinsic activity and dopamine dysfunction at rest.
Ventral Striatum Activity Correlates With Negative Symptoms
We found increased coherence of intrinsic activity in the ventral striatum during psychotic remission corresponding to blunted affect and emotional withdrawal in these patients. The ventral striatum is critically involved in emotion processing and reward-based learning.30 In schizophrenia, task-fMRI studies on reward and emotion processing also revealed a link between activity changes in the ventral striatum and negative symptoms.31–33 We interpret the increase of intrinsic connectivity in the ventral striatum with an increased frequency of negative symptom behavior. This is in accordance with rs-fMRI data of healthy subjects where repetitive sensory experiences lead to increased intrinsic connectivity in sensory systems.26 Similarly, patients with major depression (MD) suffer from frequent negative feelings or repetitive behaviors such as rumination. Accordingly, studies on resting-state networks in MD reveal increased intrinsic connectivity in associated brain regions.34
The Link Between Changed Spontaneous Activity in the Striatum and Dopamine Dysfunction in Schizophrenia
We found that increased intrinsic activity in the ventral and dorsal striatum of patients differentially predicts disorder states and symptom dimensions. Our finding of locally diverging dysfunctions in the striatum correspond well with two distinct dopaminergic pathways: dopaminergic cells in the ventral tegmentum release dopamine into the ventral striatum and dopaminergic cells of the substantia nigra project to the dorsal striatum.35 Although direct evidence for an interaction of intrinsic activity and dopamine signaling is still missing, there is experimental evidence for a relationship between dopamine levels and intrinsic activity in the dorsal striatum of animals and humans. In monkeys, iontophoretically applied dopamine modulates the spontaneous firing rate of neurons in the dorsal striatum,6 see also Goto et al.5 In patients suffering from Parkinson’s disease (PD), where dopamine levels in the dorsal striatum are reduced due to substantia nigra degeneration, resting-state fMRI data show an increase of intrinsic activity in the dorsal striatum after administration of levodopa, almost reaching levels of healthy persons.36,37 In schizophrenia, psychosis is linked to elevated striatal dopamine function in the associative part,2,3 which corresponds well to our data of increased intrinsic activity also in the dorsal part of the striatum. Together, these results from various domains suggest a potential link of intrinsic activity and dopamine signaling, at least in the dorsal striatum, which is particularly altered in patients with PD and schizophrenia. However, other neurotransmitter systems and structural changes might also impact on striatal dysfunction and should therefore be investigated with respect to intrinsic brain activity.
Control Parameters and Limitations
The BGN consistently shows low-frequency BOLD signal fluctuations across both groups, a typical characteristic of functionally relevant intrinsic brain networks.19 However, we also found slight differences in the low-frequency power between healthy subjects and patients (supplementary figure S2). While the meaning of alterations in the frequency range is still a matter of discussion,38 a recent study reported decreased low-frequency power in a cortical IC of patients with schizophrenia.14 Together, our data provide evidence for consistent changes of spatial and temporal characteristics of intrinsic striatal activity in schizophrenia.
We also aimed at controlling for several parameters related to the disease that might impact on our findings. We therefore included medication and striatal volume as confounding effects into the analyses.
Medication.
It is important to note that the remitted subsample that we investigated approximately 9 months later only differed with respect to symptom dimensions (PANSS) but not with respect to medication and demographical aspects. Still, drug effects might confound our results from the group analysis compared with HCs. However, 2 recent studies found evidence that (1) the resting-state signal in the striatum of drug-naïve schizophrenic patients is also increased16 suggesting that rs-fMRI effects are rather due to the pathology than due to drug effects. And that (2) dopaminergic substitution in PD increases resting-state connectivity in the striatum.36,37 Therefore, any antidopaminergic medication in our patients should even have a contrary effect on the coherent activity in the striatum.39 Finally, (3) both direction and localization of change in spontaneous activity were in accordance with the literature.3,16,36,37
Striatal Volume.
It is also unlikely that increased striatal spontaneous activity is due to volumetric changes; again, we included striatal volume from a VBM analysis as variable of no interest into statistical analyses. Although a previous study observed volume reduction in the striatum of schizophrenic patients depending on disorder state, medication, and duration of disorder,40 we did not find any changes of striatal volume in our patient group.
A limitation of the study is the fact that we were not able to recruit all 21 patients for another reexamination and rescan during remission. Although all patients were in remission at the time of contact, 8 subjects did not agree to return to the clinic due to several reasons. Furthermore, we did not rescan HCs a second time as various test-retest studies with rs-fMRI data proved the consistency of the intrinsic functional architecture across days and months.24,25 Although we cannot rule out any order effects in the patient group, we conclude that the long-term shift that exclusively occurred in the BGN and not in any other network is unlikely to be caused simply by changes over time.
Conclusions
Overall, we found intriguing similarities between changes of intrinsic activity in the striatum, behavioral symptoms, and previous dopamine findings. Increased coherent intrinsic activity in the dorsal striatum during psychosis is predictive for delusion and hallucination; increased activity during psychotic remission in the ventral striatum is predictive for blunted affect and emotional withdrawal in the same patients. However, further studies exploring both a patient’s dopamine function and intrinsic activity at the same time would be needed to reveal any direct relation between aberrant striatal dopamine function and intrinsic activity.
Funding
This work was supported by the German Federal Ministry of Education and Research (BMBF 01EV0710 to A.M.W.), the Alzheimer Forschung Initiative (AFI 08860 to V.R.), the Kommission für Klinische Forschung of the Klinikum Rechts der Isar der Technischen Universität München (KKF 8765162 to C.S.), and through a bilatgrunn grant from the Norwegian Research Council (to T.E.).
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
We are grateful to the participants of the study and the staff of the Department of Psychiatry and Neuroradiology for their help in recruitment and data collection. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 3.Kegeles LS, Abi-Dargham A, Frankle WG, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 4.Agid O, Mamo D, Ginovart N, et al. Striatal vs extrastriatal dopamine D2 receptors in antipsychotic response—a double-blind PET study in schizophrenia. Neuropsychopharmacology. 2007;32:1209–1215. doi: 10.1038/sj.npp.1301242. [DOI] [PubMed] [Google Scholar]
- 5.Goto Y, Otani S, Grace AA. The Yin and Yang of dopamine release: a new perspective. Neuropharmacology. 2007;53:583–587. doi: 10.1016/j.neuropharm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rolls ET, Thorpe SJ, Boytim M, Szabo I, Perrett DI. Responses of striatal neurons in the behaving monkey. 3. Effects of iontophoretically applied dopamine on normal responsiveness. Neuroscience. 1984;12:1201–1212. doi: 10.1016/0306-4522(84)90014-9. [DOI] [PubMed] [Google Scholar]
- 7.Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- 8.Rolls ET, Loh M, Deco G, Winterer G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat Rev Neurosci. 2008;9:696–709. doi: 10.1038/nrn2462. [DOI] [PubMed] [Google Scholar]
- 9.Corlett PR, Taylor JR, Wang XJ, Fletcher PC, Krystal JH. Toward a neurobiology of delusions. Prog Neurobiol. 2010;92:345–369. doi: 10.1016/j.pneurobio.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher PC, Frith CD. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci. 2009;10:48–58. doi: 10.1038/nrn2536. [DOI] [PubMed] [Google Scholar]
- 11.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 12.Lynall ME, Bassett DS, Kerwin R, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bluhm RL, Miller J, Lanius RA, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 15.Lui S, Li T, Deng W, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67:783–792. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
- 16.Huang XQ, Lui S, Deng W, et al. Localization of cerebral functional deficits in treatment-naive, first-episode schizophrenia using resting-state fMRI. Neuroimage. 2010;49:2901–2906. doi: 10.1016/j.neuroimage.2009.11.072. [DOI] [PubMed] [Google Scholar]
- 17.Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson S, Basso G, Soldati N, et al. A resting state network in the motor control circuit of the basal ganglia. BMC Neurosci. 2009;10:137. doi: 10.1186/1471-2202-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders—DSM IV—TR. Washington, DC: American Psychiatric Association; [Google Scholar]
- 21.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 22.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 23.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 24.Meindl T, Teipel S, Elmouden R, et al. Test-retest reproducibility of the default-mode network in healthy individuals. Hum Brain Mapp. 2010;31:237–246. doi: 10.1002/hbm.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shehzad Z, Kelly AM, Reiss PT, et al. The resting brain: unconstrained yet reliable. Cereb Cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riedl V, Valet M, Woller A, et al. Repeated pain induces adaptations of intrinsic brain activity to reflect past and predict future pain. Neuroimage. 2011;57:206–213. doi: 10.1016/j.neuroimage.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Martinez D, Slifstein M, Broft A, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 28.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 30.Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 31.Dowd EC, Barch DM. Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biol Psychiatry. 2010;67:902–911. doi: 10.1016/j.biopsych.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goghari VM, Sponheim SR, MacDonald AW. The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neurosci Biobehav Rev. 2010;34:468–486. doi: 10.1016/j.neubiorev.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen J, Willeit M, Zipursky RB, et al. The formation of abnormal associations in schizophrenia: neural and behavioral evidence. Neuropsychopharmacology. 2008;33:473–479. doi: 10.1038/sj.npp.1301437. [DOI] [PubMed] [Google Scholar]
- 34.Greicius MD, Flores BH, Menon V, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the dopamine systems. Annu Rev Neurosci. 1978;1:129–169. doi: 10.1146/annurev.ne.01.030178.001021. [DOI] [PubMed] [Google Scholar]
- 36.Wu T, Long X, Zang Y, et al. Regional homogeneity changes in patients with Parkinson's disease. Hum Brain Mapp. 2009;30:1502–1510. doi: 10.1002/hbm.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu T, Wang L, Chen Y, Zhao C, Li K, Chan P. Changes of functional connectivity of the motor network in the resting state in Parkinson's disease. Neurosci Lett. 2009;460:6–10. doi: 10.1016/j.neulet.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 38.Niazy RK, Xie J, Miller K, Beckmann CF, Smith SM. Spectral characteristics of resting state networks. Prog Brain Res. 2011;193:259–276. doi: 10.1016/B978-0-444-53839-0.00017-X. [DOI] [PubMed] [Google Scholar]
- 39.Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1081–1090. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.