Abstract
Individuals with schizophrenia have high levels of medical comorbidity and cardiovascular risk factors. The presence of 3 or more specific factors is indicative of metabolic syndrome, which is a significant influence upon future morbidity and mortality. We aimed to clarify the prevalence and predictors of metabolic syndrome (MetS) in adults with schizophrenia and related disorders, accounting for subgroup differences. A PRISMA systematic search, appraisal, and meta-analysis were conducted of 126 analyses in 77 publications (n = 25 692). The overall rate of MetS was 32.5% (95% CI = 30.1%–35.0%), and there were only minor differences according to the different definitions of MetS, treatment setting (inpatient vs outpatient), by country of origin and no appreciable difference between males and females. Older age had a modest influence on the rate of MetS (adjusted R 2 = .20; P < .0001), but the strongest influence was of illness duration (adjusted R 2 = .35; P < .0001). At a study level, waist size was most useful in predicting high rate of MetS with a sensitivity of 79.4% and a specificity of 78.8%. Sensitivity and specificity of high blood pressure, high triglycerides, high glucose and low high-density lipoprotein, and age (>38 y) are shown in supplementary appendix 2 online. Regarding prescribed antipsychotic medication, highest rates were seen in those prescribed clozapine (51.9%) and lowest rates of MetS in those who were unmedicated (20.2%). Present findings strongly support the notion that patients with schizophrenia should be considered a high-risk group. Patients with schizophrenia should receive regular monitoring and adequate treatment of cardio-metabolic risk factors.
Keywords: metabolic syndrome, cardiovascular risk, diabetes, lipids, glucose, schizophrenia, waist, obesity, smoking
Introduction
Increased rates of cardiovascular disease (CVD)1–5 and associated premature mortality6,7 have become a major concern in patients with schizophrenia. In order to help psychiatric clinicians to focus more on these CVD risks in patients with schizophrenia, the concept of MetS has found its way into the psychiatric literature. The metabolic syndrome (MetS) brings together a collection of abnormal clinical and metabolic findings that are predictive for CVD.8,9 These abnormal findings include visceral adiposity, insulin resistance, increased blood pressure, elevated triglyceride levels, and low high-density lipoprotein (HDL) cholesterol levels. The most common definitions for the MetS are the Adult Treatment Panel III (ATP III) of the National Cholesterol Education Program,10 and the adapted Adult Treatment Panel (ATP III-A) proposed by the American Heart Association11 which allows for a lower threshold for impaired fasting glucose of 100 mg/dl (table 1). The International Diabetes Federation (IDF) stresses the importance of waist circumference as a mandatory feature.12 Recently, more stringent and ethnic/race specific criteria were defined with, eg, slightly modified criteria of waist circumference for Asians (90 cm for male and 80 cm for female).13
Table 1.
Common Definitions of Metabolic Syndrome
| ATP III Definition (3 of 5 Required) | ATP III-A Definition (3 of 5 Required) | IDF Consensus Definition (Waist Plus 2 Required) | |
| Waist circumference | Men > 102 cm (>40 in) | Men > 102 cm (>40 in) | Male ≥ 94 cm (>37 in) |
| Women > 88 cm (>35 in) | Women > 88 cm (>35 in) | Female ≥ 80 cm (>31.5 in) | |
| Blood pressure | ≥130/≥85 mmHg | ≥130/≥85 mmHg | ≥130/≥85 mmHg |
| HDL cholesterol | Men < 40 mg/dl (1.04 mmol/l) | Men < 40 mg/dl (1.04 mmol/l) | Men < 40 mg/dl (1.04 mmol/l) |
| Women < 50 mg/dl (1.29 mmol/l) | Women < 50 mg/dl (1.29 mmol/l) | Women < 50 mg/dl (1.29 mmol/l) | |
| Triglycerides | ≥150 mg/dl (1.7 mmol/l) | ≥150 mg/dl (1.7 mmol/l) | ≥150 mg/dl (1.7 mmol/l) |
| Fasting glucose | ≥110 mg/dl (6.1 mmol/l) | ≥100 mg/dl (5.6 mmol/l) | ≥100 mg/dl (5.6 mmol/l) |
Note: IDF, International Diabetes Federation; ATP, Adult Treatment Panel; HDL, high-density lipoprotein.
In the general population, MetS is associated with a 4 times relative risk of developing diabetes14 and approximately a 2-fold risk of coronary heart disease, stroke, and premature mortality.8 As a result, MetS has been proposed as an alternative or an augmentation to the Framingham15 or systematic coronary risk evaluation16 calculations to assess the risk of CVD and death. Similar results for the MetS as a predictor of the risk of coronary heart disease have been found previously in schizophrenia.17
In part, the development of the MetS is attributable to genetic risk,18,19 limited access to general somatic health care,20 and unhealthy lifestyle choices including physical inactivity,21,22 poor diet,23 and high rates of cigarette smoking.24 In the general population, genetic and geographical environmental differences might partially be responsible for the observation that estimated rates of MetS alter to different countries of origin. For example, adopting the ATP III definition, the age-adjusted rates are 18.4% for men and 14.4% for women in Europeans, 28.8% for men and 31.8% for women in South Asians, 15.5% for men and 23.4% for women in African-Caribbeans,25 while the rate was 15.7% in Taiwan26 and 23.7% in the United States,27,28 respectively. Although previous narrative reviews29–32 reported on differences in MetS rates between different countries, to the best of our knowledge, meta-analytic data comparing MetS rates across different countries in patients with schizophrenia are lacking. In the same way, it remains to be explored if MetS rates differ between different treatment settings, ie, in order to identify patients at risk psychiatric clinicians should know if patients in particular treatment settings are at higher risk or not.
In recent years, it has become apparent that in patients with schizophrenia particular antipsychotic agents (AP) can have a negative impact on some of the modifiable MetS risk factors.4,5 The differential effects of various AP on weight are well described, with clozapine and olanzapine associated with the highest weight gain.5 Previous systematic reviews on metabolic side effects of AP were of AP have only focussed upon metabolic changes after exposure following convenience testing.33,34 A recent meta-analysis suggested a typical weight gain of 3.8 kg in drug-naïve patients after starting antipsychotic treatment in the first 3 months of treatment.35 No data on differences in MetS rates between patients taking different APs are available.
In the same way, no systematic data on differences in MetS rates between patients with and without AP and between first-episode and chronic patients are available. Knowledge of all these differences would guide psychiatric clinicians in identifying patients at risk for CVD. Given this uncertainty, we conducted a systematic review and meta-analysis with an objective to clarify prevalence rate of MetS in schizophrenia and related disorders taking into account variations in country, setting, stage of illness, AP treatment, and illness duration. Our secondary aim was to clarify the prevalence rates of every individual MetS risk factor in those systematically monitored. A tertiary aim was to examine which individual MetS risk factors would be useful markers of the full MetS at the study level.
Methods
Inclusion and Exclusion Criteria
The systematic review was written up according to the PRISMA standard (a protocol used to evaluate systematic reviews).36 Our focus was on patients with defined schizophrenia (with or without related psychosis). We excluded all studies without subjects with schizophrenia. Studies were included that examined MetS using ATP III,10 ATP III-A,11 or IDF12 criteria. We excluded studies using nonstandardized definitions of metabolic syndrome. We included only studies on adults (excluding studies in those under 18 y and over 65 y) as variations in MetS in older and younger patients may warrant separate analysis. However, to further account for the effect of age in adults, we analyzed the effect of mean age across studies. We were particularly interested in the prevalence rate of MetS in each clinical setting (inpatient, outpatient, or mixed settings), according to disease stage, country of origin, and prescribed medication. Finally, we excluded studies with inadequate data for extraction.
Search Criteria and Critical Appraisal
A systematic literature search, critical appraisal of the collected studies, and meta-analysis were conducted. The following abstract databases were searched from inception to July 2011—Medline, PsycINFO, and Embase. Four full text collections namely Science Direct, Ingenta Select, Ovid Full text, Blackwell/Wiley Interscience were searched. The abstract database Web of Knowledge (4.0, Insitute of Scientific Information) was searched, using the above terms as a text word search and using key papers in a reverse citation search. The search terms “metabolic or diabetes or cardiovascular or blood pressure or glucose or lipid” and “psychosis or psychotic or schizophrenia or schizoaffective” yield 1092 hits, of which 933 were not relevant. We also contacted many experts in the field for papers in preparation and additional data and received data from 20 groups (see Acknowledgments). Methodological appraisal of each study was conducted according to PRISMA standard including evaluation of bias (confounding, overlapping data, and publication bias).30
Statistical Analysis
We pooled individual study data using DerSimonian-Laird proportion meta-analysis.37 Heterogeneity was invariably moderate to high therefore a random effects meta-analysis was performed using StatsDirect 2.6.2. For comparative analyses (eg, by country of origin, rate per prescribed medication), we required a minimum of 3 independent studies to justify analysis according to convention. In order to elucidate subgroup effects, we stratified data by disorder, MetS definition, clinical setting, and gender, where appropriate. We analyzed several predictors of MetS including age, publication year, and duration of illness using regression analysis, and examined components of MetS were most predictive of the full syndrome at a study level using Receiver Operator Characteristic curve analysis. We excluded 36 publications containing 71 analyses with data, which were either duplicates from the same group as well as 10 publications that did not include any patients with schizophrenia.
Results
Search Results
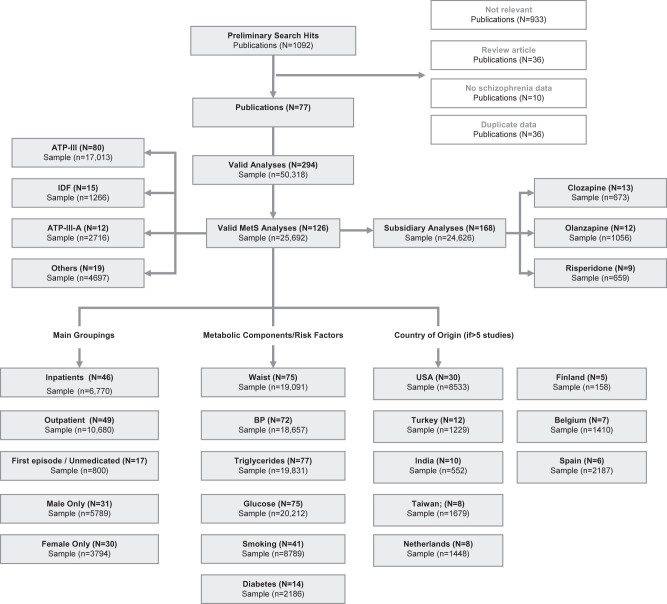
From 160 candidate publications following exclusions, our search generated 77 publications containing 294 analyses (126 main group analyses and 168 subgroup analyses). Subgroup analyses were generally those containing analyses of patients on individual antipsychotic drugs (supplementary appendix 3). The dataset comprised 25 692 unique patients from 27 countries or regions (figure 1). Of 126 main analyses, 84 (66.7%) used Diagnostic and Statistical Manual of Mental Disorders criteria to define schizophrenia and related psychosis, and 18 of 126 (14.3%) used ICD10 criteria but 24 used clinical expert judgment or miscellaneous criteria. A subset of 66 analyses examined only those with pure schizophrenia and no related psychoses.
Fig. 1.
Quality of reporting of meta-analyses (Quorom) search results.
Of 126 main analyses, 46 were conducted among inpatients (n = 6770), 49 were conducted in outpatient settings (n = 10 680), and 29 were conducted in mixed samples (n = 8186). Seventeen studies examined individuals who were either in their first-episode drug naïve or both (n = 800). The remainder examined patients in mixed stages, taking antipsychotic medication although sample sizes for those taking individual AP were modest. Only 31 analyses reported data on males and females separately, and only 33 analyses reported data on ethnicity.
Prevalence of Metabolic Syndrome in Schizophrenia
Across all 126 analyses involving 25 692 patients with schizophrenia, 64.0% of patients were male and their the mean length of illness was 10.4 years. There was no publication bias (supplementary appendix 1). The overall rate of MetS was 32.5% (95% CI = 30.1%–35.0%) (figure 2, table 2) using any standardized MetS criteria. The rates using the ATP III, adapted ATP III, or IDF definitions were 32.8% (N = 80, n = 17005, 95% CI = 30.0%–35.7%); 28.6% (N = 12, n = 2716, 95% CI = 19.8%–38.3%); and 35.3% (N = 15, n = 1266, 95% CI = 23.1%–48.6%), respectively.
Fig. 2.
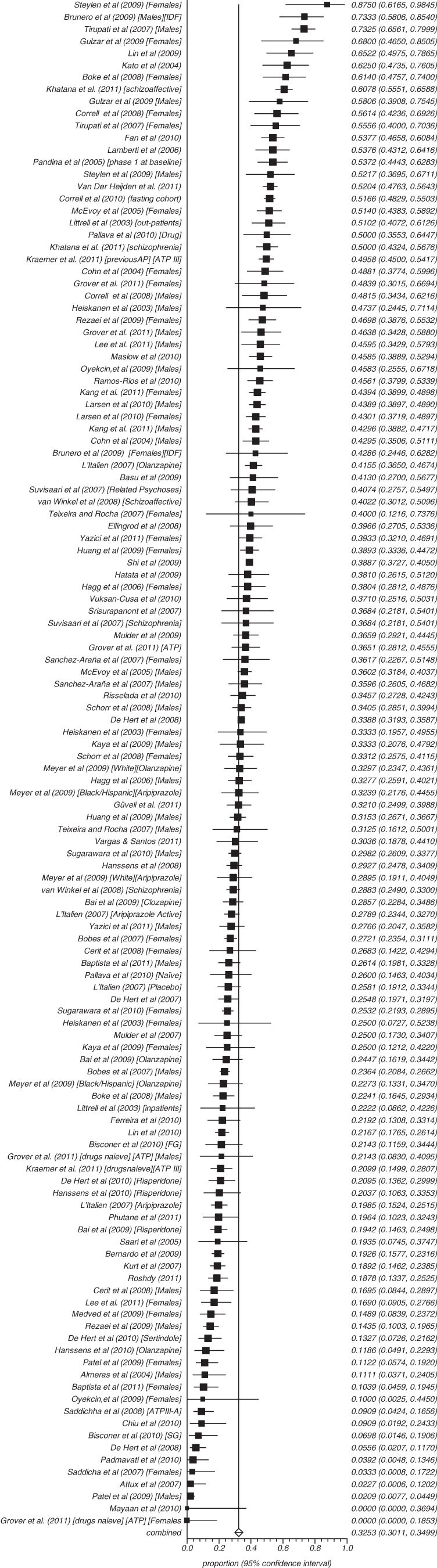
Summary rate of metabolic.
Table 2.
Summary of Main Results of Meta-Analytic Rates of Metabolic Abnormalities in Schizophrenia and Related Psychoses
| MetS by ATP III (%) | Waist (ATP) (M > 102, F > 88) | Blood pressure, >130/85 | Triglycerides (>150 mg/dl) | Glucose (ATP III) (>110 mg/dl) | Glucose (>100 mg/dl) | HDL (M < 40 mg/dl, F < 50 mg/dl) | Smoking | Diabetes | |
| Number of studies (n) | 80 | 53 | 72 | 77 | 47 | 28 | 76 | 41 | 14 |
| Sample size (n) | 17 005 | 14 305 | 18 657 | 19 831 | 13 784 | 6499 | 19 280 | 8789 | 2186 |
| Pooled proportion (%) | 32.8 | 49.4 | 38.7 | 39.3 | 19.5 | 18.8 | 42.6 | 54.3 | 10.9 |
| 95% CI | 30.0% –35.7% | 44.8% - 53.3% | 35.6%–41.9% | 35.0%–43.6% | 16.9%–22.2% | 15.5%–22.4% | 39.3%–46.0% | 50.9% −57.5% | 7.1% −15.5% |
Note: M, male; F, female.
We examined whether rates varied in several potentially important subgroups. There were only minor differences in MetS according to country of study origin. For example, the rate of ATPIII MetS was 32.5% (N = 26, n = 7037, 95% CI = 26.6%–38.7%) in the United States; 34.5% (N = 5, n = 158, 95% CI = 25.4%–44.2%) in Finland; 30.1% (N = 9, n = 702, 95% CI = 24.7%–35.8%) in Turkey; and 30.2% (N = 6, n = 2187, 95% CI = 23.6%–37.2%) in Spain. Among inpatients, the rate of MetS was 30.4% (N = 46, n = 6770, 95% CI = 26.7%–34.2%), and in outpatients, it was 31.8% (N = 49; n = 10 680, 95% CI = 27.5%–36.2%). In males, MetS was present in 34.8% (N = 31, n = 5789, 95% CI = 29.2%–40.6%), and in females, it was diagnosed in 34.8% (N = 30, n = 3794, 95% CI = 29.5%–40.3%). In those studies of patients who were drug naïve or in their first episode, the rate was 11.3% (N = 14, n = 800, 95% CI = 7.3%–16.1%). After including all subgroups with first-episode patients, the rate in pure first-episode patients was 13.0% (N = 7, n = 490, 95% CI = 7.2%–20.1%). In those who were neither drug naïve nor in their first episode, it was 35.3% (N = 112, n = 24 892, 95% CI = 32.8%–37.8%). In studies recruiting older patients with a mean age of 50 years or higher, MetS was present in 39.2% (N = 14, n = 6396, 95% CI = 32.6%–46.1%).
Individual Metabolic Abnormalities in Schizophrenia
Fifty-three studies reported on the rate of obesity defined as a waist (cm) more than 102 in males and 88 cm in females (ATP) and 8 reported those with more than 94 cm in males and 80 cm in females (IDF) (figures 3–8). The proportion overweight by ATP definition was 49.4% (N = 53; n = 14 305, 95% CI = 44.8%–53.3%) and 44.4% according to IDF (N = 8, n = 263, 95% CI = 32.3%–56.8%). Of studies reporting the rate of hyperglycaemia (>110 mg/dl; ATP), the rate was 19.5% (N = 47, n = 13784, 95% CI = 16.9%–22.2%), and it was 18.8% (N = 28, n = 6499, 95% CI = 15.5%–22.4%) for those with more than 100 mg/dl. The rate of hypertriglyceridemia was 39.3% (N = 77, n = 19 831, 95% CI = 35.0%–43.6%), and the proportion of those with low HDL was 42.6% (N = 76, n = 19 280, 95% CI = 39.3%–46.0%). Additionally, 38.7% (N = 72; n = 18 657, 95% CI = 35.6%–41.9%) of those with schizophrenia had high blood pressure and 54.2% (N = 41, n = 8789, 95% CI = 50.9%–57.5%) were smokers (table 2) and 10.9% (N = 14, n = 2186, 95% CI = 7.0%–15.5%) had diabetes. Of note, in studies from United States rates of high waist size, blood pressure, smoking, and low HDL were all above 50% (see online supplementary table 3).
Fig. 3.
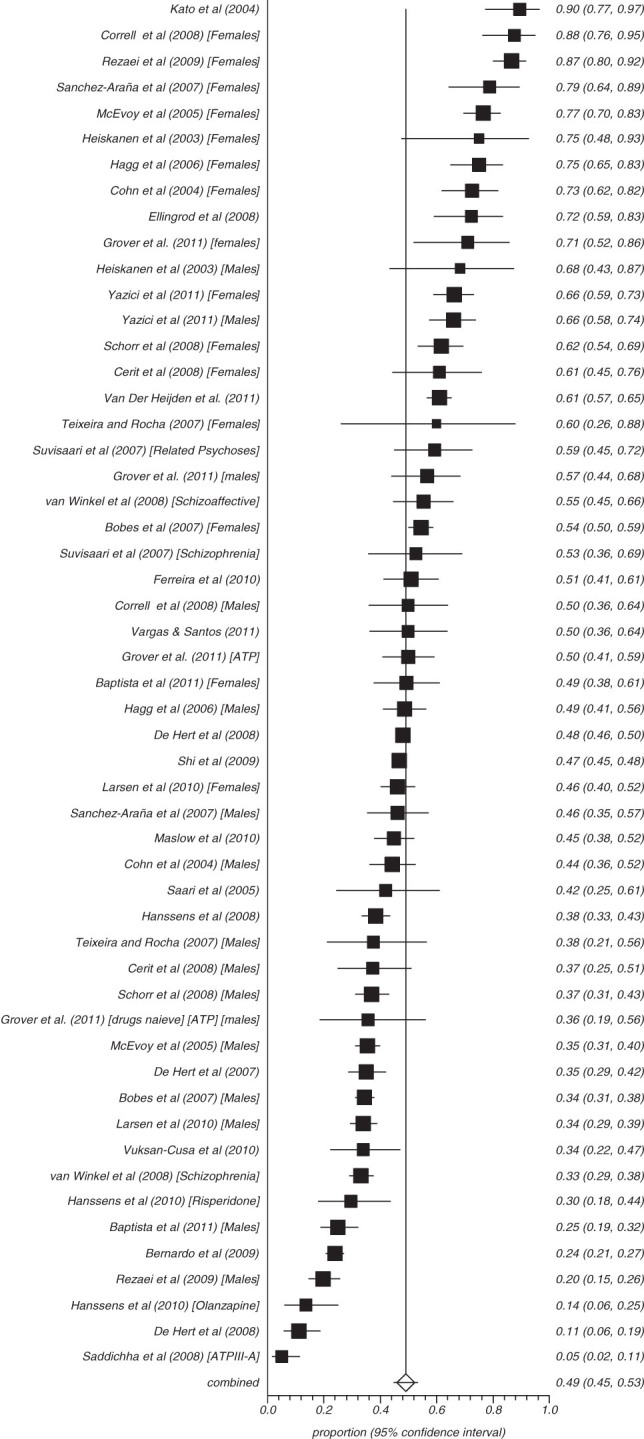
Summary rate of obesity by waist size.
Fig. 4.
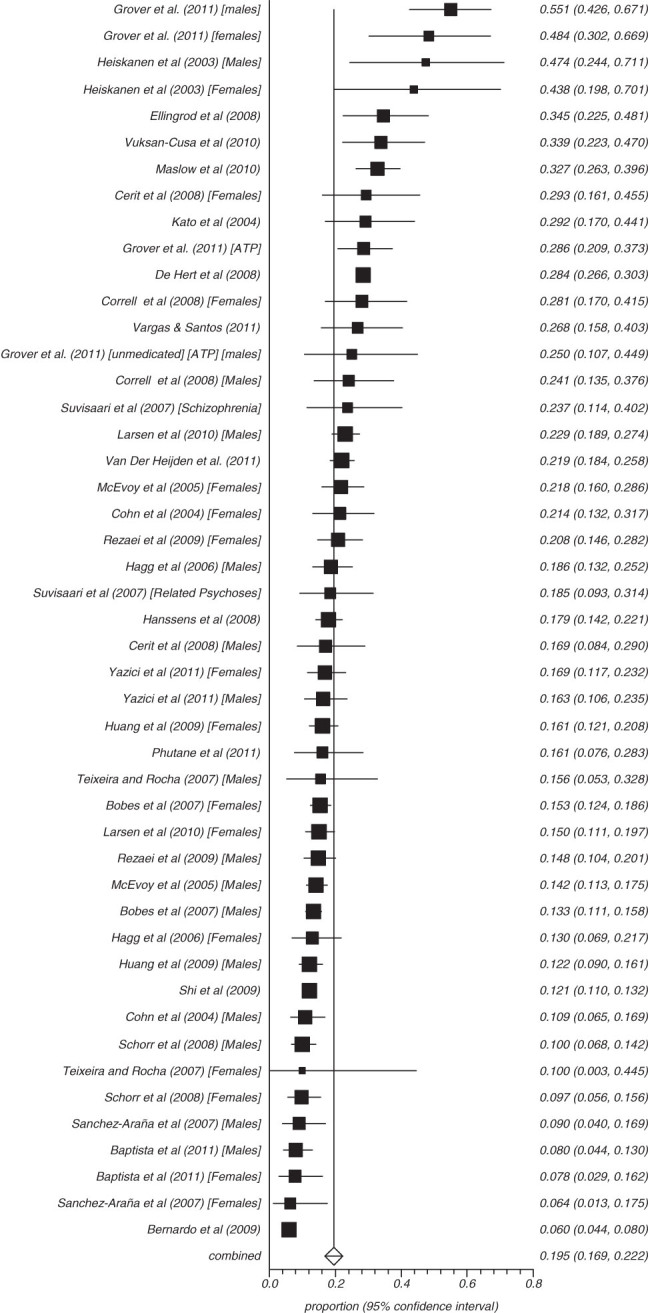
Summary rate of hyperglycaemia.
Fig. 5.
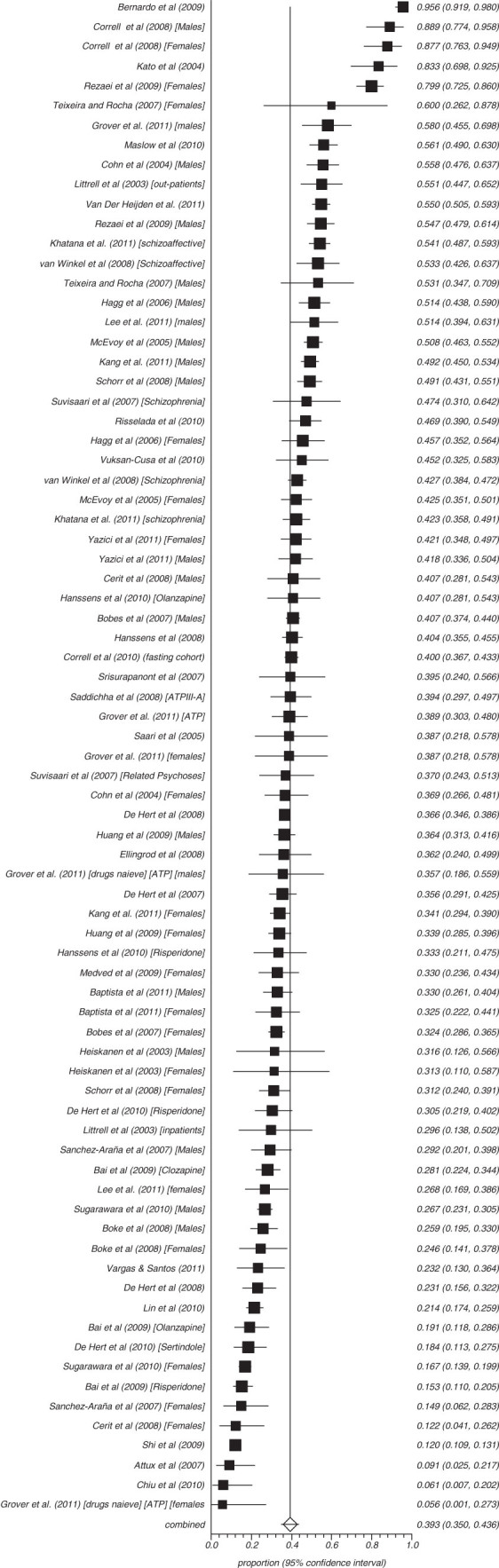
Summary rate of hypertriglyceridemia in schizophrenia (random effects).
Fig. 6.
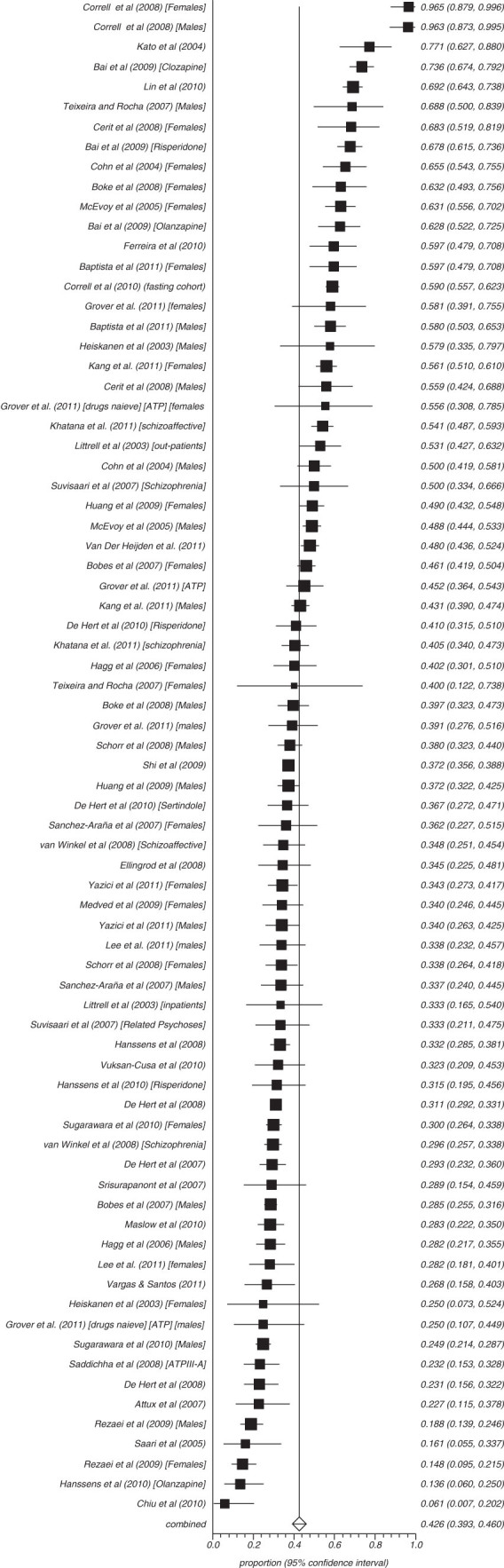
Summary rate of high-density lipoprotein (random effects).
Fig. 7.
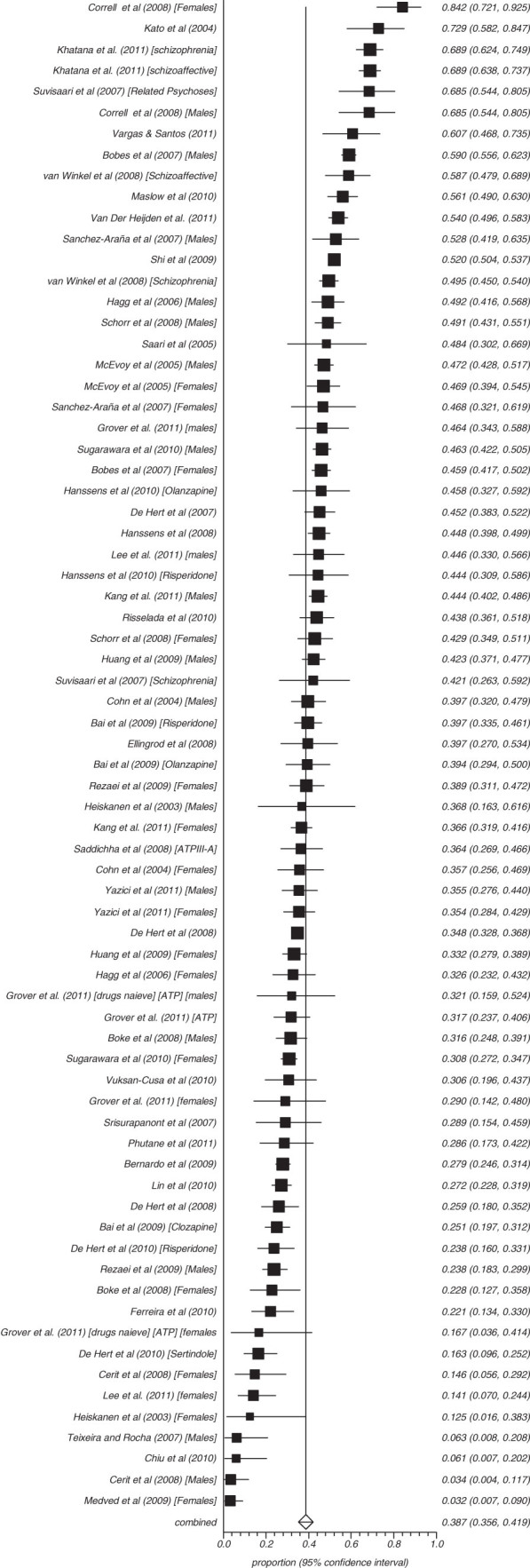
Summary rate of hypertension in schizophrenia (random effects).
Fig. 8.
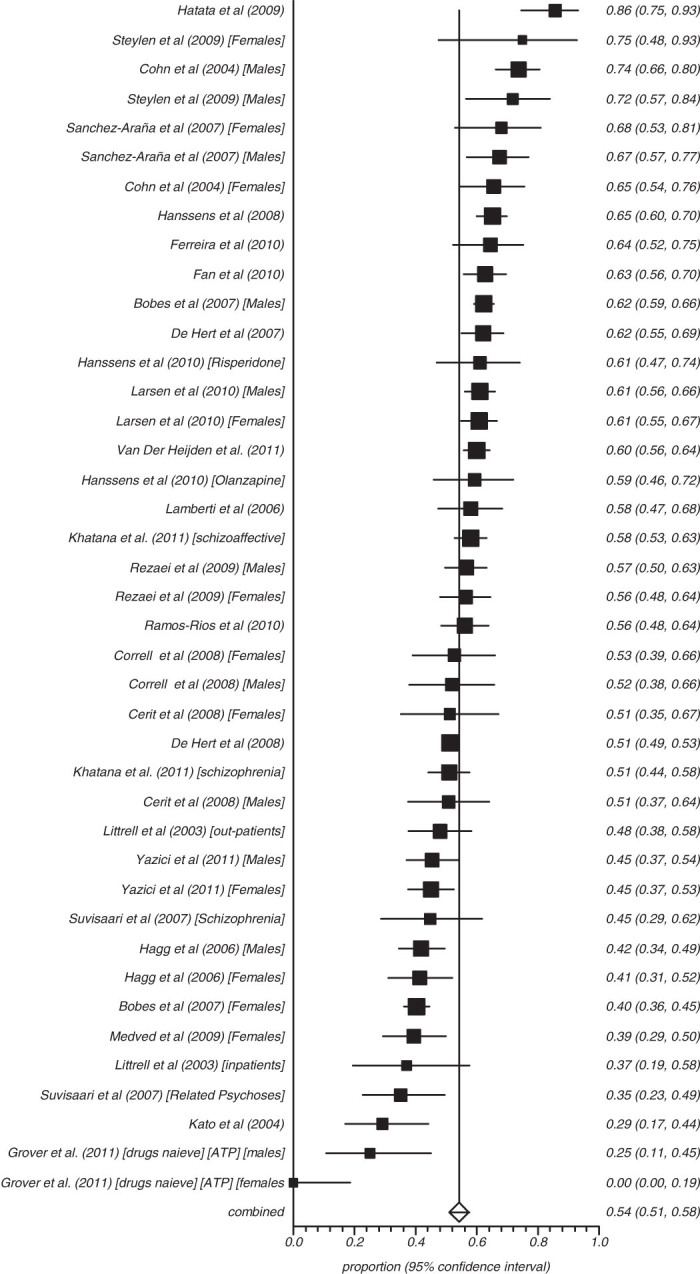
Summary rate of smoking in schizophrenia (random effects).
Predictors of Metabolic Syndrome in Schizophrenia
On regression analysis, there was a modest association of high rates of MetS in studies with older participants (adjusted R 2 = .20; t = 5.5; P < .0001) and a trend associated with older year of publication (adjusted R 2 = .7; t = −1.8; P = .08). When studies from USA alone were considered only the effect of age remained significant. In the whole sample, there was a strong effect of illness duration (adjusted R 2 = .35; t = 4.9; P < .0001). Using the median value of >32.8% to define a high rate of MetS, we examined which metabolic components were most predictive of the full syndrome. At a study level, waist size was most useful in predicting high rate of MetS with an area under curve (AUC) of 0.848 (sensitivity =79.4%; specificity 78.8%). Illness duration (>7.8 y) was also a useful predictor. Sensitivity and specificity of high blood pressure, high triglycerides, high glucose and low HDL, and age (>38 y) are shown in supplementary appendix 2 online.
Metabolic Syndrome in Schizophrenia According to Current Antipsychotic
Given the limitations in the sample, only 4 comparisons were possible: unmedicated, those taking clozapine, olanzapine, or risperidone alone. Using the same criteria (ATP III), the rate of MetS was 51.9% (N = 13, n = 673, 95% CI = 45.8%–57.9%) for clozapine; 28.2% (N = 12, n = 1056, 95% CI = 19.1%–38.4%) for olanzapine; and 27.9% (N = 9, n = 659, 95% CI = 12.6%–46.5%) for risperidone. In unmedicated patients, the rate of MetS was 20.2% (N = 7, n = 297, 95% CI = 15.9%–24.9%) using ATPIII criteria. However, we cannot exclude that certain prescriptions such as clozapine were likely to be linked with covariates of age and illness duration.
Discussion
General Findings
To the authors’ knowledge, the present large-scale meta-analysis is the first to demonstrate that almost 1 in 3 of unselected patients with schizophrenia suffer from MetS. We found 126 valid analyses in 77 publications within the period 2003 to July 2011 (see figure 1). This indicates that the cardio-metabolic risk in patients with schizophrenia is clearly becoming recognized as a key consideration in the long-term health of these patients. Examining individual cardio-metabolic risk abnormalities, 1 in 2 patients with schizophrenia are overweight, 1 in 5 appear to have significant hyperglycaemia, and at least 2 in 5 have lipid abnormalities when systematically tested (see table 2). The MetS rates appear consistently high regardless of definition of MetS (rates were nonsignificantly lower in ATP III-A) and population norms although the magnitude of the latter effect are not entirely clear. When looking at the overall rate of MetS, only minor differences were apparent between United States, Finland, Turkey, and Spain, and there was considerable heterogeneity in data from other countries. A subanalysis of studies from the United States (see online supplementary table 3) suggests significantly higher rates of obesity (by waist size), hypertension, and abnormal HDL cholesterol (all P < .0001) compared with other countries.
When considering the MetS, identifying patients who currently have or are at high risk for metabolic disorders is a first step. Knowledge on which factors are associated with the highest MetS rates helps to identify these patients at greater risk. Consistent with population studies,25–28 there was no appreciable difference in men and women indicating that both sexes need the same care. Despite the large sample size (n = 25 692), there was modest data on ethnic minority nonwhite populations. Consequently, no meta-analytic conclusions can be drawn on the differences in MetS between different ethnic populations. In contrast, prevalence of MetS was higher in those receiving antipsychotic drugs compared with those who were unmedicated. We also attempted to examine differences by prescribed antipsychotic drug for MetS, but our findings were limited by small sample size for each drug. High rates were most notably seen on clozapine (51.9%), although this could be influenced by subject age and duration of illness or indeed previous antipsychotic exposure. The present meta-analytic findings demonstrate that MetS rates are increasing with older age. Lowest MetS rates were seen in first-episode patients. However, one of the major determinants for higher MetS risk was longer illness duration (see supplementary appendix 2 online). The cumulative long-term effect of poor health behaviors and long-term exposure to antipsychotic drug places a patient with longer illness duration at a greater risk of cardio-metabolic disorders.
The lower rates of MetS observed in first-episode patients is of great interest. No study has compared these low rates with the general population, but it is quite plausible that the risk is not increased in these patients early in the course of their illness, prior to exposure to AP. Several groups have however proposed a liability for people with schizophrenia to develop metabolic abnormalities in the absence of antipsychotic medication. 38–40 In support of this, there are indications of an increased risk for diabetes in first-degree relatives.41 There is also some evidence for the underlying genetic risk for the development of metabolic abnormalities after antipsychotic treatment initiation.18,19 In studies that have assessed metabolic abnormalities in drug-naïve first-episode patients, several have found impaired glucose tolerance or insulin resistance,38–40,42–45 while others have found no appreciable effect.46–49 Raised cholesterol and/or triglyceride levels have been reported by some groups43,44 but not by others.44 Green et al50 described a significant increase in weight and total cholesterol levels but not in fasting glucose in a cohort of 263 first-episode patients treated with haloperidol or olanzapine for 2 years. In the European First-Episode Study, Kahn et al51 evaluated first-episode cases of schizophrenia during first year of exposure to antipsychotic treatment and found an increase in the number of overweight patients from 17% at baseline to 54% at 12 months and people with high cholesterol from 23% to 46.3%. Perez-Ingesias et al52 found limited abnormalities at baseline, but weight gain was positively correlated with changes in insulin levels, insulin resistance index, and triglyceride levels. Oriot et al53 found that after 9 months of first exposure, there were significant increases in body mass index (BMI), fasting glucose, and waist circumference but no effect on blood pressure or lipids.
Limitations
We wish to acknowledge several limitations in the primary data and this meta-analysis. First, there was considerable heterogeneity, which can only be partly controlled by stratification for MetS definition and setting. Second, there were limited data on nonschizophrenia psychosis, and hence, analysis of this subgroup was not possible on its own. Third, there were often missing data on duration of illness. Indeed, it is important to note that duration of illness is often a proxy of duration of medication exposure and is related to patient age, both of which may influence MetS. Fourth, there were inadequate data on individuals prescribed specific AP, particularly first-generation antipsychotic drugs. Fifth, there was inadequate measurement of population rates of MetS in each study, and as such, relative risk estimates of MetS could only be extrapolated. Finally, there was marked variation in the quality of studies, particularly sample size.
Clinical Implications
Although with limitations, our findings demonstrate that patients with schizophrenia are a high-risk group for MetS. They should therefore be routinely screened for MetS risk factors at key stages.54,55 This can be achieved by establishing a risk profile based on consideration of medical factors (eg, obesity, dyslipidaemia, hypertension, hyperglycaemia, and established diabetes) but also behavioral factors (eg, poor diet, smoking, and physical inactivity). This risk profile can then be used as a basis for ongoing monitoring, treatment selection, and management. Of the individual components of MetS, increased waist size (or abdominal obesity) was most predictive of MetS status (see supplementary appendix 2 online). At a study level, waist size predicted MetS with an AUC of 0.848. Visceral (intra-abdominal) adiposity is known to be closely associated with hyperinsulinaemia, dyslipidaemia, and impaired glucose tolerance. Several groups have proposed that waist size or BMI could be used as a simple screening test for MetS in schizophrenia.56–58 Straker et al56 found that abdominal obesity alone has 92% sensitivity and 55.6% specificity for full MetS De Hert et al59 developed a schizophrenia specific conversion method to calculate waist size from BMI Waist circumference can predicted according to the following formula: waist = 33.0 + (2.4 × BMI) for men and waist = 27.9 + (2.4 × BMI) for women.
Although measuring waist circumference and/or BMI is simple, easy to perform, and inexpensive, 50% of patients in routine care have no recorded BMI measure,60 and about 60% of inpatients receive no comprehensive physical examination.61 The American Diabetes Association62 recommends that patients receiving atypical antipsychotics should have waist circumference measured at baseline and annually and BMI at baseline, at 4, 8, and 12 weeks and then quarterly. Guidelines from the European Psychiatric Association4 recommend that monitoring should be taken at the initial presentation and before the first prescription of antipsychotic medication and (for patients with normal baseline tests) repeated at 6 and 12 weeks after initiation of treatment and at least annually thereafter. In light of the high rates of MetS observed in all settings, we propose that minimum monitoring should include BMI and waist circumference, and optimal monitoring should also include fasting glucose, lipids, and cholesterol. Additionally, psychiatrists, physicians, nurses, and other members of the multidisciplinary team can help educate and motivate patients with schizophrenia to improve their lifestyle through the use of effective behavioral interventions, including smoking cessation, dietary measures, and exercise.55,63,64 However, if lifestyle interventions do not succeed (or are unlikely to succeed) then preferential use of or switching to a lower risk medication or addition of a medication known to reduce weight and/or metabolic abnormalities should be considered.4,20,55,64–66
Future Research
Future studies should evaluate interventions that target the individual components of the metabolic syndrome especially abdominal obesity. Future research should also undertake a comprehensive assessment of MetS risk factors following, at the very least, recommended monitoring guidelines. Long-term follow-up will be required here in order to accurately document the emergence of some outcomes, such as diabetes. Thirdly, examining whether cardio-metabolic outcomes are moderated by clinical characteristics and genetic factors should become a clinical research priority.
Conclusions
Our meta-analysis clearly demonstrates that MetS risk factors are highly prevalent in patients with schizophrenia. Psychiatrists need to be aware of the potential metabolic side effects of antipsychotic medication and to include them in the risk/benefit assessment when choosing a specific antipsychotic. The treating psychiatrists should also be responsible for the implementation of the necessary screening assessments and referral for treatment. Multidisciplinary assessment of medical and behavioral conditions is needed. Psychiatric treatment facilities should offer and promote healthy lifestyle intervention early in the course of disease aiming to prevent serious metabolic adverse effects. Future research should focus on evaluating interventions that target MetS and examine if cardio-metabolic outcomes are moderated by clinical characteristics and genetic factors.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
We thank Tuula Heiskanen and Hannu Koponen, Department of Psychiatry, Kuopio University Hospital, Kuopio, Finland. Roy Chengappa, Psychiatric Institute and Clinic, University of Pittsburgh, School of Medicine, Pittsburgh, USA. Tony Cohn, Departments of Psychiatry and Nutritional Sciences, University of Toronto, Toronto, Canada. Christoph Correll, The Zucker Hillside Hospital, Glen Oaks, New York, USA and Albert Einstein College of Medicine, Bronx, New York, USA. Jonathan Meyer, Department of Psychiatry, University of California, San Diego, USA. John Crilly and J. Steven Lamberti, Department of Psychiatry, University of Rochester Medical Center, Rochester, USA. Paul Mackin, School of Neurology, Neurobiology and Psychiatry Newcastle University Newcastle upon Tyne, UK. Srinivasan Tirupati, Hunter New England Area Health Services, James Fletcher Hospital, Newcastle, New South Wales, Australia. T. Sanchez-Araña Moreno, Department Psychiatriy Hospital de la Merced, Osuna, Spain. Paulo José Teixeira, Instituto de Previdência dos Servidores do Estado de Minas Gerais, Belo Horizonte (MG), Brazil. Gilbert J. L’Italien, Yale University School of Medicine, New Haven, USA. Viki Ellingrod, University of Michigan College of Pharmacy, Department of Clinical Sciences, Ann Arbor, USA. Hung-Wen Chiu, Graduate Institute of Medical Informatics, Taipei Medical University, Taipei, Taiwan, Dan Cohen, Geestelijke Gezondheidszorg Noord-Holland Noord, The Netherlands. Hans Mulder, Division of Pharmacoepidemiology and Pharmacotherapy, Utrecht Institute for Pharmaceutical Sciences, Utrecht University, Utrecht and Department of Clinical Pharmacy, Wilhelmina Hospital Assen, Assen, The Netherlands. Jayendra K. Patel, Department of Psychiatry, University of Massachusetts Medical School, Worcester, USA. Professor de Hert he has been a consultant for, received grant/research support and honoraria from, and been on the speakers/advisory boards of Astra Zeneca, Bristol-Myers Squibb, Eli Lilly, Janssen-Cilag, Lundbeck JA, Pfizer and Sanofi-Aventis. Dr van Winkel has been a consultant for Eli Lilly, received honoraria from Eli Lilly, AstraZeneca, and Janssen-Cilag, and grant support from AstraZeneca and Eli Lilly. The remaining authors report no conflicts.
Appendix 1. Bias assessment for schizophrenia Metabolic Syndrome studies. Begg-Mazumdar: Kendall's tau b = 0.089 P = .1371; Egger: bias = 1.414 (95% CI = −0.138–2.96) P = .0739; Harbord: bias = −0.676 (92.5% CI = −1.758–0.406) P = .2644.
Appendix 2. Study level correlates of MetS using Receiver Operator Characteristic (ROC) analysis.
References
- 1.Mitchell AJ, Malone D. Physical health and schizophrenia. Curr Opin Psychiatry. 2006;19:432–437. doi: 10.1097/01.yco.0000228767.71473.9e. [DOI] [PubMed] [Google Scholar]
- 2.Fleischhacker WW, Cetkovich-Bakmas M, De Hert M, et al. Comorbid somatic illnesses in patients with severe mental disorders: clinical, policy, and research challenges. J Clin Psychiatry. 2007;69:514–519. doi: 10.4088/jcp.v69n0401. [DOI] [PubMed] [Google Scholar]
- 3.Leucht S, Burkard T, Henderson J, et al. Physical illness and schizophrenia: a review of the literature. Acta Psychiatr Scand. 2007;116:317–333. doi: 10.1111/j.1600-0447.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- 4.De Hert M, Dekker JM, Wood D, et al. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC) Eur Psychiatry. 2009;24:412–424. doi: 10.1016/j.eurpsy.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 5.De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10:52–77. doi: 10.1002/j.2051-5545.2011.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64:1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- 7.Capasso RM, Lineberry TW, Bostwick JM, Decker PA, St Sauver J. Mortality in schizophrenia and schizoaffective disorder: an Olmsted County, Minnesota cohort: 1950-2005. Schizophr Res. 2008;98:287–294. doi: 10.1016/j.schres.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incidence cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 9.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 10.Expert Panel on Detection and Evaluation of Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/ National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 12.Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome —a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabetic Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 13.Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome. A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 14.Hanley AJ, Karter AJ, Williams K, et al. Prediction of type 2 diabetes mellitus with alternative definitions of the metabolic syndrome: the Insulin Resistance Atherosclerosis Study. Circulation. 2005;112:3713–3721. doi: 10.1161/CIRCULATIONAHA.105.559633. [DOI] [PubMed] [Google Scholar]
- 15.Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38:46–51. doi: 10.1016/0002-9149(76)90061-8. [DOI] [PubMed] [Google Scholar]
- 16.Conroy RM, Pyörälä K, Fitzgerald AP, et al. SCORE project group. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 17.Correll CU, Frederickson AM, Kane JM, Manu P. Metabolic syndrome and the risk of coronary heart disease in 367 patients treated with second-generation antipsychotic drugs. J Clin Psychiatry. 2006;67:575–583. doi: 10.4088/jcp.v67n0408. [DOI] [PubMed] [Google Scholar]
- 18.van Winkel R, Rutten BP, Peerbooms O, et al. MTHFR and risk of metabolic syndrome in patients with schizophrenia. Schizophr Res. 2010;121:193–198. doi: 10.1016/j.schres.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 19.van Winkel R, Moons T, Peerbooms O, et al. MTHFR genotype and differential evolution of metabolic parameters after initiation of a second generation antipsychotic: an observational study. Int Clin Psychopharmacol. 2010;25:270–276. doi: 10.1097/YIC.0b013e32833bc60d. [DOI] [PubMed] [Google Scholar]
- 20.De Hert M, Cohen D, Bobes J, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, and recommendations at the system and individual levels. World Psychiatry. 2011;10:138–151. doi: 10.1002/j.2051-5545.2011.tb00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vancampfort D, Knapen J, Probst M, et al. Considering a frame of reference for physical activity research related to the cardiometabolic risk profile in schizophrenia. Psychiatry Res. 2010;177:271–279. doi: 10.1016/j.psychres.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Vancampfort D, Sweers K, Probst M, et al. The association of metabolic syndrome with physical activity performance in patients with schizophrenia. Diabetes Metab. 2011;37:318–323. doi: 10.1016/j.diabet.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Strassnig M, Brar JS, Ganguli R. Nutritional assessment of patients with schizophrenia: a preliminary study. Schizophr Bull. 2003;29:393–397. doi: 10.1093/oxfordjournals.schbul.a007013. [DOI] [PubMed] [Google Scholar]
- 24.Bobes J, Arango C, Garcia-Garcia M, Rejas J. Healthy lifestyle habits and 10-year cardiovascular risk in schizophrenia spectrum disorders: an analysis of the impact of smoking tobacco in the CLAMORS schizophrenia cohort. Schizophr Res. 2010;119:101–109. doi: 10.1016/j.schres.2010.02.1030. [DOI] [PubMed] [Google Scholar]
- 25.Tillin T, Forouhi N, Johnston DG, et al. Metabolic syndrome and coronary heart disease in South Asians, African-Caribbeans and white Europeans: a UK population-based cross-sectional study. Diabetologia. 2005;48:649–656. doi: 10.1007/s00125-005-1689-3. [DOI] [PubMed] [Google Scholar]
- 26.Hwang LC, Bai CH, Chen CJ. Prevalence of obesity and metabolic syndrome in Taiwan. J Formos Med Assoc. 2006;105:626–635. doi: 10.1016/S0929-6646(09)60161-3. [DOI] [PubMed] [Google Scholar]
- 27.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 28.Park YW, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer JM, Koro CE, L’Italien G. The metabolic syndrome and schizophrenia: a review. Int Rev Psychiatr. 2005;17:173–180. doi: 10.1080/09540260500071798. [DOI] [PubMed] [Google Scholar]
- 30.Van Gaal LF. Long-term health considerations in schizophrenia: metabolic effects and the role of abdominal adiposity. Eur Neuropsychopharmacol. 2006;16:S142–S148. doi: 10.1016/j.euroneuro.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 31.De Hert M, Schreurs V, Vancampfort D, van Winkel R. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8:15–22. doi: 10.1002/j.2051-5545.2009.tb00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer JM, Stahl SM. The metabolic syndrome and schizophrenia. Acta Psychiatr Scand. 2009;119:4–14. doi: 10.1111/j.1600-0447.2008.01317.x. [DOI] [PubMed] [Google Scholar]
- 33.Tarricone I, Gozzi BF, Serretti A, et al. Weight gain in antipsychotic-naive patients: a review and meta-analysis. Psychol Med. 2010;40:187–200. doi: 10.1017/S0033291709990407. [DOI] [PubMed] [Google Scholar]
- 34.Alvarez-Jiménez M, González-Blanch C, Crespo-Facorro B, et al. Antipsychotic-induced weight gain in chronic and first-episode psychotic disorders: a systematic critical reappraisal. CNS Drugs. 2008;22:547–562. doi: 10.2165/00023210-200822070-00002. [DOI] [PubMed] [Google Scholar]
- 35.Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123:225–233. doi: 10.1016/j.schres.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred reporting items for systematic reviews and meta-Analyses: the PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 38.Ryan MC, Collins P, Thakore JH. Impaired fasting glucose tolerance in first-episode, drug-naïve patients with schizophrenia. Am J Psychiatry. 2003;160:284–289. doi: 10.1176/appi.ajp.160.2.284. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen J, Skadhede S, Correll CU. Antipsychotics associated with the development of type 2 diabetes in antipsychotic-naïve schizophrenia patients. Neuropsychopharmacol. 2010;35:1997–2004. doi: 10.1038/npp.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen D, De Hert M. Endogenic and iatrogenic diabetes mellitus in drug-naive schizophrenia: the role of olanzapine and its place the psychopharmacological treatment algorithm. Neuropsychopharmacol. 2011;36:2368–2369. doi: 10.1038/npp.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohen D. Diabetes mellitus and schizophrenia: historical perspective. Br J Psychiatry. 2004;184:64–66. doi: 10.1192/bjp.184.47.s64. [DOI] [PubMed] [Google Scholar]
- 42.Cohn TA, Remington G, Zipursky RB, et al. Insulin resistance and adiponectin levels in drug-free patients with schizophrenia: a preliminary report. Can J Psychiatry. 2006;51:382–386. doi: 10.1177/070674370605100608. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez-Egea E, Bernardo M, Donner T, et al. Metabolic profile of antipsychotic-naive individuals with non-affective psychosis. Br J Psychiatry. 2009;194:434–438. doi: 10.1192/bjp.bp.108.052605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spelman LM, Walsh PI, Sharifi N, et al. Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia. Diabet Med. 2007;24:481–485. doi: 10.1111/j.1464-5491.2007.02092.x. [DOI] [PubMed] [Google Scholar]
- 45.Verma SK, Subramaniam M, Liew A, et al. Metabolic risk factors in drug-naive patients with first-episode psychosis. J Clin Psychiatry. 2009;70:997–1000. doi: 10.4088/JCP.08m04508. [DOI] [PubMed] [Google Scholar]
- 46.Graham KA, Perkins DO, Edwards LJ, et al. Effect of olanzapine on body composition and energy expenditure in adults with first-episode psychosis. Am J Psychiatry. 2005;162:118–123. doi: 10.1176/appi.ajp.162.1.118. [DOI] [PubMed] [Google Scholar]
- 47.Wu RR, Zhao JP, Liu ZN, et al. Effects of typical and atypical antipsychotics on glucose-insulin homeostasis and lipid metabolism in first-episode schizophrenia. Psychopharmacol. 2006;186:572–578. doi: 10.1007/s00213-006-0384-5. [DOI] [PubMed] [Google Scholar]
- 48.Lieberman JA, Tollefson G, Tohen M, et al. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160:1396–1404. doi: 10.1176/appi.ajp.160.8.1396. [DOI] [PubMed] [Google Scholar]
- 49.Sengupta SM, Klink R, Stip E, et al. Weight gain and lipid metabolic abnormalities induced by olanzapine first-episode, drug-naive patients with psychotic disorders. Schizophr Res. 2005;80:131–133. doi: 10.1016/j.schres.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 50.Green AI, Lieberman JA, Hamer RM, et al. Olanzapine and haloperidol in first episode psychosis: two-year data. Schizophr Res. 2006;86:234–243. doi: 10.1016/j.schres.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 51.Kahn RS, Fleischhacker WW, Boter H, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371:1085–1097. doi: 10.1016/S0140-6736(08)60486-9. [DOI] [PubMed] [Google Scholar]
- 52.Perez-Iglesias R, Mata I, Pelayo-Teran JM, Amado JA. Glucose and lipid disturbances after 1 year of antipsychotic treatment in a drug-naïve population. Schizophr Res. 2009;107:115–121. doi: 10.1016/j.schres.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 53.Oriot P, Feys JL, Mertens de Wilmars S, et al. Insulin sensitivity, adjusted beta-cell function and adiponectinaemia among lean drug-naive schizophrenic patients treated with atypical antipsychotic drugs: a nine-month prospective study. Diabetes Metab. 2008;34:490–496. doi: 10.1016/j.diabet.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell AJ, Delaffon V, Vancampfort D, et al. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med. 2011;10:1–23. doi: 10.1017/S003329171100105X. [DOI] [PubMed] [Google Scholar]
- 55.De Hert M, Vancampfort V, Correll C, et al. A systematic evaluation and comparison of the guidelines for screening and monitoring of cardiometabolic risk in people with schizophrenia. Br J Psychiatry. 2011;199:99–105. doi: 10.1192/bjp.bp.110.084665. [DOI] [PubMed] [Google Scholar]
- 56.Straker D, Correll CU, Kramer-Ginsberg E, et al. Cost-effective screening for the metabolic syndrome in patients treated with second-generation antipsychotic medications. Am J Psychiatry. 2005;162:1217–1221. doi: 10.1176/appi.ajp.162.6.1217. [DOI] [PubMed] [Google Scholar]
- 57.Srinivasan T, Ling-Ern C. Body mass index as a screening test for metabolic syndrome in schizophrenia and schizoaffective disorders. Australas Psychiatry. 2007;15:470–473. doi: 10.1080/10398560701636906. [DOI] [PubMed] [Google Scholar]
- 58.Szarek BL, Goethe JW, Woolley SB. Assessing metabolic syndrome: waist circumference versus BMI. Schizophr Res. 2009;108:295–296. doi: 10.1016/j.schres.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 59.De Hert M, Schreurs V, Sweers K, et al. Typical and atypical antipsychotics differentially affect long-term incidence rates of the metabolic syndrome in first-episode patients with schizophrenia: a retrospective chart review. Schizophr Res. 2008;101:295–303. doi: 10.1016/j.schres.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 60.Shi L, Ascher-Svanum H, Chiang Y, et al. Predictors of metabolic monitoring among schizophrenia patients with a new episode of second-generation antipsychotic use in the Veterans Health Administration. BMC Psychiatry. 2009;9:80. doi: 10.1186/1471-244X-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hodgson R, Adeyemo O. Physical examination performed by psychiatrists. Int J Psychiatr Clin Pract. 2004;8:57–60. doi: 10.1080/13651500310004830. [DOI] [PubMed] [Google Scholar]
- 62.American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinology, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65:267–272. doi: 10.4088/jcp.v65n0219. [DOI] [PubMed] [Google Scholar]
- 63.Vancampfort D, Knapen J, De Hert M, et al. Cardiometabolic effects of physical activity interventions for people with schizophrenia. Phys Ther Rev. 2009;14:388–398. [Google Scholar]
- 64.Mukundan A, Faulkner G, Cohn T, Remington G. Antipsychotic switching for people with schizophrenia who have neuroleptic-induced weight or metabolic problems. Cochrane Database Syst Rev. 2010;(12):CD006629. doi: 10.1002/14651858.CD006629.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Hert M, Detraux J, van Winkel R, et al. Antipsychotic medications metabolic, cardiovascular and cardiac risks [published online ahead of print October 18, 2011] Nat Rev Endocrinol. doi:10.1038/nrendo.2011.156. [Google Scholar]
- 66.Stroup TS, McEvoy JP, Ring KD, et al. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP) Am J Psychiatry. 2011;168:947–956. doi: 10.1176/appi.ajp.2011.10111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.