Abstract
Background: Numerous studies have demonstrated sensory gating deficits in schizophrenia. However, only a few longitudinal studies report on the effects of antipsychotic treatment on sensory gating deficits and their results are inconsistent. In the present study, P50 suppression and its neural generators were investigated in antipsychotic-naïve first-episode patients with schizophrenia before and after 6 months of treatment with quetiapine. Methods: Thirty-four antipsychotic-naïve first-episode schizophrenia patients and age and gender matched healthy controls were tested in an auditory sensory gating paradigm at baseline and after 6 months. During this period, the patients were treated with quetiapine, while controls received no treatment. Sixteen patients completed the study. Results: Patients showed significant reduced P50 suppression compared with controls at baseline but not at follow-up. Furthermore, a significant positive correlation between baseline P50 suppression and dose of quetiapine at follow-up was found. P50 suppression in patients receiving above median dosages of quetiapine increased significantly from baseline to follow-up. At baseline, a frontocentral source was significantly more active in patients than in controls at the time of the testing stimulus. Conclusions: The present findings suggest that P50 suppression deficits are already present at an early stage of schizophrenia. Furthermore, particularly those patients with more severe gating deficits appeared to need higher dosages of quetiapine, although their clinical symptoms did not seem to indicate this. Quetiapine treatment significantly improved these gating deficits. Furthermore, a frontocentral source in the brain appeared to be involved in the deficient P50 gating of the patients.
Keywords: antipsychotic-naïve, first episode, quetiapine, schizophrenia, sensory gating, source localization
Introduction
The ability to successfully filter or gate sensory information is an important feature of a healthy individual. Patients with schizophrenia show deficits in sensory gating which theoretically may contribute to a state of flooding of higher brain functions and ultimately may lead to the formation of psychoses.1,2 Early aspects of sensory gating can be quantified with a paired stimulus paradigm, in which 2 identical clicks of sound are presented with a 500 ms interstimulus interval. A healthy subjects’ P50 amplitude is usually significantly decreased in response to the second of these clicks. This decreased response is assumed to be the result of the first stimulus being processed by the brain, which effectively gates the processing of the second stimulus. There are many studies showing that P50 suppression is reduced in patients with schizophrenia.2–4
P50 suppression has generally been studied in medicated and/or chronically ill schizophrenia patients.3,5,6 Only a few studies have investigated P50 gating deficits in the early stages of illness or in first-episode schizophrenia patients.7–10 However, only 2 of these first-episode studies included substantial numbers (n > 45, compared with n < 5 in the other 2 studies) of antipsychotic-naïve patients.9,10 Except for one,8 all these studies reported reduced P50 suppression in patients compared with healthy controls.
Literature on the effects of antipsychotic treatment on P50 suppression is sparse and results are inconsistent. Some authors point toward a positive effect of atypical antipsychotics, in particular clozapine,7,11–14 but this is not always found, for reviews, see ref.4,15 The inconsistencies might be related to differences in receptor profile of the antipsychotics that were used or more specifically related to differences in their affinities for the D2 and 5HT2A receptors: Studies in healthy volunteers indicate that reduced D2 activity as well as increased serotonergic activity disrupts P50 suppression.16–18
The sources of P50 suppression are largely unknown, but a hippocampal involvement has frequently been proposed.2,19,20 Studies from our own laboratory using electroencephalography (EEG) source localization point toward a frontal component in P50 suppression,21,22 which is in agreement with other EEG source localization studies.23,24 To our knowledge, no previous EEG based P50 source localization studies have been performed in schizophrenia patients.
The aims of the present study were to investigate P50 suppression in antipsychotic-naïve first-episode patients with schizophrenia and to examine the subsequent effect of 6 months treatment with quetiapine on deficient P50 suppression and its sources in the brain. Quetiapine was chosen because it has a more pronounced (higher and longer lasting affinity) effect on 5HT2A than on D2 receptors (lower affinity and only a transient effect) in its clinically effective dosage range.25,26 Given these specific characteristics, it was expected that quetiapine would ameliorate P50 suppression deficits in the patients. Furthermore, based on our previous studies, a source in the frontal areas of the brain was expected to be involved in P50 suppression. Finally, based on our hypothesis that quetiapine will ameliorate P50 suppression deficits, it was expected that this frontal source in schizophrenia patients would be more active in their antipsychotic-naïve state and less active following 6 months treatment with quetiapine at the time of their response to testing stimuli.
Methods
The study was approved by the Ethics Committee of the Capital Region (H-KF-01-78/97), Copenhagen, with regards to the ethical principles for medical research involving human subjects as stated in the declaration of Helsinki (amendment of Washington, 2002). Written and oral informations were given, after which written informed consent was obtained from all subjects.
Subjects
Antipsychotic-naïve first-episode schizophrenia patients between 18 and 45 years of age were recruited from psychiatric hospitals and related outpatient clinics in the Copenhagen municipality and county. Diagnoses (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [DSM-IV]) were ascertained with the Schedule for Clinical Assessment in Neuropsychiatry, version 2.1 (SCAN).27 Each patient was matched on age and gender to a healthy control subject (on a one-to-one basis) with no personal (ascertained by a SCAN interview) or first-degree family history of psychiatric illness. Both patients and healthy controls passed a physical examination before inclusion to ascertain that they were all physically healthy. In addition, the subjects’ medical history and history of drug and alcohol abuse were collected. The patients’ symptomatology was rated with the Positive and Negative Syndrome Scale (PANSS).28 Exclusion criteria were coercive measures, a history of mental retardation, organic brain damage, organic psychosis, and substance dependence. To screen for hearing deficits, subjects were tested at 500, 1000, and 6000 Hz (40 dB). Among the included subjects, 11 patients fulfilled DSM-IV criteria for former or ongoing substance abuse. Concomitant treatment with benzodiazepines was allowed on an “if needed basis,” and 14 patients were in either episodic or regular treatment with benzodiazepines (oxazepam) at the time of the baseline investigations. Two patients were treated with antidepressants at the time of the baseline assessments. More than 60% of the patients were smokers, whereas 20% of the controls were smokers. In total, 34 patients (mean age 25.2 y, SD 4.9) and 34 matched healthy control subjects (mean age 26.2 y, SD 5.1) completed the psychophysiological test battery at baseline. Due to hardware failure and missing data, P50 assessments of 3 patients and 2 controls were lost at baseline, while 1 patient did not show a P50 waveform to conditioning stimuli at baseline. Table 1 shows the characteristics of the remaining 30 patients and 32 healthy control subjects at baseline, including their age and sex distribution.
Table 1.
Demographics. Characteristics of Patients and Matched Healthy Controls at Baseline and Follow-Up
| Patients Baseline | Controls Baseline | Patients Follow-Up | Controls Follow-Up | ||
| Mean age (SD) | |||||
| All | 25.2 (5.0) | 25.8 (4.9) | 26.7 (5.6) | 27.4 (5.8) | |
| Male | 24.9 (4.4) | 25.4 (4.2) | 26.2 (5.0) | 27.0 (4.4) | |
| Female | 25.7 (6.5) | 26.6 (6.6) | 27.8 (7.5) | 28.3 (8.4) | |
| Gender N | |||||
| Male | 22 | 23 | 11 | 11 | |
| Female | 8 | 9 | 5 | 5 | |
| Total | 30 | 32 | 16 | 16 | |
| Average PANSS score (SEM) | |||||
| Positive | 20.9 (1.0) | 16.8 (1.4)* | |||
| Negative | 22.8 (1.4) | 21.6 (1.2) | |||
| General | 41.7 (2.0) | 39.7 (2.3) | |||
| Total | 85.4 (3.5) | 78.1 (4.5) | |||
| Patients who completed | |||||
| Positive | 21.6 (1.1) | ||||
| Negative | 23.6 (1.6) | ||||
| General | 43.3 (2.2) | ||||
| Total | 88.4 (3.9) | ||||
| Patients who dropped out | |||||
| Positive | 18.3 (0.8)** | ||||
| Negative | 21.5 (1.2) | ||||
| General | 39.2 (1.8) | ||||
| Total | 78.9 (2.9) | ||||
Note: PANSS, Positive and Negative Syndrome Scale.
*Significantly reduced compared with baseline, P = 0.001; **Significantly lower than patients who completed.
After a period as close to 6 months as possible (average: 6.2, SD = 3.1), all assessments were repeated. During this period, the patients were treated with quetiapine in flexible doses, while the controls received no treatment. At follow-up, the average quetiapine dose was 509.4 mg (SD = 281.8 mg, range 100–1200 mg; the median dose was 500.0 mg). Three patients were excluded due to noncompliance, while 3 patients were excluded because they never started treatment. In addition, 3 patients discontinued the treatment, either because of intolerable side effects or due to lack of efficacy. Finally, 7 patients refused to be retested or dropped out for unknown reasons. Because the baseline assessment of 2 patients was missing, this resulted in a total of 16 patients with their matched controls who had a data set at both baseline and follow-up (none of their controls dropped out) (see table 1). Of 3 subjects, 2 patients (1 at baseline and 1 at follow-up) and 1 control (at follow-up), no P50 waveform, could be identified.
Experimental Design
All subjects were tested in the Copenhagen Psychophysiological Testbattery16,22,29 on 2 occasions separated by a period as close to 6 months as possible. In order to keep the article focused, only the results on P50 suppression will be reported. Although we acknowledge the relevance of investigating later components, eg, the N100 and P200 in a sensory gating paradigm in schizophrenia research as well, we neither scored nor analyzed those data in the current study. To avoid acute and/or withdrawal effects of nicotine, smoking was not allowed 1 hour prior to testing.
P50 Suppression Paradigm
The method has been described before.16,22 In short, subjects were seated in a room with a sound level below 40 dB. Subjects were instructed to sit still, to keep their eyes fixed on a spot on the wall directly in front of them, and were asked to stay awake. During P50 gating testing, 3 experimental blocks were presented, each consisting of 40 pairs of bursts of (1.5 ms and 80 dB) white noise, with an instantaneous rise time, an interstimulus interval of 500 ms, and a fixed intertrial interval of 10 seconds.
Signal Recording
EEG recordings were performed with BioSemi hardware, using a cap with 64 active electrodes.30 All signals were digitized online by a computer with a rate of 4096 Hz.
P50 Suppression Assessment
BESA software (version 5.2.4, MEGIS Software GmbH) was used for further processing of the data. First, the EEG data was downsampled to a rate of 250 Hz after which it was corrected for eye movement by applying the surrogate model of BESA.31 Thereafter, correction of movement and other non–paradigm-related artifacts was performed by removing those epochs from the database that exceeded amplitude differences between maximum and minimum in the epoch of 100 μV. Data was epoched and averaged between 100 ms prestimulus and 400 ms poststimulus. Averaged epochs were then filtered (high pass 1.6 Hz, 24 dB/octave and low pass at 70 Hz, 24 dB/octave). P50 amplitudes were scored from electrode Cz only, with average reference. P50 amplitude was defined as the largest trough to peak amplitude within an interval of 40–90 ms following the first (conditioning or “C”) stimulus in each paired click. The P50 amplitude following the second (testing or “T”) stimulus was identified as the largest trough to peak amplitude within an interval of ±10 ms of the latency of the maximum P50 amplitude to the C-stimulus. P50 suppression was expressed as the ratio “T/C.”
Source Analysis P50
Source analysis was performed with BESA software. The P50 source model as previously found in our laboratory21,22 was used as a basis. The sources of this model were set to regional, after which the model (excluding the eye sources) was refitted to the grand-average data of the C-stimuli (filters: 0.5—70 Hz) of all healthy controls at baseline, within an interval between 40 and 66 ms (coinciding with the beginning and end of the P50 waveform, see figure 1). This model was used to analyze the individual data: the first component of each of the regional sources was oriented to maximum, at the exact latency of a subject’s maximum P50 amplitude to the C-stimuli (filters: 0.5—70 Hz), after which its amplitude (source strength) was scored. Following this, the model was applied (unaltered) to the subject’s response to the T-stimuli (filters: 0.5—70 Hz) either at the latency of maximum P50 amplitude or, in the case the subject did not show a P50 amplitude to the T-stimulus, at the latency of the subject’s maximum P50 amplitude to the C-stimulus. After which, again, the amplitude of the first component (source strength) was scored.
Fig. 1.
Grand average data showing the subjects' responses to conditioning (C-stim) and testing (T-stim) stimuli, specified for controls and patients, and for baseline (30 patients and 32 controls) and follow-up (15 patients and 15 controls).
Statistical Analysis
All statistical analyses were performed with SPSS (version 11.0, SPSS Inc.). Initially, the P50 amplitude and P50 suppression data were analyzed with a univariate ANCOVA in which gender, smoking, substance abuse, and benzodiazepine were used as covariates. Because the P50 suppression data (ratio T/C) were non-normally distributed (Kolmogorov-Smirnov z = 2.01, P = .001), we normalized the T/C scores by Winsorizing them to 1.5 SD above the mean. Furthermore, to reduce loss of power, we replaced the 3 empty cells (see “subjects” above) with their group means. This last procedure and the Winsorized data were solely used for the purpose of performing the (parametric) AN(C)OVAs, ie, all nonparametric tests were performed on the unaltered data set, including the 3 empty cells. Since smoking has a known, yet transient, effect on P50 suppression,32,33 we tried 2 different approaches in our analyses: a dichotomized (yes/no smoking) and a categorical approach (no smoking, light [1–9/d], medium [10–19/d], and heavy [above 20/d]). However, none of the smoking or any of the other covariates reached statistical significance and were therefore removed from the analyses. The data were further analyzed with nonparametric Mann-Whitney tests. The source strengths of component 1 of the regional sources were analyzed stepwise in much the same way as the P50 amplitude and P50 suppression data. Further analyses were performed with independent samples Student’s t tests because the source strength data were normally distributed.
The effect of medication on psychopathology (PANSS positive, negative, general, and total scores) was analyzed with paired samples Student’s t tests. Correlations between P50 suppression and symptomatology were analyzed by means of Spearman correlation tests.
Results
Dropout
There were no significant differences in age between those patients and controls who completed the test and those who dropped out. Furthermore, there were no significant differences in psychopathology between those patients who completed the tests and those who dropped out, except that the patients who dropped out scored significantly lower ( = 18.3) on the PANSS positive scale than those who completed ( = 21.6) the study (t = 2.39, df = 30, P = .024; see table 1). To analyze the effect of dropouts on or our key dependent variable of interest, P50 suppression, a univariate analysis with factor group (completer patients, dropout patients, and their matched healthy controls) was performed, showing a trend level of significance (F 2,62 = 2.67, P = .078). Further testing revealed that only those patients who completed the study showed significant differences in T/C ratio ( = 0.58) compared with controls ( = 0.23) (z = 2.51, P = .012), whereas those patients who dropped out did not ( = 0.30) (z = 1.83, P = .067).
P50 Amplitude and P50 Suppression Data
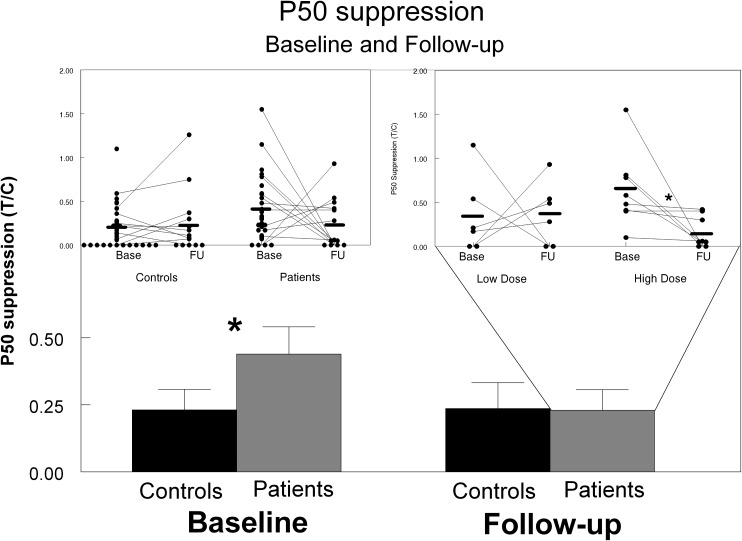
The analysis of the P50 suppression data (see figure 1) revealed a highly significant increased T/C ratio in patients compared with controls at baseline (z = 2.66, P = .008). This increased T/C ratio appeared to be based on a significantly higher P50 amplitude to T-stimuli in the patients compared with the controls (z = 2.40, P = .016), while the P50 amplitude to the C-stimuli were not significantly different between the groups (z = 0.22, P = .827). At follow-up, the ANOVA revealed a main effect of group (F 1,30 = 8.34, P = .01) and a near significant group × treatment interaction (F 1,30 = 3.98, P = .055). Further testing showed a significant group difference in baseline P50 suppression (z = 2.36, P = .019) also in this much smaller group of subjects of whom both baseline and follow-up data were available (14 patients and 15 controls), indicating the robustness of the results. At follow-up, however, no significant group differences in P50 suppression were found (z = 0.12; P = .902). These further analyses indicate that the main effect of group as found in the ANOVA was predominantly driven by the baseline data which is also reflected in the average T/C ratios, showing a large difference between patients and controls at baseline, but virtually no difference at follow-up (see figure 2). The increase in P50 suppression of the patients from baseline to follow-up did not correlate with their treatment period (ranging from 5.7 to 8 mo). Interestingly, although P50 suppression did not significantly change in the patient group as a whole from baseline to follow-up, it did improve significantly in those patients receiving equal or higher than the median dose (500 mg) of quetiapine (z = 2.52, P = .012; figure 2), by significantly decreasing their P50 amplitudes to T-stimuli (z = 1.96, P = .050) while not affecting their P50 amplitudes to C-stimuli (z = 1.68, P = .092). It has to be noted, however, that only this subgroup of high-dosed patients had P50 suppression deficits at baseline compared with the controls (high-dosed patients: z = 3.08, P = .001 and low-dosed patients: z = 0.77, P = .49).
Fig. 2.
P50 suppression (T/C ratio, ± SEM) in patients and healthy controls at baseline and follow-up, indicating significantly reduced P50 suppression in the patients compared with the controls. Inlay left: scatterplot of the individual ratio data; Inlay right: individual ratio of patients split on the median dosage (500 mg) of quetiapine.
P50 Source Localization Data
Fitting the P50 model as previously found in our laboratory21,22 to the grand average data of the control subjects’ responses to the C-stimuli at baseline resulted in a residual variance of 5.48% with a best fit of 1.83% (see figure 3). Only the strength of the source with the frontocentral location correlated significantly with the P50 amplitude to the C-stimuli both in healthy controls (r P = .62, P < .001) and patients (r P = .62, P < .001), the other 2 sources did not (see online supplementary figure 1). No group difference was found in any of the 3 source strengths at the time of maximum P50 amplitude to the C-stimuli. However, the strength of the frontocentral source at the time of maximum P50 amplitude to the T-stimuli was significantly higher in patients than in the healthy controls (t = 3.04, P = .04). At follow-up, no significant group differences were found, neither did the source strengths significantly change between baseline and follow-up within the groups (see table 2).
Fig. 3.
Source model of P50 amplitude (controls) at baseline, showing (A) 3D view (B) coronal view.
Table 2.
Source Strengths. Average Source Strength (SEM) Specified for Controls and Patients, for Baseline and Follow-Up, and for Conditioning (C-stim) and Testing (T-stim) Stimuli
| Controls | Patients | |||||||
| Baseline (N = 32) | Follow-Up (N = 16) | Baseline (N = 30) | Follow-Up (N = 16) | |||||
| C-stim | T-stim | C-stim | T-stim | C-stim | T-stim | C-stim | T-stim | |
| Frontocentral | 30.6 (3.1) | −5.1 (2.4) | 25.4 (4.3) | 1.4 (3.3) | 26.6 (3.1) | 4.6* (2.1) | 28.4 (4.3) | −1.2 (3.4) |
| Right temporal | 21.4 (2.5) | 3.4 (2.6) | 18.6 (2.4) | 3.8 (2.6) | 23.2 (2.1) | 2.6 (2.7) | 19.3 (2.8) | 0.9 (3.8) |
| Left temporal | 23.2 (3.0) | 3.2 (2.1) | 17.3 (2.2) | 5.7 (2.9) | 22.7 (1.7) | 4.0 (2.0) | 20.4 (2.8) | 2.0 (4.5) |
*Significantly higher than controls (P = .04).
Psychopathology
A significant reduction in the positive symptom subscale of the PANSS was found in the patients from baseline to follow-up (t = 3.14, P = .001). The other subscales of the PANSS (negative, general, and total) showed no significant changes (see table 1).
Correlations
The baseline P50 ratio of the patients appeared to be predictive of the dose of quetiapine at follow-up (rS= .59, P = .026) and was based on the correlation between the dose of quetiapine and the patients’ response to the T-stimuli (r S = .70, P = .005, see online supplementary figure 2). The P50 suppression ratio at baseline was neither predictive for weight gain, body mass index (BMI) nor occurrence of extrapyramidal side effects (EPS). None of the PANSS (sub)scores correlated significantly with any of the P50 suppression variables (C, T, and T/C) nor with the dose of quetiapine. Furthermore, no significant differences in PANSS (sub)scores were found between those patients with a baseline T/C ratio above the median (T/C = 0.30) and those below. However, a significant negative correlation was found between the difference in T/C ratio at baseline and follow-up and the PANSS general score (r P = .60, P = .030, see online supplementary figure 3).
Discussion
This is the first longer lasting longitudinal study on auditory P50 suppression in antipsychotic-naïve first-episode schizophrenia patients and matched healthy controls.
We found significantly impaired P50 suppression in schizophrenia patients compared with healthy controls at baseline, which is consistent with 2 previous reports on P50 suppression in antipsychotic-naïve first-episode schizophrenia.9,10 No previous longitudinal studies have reported on the effects of quetiapine treatment on P50 suppression deficits in antipsychotic-naïve first-episode schizophrenia patients. In the current study, a 6-month quetiapine treatment normalized the P50 gating deficits of the patients. Our results are in contrast with the results obtained by Adler et al14 who found P50 suppression deficits in a rather small group (n = 9) of patients who were treated with quetiapine. Besides the rather low number of included quetiapine treated patients, there were a number of other methodological differences between the study of Adler et al14 and our study that could have accounted for the discrepancy in results: they used a cross-sectional design, where patients were clinically stable for a minimum of 1 month before testing, of whom only a small proportion were medication free for 2 months prior to testing. Furthermore, several of their patients were treated with additional other antipsychotics. Our current study had a longitudinal design and included only antipsychotic-naïve first-episode patients who were subsequently treated with antipsychotic monotherapy for 6 months.
Only 3 longitudinal studies have investigated the effects of other atypical antipsychotics on P50 suppression. Hong et al9 found no effects of treatment with either risperidone or clozapine on P50 suppression in antipsychotic-naïve first-episode schizophrenia patients, while Arango et al34 found neither an effect of olanzapine nor of haloperidol on P50 gating deficits in treatment resistant schizophrenia patients. In contrast, Nagamoto et al12,35 found significantly increased P50 suppression in a small group (n = 6) of treatment resistant schizophrenia patients following clozapine treatment. However, clozapine appeared to improve P50 suppression by increasing amplitudes to C-stimuli, instead of decreasing those to T-stimuli, as reviewed by Potter, Summerfelt, Gold, and Buchanan,15 which is not indicative of truly enhanced P50 suppression as suggested by the hypothesis of Adler et al.3 In the current study, we found quetiapine to improve P50 suppression by significantly decreasing the patients’ response to T-stimuli, while not affecting their response to C-stimuli which, according to the hypothesis of Adler et al,3 would be indicative of truly enhanced P50 suppression.
The patients’ baseline P50 suppression (T/C ratio) appeared to be predictive for the dose of quetiapine at follow-up. In fact, only those patients treated with equal or above median (500 mg) dosages of quetiapine showed significantly decreased P50 suppression at baseline, while the ones treated with dosages below the median showed near normal levels of gating. It is not likely that these findings are a reflection of patients with higher T/C ratios just being less susceptible for quetiapine’s side effects: The T/C ratio at baseline was not related to those side effects that were assessed in our study: weight gain, BMI, and level of EPS. However, we cannot fully exclude that patients with lower P50 suppression were less sensitive for other relevant side effects, such as sedation and dizziness. Nevertheless, it is more likely that these patients were in genuine clinical need for higher levels of medication, although there was no clear indication for this on a symptomatological level: At baseline, T/C ratio did neither correlate with any of the PANSS (sub)scores nor did the baseline PANSS (sub)score differ significantly between those subjects with above median T/C ratios and those below. Taking all this together, our data suggest that the more severe a patient's P50 gating deficit was, the higher the dose of quetiapine he or she apparently needed to reach a clinical satisfactory level. Counterintuitively, however, the more a patient's P50 suppression increased from baseline to follow-up, the less his/her total PANSS general score decreased during that same time period. However, the data from the current study cannot explain this finding, so further research is necessary.
It is not clear from the current study which of quetiapine’s pharmacologic actions might be the mechanism by which it improved P50 suppression in our patients with schizophrenia. In general, however, both haloperidol (D2 antagonist) and high doses of escitalopram (highly specific selective serotonin reuptake inhibitor or SSRI) disrupt (high) P50 suppression in healthy volunteers.16,18,22 Therefore, it may be the combination of low D2 with high 5HT2A blockage of quetiapine that is behind its ability to improve P50 suppression as found in the current study.
In the present study, the same 3 generators of the P50 evoked potential as previously found in our earlier studies21,22 explained most of its variance: 2 symmetrically located sources in the temporal cortex and 1 located in a frontocentral position. Similar to these previous results, the current results showed that the P50 amplitude was highly positively correlated in both patients and controls with the strength of the frontocentral source only. Our results appear to confirm those of 2 studies in which in-depth electrodes were implanted in epilepsy patients undergoing invasive presurgical evaluation,36,37 and a later magnetic encephalography study from that same group,38 all pointing toward a crucial role of the frontal lobe in sensory gating. In a recent study from our laboratory with concurrent EEG and functional magnetic resonance imaging methodology, we identified, among others, a source of P50 gating in the medial frontal gyrus.39 Interestingly in the current study, at baseline, the group of patients showed a significantly higher activity in the frontocentral source strength at the time of the T-stimuli, which had disappeared at follow-up, coinciding with the disappearance of the P50 suppression deficit. It is therefore tempting to assume that the higher activity of this source is somehow related to the reduced level of P50 suppression of the patients at baseline. However, any further assumptions would be pure speculation.
The current study may have several clinical implications. The results indicate that P50 suppression deficits disappear by 6-month quetiapine treatment. Since deficient sensory gating in theory may contribute to psychosis,1,2 our results may indicate that the patients are less susceptible for relapse over time. Of particular interest is that despite a similar symptomatological profile (indicated by the PANSS scores), patients with more severe P50 suppression deficits appeared to need higher dosages of quetiapine compared with those patients with less severe gating deficits. Further research is needed to investigate the symptomatological profile of patients with severe P50 suppression deficits in more detail, eg, with global assessment of functioning scores.
A limitation in the current study was that adjunctive antidepressants, anxiolytic medication, and substance abuse were no exclusion criteria. However, these possible confounders did not significantly covariate with P50 suppression, so it is unlikely that they have affected the current results.
Summarized, the present findings suggest that P50 suppression deficits are already present at an early stage of schizophrenia. Furthermore, our data show that particularly those patients with more severe P50 gating deficits appeared to need higher dosages of quetiapine, although their clinical symptoms did not seem to indicate this. Evidence was found that a frontocentrally located source in the brain was more active at baseline in the patients’ response to testing stimuli than in the controls. Six months of quetiapine treatment improved the P50 suppression deficits, especially in those patients who were treated with higher dosages of quetiapine.
Funding
The study was sponsored by the Danish Medical Research Council; the Copenhagen Hospital Cooperation Research Council; Copenhagen University Hospital Bispebjerg; the Faculty of Health Sciences of the University of Copenhagen; the Copenhagen Council Research Foundation (1123); The Lundbeck Foundation (192/05, 192/04, R25-A2701) and a nonrestricted grant from AstraZeneca.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Braff DL, Geyer MA. Sensorimotor gating and schizophrenia: human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- 2.Freedman R, Waldo MC, Bickford-Wimer PC, Nagamoto H. Elementary neuronal dysfunctions in schizophrenia. Schizophr Res. 1991;4:233–243. doi: 10.1016/0920-9964(91)90035-p. [DOI] [PubMed] [Google Scholar]
- 3.Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- 4.Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res. 2004;70:315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Arnfred SM, Chen AC, Glenthoj BY, Hemmingsen RP. Normal p50 gating in unmedicated schizophrenia outpatients. Am J Psychiatry. 2003;160:2236–2238. doi: 10.1176/appi.ajp.160.12.2236. [DOI] [PubMed] [Google Scholar]
- 6.Freedman R, Adler LE, Waldo MC, Pachtman E, Franks RD. Neurophysiological evidence for a defect in inhibitory pathways in schizophrenia: comparison of medicated and drug-free patients. Biol Psychiatry. 1983;18:537–551. [PubMed] [Google Scholar]
- 7.Yee CM, Nuechterlein KH, Morris SE, White PM. P50 suppression in recent-onset schizophrenia: clinical correlates and risperidone effects. J Abnorm Psychol. 1998;107:691–698. doi: 10.1037//0021-843x.107.4.691. [DOI] [PubMed] [Google Scholar]
- 8.de Wilde OM, Bour LJ, Dingemans PM, Koelman JH, Linszen DH. Failure to find P50 suppression deficits in young first-episode patients with schizophrenia and clinically unaffected siblings. Schizophr Bull. 2007;33:1319–1323. doi: 10.1093/schbul/sbm001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong X, Chan RC, Zhuang X, et al. Neuroleptic effects on P50 sensory gating in patients with first-episode never-medicated schizophrenia. Schizophr Res. 2009;108:151–157. doi: 10.1016/j.schres.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Brockhaus-Dumke A, Schultze-Lutter F, Mueller R, et al. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biol Psychiatry. 2008;64:376–384. doi: 10.1016/j.biopsych.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Boutros NN, Zouridakis G, Rustin T, Peabody C. The P50 component of the auditory evoked potential and subtypes of schizophrenia. Psychiatry Res. 1993;47:243–254. doi: 10.1016/0165-1781(93)90082-r. [DOI] [PubMed] [Google Scholar]
- 12.Nagamoto HT, Adler LE, Hea RA, Griffith JM, McRae KA, Freedman R. Gating of auditory P50 in schizophrenics: unique effects of clozapine. Biol Psychiatry. 1996;40:181–188. doi: 10.1016/0006-3223(95)00371-1. [DOI] [PubMed] [Google Scholar]
- 13.Light GA, Geyer MA, Clementz BA, Cadenhead KS, Braff DL. Normal P50 suppression in schizophrenia patients treated with atypical antipsychotic medications. Am J Psychiatry. 2000;157:767–771. doi: 10.1176/appi.ajp.157.5.767. [DOI] [PubMed] [Google Scholar]
- 14.Adler LE, Olincy A, Cawthra EM, et al. Varied effects of atypical neuroleptics on P50 auditory gating in schizophrenia patients. Am J Psychiatry. 2004;161:1822–1828. doi: 10.1176/ajp.161.10.1822. [DOI] [PubMed] [Google Scholar]
- 15.Potter D, Summerfelt A, Gold J, Buchanan RW. Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Schizophr Bull. 2006;32:692–700. doi: 10.1093/schbul/sbj050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oranje B, Wienberg M, Glenthoj BY. A single high dose of escitalopram disrupts sensory gating and habituation, but not sensorimotor gating in healthy volunteers. Psychiatry Res. 2011;186:431–436. doi: 10.1016/j.psychres.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Oranje B, Gispen-de Wied CC, Verbaten MN, Kahn RS. Modulating sensory gating in healthy volunteers. The effects of ketamine and haloperidol. Biol Psychiatry. 2002;52:887–895. doi: 10.1016/s0006-3223(02)01377-x. [DOI] [PubMed] [Google Scholar]
- 18.Csomor PA, Stadler RR, Feldon J, Yee BK, Geyer MA, Vollenweider FX. Haloperidol differentially modulates prepulse inhibition and p50 suppression in healthy humans stratified for low and high gating levels. Neuropsychopharmacology. 2008;33:497–512. doi: 10.1038/sj.npp.1301421. [DOI] [PubMed] [Google Scholar]
- 19.Bickford-Wimer PC, Nagamoto H, Johnson R, Adler LE. Auditory sensory gating in hippocampal neurons: A model system in the rat. Biol Psychiatry. 1990;27:183–192. doi: 10.1016/0006-3223(90)90648-l. [DOI] [PubMed] [Google Scholar]
- 20.Freedman R, Olincy A, Ross RG, et al. The genetics of sensory gating deficits in schizophrenia. Curr Psychiatry Rep. 2003;5:155–161. doi: 10.1007/s11920-003-0032-2. [DOI] [PubMed] [Google Scholar]
- 21.Oranje B, Geyer MA, Kenemans JL, Verbaten MN. Prepulse inhibition and P50 suppression: commonalities and dissociations. Psychiatry Res. 2006;143:147–158. doi: 10.1016/j.psychres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Jensen KS, Oranje B, Wienberg M, Glenthoj BY. The effects of increased serotonergic activity on human sensory gating and its neural generators. Psychopharmacology (Berl) 2008;196:631–641. doi: 10.1007/s00213-007-1001-y. [DOI] [PubMed] [Google Scholar]
- 23.Knott V, Millar A, Fisher D. Sensory gating and source analysis of the auditory P50 in low and high suppressors. Neuroimage. 2009;44:992–1000. doi: 10.1016/j.neuroimage.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Weisser R, Weisbrod M, Roehrig M, Rupp A, Schroeder J, Scherg M. Is frontal lobe involved in the generation of auditory evoked P50? Neuroreport. 2001;12:3303–3307. doi: 10.1097/00001756-200110290-00031. [DOI] [PubMed] [Google Scholar]
- 25.Kapur S, Zipursky R, Jones C, Shammi CS, Remington G, Seeman P. A positron emission tomography study of quetiapine in schizophrenia: a preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch Gen Psychiatry. 2000;57:553–559. doi: 10.1001/archpsyc.57.6.553. [DOI] [PubMed] [Google Scholar]
- 26.Tauscher J, Hussain T, Agid O, et al. Equivalent occupancy of dopamine D1 and D2 receptors with clozapine: differentiation from other atypical antipsychotics. Am J Psychiatry. 2004;161:1620–1625. doi: 10.1176/appi.ajp.161.9.1620. [DOI] [PubMed] [Google Scholar]
- 27.Wing JK, Babor T, Brugha T, et al. SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry. 1990;47:589–593. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]
- 28.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 29.Oranje B, Jensen K, Wienberg M, Glenthoj BY. Divergent effects of increased serotonergic activity on psychophysiological parameters of human attention. Int J Neuropsychopharmacol. 2008;11:453–463. doi: 10.1017/S1461145707008176. [DOI] [PubMed] [Google Scholar]
- 30.Metting van Rijn AC, Kuiper AP, Dankers TE, Grimbergen CA. Low-cost active electrode improves the resolution in biopotential recordings. 1996 Proceedings of the 18th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Amsterdam, The Netherlands, Track 1 2 3-3. [Google Scholar]
- 31.Berg P, Scherg M. A multiple source approach to the correction of eye artifacts. Electroencephalogr Clin Neurophysiol. 1994;90:229–241. doi: 10.1016/0013-4694(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 32.Adler LE, Hoffer LJ, Griffith J, Waldo MC. Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol Psychiatry. 1992;32:607–616. doi: 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- 33.De Luca V, Wong AH, Muller DJ, Wong GW, Tyndale RF, Kennedy JL. Evidence of association between smoking and alpha7 nicotinic receptor subunit gene in schizophrenia patients. Neuropsychopharmacology. 2004;29:1522–1526. doi: 10.1038/sj.npp.1300466. [DOI] [PubMed] [Google Scholar]
- 34.Arango C, Summerfelt A, Buchanan RW. Olanzapine effects on auditory sensory gating in schizophrenia. Am J Psychiatry. 2003;160:2066–2068. doi: 10.1176/appi.ajp.160.11.2066. [DOI] [PubMed] [Google Scholar]
- 35.Nagamoto HT, Adler LE, McRae KA, et al. Auditory P50 in schizophrenics on clozapine: improved gating parallels clinical improvement and changes in plasma 3-methoxy-4-hydroxyphenylglycol. Neuropsychobiology. 1999;39:10–17. doi: 10.1159/000026553. [DOI] [PubMed] [Google Scholar]
- 36.Grunwald T, Boutros NN, Pezer N, et al. Neuronal substrates of sensory gating within the human brain. Biol Psychiatry. 2003;53:511–519. doi: 10.1016/s0006-3223(02)01673-6. [DOI] [PubMed] [Google Scholar]
- 37.Korzyukov O, Pflieger ME, Wagner M, et al. Generators of the intracranial P50 response in auditory sensory gating. Neuroimage. 2007;35:814–826. doi: 10.1016/j.neuroimage.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiland BJ, Boutros NN, Moran JM, Tepley N, Bowyer SM. Evidence for a frontal cortex role in both auditory and somatosensory habituation: a MEG study. Neuroimage. 2008;42:827–835. doi: 10.1016/j.neuroimage.2008.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bak N, Glenthoj BY, Rostrup E, Larsson H, Oranje B. Source localization of sensory gating: a combined EEG and fMRI study in healthy volunteers. Neuroimage. 2010;54:2711–2718. doi: 10.1016/j.neuroimage.2010.11.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.