Abstract
We examined the antihypertensive effects of valsartan, aliskiren or both drugs combined on circulating, cardiac and renal components of the renin-angiotensin system (RAS) in congenic mRen2.Lewis hypertensive rats assigned to: vehicle (n=9), valsartan (via drinking water, 30 mg/kg/day; n=10), aliskiren (s.c. by osmotic mini-pumps, 50 mg/kg/day; n=10), or valsartan (30 mg/kg/day) combined with aliskiren (50 mg/kg/day; n=10). Arterial pressure and heart rate were measured by telemetry before and during two weeks of treatment; trunk blood, heart, urine and kidneys were collected for measures of RAS components. Arterial pressure and left ventricular weight/tibia length ratio were reduced by monotherapy of valsartan, aliskiren and further reduced by the combination therapy. Urinary protein excretion was reduced by valsartan and further reduced by the combination. The increases in plasma Ang II induced by valsartan were reversed by the treatment of aliskiren and partially suppressed by the combination. The decreases in plasma Ang-(1–7) induced by aliskiren recovered in the combination group. Kidney Ang-(1–12) was increased by the combination therapy while the increases in urinary creatinine mediated by valsartan were reversed by addition of aliskiren. The antihypertensive and antiproteinuric actions of the combined therapy were associated with marked worsening of renal parenchymal disease and increased peritubular fibrosis. The data show that despite improvements in the surrogate endpoints of blood pressure, ventricular mass and proteinuria, dual blockade of Ang II receptors and renin activity is accompanied by worsening of renal parenchymal disease reflecting a renal homeostatic stress response due to loss of tubuloglomerular feedback by Ang II.
Keywords: angiotensin-(1–12), aliskiren, arterial remodeling, direct renin inhibitors, urinary protein, valsartan
Introduction
The lessons learned from studies in humans and animals using angiotensin converting enzyme (ACE) inhibitors showed the existence of alternate mechanisms for angiotensin II (Ang II) generation, through either ACE escape or direct conversion of Ang I to Ang II by chymase. These findings led to exploring the utility of therapies in which ACE inhibitors and Ang II type1 receptor (AT1R) blockers (ARBs) were combined to optimize renin-angiotensin system (RAS) blockade.1 Although the rationale for blocking the system at multiple foci was compelling, the increased risk of acute dialysis and hyperkaliemia found in the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET)2 discouraged this therapeutic approach.3 The introduction of an orally active direct renin inhibitor (DRI) created an opportunity to bypass the large reactive increase in renin secretion induced by interruption of the Ang II negative feedback on renin release.4 Although aliskiren hemifumarate, the first orally effective DRI for the treatment of hypertension, showed efficacy as an antihypertensive agent and in target organ protection, it failed to suppress Ang II formation as the valsartan/aliskiren combination was more effective than each drug alone.5 Enthusiasm for combined blockade of either ACE inhibitors or DRI with ARB is now further questioned given the recent halting of the Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints (ALTITUDE) trial6 in which patients receiving the combination of aliskiren with either valsartan or an ACE inhibitor (http://www.theheart.org/article/1331173.do) experienced an increased incidence of nonfatal stroke, renal complications, hyperkalemia, and hypotension over 18 to 24 months of follow-up.7
The complexity of the physiological mechanisms that participate in the expression of angiotensin peptides is further underscored by the identification of an extended form of Ang I –proangiotensin 12 [Ang-(1–12)]– by Nagata et al.8 Evidence that Ang-(1–12) acts as an alternate pathway for Ang II production in rodents8–13 and human tissue14 led us to conduct a rigorous investigation of the effect of combining aliskiren with valsartan on blood pressure, the circulating and tissue RAS, and renal excretory function. These studies were done in a congenic model of hypertension expressing increased tissue renin.15
Methods
Animals
Experiments in male 10 week-old mRen2.Lewis rats were performed in accordance with National Institutes of Health guidelines and were approved by the Wake Forest University animal care and use committee.
Arterial pressure, heart rate and locomotive activity measurements
Continuous measures of arterial pressure and heart rate were obtained from telemetry probes chronically implanted as reported previously.16 Delta mean arterial pressure (MAP) changes were calculated as the difference between the 3-day MAP averages obtained during the baseline period and the 7-day MAP average during the last treatment-week.
Treatment protocol
After a 2 weeks recovery period from telemetry probe implantation, rats were randomized to receive either: (a) vehicle, (b) valsartan (30 mg/kg/day), (c) aliskiren (50 mg/kg/day), or (d) both drugs [valsartan (30 mg/kg/day) and aliskiren (50 mg/kg/day)]. Valsartan was given in the drinking water and aliskiren was subcutaneously delivered by osmotic mini-pumps (ALZET® model 2M2L; DURECT Corp., Cupertino, CA). Pumps containing saline were implanted to vehicle and valsartan groups. After two weeks treatment, animals were decapitated and trunk blood, heart and kidneys were collected and processed as previously described.15 Doses of aliskiren alone or combined with valsartan were shown to reverse hypertension and cardiac hypertrophy in both transgenic m(Ren2)2717 and congenic mRen2.Lewis hypertensive rats.16 Valsartan and aliskiren were a kind gift from Novartis, Inc.
Biochemistry
Plasma renin activity (PRA), defined as the rate of Ang I generation from endogenous substrate, was measured in incubated plasma treated with EDTA and PMSF to prevent the degradation of the generated peptide. Plasma renin concentration (PRC) was defined as the rate of Ang I generation from renin in samples incubated at pH 6.5 for 90 min with excess exogenous angiotensinogen (Aogen) obtained from nephrectomized rat plasma.16
Ang-(1–12) in plasma, left ventricle and kidney cortex (groups: a, b, and d) were measured by radioimmunoassay (RIA) by our collaborators (SN and JK), as described elsewhere8 while urinary Ang-(1–12), Ang II and Ang-(1–7) were measured in our laboratory.15,18 RIA for urinary Ang-(1–12) was performed utilizing an antibody produced to the unique C-terminus of rat Ang-(1–12) by coupling the N-terminal region to KLH via cysteine linkage. The primary antibody was diluted to 1:20,000 in 50 mM HEPES (pH 7.4) 125 mM NaCl, 5 mM EDTA containing 0.5% BSA and 0.5% Triton-X to reduce non-specific absorption. Varying concentrations of standard Ang-(1–12) [0.1 to 500 fmol] are incubated with the antibody and 10,000 cpm of 125I-Ang-(1–12) in a total volume of 0. 5 ml overnight at 4°C. The antibody-peptide complex was precipitated by addition of anti-rabbit IgG. The sensitivity of the RIA for Ang-(1–12) is 2 fmol. Cross-reactivities of the Ang-(1–12) RIA for Ang I, Ang-(1–9), Ang II and Ang-(1–7) are less than 0.01%. Urinary Aogen concentration was measured by the enzyme-linked immunosorbent assay (ELISA) with a total Aogen assay kit (Immuno Biological Laboratories, Minneapolis, MN).19 Serum ACE activity was determined by incubation of the sample with radiolabeled 3H-Hip-Gly-Gly (pH 8.0) for 1 hour at 37° C.18
Analysis of gene expression by quantitative real-time PCR
Real-time PCR was performed to detect cardiac and renal mRNA expression levels for ACE and ACE2 in group a, b, and d as described previously.20 Sequence-specific oligonucleotide primers were designed according to published GenBank (www.ncbi.nlm.nih.gov/Genbank) sequences. Expression levels are given relative to the geometric mean of the control group.
Histology
Harvested kidney tissues were fixed in 4% paraformaldehyde for 24 h and then transferred into 70% ethanol. After dehydration, the tissues were embedded into paraffin blocks, and sectioned at 4 μm for histological examination. Paraffin sections of kidney tissue were stained with Hematoxylin and Eosin (H-E). Immunostaining for α-SMA was performed as previously described.21
Statistical analysis
All values are expressed as mean ± SEM. Mean arterial pressure and urinary parameters were analyzed by two-way analysis of variance (ANOVA) followed by the Bonferroni’s post hoc test. Other data were analyzed by one-way ANOVA followed by the Turkey’s post hoc test. All data were analyzed using GraphPad PRISM (GraphPad, San Diego, CA,) with P<0.05 considered statistically significant.
Results
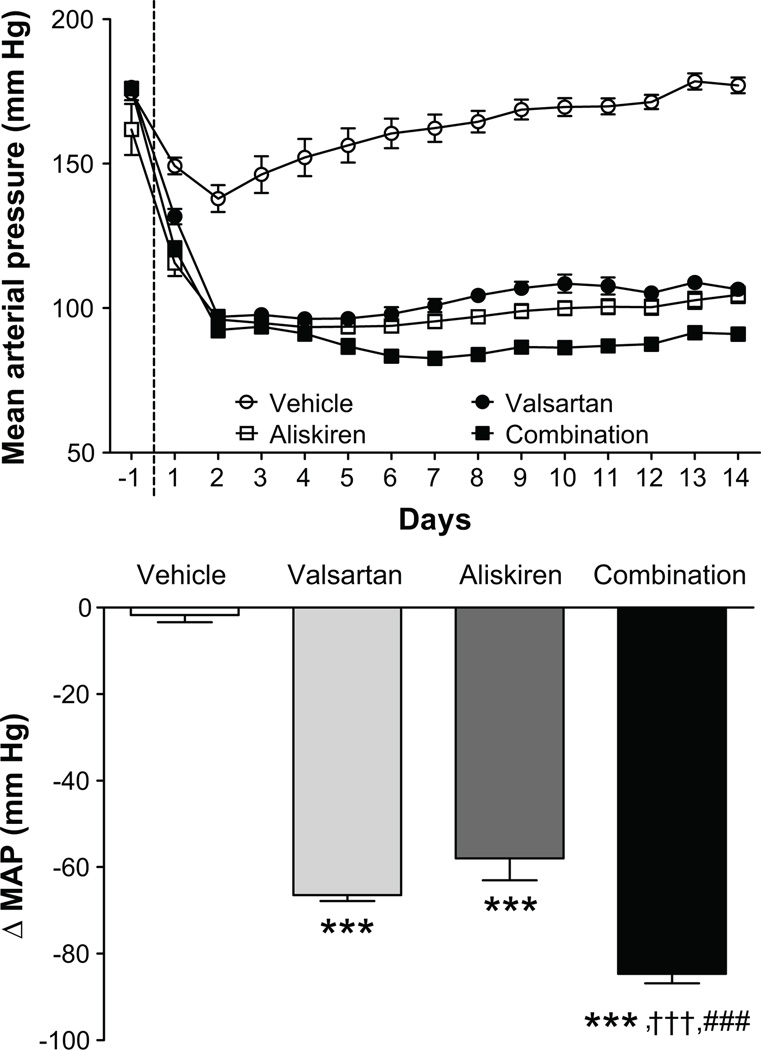
The reductions in mean arterial pressure induced by the combination therapy were greater than those produced by valsartan or aliskiren monotherapy (Figure 1). No treatment had an effect on heart rate. While all three treatments reduced whole heart weight/tibia length (WHW/TL) ratio and LV weight/TL (LVW/TL) ratios compared to vehicle-treated mRen2.Lewis rats (Table 1), the combination treatment reduced LVW/TL ratio more than each agent alone (Table 1). On the other hand, the combination of valsartan/aliskiren increased kidney weight/body weight (KW/BW) and KW/TL ratios by 14% and 11%, respectively, when compared to vehicle (Table 1).
Figure 1.
Time course of changes in mean arterial pressure in response to administration of vehicle, valsartan, aliskiren, and valsartan/aliskiren (upper panel) and changes in mean arterial pressure from baseline (lower panel) Values are means ± SEM. ***: P<0.001 vs vehicle, †††: P<0.001 vs valsartan, §§§: P<0.001 vs aliskiren.
Table 1.
Effects of treatments on heart and kidney weight
| Variable | Vehicle (n=9) |
Valsartan (n=10) |
Aliskiren (n=10) |
Combination (n=10) |
|---|---|---|---|---|
| WHW/BW (mg/g) | 3.85 ± 0.06 | 3.10 ± 0.03*** | 3.25 ± 0.08*** | 3.02 ± 0.04***,§ |
| WHW/TL (mg/mm) | 36.8 ± 0.5 | 29.0 ± 0.4*** | 30.7 ± 0.2***,† | 28.1 ± 0.4***,§§§ |
| LVW/BW (mg/g) | 2.74 ± 0.04 | 2.10 ± 0.03*** | 2.16 ± 0.05*** | 1.97 ± 0.03***,§§§ |
| LVW/TL (mg/mm) | 26.2 ± 0.41 | 19.6 ± 0.24*** | 20.4 ± 0.20*** | 18.3 ± 0.29***,†,§§§ |
| KW/BW (mg/g) | 3.82 ± 0.05 | 3.95 ± 0.10 | 4.17 ± 0.12 | 4.35 ± 0.11**,† |
| KW/TL (mg/mm) | 36.5 ± 0.4 | 36.9 ± 0.9 | 39.5 ± 1.1 | 40.5 ± 0.9*,† |
WHW: whole heart weight, BW: body weight, TL: tibia length, LVW: left ventricular weight, KW: kidney weight. Values are means ± SEM.
: P<0.05 vs vehicle,
: P<0.05 vs valsartan,
: P<0.05 vs aliskiren,
: P<0.01 vs vehicle,
: P<0.001 vs vehicle,
: P<0.001 vs aliskiren
Effects of Valsartan and Combination Therapy on RAS Components
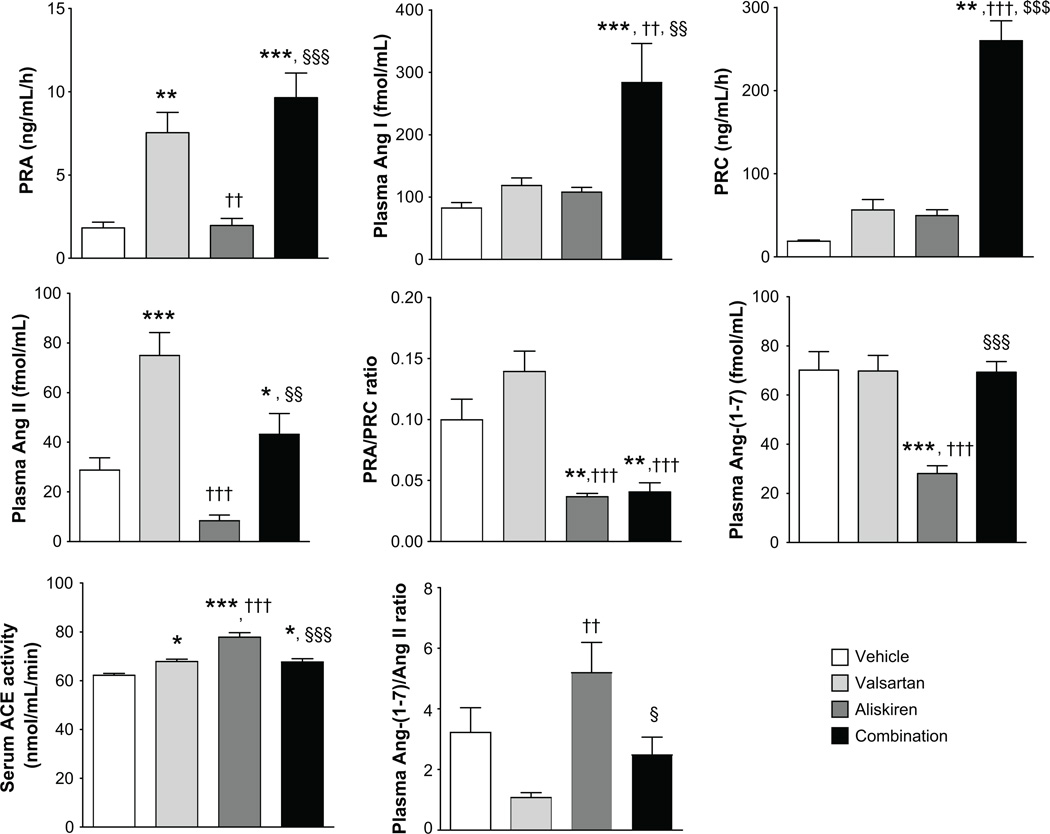
Figure 2 shows the effects of the treatments on systemic RAS components. Valsartan and combination therapy increased PRA compared to vehicle and aliskiren. Combination therapy increased PRC by 14-fold compared to vehicle and this change contrasted markedly with the reductions in PRA/PRC ratio found in rats treated with either aliskiren or valsartan alone. Only the combination therapy induced increases in plasma Ang I levels while the large increases in plasma Ang II produced by valsartan were suppressed in rats medicated with aliskiren and partially reversed during combination therapy (Figure 2). Aliskiren reduced plasma Ang-(1–7) level compared to other groups. Plasma Ang-(1–7)/Ang II ratio was highest in the aliskiren group. Plasma Ang-(1–12) and tissue RAS components in group a, b, and d are shown in Table S1. There were no differences in plasma Ang-(1–12) levels among the three groups. Tissue concentrations of Ang-(1–12), Ang II and Ang-(1–7) in the LV were not different among rats given vehicle, valsartan or the combined therapy (Table S1). On the other hand, kidney cortex content of Ang-(1–12) was higher in rats given the combination therapy compared to valsartan-treated rats, whereas both valsartan and the combination therapy led to marked decreases in renal Ang II content (Table S1). The treatments had no effect on renal Ang-(1–7) content (Table S1). Exposure to either valsartan or the combination therapy had no effect on the cardiac expression of ACE and ACE2 mRNAs. Renal cortex ACE2 gene expression was not altered by valsartan and decreased in rats medicated with valsartan/aliskiren.
Figure 2.
Effects of each treatment on systemic RAS components. Values are means ± SEM. *: P<0.05 vs vehicle, **: P<0.01 vs vehicle, ***: P<0.001 vs vehicle, ††: P<0.01 vs valsartan, †††: P<0.001 vs valsartan, §: P<0.05 vs aliskiren, §§: P<0.01 vs aliskiren, §§§: P<0.001 vs aliskiren.
Figure S1 shows a marked reduction in the urinary excretion of Aogen by valsartan, aliskiren, or valsartan/aliskiren when compared to baseline values obtained either before initiation of treatment or the vehicle-treated groups. Urinary Ang-(1–12) excretion was similarly reduced by valsartan and the combination therapy and not changed in rats receiving aliskiren. Only aliskiren reduced urinary Ang II levels compared to valsartan. The decreases in urinary Ang-(1–7) excretion were similarly reduced in rats medicated with valsartan, aliskiren, or valsartan/aliskiren. In addition, urinary protein/creatinine ratio correlated positively with kidney Ang II level and negatively with kidney Ang-(1–7)/Ang II ratio (Figure S2).
Effects of RAS Blockade on Renal Excretory Function
The effects of treatments on renal excretory function are shown in Table 2. All drug-treatments caused a mild reduction in water intake and comparative decreases in urinary output. While valsartan or aliskiren increased urinary creatinine levels, the combination treatment reversed these changes. 24 hours urinary protein excretion was decreased only in the combination group, whereas urinary protein/creatinine ratio was reduced in valsartan and the combination group but not in rats medicated with aliskiren. Moreover, the values of urinary protein creatinine ratio in the combination group were significantly lower than that in aliskiren group. Serum urea nitrogen and potassium was augmented only in rats medicated with aliskiren or the combined treatment.
Table 2.
Renal Excretory Response to the Treatments
| Variable | Vehicle (n=9) |
Valsartan (n=10) |
Aliskiren (n=10) |
Combination (n=10) |
|---|---|---|---|---|
| Water intake, mL | ||||
| Baseline | 32.0 ± 1.2 | 30.7 ± 1.7 | 32.6 ± 3.3 | 30.9 ± 1.1 |
| 2-wks. | 37.0 ± 2.4 | 24.0 ± 1.0***,# | 23.7 ± 1.2*** | 26.1 ± 0.6***,## |
| Urine volume, mL | ||||
| Baseline | 20.7 ± 1.1 | 20.4 ± 1.2 | 19.9 ± 3.2 | 20.4 ± 1.5 |
| 2-wks. | 23.7 ± 2.9 | 14.8 ± 0.4** | 14.2 ± 0.9** | 15.4 ± 0.7*** |
| Urine creatinine, mg/dL | ||||
| Baseline | 70.8 ± 7.7 | 67.1 ± 7.0 | 74.3 ± 7.1 | 70.6 ± 8.2 |
| 2-wks | 88.0 ± 9.1 | 126.3 ± 10.8**,### | 100.5 ± 2.7** | 94.6 ± 6.2† |
| Urine protein, mg/24h | ||||
| Baseline | 21.0 ± 2.1 | 20.5 ± 1.7 | 23.3 ± 2.8 | 21.7 ± 2.0 |
| 2-wks. | 23.8 ± 1.9 | 17.9 ± 2.3 | 23.5 ± 3.1 | 10.7 ± 0.9***,†,§§§,## |
| Protein/creatinine, mg/mg/24h | ||||
| Baseline | 32.1 ± 4.1 | 33.6 ± 4.3 | 31.8 ± 2.9 | 32.4 ± 3.5 |
| 2-wks | 25.9 ± 1.7 | 14.8 ± 2.1*,### | 23.7 ± 3.4 | 11.4 ± 0.8**,§,### |
| Serum urea nitrogen, mg/dL | ||||
| Baseline | NA | NA | NA | NA |
| 2-wks. | 35.8 ± 2.7 | 38.0 ± 1.0 | 44.9 ± 2.1* | 46.0 ± 2.4**,† |
| Serum creatinine, mg/dL | ||||
| Baseline | NA | NA | NA | NA |
| 2-wks. | 0.45 ± 0.03 | 0.42 ± 0.03 | 0.42 ± 0.03 | 0.51 ± 0.04 |
| Serum Na+ mmol/L | ||||
| Baseline | NA | NA | NA | NA |
| 2-wks. | 140.3 ± 3.6 | 138.5 ± 1.3 | 139.0 ± 2.4 | 138.9 ± 0.8 |
| Serum K+ mmol/L | ||||
| Baseline | NA | NA | NA | NA |
| 2-wks. | 6.07 ± 0.19 | 6.55 ± 0.16 | 6.83 ± 0.13** | 7.02 ± 0.16* |
| Creatinine clearance, mL/min | ||||
| Baseline | NA | NA | NA | NA |
| 2-wks. | 2.83 ± 0.25 | 3.49 ± 0.63 | 2.37 ± 0.18 | 2.37 ± 0.18 |
Values are means ± SEM.
: P<0.05 vs vehicle,
: P<0.05 vs valsartan,
: P<0.05 vs aliskiren,
: P<0.05 vs basal,
: P<0.01 vs vehicle,
: P<0.01 vs basal,
: P<0.001 vs vehicle,
: P<0.001 vs basal,
: P<0.001 vs aliskiren,
: not applicable.
Renal Histological Changes Induced by the Treatments
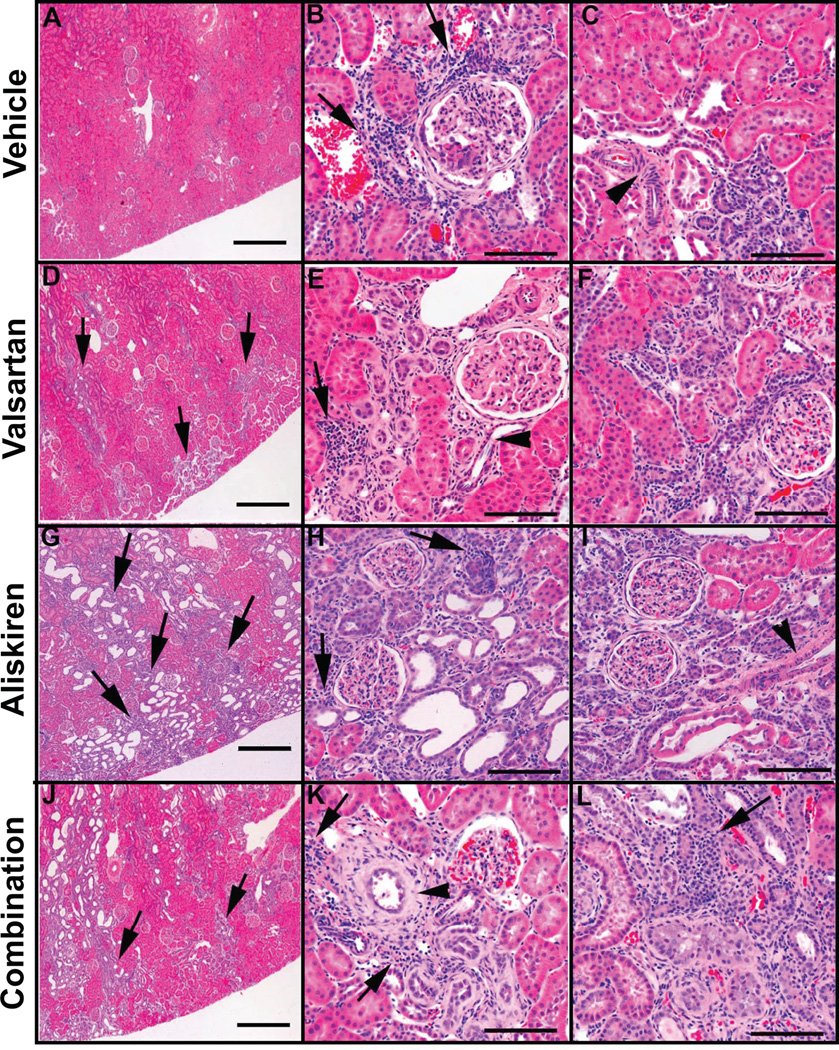
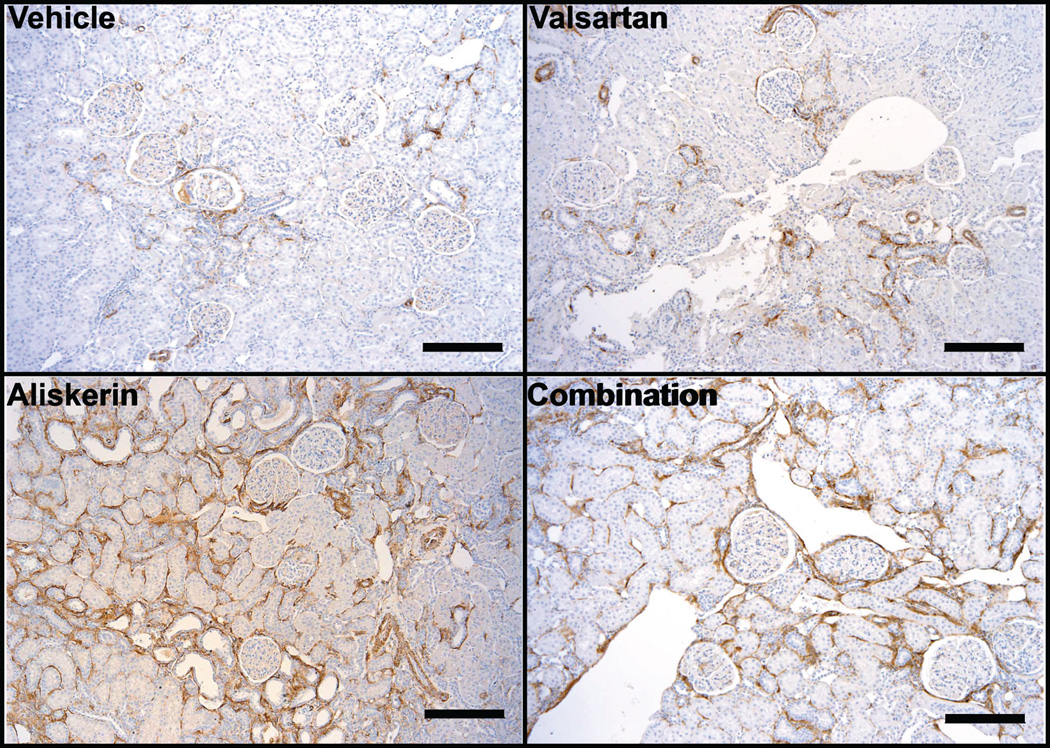
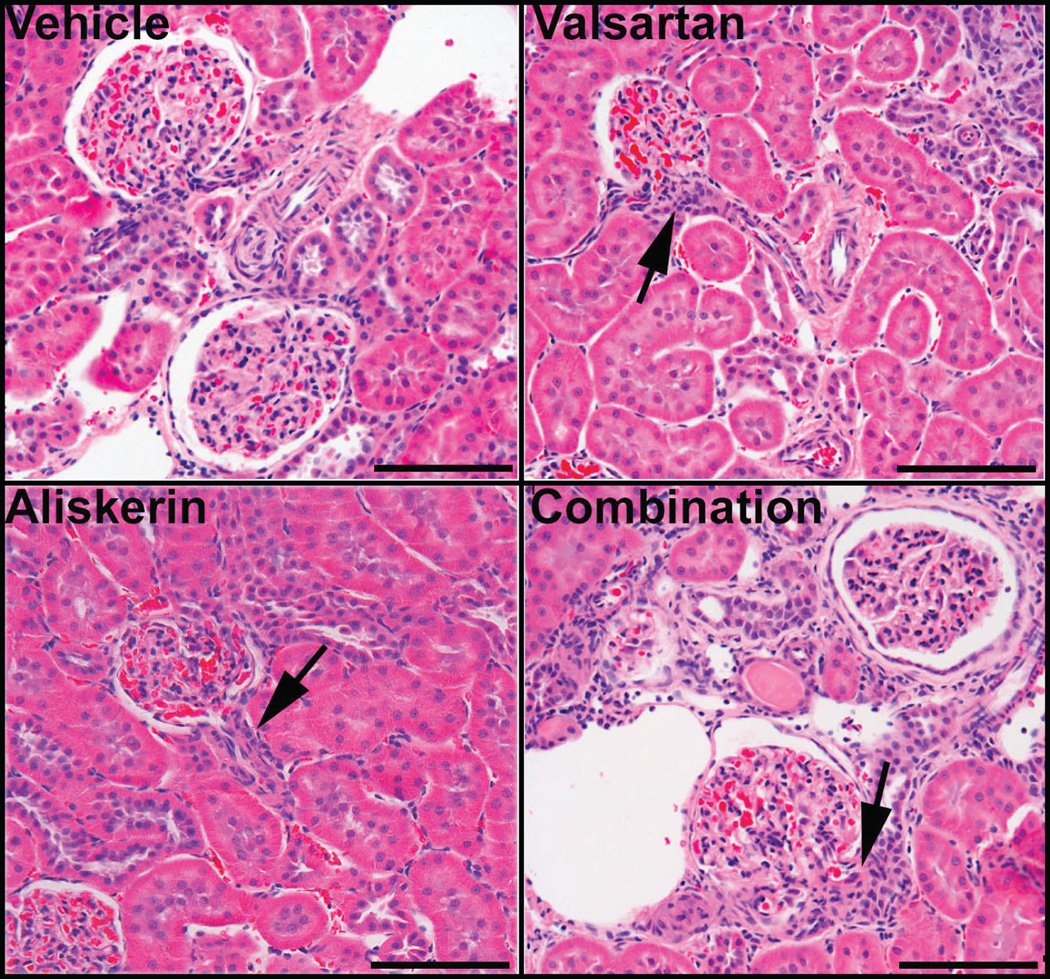
Representative changes of the alterations present in the kidney of vehicle treated mRen2.Lewis hypertensive rats and of those receiving valsartan, aliskiren or the combined treatment are illustrated in Figure 3. The extension and number of basophilic areas with tubular dilatation, signs of proliferation such as high nuclear to cytoplasm ratio and increased mitotic figures are augmented in mRen2.Lewis rats treated with valsartan and more drastically in rats with either aliskiren or the combination therapy. Although the combination therapy was consistently associated with marked renal abnormalities, the aliskiren group showed them as well although variability was noted among animals. Figure 5 also shows that the peritubular interstitium in the treated rats is expanded and occupied by proliferating activated pericytes expressing α-smooth muscle actin leading to fibrosis. There is also evidence of tubular proliferation and lymphocytic infiltrates that becomes more prominent in mRen2.Lewis rats receiving aliskiren alone and the combination therapy. Combination therapy is associated with extensive cortical (outer and inner cortex) areas consisting of peritubular and periglomerular fibrosis, disorganized tubular proliferation, and tubular dilatation/atrophy. Many congestive, enlarged glomeruli with aneurysmatic capillary dilatations as well as some non-perfused (sclerotic) glomeruli are also observed in this group. Abnormal glomeruli are visualized undergoing periglomerular and glomerular sclerosis (Figure 3, panels B, E, H and I) and mesangial proliferation. Tubular dilation was more prominent in the aliskiren and combined therapy groups (Figure 3, panels G, H and J). In addition, Figure 4 shows increase in the number of cells in the afferent arterioles of the treated groups, corresponding to renin cells.
Figure 3.
Hematoxylin-eosin stained sections of renal cortical tissue obtained from representative vehicle (panels A, B, and C), valsartan (panels D, E, F), aliskiren (panels G, H, and I), and valsartan/aliskiren (panels J, K, and L)-treated mRen2.Lewis hypertensive rats. At low magnification vehicle treated mRen2.Lewis hypertensive rats shows an overall conserved renal architecture (A), whereas valsartan (D and G) -treated mRen2.Lewis rats show abnormal areas (arrows) with tubular proliferation, periglomerular and peritubular fibrosis and tubular dilatation, more extensive in the aliskiren or the combined treatment groups (J). Lymphocytic infiltrates surrounding tubules and vessels are observed in all groups (arrows in panels B, E, H and K). Examples of periglomerular fibrosis and signs of glomerulosclerosis are shown in panels B, E, F, H and I. Areas consisting of high nuclear to cytoplasm ratio, peritubular and periglomerular fibrosis and disorganized tubular proliferation (basophilic areas) are small in the vehicle group (B and C) but are more frequent and extended in the valsartan (E and F), aliskiren (H and I) and combined treatment group (K and L). Most of the arteries/arterioles are fairly conserved (C, E and I, arrowheads) but some are very abnormal denoting a hypertrophic response with thickening of the media (panel K, arrowhead). Scale bar: A, D, G and J: 500 µm; B, C, E, F, H, I, K and L: 100 µm.
Figure 5.
Immunohistochemical staining for α-SMA (brown) on kidneys sections obtained from vehicle, valsartan, aliskiren, and combination-treated mRen2.Lewis hypertensive rats. Kidneys from vehicle treated mRen2.Lewis rats showed the expected α-SMA expression in the mural cells of the vasculature and occasionally in the peritubular interstitium whereas in the treated groups there was an increase in the expression of α-SMA in the interstitium (peritubular and periglomerular) indicating the activation of myofibroblasts leading to fibrosis. Remarkably, the increase in interstitial expression of α-SMA was more significant in the aliskiren and combined therapy groups. Scale bar: 200 µm.
Figure 4.
Hematoxylin-eosin stained sections of renal cortical tissue obtained from mRen2.Lewis rats. Arrows point to juxtaglomerular hypertrophy presumably due to increase in the number of renin cells in the afferent arterioles of the treated rats. Scale bar: 100 µm.
Discussion
While confirming a beneficial effect of the combination therapy on the surrogate end-points of blood pressure, cardiac hypertrophy, and proteinuria, we now show that these treatments are associated with worsening of renal injury. The apparent improvement in the surrogate end-point of protein excretion contrasted markedly with the increases in kidney weight, the elevations in serum urea nitrogen and histological findings of worsening of glomerulosclerosis with all three treatments, especially with the combination of valsartan/aliskiren. We suggest that these new findings reflect the kidneys inability to autoregulate renal blood flow following the "relative" hypotension and blockade of the actions of Ang II on tubuloglomerular feedback mechanisms.22 We further suggest that the larger antihypertensive response produced by the combination therapy may make the kidneys more susceptible to hypoperfusion leading to regional tissue ischemia and fibrosis. In keeping with this interpretation, Gomez and colleagues23–25 showed that progression of renal disease is associated with deterioration of the kidney microvasculature and/or the reenactment of embryonic pathways, features that were present in the kidney of valsartan/aliskiren treated rats. In agreement with these findings a previous study showed no additional renoprotective actions of the combination therapy in m(Ren2)27 transgenic hypertensive rats.26 Thus, these new results agree with clinical findings in which a multiloci blockade of RAS components has a detrimental impact on renal function despite the apparent beneficial effects on surrogate endpoints such as blood pressure and proteinuria. Altogether, the detailed characterization of the effects of valsartan/aliskiren over those induced by valsartan or aliskiren alone is in keeping with emerging data demonstrating that blockade of AT1Rs together with either ACE or renin inhibition may negate the beneficial effects of selective blockade of one of these pathways.2,3,7,26,27
As an experimental model of hypertension, the mRen2.Lewis rat retains the increased tissue renin transgene expression derived from the backcross of the original m(Ren2)27 transgenic hypertensive rat.15 The use of telemetry for assessment of the effects of therapies on blood pressure and heart rate, a comprehensive quantitative evaluation of changes in RAS components using procedures firmly established in our laboratory, and an independent and blind assessment of histological findings are additional strengths of the reported research. While the generalizability of our findings to human hypertensive disease has obvious limitations, our data provides a window for understanding why dual RAS inhibition may negate clinically the beneficial effects of selective blockade of Ang II or renin.2,3,7,26,27
The demonstration that valsartan/aliskiren reduced arterial pressure more than valsartan or aliskiren alone in mRen2. Lewis hypertensive rats agree with previous human and animal findings.5 Partial blunting of the increased plasma Ang II induced by valsartan and recover of reduced plasma Ang-(1–7) induced by aliskiren in the combination group, may explain the stronger antihypertensive and cardiac anti-hypertrophic actions of the combination therapy. Overall, the effects of the treatments on plasma and tissue RAS components agree with the existence of separate regulatory expression of the components in the circulating and tissue compartments. In this study, the increases in both plasma Ang I and serum ACE activity induced by the combined treatment may reflect a compensatory rise in renin secretion due to blockade of the AT1R mediated negative feedback to renin production. Since the increased serum ACE activity was associated with a reduction in the plasma Ang II/Ang I ratio, these data confirm that the combination of valsartan/aliskiren causes an upregulation of circulating ACE. Aliskiren is reported to have high affinity with human and mouse renin but very low affinity with rat renin.28 Due to the release of the negative feedback from AT1R, the combined therapy of valsartan/aliskiren causes a reactive rise in secretion of rat renin, which is much less susceptible to inhibition by aliskiren as reported in m(Ren2)27 transgenic hypertensive rats.28 In addition, this reactively increased renin acts to attenuate the suppression of Ang I by aliskiren.29,30 The demonstration of increased number of renin cells in the afferent arterioles of the treated groups (Figure 4) agree with the observed increases in PRA, PRC and plasma Ang I found with the combination therapy. On the other hand, the large increases in Ang I mediated by the combination therapy did not result in augmented plasma Ang II levels since aliskiren effectively suppressed the increases in plasma Ang II levels induced by valsartan. This may be explained through increased ACE2 activity by aliskiren, reflecting the increase in plasma Ang-(1–7)/Ang II ratio.
The existence of non-renin dependent pathways for angiotensin peptides formation led us to determine the effect of the treatments on plasma, cardiac and renal concentrations of Ang-(1–12). Recent studies from this laboratory showed that both ACE and chymase account for the production of Ang II from Ang-(1–12).9,14 Measurements of Ang-(1–12) in plasma and cardiac tissue confirmed that its presence is not altered by administration of the treatments, additional evidence for its independent regulation from renin.13 The increase in renal Ang-(1–12) induced by the combination therapy compared to valsartan-treated rats suggest that renin inhibition upregulates alternative processing of Aogen to Ang-(1–12) by as a yet to be identified enzymatic pathway. Further work is necessary to determine whether the increases in Ang-(1–12) might be associated with the worsening of kidney injury.
The effects of the therapies on renal cortex content of the angiotensins showed a significant reduction of Ang II in both valsartan and valsartan/aliskiren treated rats. Consistently, Kobori et al.31 showed that Aogen is produced in proximal tubular cells as well as in the liver and also found that systemic Ang II infusion increase Aogen in kidney and urine. The decrease of renal ACE2 mRNA in the combination group may be explained by the worsening of renal parenchymal disease as reported in human renal biopsies from patients with chronic kidney disease.32
We conclude that while the combination of valsartan/aliskiren exerted a greater effect in reducing blood pressure, cardiac hypertrophy, and proteinuria, improvements in these surrogate endpoints are associated with aggravation of renal disease. The discrepant results in terms of the surrogate end-points of blood pressure, cardiac hypertrophy, and proteinuria compared to the morphological changes in the kidney provides now a mechanistic window for understanding the adverse clinical outcomes reported with the use of dual RAS inhibitors.
Perspective
The theoretical advantages of dual inhibition of RAS components using ACE or direct renin inhibitors with ARBs is a logical approach for the management of hypertension as they act synergistically in suppressing the reactive rise in plasma renin activity resulting from blockade of AT1Rs. While previous studies in animals and hypertensive subjects suggested that such combinations favorably affect blood pressure, adverse cardiac remodeling, and proteinuria, their long-term effects on renal function and clinical events has failed to support the merits of this therapeutic combination. Our detailed investigation of the effect of combination therapy with valsartan and aliskiren in a renin-dependent hypertension model showed a significant discrepancy among the merit of this combination in terms of the surrogate end-points of blood pressure, cardiac hypertrophy, and proteinuria and the progression of hypertension-induced renal damage. The results of this study suggest that dual blockade of renin activity and AT1Rs prevents the activation of intrinsic renoprotective mechanisms to compensate for the decreases in renal perfusion pressure.
Supplementary Material
Novelty and Significance.
1) What Is New?
The first direct demonstration in an experimental model of renin-dependent hypertension that the merits of combining the AT1 receptor antagonist valsartan with a direct renin inhibitor aliskiren is associated with worsening of renal parenchymal disease despite the beneficial effects of this treatment on blood pressure, cardiac hypertrophy, and proteinuria.
2) What Is Relevant?
These experimental findings now provide an explanation for the absence of any additional cardiovascular protection and even worsening of renal function during dual blockade of the renin angiotensin system.
3) Summary
Our studies in mRen2.Lewis congenic hypertensive rats medicated with valsartan or the combination of valsartan/aliskiren demonstrated that the additional antihypertensive and antiproteinuric effect of preventing a reactive rise in plasma renin activity does not suppress cardiac content of angiotensin peptides while markedly lowering the renal content of Ang II but not Ang-(1–7). In addition, the combination therapy is associated with a mild increase in renal cortical content of Ang-(1–12) as well as causing large decreases in urinary excretion rates of angiotensinogen, Ang-(1–12) and Ang-(1–7). The deactivation of renin angiotensin system components in the kidney of valsartan/aliskiren-treated rats is associated with worsening of renal parenchymal disease above the structural changes that are induced by the hypertensive process in the vehicle-treated rats.
Acknowledgments
Sources of Funding
This study was supported by a grant from Novartis, Inc. Additional funding for the performance of the study was obtained by NIH grants HL-051952 (CMF) and AG-033727(LG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict(s) of Interest/Disclosure(s) Statement
CM Ferrario is the recipient of a grant from Novartis, Inc. He is also a member of the Novartis Advisory Board.
References
- 1.Azizi M, Menard J. Combined blockade of the renin-angiotensin system with angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists. Circulation. 2004;109:2492–2499. doi: 10.1161/01.CIR.0000131449.94713.AD. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 3.Harel Z, Gilbert C, Wald R, Bell C, Perl J, Juurlink D, Beyene J, Shah PS. The effect of combination treatment with aliskiren and blockers of the renin-angiotensin system on hyperkalaemia and acute kidney injury: systematic review and meta-analysis. BMJ. 2012;344:e42. doi: 10.1136/bmj.e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood JM, Cumin F, Maibaum J. Pharmacology of renin inhibitors and their application to the treatment of hypertension. Pharmacol Ther. 1994;61:325–344. doi: 10.1016/0163-7258(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 5.Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet. 2007;370:221–229. doi: 10.1016/S0140-6736(07)61124-6. [DOI] [PubMed] [Google Scholar]
- 6.Parving HH, Brenner BM, McMurray JJ, de ZD, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Nicolaides M, Richards A, Xiang Z, Armbrecht J, Pfeffer MA. Baseline characteristics in the Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (ALTITUDE) J Renin Angiotensin Aldosterone Syst. 2012;13:387–393. doi: 10.1177/1470320311434818. [DOI] [PubMed] [Google Scholar]
- 7.Azizi M, Menard J. Renin Inhibitors and Cardiovascular and Renal Protection: An Endless Quest? Cardiovasc Drugs Ther. 2012 doi: 10.1007/s10557-012-6380-6. In Press. [DOI] [PubMed] [Google Scholar]
- 8.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun. 2006;350:1026–1031. doi: 10.1016/j.bbrc.2006.09.146. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad S, Varagic J, Westwood BM, Chappell MC, Ferrario CM. Uptake and metabolism of the novel peptide angiotensin-(1–12) by neonatal cardiac myocytes. PLoS One. 2011;6:e15759. doi: 10.1371/journal.pone.0015759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagata S, Kato J, Kuwasako K, Asami M, Kitamura K. Plasma and tissue concentrations of proangiotensin-12 in rats treated with inhibitors of the reninangiotensin system. Hypertens Res. 2012;35:234–238. doi: 10.1038/hr.2011.165. [DOI] [PubMed] [Google Scholar]
- 11.Prosser HC, Forster ME, Richards AM, Pemberton CJ. Cardiac chymase converts rat proAngiotensin-12 (PA12) to angiotensin II: effects of PA12 upon cardiac haemodynamics. Cardiovasc Res. 2009;82:40–50. doi: 10.1093/cvr/cvp003. [DOI] [PubMed] [Google Scholar]
- 12.Prosser HC, Richards AM, Forster ME, Pemberton CJ. Regional vascular response to ProAngiotensin-12 (PA12) through the rat arterial system. Peptides. 2010;31:1540–1545. doi: 10.1016/j.peptides.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Trask AJ, Jessup JA, Chappell MC, Ferrario CM. Angiotensin-(1–12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol Heart Circ Physiol. 2008;294:H2242–H2247. doi: 10.1152/ajpheart.00175.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad S, Simmons T, Varagic J, Moniwa N, Chappell MC, Ferrario CM. Chymase-dependent generation of angiotensin II from angiotensin-(1–12) in human atrial tissue. PLoS One. 2011;6:e28501. doi: 10.1371/journal.pone.0028501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jessup JA, Gallagher PE, Averill DB, Brosnihan KB, Tallant EA, Chappell MC, Ferrario CM. Effect of angiotensin II blockade on a new congenic model of hypertension derived from transgenic Ren-2 rats. Am J Physiol Heart Circ Physiol. 2006;291:H2166–H2172. doi: 10.1152/ajpheart.00061.2006. [DOI] [PubMed] [Google Scholar]
- 16.Moniwa N, Varagic J, Ahmad S, Voncannon JL, Ferrario CM. Restoration of the blood pressure circadian rhythm by direct renin inhibition and blockade of angiotensin II receptors in mRen2.Lewis hypertensive rats. Ther Adv Cardiovasc Dis. 2012;6:15–29. doi: 10.1177/1753944711434039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whaley-Connell A, Habibi J, Cooper SA, DeMarco VG, Hayden MR, Stump CS, Link D, Ferrario CM, Sowers JR. Effect of renin inhibition and AT1R blockade on myocardial remodeling in the transgenic Ren2 rat. Am J Physiol Endocrinol Metab. 2008;295:E103–E109. doi: 10.1152/ajpendo.00752.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrario CM, Jessup J, Gallagher PE, Averill DB, Brosnihan KB, Ann TE, Smith RD, Chappell MC. Effects of renin-angiotensin system blockade on renal angiotensin-(1–7) forming enzymes and receptors. Kidney Int. 2005;68:2189–2196. doi: 10.1111/j.1523-1755.2005.00675.x. [DOI] [PubMed] [Google Scholar]
- 19.Cohen JA, Lindsey SH, Pirro NT, Brosnihan KB, Gallagher PE, Chappell MC. Influence of estrogen depletion and salt loading on renal angiotensinogen expression in the mRen(2).Lewis strain. Am J Physiol Renal Physiol. 2010;299:F35–F42. doi: 10.1152/ajprenal.00138.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrario CM, Varagic J, Habibi J, Nagata S, Kato J, Chappell MC, Trask AJ, Kitamura K, Whaley-Connell A, Sowers JR. Differential regulation of angiotensin-(1–12) in plasma and cardiac tissue in response to bilateral nephrectomy. Am J Physiol Heart Circ Physiol. 2009;296:H1184–H1192. doi: 10.1152/ajpheart.01114.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell. 2004;6:719–728. doi: 10.1016/s1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- 22.Cupples WA. Interactions contributing to kidney blood flow autoregulation. Curr Opin Nephrol Hypertens. 2007;16:39–45. doi: 10.1097/MNH.0b013e3280117fc7. [DOI] [PubMed] [Google Scholar]
- 23.Gomez RA, Sequeira Lopez ML, Fernandez L, Chernavvsky DR, Norwood VF. The maturing kidney: development and susceptibility. Ren Fail. 1999;21:283–291. doi: 10.3109/08860229909085090. [DOI] [PubMed] [Google Scholar]
- 24.Sequeira Lopez ML, Gomez RA. The role of angiotensin II in kidney embryogenesis and kidney abnormalities. Curr Opin Nephrol Hypertens. 2004;13:117–122. doi: 10.1097/00041552-200401000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Sequeira Lopez ML, Gomez RA. Development of the renal arterioles. J Am Soc Nephrol. 2011;22:2156–2165. doi: 10.1681/ASN.2011080818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whaley-Connell A, Habibi J, Nistala R, Hayden MR, Pulakat L, Sinak C, Locher B, Ferrario CM, Sowers JR. Combination of direct renin inhibition with angiotensin type 1 receptor blockade improves aldosterone but does not improve kidney injury in the transgenic Ren2 rat. Regul Pept. 2012;176:36–44. doi: 10.1016/j.regpep.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrario CM. Addressing the theoretical and clinical advantages of combination therapy with inhibitors of the renin-angiotensin-aldosterone system: antihypertensive effects and benefits beyond BP control. Life Sci. 2010;86:289–299. doi: 10.1016/j.lfs.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldman DL, Jin L, Xuan H, Contrepas A, Zhou Y, Webb RL, Mueller DN, Feldt S, Cumin F, Maniara W, Persohn E, Schuetz H, Jan Danser AH, Nguyen G. Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen-2)27 rats. Hypertension. 2008;52:130–136. doi: 10.1161/HYPERTENSIONAHA.107.108845. [DOI] [PubMed] [Google Scholar]
- 29.Nussberger J, Wuerzner G, Jensen C, Brunner HR. Angiotensin II suppression in humans by the orally active renin inhibitor Aliskiren (SPP100): comparison with enalapril. Hypertension. 2002;39:E1–E8. doi: 10.1161/hy0102.102293. [DOI] [PubMed] [Google Scholar]
- 30.Azizi M, Menard J, Bissery A, Guyenne TT, Bura-Riviere A, Vaidyanathan S, Camisasca RP. Pharmacologic demonstration of the synergistic effects of a combination of the renin inhibitor aliskiren and the AT1 receptor antagonist valsartan on the angiotensin II-renin feedback interruption. J Am Soc Nephrol. 2004;15:3126–3133. doi: 10.1097/01.ASN.0000146686.35541.29. [DOI] [PubMed] [Google Scholar]
- 31.Kobori H, Ozawa Y, Suzaki Y, Prieto-Carrasquero MC, Nishiyama A, Shoji T, Cohen EP, Navar LG. Young Scholars Award Lecture: Intratubular angiotensinogen in hypertension and kidney diseases. Am J Hypertens. 2006;19:541–550. doi: 10.1016/j.amjhyper.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reich HN, Oudit GY, Penninger JM, Scholey JW, Herzenberg AM. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int. 2008;74:1610–1616. doi: 10.1038/ki.2008.497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.