Abstract
The aim of this study was to investigate the role of the XRCC1 Arg399Gln polymorphism in the susceptibility of a Kashmiri population to colorectal cancer (CRC). We investigated the genotype distribution of the XRCC1 gene in 130 CRC cases in comparison with that of 150 healthy subjects. There was no direct significant association between the XRCC1 genotypes and CRC; however, the Arg/Gln genotype was associated with an elevated risk of CRC (OR>1.47) and the Gln/Gln variant genotype was associated with an increased risk of CRC in various clinicopathological parameters. This study suggests that the XRCC1 polymorphism is associated with an increased risk of CRC.
Keywords: colorectal cancer, XRCC1, polymorphism, Kashmir
Introduction
Colorectal cancer (CRC) is the third most common cancer in males and the second most common cancer in females worldwide (1). In the Kashmir valley, CRC represents the third most common gastrointestinal cancer following esophageal and gastric cancer (2–4).
The Kashmiri population is exposed to a particular set of environmental and dietary risks, including exposure to nitroso compounds, amines and nitrates reported to be present in local foodstuffs, the majority of which have been shown to contain significant irritants and carcinogens (2,3,5,6).
Genetic polymorphisms in DNA repair genes, which lead to amino acid substitution, may influence individual capacity to repair DNA damage, which may be associated with increased genetic instability and carcinogenesis (7). In mammalian cells, four major DNA repair pathways have been identified: base excision repair (BER), nucleotide excision repair (NER), double-strand break repair and mismatch repair (8,9).
The DNA repair gene XRCC1, located at 19q13.2, codes for a scaffolding protein physically associated with DNA polymerase β, DNA ligase III, human AP endonuclease, polynucleotide kinase and poly (ADP-ribose) polymerase (8,10,11), which function in a complex to facilitate BER and single-strand break repair processes. The BER pathway mainly removes non-bulky base adducts produced by methylation, oxidation or reduction by ionizing radiation or oxidative damage (12). The XRCC1 protein is capable of binding directly to both gapped and nicked DNA, as well as to gapped DNA associated with DNA polymerase β, suggesting that this protein may be independently involved in DNA damage recognition (13).
Three polymorphisms occurring at conserved sequences in the XRCC1 gene have been reported, and amino acid substitutions were detected at codons 194 (Arg>Trp), 280 (Arg>His) and 399 (Arg>Gln) (14). Out of the 3 polymorphisms, the XRCC1 codon 399 polymorphism has been studied most widely in a number of cancers but with varied results (15–23). While an increased risk has been reported in lung cancer (22) and breast cancer (17,18), there is no definite correlation between XRCC1 339 status and the risk of CRC. Abdel-Rahman et al(15) were the first to report that the XRCC1 399Gln allele, similarly to the XRCC1 399Arg/Arg genotype, was associated with an increased risk of developing CRC, particularly amongst young urban residents; however, these results were not reproducible and have not been demonstrated in any other population since. Stern et al(24) reported that the 399SNP of the XRCC1 gene plays a role in modifying the risk of CRC by interactions with fatty acids of the diet. There are also some contradictory reports such as that of Mort et al(20), who reported no correlation between CRC risk and polymorphisms in any of the 4 NER genes or in XRCC1. Yeh et al(21) investigated the involvement of the DNA repair pathway genes in modulating the risk of CRC in a Taiwanese population and found the Arg 399 form to cause an increased cancer risk. Similarly, Skjelbred et al(2006)(23) reported a decreased risk of CRC with the Gln399 genotype. More recently, in a study by Wang et al(2010) in a South East Indian population (25), the XRCC1 Gln399 allele was found to significantly increase the risk of rectal cancer. In the present study, we conducted a hospital-based case-controlled investigation to evaluate the potential impact of the XRCC1 Arg399Gln gene polymorphism on the risk of CRC in a Kashmiri population. We also investigated whether there was a correlation between the clinicopathological variables and the XRCC1 variant genotype (Gln/Gln), and hence its role in modulating the risk of CRC.
Materials and methods
Study population
This study included 130 CRC cases. All patients were recruited from the Department of General Surgery, Sher-I-Kashmir Institute of Medical Sciences, Kashmir, India. Blood samples were collected from 160 ageand gender-matched individuals with no signs of any malignancy to serve as external controls. The mean age in the patient and control groups was 53 years (Table I).
Table I.
Genotype frequencies of the XRCC1 gene polymorphism in CRC cases and controls.
| XRCC1 genotype | CRC cases (n=130) | Controls (n=150) | OR (95% CI); P¥; Fψ | χ2; P-value (overall) |
|---|---|---|---|---|
| GG (wild) | 63 (48.5%) | 75 (50.0%) | 1 | 0.40; 0.81 |
| AG (heterozygous) | 37 (28.5%) | 30 (20.0%) | 1.47 (0.82–2.64); 0.19; 0.23 | |
| AA (variant) | 30 (23.0%) | 45 (30.0%) | 0.79 (0.44–1.4); 0.43; 0.47 |
CRC, colorectal cancer; ¥, Pearson’s P-value; ψ, Fisher’s exact P-value.
Data on all CRC patients were obtained from personal interviews with patients and/or their guardians, and their medical records. All patients and/or guardians were informed about the study and their willingness to participate was recorded on a predesigned questionnaire (available on request). The collection and use of blood samples (from patients and controls) for this study had been previously approved by the appropriate institutional ethics committee.
DNA extraction and polymerase chain reaction
DNA extraction was performed using the ammonium precipitation method. Genotyping for the XRCC1 R399Q polymorphism was determined using a method described previously (25). The oligonucleotide primers used for the amplification of the target region were: forward F-5′-TTG TGC TTT CTC TGT GTC CA-3′ and reverse R-5′-TCC TCC AGC CTT TTC TGA TA-3′, which generated a 615-bp fragment.
The polymerase chain reaction (PCR) was carried out in a final volume of 20 μl, containing 50 ng genomic DNA template, 1X PCR buffer (Fermentas, Glen Burnie, MD, USA), with 2 mM MgCl2, 0.5 μM of each primer (Sigma-Aldrich, Bangalore, India), 50 μM deoxynucleotide triphosphates (dNTPs; Cinnagen, Tehran, Iran) and 0.25 units of DNA polymerase (Invitrogen, Bangalore, India). For PCR amplification, the standard programme was used as follows: one initial denaturation step at 94°C for 7 min, followed by 40 denaturation cycles of 30 sec at 94°C, 30 sec of annealing at 57°C and 30 sec of extension at 72°C for 40 cycles, followed by a final elongation cycle at 72°C for 7 min.
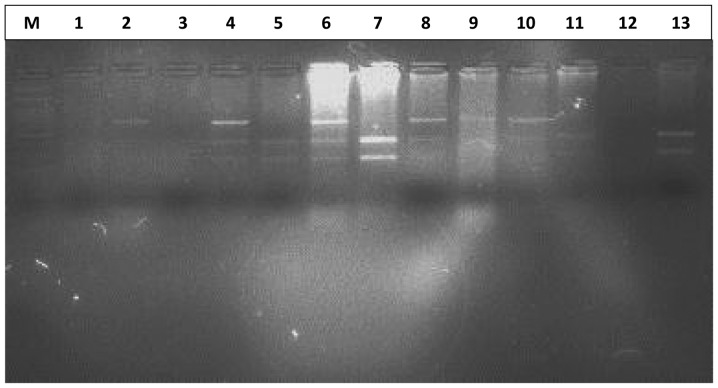
The PCR product of XRCC1 was then digested with 2 units of MspI in a reaction mixture of 20 μl for 3 h at 37°C. The Arg allele revealed 374 and 241-bp fragments, while the Gln allele was not digested (indicative of the absence of the MspI cutting site; Fig. 1).
Figure 1.
Representative gel of XRCC1 Arg399Gln polymorphism, showing MspI digested amplicons. The Arg allele is represented by 374 and 241-bp fragments, while the Gln allele is represented by a 615-bp band. Lane M, 100-bp ladder; lanes 2, 9 and 10, homozygous (Gln/Gln) genotype (615 bp); lanes 5, 7, 11 and 13, homozygous (Arg/Arg) genotype (374 and 241 bp); lanes 4, 6 and 8, homozygous (CC) genotype (615, 374 and 241 bp).
DNA amplicons, as well as the digestion products, were electrophoresed through a 2–3% agarose gel (Genie, Bangalore, India) for resolution. The genotypes of >20% of the samples were reassessed in a double-blind manner by two independent researchers, to confirm the results. A random sample of 10% of each genotype was re-checked with sequencing to confirm the results.
Statistical analysis
The observed frequencies of genotypes in CRC patients were compared with controls using the Chi-square test, or Fisher’s exact test when the expected frequencies were small. The Chi-square test was used to verify whether the genotype distributions were in Hardy-Weinberg equilibrium. P≤0.05 was considered to indicate a statistically significant result. Statistical analyses were performed using PASW version 18 software.
Results
A total of 130 CRC cases and 150 control subjects were included in this study. The CRC patients consisted of 76 males and 54 females (M/F ratio, 1.41) and the control subjects consisted of 88 males and 72 females (M/F ratio, 1.2; data not shown). The mean age in both the patient and control groups was 53 years. No significant gender- or age-related differences were observed between the groups (P>0.05). Furthermore, out of 130 confirmed cases of CRC, 125 cases were sporadic, 4 were familial adenomatous polyposis and one case was hereditary non-polyposis (Lynch syndrome) CRC. All but one CRC case had adenocarcinoma and one had squamous cell carcinoma of the basal cell type. Fifty-two cases had carcinoma in the colon and 78 had carcinoma in the rectum; 78 were rural and 39 urban, and 81 were smokers and 49 non-smokers (Table II).
Table II.
Association between the XRCC1 polymorphism and clinicopathological characteristics.
| No. of cases (n=130)a
|
|||||
|---|---|---|---|---|---|
| Variables | No. (%) | GG (n=63) | AG (n=37) | AA (n=30) | χ2; P-value |
| Age group | |||||
| ≤50 | 48 (36.9) | 14 | 20 | 14 | 11.73; 0.002 |
| >50 | 82 (63.1) | 49 | 17 | 16 | |
| Gender | |||||
| Female | 54 (41.54) | 22 | 12 | 20 | 10.20; 0.006 |
| Male | 76 (58.46) | 41 | 25 | 10 | |
| Dwelling | |||||
| Rural | 91 (70.0) | 36 | 33 | 22 | 11.61; 0.003 |
| Urban | 39 (30.0) | 27 | 4 | 8 | |
| Smoking status | |||||
| Ever | 81 (62.3) | 41 | 23 | 17 | 0.61; 0.737 |
| Never | 49 (37.7) | 22 | 14 | 13 | |
| Tumor location | |||||
| Colon | 52 (40.0) | 17 | 22 | 13 | 10.42; 0.005 |
| Rectum | 78 (60.0) | 46 | 15 | 17 | |
| Nodal status | |||||
| Involved | 88 (67.7) | 50 | 18 | 20 | 10.08; 0.006 |
| Not involved | 42 (32.3) | 13 | 19 | 10 | |
| Tumor grade | |||||
| WD | 98 (75.4) | 48 | 28 | 22 | 0.09; 0.956 |
| MD+PD | 32 (24.6) | 15 | 9 | 8 | |
One was squamous cell carcinoma. Significant P-values are shown in bold. WD, well-differentiated; MD, moderately differentiated; PD, poorly differentiated.
Among the CRC cases, we found the frequency of the XRCC1 genotype to be 48.5% GG (63/130), 28.5% AG (37/130) and 23.0% AA (30/130), while the frequency in the general control population was 50.0% GG (75/150), 20.0% AG (30/150) and 30.0% AA (45/150). The overall association between the XRCC1 polymorphism and the CRC cases was found to be non-significant (P>0.05; Table II). Furthermore, an independent analysis for the AG and AA genotypes revealed a significant correlation with the risk of CRC (P<0.05). The overall hazard ratio of the XRCC1 A allele in CRC was 0.79 (95% CI, 0.44–1.4).
The correlation of the XRCC1 polymorphic status with the clinicopathological characteristics was also carefully analyzed. We found a significant association (P<0.05) of the A allele with the age, gender, dwelling, tumor location, nodal status and tumor grade of the patients (P<0.05; Table II).
Discussion
In this hospital-based case-control study of CRC patients in Kashmir, we analyzed the polymorphism of the XRCC1 R399Q gene, a DNA repair gene, and its concomitant role in modulating the risk of CRC.
The XRCC1 gene belongs to a DNA repair gene family and encodes for a protein which plays a role in the repair of single-strand breaks (SSB) and in base excision repair (BER) (23). Shen et al(14) first of all reported the three common polymorphisms for the XRCC1 gene occurring at its conserved sequences, all of which affect the coding region of the gene. These coding polymorphisms result in amino acid substitutions affecting the overall activity of the synthesized proteins, which are reported to affect codons 194 (Arg>Trp), 280 (Arg>His) and 399 (Arg>Gln) (23).
We found noteworthy results in our population comparable to those of Abdel-Rahman et al(15), since we found the frequency of the XRCC1 genotype to be 48.5% GG (63/130), 28.5% AG (37/130) and 23.0% AA (30/130) in CRC cases. This frequency distribution is also comparable to that observed in the study of Wang et al(25).
In the case of the XRCC1 Arg194Trp polymorphism, a few studies reported a reduced risk of cancer associated with the 194Trp variant form (16) while another study by Skjelbred et al(23) reported no association of the codon 194 polymorphism with the risk of CRC. In one of the studies, which involved the investigation of the XRCC1 Arg280His allele and the risk of cancer, no association was observed (19). The XRCC1 Arg399Gln polymorphism has been well studied in many cancers and positive correlations have been established; however, the results from these studies are not consistent (15,17,19–21,26).
The Arg399Gln polymorphism of the XRCC1 gene resides at the C-terminal side of the poly (ADP-ribose) polymerase interacting domain which has been indicated as a protein-protein interaction module in many proteins involved in DNA repair mechanisms in the cell (27).
The majority of epidemiological case-control studies did not find any significant correlation between the XRCC1 399Gln variant and the risk of CRC (23,24,28,29), However, a case-control study carried out on a Taiwanese population found that an increased risk of CRC correlated with the XRCC1 399Arg/Arg genotype when compared with the XRCC1 399Gln in younger subjects (21). Although a study on a Norwegian population by Skjelbred et al(23) reported the XRCC1 399Gln allele to be related to a reduction in the incidence of high-risk adenomas, there was no association with any risk of carcinomas. In contrast to these reports, Abdel-Rahman et al(15) observed a significantly increased risk of CRC with the XRCC1 399Gln allele in their study on an Egyptian population and this effect was shown to be more significant among urban residents. In addition, Hong et al(19) demonstrated a positive association for this polymorphism in their study on a South Korean population.
Although our study on a Kashmiri population did not find any significant correlation between the XRCC1 Arg399Gln polymorphism and an increased risk of CRC, similarly to many of the already reported studies, we did find an increased risk of CRC among Arg/Gln heterozygous cases compared to the controls (OR=1.47; 95% CI, 0.82–2.64). We also found a significant correlation between the 399Gln allele and the various clinicopathological parameters (Table II), particularly for gender (females), dwelling (rural) and tumor location (rectum). As already noted by Wang et al(25), differences due to gender may arise either due to physiologically different effects of the XRCC1 399Gln allele or due to different dietary habits, lifestyles and other genetic factors.
The interaction of the polymorphism with various environmental factors causes an increase in the overall susceptibility to CRC in any population (30,31). Therefore, we also hypothesize that since our population is exposed to certain environmental and dietary risks, including the consumption of sun-dried and smoked fish and meat, dried and pickled vegetables, red chilli, hakh (a leafy vegetable of the Brassica family), hot noon chai (salted tea) and hukka (water pipe) smoke (2,3,5), these may play a significant role in modulating the effect of the polymorphism in a dominant model of inheritance. As previously reported, the etiology and incidence of various gastrointestinal cancers in this population has been attributed to probable exposure to nitroso compounds, amines and nitrates, reported to be present in local foodstuffs, the majority of which have been shown to contain significant irritants and carcinogens (6).
The effect of 399Gln is also increasingly amplified in the carriers due to the impairment (or reduction) of the repair pathways, which in turn is reflected in significantly higher DNA adduct levels and an increased sister chromosome exchange frequency when compared with 399Arg (32).
We conclude that there is a significant correlation between the XRCC1 A870G polymorphism and the risk of CRC in the ethnic Kashmiri population. These correlations now need to be authenticated in a large sample study, in order to discern racial differences and determine the aggressiveness of CRC.
Acknowledgments
The authors wish to thank the CRC patients who took part in this study and cooperated during the interview and sample collection. The authors gratefully acknowledge the financial support provided by the Sher-I-Kashmir Institute of Medical Sciences, Kashmir, for this study. We also thank the head and technical staff of the operating theatre in the Department of General Surgery at Sher-I-Kashmir Institute of Medical Sciences who helped us with tissue procurement, and the anonymous pathologists in the Department of Pathology, Sher-I-Kashmir Institute of Medical Sciences, for the histo-pathological assessment of the tumor tissues.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Sameer AS, Nissar S, Abdullah S, Chowdri NA, Siddiqi MA. DNA repair gene 8-oxoguanine DNA glycosylase Ser326Cys polymorphism and colorectal cancer risk in a Kashmiri population. DNA Cell Biol. 2012;31:541–546. doi: 10.1089/dna.2011.1349. [DOI] [PubMed] [Google Scholar]
- 3.Sameer AS, Shah ZA, Nissar S, Mudassar S, Siddiqi MA. Risk of colorectal cancer associated with the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in the Kashmiri population. Genet Mol Res. 2011;10:1200–1210. doi: 10.4238/vol10-2gmr1067. [DOI] [PubMed] [Google Scholar]
- 4.Javid G, Zargar SA, Rather S, Khan AR, Khan BA, et al. Incidence of colorectal cancer in Kashmir valley, India. Indian J Gastroenterol. 2011;30:7–11. doi: 10.1007/s12664-010-0071-7. [DOI] [PubMed] [Google Scholar]
- 5.Rasool S, Ganai BA, Kadla SA, Ahanger AG, Qazi F, et al. The ECRG1 290Arg/Gln polymorphism is related to risk of esophageal squamous cell carcinoma in Kashmir. Asian Pac J Cancer Prev. 2011;12:265–269. [PubMed] [Google Scholar]
- 6.Siddiqi M, Kumar R, Fazili Z, Spiegelhalder B, Preussmann R. Increased exposure to dietary amines and nitrate in a population at high risk of oesophageal and gastric cancer in Kashmir (India) Carcinogenesis. 1992;13:1331–1335. doi: 10.1093/carcin/13.8.1331. [DOI] [PubMed] [Google Scholar]
- 7.de Boer JG. Polymorphisms in DNA repair and environmental interactions. Mutat Res. 2002;509:201–210. doi: 10.1016/s0027-5107(02)00217-8. [DOI] [PubMed] [Google Scholar]
- 8.Yu Z, Chen J, Ford BN, Brackley ME, Glickman BW. Human DNA repair systems: an overview. Environ Mol Mutagen. 1999;33:3–20. doi: 10.1002/(sici)1098-2280(1999)33:1<3::aid-em2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 9.Christmann M, Tomicic MT, Roos WP, Kaina B. Mechanisms of human DNA repair: an update. Toxicology. 2003;193:3–34. doi: 10.1016/s0300-483x(03)00287-7. [DOI] [PubMed] [Google Scholar]
- 10.Wood RD, Mitchell M, Sgouros J, Lindahl T. Human DNA repair genes. Science. 2001;291:1284–1289. doi: 10.1126/science.1056154. [DOI] [PubMed] [Google Scholar]
- 11.Caldecott KW, Aoufouchi S, Johnson P, Shall S. XRCC1 polypeptide interacts with DNA polymerase beta and possibly poly (ADP-ribose) polymerase, and DNA ligase III is a novel molecular ‘nick-sensor’ in vitro. Nucleic Acids Res. 1996;24:4387–4394. doi: 10.1093/nar/24.22.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duarte MC, Colombo J, Rossit AR, Caetano A, Borim AA, Wornrath D, Silva AE. Polymorphisms of DNA repair genes XRCC1 and XRCC3, interaction with environmental exposure and risk of chronic gastritis and gastric cancer. World J Gastroenterol. 2005;11:6593–6600. doi: 10.3748/wjg.v11.i42.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marintchev A, Mullen MA, Maciejewski MW, Pan B, Gryk MR, Mullen GP. Solution structure of the single-strand break repair protein XRCC1 N-terminal domain. Nat Struct Biol. 1999;6:884–893. doi: 10.1038/12347. [DOI] [PubMed] [Google Scholar]
- 14.Shen MR, Jones IM, Mohrenweiser H. Nonconservative amino acid substitution variants exist at polymorphic frequency in DNA repair genes in healthy humans. Cancer Res. 1998;58:604–608. [PubMed] [Google Scholar]
- 15.Abdel-Rahman SZ, Soliman AS, Bondy ML, Omar S, El-Badawy SA, Khaled HM, Seifeldin IA, Levin B. Inheritance of the 194Trp and the 399Gln variant alleles of the DNA repair gene XRCC1 are associated with increased risk of early-onset colorectal carcinoma in Egypt. Cancer Lett. 2000;159:79–86. doi: 10.1016/s0304-3835(00)00537-1. [DOI] [PubMed] [Google Scholar]
- 16.Goode EL, Ulrich CM, Potter JD. Polymorphisms in DNA repair genes and associations with cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:1513–1530. [PubMed] [Google Scholar]
- 17.Nexo BA, Vogel U, Olsen A, Ketelsen T, Bukowy Z, Thomsen BL, Wallin H, Overvad K, Tjonneland A. A specific haplotype of single nucleotide polymorphisms on chromosome 19q13.2-3 encompassing the gene RAI is indicative of post-menopausal breast cancer before age 55. Carcinogenesis. 2003;24:899–904. doi: 10.1093/carcin/bgg043. [DOI] [PubMed] [Google Scholar]
- 18.Moullan N, Cox DG, Angele S, Romestaing P, Gerard JP, Hall J. Polymorphisms in the DNA repair gene XRCC1, breast cancer risk, and response to radiotherapy. Cancer Epidemiol Biomarkers Prev. 2003;12:1168–1174. [PubMed] [Google Scholar]
- 19.Hong YC, Lee KH, Kim WC, Choi SK, Woo ZH, Shin SK, Kim H. Polymorphisms of XRCC1 gene, alcohol consumption and colorectal cancer. Int J Cancer. 2005;116:428–432. doi: 10.1002/ijc.21019. [DOI] [PubMed] [Google Scholar]
- 20.Mort R, Mo L, McEwan C, Melton DW. Lack of involvement of nucleotide excision repair gene polymorphisms in colorectal cancer. Br J Cancer. 2003;89:333–337. doi: 10.1038/sj.bjc.6601061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeh C-C, Sung F-C, Tang R, Chang-Chieh C, Hsieh L-L. Polymorphisms of the XRCC1, XRCC3, & XPD genes, and colorectal cancer risk: a case-control study in Taiwan. BMC Cancer. 2005;5:12. doi: 10.1186/1471-2407-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratnasinghe D, Yao SX, Tangrea JA, Qiao YL, Andersen MR, Barrett MJ, Giffen CA, Erozan Y, Tockman MS, Taylor PR. Polymorphisms of the DNA repair gene XRCC1 and lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2001;10:119–123. [PubMed] [Google Scholar]
- 23.Skjelbred CF, Saebø M, Wallin H, Nexø BA, Hagen PC, Lothe IM, Aase S, Johnson E, Hansteen IL, Vogel U, Kure EH. Polymorphisms of the XRCC1, XRCC3 and XPD genes and risk of colorectal adenoma and carcinoma, in a Norwegian cohort: a case control study. BMC Cancer. 2006;6:67. doi: 10.1186/1471-2407-6-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stern MC, Siegmund KD, Corral R, Haile RW. XRCC1 and XRCC3 polymorphisms and their role as effect modifiers of unsaturated fatty acids and antioxidant intake on colorectal adenomas risk. Cancer Epidemiol Biomarkers Prev. 2005;14:609–615. doi: 10.1158/1055-9965.EPI-04-0189. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Zhao Y, Jiang J, Gajalakshmi V, Kuriki K, Nakamura S, Akasaka S, Ishikawa H, Suzuki S, Nagaya T, Tokudome S. Polymorphisms in DNA repair genes XRCC1, XRCC3 and XPD, and colorectal cancer risk: a case-control study in an Indian population. J Cancer Res Clin Oncol. 2010;136:1517–1525. doi: 10.1007/s00432-010-0809-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogel U, Nexo BA, Wallin H, Overvad K, Tjonneland A, Raaschou-Nielsen O. No association between base excision repair gene polymorphisms and risk of lung cancer. Biochem Genet. 2004;42:453–460. doi: 10.1023/b:bigi.0000043957.03420.7e. [DOI] [PubMed] [Google Scholar]
- 27.Masson M, Niedergang C, Schreiber V, Muller S, Menissier-de Murcia J, de Murcia G. XRCC1 is specifically associated with poly (ADP-ribose) polymerase and negatively regulates its activity following DNA damage. Mol Cell Biol. 1998;18:3563–3571. doi: 10.1128/mcb.18.6.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sliwinski T, Krupa R, Wisniewska-Jarosinska M, Lech J, Morawiec Z, Chojnacki J, Blasiak J. No association between the Arg194Trp and Arg399Gln polymorphisms of the XRCC1 gene and colorectal cancer risk and progression in a Polish population. Exp Oncol. 2008;30:253–254. [PubMed] [Google Scholar]
- 29.Improta G, Sgambato A, Bianchino G, Zupa A, Grieco V, La Torre G, Traficante A, Cittadini A. Polymorphisms of the DNA repair genes XRCC1 and XRCC3 and risk of lung and colorectal cancer: a case-control study in a Southern Italian population. Anticancer Res. 2008;28:2941–2946. [PubMed] [Google Scholar]
- 30.Zhang LQ, Huang X, Wang J, Shang JQ, Bai J, et al. The cyclin D1 G870A polymorphism and colorectal cancer susceptibility: a meta-analysis of 20 populations. Asian Pacific J Cancer Prev. 2011;12:81–85. [PubMed] [Google Scholar]
- 31.Palmqvist R, Stenling R, Oberg A, et al. Expression of cyclin D1 and retinoblastoma protein in colorectal cancer. Eur J Cancer. 1998;34:1575–1581. doi: 10.1016/s0959-8049(98)00162-2. [DOI] [PubMed] [Google Scholar]
- 32.Lunn RM, Langlois RG, Hsieh LL, Thompson CL, Bell DA. XRCC1 polymorphisms: effects on aflatoxin B1-DNA adducts and glycophorin A variant frequency. Cancer Res. 1999;59:2557–2561. [PubMed] [Google Scholar]