Abstract
Objective
To analyze the impact of the lymph node ratio (LNR, ratio of metastatic to examined nodes) on the prognosis of hypopharyngeal cancer patients.
Methods
SEER (Surveillance, Epidemiology and End Results)-registered hypopharyngeal cancer patients with lymph node metastasis were evaluated using multivariate Cox regression analysis to identify the prognostic role of the LNR. The categorical LNR was compared with the continuous LNR and pN classifications to predict cause-specific survival (CSS) and overall survival (OS) rates of hypopharyngeal cancer patients.
Results
Multivariate analysis of 916 pN+ hypopharyngeal cancer cases identified race, primary site, radiation sequence, T classification, N classification, M classification, the number of regional lymph nodes examined, the continuous LNR (Hazard ratio 2.415, 95% CI 1.707–3.416, P<0.001) and age as prognostic variables that were associated with CSS in hypopharyngeal cancer. The categorical LNR showed a higher C-index and lower Akaike information criterion (AIC) value than the continuous LNR. When patients (n = 1152) were classified into four risk groups according to LNR, R0 (LNR = 0), R1 (LNR ≤0.05), R2 (LNR 0.05–0.30) and R3 (LNR >0.30), the Cox regression model for CSS and OS using the R classification had a higher C-index value and lower AIC value than the model using the pN classification. Significant improvements in both CSS and OS were found for R2 and R3 patients with postoperative radiotherapy.
Conclusions
LNR is a significant prognostic factor for the survival of hypopharyngeal cancer patients. Using the cutoff points 0.05/0.30, the R classification was more accurate than the pN classification in predicting survival and can be used to select high risk patients for postoperative treatment.
Introduction
Hypopharyngeal cancer accounts for 2–6% of head neck cancers. [1], [2] The prognosis of patients with cancer of the hypopharynx can be poor despite aggressive combined modality treatment. [3] Traditionally, laryngopharyngectomy with reconstruction of the pharynx has been the preferred initial treatment modality for hypopharyngeal cancers. In an attempt to limit the morbidity of surgical therapy, nonsurgical treatments have gained popularity. [2] While for selected patients with early T classification hypopharyngeal cancer, transoral laser microsurgery in combination with neck dissection and postoperative radiotherapy shows results comparable to those of open surgical procedures and radiotherapy, morbidity and complication rates tend to be lower. [4] For patients with a T4a classification (tumor invasion of thyroid/cricoid cartilage, hyoid bone, thyroid gland or the central compartment of soft tissue), surgery plus neck dissection followed by adjuvant chemotherapy/radiotherapy or radiotherapy has been preferred in accordance to the NCCN guidelines. [3]
When primary surgery is the selected management path for resectable hypopharyngeal cancer cases, postoperative chemotherapy/radiotherapy is recommended (level I evidence) for the adverse pathologic features of extracapsular nodal spread and/or a positive mucosal margin. [3], [5] For other risk features, clinical judgment should be used when deciding to use radiotherapy alone or when considering including chemotherapy with radiotherapy. A better postoperative staging system for hypopharyngeal cancer would be helpful in selecting suitable patients for more intensified postoperative therapy and in the design of clinical trials. The lymph node ratio (LNR), defined as the number of involved nodes divided by the number of lymph nodes examined, was found to improve prognostic information in breast cancer, gastric cancer, colorectal cancer, melanoma and others. [6], [7], [8], [9], [10], [11], [12] Approximately 60% to 80% of patients with hypopharyngeal cancer have locally advanced disease with spread to regional nodes at diagnosis. [13] However, there have been no previous studies on the impact of the LNR on predicting the prognosis of hypopharyngeal cancer.
In the current study, the prognostic role of LNR was analyzed in SEER (Surveillance, Epidemiology and End Results)-registered hypopharyngeal cancer patients with lymph node metastasis. In addition, the cutoff points for the LNR in defining patients as high, medium or low risk groups were identified. The predictive accuracy for survival of the categorical LNR was also compared with the pN classification of hypopharyngeal cancer.
Materials and Methods
Patients
The SEER Cancer Statistics Review (http://seer.cancer.gov/), a report on the most recent cancer incidence, mortality, survival, prevalence and lifetime risk statistics, is published annually by the Data Analysis and Interpretation Branch of the National Cancer Institute, MD, USA. [14] SEER collects and publishes these statistics from population-based registries covering 26% of the US population. The 17 SEER registries routinely collect data on patient demographics, primary tumor site, tumor morphology, extent of disease, first course of treatment and active follow-up for vital status. [14] The SEER set has been widely used for the analysis of LNR staging of breast, colon, gastric and other cancers. [11], [15], [16].
Cases of hypopharyngeal carcinoma from 1988 to 2008 were extracted from the SEER database (SEER*Stat 7.0.5) according to the Site Recode classifications. The data were standardized according to the schema of the International Classification of Disease for Oncology. Cancers were limited to the hypopharynx, which were defined as the pyriform sinus (C12.9), postcricoid region (C13.0), hypopharyngeal aryepiglottic fold (C13.1), posterior wall of the hypopharynx (C13.2), overlapping lesion of the hypopharynx (C13.8) and hypopharynx, NOS (C13.9). Histology was limited to squamous cell carcinoma (histology recode - broad groupings 8050–8090). Only hypopharyngeal carcinomas as a single primary tumor or the first diagnosed cancer type of two or more primary tumors were included in current study due to the available information for cause specific survival analysis in SEER database. The LNR was calculated as the number of positive regional nodes (1988+) divided by the number of regional nodes examined (1988+). The cases with disconcordant N classification information and the number of positive regional lymph nodes recorded in SEER database were rejected (N = 116). Finally, a total of 1152 cases of hypopharyngeal carcinoma were collected as the analysis set, and among them 916 cases had pathological lymph node involvement (pN+). This study was based on public use de-identified data from the U.S. SEER database and did not include interaction with human subjects or use personal identifying information. The study did not require informed consent and was approved by the Review Board of Fudan Univesity Shanghai Cancer Center, Shanghai, China. The authors, however, obtained Limited-Use Data Agreements from SEER.
Statistical Analysis
The categorical and continuous variables of hypopharyngeal cancer cases were retrieved from the SEER database and rechecked. TNM staging information was restaged according to the 2010 American Joint Committee on Cancer (AJCC) staging system, and cases with insufficient classification were defined as X. The CSS (SEER cause-specific survival, http://seer.cancer.gov/causespecific/) was a net survival measure representing survival of a specified cause of death in the absence of other causes of death. The ‘SEER cause-specific death classification’ variable is used to obtain cancer-specific survival probability for a given cohort of cancer patients. Deaths attributed to the cancer of interest are treated as events and deaths from other causes are treated as censored observation. [14] The overall survival (OS) was calculated based on the Vital status recode. [14].
The analysis was carried out in three stages. First, we evaluated the prognostic value of LNR as a continuous variable, adjusting for other covariates associated with CSS in 916 pN+ hypopharyngeal cancer cases. In the second stage, we proceeded to determine the most appropriate cutoff points for categorizing the LNR into high, medium and low risk groups, and compared the predictive accuracy for survival between the categorical LNR and the continuous LNR. The fist pair of cutoff points was identified by means of tertiles of continuous LNR. The second pair of cutoff points were analyzed using the X-tile program (http://www.tissuearray.org/rimmlab/), which identified the cutoff with the minimum P values from log-rank χ2 statistics for the categorical LNR in terms of survival. [8], [17] The third pair of cutoff points were calculated according to Akaike information criterion (AIC) selection for the multivariate Cox regression model. [7] Using R software with the MASS, Boot and Survival package, the possible pair of cutoff points with the maximum likelihood associated with the multivariate Cox regression model, which ranged from 0.05 to 0.95 at intervals of 0.05, was identified as the third pair of cutoff points. Patients with hypopharyngeal cancer (N = 1152) were classified into four categories (R0-3) according to the identified LNR cutoff points. The prognostic significance of categorical LNR (R classification) was compared with the pN classification.
Survival curves were plotted and survival rates were calculated using the Kaplan-Meier method. Statistical comparisons of different factors related to mortality were undertaken using the Log-rank χ2 test. In multivariate analysis, forward stepwise regression analysis was carried out using a Cox proportional hazards model. Harrell’s concordance index (C-index) and AIC values related to the Cox regression model were analyzed to compare the predictive ability of the continuous LNR and categorical LNR for the identification of the optimal cutoff points for the LNR, and the R and pN classifications. [18], [19] A higher C-index value and a lower AIC value indicated a more desirable model for predicting outcome. A P value of 0.05 was considered statistically significant. All statistical analyses were carried out using SPSS software version 17.0 (SPSS Inc., Chicago, IL) and R2.14.0 software with packages. [20].
Results
Lymph Node Ratio as a Prognostic Factor for Survival in Hypopharyngeal Cancer
The clinical characteristics of the 916 SEER patients with pN+ hypopharyngeal cancer are shown in Table 1. The median age of patients was 61 years (range 35–93 years). The median number of lymph nodes examined, positive lymph nodes and lymph node ratio were 25 (range 1–90), 2 (range 1–90) and 0.14 (range 0.01–1.00), respectively. The median follow-up time was 23 months (range, 0–238 months). There were 495 cases with cancer caused death, 181 cases with non-cancer caused death, 238 alive cases and 2 cases lost during follow-up at the end of follow-up. Kaplan-Meier estimates of 1-, 3-, 5- and 10-year CSS were 76.3%, 51.0%, 42.3% and 33.6%, respectively, and estimates of 1-, 3-, 5- and 10-year OS were 76.3%, 43.9%, 30.9% and 18.7%, respectively. The case number and 5-year CSS of patients diagnosed at 1988–1994, 1995–2001, 2002–2008 were 282, 298, 336 and 42.1%, 43.1%, 41.4%, respectively. There was no changes of the survival for pN+ hypopharyngeal cancer cases diagnosed at different period (Log-rank χ2 0.156, P = 0.925). The univariate analysis showed that race, primary site, radiation sequence, cancer directed surgery, T classification, N classification and M classification were all associated with CSS in 916 pN+ hypopharyngeal cancer cases. When these factors were assessed using multivariate Cox regression analysis, we found that race, primary site, radiation sequence, T classification, N classification, M classification, the number of regional lymph nodes examined, continuous LNR and age were all independent variables (Table 2).
Table 1. Clinicopathological characteristics and Kaplan-Meier cause specific survival (CSS) analysis of SEER hypopharyngeal cancer cases with pathological lymph node involvement.
| Variables | No. | 5-year CSS | Log-rank χ2 | P value |
| Race | 29.840 | <0.001 | ||
| White | 696 | 46.2% | ||
| Black | 155 | 26.7% | ||
| Other | 65 | 37.5% | ||
| Gender | 0.303 | 0.582 | ||
| Male | 750 | 41.7% | ||
| Female | 166 | 44.8% | ||
| Year of diagnosis | 0.156 | 0.925 | ||
| 1988–1994 | 282 | 42.1% | ||
| 1995–2001 | 298 | 43.1% | ||
| 2002–2008 | 336 | 41.4% | ||
| Primary site | 17.637 | 0.003 | ||
| Pyriform sinus | 664 | 43.2% | ||
| Postcricoid region | 20 | 28.7% | ||
| Aryepiglottic fold | 25 | 63.4% | ||
| Posterior wall | 36 | 42.0% | ||
| Overlapping lesion | 24 | 13.8% | ||
| Hypopharynx, NOS | 147 | 42.1% | ||
| Histological grade | 3.230 | 0.520 | ||
| I | 28 | 36.5% | ||
| II | 370 | 44.6% | ||
| III | 406 | 40.8% | ||
| IV | 16 | 0 | ||
| Unknown | 96 | 39.9% | ||
| Surgery of primary site | 1.317 | 0.725 | ||
| None | 216 | 40.0% | ||
| Local excision | 92 | 48.4% | ||
| Pharyngectomy | 595 | 42.4% | ||
| Unknown | 13 | 0 | ||
| Radiation sequence | 25.588 | <0.001 | ||
| No radiation | 226 | 34.5% | ||
| Pre-operative | 50 | 35.1% | ||
| Post-operative | 620 | 46.1% | ||
| Other | 20 | 0 | ||
| Cancer directed surgery | 7.519 | 0.006 | ||
| Yes | 747 | 43.2% | ||
| Other | 169 | 38.9% | ||
| T stage | 38.547 | <0.001 | ||
| T1 | 81 | 65.9% | ||
| T2 | 152 | 56.1% | ||
| T3 | 144 | 39.6% | ||
| T4 | 376 | 33.2% | ||
| Tx | 163 | 41.8% | ||
| N stage | 15.483 | <0.001 | ||
| N1 | 194 | 51.4% | ||
| N2 | 653 | 41.5% | ||
| N3 | 69 | 23.0% | ||
| M stage | 46.848 | <0.001 | ||
| M0 | 871 | 43.8% | ||
| M1 | 31 | 5.4% | ||
| Mx | 14 | 24.2% |
Table 2. Multivariate analysis of the LNR and covariates associated with SEER cancer cause-specific survival of hypopharyngeal cancer cases with lymph node metastasis.
| Variables | HR (95% CI) | P value |
| Race (White as reference) | ||
| Black | 1.678(1.334–2.112) | <0.001 |
| Other | 1.392(0.999–1.940) | 0.051 |
| Primary site (Pyriform sinus as reference) | ||
| Postcricoid region | 1.088(0.619–1.911) | 0.769 |
| Aryepiglottic fold | 0.664(0.338–1.305) | 0.235 |
| Posterior wall | 2.327(1.459–3.711) | <0.001 |
| Overlapping lesion | 2.140(1.327–3.453) | 0.002 |
| Hypopharynx, NOS | 1.206(0.935–1.555) | 0.149 |
| Radiation sequence (no radiation as reference) | ||
| Pre-operative | 0.976(0.642–1.482) | 0.908 |
| Post-operative | 0.643(0.512–0.807) | <0.001 |
| Other | 1.060(0.580–1.936) | 0.850 |
| T classification (T1 as reference) | ||
| T2 | 1.441(0.906–2.292) | 0.122 |
| T3 | 2.504(1.597–3.925) | <0.001 |
| T4 | 2.965(1.954–4.497) | <0.001 |
| Tx | 1.671(1.055–2.648) | 0.029 |
| N classification (N1 as reference) | ||
| N2 | 1.352(1.064–1.717) | 0.014 |
| N3 | 2.224(1.544–3.202) | <0.001 |
| M classification (M0 as reference) | ||
| M1 | 4.195(2.679–6.569) | <0.001 |
| MX | 1.289(0.636–2.613) | 0.482 |
| No. of regional lymph node examined | 1.009(1.003–1.014) | 0.002 |
| LNR as continuous variable | 2.415(1.707–3.416) | <0.001 |
| Age | 1.017(1.007–3.416) | <0.001 |
Identification of LNR Cutoff Points
The complexity of the underlying computations for obtaining the hazard ratios for the continuous LNR ensures that the immediately readable hazard ratios for the categorized LNR are preferred in daily clinical practice. To stratify the patients with lymph node metastasis as high, medium and low risk groups associated with CSS, the upper and lower tertiles of continuous LNR that corresponded to 0.09 and 0.27 were defined as the first pair of cutoff points. The X-tile, which can control the inflated type I error problem and minimize the loss of information due to multiple testing through cross-validation, identified 0.08/0.33 as the second pair of cutoff points. [8], [17] After recomputing the likelihood associated with all pairs of LNR cutoffs (dividing patients into three risk groups) using the multivariate regression model (Table 2), 0.05/0.30 was identified as the third pair of cutoff points with the maximum likelihood associated with the Cox regression model. The SEER cases with lymph node metastasis were stratified as high, medium and low risk groups according to the three pairs of cutoff points identified above. The case numbers, the 5-year CSS and 5-year OS of the different risk groups were summarized in Table 3. To compare the predictive ability of the categorical LNR and the continuous LNR, the C-index and AIC value of the Cox regression model (Table 2) with substitution of the continuous LNR with the categorical LNR were calculated. As listed in Table 3, all three pairs of the categorical LNRs showed superior predictive ability to that of the continuous LNR, with the lower AIC value and higher C-index value associated with the Cox regression model. For the sake of convenience in clinical interpretation and application, the cutoff points 0.05/0.30 with the lowest AIC values were used for further analysis.
Table 3. Univariate and multivariate analysis of the categorical and continuous LNR with cause-specific survival (CSS) and overall survival (OS) of SEER hypopharyngeal cancer patients with lymph node metastasis.
| LNR classification | No. | 5-y CSS(%) | Log-rank χ2 † | HR (95% CI) ‡ | C-index‡ | AIC‡ | 5-y OS(%) | Log-rank χ2 # | HR (95% CIs) * | C-index* | AIC* |
| Continuous LNR | 2.415(1.707–3.416) | 0.688 | 5946.70 | 2.005(1.483–2.712) | 0.668 | 8001.08 | |||||
| Cutpoints 0.09/0.27 | 39.326 | 0.695 | 5943.40 | 28.887 | 0.672 | 7997.18 | |||||
| R1∶0–0.09 | 330 | 51.6 | Reference | 38.9 | Reference | ||||||
| R2∶0.09–0.27 | 285 | 40.9 | 1.451(1.131–1.861) | 30.0 | 1.328(1.076–1.639) | ||||||
| R3: >0.27 | 301 | 32.6 | 2.150(1.631–2.845) | 22.4 | 1.855(1.463–2.353) | ||||||
| Cutpoints 0.08/0.33 | 35.427 | 0.698 | 5937.27 | 37.160 | 0.675 | 7988.71 | |||||
| R1∶0–0.08 | 301 | 52.0 | Reference | 39.5 | Reference | ||||||
| R2∶0.08–0.33 | 357 | 41.9 | 1.523(1.190–1.948) | 30.1 | 1.343(1.090–1.653) | ||||||
| R3: >0.33 | 258 | 30.3 | 2.488(1.848–3.350) | 21.3 | 2.160(1.672–2.790) | ||||||
| Cutpoints 0.05/0.30 | 33.768 | 0.697 | 5934.87 | 33.894 | 0.674 | 7988.71 | |||||
| R1∶0–0.05 | 177 | 55.6 | Reference | 43.1 | Reference | ||||||
| R2∶0.05–0.30 | 458 | 43.6 | 1.686(1.262–2.252) | 31.6 | 1.461(1.151–1.856) | ||||||
| R3: >0.30 | 281 | 30.7 | 2.822(2.011–3.959) | 21.3 | 2.301(1.729–3.062) |
The P value for Log-rank χ2 tests of cause specific survival was less than 0.001.
Being calculated using the multivariates Cox regression model for cause specific survival with either continuous or categorical LNR and variates listed at Table 2 as covariates.
The P value for Log-rank χ2 tests of overall survival was less than 0.001.
Being calculated using the multivariates Cox regression model for overall survival with either continuous or categorical LNR and variates listed at Table 2 as covariates.
Superiority of the R Classification Over the pN Classification in the Prediction of Survival
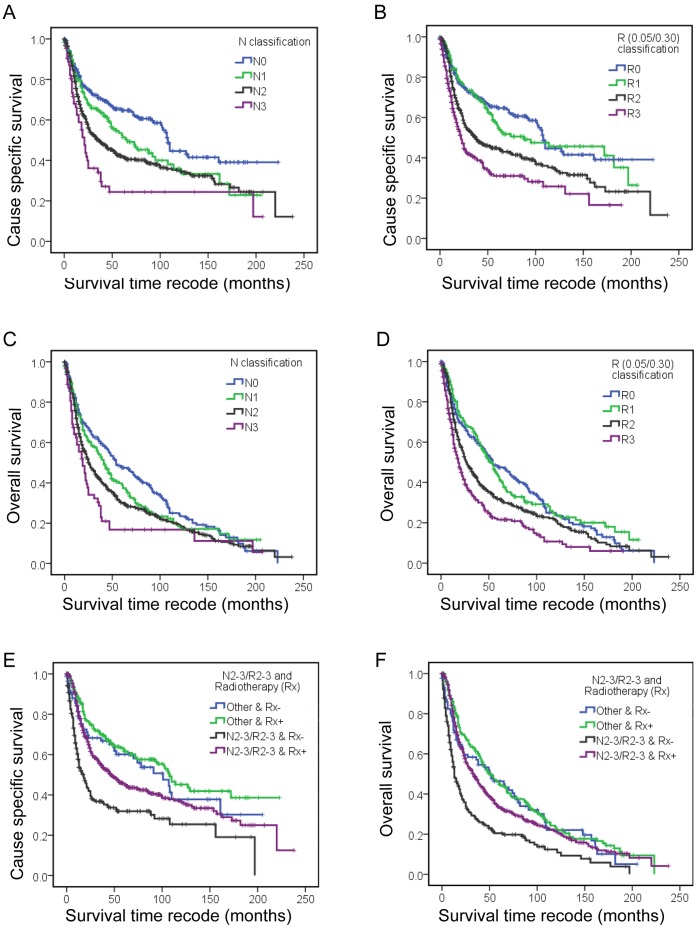
Since the N classification and LNR are both lymph node staging system, the 1152 SEER cases of hypopharyngeal carcinoma were classified as the four risk groups, R0 (LNR, 0), R1 (LNR, 0–0.05), R2 (LNR, 0.05–0.30), R3 (LNR, >0.30) and defined as the R classification to compare with traditional N classification. Univariate analysis (Table 4) and multivariate Cox regression analysis (Table 5) indicated that race, primary site, T classification, N classification, M classification, radiation sequence, cancer directed surgery and age had a significant association with CSS. When substituting the N classification with the R classification, the Cox regression model of CSS for the R classification showed a lower AIC value and higher C-index value, which suggested that Cox regression model with R classification had better predictive ability (Table 5). The Cox regression model for the R classification was also superior to the N classification in predicting OS (Table 5). Table 5 and Figure 1 show the univariate Kaplan-Meier CSS and OS estimates according to risk groups, defined using the pN (Fig. 1A and C) or R classification (Fig. 1B and D). As shown at Figure 1, for the 5-year CSS or OS, both the N and R classifications showed good discriminatory ability among each group. For the 10-year CSS and OS, the survival curves for N1 and N2 crossed at 35% and 19%. The 15-year CSS and OS survival curves for N1, N2 and N3 classifications converged at 23% and 10%, respectively (Table 5). In comparison, the 10-year CSS and OS curves for the R1-3 classification were clearly separated even with a follow-up time exceeding 15 years. For the R classification, similar survival curves were obtained for the R1 (lower LNR) and R0 (no lymph node metastasis) groups (Fig. 1B and D), and the adjusted hazard ratio (95% CIs) of the Cox regression model for the R1 group was 1.134 (0.901–1.439) for CSS and 1.072 (0.850–1.352) for OS, when using R0 as the reference group (Table 5).
Table 4. Univariate analysis of clinicopathological variables associated with SEER cause-specific survival (CSS) and overall survival (OS) of hypopharyngeal cancer patients (n = 1152).
| Patients’ variables | No. | 5-y CSS (%) | Log-rank χ2 | P value | 5-y OS(%) | Log-rank χ2 | P value |
| Race | 28.206 | <0.001 | 26.402 | <0.001 | |||
| White | 872 | 50.5 | 37.1 | ||||
| Black | 198 | 33.8 | 23.9 | ||||
| Other | 82 | 42.1 | 33.9 | ||||
| Primary site | 18.132 | 0.003 | 9.588 | 0.088 | |||
| Pyriform sinus | 829 | 48.0 | 34.8 | ||||
| Postcricoid region | 24 | 33.4 | 23.2 | ||||
| Aryepiglottic fold | 38 | 69.6 | 52.0 | ||||
| Posterior wall | 48 | 39.9 | 27.6 | ||||
| Overlapping lesion | 27 | 21.6 | 18.3 | ||||
| Hypopharynx, NOS | 186 | 48.2 | 34.1 | ||||
| T classification | 37.299 | <0.001 | 36.991 | <0.001 | |||
| T1 | 93 | 68.7 | 53.9 | ||||
| T2 | 203 | 61.5 | 47.0 | ||||
| T3 | 181 | 43.0 | 32.5 | ||||
| T4 | 484 | 39.5 | 28.1 | ||||
| Tx | 191 | 44.5 | 30.4 | ||||
| N classification | 41.026 | <0.001 | 22.437 | <0.001 | |||
| N0 | 236 | 64.7 | 47.3 | ||||
| N1 | 194 | 51.4 | 38.8 | ||||
| N2 | 653 | 41.5 | 29.8 | ||||
| N3 | 69 | 23.0 | 15.8 | ||||
| M classification | 49.844 | <0.001 | 46.822 | <0.001 | |||
| M0 | 1099 | 48.7 | 35.5 | ||||
| M1 | 36 | 12.3 | 7.0 | ||||
| Mx | 17 | 30.5 | 20.2 | ||||
| Radiation sequence | 24.501 | <0.001 | 26.596 | <0.001 | |||
| No radiation | 292 | 40.4 | 28.3 | ||||
| Pre-operative | 61 | 40.3 | 35.8 | ||||
| Post-operative | 776 | 50.8 | 36.9 | ||||
| Other | 23 | 35.9 | 21.7 | ||||
| Cancer directed surgery | 18.421 | <0.001 | 14.241 | <0.001 | |||
| Yes | 970 | 48.6 | 35.4 | ||||
| Other | 182 | 38.5 | 28.8 |
Table 5. Different staging systems for SEER cause-specific survival (CSS) and overall survival (OS) of SEER patients with hypopharyngeal cancer.
| Staging system | No. | 5/10/15-y CSS(%) | log-rank χ2 † | HR (95% CI) ‡ | C-index‡ | AIC‡ | 5/10/15-y OS(%) | log-rank χ2# | HR (95% CIs) * | C-index* | AIC* |
| N classification | 41.026 | 0.683 | 7359.73 | 22.437 | 0.657 | 10428.44 | |||||
| N0 | 236 | 64.7/45.7/40.3 | Reference | 47.3/24.5/12.4 | Reference | ||||||
| N1 | 194 | 51.4/35.6/22.8 | 1.406(1.048–1.887) | 38.8/19.2/11.2 | 1.134(0.901–1.439) | ||||||
| N2 | 653 | 41.5/34.1/26.5 | 1.926(1.523–2.435) | 29.8/19.0/9.6 | 1.515(1.266–1.812) | ||||||
| N3 | 69 | 23.0/23.0/23.0 | 2.869(1.984–4.148) | 15.8/15.8/10.6 | 1.954(1.422–2.683) | ||||||
| R classification | 61.697 | 0.686 | 7352.23 | 49.499 | 0.661 | 10417.85 | |||||
| R0 | 236 | 64.7/45.7/40.3 | Reference | 47.3/24.5/12.4 | Reference | ||||||
| R1∶0–0.05 | 177 | 55.6/45.4/41.3 | 1.256(0.930–1.698) | 43.1/24.7/18.1 | 1.072(0.850–1.352) | ||||||
| R2∶0.05–0.30 | 458 | 43.6/33.3/22.5 | 1.922(1.507–2.452) | 31.6/20.7/8.1 | 1.470(1.218–1.775) | ||||||
| R3: >0.30 | 281 | 30.7/25.8/16.9 | 2.497(1.882–3.314) | 21.3/11.0/6.4 | 1.952(1.556–2.448) |
The P value for Log-rank χ2 tests of cause specific survival was less than 0.001.
Being calculated using the multivariates Cox regression model for cause specific survival with either N classification or R classification and variates listed at Table 4 as covariates.
The P value for Log-rank χ2 tests of overall survival was less than 0.001.
Being calculated using the multivariates Cox regression model for overall survival with N classification or R classification and variates listed at Table 4 as covariates.
Figure 1. Kaplan-Meier survival estimates for different patient groups.
SEER cause-specific survival of the hypopharyngeal cancer cases with different pN classification (A) and R classification (B). Overall survival for different pN classifications (C) and R classifications (D). The SEER cause-specific survival (E) and overall survival (F) differences observed in N2-3/R2-3 group and other hypopharyngeal cancer patients with and without postoperative radiotherapy.
R Classification as an Index for Selecting High Risk Patients for Postoperative Radiotherapy
To analysis the role of the R classification in the management of hypopharyngeal cancer, the cases with (n = 776) and without (n = 292) postoperative radiotherapy according to the Radiation Sequence with Surgery recorded in SEER database were used for further study. Surgery and postoperative radiotherapy improved the CSS for N2 and N3 cases, whereas increased OS was only observed in N2 patients (Table 6). In the case of the R classification, an improvement in both CSS and OS was found for R2 and R3 patients who had received postoperative radiotherapy. To select high risk patients for postoperative radiotherapy, we defined the patients with a N2/N3 classification (n = 669/1068, 62.6%) and R2/R3 classification (n = 682/1068, 63.9%) as the N2-3/R2-3 group (n = 763/1068, 71.4%), a significant improvement (P<0.001) in both the CSS and OS was observed in the N2-3/R2-3 patients who had received postoperative radiotherapy (Table 6; Fig. 1E and F).
Table 6. The cause-specific survival (CSS) and overall survival (OS) differences between different N classification or R classification patients with and without postoperative radiotherapy (Rx).
| Patients’ variables | No. | 5/10-y CSS (%) | Log-rank χ2 † | P value | 5/10-y OS(%) | Log-rank χ2 † | P value |
| N classification | |||||||
| N0 & Rx – | 66 | 60.1/31.7 | 3.507 | 0.061 | 45.5/17.2 | 3.139 | 0.076 |
| N0 & Rx + | 156 | 66.7/50.2 | 48.3/27.2 | ||||
| N1 & Rx – | 72 | 51.5/34.1 | 1.360 | 0.243 | 33.9/21.0 | 1.567 | 0.211 |
| N1 & Rx + | 105 | 53.9/37.5 | 42.6/17.9 | ||||
| N2 & Rx – | 129 | 28.6/28.6 | 24.185 | <0.001 | 18.4/11.7 | 33.221 | <0.001 |
| N2 & Rx + | 476 | 45.7/36.4 | 33.3/21.0 | ||||
| N3 & Rx – | 25 | 18.4/18.4 | 5.591 | 0.018 | 17.6/17.6 | 2.602 | 0.107 |
| N3 & Rx + | 39 | 28.3/28.3 | 15.5/15.5 | ||||
| R classification | |||||||
| R0 & Rx – | 66 | 60.1/31.7 | 0.984 | 0.061 | 45.5/17.2 | 3.139 | 0.076 |
| R0 & Rx + | 156 | 66.7/50.2 | 48.3/27.2 | ||||
| R1 & Rx – | 34 | 64.0/64.0 | 13.402 | 0.321 | 50.9/42.0 | 1.037 | 0.308 |
| R1 & Rx + | 130 | 53.5/40.8 | 40.8/19.3 | ||||
| R2 & Rx – | 68 | 27.4/27.4 | 8.842 | <0.001 | 13.7/11.8 | 23.053 | <0.001 |
| R2 & Rx + | 350 | 47.9/35.8 | 35.4/22.9 | ||||
| R3 & Rx – | 124 | 29.2/17.2 | 17.632 | 0.003 | 20.4/7.9 | 7.398 | 0.007 |
| R3 & Rx + | 140 | 32.4/32.4 | 22.3/12.7 | ||||
| N+R classification | |||||||
| Other & Rx – | 87 | 60.1/37.8 | 1.068 | 0.301 | 46.4/22.0 | 0.547 | 0.459 |
| Other & Rx + | 218 | 61.7/45.1 | 45.9/22.7 | ||||
| N2-3/R2-3 & Rx – | 205 | 31.9/25.4 | 30.584 | <0.001 | 20.4/12.4 | 37.565 | <0.001 |
| N2-3/R2-3 & Rx + | 558 | 45.7/36.5 | 33.6/21.1 |
For Log-rank χ2 test, postoperative radiotherapy (Rx) was defined as factor, the individual R, N and N+R classification were defined as Stratum.
Discussion
The hypopharyngeal cancer cases with lymph node metastasis were staged as III/IV according to the AJCC TNM staging system. Approximately 70 to 85% of the patients reported in larger series were diagnosed as having stage III or IV disease at presentation. [3] The management of stages III/IV hypopharyngeal cancer has been relatively unsatisfactory owing to local recurrence and distant metastasis. Optimal therapy has remained a matter of debate, especially with regard to treatment intensity and sequencing. [2] Although treatment strategies of cancer advance tremendously, no significant survival improvements were observed in pN+ hypopharyngeal cancer patients diagnosed at different periods as shown in current results, which was also observed for laryngeal cancer and supposed to be caused by inappropriate patients selecting and lack of better index for guiding postoperative therapy. [21], [22], [23] It is plausible that selecting high risk patients is important in the choice of optimal treatment strategies. Current study clearly identified that the LNR is an important prognostic factor for CSS in hypopharyngeal cancer patients with lymph node metastasis. As compared with the N classification, the R classification using the cutoff points 0.05/0.30 showed better predictive ability for CSS and OS, with a lower AIC value and a higher C-index value. The survival curves for R1, R2 and R3 patients were clearly separate even after a 15-year follow-up period, whereas intersection of the survival curves for N 1–3 patients was found after a 10-year follow-up period. The similar survival estimates for R1 and R0 patients further confirmed the role of LNR in stratifying patients (a lower LNR to select lower risk patients).
Over the past three decades, more attention has been paid to the identification of factors that might help the surgeon to assess the precise risk of failure in individual patients. [20], [24] The best predictive index should be easily assessed and dependent on the target measured. For example in primary radiotherapy, volume of the primary tumor may be a predictive factor. [25], [26] For surgical approaches to organ preservation, anatomic sub-localization and extension of the primary tumor are more relevant factors. In the case of postoperative radiotherapy for hypopharyngeal cancer, adverse features were defined as extracapsular nodal spread, positive margins, pT3 or pT4 primary tumor, N2 or N3 nodal disease, selected pT1-T2, N0-N1, perineural invasion and vascular embolism according to the NCCN guidelines. [3] When the CSS and OS of individual R classification patients, with or without postoperative radiotherapy, were compared in the current study, survival benefits were observed for R2 and R3 patients. Combining the N and R classifications together can also select more high risk patients for postoperative radiotherapy than using the N classification alone (62.6% vs. 71.4%). All these results support the fact that R2 and R3 should be defined as adverse features for the postoperative management of hypopharyngeal cancer. Because information regarding the details of radiotherapy (the radiation techniques, dose delivered and fractions, and use of concurrent chemotherapy, etc) and pathological reports (margin status, extracapsular extension, perineural invasion and vascular embolism, etc) are not available in the SEER database, the definitive role of the R classification in postoperative staging and selecting high risk patients for postoperative concurrent chemoradiotherapy could not be discussed in the current study, and is deserving of further research.
Although we have adjusted for all available patient and tumor characteristics in our analysis, as with all SEER based studies, our study was limited by the information available in the database. The SEER database is dependent on the individual physician reporting on the classification of the patient, tumor and treatment characteristics. In this way, it is representative of national patterns of cancer care and has a large population base; however, it is also subject to inconsistencies. Although the number of lymph nodes examined and the number of positive nodes is information that can easily be reported with accuracy, there are still cases that were rejected for inclusion in the current study because of disconcordant information with regard to the N classification and the number of lymph node metastases. The quality of neck dissection achieved by the surgeon, and the quantity of lymph nodes harvested and examined by the pathologist will substantially change the LNR and the results. On the other hand, patients who have a lower number of lymph nodes resected (a relatively lower quality of operation), which may cause inflation of the LNR and a higher R classification, will be assessed as a high risk group. The LNR is an index not only of disease burden but also of surgical standards. This is the reason why the current study, together with studies on cancer from other sites, showed that the LNR improved the predictive ability of survival relative to the N classification. [8], [9], [16].
In conclusion, our analysis of the SEER database revealed a significant association between the LNR and survival of hypopharyngeal cancer patients. Using the cutoff points 0.05/0.30, the hypopharyngeal cancer patients with lymph node metastasis were classified into R1, R2 and R3 risk groups. The R classification can be used together with the N classification to select high-risk patients for postoperative treatment. However, more and prospective studies were still needed to confirm the prognostic role of LNR for hypophayryngeal cancer and more clinical evidence should be obtained.
Acknowledgments
The authors would like to thank SEER for open access to the database and Dr. Minghui Wang from School of Biosciences, The University of Birmingham, UK for his critical discussion of statistical methods.
Funding Statement
This work was supported by the National Science Foundation of China (81001204 to Y-L Wang). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ligier K, Belot A, Launoy G, Velten M, Bossard N, et al. (2011) Descriptive epidemiology of upper aerodigestive tract cancers in France: incidence over 1980–2005 and projection to 2010. Oral Oncol 47: 302–307. [DOI] [PubMed] [Google Scholar]
- 2. Takes RP, Strojan P, Silver CE, Bradley PJ, Haigentz M Jr, et al. (2012) Current trends in initial management of hypopharyngeal cancer: the declining use of open surgery. Head Neck 34: 270–281. [DOI] [PubMed] [Google Scholar]
- 3. Pfister DG, Ang KK, Brizel DM, Burtness BA, Cmelak AJ, et al. (2011) National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Head and neck cancers. J Natl Compr Canc Netw 9: 596–650. [DOI] [PubMed] [Google Scholar]
- 4.Suarez C, Rodrigo JP, Silver CE, Hartl DM, Takes RP, et al.. (2012) Laser surgery for early to moderately advanced glottic, supraglottic, and hypopharyngeal cancers. Head Neck. [DOI] [PubMed] [Google Scholar]
- 5. Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, et al. (2005) Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 27: 843–850. [DOI] [PubMed] [Google Scholar]
- 6. Spillane AJ, Cheung BL, Winstanley J, Thompson JF (2011) Lymph node ratio provides prognostic information in addition to american joint committee on cancer N stage in patients with melanoma, even if quality of surgery is standardized. Ann Surg 253: 109–115. [DOI] [PubMed] [Google Scholar]
- 7. Vinh-Hung V, Verkooijen HM, Fioretta G, Neyroud-Caspar I, Rapiti E, et al. (2009) Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol 27: 1062–1068. [DOI] [PubMed] [Google Scholar]
- 8. Wang W, Xu DZ, Li YF, Guan YX, Sun XW, et al. (2011) Tumor-ratio-metastasis staging system as an alternative to the 7th edition UICC TNM system in gastric cancer after D2 resection–results of a single-institution study of 1343 Chinese patients. Ann Oncol 22: 2049–2056. [DOI] [PubMed] [Google Scholar]
- 9. Ceelen W, Van Nieuwenhove Y, Pattyn P (2010) Prognostic value of the lymph node ratio in stage III colorectal cancer: a systematic review. Ann Surg Oncol 17: 2847–2855. [DOI] [PubMed] [Google Scholar]
- 10. Deng J, Sun D, Pan Y, Zhang L, Zhang R, et al. (2012) Ratio between Negative and Positive Lymph Nodes Is Suitable for Evaluation the Prognosis of Gastric Cancer Patients with Positive Node Metastasis. PLoS One 7: e43925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao P, Song YX, Wang ZN, Xu YY, Tong LL, et al. (2012) Integrated ratio of metastatic to examined lymph nodes and number of metastatic lymph nodes into the AJCC staging system for colon cancer. PLoS One 7: e35021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song YX, Gao P, Wang ZN, Tong LL, Xu YY, et al. (2012) Which is the most suitable classification for colorectal cancer, log odds, the number or the ratio of positive lymph nodes? PLoS One 6: e28937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kotwall C, Sako K, Razack MS, Rao U, Bakamjian V, et al. (1987) Metastatic patterns in squamous cell cancer of the head and neck. Am J Surg 154: 439–442. [DOI] [PubMed] [Google Scholar]
- 14.Surveillance, Epidemiology and End Results (SEER) website. Howlader N, Noone A, Krapcho M, Neyman N, Aminou R, et al. SEER Cancer Statistics Review, 1975–2008, National Cancer Institute. Bethesda, MD. Based on November 2010 SEER data submission, posted to the SEER web site, 2011. Available: http://seer.cancer.gov/csr/1975_2008/. Accessed 2011 Oct 7.
- 15. Vinh-Hung V, Joseph SA, Coutty N, Ly BH, Vlastos G, et al. (2010) Age and axillary lymph node ratio in postmenopausal women with T1–T2 node positive breast cancer. Oncologist 15: 1050–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang J, Dang P, Raut CP, Pandalai PK, Maduekwe UN, et al. (2012) Comparison of a lymph node ratio-based staging system with the 7th AJCC system for gastric cancer: analysis of 18,043 patients from the SEER database. Ann Surg 255: 478–485. [DOI] [PubMed] [Google Scholar]
- 17. Camp RL, Dolled-Filhart M, Rimm DL (2004) X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10: 7252–7259. [DOI] [PubMed] [Google Scholar]
- 18. Bradburn MJ, Clark TG, Love SB, Altman DG (2003) Survival analysis Part III: multivariate data analysis – choosing a model and assessing its adequacy and fit. Br J Cancer 89: 605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harrell FE Jr, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15: 361–387. [DOI] [PubMed] [Google Scholar]
- 20.The R Project for Statistical Computing websit. R Development Core Team (2011). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. Available: http://www.R-project.org/. Accessed 2012 Dec 7.
- 21. Glynn RW, Lowery AJ, Scutaru C, O’Dwyer T, Keogh I (2012) Laryngeal cancer: Quantitative and qualitative assessment of research output, 1945–2010. Laryngoscope 122: 1967–1973. [DOI] [PubMed] [Google Scholar]
- 22. Hoffman HT, Porter K, Karnell LH, Cooper JS, Weber RS, et al. (2006) Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope 116: 1–13. [DOI] [PubMed] [Google Scholar]
- 23. Chen AY, Fedewa S, Zhu J (2011) Temporal trends in the treatment of early- and advanced-stage laryngeal cancer in the United States, 1985–2007. Arch Otolaryngol Head Neck Surg 137: 1017–1024. [DOI] [PubMed] [Google Scholar]
- 24. Bernier J, Cooper JS (2005) Chemoradiation after surgery for high-risk head and neck cancer patients: how strong is the evidence? Oncologist 10: 215–224. [DOI] [PubMed] [Google Scholar]
- 25. Bisdas S, Nguyen SA, Anand SK, Glavina G, Day T, et al. (2009) Outcome prediction after surgery and chemoradiation of squamous cell carcinoma in the oral cavity, oropharynx, and hypopharynx: use of baseline perfusion CT microcirculatory parameters vs. tumor volume. Int J Radiat Oncol Biol Phys 73: 1313–1318. [DOI] [PubMed] [Google Scholar]
- 26. Mendenhall WM, Morris CG, Amdur RJ, Hinerman RW, Mancuso AA (2003) Parameters that predict local control after definitive radiotherapy for squamous cell carcinoma of the head and neck. Head Neck 25: 535–542. [DOI] [PubMed] [Google Scholar]