Abstract
Herpetic eye disease, termed herpetic stromal keratitis (HSK), is a potentially blinding infection of the cornea that results in over 300,000 clinical visits each year for treatment. Between 1 and 2 percent of those patients with clinical disease will experience loss of vision of the infected cornea. The vast majority of these cases are the result of reactivation of a latent infection by herpes simplex type I virus and not due to acute disease. Interestingly, the acute infection is the model most often used to study this disease. However, it was felt that a recurrent model of HSK would be more reflective of what occurs during clinical disease. The recurrent animal models for HSK have employed both rabbits and mice. The advantage of rabbits is that they experience reactivation from latency absent any known stimulus. That said, it is difficult to explore the role that many immunological factors play in recurrent HSK because the rabbit model does not have the immunological and genetic resources that the mouse has. We chose to use the mouse model for recurrent HSK because it has the advantage of there being many resources available and also we know when reactivation will occur because reactivation is induced by exposure to UV-B light. Thus far, this model has allowed those laboratories using it to define several immunological factors that are important to this disease. It has also allowed us to test both therapeutic and vaccine efficacy.
Keywords: Infection, Issue 70, Immunology, Virology, Medicine, Infectious Diseases, Ophthalmology, Herpes, herpetic stromal keratitis, HSK, keratitis, pathogenesis, clinical evaluation, virus, eye, mouse, animal model
Protocol
Animal Care Note: Analgesics are not used in this model as they are anti-inflammatory and thus would invalidate the model.
1. Preparation of Virus
Grow HSV-1 on Vero cells (80% confluent) using a starting dilution of 0.01 multiplicity of infection (in T-150 flask, about 5 x 105 plaque forming units) for 3-4 days until all of cells have rounded-up and are easily dislodged by hitting the flask.
Remove media to sterile centrifuge tubes and centrifuge in Sorvall Legend RT at 4 °C and 3,500 rpm for 15 min. After removing supernatant (see 1.3), resuspend large pellet from each 50 ml tube with 5 ml of media total. Sonicate for 1 min in two 30 sec bursts.
Remove supernatant and put into 50 ml sterile Oak Ridge tubes and centrifuge at 9,000 rpm, for 1 hr at 4 °C in a Beckman J2-21 centrifuge. This is to produce a small viral pellet. Discard the supernatant.
Combine sonicate from 1.2 with pellet from 1.3 and sonicate again for 45 sec. Centrifuge in Sorvall Legend RT at 1,500 rpm for 5 min at 4 °C. Save the supernatant. This constitutes the virus stock which is aliquoted and stored at -70 °C.
Titer virus on Vero cells. I usually plate 100 out to 10-8 dilution (each being a 10-fold dilution) 2x from 2 separate dilution sets. Titration of virus is performed on 12-well sterile plates.
2. Mouse Infection
Inject each mouse with 0.1 ml of anesthetic cocktail/20 g mouse body weight (Ketamine, 60 mg/kg + Xylazine 5 mg/kg which is diluted in HBSS). This cocktail is used because it is one of the safest means of anesthesia when proper dosages are used and the effects of this drug are very short-lived.
When mice are anesthetized scratch the cornea of the right eye with a 30 gauge needle using a dissecting microscope to ensure that the cornea is not perforated.
Each mouse received an intraperitoneal injection of 1 ml of pooled human serum (Sigma, Temecula, Calif.; anti-HSV reactivity with an effective dose for 50% viral neutralization of 1:800) in order to protect corneas from damage during primary infection.
Number the mice with either ear clips or by ear punch.
After all of the mice have been numbered; using a 20 μl pipettor, we infect each eye with 106 PFU (5 μl). We infect only one eye and typically the right eye. We also place 5 μl of HBSS to the other eye to keep it moist while they are anesthetized. 5 ul is sufficient to maintain hydration of the eyes since the procedures are performed in less than 10 min, and the animals are dosed with anesthetic accordingly. Infection is confirmed by swabbing mice 3 days post-infection with a sterile cotton applicator that was hydrated in 1 ml of Vero media. Then 100 μl is added to Vero cells grown in 48-well plates. These plates are monitoring daily for cytopathic effects (cpe).
3. Reactivation of Mice from Latency
Mice are anesthetized at least 5 weeks following primary infection. It should be noted that we have reactivated mice up to one year following primary infection and have not observed significant differences in response.
Once anesthetized they are placed TM20 Chromato-Vu transilluminator, which emits UV-B at a peak wavelength of 302 nm such that only a single eye is exposed to the UV-B light. Each mouse is exposed to 250 mJ of UV-B light cm2.
Reactivation is determined by swabbing eyes with sterile cotton applicator that is first placed in 1 ml of Vero media on Day 0 (Before UV-B exposure to control for spontaneous shedders) and then on days 1 to 7 post-UV-B irradiation. The swab material is evaluated as described in 2.5). For those swabs that have infectious virus the amount of virus is tittered by a standard plaque assay using 12 well plates.
4. Clinical Evaluation
Mice are evaluated for clinical eye disease by a masked observer using a binocular dissecting microscope.
Corneal opacity is rated on a scale of 0 to 4, where 0 indicates clear stroma, 1 indicates mild stromal opacification, 2 indicates moderate opacity with discernible iris features, 3 indicates dense opacity with loss of defined iris detail except pupil margins, and 4 indicates total opacity with no posterior view.
Neovascularization is rated on a scale of 1 to 8 by dividing the cornea into 4 equal quadrants and determining the extent of vascularization in each of these quadrants.
Blepharitis is measured as follows: 0, no lesions; 1, minimal eyelid swelling; 2, moderate swelling and crusty ocular discharge; 3, severe swelling, moderate periocular hair loss, and skin lesions; and 4, severe swelling with eyes crusted shut, severe periocular hair loss, and skin lesions.
Evidence of encephalitis included hunched back, ruffled hair, and obvious neurologic abnormalities. Mice are euthanized when they display significant signs of encephalitis.
5. Representative Results
The recurrent model which was described above was first published by Shimeld, et al. 1-3. They tested and our laboratory has been using a modified version of this model to publish many manuscripts over the years 4-15. The model was used to define the role that the cytokine interferon-γ plays in recurrent HSV-1 disease 10. As shown in Figure 3, mice lacking IFNγ displayed worse corneal disease than did wild-type mice 14. It was also used successfully to define the therapeutic value of several different vaccine constructs 6,10,11. Note that in one case the vaccine tested worked well prophylactically but did not prove to be therapeutically effective 6. However, when a replication-incompetent viral vaccine was tested, it was not only effective prophylactically, but was also very effective therapeutically 10,11, see Figure 5 in reference 10. More recently we have used this model to determine if gender differences were detectable in mice undergoing recurrent HSK. As Figure 1 demonstrates, male C57BL/6 mice display significantly less corneal opacity than do female C57BL/6 mice. Likewise, when corneal neovascularization was evaluated, male mice demonstrated significantly less new vessel growth in the cornea than did female mice (Figure 2). We are currently testing whether this phenotype is strain specific by performing similar analysis in other strains of mice. We will also determine whether the difference is due to the lack of testosterone or the presence of estrogen.
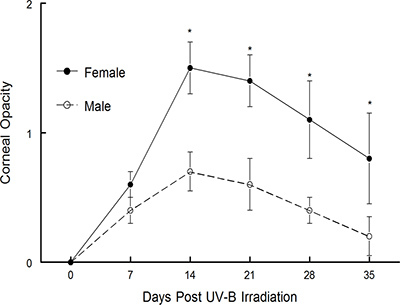 Figure 1. Corneal disease in male and female C57BL/6 mouse strains following UV-B induced reactivation. Latently infected mice were induced to reactivate with UV-B irradiation and mice were monitored for corneal opacity for 35 days. The numbers of mice used for these studies were as follows: males, n = 15; females, n = 15. Results indicate mean ± SEM. *There was significantly greater virus-induced disease in female C57BL/6 mice for days 14-35 (P = 0.001 to 0.01).
Figure 1. Corneal disease in male and female C57BL/6 mouse strains following UV-B induced reactivation. Latently infected mice were induced to reactivate with UV-B irradiation and mice were monitored for corneal opacity for 35 days. The numbers of mice used for these studies were as follows: males, n = 15; females, n = 15. Results indicate mean ± SEM. *There was significantly greater virus-induced disease in female C57BL/6 mice for days 14-35 (P = 0.001 to 0.01).
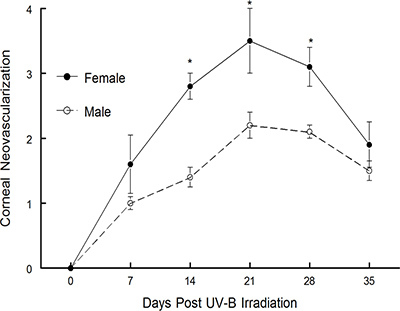 Figure 2. Corneal disease in male and female C57BL/6 mouse strains following UV-B induced reactivation. Latently infected mice were induced to reactivate with UV-B irradiation and mice were monitored for corneal neovascularization for 35 days. The numbers of mice used for these studies were as follows: males, n = 15; females, n = 15. Results indicate mean ± SEM. *There was significantly greater virus-induced disease in female C57BL/6 mice for days 14-28 (P = 0.001 to 0.01).
Figure 2. Corneal disease in male and female C57BL/6 mouse strains following UV-B induced reactivation. Latently infected mice were induced to reactivate with UV-B irradiation and mice were monitored for corneal neovascularization for 35 days. The numbers of mice used for these studies were as follows: males, n = 15; females, n = 15. Results indicate mean ± SEM. *There was significantly greater virus-induced disease in female C57BL/6 mice for days 14-28 (P = 0.001 to 0.01).
Discussion
The recurrent model of herpetic stromal keratitis (HSK) described here enables the investigator to study human HSK in a model that is more consistent with that which is observed during human disease. Thus, the model's strengths are that disease occurs in the context of an immune system that has already by stimulated during primary disease. Since these mice already have an immune response to HSV-1, the factors that reactivate that immune response are likely different from those that originally generated it. Therefore, therapeutic intervention to prevent primary HSK will not always lead to amelioration of recurrent HSK, which we have shown in various publications when evaluating differences between primary and recurrent HSK 6,8,10-13,15. As indicated previously, this is particular the case when developing vaccines to prevent human HSK. Most vaccines will prevent the development of primary HSK but very few have been shown to be effective in reducing recurrent HSK 6,10,11.
The weaknesses of this model are that it takes much longer to perform an experiment. There is an almost 6 week period following primary infection during which the mice become latently infected and the administered human serum is metabolized. Furthermore, not all mice that are latently infected will shed detectable virus into the tear film of the infected eye. This does not mean that they did not reactivate, only that we cannot detect their reactivation by culturing virus. It is highly likely that most, if not all mice do reactivate but that they do not produce sufficient numbers of virus to be detected by plaque assay.
Disclosures
No conflicts of interest declared.
Acknowledgments
The authors would like to thank Dr. Jay Pepose for helping teaching the model to us. This work was supported by National Institutes of Health Grants EY11885 (PMS), EY21247 (PMS) and an unrestricted grant from Research to Prevent Blindness to Department of Ophthalmology.
References
- Shimeld C, Hill TJ, Blyth B, Easty D. An improved model of recurrent herpetic eye disease in mice. Curr. Eye Res. 1989;8:1193–1205. doi: 10.3109/02713688909000044. [DOI] [PubMed] [Google Scholar]
- Shimeld C, Hill TJ, Blyth WA, Easty DL. Reactivation of latent infection and induction of recurrent herpetic eye disease in mice. J. Gen. Virol. 1990;71:397–404. doi: 10.1099/0022-1317-71-2-397. [DOI] [PubMed] [Google Scholar]
- Shimeld C, Hill TJ, Blyth WA, Easty DL. Passive immunization protects the mouse eye from damage after herpes simplex virus infection by limiting spread of virus in the nervous system. J. Gen. Virol. 1990;71:681–687. doi: 10.1099/0022-1317-71-3-681. [DOI] [PubMed] [Google Scholar]
- Laycock KA, Lee SF, Brady RH, Pepose JS. Characterization of a murine model of recurrent herpes simplex viral keratitis induced by ultraviolet B radiation. Invest. Ophthalmol. Vis. Sci. 1991;32:2741–2746. [PubMed] [Google Scholar]
- Keadle TL, Stuart PM. IL-10 ameliorates corneal disease in a mouse model of recurrent herpetic keratitis. Microbial Path. 2005;38:13–21. doi: 10.1016/j.micpath.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Keadle TL, Laycock KA, Miller JK, Hook KK, Fenoglio ED, Francotte M, Slaoui M, Stuart PM, Pepose JS. Efficacy of a recombinant glycoprotein D subunit vaccine on the development of primary and recurrent ocular infection with herpes simplex virus type 1 in mice. J. Inf. Dis. 1997;176:331–338. doi: 10.1086/514049. [DOI] [PubMed] [Google Scholar]
- Keadle TL, Laycock KA, Pepose JS, Stuart PM. Proinflammatory cytokines IL-1 and TNF-a are required for recurrent herpetic keratitis in NIH mice. Invest. Ophth. Vis. Sci. 2000;41:96–102. [PubMed] [Google Scholar]
- Keadle TL, Usui N, Laycock KA, Kumano Y, Pepose JS, Stuart PM. Corneal cytokine expression in a murine model recurrent herpetic stromal keratitis. Ocular Immunol. Inflam. 2001;9:193–205. doi: 10.1076/ocii.9.3.193.3967. [DOI] [PubMed] [Google Scholar]
- Keadle TL, Morris JL, Pepose JS, Stuart PM. CD4+ and CD8+ cells are key participants in the development of recurrent herpetic stromal keratitis in mice. Microbial Path. 2002;32:255–262. doi: 10.1006/mpat.2002.0506. [DOI] [PubMed] [Google Scholar]
- Keadle TL, Morrison LA, Morris JL, Pepose JS, Stuart PM. Therapeutic immunization with a virion host shutoff-defective, replication-incompetent herpes simplex virus type 1 strain limits recurrent herpetic ocular infection. J. Virol. 2002;76:3615–3625. doi: 10.1128/JVI.76.8.3615-3625.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keadle TL, Laycock KA, Morris JL, Leib DA, Morrison LA, Pepose JS, Stuart PM. Therapeutic vaccination with vhs(-) herpes simplex virus reduces the severity of recurrent herpetic stromal keratitis in mice. J. Gen. Virol. 2002;83:2361–2365. doi: 10.1099/0022-1317-83-10-2361. [DOI] [PubMed] [Google Scholar]
- Keadle TL, Morris JL, Stuart PM. The effects of aminoguanidine on primary and recurrent ocular herpes simplex virus infection. Nitric Oxide. 2005;13:247–253. doi: 10.1016/j.niox.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Stuart PM, Morris JE, Sidhu M, Keadle TL. CCL3 protects mice from corneal pathology during recurrent HSV-1 infection. Front. Biosci. 2008;13:4407–4415. doi: 10.2741/3013. [DOI] [PubMed] [Google Scholar]
- Keadle TL, Alexander DE, Leib DA, Stuart PM. Interferon gamma is not required for recurrent herpetic stromal keratitis. Virology. 2008;380:46–51. doi: 10.1016/j.virol.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DJ, Austin BA, Halford WP, Stuart PM. Delivery of Interferon-gamma by an adenovirus vector blocks herpes simplex virus Type 1 reactivation in vitro and in vivo independent of RNase L and double-stranded RNA-dependent protein kinase pathways. J. Neuroimmunol. 2009;206:39–43. doi: 10.1016/j.jneuroim.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


