Abstract
Our previously reported phase I clinical trial with the allogeneic gene–modified tumor cell line RCC-26/CD80/IL-2 showed that vaccination was well tolerated and feasible in metastatic renal cell carcinoma (RCC) patients. Substantial disease stabilization was observed in most patients despite a high tumor burden at study entry. To investigate alterations in immune responses that might contribute to this effect, we performed an extended immune monitoring that included analysis of reactivity against multiple antigens, cytokine/chemokine changes in serum and determination of the frequencies of immune suppressor cell populations, including natural regulatory T cells (nTregs) and myeloid-derived suppressor cell subsets (MDSCs). An overall immune response capacity to virus-derived control peptides was present in 100% of patients before vaccination. Vaccine-induced immune responses to tumor-associated antigens occurred in 75% of patients, demonstrating the potent immune stimulatory capacity of this generic vaccine. Furthermore, some patients reacted to peptide epitopes of antigens not expressed by the vaccine, showing that epitope-spreading occurred in vivo. Frequencies of nTregs and MDSCs were comparable to healthy donors at the beginning of study. A significant decrease of nTregs was detected after vaccination (p = 0.012). High immune response rates, decreased frequencies of nTregs and a mixed T helper 1/T helper 2 (TH1/TH2)-like cytokine pattern support the applicability of this RCC generic vaccine for use in combination therapies.
INTRODUCTION
Renal cell carcinoma (RCC) accounts for 2–3% of all adult malignancies worldwide with increasing incidences. Up to 30% of RCC patients have metastases at the time of diagnosis, and metastases develop metachronously in 20–40% of patients undergoing partial or radical nephrectomy. Metastatic renal cell carcinoma (mRCC) is resistant to both conventional chemotherapy and radiotherapy. The response rate is very low, and the 5-year survival of patients with mRCC is <10%. Historically, a cytokine-based immunotherapy with interleukin (IL)-2 and interferon (IFN)-α was the only therapeutic option for mRCC, with response rates up to 20% and a median survival of 5–25 months, depending on drug combination and patient selection.
Over the last decade, the development of targeted molecular therapies as both first- and second-line treatments has substantially improved the prognosis for patients with mRCC. These molecular agents are mostly directed against signaling pathways that foster angiogenesis. They include receptor tyrosine kinase inhibitors (for example, sunitinib, sorafenib, pazopanib, axitinib, cediranib and tivozanib), monoclonal antibodies (for example, bevacizumab) and mammalian target of rapamycin (mTOR) inhibitors (for example, temsirolimus and everolimus). Although some tumors show regression, most patients develop therapy resistance over time. Interestingly, some molecular therapies may enhance antitumor responses; therefore, immunotherapy in combination with tyrosine kinase inhibitors has become a recent research focus (1–3).
Among cancer patients with solid tumors, individuals with mRCC showed some of the most favorable responses to immunotherapy (4). Tumor cell vaccines are of special interest, and there is evidence that immunization against tumors can reduce or even eliminate some lesions and induce long-lasting T-cell memory responses, with a capacity to control tumor relapse. One approach is to use autologous tumor cells, either alone or combined with adjuvants, often after introduction of immunologically relevant genes to enhance tumor cell immunogenicity. The first phase I trial in RCC implementing this strategy, by expressing the gene encoding granulocyte-macrophage colony stimulating factor (GM-CSF) in autologous tumor cells, demonstrated induction of specific T-cell immunity and clinical benefit (5,6). An autologous gene-modified tumor cell vaccine expressing the costimulatory molecule CD80 was tested in patients with mRCC in combination with systemic IL-2 (7). Even fusion vaccines of autologous tumor cells and allogeneic dendritic cells (DCs) induced immune responses in a significant number of patients (8,9). Severe toxicities were not seen; however, strong limitations in feasibility and high costs were incurred with the production of individualized vaccines. Generic tumor cell vaccines that could be applied to many patients would reduce production costs, thereby enabling treatment of more patients. Our previously reported clinical trial used an allogeneic gene–modified RCC tumor cell vaccine that acquired improved immunogenicity through coexpression of CD80 and IL-2 (10). Vaccination was well tolerated, and substantial disease stabilization was observed in most patients. Preliminary immune monitoring demonstrated vaccine-induced responses against tumor lysates and a small set of tumor-associated antigens (TAAs) in the majority of the patients.
Here we report extended immune monitoring of these study patients, including assessment of enzyme-linked immunosorbent spot (ELISPOT) reactivity against numerous new RCC-associated antigens, and analyses of cytokines/chemokines in serum and culture supernatants of skin biopsies taken from vaccine challenge sites. Immune suppression in cancer patients often result from high numbers of immune suppressor cell populations, including natural regulatory T cells (nTregs) and myeloid-derived suppressor cells (MDSCs). For this reason, we also determined the frequencies of Tregs and MDSC subsets throughout vaccination.
MATERIALS AND METHODS
Patients
Fifteen patients with histologically proven clear-cell RCC and at least one evaluable metastasis were enrolled in the clinical trial (10). Immune monitoring was done for 12 patients. The study is registered with the German Somatic Gene Transfer Clinical Trial Database (DeReG, reference number 47) and the German Clinical Trial Register, partner register of the World Health Organization primary register (reference number DRKS00000249). Only patients with an HLA-A*02:01 allotype were included, matching one major histocompatibility complex class I molecule with the vaccine. Patients gave written informed consent before the study. The clinical protocol was approved by the Ethics Committee of the Ludwig Maximilians University Munich, and good manufacturing practice (GMP)-certified vaccine production was approved by local, state and national authorities.
Vaccination and Study Schedule
The vaccine cell line RCC-26/CD80/IL-2 was generated as described previously (11). Vaccine cells were thawed immediately before intradermal injection into the inguinal region. Graded doses of cells were applied up to 10 times over a 22-wk period (Supplementary Figure S1). Clinical examination and routine blood checks were performed at every visit. Patients were withdrawn from the study if evidence of tumor progression appeared according to RECIST criteria (response evaluation criteria in solid tumors). In four patients (MR-6, MR-7, MR-8 and MR-9), the lowest vaccine dose (2.5 × 106 cells) was omitted and vaccination was initiated with a dose of 1 × 107 cells.
Enzyme-linked Immunosorbent Spot (ELISPOT)
Patient blood samples for ELISPOT were obtained at wks 1, 6, 14, 22 and 36 (see Supplementary Figure S1), and peripheral blood mononuclear cells (PBMCs) were isolated and cryopreserved. Additional samples from five patients were taken on d 15 after the tenth vaccination, and samples were available from two patients during longer follow-up. The IFN-γ ELISPOT assay was performed as described (10). PBMCs were directly stimulated with selected peptides (each peptide, 5 μg/mL) in serum-free medium (CTL Test medium; Cellular Technology Europe, Bonn, Germany), supplemented with anti-CD28 antibody (1 μg/mL; BD Biosciences, San Jose, CA, USA) and recombinant IL-2 (Proleukin, 2 U/mL; Chiron, Emeryville, CA, USA). Spots were counted with the AID reader system ELR03 with software versions 4.0 and 5.0 (Autoimmun Diagnostika [AID], Strassberg, Germany) and controlled by human audit. The mean spot number of quadruplicates for a given peptide had to be at least two-fold over the mean background spot number. A response was considered to be vaccine induced, if the ratio of peptide mean to background mean for a given vaccination time point was at least two-fold over the corresponding pre-vaccination ratio. Surrogate peptides for immune monitoring were selected from sequences of TAAs shown to be overexpressed in metastatic RCC lesions and/or the vaccine cells, by using HLA-A*02:01 motif-based epitope predictions available on the web (http://www.syfpeithi.de), or as published in the literature. Several peptides were identified by elution from the RCC-26 cell line. Peptide sequences are given in the supplementary Material and Methods section ELISPOT.
Flow Cytometry
Surface immunostaining used directly labeled monoclonal antibodies (mAbs) from BD Biosciences: CD3, CD4, CD8, CD25, CD39, CD127, CD11b, CD15, CD14, CD33, CD124, HLA-DR and CD19. The FoxP3 mAbs (eBiosciences, Frankfurt, Germany) was used for nTreg analysis. The LIVE/DEAD® Fixable Blue Dead Cell Stain Kit, for UV excitation (Molecular Probes®; Life Technologies, Carlsbad, CA, USA) was applied for discrimination of live/dead cells.
Flow cytometry was performed as described in detail in the supplementary Material and Methods section Flow Cytometry. Briefly, isolated PBMCs were incubated first with the LIVE/DEAD Fixable Blue dye, washed and stained with directly labeled mAbs. Fix/Perm buffer (eBiosciences) was used for intracellular staining with FoxP3 antibody. Cells were analyzed by using an LSRII (BD Biosciences). Data were processed by using FlowJo software (version 8.8.6; Tree Star, Ashland, OR, USA).
Cytokine/Chemokine Assays
Commercial ELISA kits and protocols (R&D Systems, Wiesbaden, Germany) were used for vascular endothelial growth factor (VEGF), VEGF-C, VEGF-D, transforming growth factor (TGF)-β1 and prostaglandin E2 (PGE2). For all other cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-13, IL-17, tumor necrosis factor [TNF]-α, IFN-γ, GM-CSF, G-CSF) and chemokines (CXCL8 [IL-8], CXCL9 [monokine induced by gamma-interferon {MIG}], CXCL10 [IP-10], CCL2 [monocyte chemotactic protein-1 {MCP-1}], CCL4 [macrophage inflammatory protein-1β {MIP-1β}] and CCL5 [regulated upon activation normal T cell expressed and presumably secreted {RANTES}]), the Luminex (BioPlex; Bio-Rad, Munich, Germany) and the multiplex cytokine bead array systems from BD Biosciences (CBA kit for chemokines and CBA Flex Set) were used according to the manufacturers’ instructions.
Serum samples were obtained before and after the first, fourth, fifth, eighth, ninth and tenth vaccinations (see Supplementary Figure S1). Prevaccination PBMCs (wk 1) and PBMCs from wk 22 were cultured with parental RCC-26 cells and with RCC-26/CD80/IL-2 vaccine cells. Skin-infiltrating lymphocytes grown from biopsies (wks 6, 14 and 22) for 10–19 d were measured for cytokine/chemokine secretion at 24–48 h after stimulation with RCC-26 and vaccine cells. The FACSCalibur was used for the CBA assays. The Luminex 100 Reader was used for the BioPlex assays. Details for sample processing, culturing and analysis are described in the supplementary Material and Methods section Cytokine/Chemokine Assays.
Analysis of the Demethylation of the Foxp3 Gene
Genomic DNA was isolated by using the DNeasy blood and tissue kit (Qiagen, Hilden, Germany). Bisulphite treatment of genomic DNA was performed as described (12) with minor modifications. A quantitative real-time (qRT) polymerase chain reaction (PCR)-based methylation assay with methylation- and demethylation-specific amplification primers was used to analyze the Foxp3 Treg-specific demethylated region DNA, as described (13). Samples were analyzed in triplicate by using a LightCycler 480 System (Roche, Mannheim, Germany). Cycling conditions and data analysis are described in the supplementary Material and Methods section Analysis of the Demethylation of the Foxp3 Gene.
Microarray Analysis
Tissue samples from 32 patients with clear-cell RCC metastases were collected, snap-frozen and stored in liquid nitrogen after written informed consent was obtained. The study was approved by the ethics committee of the Ludwig Maximilians University Munich. Cryostat sections were made, and laser microdissection was used to isolate tumor cells (PALM MicroBeam; Zeiss, Munich, Germany). Processing of the probes is described in detail in the supplementary Material and Methods section Microarray Analysis.
All supplementary materials are available online at www.molmed.org.
RESULTS
Study Patients Had Advanced Disease
Characteristics of study patients, individual vaccination courses and some clinical parameters are summarized in Table 1 and Supplementary Table S1. Study details were described previously (10). More than half of the patients had poor prognosis scores (>0) according to Palmer et al.(14), revealing their advanced states of disease. All patients had good performance status (Eastern Cooperative Oncology Group scores of 0 or 1; data not shown). Of the 12 patients evaluated here, 8 were treated before study entry with other immunotherapies, with the majority receiving subcutaneous IFN-α and IL-2, with or without 5-fluorouracil. The interval between the last therapy course and enrollment in this study was at least 3 months. All patients progressed during the study, except for patients MR-11 and MR-15.
Table 1.
Patient characteristics, vaccination course and clinical outcome.
| ID | Prognostic scorea | Therapy before study entry | Site(s) of metastases | 2.5 × 106 cells | 10 × 106 cells | 40 × 106 cells | Site(s) of progression | TTP (wks) | Survival (wks) |
|---|---|---|---|---|---|---|---|---|---|
| MR-1 | 2 | — | Lung, bone | 4 | 1 | — | Bone, brainb | 7 | 31 |
| MR-2 | 1 | Cytokine,c G250d | Lung, LN | 4 | 4 | 2 | Pleura,b boneb | 23 | 79 |
| MR-4 | 1 | Cytokine, G250 | Lung, LN, adrenal gland | 4 | 4 | 2 | Lung | 35 | 68 |
| MR-5 | 1 | Cytokine | Lung | 4 | 4 | 2 | LN,b adrenal glandb | 7 | 76 |
| MR-6 | 2 | — | Bone | — | 4 | 2 | Bone | 6 | 18 |
| MR-7 | 1 | — | LN, pleura, local recurrence | — | 4 | 2 | Pleura, liverb | 28 | 84 |
| MR-9 | 2 | Cytokine | Lung, pancreas | — | 4 | 2 | Pancreas, liverb | 39 | 79 |
| MR-10 | 0 | Sorafenib | Lung | 4 | 4 | 2 | Brainb | 23 | 55 |
| MR-11 | 2 | G250 | Lung, LN, spinal cord | 4 | 4 | — | — | SD (7)e | 17f |
| MR-13 | 1 | DC, multi-peptide vaccine | Lung, LN, brain | 4 | 4 | 2 | Brain | 33 | 104 |
| MR-14 | 2 | Multi-peptide vaccine | Lung, LN, adrenal gland | 4 | 4 | 2 | LN | 35 | 107 |
| MR-15 | 1 | Cytokine | Lung, LN | 4 | 4 | 2 | — | SD (131)e | 151 (alive) |
TTP, time to progression; LN, lymph node; DC, dendritic cell; SD, stable disease.
Palmer score from 0 (very low risk) to 3 (high risk) based on performance status, number of metastatic sites and the time from first diagnosis to study entry (14).
New metastases.
Cytokine: IFN-α, IL-2, 5-fluorouracil.
G250: monoclonal antibody directed against carbonic anhydrase IX.
SD (7) and SD (131) indicate stable disease at wks 7 and 131, respectively.
Death of patient MR-11, not related to therapy or disease.
Routine blood parameters and common side effects were described previously (10). Peripheral blood leukocyte populations were not changed significantly during vaccination (Supplementary Figure S2). Patients MR-5, MR-11 and MR-14 showed transient increase of eosinophils (>500 cells/μL blood). Most patients (67%) showed transiently increased neutrophil granulocyte to lymphocyte ratios during vaccination (ratio >4). Two patients demonstrated extremely high ratios at the end of their vaccination courses, at time of progression (MR-7, MR-10; ratio >21; data not shown).
High Rates of Immune Response Occurred after Vaccination
PBMCs were obtained throughout vaccination for IFN-γ ELISPOT analyses to compare lymphocyte responses to a variety of peptide antigens before and after vaccination (see Supplementary Figure S1). Three patients (MR-3, MR-8 and MR-12) were not included because of early progression and removal from study.
In our previous trial report, we demonstrated that all patients responded before vaccination to pooled control peptides from cytomegalovirus, Epstein-Barr virus and influenza virus (CEF peptides) and to vaccine cell lysate, with increases in responses to tumor cell lysate noted in 66% of patients after vaccination (10). Here, additional ELISPOT responses after vaccination were analyzed by using surrogate peptides derived from numerous TAAs that are overexpressed in RCC or other tumors. Selected TAAs were tested for prevalence and expression by using microarray analysis of 32 mRCC tumors, parental RCC-26 cells and vaccine cells (Supplementary Table S2). Nearly all selected TAAs were expressed in the majority of tumor samples. Two TAAs (carbonic anhydrase IX [CAIX] and PRUNE2) were present in metastatic lesions but were not detected in parental RCC-26 or vaccine cells. Matrix metalloproteinase 7 (MMP7) was not detected in the vaccine and NY-ESO1 was missing throughout. Several HLA-A2–restricted peptides (transcriptional intermediary factor 1 [TIF1], chloride intracellular channel protein 1 [CLIC1], elaC homolog-2, putative prostate cancer susceptibility protein 2 or heredity prostate cancer protein 2 [ELAC2], tropomyosin 1 [TPM1], ORM1-like protein 3 [ORMDL3], AHNAK-related protein [AHNAK-rel], B cell translocation gene 1 protein [BTG1], p53-induced gene 10 protein [PIG10] and LENG4) were eluted from RCC-26 cells, identifying additional TAAs. All peptides selected from these TAAs carried anchor residues for HLA-A*02:01, matching the HLA-A2 allotype of patients (see supplementary Material and Methods section ELISPOT).
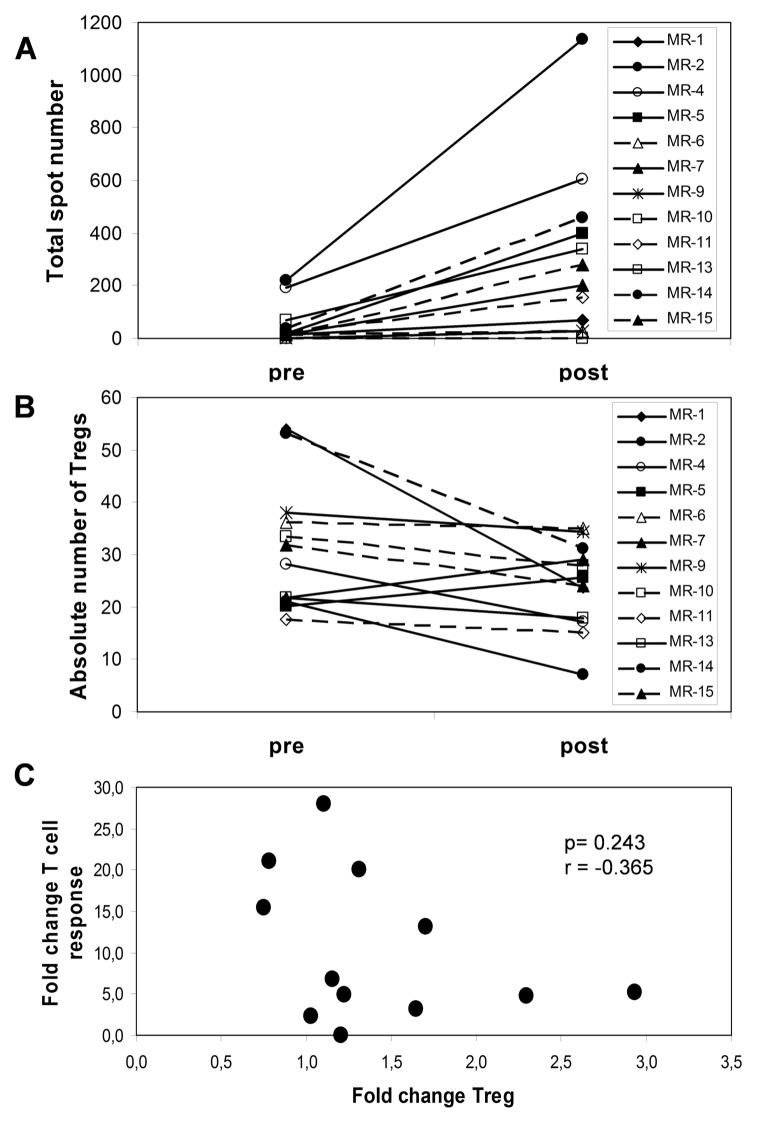
The summary of ELISPOT results for 12 patients is shown with values before treatment juxtaposed to the strongest recorded responses detected at any time after vaccination (Table 2). The delayed-type of hypersensitivity reactions (DTH) are also indicated here. All patients except MR-6 developed DTH responses after a challenge with 2.5 × 106 vaccine cells, applied intradermally in the alternate inguinal region at wks 6, 14 and 22. In previous studies, we demonstrated patient ELISPOT responses to peptides derived from survivin, cyclin D1, adipophilin, c-Met and vimentin (10). Here, our analyses were extended to include thymidylate synthetase (TYMS), insulin-like growth factor binding protein 3 (IGF-BP3), PRUNE2, transcriptional intermediary factor 1 (TIF1), regulator of G protein signalling 5 (RGS5), VEGF, MMP7, CAIX and NY-ESO1, in addition to two pools, including 10 additional peptides. Interestingly, 100% of the patients reacted to at least one peptide in the entire set of analyzed epitopes. Greater than two-fold increases in reactivity to at least two peptides were detected in 75% of patients after vaccination (Table 3). The time course of responses varied as illustrated for three patients (Supplementary Figure S3). Faster responses in MR-13 and MR-14 may be due to prior multi-peptide vaccination given more than 4 months before study entry. MR-2 showed a pattern representative of most patients, whereby immune responses increased later in treatment. Figure 1A shows the total number of IFN-γ– secreting T cells, given as total number of spots for all tested antigens. This result demonstrates the magnitude of vaccine-induced immune responses. In general, patients with a higher total number of spots showed a longer survival, and the same was observed for patients recognizing higher numbers of peptides (Figure 1B). The survival probability for patients reacting to 0–2 versus ≥3 peptides demonstrated a trend for increased length of survival in patients achieving broader immune responses (Figure 1C).
Table 2.
Absolute numbers of peptide-reactive T cells in the periphery (per 150,000 cells pre-/postvaccination).
| Patient | TYMS | IGF-BP3 | RGS-5 | VEGF | MMP7 | CA IX | PRUNE | TIF1 | NY-ESO | Pool Ca | Pool Eb | DTH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MR-1 | 51/41c | 43/32 | d−/16 | 45/85 | 14/43e | −/24 | nd/nd | 22/nd | 81/112 | −/15 | 12/51 | + |
| MR-2 | 12/45 | 64/183 | 49/135 | 22/124 | 48/162 | −/65 | −/66 | 50/82 | 24/173 | 19/107 | 39/157 | ++ |
| MR-4 | 23/70 | 38/107 | 123/40 | 80/70 | 76/157 | −/29 | 105/58 | nd/73 | 40/63 | 66/71 | 99/97 | + |
| MR-5 | −/17 | −/60 | 19/41 | 32/41 | 24/42 | −/23 | nd/nd | −/37 | −/64 | −/64 | 13/73 | + |
| MR-6f | 12/27 | 30/48 | 49/18 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/11 | − |
| MR-7f | −/− | −/− | −/50 | −/13 | −/46 | −/− | nd/nd | 13/55 | −/− | 22/126 | 15/46 | + |
| MR-9f | −/− | −/− | −/− | −/− | −/28 | −/− | −/− | −/− | −/12 | 15/31 | 39/37 | (+) |
| MR-10 | 60/20 | 64/13 | 82/49 | 77/33 | 56/45 | 32/− | 32/19 | 89/57 | 69/29 | 69/54 | 123/155 | (+) |
| MR-11 | −/39 | 99/37 | −/43 | 51/nd | −/− | 42/− | −/− | 29/46 | 142/255 | 52/107 | 128/210 | + |
| MR-13 | 28/46 | 71/80 | 40/62 | 61/32 | 111/17 | −/− | −/− | 40/101 | 80/32 | 41/74 | 157/125 | ++ |
| MR-14 | 23/36 | 76/141 | 39/49 | −/126 | −/64 | 28/33 | −/14 | 52/67 | 16/44 | −/63 | 12/70 | ++ |
| MR-15 | 11/− | −/28 | −/15 | −/59 | 15/25 | −/− | −/25 | 4/53 | 14/31 | −/14 | −/67 | + |
DTH, delayed-type hypersensitivity reaction; nd, not determined (not enough cells).
Pool C contains the peptides for ORMDL3, AHNAK-rel, BTG1, PIG10, LENG4, CP and PRAME.
Pool E contains the peptides for TIF1, CLIC1, ELAC2 and TMP1.
The values represent absolute numbers of spots for pre- and postvaccination (pre/post) responses. These values are given after subtraction of background responses (that is, without peptide or with irrelevant Bcr/Abl peptide). The highest postvaccination response is given irrespective of the time point postvaccination (that is, at 6, 14, 22 or 36 wks).
No values mean no reactivity (≤10 spots above background, which corresponds to a frequency of ≤1 = 1.5 × 104 IFN-γ–secreting peptide-specific T cells).
Values representing a greater than two-fold increase in reactivity against the peptides post-versus prevaccination are indicated in bold. With the larger peptide pools, a reactivity of ≥40 spots above background was considered positive.
Short protocol of vaccination starting with the middle dose of 107 vaccine cells. DTH reactivity: −, negative; (+), weak; +, positive; ++, positive (>2 cm redness and/or induration).
Table 3.
Peptide reactivity, immune response rate and clinical response.
| Peptide reactivity (number of tested peptides) | ||||
|---|---|---|---|---|
|
|
||||
| Patient ID | Prevaccination | Postvaccination | TTP (wks) | Survival (wks) |
| MR-1 | a10/15b | 7/13 (2)c | 7 | 31 |
| MR-2 | 12/16 | 15/17 (11) | 23 | 79 |
| MR-4 | 13/14 | 15/17 (7) | 35 | 68 |
| MR-5d | 3/14 | 15/15 (11) | 7 | 76 |
| MR-6d | 8/16 | 3/17 (1) | 6 | 18 |
| MR-7d | 2/16 | 5/15 (5) | 28 | 84 |
| MR-9 | 3/18 | 4/17 (1) | 39 | 79 |
| MR-10 | 16/19 | 13/19 (0) | 23 | 55 |
| MR-11 | 7/17 | 7/15 (3) | SD (7)e | 17 |
| MR-13 | 13/18 | 13/18 (4) | 33 | 104 (alive) |
| MR-14 | 9/19 | 17/19 (9) | 35 | 107 |
| MR-15 | 6/19 | 15/19 (8) | SD (131)e | 151 (alive) |
| Immune response rate (%) | 100f | 100 (75)f | ||
| Clinical response (wks) | 25.5g | 77.5g | ||
TTP, time to progression; SD, stable disease.
Numbers of peptides to which reactivity was observed (>10 spots above background).
Numbers of peptides tested (survivin, cyclin D1, adipophilin, c-MET, vimentin, PRUNE2, VEGF, NY-ESO, MMP7, TYMS, IGF-BP3, RGS-5, CA IX, CP, TIF-1, ORMDL3, CLIC1, ELAC2 and PIG-10).
Numbers of peptides (in bold) to which a greater-than-two-fold increase after versus before vaccination was detectable.
Short protocol of vaccination starting with the middle dose of 107 vaccine cells.
SD (7) and SD (131) indicate stable disease at wks 7 and 131, respectively.
Patients reacting to more than one peptide in %.
Median PD and median survival in weeks.
Figure 1.
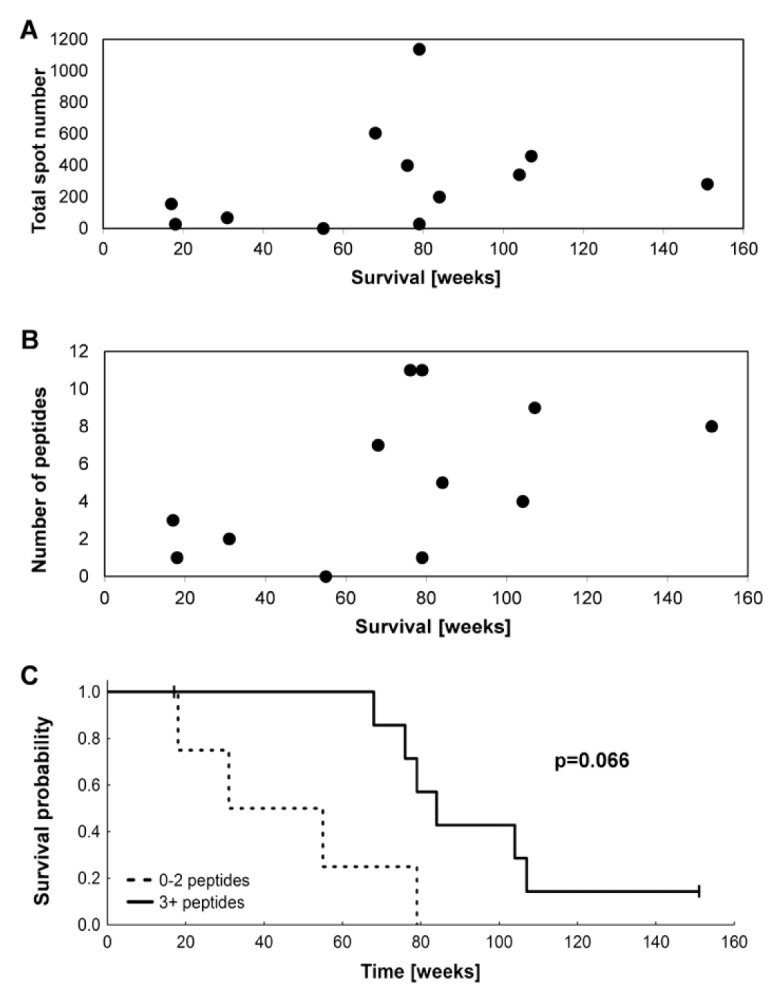
Correlation of immune response to survival time. (A) Total spot number is shown for each patient (n = 12) (y axis) to demonstrate the magnitude of the vaccine-induced immune response in correlation to survival (x axis). (B) The number of peptides (y axis) to which each patient responded is shown in relation to survival (x axis). (C) Survival probability of the patients with immune responses to 0–2 peptides (dashed line) and immune responses to ≥3 peptides (solid line). p = 0.066; log-rank test.
Frequencies of Tregs Decreased after Vaccination
Immune suppression in cancer patients is often discussed to result from higher numbers of Tregs or MDSCs. For this reason, we analyzed frequencies of CD4+CD25highCD127low/negativeFoxp3+CD3 9+ cells in patient PBMCs. Supplementary Figure S4 shows the gating strategy for patient MR-1. In general, frequency of Tregs before vaccination (median 2.60, minimum 0.93, maximum 3.56) was similar to that in healthy controls (median 2.71). A decrease of Tregs was observed after vaccination (p = 0.065, Supplementary Table S3). This change with respect to vaccination could even be demonstrated with absolute numbers of Tregs (p = 0.034, Figure 2A). To quantify nTregs more precisely, we used a real-time PCR-based methylation assay using methylation- and demethylation-specific primers. Baseline levels of stable Foxp3-expressing nTregs in the patient cohort did not differ from healthy controls. Interestingly, significant decreases of nTregs (p = 0.012) were detected in patients after vaccination, as illustrated in Figure 2B. A combined analysis of total numbers of responding T cells before and after vaccination (Figure 3A) and absolute numbers of Tregs before and after vaccination (Figure 3B) suggests a reciprocal relationship, as might be expected, but this result did not reach significance owing to the small number of patients (Figure 3C; p = 0.243, r = −0.365).
Figure 2.
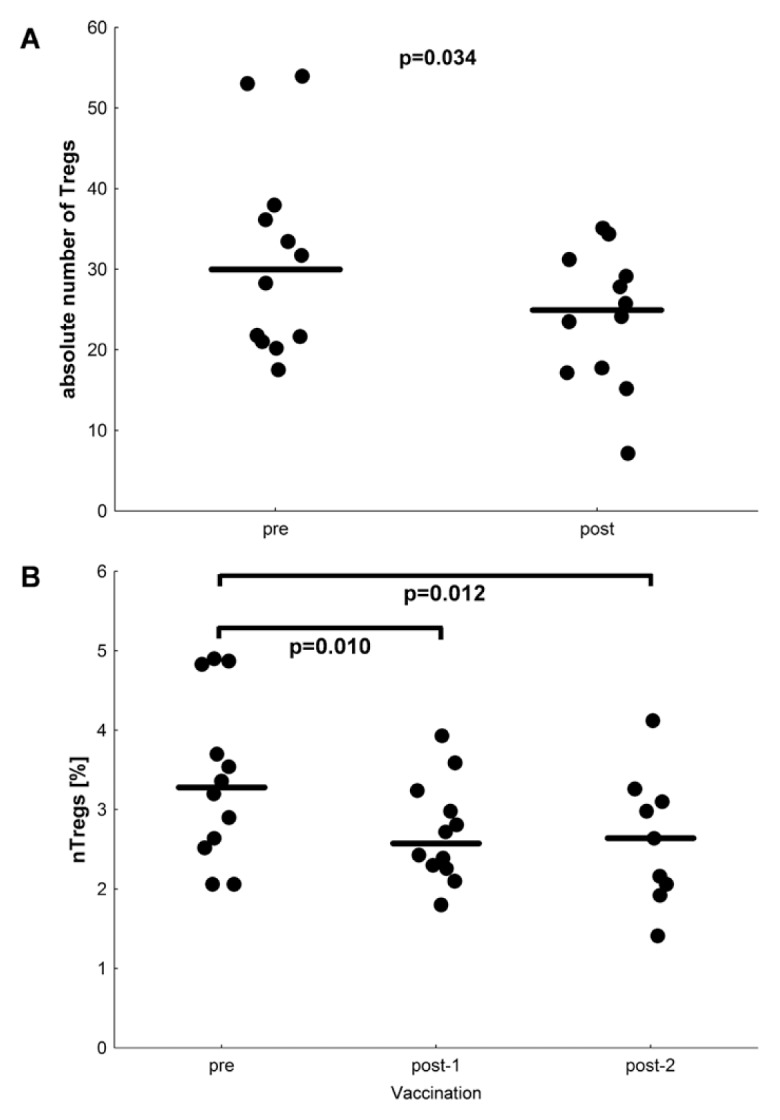
Decreased frequencies of nTregs during vaccination. (A) Absolute number of CD4+CD25highCD127low/negativeFoxP3+ CD39+ Tregs per μL blood measured by flow cytometry before and after vaccination for each patient (n = 12). The median is indicated. p = 0.034; Wilcoxon signed-rank test. (B) The nTregs were quantified with a real-time PCR-based methylation assay with methylation- and demethylation-specific primers. The y axis shows the percent of nTregs, and the x axis shows three different time points of the study protocol. Post-2 is the same time point as the postvaccination time point in (A). The decrease of nTregs is statistically significant with p < 0.05 (Wilcoxon signed rank test).
Figure 3.
Reciprocal correlation between vaccine-induced immune response and Treg numbers. (A) Total number of spots before and after vaccination for each patient (IFN-γ ELISPOT). (B) Absolute number of CD4+CD25highCD127low/−Foxp3+CD39+ Tregs before and after vaccination for each patient (flow cytometry). (C) Fold-change of T-cell response (total spot number) versus fold-change of Treg number (absolute number of CD4+CD25highCD127low/−Foxp3+CD39+ Tregs). p = 0.243; correlation coefficient r = −0.365, Spearman rank correlation.
Patients with Fewer MDSCs Trended to Longer Survival
MDSCs represent a second cell population with suppressive function, which was also described in RCC patients (15). These cells were immunophenotyped by using antibodies specific for CD3, CD19, CD14, CD15, CD11b, CD33, HLA-DR and CD124. Supplementary Figure S5 shows the gating strategy for different MDSC cell subpopulations for patient MR-15, as a representative example. Five subpopulations were detected in RCC patients and healthy donors: MDSC1 (CD14+ CD124+), MDSC2 (CD15+ CD124+), MDSC3 (Lin− HLA-DR−, CD33+, SSChigh), MDSC4 (SSCim CD14+ HLA-DR−) and MDSC5 (CD14−CD15+ CD11b+). Results for all patients are given in Supplementary Table S4. Slightly increased frequencies of MDSC4 and MDSC5 were observed in 58% of patients compared with healthy donors. Significant increases or decreases in any of the five MDSC sub-populations were not observed before and after vaccination, even when calculated for absolute numbers. However, there was a trend for better immune responses in patients with lower frequencies of MDSC4 and MDSC5. Figure 4 indicates a slightly higher survival probability for patients with lower frequencies of MDSC4 (p = 0.091), although these differences did not reach significance because of the small number of patients.
Figure 4.
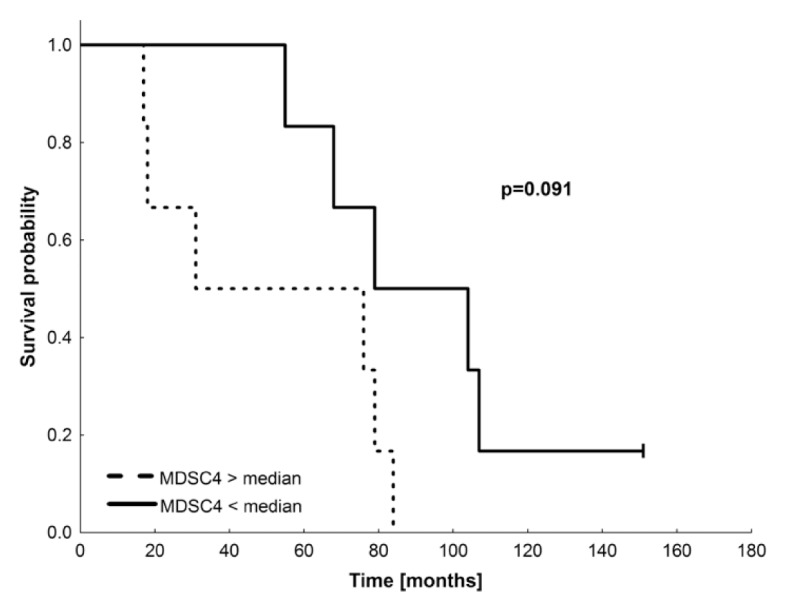
Survival probability of patients with high versus low frequencies of MDSC4. Survival probability of the patients (n = 12) with MDSC4 in %/lymphocytes < median (solid line) and MDSC4 in %/lymphocytes > median (dashed line). Median = 12.13%; p = 0.091; log-rank test.
Effector T Cells Displayed Mixed TH1/TH2-Like Cytokine Profiles
Cytokine profiles also provide useful information about the effects of immunotherapeutic vaccination. Therefore, we analyzed the general cytokine profile in PBMC responses to vaccine cells. In unstimulated controls, a predominant secretion of IL-10 and the proinflammatory cytokines IL-1β, IL-6, IL-8 and TNF-α was found, whereas there was no or only low detection of IL-2, IL-4, IL-5 and IFN-γ (Supplementary Table S5). After stimulation with vaccine cells, much more IFN-γ, IL-2, IL-4 and IL-5 were measured, which increased further after a second restimulation. Also, IL-10 increased after a second stimulation. In contrast, secretion of IL-1β, IL-6, IL-8 and TNF-α decreased after stimulation. This result can be interpreted as a mixed T helper 1/T helper 2 (TH1/TH2)-like profile, reflecting decreases of proinflammatory cytokines after in vitro restimulation with vaccine cells.
Challenge Site Infiltrating Lymphocytes Produced IL-10 and IFN-γ–Dependent Chemokines
Cytokines secreted by skin-infiltrating lymphocytes were analyzed in skin biopsies taken 48 h after an intradermal (i.d.) vaccine challenge at wks 6, 14 and 22. Small pieces of biopsies were cultured for 10–19 d, depending on the number of outgrowing cells. The composition of infiltrating cell populations differed from biopsy to biopsy and depended on the cultivation period. After 10 d, up to 95% of infiltrating cells were T cells and NK cells with residual B cells, eosinophils, macrophages and monocytes (data not shown).
Substantial differences were noted in the cytokine/chemokine profiles of the skin-infiltrating lymphocytes taken at different times (Supplementary Table S6). IL-5 and IL-13 were greatest in the first biopsy at 6 wks and declined substantially thereafter. Most factors were at a maximum in cells isolated from wk 14 biopsies, after patients had received eight vaccine doses. IL-10 increased throughout vaccination for most patients, in addition to three other factors (RANTES, IP-10 and MIG), all of which are induced by IFN-γ.
Multiple Serum Cytokines Increased During Vaccination
Supplementary Table S7 shows serum cytokine levels. All cytokines increased slightly during vaccination, however, sometimes only transiently. Small increases in IFN-γ were observed in 64% of patients, with maximum levels reaching >20 pg/mL for several patients (MR-11, MR-13 and MR-15 [data not shown]). These same patients also showed increases of IL-10. Enhanced IL12p70 secretion was only detected in patients who had longer survival times (MR-13, MR-14 and MR-15). IL-5 but not IL-4 was slightly enhanced in most patients, whereas IL-17 was only detected in one patient (MR-11). Increases in IL-5 were not associated with higher numbers of eosinophils (Supplementary Figure S6). Between 40% and 60% of patients showed enhanced levels of IL-6, IL-8 and C-reactive protein, which have been reported to be elevated in patients with advanced disease. Changes were not detected in levels of VEGF-C, VEGF-D and PGE2, whereas VEGF-A and TGF-β1 decreased slightly over time (Supplementary Table S8).
DISCUSSION
Extensive preclinical studies demonstrated that RCC-26 displays strong immunogenic potential that was enhanced by gene modification to allow the RCC-26 vaccine cells to express surface CD80 and to secrete IL-2 (11). On the basis of its improved stimulatory capacity, a phase I vaccine trial was performed in HLA-A2–matched patients with modified RCC-26 cells (10).
The majority of patients had multiple metastases at two or more sites at enrollment, and 42% of patients had poor prognosis scores (10,14), revealing their advanced state of disease. Nevertheless, 50% of patients achieved a state of stable disease ranging from 23 to 131 wks, with a median time to progression of 5.3 months and median tumor-specific survival time of 15.6 months (10). Studies of larger numbers of patients with similar advanced disease showed progression-free survival of 5.5 months with the angiogenesis inhibitor sorafenib (16) and 5.0 months with IFN-α (17); however, the side effects of these therapies were much greater.
All patients but one showed DTH reactions that increased in magnitude over time, indicating strong induction or reactivation of T-cell responses in vivo through vaccination. To better understand the basis of vaccine-induced immune responses that may have contributed to stable disease, we analyzed numerous immune parameters in patient blood lymphocytes, serum and vaccine-site biopsy samples. Among the numerous parameters that were assessed, the results of antigen-specific T-cell responses and the frequencies of Tregs and MDSCs were most informative.
IFN-γ–secreting cells were measured after ex vivo stimulation with numerous new RCC-associated antigens. Here, a large panel of surrogate peptides, representing epitopes derived from TAAs known to be overexpressed in RCC, were used in addition to several peptides eluted from RCC-26 cells. Peripheral blood lymphocytes (PBL) of all but one patient responded to more than one peptide, and the vaccine-induced immune response rate was 75%. Some patients reacted to epitopes (for example, PRUNE2 and CAIX) that were not expressed by vaccine cells, demonstrating the occurrence of epitope spreading. Over the full survival time range of 160 wks, it was apparent that a higher magnitude of T-cell response was seen in most patients who survived longer. Likewise, complex responses to several different peptides were more common in patients with longer survival. Thus, three of four patients responding to fewer than three peptides survived <15 months; their total number of T cells responding to the peptide panel was <200. In contrast, seven of eight patients responding to three or more peptides survived beyond 15 months and had total T-cell responses ranging from 200 to 1,138 spots.
Poor clinical outcome in many cancer patients is associated with higher numbers of Tregs or MDSCs. Thus, elevated Treg numbers in peripheral blood, tumors or lymph nodes were associated with poor prognosis (18,19). In some cases, decreased numbers of Tregs were observed after sunitinib treatment, and this result correlated with improved overall survival (20,21). Treg depletion may support enhanced vaccine-induced antitumor immunity (22). In our cohort, baseline levels of stable Foxp3-expressing nTregs in patients did not differ from healthy controls, as reported for a related vaccine study (23). In contrast, significant decreases of nTregs (p = 0.012) were detected in this patient cohort after vaccination, and to some degree, these decreases were matched with increases in antigen-specific T-cell responses. Thus, this IL-2–secreting vaccine did not cause higher frequencies of nTregs, as seen with systemic IL-2 treatment in melanoma and RCC (24,25) or during treatment of mRCC patients with dendritic cells and low-dose IL-2 (26). Our vaccine strategy appears to have a favorable profile with respect to impact on Treg frequencies. The mechanism underlying this effect is unknown but may be related to the effect of vaccination on the cytokine network. Furthermore, the IL-2 from our vaccine is unlikely to have systemic effects, since the cells are applied intradermally.
MDSCs can also interfere with immune responses by several different mechanisms (15,27–32), and increased numbers of MDSCs have been correlated with cancer stage and metastatic spread (33). MDSCs were reported in RCC (15), and enhanced antitumor immunity was obtained by reversing MDSC-mediated immune suppression after treatment with sunitinib or all-trans-retinoic acid (34,35). In our patient cohort, increased levels of MDSC4 and MDSC5 were found in 58% of patients compared with healthy donors. These values did not significantly change before and after vaccination. A trend for better survival was seen for patients who had lower frequencies of the MDSC4 subset.
Cytokine profiles may also provide useful information for understanding clinical effects of immunotherapy. The presence of elevated systemic levels of proinflammatory cytokines may indicate unfavorable clinical outcomes in patients with advanced solid tumors (36). The cytokine balance gives an indication of the overall inflammatory milieu. Kyte et al.(37) reported that mixed TH1/TH2 cytokine patterns in vaccinated melanoma patients, even at the clonal T-cell level, were associated with long-term survival.
In this cohort, 64% of patients showed increases in serum IFN-γ during vaccination, but the same patients also demonstrated a TH2-like profile. In general, associations of particular serum cytokine profiles with immune response rates or survival times were not obvious, with the exception that IL-12p70 was only detected in patients who survived longer. IL-12 is known to activate TH1 cells and NK cells, and it was described by others that higher levels of IL-12p70 were associated with better survival in mRCC (38). The mechanisms by which vaccination led to increases in this cytokine are unknown, but it is possible that T cells producing IFN-γ led to IL-12p70 production by dendritic cells.
Four TH2 cytokines were prominent in cohort samples. The slightly enhanced IL-10 secretion, as measured both in serum and in skin-infiltrating lymphocytes during vaccination, did not necessarily mean immune suppression. The role of IL-10 in immune response against cancer is still controversial. One report suggests that IL-10 may favor immune-mediated rejection of cancer (39). Persistent, moderate threshold levels of IL-10 seem to be necessary for inhibition of tumor growth by activated effector TH1 cells (40).
Chemokines play a major role in leukocyte trafficking to sites of inflammation. An increase of chemokines induced by IFN-γ (RANTES, IP-10 and MIG) was observed in skin-infiltrating cells after stimulation with vaccine cells in vitro. IP-10 plays an important role in DTH reactivity and is chemotactic for T cells, NK cells, dendritic cells, macrophages and monocytes. Schwaab et al.(41) observed a treatment-related induction of IP-10 and a relationship between outcome and pretreatment serum IP-10 levels (41). IP-10 expression in RCC tumors has been described as a predictor of good outcome and was shown to be induced by IL-2 and implicated as a component of TH1 responses (42). MIG is a chemoattractant for T cells, and RANTES is chemotactic for T cells, monocytes and eosinophils and plays a role in recruiting these cells into inflamed tissues. Thus, induction of these factors may reflect a positive impact of vaccination on immune responses in patients showing this response profile.
In general, the complexity of cytokine networks makes it difficult to determine the relationships between immune response rates, systemic cytokine/chemokine levels and clinical outcome, particularly in a small cohort. It will be necessary to analyze larger patient cohorts to understand how these complex networks regulate immune responses during vaccination.
CONCLUSION
In summary, these extensive immune monitoring studies demonstrate that most patients in this trial acquired increased T-cell reactivity during vaccination to several epitopes derived from shared TAAs and vaccine-eluted peptides. Despite its allogeneic nature, this generic vaccine induced TAA-associated T-cell responses that may elicit significant immune attack of autologous tumor cells in better clinical settings. In future combination therapies or in adjuvant therapies for RCC patients with minimal residual disease, preanalysis of patients for general immune response capacity and prevalence of suppressor cell populations might guide the better use of vaccines. Only patients with an overall good immune response capacity and normal numbers of suppressor cells may benefit from tumor vaccine approaches.
Supplemental Data
ACKNOWLEDGMENTS
The authors thank Heidi Herbig and Adam Slusarski for excellent technical support. The study was funded by the Federal Ministry of Education and Research (01 GE 9624/1) and the German National Research Foundation (SFB-455 and SFB-TR36).
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
T Schwachula and S Olek are members of the company Epiontis; and S Walter is a member of the company Immatics Biotechnologies; but being part of these companies has not influenced the results and discussion in this paper.
REFERENCES
- 1.Frey K, et al. The immunocytokine F8-IL2 improves the therapeutic performance of sunitinib in a mouse model of renal cell carcinoma. J Urol. 2010;184:2540–8. doi: 10.1016/j.juro.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 2.Escudier B, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010;28:2144–50. doi: 10.1200/JCO.2009.26.7849. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Wang XY, Subjeck JR, Shrikant PA, Kim HL. Temsirolimus, an mTOR inhibitor, enhances anti-tumour effects of heat shock protein cancer vaccines. Br J Cancer. 2011;104:643–52. doi: 10.1038/bjc.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ. Renal cell carcinoma: a priority malignancy for development and study of novel therapies. J Clin Oncol. 2003;21:1193–4. doi: 10.1200/JCO.2003.12.072. [DOI] [PubMed] [Google Scholar]
- 5.Simons JW, et al. Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte-macrophage colony-stimulating factor gene transfer. Cancer Res. 1997;57:1537–46. [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou X, et al. Diverse CD8+ T-cell responses to renal cell carcinoma antigens in patients treated with an autologous granulocyte-macrophage colony-stimulating factor gene-transduced renal tumor cell vaccine. Cancer Res. 2005;65:1079–88. [PubMed] [Google Scholar]
- 7.Antonia SJ, et al. Phase I trial of a B7-1 (CD80) gene modified autologous tumor cell vaccine in combination with systemic interleukin-2 in patients with metastatic renal cell carcinoma. J Urol. 2002;167:1995–2000. [PubMed] [Google Scholar]
- 8.Zhou J, et al. Patient-derived renal cell carcinoma cells fused with allogeneic dendritic cells elicit anti-tumor activity: in vitro results and clinical responses. Cancer Immunol Immunother. 2009;58:1587–97. doi: 10.1007/s00262-009-0668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avigan DE, et al. Phase I/II study of vaccination with electrofused allogeneic dendritic cells/autologous tumor-derived cells in patients with stage IV renal cell carcinoma. J Immunother. 2007;30:749–61. doi: 10.1097/CJI.0b013e3180de4ce8. [DOI] [PubMed] [Google Scholar]
- 10.Buchner A, et al. Phase 1 trial of allogeneic gene-modified tumor cell vaccine RCC-26/CD80/IL-2 in patients with metastatic renal cell carcinoma. Hum Gene Ther. 2010;21:285–97. doi: 10.1089/hum.2008.192. [DOI] [PubMed] [Google Scholar]
- 11.Frankenberger B, et al. Influence of CD80, interleukin-2, and interleukin-7 expression in human renal cell carcinoma on the expansion, function, and survival of tumor-specific CTLs. Clin Cancer Res. 2005;11:1733–42. doi: 10.1158/1078-0432.CCR-04-1883. [DOI] [PubMed] [Google Scholar]
- 12.Olek A, Oswald J, Walter J. A modified and improved method for bisulphite based cytosine methylation analysis. Nucleic Acids Res. 1996;24:5064–6. doi: 10.1093/nar/24.24.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wieczorek G, et al. Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Res. 2009;69:599–608. doi: 10.1158/0008-5472.CAN-08-2361. [DOI] [PubMed] [Google Scholar]
- 14.Palmer PA, et al. Prognostic factors for survival in patients with advanced renal cell carcinoma treated with recombinant interleukin-2. Ann Oncol. 1992;3:475–80. doi: 10.1093/oxfordjournals.annonc.a058239. [DOI] [PubMed] [Google Scholar]
- 15.Zea AH, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–8. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 16.Escudier B, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 17.Motzer RJ, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 18.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 19.Liotta F, et al. Frequency of regulatory T cells in peripheral blood and in tumour-infiltrating lymphocytes correlates with poor prognosis in renal cell carcinoma. BJU Int. 2011;107:1500–6. doi: 10.1111/j.1464-410X.2010.09555.x. [DOI] [PubMed] [Google Scholar]
- 20.Finke JH, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14:6674–82. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 21.Adotevi O, et al. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J Immunother. 2010;33:991–8. doi: 10.1097/CJI.0b013e3181f4c208. [DOI] [PubMed] [Google Scholar]
- 22.Dannull J, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–33. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westermann J, et al. Allogeneic gene-modified tumor cells (RCC-26/IL-7/CD80) as a vaccine in patients with metastatic renal cell cancer: a clinical phase-I study. Gene Ther. 2011;18:354–63. doi: 10.1038/gt.2010.143. [DOI] [PubMed] [Google Scholar]
- 24.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–14. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Vliet HJ, et al. Effects of the administration of high-dose interleukin-2 on immunoregulatory cell subsets in patients with advanced melanoma and renal cell cancer. Clin Cancer Res. 2007;13:2100–8. doi: 10.1158/1078-0432.CCR-06-1662. [DOI] [PubMed] [Google Scholar]
- 26.Berntsen A, Brimnes MK, thor Straten P, Svane IM. Increase of circulating CD4+CD25high Foxp3+ regulatory T cells in patients with metastatic renal cell carcinoma during treatment with dendritic cell vaccination and low-dose in-terleukin-2. J Immunother. 2010;33:425–34. doi: 10.1097/CJI.0b013e3181cd870f. [DOI] [PubMed] [Google Scholar]
- 27.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24:302–6. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 28.Kasic T, et al. Modulation of human T-cell functions by reactive nitrogen species. Eur J Immunol. 2011;41:1843–9. doi: 10.1002/eji.201040868. [DOI] [PubMed] [Google Scholar]
- 29.Kusmartsev S, Gabrilovich DI. Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J Leukoc Biol. 2003;74:186–96. doi: 10.1189/jlb.0103010. [DOI] [PubMed] [Google Scholar]
- 30.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183:937–44. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang B, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 33.Diaz-Montero CM, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ko JS, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 35.Kusmartsev S, et al. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2008;14:8270–8. doi: 10.1158/1078-0432.CCR-08-0165. [DOI] [PubMed] [Google Scholar]
- 36.Callard R, George AJ, Stark J. Cytokines, chaos, and complexity. Immunity. 1999;11:507–13. doi: 10.1016/s1074-7613(00)80125-9. [DOI] [PubMed] [Google Scholar]
- 37.Kyte JA, et al. Unconventional cytokine profiles and development of T cell memory in long-term survivors after cancer vaccination. Cancer Immunol Immunother. 2009;58:1609–26. doi: 10.1007/s00262-009-0670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guida M, Casamassima A, Monticelli G, Quaranta M, Colucci G. Basal cytokines profile in metastatic renal cell carcinoma patients treated with subcutaneous IL-2-based therapy compared with that of healthy donors. J Transl Med. 2007;5:51. doi: 10.1186/1479-5876-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol. 2005;78:1043–51. doi: 10.1189/jlb.0705358. [DOI] [PubMed] [Google Scholar]
- 40.De Vleeschouwer S, Spencer Lopes I, Ceuppens JL, Van Gool SW. Persistent IL-10 production is required for glioma growth suppressive activity by Th1-directed effector cells after stimulation with tumor lysate-loaded dendritic cells. J Neurooncol. 2007;84:131–40. doi: 10.1007/s11060-007-9362-y. [DOI] [PubMed] [Google Scholar]
- 41.Schwaab T, et al. Clinical and immunologic effects of intranodal autologous tumor lysate-dendritic cell vaccine with Aldesleukin (interleukin 2) and IFN-α2a therapy in metastatic renal cell carcinoma patients. Clin Cancer Res. 2009;15:4986–92. doi: 10.1158/1078-0432.CCR-08-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panelli MC, et al. Forecasting the cytokine storm following systemic interleukin (IL)-2 administration. J Transl Med. 2004;2:17. doi: 10.1186/1479-5876-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.