Abstract
Object
The gold-standard method for determining cortical functional organization in the context of neurosurgical intervention is electrical cortical stimulation (ECS), which disrupts normal cortical function to evoke movement. This technique is imprecise, however, as motor responses are not limited to the precentral gyrus. Electrical cortical stimulation also can trigger seizures, is not always tolerated, and is often unsuccessful, especially in children. Alternatively, endogenous motor and sensory signals can be mapped by somatosensory evoked potentials (SSEPs), functional MRI (fMRI), and electrocorticography of high gamma (70–150 Hz) signal power, which reflect normal cortical function. The authors evaluated whether these 4 modalities of mapping sensorimotor function in children produce concurrent results.
Methods
The authors retrospectively examined the charts of all patients who underwent epilepsy surgery at Seattle Children’s Hospital between July 20, 1999, and July 1, 2011, and they included all patients in whom the primary motor or somatosensory cortex was localized via 2 or more of the following tests: ECS, SSEP, fMRI, or high gamma electrocorticography (hgECoG).
Results
Inclusion criteria were met by 50 patients, whose mean age at operation was 10.6 years. The youngest patient who underwent hgECoG mapping was 2 years and 10 months old, which is younger than any patient reported on in the literature. The authors localized the putative sensorimotor cortex most often with hgECoG, followed by SSEP and fMRI; ECS was most likely to fail to localize the sensorimotor cortex.
Conclusions
Electrical cortical stimulation, SSEP, fMRI, and hgECoG generally produced concordant localization of motor and sensory function in children. When attempting to localize the sensorimotor cortex in children, hgECoG was more likely to produce results, was faster, safer, and did not require cooperation. The hgECoG maps in pediatric patients are similar to those in adult patients published in the literature. The sensorimotor cortex can be mapped by hgECoG and fMRI in children younger than 3 years old to localize cortical function.
Keywords: functional mapping, electrical cortical stimulation, functional magnetic resonance imaging, epilepsy, electrocorticography, somatosensory evoked potential, high gamma activity
During resective neurosurgery, localization of the salient cortex helps minimize postoperative deficits. The gold-standard method for determining cortical functional organization in this context is ECS, which acts by intensive exogenous electric current to disrupt normal cortical function to evoke movement or create transient functional disruption.7,9,10
A drawback to this technique is that it is imprecise, as motor responses are not limited to the precentral gyrus but may be elicited by stimuli as much as 4 cm anterior to the central sulcus and up to 2 cm posterior.27 Also, ECS can trigger seizures, and it is not always tolerated. It is also not always successful, especially in children, although published data on this are scarce.
Endogenous motor and sensory signals can also be mapped using other techniques such as SSEP, fMRI, and electrocorticography. The benefit of these approaches is that they reflect normal cortical function, rather than disruption of function. However, ECS and SSEP are currently the only 2 clinically established methods for functional cortical mapping.9
Somatosensory evoked potentials map the sensorimotor cortex by eliciting intrinsic cortical physiological responses to peripheral nerve stimulation, most often of the median or posterior tibial nerves.9 The SSEP N20 waveform is recorded over the contralateral somatosensory cortex, whereas a P20 waveform with the opposite polarity is recorded over the primary motor cortex. Referential recordings from adjacent electrodes over the pre- and postcentral gyri produce a “phase reversal” between the N20 and the P20, marking the site of the central sulcus, which is directly underneath or a few millimeters anterior to the area of highest amplitude.13
By statistically comparing the hemodynamic response of the BOLD signal between task and nontask states, fMRI localizes the sensorimotor cortex during voluntary or passive movement, most commonly for contralateral finger tapping. The spatial resolution of fMRI is 8–50 mm3, which corresponds to 105 neurons.25 Correlation between fMRI findings and the results of direct electrical brain stimulation is high, although not 100%, in part because fMRI shows “participative” cortices while ECS shows sites that are critical to function.6,15 Functional MRI colocalizes with hgECoG motor mapping8 and many other testing paradigms, including auditory,22 reading, 12 working memory,11 and attentional tasks.2
Electrocorticography records normal cortical function and is most sensitive in the high gamma range, that is, between 70 and 150 Hz.4,5,14,17,18,23 Broad-band spectral increases of the hgECoG signal provide a correlate of local cortical activity but are masked by changes in band-specific peaks at low frequencies, classically named event-related desynchronization.24 In hand motor tasks, these conjugate processes cause behavioral splits at 48 ± 9 Hz (± SD) to assess local cortical function.17,18 Changes in power in high gamma activity during hand movement rapidly localize cortical hand area with only several seconds of data collection, giving a rapid, specific, and straightforward method for locating functional areas in the cortex with the hgECoG signal. Even imagining motor movement can activate these areas of cortex.20
These 4 modalities of mapping sensorimotor function should produce concurrent results, but this has not been well studied in children. Having multiple modalities to localize salient cortex prior to resective epilepsy surgery can lead to better-informed decisions about proceeding with these elective procedures, especially if some of the modalities fail to generate meaningful results.
Methods
We retrospectively examined the charts of all patients who underwent epilepsy surgery under an institutional review board–approved protocol at Seattle Children’s Hospital between July 20, 1999, and July 1, 2011. We reviewed the charts for any attempt to localize the primary motor and somatosensory cortices via 2 or more of the following tests: ECS, SSEP, fMRI, or hgECoG. Perirolandic subdural platinum electrodes (4-mm diameter, 0.75–1 cm interelectrode spacing; Ad-Tech or Integra) were placed to localize seizure onset in patients with medically refractory localization-related epilepsy. Preoperative T1-weighted MRI studies were coregistered to postoperative CT scans of intracranial electrodes by using the BioImage Suite.
Electrical cortical stimulation was performed in 36 awake patients at the bedside by using an Ojemann stimulator (Integra) to elicit motor activity by delivering up to 12 mA of current to pairs of implanted electrodes, and patients were monitored for movement or reproducible sensations of the hand, face, or leg. Monitoring of SSEPs was performed in 46 patients, stimulating the median nerve at a rate of 5.1 Hz, with a pulse width of 200 μsec and a current of 6 mA. There were 2 trials with 200 acquisitions per trial, and waveforms were evaluated for reproducible N20 and P20 peaks. The recordings were inspected for a “phase reversal” of the N20 and P20 between adjacent electrodes.
Imaging for fMRI mapping was performed in 18 patients using Siemens (either the 1.5-T [Avanto] or 3-T [Trio] scanners) or General Electric 1.5-T (Signa scanner) systems. In the former machines, a 36-slice BOLD-T2 study (TE 30 msec, TR 3820 msec) was acquired, with the following parameters: slice thickness 3 mm, gap 0.8 mm, and voxel size 3 × 3 × 3 mm. A total of 60 volumes were obtained per run, resulting in a total time of less than 4 minutes per run. In the latter scanner, only 8 slices were acquired, with a slice thickness of 8 mm. Each study was designed in an ABABAB algorithm where the 2 tasks, A and B, were performed for 10 volumes (39 seconds) each. The patients were instructed to tap their fingers or their foot. In children too young to cooperate, the somatosensory cortex was activated by passive movement with the patient sedated. Postacquisition analysis was performed using the commercially available Siemens or GE software, which performed a t-test for each voxel between acquisitions in the 2 tasks. These were projected onto the source images for analysis.28 These images were then coregistered to the previously mentioned scans.
We recorded hgECoG in 10 patients using SynAmps2 (Neuroscan) amplifiers, set to sample at 500–2000 Hz and bandpass filtered from 0.15 to 500 Hz. We looked for frequencies up to 150 Hz as our amplifiers have a built-in low-pass filter at 200 Hz. In some patients, the position of each finger was registered through a 5-df data glove device (Fifth Dimension Technologies, Inc.). Some patients were given a button-pushing task. In other patients, we compared 15 seconds of active or passive hand movement with 15 seconds of the hand at rest. Some patients also had face or leg cortex localized with focal movements. The data were notch filtered for 60, 120, and 180 Hz to eliminate line noise by using a third-order Butterworth filter. We referenced the data with respect to the common average and computed the fast Fourier transform for the t = 1- to 2.5-second interval from each t = 0- to 3-second epoch (a subinterval was used because of jitter in behavioral response). The data from these epochs were transformed using overlapping 0.256-second (256 sample) windows with 0.1-second step sizes between them. A Hann window was imposed on each data window to attenuate edge effects. Spectral coefficients were normalized with respect to a baseline period. We measured the total integrated power Pb (e; t, nt) for each electrode “e,” where “nt” is an interval of task type “t” (t can be hand movement or rest). We used the library for support vector machine (LIBSVM) implementation (http://www.csie.ntu.edu.tw/~cjlin/) of a linear support vector machine classifier29 to make pairwise class divisions between hand movement and rest data, using the projection vector Pb (e; t, nt) with 6-fold nested cross-validation. Data were collected and processed online at the bedside using the BCI2000 software on a laptop computer.26 Patients’ parents gave informed consent through a protocol approved by the Seattle Children’s Hospital Institutional Review Board.
Results
A total of 50 patients met inclusion criteria. The mean age at operation was 10.6 years (range 0.7–18.8 years), and 44% were female. Operations were performed in the left hemisphere in 50% of the patients. Our youngest patient to have hgECoG mapping was 2 years and 10 months old. Lesions were detected on neuroimaging in 37 patients (74%). Success rates of the different modalities and comparison with ECS are listed in Table 1. An example of convergent localization via all 4 modalities in 1 patient is shown in Figs. 1–4. The locations of the epileptogenic zones of our patients are listed in Table 2.
TABLE 1.
Comparison of success rates of methods of hand sensorimotor cortex mapping in children
| Modality | Hand Sensorimotor Cortex Mapping
|
|||||||
|---|---|---|---|---|---|---|---|---|
| No. Attempted | No. Failed (%) | No. w/ECS | No. Concordant w/ECS (%) | No. Concordant w/or w/in 1 Electrode of ECS (%) | No. Discordant w/ECS (%) | No. Failed w/ECS Successful (%) | No. Successful w/ECS Failed (%) | |
| ECS | 36 | 7 (20) | ||||||
| SSEP | 46 | 6 (13) | 34 | 12 (35) | 18 (53) | 3 (9) | 6 (18) | 7 (21) |
| fMRI | 18 | 3 (17) | 15 | 7 (47) | 10 (67) | 0 (0) | 1 (7) | 2 (13) |
| hgECoG | 10 | 0 (0) | 9 | 5 (56) | 6 (67) | 0 (0) | 0 (0) | 3 (33) |
Fig. 1.
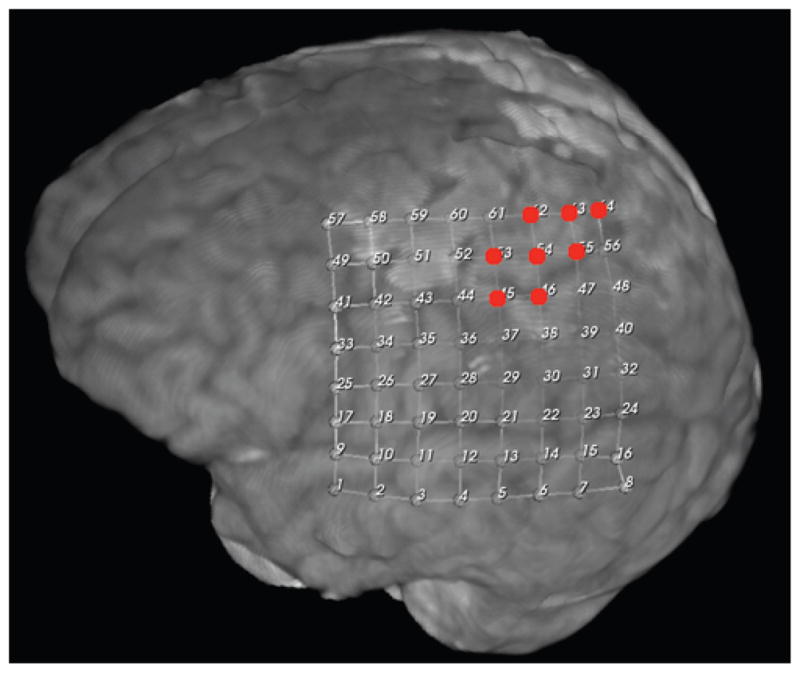
Motor cortex localization in Case 43 by ECS. Red dots denote localization.
Fig. 4.
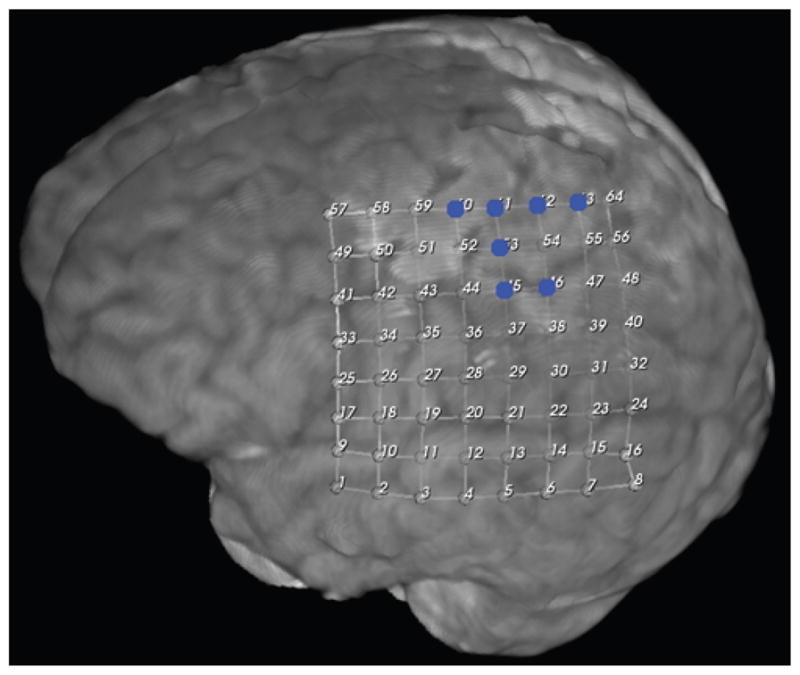
Motor cortex localization in Case 43 by increase in spectral power of hgECoG with movement. Blue dots denote localization.
TABLE 2.
Epileptogenic zone location of 50 children who underwent sensorimotor cortex mapping
| Location | No. (%) |
|---|---|
| frontal | 28 (56) |
|
| |
| pure frontal | 20 (40) |
| frontal, temporal | 3 (6) |
| frontal, temporal, parietal | 3 (6) |
| frontal, parietal | 2 (4) |
|
| |
| insula | 1 (2) |
| temporal | 21 (42) |
|
| |
| pure temporal | 10 (20) |
| frontal, temporal | 3 (6) |
| frontal, temporal, parietal | 3 (6) |
| temporal, parietal | 1 (2) |
| temporal, parietal, occipital | 3 (6) |
| temporal, occipital | 1 (2) |
|
| |
| parietal | 13 (26) |
|
| |
| pure parietal | 2 (4) |
| parietal, occipital | 2 (4) |
| frontal, temporal, parietal | 3 (6) |
| frontal, parietal | 2 (4) |
| temporal, parietal | 1 (2) |
| temporal, parietal, occipital | 3 (6) |
|
| |
| occipital | 7 (14) |
|
| |
| pure occipital | 1 (2) |
| temporal, parietal, occipital | 3 (6) |
| temporal, occipital | 1 (2) |
| parietal, occipital | 2 (4) |
Discussion
Electrical cortical stimulation, SSEP, fMRI, and hgECoG generally produced convergent localization of cortical sensorimotor function in children. Electrical cortical stimulation remains the gold standard in large part because it has the longest history of use. It also has the advantage of being easy to interpret. The disadvantages of this technique include its potential to cause seizures, and, in our experience, it is the modality most likely to have negative results. Somatosensory evoked potentials monitoring has the next longest track record, and is also easy to interpret, but is the second most likely to fail to generate results. We consistently used these modalities to try to localize the hand sensorimotor cortex since it was proximate to our respective sites in most patients. We occasionally used these modalities with the face or leg cortex and also had success, although there were too few of these patients to provide meaningful comparisons.
We continue to use fMRI in children younger than 3 years old to localize either hand or foot cortical sensorimotor function,28 and in the past 4 years the sensorimotor cortex has been accurately localized in all cases as compared with ECS. In this study, fMRI succeeded in localizing the sensorimotor cortex in 2 patients in whom ECS failed to localize it. While fMRI is harder to interpret than ECS or SSEPs, there are good commercially available software packages for many MRI machines for motor localization, as well as for coregistration.
Changes in power in high gamma activity during repeated hand movement rapidly localized the cortical hand area with only several seconds of data collection, giving a rapid, specific, and straightforward method for locating functional areas in the cortex. We had a superior success rate for localizing the motor and somatosensory cortices with hgECoG in children compared with ECS and SSEP. Like ECS and SSEP, hgECoG requires direct access to the brain to prevent the attenuation of very high frequency activity by intervening tissues and to provide better spatial resolution.
Our results of hgECoG mapping in pediatric patients are similar to those of 66 adult patients published in the literature (Table 3). In adults, hand motor hgECoG compared with ECS maps showed that 1.2% of contacts were identified by ECS but not by hgECoG, and 3.19% of contacts were identified by hgECoG but not by ECS. For hand mapping, there were no false-negative results between ECS and hgECoG and only a 0.46% false-positive result, suggesting that hgECoG may be more sensitive than ECS for mapping the hand motor cortex.3 Another advantage of this technique over ECS is that the sensorimotor cortex can be mapped using hgECoG to sample endogenous cortical function in children younger than 3 years old. This method is also much faster and better tolerated than traditional ECS. The major disadvantage of hgECoG is that it is the hardest of these modalities to interpret, requiring substantial computer literacy, specifically signal processing skills, to perform it as described in Methods and elsewhere. 16,19 Fortunately, more automated signal processing programs are on the horizon. The signal generated is quite robust in preventing interrater reliability problems, although we did not formally quantify this.
TABLE 3.
Published series of patients with somatosensory cortex located by hgECoG*
| Authors & Year | No. of Patients | Age Range (yrs) | Institution(s) |
|---|---|---|---|
| Crone et al., 1998 | 5 | 22–38 | Johns Hopkins University |
| Miller et al., 200717 | 8 | NA | University of Washington |
| Leuthardt et al., 2007 | 7 | 21–39 | University of Washington; Washington University in St. Louis |
| Miller et al., 200718 | 22 | 18–48 | University of Washington |
| Brunner et al., 2009 | 10 | 19–62 | Albany Medical College; University of Wisconsin, Madison; University Medical Center Utrecht, the Netherlands; Washington University in St. Louis |
| Miller et al., 2009 | 10 | 18–45 | University of Washington |
| Acharya et al., 2010 | 4 | 15–55 | Johns Hopkins University |
NA = not available.
Conclusions
This study is limited by its small sample size and retrospective nature, but it establishes that prospective studies from other institutions are warranted to address these limitations. These 4 modalities of mapping sensorimotor function produced concurrent results in children, as they have in adults. Having multiple modalities to localize salient cortex prior to resective epilepsy surgery can lead to better-informed decisions about proceeding with these elective procedures, especially if some of the modalities fail to generate meaningful results. It is particularly useful to have techniques that can be used to identify eloquent cortex in young children, as early epilepsy surgery is often critical for maximizing developmental potential. In addition, if future studies confirm our findings in larger series, hcECoG and fMRI may ultimately obviate the need for time-intensive, less well-tolerated techniques such as ECS.
Fig. 2.
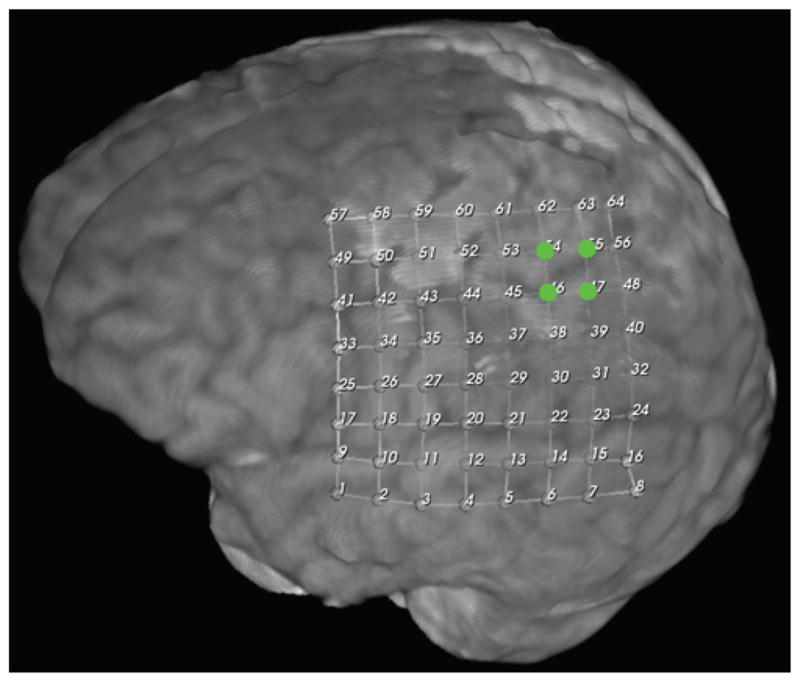
Motor cortex localization in Case 43 by SSEP. Green dots denote localization.
Fig. 3.
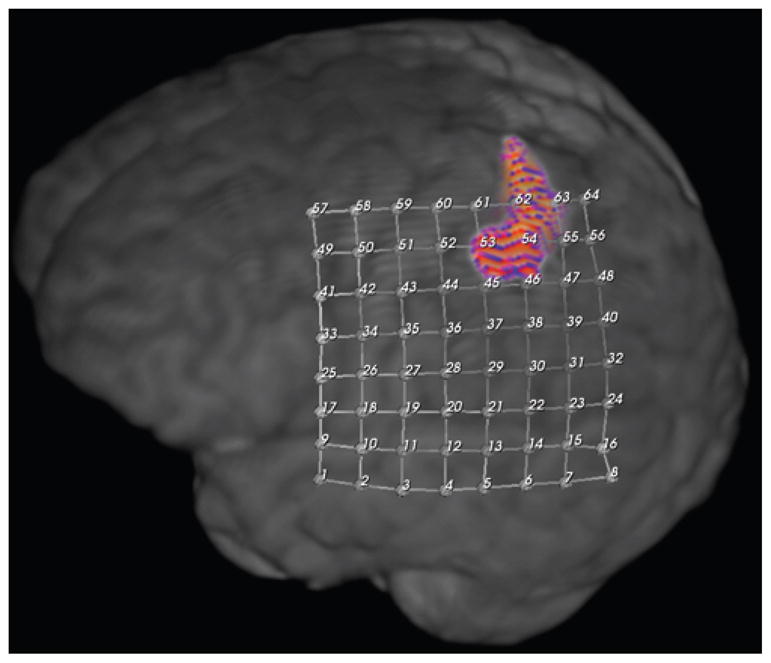
Motor cortex localization in Case 43 by fMRI finger tapping. The colored area denotes localization.
Acknowledgments
The authors wish to thank Andrew Bridges for editorial assistance.
Abbreviations used in this paper
- BOLD
blood oxygen level–dependent
- ECS
electrical cortical stimulation
- fMRI
functional MRI
- hgECoG
high gamma electrocorticography
- SSEP
somatosensory evoked potential
Footnotes
Disclosure
This work was supported in part by the National Institutes of Health/National Institute of Neurological Disorders and Stroke R01 NS065186, National Science Foundation Award No. 0930908, and The Seattle Foundation.
Author contributions to the study and manuscript preparation include the following. Conception and design: Wray, Miller, Ojemann. Acquisition of data: Wray, Blakely, Poliachik, Poliakov, Miller, Ojemann. Analysis and interpretation of data: Wray, Blakely, Poliachik, Poliakov, Ojemann. Drafting the article: Wray. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Wray. Administrative/technical/material support: Wray, Ojemann.
References
- 1.Acharya S, Fifer MS, Benz HL, Crone NE, Thakor NV. Electrocorticographic amplitude predicts finger positions during slow grasping motions of the hand. J Neural Eng. 2010;7:046002. doi: 10.1088/1741-2560/7/4/046002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brovelli A, Lachaux JP, Kahane P, Boussaoud D. High gamma frequency oscillatory activity dissociates attention from intention in the human premotor cortex. Neuroimage. 2005;28:154–164. doi: 10.1016/j.neuroimage.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 3.Brunner P, Ritaccio AL, Lynch TM, Emrich JF, Wilson JA, Williams JC, et al. A practical procedure for real-time functional mapping of eloquent cortex using electrocorticographic signals in humans. Epilepsy Behav. 2009;15:278–286. doi: 10.1016/j.yebeh.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. No. II: Event-related synchronization in the gamma band. Brain. 1998;121:2301–2315. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- 5.Crone NE, Sinai A, Korzeniewska A. High-frequency gamma oscillations and human brain mapping with electrocorticography. Prog Brain Res. 2006;159:275–295. doi: 10.1016/S0079-6123(06)59019-3. [DOI] [PubMed] [Google Scholar]
- 6.Fandino J, Kollias SS, Wieser HG, Valavanis A, Yonekawa Y. Intraoperative validation of functional magnetic resonance imaging and cortical reorganization patterns in patients with brain tumors involving the primary motor cortex. J Neurosurg. 1999;91:238–250. doi: 10.3171/jns.1999.91.2.0238. [DOI] [PubMed] [Google Scholar]
- 7.Haglund MM, Berger MS, Shamseldin M, Lettich E, Ojemann GA. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994;34:567–576. doi: 10.1227/00006123-199404000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Hermes D, Miller KJ, Vansteensel MJ, Aarnoutse EJ, Leijten FS, Ramsey NF. Neurophysiologic correlates of fMRI in human motor cortex. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21314. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda A, Shibasaki H. Cortical mapping using evoked potentials and Bereitschaftspotentials. In: Luders H, editor. Textbook of Epilepsy Surgery. London: Informa Healthcare; 2008. pp. 1036–1048. [Google Scholar]
- 10.Keles GE, Lundin DA, Lamborn KR, Chang EF, Ojemann G, Berger MS. Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients. J Neurosurg. 2004;100:369–375. doi: 10.3171/jns.2004.100.3.0369. [DOI] [PubMed] [Google Scholar]
- 11.Khursheed F, Tandon N, Tertel K, Pieters TA, Disano MA, Ellmore TM. Frequency-specific electrocorticographic correlates of working memory delay period fMRI activity. Neuroimage. 2011;56:1773–1782. doi: 10.1016/j.neuroimage.2011.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lachaux JP, Fonlupt P, Kahane P, Minotti L, Hoffmann D, Bertrand O, et al. Relationship between task-related gamma oscillations and BOLD signal: new insights from combined fMRI and intracranial EEG. Hum Brain Mapp. 2007;28:1368–1375. doi: 10.1002/hbm.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legatt AD, Kader A. Topography of the initial cortical component of the median nerve somatosensory evoked potential. Relationship to central sulcus anatomy. J Clin Neurophysiol. 2000;17:321–325. doi: 10.1097/00004691-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Leuthardt EC, Miller K, Anderson NR, Schalk G, Dowling J, Miller J, et al. Electrocorticographic frequency alteration mapping: a clinical technique for mapping the motor cortex. Neurosurgery. 2007;60 (4 Suppl 2):260–271. doi: 10.1227/01.NEU.0000255413.70807.6E. [DOI] [PubMed] [Google Scholar]
- 15.Martino J, Gabarrós A, Deus J, Juncadella M, Acebes JJ, Torres A, et al. Intrasurgical mapping of complex motor function in the superior frontal gyrus. Neuroscience. 2011;179:131–142. doi: 10.1016/j.neuroscience.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 16.Miller KJ, Abel TJ, Hebb AO, Ojemann JG. Rapid online language mapping with electrocorticography. Clinical article. J Neurosurg Pediatr. 2011;7:482–490. doi: 10.3171/2011.2.PEDS1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller KJ, denNijs M, Shenoy P, Miller JW, Rao RP, Ojemann JG. Real-time functional brain mapping using electrocorticography. Neuroimage. 2007;37:504–507. doi: 10.1016/j.neuroimage.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Miller KJ, Leuthardt EC, Schalk G, Rao RP, Anderson NR, Moran DW, et al. Spectral changes in cortical surface potentials during motor movement. J Neurosci. 2007;27:2424–2432. doi: 10.1523/JNEUROSCI.3886-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller KJ, Makeig S, Hebb AO, Rao RPN, den Nijs M, Ojemann JG. Cortical electrode localization from X-rays and simple mapping for electrocorticographic research: the “Location on Cortex” (LOC) package for MATLAB. J Neurosci Methods. 2007;162:303–308. doi: 10.1016/j.jneumeth.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Miller KJ, Schalk G, Fetz EE, den Nijs M, Ojemann JG, Rao RPN. Cortical activity during motor execution, motor imagery, and imagery-based online feedback. Proc Natl Acad Sci U S A. 2010;107:4430–4435. doi: 10.1073/pnas.0913697107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller KJ, Zanos S, Fetz EE, den Nijs M, Ojemann JG. Decoupling the cortical power spectrum reveals real-time representation of individual finger movements in humans. J Neurosci. 2009;29:3132–3137. doi: 10.1523/JNEUROSCI.5506-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- 23.Pfurtscheller G, Graimann B, Huggins JE, Levine SP, Schuh LA. Spatiotemporal patterns of beta desynchronization and gamma synchronization in corticographic data during self-paced movement. Clin Neurophysiol. 2003;114:1226–1236. doi: 10.1016/s1388-2457(03)00067-1. [DOI] [PubMed] [Google Scholar]
- 24.Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 25.Reis J, Rosenow F. Eloquent cortex and tract: overview and noninvasive evaluation methods. In: Luders H, editor. Textbook of Epilepsy Surgery. London: Informa Healthcare; 2008. pp. 869–880. [Google Scholar]
- 26.Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR. BCI2000: a general-purpose brain-computer interface (BCI) system. IEEE Trans Biomed Eng. 2004;51:1034–1043. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- 27.Schule S, McIntyre C, Luders H. General principles of cortical mapping by electrical stimulation. In: Luders H, editor. Textbook of Epilepsy Surgery. London: Informa Healthcare; 2008. pp. 961–977. [Google Scholar]
- 28.Shurtleff H, Warner M, Poliakov A, Bournival B, Shaw DW, Ishak G, et al. Functional magnetic resonance imaging for presurgical evaluation of very young pediatric patients with epilepsy. Clinical article. J Neurosurg Pediatr. 2010;5:500–506. doi: 10.3171/2009.11.PEDS09248. [DOI] [PubMed] [Google Scholar]
- 29.Vapnik VN. The Nature of Statistical Learning Theory. New York: Springer; 2000. [Google Scholar]


