Abstract
Cardiovascular collapse remains a leading cause of death in severe acute drug intoxication. Commonly prescribed medications such as antidysrhythmics, calcium channel antagonists, and beta adrenergic receptor antagonists can cause refractory cardiovascular collapse in massive overdose. Emergency cardiopulmonary bypass (ECPB), a modality originating in cardiac surgery, is a rescue technique that has been successfully implemented in the treatment of refractory cardiogenic shock and cardiac arrest unresponsive to traditional medical interventions. More recently a growing number of animal studies, case reports, and case series have documented its use in refractory hemodynamic collapse in poisoned patients. This article will review current ECPB techniques and explore its growing role in the treatment of severely hemodynamically compromised poisoned patients.
Keywords: Emergency cardiopulmonary bypass, Extracorporeal membrane oxygenation, Extracorporeal life support, Cardiogenic shock, Cardiotoxic drugs
Case Vignette
A 55-year-old man presented to the emergency department (ED) with altered mental status, hypotension, and bradycardia after an intentional overdose of carvedilol and amlodipine. Despite aggressive resuscitation and treatment with glucagon, atropine, calcium, insulin, and multiple vasopressors, his condition worsened. Cardiothoracic surgery was emergently consulted and cardiopulmonary bypass was initiated in the emergency department prior to admission to the intensive care unit. Three days later, as his cardiac function improved he was successfully weaned from bypass. On hospital day 40, after extensive rehabilitation, he was transferred, neurologically intact, to a psychiatric unit.
Introduction
Cardiovascular failure remains a leading cause of death in severe acute drug intoxication [1, 2]. Among approximately 2.8 million poisonings in the USA in 2010, cardiovascular drugs were involved in 3.5 % and accounted for 9.4 % of the 2,813 fatalities [1]. A number of clinical advancements have improved the management of toxin-induced cardiovascular compromise including an emphasis on early recognition and treatment of shock, improved hemodynamic monitoring and support, digitalis-Fab fragments [3–5]. Modalities such as hyperinsulinemia–euglycemia therapy and lipid emulsion infusions have also shown promise, though further study is needed [3, 4, 6]. In addition, changes in prescribing practices have resulted in decreased availability in the USA of tricyclic antidepressants, digitalis, and other antidysrhythmics, which were once common causes of drug-induced cardiotoxicity [3, 7, 8]. Despite these advancements, mortality remains high among patients with significant poisoning from cardiotoxic medications. Calcium channel antagonists and beta receptor antagonists now account for approximately 40 % of cardiovascular drug poisonings reported to the American Association of Poison Control Centers but represent more than 65 % of deaths from cardiovascular medications [2, 9].
Cardiopulmonary bypass has been used in cardiac surgery since the 1950s and has subsequently been applied to patients outside of the operating room with cardiopulmonary failure. A number of series have demonstrated successful treatment of refractory respiratory failure, cardiac arrest and cardiogenic shock [10–14]. This article will review current emergency cardiopulmonary bypass techniques and applications and explore its growing role in the treatment of hemodynamically compromised poisoned patients.
Overview of Emergency Cardiopulmonary Bypass
Definitions
Extracorporeal life support (ECLS) is a term used to describe a number of modalities to support both the cardiac and pulmonary systems in times of cardiac or pulmonary failure. Included under the umbrella term, ECLS is extracorporeal membrane oxygenation (ECMO), which can be either veno-arterial (VA-ECMO) or veno-venous (VV-ECMO), and emergency cardiopulmonary bypass (ECPB). Another form of cardiovascular support separate from ECLS is the intra-aortic balloon pump (IABP).
ECMO is a method of providing circulatory or pulmonary support or both: VA-ECMO provides both circulatory and pulmonary support; VV-ECMO, on the other hand, provides only pulmonary support (oxygenation and carbon dioxide removal) and is primarily used in patients with severe lung injury with preserved cardiac function. For toxin-induced hemodynamic compromise, VA-ECMO is used exclusively. Both forms of ECMO use cannulae to remove blood from the venous circulation and deliver it to a centrifugal or, less commonly, a roller pump. This acts as an extracorporeal heart to circulate blood to a membrane oxygenator, which acts as an extracorporeal lung for gas exchange. VV-ECMO requires a normally functioning heart to circulate the oxygenated blood from the circuit to the venous circulation and through the cardiopulmonary system. The centrifugal pump is the driving force of blood circulation in VA-ECMO as blood is returned via an arterial cannula. Because the devices used for ECPB and VA-ECMO are similar and both modalities offer circulatory and pulmonary support, these terms are often used interchangeably.
An IABP is a device placed in the descending aorta that decreases ventricular afterload and increases coronary perfusion. It inflates during diastole increasing coronary perfusion pressure and deflates during systole reducing afterload. IAPBs increase cardiac output by approximately 20 % and may be a useful adjunct in a poisoned patient who presents with cardiogenic shock. However, IABPs do not function in patients with cardiac arrest or ventricular tachycardia as they rely on the cardiac rhythm to trigger inflation and deflation. This limits their utility in many patients who are severely poisoned from beta adrenergic receptor and calcium channel antagonists who have suffered cardiac arrest.
Ventricular assist devices are a heterogeneous group of implantable and external devices capable of providing mechanical cardiac support and have been used primarily to bridge patients to heart transplantation.
Brief History of Emergency Cardiopulmonary Bypass
The concept of cardiopulmonary bypass (CPB) was developed and refined in the early twentieth century, and prototype pumps and oxygenators were developed in the 1920s and 1930s by Hooker, Gibbon, DeBakey and others, in order to aid in the surgical repair of cardiac defects [15]. The first known CPB-assisted operation was performed by Dr. Clarence Dennis at the University of Wisconsin in 1951. The latter half of the twentieth century brought refinements in pump and oxygenator technology, including the development of the membrane oxygenator, leading to better gas exchange and less blood damage. These advancements, as well as an improved understanding of anticoagulants and increasing portability of CPB machines, have made CPB available and utilized in hundreds of institutions worldwide.
The first reports of ECPB for refractory cardiac arrest were published in the late 1990s [13, 16]. In 2000, physicians at the University of Michigan documented their experience with 1,000 consecutive patients treated with ECPB for a variety of indications including cardiac arrest and cardiogenic shock as well as the scientific and logistical evolution of their program [10]. Since this time, a number of centers both in the USA and internationally have documented success with using ECPB for cardiogenic shock or cardiac arrest refractory to traditional therapies and a registry of these cases is maintained by the Extracorporeal Life Support Organization, centered at the University of Michigan [12, 17, 18].
ECPB Techniques
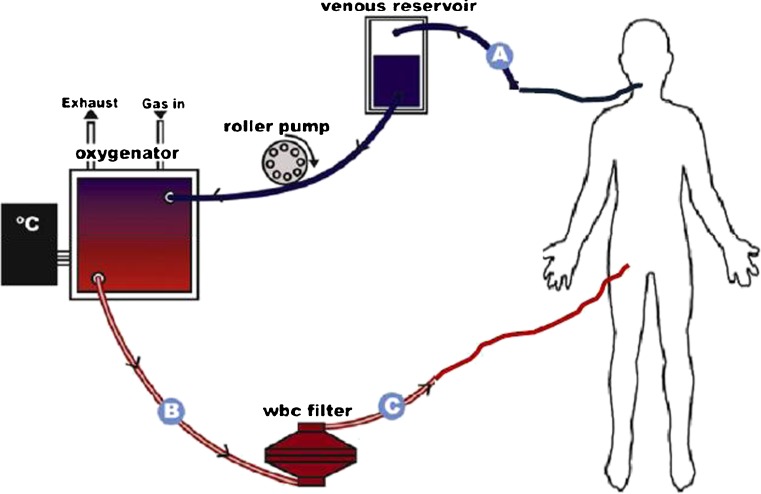
While a number of cannulation techniques for ECPB have been described, when the patient is hemodynamically unstable, a peripheral cannulation approach is typically preferred. This involves cannulation of either the femoral, internal jugular, or carotid vessels using a percutaneous Seldinger technique or surgical exposure via cutdown. Central cannulation of the heart or great vessels may also be performed for ECPB, but this is an intraoperative approach typically used during cardiac surgery or in combination with direct cardiac massage. Before the ECPB circuit can be connected to the cannulated vessels, its tubing must first be primed with blood or saline; this is a time consuming and often process-limiting step. When the catheters are connected, the patient’s blood is withdrawn from the venous circulation and propelled forward by either a centrifugal or roller pump to the oxygenator where it passes across a semi-permeable membrane, allowing for oxygen and carbon dioxide exchange. During VA-ECMO, this newly oxygenated blood returns to the patient’s arterial system via the arterial cannula (Fig. 1).
Fig. 1.
Example of ECPB configuration. Deoxygenated blood is pumped by a roller pump from the right internal jugular vein into A a venous cannula and then through the membrane oxygenator out via B arterial outflow tubing and back into the body via C an arterial cannula placed in the right femoral vein. Adapted with permission from Gaieski et al. [12]
In order to prevent clot formation in the ECPB circuit, anticoagulation with heparin is infused and titrated with regular measures of coagulation studies. Hemolysis and blood loss is common so packed red blood cells are transfused as needed. The ventilator is set to deliver low tidal volumes, moderate positive end expiratory pressure, low inspiratory pressures, and a low inspired oxygen fraction to prevent ventilator induced lung injury, oxygen toxicity, and ventilator associated hemodynamic compromise. Continuous bedside monitoring by a provider with specialized training such as a perfusionist or an intensive care unit (ICU) nurse with additional training in ECPB is required.
ECPB affects drug metabolism and elimination via a number of mechanisms including sequestration in the circuit, increased volume of distribution, and decreased drug elimination [19]. While lipophilic drugs and highly protein-bound drugs are significantly sequestered in the circuit, hydrophilic drugs are affected by hemodilution and other pathophysiologic changes that occur during ECPB. It is not yet known exactly how ECPB affects drug metabolism or elimination in poisoning, or whether it can be effectively used in conjunction with enhanced elimination techniques.
ECPB Indications, Contraindications, and Complications
ECPB is a rescue technique for patients with cardiovascular collapse refractory to traditional treatments. It is best applied as either a bridge to more definitive therapy (such as heart or lung transplantation, or pulmonary embolectomy) or to support failing hearts and lungs that are expected to recover from reversible pathologies (such as poisoning). ECPB has been most extensively used for neonatal respiratory distress syndrome with improved outcomes demonstrated for many years [10, 14, 20–22]. In adults, it has been applied to patients with severe respiratory failure, refractory cardiac arrest, cardiogenic shock, massive pulmonary embolus, traumatic injury, as well as poisoning [10–14, 23, 24]. It has also been used to preserve organs for transplantation after cardiac death [25].
ECPB is generally thought to be contraindicated in cases of irreversible multiorgan failure or in the neurologically devastated patient, including patients who have had prolonged periods of end-organ hypoperfusion.
The most common complications of ECPB are bleeding (7–36 %) and thrombosis (8–17 %) [26]. Bleeding, likely in part related to anticoagulation, most commonly occurs at surgical sites, but gastrointestinal, intracranial, and pulmonary hemorrhages have been reported. Thrombosis of the bypass circuit can have devastating consequences, and catheter-associated clots are common. Other complications include hemolysis, disseminated intravascular coagulation, vascular injury, limb ischemia, cerebral ischemia or hemorrhage, seizures, catheter-associated infection, and mechanical malfunction. Because of the large diameter of the cannulae used in ECPB, up to 30 French in adults, vascular injury at the site of cannulation may occur. In addition, distal arterial perfusion or venous flow may be impaired. A number of cases of cannula-related limb ischemia have been reported in the literature, and techniques have evolved, such as placement of a second distal arterial catheter, to allow antegrade perfusion of the cannulated limb [27]. When some native cardiac function is preserved and femoral arterial cannulation is performed, high pressures may prevent oxygenated blood from reaching the proximal aorta resulting in cerebral, cardiac, and upper body hypoxia.
There are significant financial costs associated with utilization and implementation of ECPB for medical or surgical indications. To our knowledge, there are no data documenting costs of ECPB in poisoning. A 2005 large European randomized trial in comparing ECMO with conventional management among adults respiratory failure documented an average total cost of £73,979 (approximately US$120,000) compared with £33,435 (approximately US$55,000), though lifetime quality-adjusted life years gained were greater in the ECMO group [28]. It is important that the health care community considers the financial impact of ECPB and other costly treatment modalities on patients, their families, and society.
Successful implementation of ECPB requires expert orchestration by a highly trained multidisciplinary team of clinicians and support staff. Significant coordination, training, planning, and equipment acquisition and preparation are required. A number of institutions have described their early experiences implementing ECPB and document the tremendous amount of institutional support and coordination that is required [10, 29]. The Extracorporeal Life Support Organization provides resources and training for individuals and institutions interested in starting an ECPB program [18].
Evidence-Based Support for Emergency Cardiopulmonary Bypass in Poisoning
Literature Search
A PubMed search (1948 to July 2012) was performed combining the phrases “cardiopulmonary bypass,” “extracorporeal life support,” and “extracorporeal membrane oxygenation” with the following keywords: “poisoning,” “overdose,” “intoxication,” and “ingestion.” The PubMed feature “Related Articles” was used to identify additional relevant publications. We also reviewed the references of all relevant studies to identify additional salient articles. Our initial search yielded 272 abstracts, which were reviewed by the authors. Most of these abstracts were completely unrelated, duplicates, or encompassed only one of the search terms. Of these, we identified two relevant animal studies, 11 case reports, and two case series.
Preclinical Evidence
Two animal models have demonstrated the utility of ECPB in poisoning. These models studied poisoning with the membrane stabilizers lidocaine and amitriptyline, respectively. Drugs with membrane stabilizing effects, in addition to their main pharmacologic activity, have been associated with increased mortality in poisoning [30, 31]. No literature was found describing animal models that use medications such as calcium channel antagonists or beta receptor antagonists which in overdose impair cardiac conduction and contractility, as well as cause profound vasodilation [2].
In 1986, Freedman et al. poisoned 16 dogs with a 30-mg/kg bolus of lidocaine [32]. In eight dogs, toxicity was treated with antidysrhythmic drugs, vasopressors, and cardioversion; six of these animals died within 30 min of the lidocaine bolus. In the second group of eight dogs, an extracorporeal bypass pump was used for 90 min after the lidocaine and none of these animals died.
In 1992, Larkin et al. performed a prospective randomized controlled trial in swine and showed successful application of ECPB to support circulatory collapse due to amitriptyline overdose [33]. They poisoned 20 swine with an infusion of amitriptyline at 0.5 mg/kg/min until the systolic blood pressure dropped below 30 mmHg for 1 min. The control group received aggressive supportive management including of intravenous fluids, sodium bicarbonate, vasopressors, standard advanced life support interventions, and, if needed, open-chest cardiac massage for 30 min or until the return of spontaneous circulation. The ECPB group received only mechanical support by ECPB for 90 to 120 min. All 20 animals experienced cardiac conduction delays, dysrhythmias, and progressive hypotension within 30 min of receiving amitriptyline. The 10 swine randomized to the ECPB group were able to completely correct these amitriptyline-induced abnormalities while only one of 10 control animals could be resuscitated. Nine of 10 swine treated with ECPB were weaned off bypass without any pharmacologic intervention and only one required norepinephrine. All 11 resuscitated swine were able to be salvaged.
In both models, the animals were placed onto ECPB almost immediately after hemodynamic collapse, and their duration on ECPB was short compared to most human clinical situations. Nonetheless, these models provide promise that ECPB might successfully be applied to poisoned patients.
Clinical Experience
A total of 11 case reports have been published detailing the use of ECPB in patients poisoned with flecainide [34–37], tricyclic antidepressants [38, 39], beta adrenergic receptor antagonists [40], calcium channel antagonists [41–44], digoxin [45], and buprioprion [46] (Table 1). All patients received veno-arterial support. Times elapsed until initiation of ECPB and times spent on ECPB were highly variable. A number of complications such as bleeding, hypotension, and thromboembolism were documented. Of 11 cases, eight patients survived and seven had full neurologic recovery (one patient had persistent mild ataxia). While the majority of these reports depict favorable outcomes, this may represent both reporting and publication biases in this population with a high expected mortality.
Table 1.
Summary of case reports
| Author/year | Drug(s) | Dose (mg) | Age/sex | Weight (kg) | Time to ECPB (h) | ECPB technique | Time on ECPB (h) | Outcome |
|---|---|---|---|---|---|---|---|---|
| Hendren et al. (1989) | Verapamil SR | 1,440 | 2/M | 13.5 | Unknown | Unknown | 3.75 | Died 29 h post-OD |
| Yasui et al. (1997) | Flecainide | Unknown | 20/F | Unknown | 2.5 after OD | VA femoral | 10 | Died 10 days post-OD; brain damage and renal failure |
| 2 after arrival | ||||||||
| Berhinger et al. (1998) | Digoxin | 10 | 79/M | Unknown | 5.17 from OD | VA femoral | 4 | Died 12 days post-OD; ARDS and septic shock |
| 0.37 from admission | ||||||||
| Holzer et al. (1999) | Verapamil | 4,800–6400 | 60/M | 60 | 8.10 from OD | VA femoral | 5.5 | Full recovery |
| 3.5 from admission | ||||||||
| Corkeron et al. (1999) | Flecainide | 4,000 | 20/F | Unknown | 5.15 from OD | VA femoral | 30 | Full recovery |
| 5.0 from admission | ||||||||
| Pasic et al. (2000) | Prajmalium bitartrate | 320 | 25/F | Unknown | 1.30 from OD | Ascending aorta | 16.75 | Mild ataxia, discharged 35 days post-OD |
| Durward et al. (2003) | Diltiazem SR | 12,000 | 16/F | 50 | 17 from OD | Right atrium; aorta | 48 | Full recovery |
| 10 from admission | ||||||||
| MacLaren et al. (2004) | Verapamil SR | 7,200 | 45/F | Unknown | Unknown | VA femoral | 144 | Full recovery |
| Doxepin | 1,600 | |||||||
| Quetiapine | 10,000 | |||||||
| Diazepam | 2,000 | |||||||
| Clonazepam | 100 | |||||||
| Temazepam | 200 | |||||||
| Kolcz et al. (2007) | Verapamil SR | 960 | 15/F | 60 | 2 from admission | Right atrium; aorta | 70 | Full recovery |
| Propanolol | 550 | |||||||
| Marciniak et al. (2007) | Ibuprofen | 10,000 | 14/M | 75 | 7 from OD | VA carotid; internal jugular | 96 | Full recovery |
| Shenoi et al. (2011) | Bupropion SR | 9,000 | 11 months/M | 12 | 21 from OD | VA | 71 | Full recovery |
| 18 from admission |
SR sustained release, M male, F female, OD overdose, VA veno-arterial
A series of 17 cases of poisoned patients with refractory shock or cardiac arrest who were treated with ECPB was published in 2009 by Daubin et al. [47]. Fifteen patients ingested cardiotoxic drugs, 11 of which had membrane stabilizing activity. Thirteen patients ingested multiple agents. All patients were mechanically ventilated and received vasopressor therapy. Four patients needed temporary external transthoracic pacing. Six patients required continuous veno-venous hemofiltration or conventional dialysis for acute renal failure before or immediately after ECLS implantation. After a mean 101 ± 55 min of external cardiac massage, ECPB was initiated. In 13 of the 17 cases, the cannulation was performed in the operating room and the remainder in the ICU or ED. In all of these patients, an additional catheter was placed in the distal femoral artery in order to reduce the risk of distal limb ischemia. The mean ECPB duration was 4.5 ± 2.4 days. Fifteen of 17 patients were successfully weaned off ECPB, and 13 (76 %) were discharged without significant cardiovascular or neurologic sequelae (Cerebral Performance Score [CPC] of one in nine patients and CPC of two in four patients). Reported complications included limb ischemia, thrombus formation, and cannula site bleeding. The authors concluded that ECPB as a last option in the severely poisoned patient with cardiovascular compromise is effective and relatively safe.
A recently published retrospective cohort analysis by Masson and colleagues documents the largest collection of poisoned patients treated with ECPB to date [48]. This study examined all patients admitted to two French hospitals over a 10-year period with persistent cardiac arrest or severe shock following poisoning. ECPB was preferentially performed at one of the two centers, where medical teams have extensive experience with ECPB. They enrolled a total of 62 patients (10 with persistent cardiac arrest and 52 with severe shock), 14 of whom were treated with ECPB (the remainder was treated with conventional medical management). The patients had comparable drug ingestion histories, illness severities (as measured by Simplified Acute Physiology II [SAPS II] and Sequential Organ Failure Assessment scores), Glasgow Coma Scale scores, need for mechanical ventilation, and use of renal replacement therapy. The ECPB technique was the same as the one described by Daubin et al. In the ECPB group, 12 of 14 (86 %) patients survived compared with 23 of 48 (48 %) in the conventional management group. A variety of subgroup analyses was performed. Among the 10 patients with persistent cardiac arrest (defined as absence of ROSC after continuous cardiopulmonary resuscitation for at least 30 min), none survived without ECPB support. They also found that beta adrenergic receptor antagonist poisoning was associated with lower mortality. In multivariate analysis adjusting for SAPS II and adrenergic receptor antagonists, ECPB support remained associated with lower mortality (adjusted odds ratio 0.18, 95 % confidence interval 0.03–0.96, p = 0.04). The authors concluded that, in cases of refractory cardiac arrest and severe shock unresponsive to conventional therapies, poisoned patients may benefit from ECPB.
It should be noted that both of the above reports came from the same institution, and 12 of the 17 patients presented by Daubin et al. were also included by Masson et al. [47, 48]. Furthermore, these studies illustrate the experience of a single hospital with extensive experience using ECPB for a variety of indications in critically ill patients [27, 47, 48]. Because the development and successful implementation of an ECPB program requires careful planning and extensive coordination, the outcomes presented in these studies may not parallel the experiences at less practiced institutions. In addition, all of these studies were retrospective in nature, and thus should only be considered as hypothesis generating. Despite these limitations, these studies demonstrate the potential for ECPB as a rescue therapy for the severely poisoned patients, especially in experienced hands.
In summary, approximately 30 cases of severely poisoned patients treated with ECPB have been reported in the medical literature. Although reporting and publication biases likely exist and many of these patients were treated at a single institution with a robust ECPB program, approximately 20 (66 %) of these patients survived without serious sequelae.
Conclusion
Emergency cardiopulmonary bypass is a promising tool that may be beneficial to acutely poisoned patients with cardiovascular collapse refractory to standard therapies. Although evidence is limited, we recommend early consideration of ECPB in poisoned patients with refractory cardiac arrest or hemodyamic compromise who have not yet undergone irreversible organ failure. Most of the available data, though limited to case series and single reports, demonstrate that good outcomes are attainable at experienced institutions. No prospective or randomized trials have been conducted; they would likely be both logistically and ethically challenging. Future studies might evaluate the cost effectiveness of ECPB in overdose and the experiences of less practiced institutions. IABP and veno-venous ECMO may also be useful adjuncts in patients with toxin-induced cardiogenic shock and pulmonary compromise, respectively.
Acknowledgments
Conflict of Interest
The authors report no conflicts of interest relevant to this manuscript. Nicholas J. Johnson, Steven R. Allen, Jeanmarie Perrone, and Francis DeRoos have no disclosures. David Gaieski receives research support and consulting fees from Stryker.
References
- 1.Bronstein AC, Spyker DA, Cantilena LR, Jr, et al. 2010 Annual report of the American association of poison control centers' national poison data system (NPDS): 28th annual report. Clin Toxicol (Phila) 2011;49(10):910–941. doi: 10.3109/15563650.2011.635149. [DOI] [PubMed] [Google Scholar]
- 2.DeWitt CR, Waksman JC. Pharmacology, pathophysiology and management of calcium channel blocker and beta-blocker toxicity. Toxicol Rev. 2004;23(4):223–238. doi: 10.2165/00139709-200423040-00003. [DOI] [PubMed] [Google Scholar]
- 3.Baud FJ, Megarbane B, Deye N, et al. Clinical review: aggressive management and extracorporeal support for drug-induced cardiotoxicity. Crit Care. 2007;11(2):207. doi: 10.1186/cc5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerns W., 2nd Management of beta-adrenergic blocker and calcium channel antagonist toxicity. Emerg Med Clin N Am. 2007;25(2):309–331. doi: 10.1016/j.emc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Taboulet P, Baud FJ, Bismuth C. Clinical features and management of digitalis poisoning—rationale for immunotherapy. J Toxicol Clin Toxicol. 1993;31(2):247–260. doi: 10.3109/15563659309000392. [DOI] [PubMed] [Google Scholar]
- 6.Engebretsen KM, Kaczmarek KM, Morgan J, et al. High-dose insulin therapy in beta-blocker and calcium channel-blocker poisoning. Clin Toxicol (Phila) 2011;49(4):277–283. doi: 10.3109/15563650.2011.582471. [DOI] [PubMed] [Google Scholar]
- 7.Haynes K, Heitjan D, Kanetsky P, et al. Declining public health burden of digoxin toxicity from 1991 to 2004. Clin Pharmacol Ther. 2008;84(1):90–94. doi: 10.1038/sj.clpt.6100458. [DOI] [PubMed] [Google Scholar]
- 8.Grunebaum MF, Ellis SP, Li S, et al. Antidepressants and suicide risk in the United States, 1985–1999. J Clin Psychiatry. 2004;65(11):1456–1462. doi: 10.4088/JCP.v65n1103. [DOI] [PubMed] [Google Scholar]
- 9.Lai MW, Klein-Schwartz W, Rodgers GC, et al. 2005 Annual report of the American Association of Poison Control Centers' national poisoning and exposure database. Clin Toxicol (Phila) 2006;44(6–7):803–932. doi: 10.1080/15563650600907165. [DOI] [PubMed] [Google Scholar]
- 10.Bartlett RH, Roloff DW, Custer JR, et al. Extracorporeal life support: the University of Michigan experience. JAMA. 2000;283(7):904–908. doi: 10.1001/jama.283.7.904. [DOI] [PubMed] [Google Scholar]
- 11.Wu MY, Lee MY, Lin CC, et al. Resuscitation of non-postcardiotomy cardiogenic shock or cardiac arrest with extracorporeal life support: the role of bridging to intervention. Resuscitation. 2012;83(8):976–981. doi: 10.1016/j.resuscitation.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Gaieski DF, Boller M, Becker LB. Emergency cardiopulmonary bypass: a promising rescue strategy for refractory cardiac arrest. Crit Care Clin. 2012;28(2):211–229. doi: 10.1016/j.ccc.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Younger JG, Schreiner RJ, Swaniker F, et al. Extracorporeal resuscitation of cardiac arrest. Acad Emerg Med. 1999;6(7):700–707. doi: 10.1111/j.1553-2712.1999.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 14.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 15.Stammers AH. Historical aspects of cardiopulmonary bypass: from antiquity to acceptance. J Cardiothorac Vasc Anesth. 1997;11(3):266–274. doi: 10.1016/S1053-0770(97)90095-1. [DOI] [PubMed] [Google Scholar]
- 16.Martin GB, Rivers EP, Paradis NA, et al. Emergency department cardiopulmonary bypass in the treatment of human cardiac arrest. Chest. 1998;113(3):743–751. doi: 10.1378/chest.113.3.743. [DOI] [PubMed] [Google Scholar]
- 17.Nagao K, Hayashi N, Kanmatsuse K, et al. Cardiopulmonary cerebral resuscitation using emergency cardiopulmonary bypass, coronary reperfusion therapy and mild hypothermia in patients with cardiac arrest outside the hospital. J Am Coll Cardiol. 2000;36(3):776–783. doi: 10.1016/S0735-1097(00)00779-8. [DOI] [PubMed] [Google Scholar]
- 18.Extracorporeal Life Support Organization (2012) http://www.elso.med.umich.edu. Accessed 23 July 2012
- 19.Shekar K, Fraser JF, Smith MT et al (2012) Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care 18 Apr 2012 [DOI] [PubMed]
- 20.Bartlett RH, Gazzaniga AB, Huxtable RF, et al. Extracorporeal circulation (ECMO) in neonatal respiratory failure. J Thorac Cardiovasc Surg. 1977;74(6):826–833. [PubMed] [Google Scholar]
- 21.Bartlett RH, Gazzaniga AB, Toomasian J, et al. Extracorporeal membrane oxygenation (ECMO) in neonatal respiratory failure. 100 cases. Ann Surg. 1986;204(3):236–245. doi: 10.1097/00000658-198609000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartlett RH, Roloff DW, Cornell RG, et al. Extracorporeal circulation in neonatal respiratory failure: a prospective randomized study. Pediatrics. 1985;76(4):479–487. [PubMed] [Google Scholar]
- 23.Frizelle FA, Massey R, Feint JA, et al. Tissue plasminogen activator and cardiopulmonary bypass for massive pulmonary embolus. Aust N Z J Surg. 1991;61(2):151–153. doi: 10.1111/j.1445-2197.1991.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 24.Dauphine C, McKay C, De Virgilio C, et al. Selective use of cardiopulmonary bypass in trauma patients. Am Surg. 2005;71(1):46–50. [PubMed] [Google Scholar]
- 25.Magliocca JF, Magee JC, Rowe SA, et al. Extracorporeal support for organ donation after cardiac death effectively expands the donor pool. J Trauma. 2005;58(6):1095–1101. doi: 10.1097/01.TA.0000169949.82778.DF. [DOI] [PubMed] [Google Scholar]
- 26.Gaffney AM, Wildhirt SM, Griffin MJ, et al. Extracorporeal life support. BMJ. 2010;341:c5317. doi: 10.1136/bmj.c5317. [DOI] [PubMed] [Google Scholar]
- 27.Massetti M, Bruno P, Babatasi G, et al. Cardiopulmonary bypass and severe drug intoxication. J Thorac Cardiovasc Surg. 2000;120(2):424–425. doi: 10.1067/mtc.2000.107825. [DOI] [PubMed] [Google Scholar]
- 28.Peek GJ, Elbourne D, Mugford M, et al. Randomised controlled trial and parallel economic evaluation of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR) Health Technol Assess. 2005;14(35):1–46. doi: 10.3310/hta14350. [DOI] [PubMed] [Google Scholar]
- 29.Bellezzo JM, Shinar Z, Davis DP, et al. Emergency physician-initiated extracorporeal cardiopulmonary resuscitation. Resuscitation. 2012;83(8):966–970. doi: 10.1016/j.resuscitation.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 30.Henry JA, Cassidy SL. Fatal poisoning and membrane stabilising activity. Lancet. 1986;2(8512):919. doi: 10.1016/S0140-6736(86)90438-1. [DOI] [PubMed] [Google Scholar]
- 31.Henry JA, Cassidy SL. Membrane stabilising activity: a major cause of fatal poisoning. Lancet. 1986;1(8495):1414–1417. doi: 10.1016/S0140-6736(86)91558-8. [DOI] [PubMed] [Google Scholar]
- 32.Freedman MD, Gal J, Freed CR. Extracorporeal pump assistance—novel treatment for acute lidocaine poisoning. Eur J Clin Pharmacol. 1982;22(2):129–135. doi: 10.1007/BF00542457. [DOI] [PubMed] [Google Scholar]
- 33.Larkin GL, Graeber GM, Hollingsed MJ. Experimental amitriptyline poisoning: treatment of severe cardiovascular toxicity with cardiopulmonary bypass. Ann Emerg Med. 1994;23(3):480–486. doi: 10.1016/S0196-0644(94)70066-4. [DOI] [PubMed] [Google Scholar]
- 34.Auzinger GM, Scheinkestel CD. Successful extracorporeal life support in a case of severe flecainide intoxication. Crit Care Med. 2001;29(4):887–890. doi: 10.1097/00003246-200104000-00041. [DOI] [PubMed] [Google Scholar]
- 35.Corkeron MA, van Heerden PV, Newman SM, et al. Extracorporeal circulatory support in near-fatal flecainide overdose. Anaesth Intensive Care. 1999;27(4):405–408. doi: 10.1177/0310057X9902700413. [DOI] [PubMed] [Google Scholar]
- 36.Vivien B, Deye N, Megarbane B, et al. Extracorporeal life support in a case of fatal flecainide and betaxolol poisoning allowing successful cardiac allograft. Ann Emerg Med. 2010;56(4):409–412. doi: 10.1016/j.annemergmed.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 37.Yasui RK, Culclasure TF, Kaufman D, et al. Flecainide overdose: is cardiopulmonary support the treatment? Ann Emerg Med. 1997;29(5):680–682. doi: 10.1016/S0196-0644(97)70257-9. [DOI] [PubMed] [Google Scholar]
- 38.Goodwin DA, Lally KP, Null DM., Jr Extracorporeal membrane oxygenation support for cardiac dysfunction from tricyclic antidepressant overdose. Crit Care Med. 1993;21(4):625–627. doi: 10.1097/00003246-199304000-00025. [DOI] [PubMed] [Google Scholar]
- 39.Williams JM, Hollingshed MJ, Vasilakis A, et al. Extracorporeal circulation in the management of severe tricyclic antidepressant overdose. Am J Emerg Med. 1994;12(4):456–458. doi: 10.1016/0735-6757(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 40.Rooney M, Massey KL, Jamali F, et al. Acebutolol overdose treated with hemodialysis and extracorporeal membrane oxygenation. J Clin Pharmacol. 1996;36(8):760–763. doi: 10.1002/j.1552-4604.1996.tb04247.x. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida K, Kimura K, Hibi K, et al. A patient with disopyramide intoxication rescued by percutaneous cardiopulmonary support. J Cardiol. 1998;32(2):95–100. [PubMed] [Google Scholar]
- 42.Holzer M, Sterz F, Schoerkhuber W, et al. Successful resuscitation of a verapamil-intoxicated patient with percutaneous cardiopulmonary bypass. Crit Care Med. 1999;27(12):2818–2823. doi: 10.1097/00003246-199912000-00035. [DOI] [PubMed] [Google Scholar]
- 43.Hendren WG, Schieber RS, Garrettson LK. Extracorporeal bypass for the treatment of verapamil poisoning. Ann Emerg Med. 1989;18(9):984–987. doi: 10.1016/S0196-0644(89)80465-2. [DOI] [PubMed] [Google Scholar]
- 44.Durward A, Guerguerian AM, Lefebvre M, et al. Massive diltiazem overdose treated with extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2003;4(3):372–376. doi: 10.1097/01.PCC.0000074273.50306.F5. [DOI] [PubMed] [Google Scholar]
- 45.Behringer W, Sterz F, Domanovits H, et al. Percutaneous cardiopulmonary bypass for therapy resistant cardiac arrest from digoxin overdose. Resuscitation. 1998;37(1):47–50. doi: 10.1016/S0300-9572(98)00025-2. [DOI] [PubMed] [Google Scholar]
- 46.Shenoi AN, Gertz SJ, Mikkilineni S, et al. Refractory hypotension from massive bupropion overdose successfully treated with extracorporeal membrane oxygenation. Pediatr Emerg Care. 2011;27(1):43–45. doi: 10.1097/PEC.0b013e3182045f76. [DOI] [PubMed] [Google Scholar]
- 47.Daubin C, Lehoux P, Ivascau C, et al. Extracorporeal life support in severe drug intoxication: a retrospective cohort study of seventeen cases. Crit Care. 2009;13(4):R138. doi: 10.1186/cc8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masson R, Colas V, Parienti JJ, et al. A comparison of survival with and without extracorporeal life support treatment for severe poisoning due to drug intoxication. Resuscitation. 2012;83(11):1413–1417. doi: 10.1016/j.resuscitation.2012.03.028. [DOI] [PubMed] [Google Scholar]