Abstract
Introduction
Sodium hypochlorite is the active ingredient in bleach, a ubiquitous household disinfectant, and has known toxicities depending on route of exposure and amount. Acute kidney injury due to sodium hypochlorite exposure has never been reported. Patients that did develop nephrotoxicity following bleach exposure did so due to development of other risk factors for kidney injury such as volume depletion or sepsis.
Discussion
We report a patient who presented with black urine after parenteral self-administration of a large quantity of bleach. We review the clinical presentation, laboratory and biopsy findings, and outcome as well as discuss possible mechanisms of sodium hypochlorite toxicity and management strategies.
Keywords: Bleach, Cardiac, Dialysis, Failure, Kidney, Sodium hypochlorite
Introduction
Physicians often encounter acute kidney injury (AKI) mediated by toxic substances. The commonly encountered toxins necessitating dialysis are ethylene glycol, methanol, salicylates, and lithium. Sodium hypochlorite (NaOCl) is a readily available disinfectant and an active ingredient in household bleach. There have been several reports of low toxicity of NaOCl with topical, oral, or parenteral administration [1–7]. Topical administration of large doses may cause localized inflammatory signs and symptoms. Oral ingestion is reported to have caused nausea, vomiting, and more ominously, esophageal burning, perforation, and strictures [8]. Vapor inhalation may cause an acute lung injury. Direct nephrotoxicity following oral or intravenous administration of bleach is not described. AKI may result indirectly from volume depletion, hypotension rhabdomyolysis, or hemolysis. We report an unusual case of parenteral self-administration of household bleach. This patient developed transient hemolysis and profound acute kidney injury requiring renal replacement therapy.
Case Report
An 18-year-old female was brought to the emergency room by her mother with black urine (Fig. 1, panel A) less than 2 h after self-injection of 100 mL of Clorox® Lemon Fresh Bleach (1–5 % NaOCl and 0.1–1 % sodium hydroxide) through a tunneled catheter intended for treatment of purported chronic Lyme disease. The patient felt that she had done it in a “Lyme fog” with no intention of self-harm and immediately called her mother for help. The mother witnessed an opened Clorox® container and a syringe smelling of bleach in the bathroom. The past medical history included depression, anxiety, a 3-year history of chronic Lyme disease, and occasional episodes of Lyme fog—a controversial condition, recognized by some practitioners and chronic Lyme disease advocacy groups—associated with intermittent cognitive dysfunction. The patient had been on intravenous ceftriaxone and a number of oral antibiotics, including trimethoprim-sulfamethoxazole, for over a year, as well as lithium.
Fig. 1.
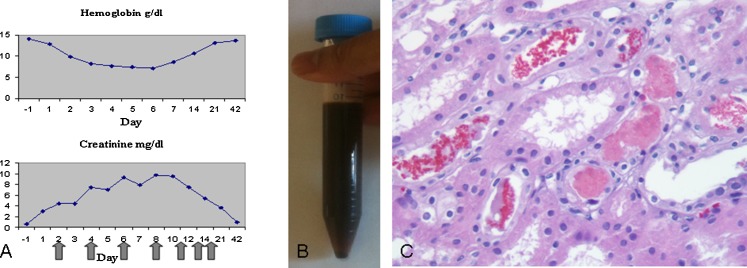
a Hemoglobin and creatinine trends. The serum creatinine and hemoglobin values are plotted over time. Self-administration of intravenous bleach through the tunneled catheter occurred on day 0. Values from day −1 were obtained from a routine follow-up visit with the pediatrician. Black arrows indicate days that hemodialysis was performed. b Patient’s black urine on the day of admission, within hours of bleach injection. c Kidney biopsy (×400) 4 days after self-administration of intravenous bleach. The kidney biopsy revealed diffuse tubular necrosis and large numbers of intratubular fragmented erythrocytes. No necrosis was seen outside of the tubules in the renal cortex or medulla
On admission, she was drowsy but arousable to voice and oriented, afebrile with a regular heart rate of 106/min, respiratory rate of 16/min, blood pressure of 124/92 mmHg, and oxygen saturation of 97 % on room air. The exam was only remarkable for the presence of a tunneled catheter with clean exit site in her left internal jugular vein. Two liters of normal saline was infused and the patient was transferred to the critical care unit. For the first 12 h after presentation, all blood chemistries performed on an automated analyzer were rejected due to hemolysis. The day before admission, at a routine visit to her doctor, the patient’s blood urea nitrogen was 10 (7–23) mg/dL (3.57 mmol/L), creatinine was 0.63 (0.5–1.2) mg/dL (55.7 μmol/L), and bilirubin was 0.2 (0.3–1.2) mg/dL (3.42 μmol/L). On the day of admission, they were 23 mg/dL (8.2 mmol/L), 2.99 mg/dL (264.3 μmol/L), and 2.3 mg/dL (39.3 μmol/L), respectively. Serum lactate dehydrogenase (LDH) was 1,984 (110–240) IU/L. Prothrombin time and activated partial thromboplastin time were 28.5 (9.6–12.5) and 46 (22.3–34) s, respectively. Haptoglobin was 16 (36–195) mg/dL (0.16 g/L). Creatine phosphokinase was 87 (38–206) u/L and troponin was 1.88 (<0.03) ng/mL. These data indicated AKI, intravascular hemolysis, and mild myocardial injury; rhabdomyolysis was excluded. Hemoglobin and creatinine during recovery are trended in Fig. 1a. Urine toxicology was only positive for her prescription medications, such as trazadone, diphenhydramine, citalopram, diazepam, trimethoprim, as well as caffeine; serum salicylate and acetaminophen were undetectable, and the lithium level was in the normal range. Electrocardiography showed sinus tachycardia with normal intervals. Her urine was black in color (Fig. 1b) and was strongly positive for blood by dipstick. The urine sediment demonstrated several monomorphic red cells and cellular debris but no casts. The initial urinary output was 50 mL an hour. She became anuric over the next 3 h despite aggressive fluid resuscitation. Hemolysis resolved over the next 96 h with LDH trending down to 623 IU/L. The myocardial injury resolved with a troponin of 0.7 ng/mL at 72 h. However, the patient remained anuric, volume overloaded, and azotemic. Hemodialysis was initiated on the second day of hospitalization. A kidney biopsy was performed on the fourth day to assess the nature and extent of kidney injury and its prognosis (Fig. 1c). Light microscopy showed extensive loss of the proximal tubular epithelium with anucleate cellular debris compatible with hemolyzed erythrocytes. The interstitium contained focal acute inflammatory infiltrates surrounding the necrotic tubules. There were no significant chronic parenchymal changes or necrosis outside of the tubular compartment.
The patient received seven hemodialysis treatments before recovering sufficient renal function. The hemodialysis and tunneled catheters were removed. The patient was subsequently discharged to a psychiatric unit for further management.
Discussion
Sodium hypochlorite is a strong alkali (pH ∼11) manufactured by passing chlorine gas through dilute sodium hydroxide. When exposed to water, it forms hypochlorous acid (HOCl) that generates superoxide radicals that lead to oxidative injury and cell or pathogen death [9]. Medical and dental uses include disinfection of dialysis machines and tunneled catheters, surgical equipment, wound cleaning, hand hygiene, and root canal sterilization [6]. Previously, intravenous drug users sharing needles and syringes were encouraged to use NaOCl to disinfect their injection equipment, as it is effective against the human immunodeficiency virus [10]. Commercially, NaOCl has been used for water purification, cleansing of textiles, and as domestic bleach. Household bleach contains 1–6 % solution of sodium hypochlorite.
AKI due to NaOCl is likely multifactorial [9]. NaOCl converts to HOCl upon contact with plasma and generates superoxide radicals causing oxidative cell death, hemolysis, and rhabdomyolysis. Hemolysis can also occur due to rapid protein degradation by this hypertonic and strongly alkaline solution. Massive hemolysis leads to excessive hemoglobin breakdown, overwhelming haptoglobin stores causing generation of free heme proteins. These heme breakdown products cause kidney injury by direct toxicity, reduced kidney blood flow, pigment cast formation, and subsequent tubular obstruction [11, 12].
There are no known antidotes to NaOCl. Sodium thiosulfate is a reducing agent that has been used for neutralizing NaOCl, but its efficacy is not established [2]. Vigorous volume repletion should be undertaken with normal saline to minimize ischemic injury, increase tubular flow, reduce cast formation, and expedite potassium excretion. Forced alkaline diuresis with an infusion of bicarbonate to keep urinary pH above 6.5 has also been recommended to reduce precipitation of heme protein casts in hemolysis and decrease generation of free iron from myoglobin in rhabdomyolysis. However, bicarbonate administration has no proven benefit over normal saline infusion in the setting of rhabdomyolysis [13].
Exchange transfusion may be helpful in ameliorating sodium hypochlorite-induced hemolysis; this approach needs validation and was not utilized in our patient. Sodium hypochlorite molecule is easily dialyzable due to its low molecular weight and small volume of distribution. Early institution of dialysis, however, is unlikely to be practical in prevention of the toxic effects of bleach exposure as it converts to HOCl upon contact with plasma with rapid development of cellular toxicity.
An important consideration is the differential of black discoloration of urine (Table 1) [14–16]. Black urine and hemolyzed blood specimens should raise suspicion for in vivo hemolysis. In addition to bleach, another common household substance with the potential to cause hemolysis is hydrogen peroxide. Preparations containing chlorates, bromates, or nitrite-based cleaners, while capable of causing hemolysis, are not found in a typical residence. This patient was not tested for glucose-6-phosphate dehydrogenase deficiency (G6PD)—an X-linked condition. Individuals taking trimethoprim, such as this patient, would be predisposed to hemolysis if also G6PD deficient, although severe hemolysis is more typically seen in males of African, South-East Asian, or Mediterranean descent. Significant hemolysis may occur in females due to X chromosome lionization. However, G6PD is unlikely in this patient based on her ethnicity, family history, and prior exposure to trimethoprim with no evidence of hemolysis. Nevertheless, while the direct effect of bleach is the most likely cause, hydrogen peroxide and trimethoprim could have been involved. The ubiquitous nature of bleach, as well as its frequent use as an antiseptic in medicine, dentistry, and intravenous drug abuse, should warrant inclusion of sodium hypochlorite toxicity among the etiologies of black urine, especially since such patients may not present with an obvious or verifiable history of exposure as was present in our case.
Table 1.
Causes of black/brown urine
| Pigment excretion |
| Hemoglobina, myoglobin, bilirubin, and melanin (melanoma patients) |
| Ingestion of food products |
| Aloe, fava beansb, rhubarb |
| Poisoninga |
| Sodium hypochlorite, copper, and phenol |
| Drugs |
| Iron, levodopa, methyldopa, metronidazole , nitrofurantoinb, primaquineb, senna, and sorbitol |
| Hereditary syndromes |
| Alkaptonuria, porphyrinuria (reddish brown, pink in UV light), tyrosinosis, sickle cell diseasea |
| Falciparum malariaa (blackwater fever) |
aAssociated with hemolysis
bAssociated with glucose-6-phosphate dehydrogenase deficiency. Other poisons such as chlorates, bromates, and nitrites may cause hemolysis, but black urine has not been reported
Conclusion
This is a unique presentation of large volume injection of bleach directly into a central vein. It illustrates previously unreported severe nephrotoxicity from domestic bleach that is usually considered benign. It suggests that kidneys are vulnerable to the effects of massive hemolysis induced by oxidative stress due to sodium hypochlorite. This patient recovered kidney function in spite of significant hemolysis and extensive tubular necrosis that required supportive measures and renal replacement therapy. This suggests a relatively good prognosis from intravenous injection of 100 mL of bleach. Since bleach is readily available, clinicians should recognize the potential nephrotoxicity of intravenous hypochlorite and be aware of its differential diagnosis, prognosis, and therapeutic interventions to minimize the duration and severity of renal injury.
Hypochlorite toxicity would be an important differential in a patient with black urine or other signs of hemolysis and acute kidney injury. Physicians need to be familiar with the differential diagnosis of “black urine” as it may be the only clue in an uncooperative or obtunded patient.
Acknowledgments
Conflict of Interest
None for any of the authors.
References
- 1.Eilers MA, Garrison TE. General management principles. In: Rosen P, Barkin RM, editors. Emergency medicine concepts and clinical practice. 3. St Louis: Mosby; 1992. pp. 2472–2473. [Google Scholar]
- 2.Hoy RH. Accidental systemic exposure to sodium hypochlorite during hemodialysis. Am J Hosp Pharm. 1981;38:1512–1514. [PubMed] [Google Scholar]
- 3.Froner GA, Rutherford GW, Rokeach M. Injection of sodium hypochlorite by intravenous drug users. JAMA. 1987;258:325. doi: 10.1001/jama.258.3.325. [DOI] [PubMed] [Google Scholar]
- 4.Marroni M, Menichetti F. Accidental intravenous infusion of sodium hypochlorite. DICP. 1991;25:1008. doi: 10.1177/106002809102500919. [DOI] [PubMed] [Google Scholar]
- 5.Morgan DL. Intravenous injection of household bleach. Ann Emerg Med. 1992;21:1394–1395. doi: 10.1016/S0196-0644(05)81909-2. [DOI] [PubMed] [Google Scholar]
- 6.Motta MV, Chaves-Mendonca MAL, Stirton CG, Cardozo HF. Accidental injection with sodium hypochlorite: report of a case. Int Endod J. 2009;42(2):175–182. doi: 10.1111/j.1365-2591.2008.01493.x. [DOI] [PubMed] [Google Scholar]
- 7.Bruch MK. Toxicity and safety of topical sodium hypochlorite. Contrib Nephrol. 2007;154:24–38. doi: 10.1159/000096812. [DOI] [PubMed] [Google Scholar]
- 8.Arevalo-Silva C, Eliashar R, Wohlgelernter J, et al. Ingestion of caustic substances: a 15-year experience. Laryngoscope. 2006;116:1422–1426. doi: 10.1097/01.mlg.0000225376.83670.4d. [DOI] [PubMed] [Google Scholar]
- 9.Peck B, Workeneh B, Kadikoy H, Patel SJ, Abdellatif A. Spectrum of sodium hypochlorite toxicity in man—also a concern for nephrologists. NDT Plus. 2011;4(4):231–235. doi: 10.1093/ndtplus/sfr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control Recommendations for preventing transmission of infection with human T-lymphotropic virus type III/lymphadenopathy-associated virus in the workplace. MMWR. 1985;34:685–694. [PubMed] [Google Scholar]
- 11.Holt S, Moore K. Pathogenesis of renal failure in rhabdomyolysis: the role of myoglobin. Exp Nephrol. 2000;8(2):72. doi: 10.1159/000020651. [DOI] [PubMed] [Google Scholar]
- 12.Qian Q, Nath K, Wu Y, Daoud TM, Sethi S. Hemolysis and acute kidney failure. Am J Kidney Dis. 2010;56:780–784. doi: 10.1053/j.ajkd.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9(2):158–169. doi: 10.1186/cc2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aycock RD, Kass DA. Abnormal urine color. South Med J. 2012;105(1):43–47. doi: 10.1097/SMJ.0b013e31823c413e. [DOI] [PubMed] [Google Scholar]
- 15.Slawson M. Thirty-three drugs that discolor urine and/or stools. RN. 1980;43(1):40–41. [PubMed] [Google Scholar]
- 16.Gambichler T, Stucker M, Kerner K, Weiner S, Waldherr R, Altmeyer P, Krueter A. Acute kidney injury in a patient with melanuria, diffuse melanosis, and metastatic malignant melanoma. Am J Clin Dermatol. 2008;9:267–270. doi: 10.2165/00128071-200809040-00007. [DOI] [PubMed] [Google Scholar]