Abstract
Background
During the summer of 2005, multiple cities in the United States began to report outbreaks of fentanyl-associated fatalities among illicit drug users. The objectives of this study were to (1) determine if an outbreak of fentanyl-associated fatalities occurred in mid-2005 to mid-2006 and (2) to examine trends and compare features of fentanyl-contaminated heroin-associated fatalities (FHFs) with non-fentanyl, heroin-associated fatalities (NFHFs) among illicit drug users.
Methods
Baseline prevalence of fentanyl- and heroin-associated deaths was estimated from January to May 2005 based on recorded cause of death (determined by the medical examiner (ME)) using the Wayne County, MI, USA toxicology database. The database was then queried for both FHFs and NFHFs between July 1, 2005 and May 12, 2006. A FHF was defined as having fentanyl or norfentanyl (metabolite) detected in any postmortem biological sample and either (1) detection of heroin or its metabolite (6-acetylmorphine) and/or cocaine or its metabolite (benzoylecgonine) in a postmortem biological specimen or (2) confirmation of fentanyl abuse as the cause of death by the ME or a medical history available sufficient enough to exclude prescription fentanyl or other therapeutic opioid use. A NFHF was defined as detection of heroin, 6-acetylmorphine (heroin metabolite) or morphine in any postmortem biological specimen, heroin overdose listed as the cause of death by the ME, and absence of fentanyl detection on postmortem laboratory testing. Information was systematically collected, trended for each group and then compared between the two groups with regard to demographic, exposure, autopsy, and toxicology data. Logistic regression was performed using SAS v 9.1 examining the effects of age, gender, and marital status with fentanyl group status.
Results
Monthly prevalence of fentanyl-associated fatalities among illicit drug users increased from an average of two in early 2005 to a peak of 24 in May, 2006. In total, 101 FHFs and 90 NFHFs were analyzed. The median age of decedents was 46 and 45 years for the fentanyl and non-fentanyl groups, respectively. Fentanyl-contaminated heroin-associated fatalities (FHFs) were more likely to be female (p = 0.003). Women aged over 44 years (OR = 4.67;95 % CI = 1.29–16.96) and divorced/widowed women (OR = 14.18;95 % CI = 1.59–127.01) were more likely to be FHFs when compared to women aged less than 44 years and single, respectively. A significant interaction occurred between gender and age, and gender and marital status. Most FHFs had central (heart) blood samples available for fentanyl testing (n = 96; 95 %): fentanyl was detected in most (n = 91; 95 %). Of these, close to half had no detectable heroin (or 6-acetylmorphine) concentrations (n = 37; 40.7 %). About half of these samples had detectable cocaine concentrations (n = 20; 54 %). Median fentanyl concentration in central blood samples was 0.02 μg/ml (n = 91, range <0.002–0.051 μg/ml) and 0.02 μg/ml (n = 32, range <0.004–0.069 μg/ml) in peripheral blood samples. The geometric mean of the ratio of central to peripheral values was 2.10 (median C/P = 1.75). At autopsy, pulmonary edema was the most frequently encountered finding for both groups (77 %).
Conclusion
Illicit drugs may contain undeclared ingredients that may increase the likelihood of fatality in users. Gender differences in fentanyl-related mortality may be modified by age and/or marital status. These findings may help inform public health and prevention activities if fatalities associated with fentanyl-contaminated illicit drugs reoccur.
Introduction
Fentanyl is a synthetic opioid analgesic that is 50 to 100 times more potent than morphine [1]. As a pharmaceutical, fentanyl is or has been available as a patch, a lozenge, lollipop, and an intravenous formulation. Fentanyl is commonly used in medicine as an analgesic and anesthetic regardless of formulation. As with other prescription opioids, fentanyl's euphoric properties are often exploited and the drug has been abused by addicts seeking opioid formulations with greater potencies and a better “high.” Unfortunately, fentanyl can be deadly if not used properly. Street drugs often have various substances added by their manufacturers to increase potency/euphoria (e.g., fentanyl added to heroin) or increase profit (e.g., starch to heroin).
The first reports of overdose and deaths from illicitly manufactured fentanyl were in the early 1980s, with most cases occurring in California. An analysis of 112 deaths during that time period identified alcohol coingestion and a probable history of opioid intolerance as potential risk factors for fatal outcomes [2]. Over the next 10 years, sporadic clusters of fentanyl-associated fatalities were encountered on the United States eastern coast. In 1988, 16 deaths were associated with the use of heroin contaminated with 3-methylfentanyl, a fentanyl analogue, in Pittsburgh, PA, USA [3]. Thirty deaths associated with illicit fentanyl use were reported in Baltimore, MD, USA in 1992 [4].
A total of 1,013 fentanyl-associated fatalities in six states were reported during April 4, 2005–March 28, 2007. Decedents were mostly male (80.1 %) and white (55.4 %) with 58.6 % between 35 and 54 years of age. Community outreach efforts were initiated by public health officials to train drug users in overdose prevention, cardiopulmonary resuscitation (CPR), and the use of take-home naloxone. Officials from the United States Drug Enforcement Administration (DEA) and other law enforcement organizations began arresting sellers of nonpharmaceutical fentanyl (NPF) intended for illicit use. This finally led to the closure of an illegal NPF production facility in Toluca, Mexico in May 2006. In April 2007, the DEA began regulating access to a chemical used to manufacture NPF, N-phenethyl-4-piperidone [5]. The number of monthly NPF-related deaths reported then dropped from a high of nearly 150 per month in June 2006 to zero in April 2007. The public health response to this epidemic illustrated how a concerted effort among clinicians, public health officials, and law enforcement authorities can effectively respond to an epidemic [5–8]. Drug users, specifically fentanyl users, are a difficult population to study and characterize because of clandestine production, illicit use, nonspecific clinical presentation, and lack of a readily available clinical laboratory test to confirm exposure. In this article, we present detailed data from a joint investigation conducted in Wayne County, MI, USA among the offices of the Wayne County Medical Examiner (Detroit, MI, USA), the Bureau of Epidemiology within the Michigan Department of Community Health (Lansing, MI, USA), and the Centers for Disease Control and Prevention (CDC). Although limited data from this investigation was included in a larger, previously published report, we present more detailed findings that were not included [5].
Between July 2005 and May 2006, the presence of fentanyl was confirmed by the Wayne County Medical Examiner's office (ME) in postmortem biological specimens from more than 100 fatalities. Prior to this time period, Wayne County averaged 15–20 fentanyl-associated fatalities annually, which were primarily associated with prescription fentanyl use (Fig. 1). The Michigan Department of Community Health invited the National Center for Environmental Health (NCEH) and the National Center for Injury Prevention and Control from the CDC to participate in an epidemiologic investigation of fentanyl-associated fatalities among illicit drug users in Wayne County. The objectives of this study were (1) to determine if an outbreak of fentanyl-associated fatalities occurred in mid-2005 to mid-2006 and (2) to trend and compare features of fentanyl-containing, heroin-associated fatalities with non-fentanyl, heroin-associated fatalities (NFHFs) among illicit drug users in order to identify fentanyl-specific features that might help guide future public health interventions.
Fig. 1.
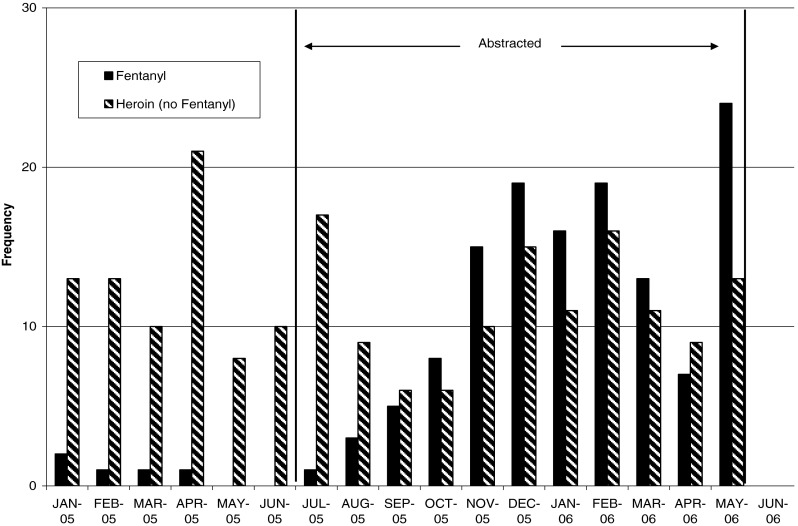
Estimated monthly prevalence of fentanyl and heroin-associated fatalities from January 2005 to May 2006 in Wayne County, MI, USA. From January 2005 to June 2005, the baseline prevalence of fentanyl and heroin-associated fatalities was estimated based on cause of death (fentanyl or heroin) data from medical examiner records. From July 2005 to May 2006, prevalence of fentanyl-associated and heroin-associated fatalities was determined by employing specific case definitions to medical examiner data. These case definitions were employed primarily to facilitate comparison of data abstracted from the medical examiners' record between the two groups with each other during the study period (July 2005–May 2006), hence the label “Abstracted” for this period. The data presented in this graph illustrates the outbreak's epidemiological curve over the entire time period that any data was collected for this outbreak. Data collected on total cases from other regions as well as during this time show a peak case count (nationally) in June 2006 and a subsequent drop that corresponded to closure of a fentanyl manufacturing plant in Mexico during May 2006. (5)
Methods
Study Site
The Wayne County ME's office conducts investigations (including autopsies) to identify the cause and manner of death in fatalities resulting from violence or in persons without recent medical attendance and in deaths under other circumstances outlined in the laws of the State of Michigan [9]. The Wayne County ME's office is responsible for all such fatalities that occur within Wayne County, which includes metropolitan Detroit. The ME's office maintains comprehensive written and electronic records on all autopsies including pathology and toxicology reports, death certificates, identification data sheets, and case investigation summaries. This investigation was exempt from review by CDC's Internal Review Board.
Data Collection: Baseline Prevalence
Medical examiner (ME) records were reviewed for the 6 months prior (January–July, 2005) to this outbreak in order to determine the overall baseline prevalence of fentanyl- and heroin-associated fatalities in Wayne County, MI, USA. Records were included for this part of the project if fentanyl or heroin was listed on the death certificate as the cause of death.
Data Collection: Study Period
ME records were reviewed for the study period (July 2005 to May 12, 2006) to identify cases meeting specific inclusion criteria for FHFs. The ME's toxicology database was initially screened for any fatalities in which fentanyl was mentioned. From there, a FHF was defined in one of the following two ways: (1) presence of fentanyl or its metabolite (norfentanyl) and detection of either heroin or cocaine or their metabolites (6-acteylmorphine or benzoylecgonine) in any postmortem biological specimen or (2) the presence of fentanyl or norfentanyl in any postmortem biological specimen and at least one of the following: (1) documentation of drug paraphernalia consistent with intravenous opioid abuse on the scene (e.g., needle in vein, full syringe, and other cases of opioid overdose at the scene), (2) absence of known history of therapeutic opioid or prescription fentanyl use, (3) cause of death listed as “multiple drug intoxication” in an individual with a history of drug abuse or no history of therapeutic opioid use, or (4) cause of death listed as “fentanyl intoxication” or “fentanyl abuse.” This second definition, thus, included fatalities in which there was no laboratory confirmation of either heroin or cocaine, but for which evidence at the scene of the death strongly suggested illicit fentanyl use. Among the fatalities abstracted were patients with fentanyl prescriptions and others whose deaths were not directly associated with illicit drugs. Since we were primarily interested in fatalities involving illicit fentanyl use, we excluded from the analysis fatalities in which (1) death occurred outside a medical setting and there was evidence of possible legal or prescription use of fentanyl (e.g., fentanyl patch), (2) there was evidence of use of fentanyl in a nonillicit manner (e.g., pain control in a medical setting), and (3) there was evidence of illicit drug use but the ME determined that the cause and manner of death was not consistent with drug toxicity (e.g., motor vehicle collision and gunshot wound).
In order to identify unique features among FHFs that might be targeted for public health interventions, we abstracted the records of 100 randomly selected deaths in which heroin or its metabolite 6-acetylmorphine (but no fentanyl or fentanyl metabolites) were detected in any postmortem biological specimen during the same study period. This number was chosen based on available resources and time allotted for the project. Inclusion criteria for NFHFs included: the presence of heroin, morphine, or 6-acetylmorphine in any postmortem biological specimen; absence of fentanyl or norfentanyl in postmortem biological specimens; and heroin abuse listed as the cause of death as determined by the ME. NFHFs were selected as the comparison group because both fentanyl and heroin are opioids and toxicity results in similar clinical manifestations (euphoria, respiratory depression, miosis, etc.). Furthermore, heroin and fentanyl are often both parenterally administered. Thus, it seems likely that the drug users who use illicit fentanyl most likely, knowingly or unknowingly, are probably heroin users, too.
Standardized data collection forms were developed and used to systematically abstract information on multiple variables including demographics, date of death, illicit drug use history, scene investigation findings, place of death, autopsy findings, toxicology data, and route of administration for both types of fatalities. All chart abstractors were trained by two of the principal investigators on use of the abstraction sheet and were provided definitions for the abstracted variables.
Toxicology
The selection of a specific matrix for laboratory testing was case-dependent and may have included blood, bile, urine, vitreous humor, liver tissue, or kidney tissue. Matrices collected or tested were based on the discretion of the ME's office and/or the availability or adequacy of biological specimens for testing. In some cases, the Wayne County ME's office tested multiple postmortem biological specimens for the presence of drugs. Blood specimens most often were obtained centrally, usually from the heart or a great vessel. Additional blood specimens were obtained peripherally from the femoral, iliac, or popliteal vein depending on accessibility.
Specimens from fatalities without any obvious cause of death are routinely analyzed for prescription medications and drugs of abuse using standardized toxicology assays. For prescription medications, blood is analyzed using gas chromatography/mass spectrometry (GC/MS), while an enzyme-linked immunosorbent assay (ELISA) is used for screening blood for drugs of abuse. ELISA results are then confirmed with GC/MS. If a drug was detected in any matrix from a case, we considered the screening test for that case to be positive. Since 1999, the Wayne County ME's office has been using GC/MS testing for fentanyl as part of its routine toxicology assay. Testing methods have remained consistent and unchanged during this time. An ELISA screen for fentanyl was introduced in May 2006 to allow for more rapid screening of biological samples; the current level of detection for fentanyl in blood is 0.002 μg/ml for ELISA and 0.005 μg/ml for the full-scan GC/MS qualitative screening method (when applied) and 0.002 μg/ml for Selected Ion Monitoring (SIM) GC/MS confirmation quantitation. Other matrices (e.g., bile and liver) are tested and used for quantification of drug concentrations when blood is unavailable or inadequate.
Statistical Analysis
Variables for the analysis were chosen based upon potential clinical significance. Demographic factors examined included age, race/ethnicity, city of residence, history of drug use, marital status, and anthropometry. Information recorded at the scene of death included location of the scene, evidence of drug paraphernalia, and the presence of others at the scene. Autopsy and laboratory findings included cause of death and contributing factors, the presence of chronic medical conditions, and concentrations of alcohol and specific drugs and their metabolites identified in toxicological screening. Characteristics of the fentanyl and non-fentanyl groups were compared using Student's t test (continuous variables) and chi-square statistic (categorical variables) or Fisher's exact test when appropriate. Based upon the bivariate analyses, logistic regression analysis was used to model associations between specific variables and fentanyl-associated fatalities and to examine gender-specific associations. Further modeling explored effect of measure modification, examining the statistical significance of interactions between gender and age and gender and marital status. All data were analyzed using SAS statistical software version 9.1 (SAS Institute Inc., Cary, NC, USA), Microsoft Office Excel 2003 (Microsoft Corp., Redmond, WA, USA), and ArcGIS version 9.1 (ERIS, Redlands, CA, USA). Median drug concentrations in a given matrix were compared. The geometric mean of central to peripheral fentanyl concentration ratios was calculated.
Results
Between January 1 and June 30, 2005, there had been five (0.83 per month) fentanyl-associated fatalities in Wayne County. Beginning July 1, 2005 through May 12, 2006, there were 121 (11.5 per month) FHFs with laboratory confirmed presence of fentanyl in Wayne County and 160 (15.2 per month) deaths with laboratory confirmed presence of the heroin metabolite 6-acetylmorphine (and no fentanyl).
We excluded from the statistical analyses 20 of the 121 FHFs and nine of the 100 randomly selected fatalities selected as part of the non-fentanyl, heroin-associated group because further review supported that the deaths were not due to illicit drugs (e.g., pain relief from trauma and opioid prescription use). An additional NFHF was excluded because of insufficient data. After these exclusions, 101 (83 %) fentanyl-containing heroin-associated fatalities and 90 NFHFs were used in the analyses.
FHFs were more likely to be female (p = 0.003) (Table 1). Among this group, 57 % were white and 41 % were black: proportions were similar among the NFHFs. The median ages of the fentanyl group and the non-fentanyl group were 46 (range, 18–60 years) and 45 (range, 17 to 64 years), respectively. Female FHFs were significantly older (median age, 47; range, 18–58 years) than female NFHFs (median age, 32; range, 18–59 years) at death, but no significant difference in age was noted between the two groups among males. Half of all fatalities (53 %) were in their own home at the time of death. Overall, only 11 % had evidence of needle tracks (i.e., scarring from chronic intravenous drug use), suggesting the possibility of inhalation or insufflation as a route of exposure or that the intravenous route was used infrequently by the decedent.
Table 1.
Selected characteristics of fentanyl-contaminated heroin-associated and non-fentanyl, heroin-associated fatalities
| Fentanyla (n = 101) | Non-fentanylb (n = 90) | p c | |
|---|---|---|---|
| n (%) | n (%) | ||
| Gender | 0.003 | ||
| Female | 39 (38.6) | 17 (18.9) | |
| Male | 62 (61.4) | 73 (81.1) | |
| Race/ethnicity | 0.88 | ||
| White | 58 (57.4) | 50 (55.6) | |
| African American | 41 (40.6) | 37 (41.1) | |
| Hispanic/Latino | 1 (1.0) | 1 (1.1) | |
| Missing | 1 (1.0) | 2 (2.2) | |
| Age | 0.61 | ||
| 1–19 | 3 (2.9) | 2 (2.2) | |
| 20–29 | 15 (14.9) | 15 (16.7) | |
| 30–39 | 15 (14.9) | 16 (17.8) | |
| 40–49 | 42 (41.6) | 32 (35.6) | |
| 50–59 | 24 (23.8) | 22 (24.4) | |
| 60 + | 2 (2.0) | 3 (3.3) | |
| Marital status | 0.04 | ||
| Single | 44 (43.6) | 49 (54.4) | |
| Married | 14 (13.9) | 13 (14.4) | |
| Divorced/widowed | 24 (23.8) | 9 (10.0) | |
| Missing | 19 (18.8) | 19 (21.1) | |
| History of drug use | |||
| Yes | 71 (70.3) | 59 (65.6) | |
| Unknown | 30 (29.7) | 31 (34.4) | |
| Place of death | 0.26 | ||
| Home | 56 (55.5) | 45 (50.0) | |
| Public place | 1 (1.0) | 7 (7.8) | |
| Emergency department | 20 (19.8) | 15 (16.7) | |
| Hospital (not ED) | 1 (1.0) | 1 (1.1) | |
| Car | 5 (5.0) | 5 (5.6) | |
| Other | 16 (15.8) | 17 (18.9) | |
| Missing | 2 (2.0) | – | |
| Scene characteristics | |||
| Drug paraphernalia | 33 (32.7) | 26 (28.9) | |
| Needle in vein | 3 (3.0) | – | |
| Full syringe injected | 2 (2.0) | 1 (1.1) | |
| Others overdosed in the same incident | 5 (5.0) | – | |
| Evidence of shared drugs | 1 (1.0) | 1 (1.1) | |
| Evidence of naloxone | – | – | |
| Nonprofessional resuscitation attempted | 1 (1.0) | – | |
| Died alone (not in hospital) | 53 (52.5) | 46 (51.1) | |
| Trauma | 6 (5.9) | 3 (3.3) | |
| Evidence of tracks | 12 (11.9) | 9 (10.0) |
aFentanyl found on at least one toxicology screen and listed as cause of death
bHeroin, but not fentanyl, found on at least one toxicology screen
c p value for Chi-square (race, gender, marital status, and residence), Fisher's exact test (place of death), or nonparametric Wilcoxon test comparing medians (age). Because of the missing data for death scene and drug history variables, statistical comparisons were not calculated
FHFs were more likely to be women when compared to the non-fentanyl group (Odds Ratio (OR) = 2.70, 95 % Confidence Interval (CI) = 1.39–5.24). The results of bivariate logistic regression models examining factors associated with the fentanyl group when compared to the non-fentanyl group are presented in Table 2. The median age for all women was 44 years. The odds of being a FHF for women over 44 were four times greater than the odds of women less than 44 (OR = 4.67; 95 % CI = 1.29–16.96). The odds of being a FHF among divorced/widowed women were 14 times greater than the odds among single women (95 % CI = 1.59–127). Among women, there was no clear trend in association between body mass and fentanyl group status. However, among men, each higher quartile body mass index (BMI) was more negatively associated with being in the fentanyl group. Men in the highest quartile of BMI were 70 % less likely to be a FHF than those in the lowest quartile (OR = 0.31; 95 % CI = 0.12–0.84).
Table 2.
Associations between selected variables in bivariate models comparing fentanyl-contaminated heroin-associated fatalities and non-fentanyl, heroin-associated fatalities
| All | Men | Women | |
|---|---|---|---|
| OR (CI) | OR (CI) | OR (CI) | |
| Gender | |||
| Male | 1.0 | ||
| Female | 2.70 (1.39–5.24) | ||
| Age | |||
| ≤Medianb (ref) | 1.0 | 1.0 | 1.0 |
| >Median | 1.51 (0.85–2.68) | 1.02 (0.52–2.00) | 4.67 (1.29–16.96) |
| Race/ethnicity | |||
| Non-white/Hispanic (ref) | 1.0 | 1.0 | 1.0 |
| White | 1.08 (0.61–1.91) | 1.14 (0.58–2.26) | 0.91 (0.29–2.88) |
| Marital status | |||
| Single (ref) | 1.0 | 1.0 | 1.0 |
| Married | 1.20 (0.51–2.83) | 0.55 (0.18–1.62) | 8.27 (0.88–78.01) |
| Divorced/widowed | 2.97 (1.25–7.07) | 1.64 (0.60–4.50) | 14.18 (1.59–127.01) |
| BMIb | |||
| 1st quartile (ref) | 1.0 | 1.0 | 1.0 |
| 2nd quartile | 1.04 (0.46–2.34) | 0.61 (0.24–1.55) | 1.02 (0.21–4.98) |
| 3rd quartile | 0.50 (0.23–1.12) | 0.53 (0.20–1.38) | 0.82 (0.18–3.74) |
| 4th quartile | 0.52 (0.23–1.16) | 0.31 (0.12–0.84) | 1.51 (0.29–8.03) |
OR odds ratio, CI confidence interval
aThe median ages are: overall 45, men 45, women 44
bQuartile cut points for BMI: all 22.6, 25.6, 30.0; men 22.9, 26.7, 30.1; women 20.9, 24.4, 28.7
Overall, FHFs were one-half as likely to have been found in the city of Detroit than in Wayne County outside the city limits (OR = 0.55; 95 % CI = 0.31–0.98). Interestingly, males in the fentanyl group were 62 % less likely than males in the non-fentanyl group to be found within the city limits (OR 0.38, 95 % CI = 0.19–0.76), while females in the fentanyl group were 46 % more likely to be found in the city (OR 1.46, 95 % CI = 0.46–4.57).
In a multivariable (gender, age, marital status, and the interaction terms: gender × age, gender × marital status) logistic model comparing FHFs to NFHFs, interaction terms remained statistically significant (Table 3). Detection of multiple drugs of abuse was common in both groups (Table 4). Although fentanyl detection in any biologic medium met the laboratory criterion for being a case in this study, most subjects in the fentanyl group had central (heart) blood samples available for fentanyl testing (n = 96; 95 %) and fentanyl was detected in most (n = 91; 95 %). Of these, close to half had no detectable heroin (or heroin metabolite) concentrations (n = 37; 40.7 %) and more than half of these samples had detectable cocaine concentrations (n = 20; 54 %) (data not shown). Among those fatalities with quantitative results, drug concentrations were not statistically different among the two groups (data not shown). The median fentanyl concentration in central blood samples was 0.02 μg/ml (n = 100, range = 0.002–0.051 μg/ml). In peripheral blood samples, the median fentanyl concentration was 0.02 μg/ml (n = 32, range = 0.004–0.069 μg/ml). Twenty-one of the FHFs had both central and peripheral blood fentanyl concentrations recorded. The geometric mean of the ratio of central to peripheral values was 2.10 (median C/P = 1.75).
Table 3.
Associations between selected variables in a multivariable model comparing fentanyl-contaminated heroin-associated fatalities and non-fentanyl, heroin-associated fatalities
| OR (CI) | p value | |
|---|---|---|
| Gender | 0.52 (0.17–1.62) | 0.26 |
| Age (≤45= ref) | 0.94 (0.42–2.08) | 0.87 |
| Gender × age | 7.51 (1.08–52.46) | 0.04 |
| Marital status | 0.92 | |
| Married | 0.55 (0.18–1.63) | |
| Divorced/widowed | 1.67 (0.59–4.78) | |
| Gender × marital status | 0.02 | |
| 11.49 (0.86–153.83) | ||
| 7.50 (0.61–91.55) |
OR odds ratio, CI confidence interval
Table 4.
Frequency of positive drug tests in body fluids among deceased fentanyl-containing heroin-associated and non-fentanyl, heroin-associated fatalities
| Case group | Central blood | Peripheral blood | Bile | Urine | Vitreous | |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Alcohol screen | ||||||
| Fentanyl | 21/100 (21) | 0/33 (0) | 0/24 (0) | 21/76 (28) | 18/52 (35) | |
| Non-fent | 26/86 (30) | 1/7 (14) | 1/21 (5) | 24/73 (33) | 13/46 (14) | |
| Benzodiazepine screen | ||||||
| Fentanyl | 32/100 (32) | 1/33 (3) | 10/24 (42) | 13/76 (17) | 0/52 (0) | |
| Non-fent | 19/86 (22) | 2/7 (29) | 6/21 (29) | 8/73 (11) | 0/46 (0) | |
| Cocaine screen | ||||||
| Cocaine | Fentanyl | 37/49 (76) | 1/2 (50) | 9/9 (100) | 38/42 (90) | – |
| Non-fent | 25/34 (74) | 1/1 (100) | 7/7 (00) | 32/36 (89) | – | |
| Benzoylecognine | Fentanyl | 48/49 (98) | 2/2 (100) | 0/9 (0) | 1/42 (2) | – |
| Non-fent | 34/34 (100) | 0/1 (0) | 0/7 (0) | 3/36 (8) | – | |
| Ethylcocaine | Fentanyl | 6/49 (12) | 1/2 (50) | 5/9 (56) | 20/42 (48) | – |
| Non-fent | 6/34 (18) | 0/1 (0) | 1/7 (14) | 13/36 (36) | – | |
| Opioid screen | ||||||
| Heroin | Fentanyl | 0/96 (0) | 0/33 (0) | 0/23 (0) | 0/73 (0) | 1/34 (3) |
| Non-fent | 0/80 (0) | 5/7 (71) | 0/2 (0) | 1/60 (2) | 0/36 (0) | |
| Morphine | Fentanyl | 58/96 (60) | 1/33 (3) | 0/23 (0) | 2/73 (3) | 26/34 (76) |
| Non-fent | 80/80 (100) | 5/7 (71) | 1/17 (6) | 4/60 (7) | 36/36 (100) | |
| 6-Acetyl morphine | Fentanyl | 17/96 (18) | 0/33 (0) | 0/23 (0) | 35/73 (48) | 31/34 (91) |
| Non-fent | 47/80 (59) | 3/7 (43) | 7/17 (41) | 55/60 (92) | 34/36 (94) | |
| Fentanyl | Fentanyl | 91/96 (95) | 29/33 (88) | 23/23 (100) | 71/73 (99) | 1/34 (3) |
| Non-fent | 0/80 (0) | 0/7 (0) | 0/17 (0) | 0/60 (0) | 0/36 (0) | |
| Norfentanyl | Fentanyl | 5/96 (5) | 0/33 (0) | 3/23 (13) | 43/73 (59) | 0/34 (0) |
| Non-fent | 0/80 (0) | 0/7 (0) | 0/17 (0) | 0/60 (0) | 0/36 (0) | |
| Methadone | Fentanyl | 9/96 (9) | 3/33 (9) | 4/24 (17) | 6/68 (9) | 0/33 (0) |
| Non-fent | 6/80 (8) | 0/7 (0) | 2/20 (10) | 9/70 (13) | 0/32 (0) | |
| Codeine | Fentanyl | 18/96 (19) | 2/33 (6) | 1/23 (4) | 26/73 (36) | 9/34 (26) |
| Non-fent | 54/80 (68) | 5/7 (71) | 17/17 (100) | 47/60 (78) | 21/36 (58) | |
| Hydrocodone | Fentanyl | 9/96 (9) | 1/33 (3) | 2/23 (8) | 20/73 (27) | 7/34 (21) |
| Non-fent | 8/80 (10) | 2/7 (29) | 0/17 (0) | 9/60 (15) | 5/36 (14) | |
| Oxycodone | Fentanyl | 2/96 (2) | 0/33 (0) | 0/24 (0) | 2/72 (3) | 0/33 (0) |
| Non-fent | 2/80 (3) | 1/7 (14) | 0/20 (0) | 2/68 (3) | 1/34 (3) | |
n Number positive/total number tested (percentages)
With regard to autopsy findings, pulmonary edema was the most frequently encountered finding and was present in 77 % of both groups (data not shown). Coronary artery disease was present in 17 (17 %) FHFs and 21 (23 %) NFHFs. No statistically significant differences in type or number of pathologic findings at autopsy were noted between the two groups.
Discussion
Epidemics resulting from use of illicit fentanyl and fentanyl analogues have occurred sporadically in the United States in the past 25 years [2–5, 10]. The deaths in this report contributed to one of the largest, recognized multistate outbreaks of fentanyl-associated fatalities reported in the United States to date. The number of cases associated with this multistate outbreak peaked in June 2006, at which point case volume began to fall slowly to zero by April, 2007. This drop corresponded with the closure of a fentanyl-manufacturing plant in Mexico (May 2006) [5]. The results from our investigation demonstrate that the Detroit area encountered a resurgence of illicit fentanyl use during 2005–2006 during this larger, multistate outbreak. Our investigation identified several findings with potential public health implications.
A high proportion of FHFs in this report were female. Nearly 40 % were women compared to previous outbreak reports in which women accounted for 0–22 % of the victims [2–4, 10, 11]. The reasons for the increased prevalence of women in the fentanyl contaminated heroin-associated fatality group is unclear. There is evidence of a gender difference in the association between body mass and fentanyl status. Although the highest quartile of BMI was associated with fentanyl use among female fatalities and the opposite effect was seen among male fatalities in our study, we do not have data regarding the dose of fentanyl used and, therefore, were not able to determine if a dose–response effect existed. Emerging evidence demonstrates that there are sex-related differences in opioid response that could result in women having a more pronounced clinical effect and greater risk of toxicity [12]. There could be other, as of yet unidentified, pharmacokinetic or pharmacodynamic interactions resulting in women being more susceptible to fentanyl. Finally, the prevalence of fentanyl and fentanyl-contaminated product use may simply be increasing in women compared to men for unclear reasons. Further complicating interpretation of the effects of gender among FHFs is evidence of effect modification by age and marital status. These interactions may be of interest in future studies.
A substantial number of victims in our study had laboratory evidence of recent cocaine (but not heroin) and fentanyl use, which suggests that cocaine users may have been using a product adulterated with fentanyl, although the possibility that both individual agents were used separately and simultaneously cannot be definitively excluded. These findings suggest that persons using street drugs other than heroin may be at risk for fentanyl-associated adverse events as well, although more work is needed on this issue. Furthermore, non-opioid users of illicit drugs may be at greater risk for fentanyl-associated toxicity when compared to opioid users, since they are opioid-naïve.
Our results highlighted physical evidence of injection (as a route of exposure) on physical examination in a relatively small number of fatalities. It is often assumed by heroin drug users that the risk of death is significantly decreased with noninjection routes of administration [13]. However, given fentanyl's potency, all routes of administration place the user at risk of an overdose and an adverse event.
There are a number of ways to possibly decrease mortality from these agents in those who refuse to stop usage. Opioid poisoning intervention programs, such as provision of naloxone (an opioid receptor antagonist and antidote for poisoning), aimed at recognized heroin users have been successful in preventing fatal heroin overdoses and would be expected to have similar results with fentanyl overdoses regardless of route of administration and concomitant drug exposure [14, 15]. Many heroin users have reported seeing another individual overdose [16–18]. Thus, it appears that there are often individuals present that could intervene and summon help. However, bystander response rates in overdose situations are uniformly poor [18–20]. Fear of police involvement is commonly cited as a reason for not activating the emergency response system. Educational efforts should focus on encouraging bystanders to immediately activate the emergency response system in cases of drug overdose. Further research could address potential interventions to improve emergency medical service (EMS) activation such as assuring bystanders immunity from arrest or prosecution [16–18].
Fentanyl blood concentrations measured in this outbreak were similar, if not slightly higher than, blood concentrations from previous outbreaks of fentanyl-associated fatalities (mean = 0.0083 μg/ml; range, 0.003–0.028 μg/ml) for both central and peripheral blood testing results [21]. Many drugs undergo postmortem redistribution from tissues into the blood, which can complicate interpretation of postmortem blood concentrations. Some studies have found that fentanyl may undergo postmortem redistribution (defined by an average central blood fentanyl concentration/peripheral blood fentanyl concentration >1) [21]. We found a geometric mean-based central/peripheral blood fentanyl concentration ratio of 2.10 that is consistent with other studies [21]. Another possible explanation for the elevated central/peripheral fentanyl concentration ratio is a lack of distribution. This may have occured if deaths occured rapidly after exposure.
The reason for fentanyl adulteration of illicit street drugs is unclear but might occur for various reasons. The production cost for illicit fentanyl might be less than that of heroin. If so, mixing fentanyl with other drugs might be more profitable for the drug manufacturers. The costs of transporting fentanyl into the US (e.g., per kilogram) may be less than heroin given its greater potency. Therefore, distributors may have had access to greater amounts of fentanyl for a period of time and as a result were substituting it, in part, for some of their product (e.g., heroin). Alternatively, fentanyl could be introduced into street drugs to make a more potent product. Drug users reportedly seek out dealers who sell drugs of high purity; thus, drug dealers may exploit the overdoses as an indicator of purity and potentially increase the demand for their product. In a case series out of Bethlehem, PA, USA from 2006, nine out of 30 overdose patients stated they had knowingly used “blue bag” heroin (a street name for fentanyl-laced heroin), probably hoping to obtain a greater euphoria [22]. An ethnographer interviewing drug abusers in Patterson, NJ, USA during the fentanyl-heroin epidemic of 2006 found that increased media coverage about the increased risk of overdose “only confirmed this subculture's belief in the purity of heroin.” [23] Thus, publicity and education regarding the dangers of fentanyl-tainted heroin may, counterintuitively, only serve to highlight and encourage a specific product for use.
Early detection of outbreaks, regardless of cause, is essential to protecting the public health. Unfortunately, fentanyl detection requires specific testing and is not readily detected on most routine opioid drug screens used in clinical settings [24–26]. Thus, if a lab is not testing for fentanyl, its presence will go undetected. This undoubtedly results in underrecognition of fentanyl poisoning and death. Development of guidelines to help standardize and improve availability of forensic toxicology testing with regard to fentanyl may improve the capacity of state public health departments to study fentanyl-associated fatalities further. Ongoing surveillance is also a critical aspect of outbreak detection. Health care professionals are encouraged to report all exposures and drug overdoses to their regional poison center. When suspicious clusters of deaths due to drug overdose are identified through an ME's office, reporting to appropriate public health authorities and poison centers should be encouraged. This may help in the detection of a larger, more widespread problem by surveillance systems such as the Drug Abuse Warning Network or the National Poison Data System [27, 28].
Limitations
This investigation had several limitations. Retrospective chart reviews rely upon what data are available and reported. The past medical history and scene information in the ME's records was often limited, and emergency medical services and hospital medical records were not accessible. The possibility that misclassification of fentanyl status might have affected our results also must be acknowledged. Caution should be taken when interpreting our geographic findings, as persons may die in places other than where they live or where they use their drug. The baseline prevalence analyses used the recorded cause of death as the sole determining factor for being a fentanyl or heroin-associated death, whereas stricter criteria were used for the study period. This may have resulted in a slight underestimation of the baseline prevalence. The choice to use heroin-associated deaths as a comparison group may mask certain opioid-specific characteristics that could not be studied. Further work in this issue should consider the use of a comparison group that uses illicit drugs other than opioids. It is possible that non-fentanyl, heroin users are different from fentanyl-containing heroin users and that they may not be a good control group. Data collection and subject enrollment for both the baseline prevalence and study period were limited by available time, personnel, and resources of personnel deployed to the field for this investigation. Our baseline prevalence determination was relatively short (6 months). This may have complicated interpretation of our results as longer baseline prevalence would have provided better data and revealed potential biases (e.g., seasonal variability). Some point estimates had corresponding wide CIs reflecting data variability that complicates interpretation of significant point estimates. This may be due to a number of different reasons such as sample size or difficulty in evaluating certain variables posthumously. Fentanyl concentrations are difficult to interpret given they were not obtained from uniform sites. Furthermore, normal postmortem physiologic processes could affect accurate quantitation of fentanyl in biological samples. We did not have information on interval between death and biological sampling, which can influence postmortem measurements; this may have affected our C/P ratio calculations. Formal interindividual analysis was not performed to assess the accuracy of chart abstraction.
Conclusion
The FHFs reviewed during this investigation in Michigan were a part of the largest, multistate outbreak reported in the United States to date. Illicit drugs may contain undeclared ingredients for different reasons; the risk of fatality or other adverse health effect from such ingredients will depend on type and dose. Fentanyl exposure may occur through routes other than intravenous injection and users are likely using other illicit drugs. This study found that gender differences in fentanyl-related mortality may be modified by age and/or marital status. Although not surprising, this is the first report of such a finding with regard to fentanyl. Outbreaks of illicit drug-related illness may differ from past outbreaks with regard to epidemiological characteristics that, in turn, if identified, may help target national or region-specific interventions, inform public health response efforts, and raise awareness among drug users and health care providers. If a resurgence of illicit fentanyl-associated illness occurs, the findings of this study may help to inform public health response activities. These efforts may allow for the development of local and national prevention strategies that, ideally, can decrease morbidity and mortality from fentanyl overdoses resulting in fatality.
Disclamer
The findings and conclusions in this presentation are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC).
Contributor Information
D. Adam Algren, Email: Douglas.Algren@tmcmed.org.
Carolyn P. Monteilh, Email: cmonteilh@bellsouth.net
Mohan Punja, Email: mpunja@cdc.gov.
Joshua G. Schier, Email: jschier@cdc.gov
Martin Belson, Email: mbelson1260@comcast.net.
Bradford R. Hepler, Email: bhep2001@sbcglobal.net
Carl J. Schmidt, Email: cjschmidt@earthlink.net
Corinne E. Miller, Email: MillerC39@michigan.gov
Manish Patel, Email: aul3@cdc.gov.
Leonard J. Paulozzi, Email: lbp4@cdc.gov
Masja Straetemans, Email: M.Straetemans@kit.nl.
Carol Rubin, Email: chr1@cdc.gov.
References
- 1.Poklis A. Fentanyl: a review for clinical and analytical toxicologists. J Toxicol Clin Toxicol. 1995;33:439–447. doi: 10.3109/15563659509013752. [DOI] [PubMed] [Google Scholar]
- 2.Henderson GL. Fentanyl-related deaths: demographics, circumstances, and toxicology of 112 cases. J Forensic Sci. 1991;36:422–433. [PubMed] [Google Scholar]
- 3.Hibbs J, Perper J, Winek CL. An outbreak of designer drug-related deaths in Pennsylvania. JAMA. 1991;265:1011–1013. doi: 10.1001/jama.1991.03460080081037. [DOI] [PubMed] [Google Scholar]
- 4.Smialek JE, Levine B, Chin L, Wu SC, Jenkins AJ. A fentanyl epidemic in Maryland 1992. J Forensic Sci. 1994;39:159–164. [PubMed] [Google Scholar]
- 5.MMWR Nonpharmaceutical fentanyl-related deaths—multiple states, April 2005-March 2007. MMWR. 2008;57(29):793–6. [PubMed] [Google Scholar]
- 6.Boddiger D. Fentanyl-laced street drugs “kill hundreds”. Lancet. 2006;368:569–570. doi: 10.1016/S0140-6736(06)69181-2. [DOI] [PubMed] [Google Scholar]
- 7.A look at a drug cocktail problem around the country. (2006, May 27). The Associated Press News Service
- 8.Karush S (2006) Bad heroin sparks a series of overdoses. The Associated Press News Service
- 9.County Medical Examiners (Excerpt) Act of 1953. Michigan legislature. Available at: http://legislature.mi.gov/doc.aspx?mcl-52-202. Accessed on 9/29/2011.
- 10.Martin M, Hecker J, Clark R, et al. China white epidemic: an eastern United States emergency department experience. Ann Emerg Med. 1991;20:158–164. doi: 10.1016/S0196-0644(05)81216-8. [DOI] [PubMed] [Google Scholar]
- 11.Kronstrand R, Henrik D, Holmgren P, Rajs J. A cluster of fentanyl-related deaths among drug addicts in Sweden. Forensic Sci Int. 1997;88:185–195. doi: 10.1016/S0379-0738(97)00068-6. [DOI] [PubMed] [Google Scholar]
- 12.Fillingim RB, Gear RW. Sex differences in opioid analgesia: clinical and experimental findings. Eur J Pain. 2004;8:413–425. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Darke S, Hall W. Heroin overdose: research and evidence-based intervention. J Urban Health. 2003;80:189–200. doi: 10.1093/jurban/jtg022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baca CT, Grant KJ. Take-home naloxone to reduce heroin death. Addiction. 2005;100:1823–1831. doi: 10.1111/j.1360-0443.2005.01259.x. [DOI] [PubMed] [Google Scholar]
- 15.Sporer KA, Kral AH. Prescription naloxone: a novel approach to heroin overdose prevention. Ann Emerg Med. 2007;49:172–177. doi: 10.1016/j.annemergmed.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 16.McGregor C, Darke S, Christie P, Ali R. Experience of non-fatal overdose among heroin users in Adelaide: circumstances and risk perception. Addiction. 1998;93:701–711. doi: 10.1046/j.1360-0443.1998.9357016.x. [DOI] [PubMed] [Google Scholar]
- 17.Strang J, Best D, Man L, Noble A, Gossop M. Peer-initiated overdose resuscitation: fellow drug users could be mobilised to initiate resuscitation. Int J Drug Policy. 2000;11:437–445. doi: 10.1016/S0955-3959(00)00070-0. [DOI] [PubMed] [Google Scholar]
- 18.Davidson PJ, McLean RL, Kral AH, Gleghorn AA, Edlin BR, Moss AR. Fatal heroin-related overdose in San Francisco, 1997–2000: a case for target intervention. J Urban Health. 2003;80:261–273. doi: 10.1093/jurban/jtg029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darke S, Ross J, Zador D, Sunjic S. Heroin-related deaths in New South Wales, Australia, 1992–1996. Drug Alcohol Depend. 2000;60:141–150. doi: 10.1016/S0376-8716(99)00147-7. [DOI] [PubMed] [Google Scholar]
- 20.McGregor C, Ali R, Lokan R, Christie P, Darke S. Accidental fatalities among heroin users in South Australia, 1994–1997: toxicological findings and circumstances of death. Addict Res. 2002;10:335–346. doi: 10.1080/1606635021000010261. [DOI] [Google Scholar]
- 21.Baselt RC. Disposition of toxic drugs and chemicals in man: fentanyl. Seal Beach: Biomedical Publications; 2011. pp. 675–8. [Google Scholar]
- 22.Turock MK, Watts DJ, Mude H, Prestosh J, Stoltzfus J. Fentanyl-laced heroin: a report from an unexpected place. Am J Emerg Med. 2009;27(2):237–239. doi: 10.1016/j.ajem.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 23.Fernando D. Fentanyl-laced heroin. JAMA. 1991;265(22):2962. doi: 10.1001/jama.1991.03460220050029. [DOI] [PubMed] [Google Scholar]
- 24.Henderson GL, Harkey MR, Jones AD. Rapid screening of fentanyl (China white) powder samples by solid-phase radioimmunoassay. J Anal Toxicol. 1990;14:172–175. doi: 10.1093/jat/14.3.172. [DOI] [PubMed] [Google Scholar]
- 25.Silverstein JH, Rieders MF, McMullin M, Schulman S, Zahl K. An analysis of the duration of fentanyl and its metabolites in urine and saliva. Anesth Analg. 1993;76:618–621. doi: 10.1213/00000539-199303000-00030. [DOI] [PubMed] [Google Scholar]
- 26.Heit HA, Gourlay DL. Urine drug testing in pain medicine. J Pain Symptom Manage. 2004;27:260–267. doi: 10.1016/j.jpainsymman.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Drug Abuse Warning Network. Available at: http://dawninfo.samhsa.gov. Accessed on 9/29/2011.
- 28.Wolkin AF, Martin CA, Law RK, Schier JG, Bronstein AC. Using poison center data for national public health surveillance for chemical and poison exposure and associated illness. Ann Emerg Med. 2011 Sep 19. [Epub ahead of print] [DOI] [PubMed]


