Abstract
Introduction
In the treatment of acetaminophen toxicity, clinicians believe that N-acetylcysteine (NAC) artificially elevates prothrombin time (PT). However, the effect of NAC on human blood coagulation remains unverified. In a previous study, we show that NAC had a dose-dependent effect on PT. To our knowledge, there are no studies that specifically examine the mechanism by which NAC affects PT. This study evaluates the effect from a therapeutic NAC dose on the activity of coagulation factors II, VII, IX, and X in human plasma.
Method
We obtained blood samples from ten volunteer subjects. After centrifugation of each volunteer's blood sample, the plasma was pipetted and divided into two 1-mL aliquots. We used the first-1 mL sample as a control. The second 1-mL plasma sample had 5 μL of 20 % NAC, added to make a final concentration of 1,000 mg of NAC per L of plasma. This concentration of NAC approximates the plasma levels achieved after a 150-mg/kg dose. We incubated the two samples for each subject (control and 1,000 mg/L) at 37°C for 1 h and measured the activity of coagulation factors II, VII, IX, and X. We compared factor activity using the paired student t test.
Results
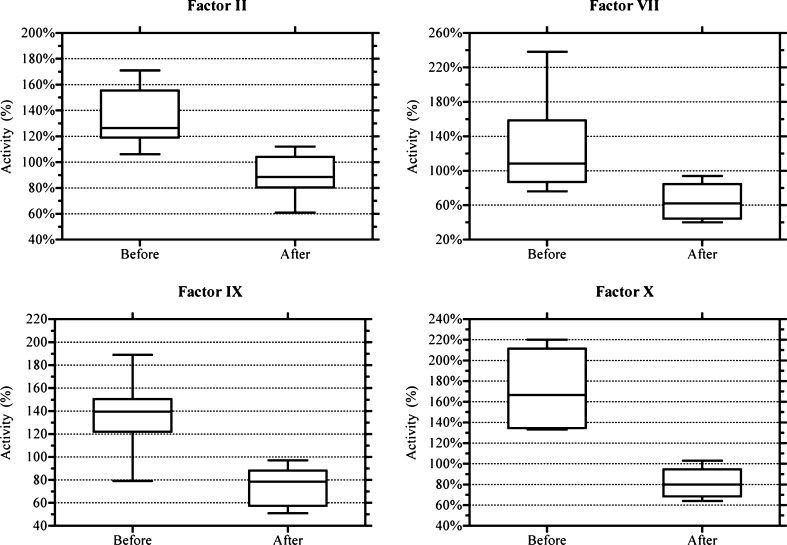
Participants included ten healthy subjects; six males, four females, median age 31 years. Mean values of the control samples for factors II, VII, IX, and X were 134 (CI 119–149), 126 (CI 90–163), 137 (CI 117–157), and 170 (CI 144–196) %, respectively. Mean values of the NAC-containing samples for factors II, VII, IX, and X were 90 (CI 79–100), 66 (CI 51–80), 74 (CI 63–85), and 81 (CI 71–90) %, respectively. All samples containing NAC had significantly lower coagulation factor activity level than their controls with a p < 0.001.
Discussion
In a previous study, we were able to demonstrate that NAC had a dose-dependent effect on PT. In this study, we compared activity of factors II, VII, IX, and X at baseline and for samples that received NAC. All factor activity had a significant decrease with the addition of NAC. This fall in factor activity is not explained by the dilution of adding NAC to the test samples.
Conclusion
We are able to demonstrate a significant decrease in the activity of coagulation factors II, VII, IX, and X with the addition of NAC. This may be the mechanism by which PT increased in our previous study.
Keywords: Acetadote, Coagulation, NAC
Introduction
N-acetylcysteine (NAC) is most commonly used for the treatment of acetaminophen-related toxicity [1]. NAC is also used for a variety of other indications such as the prevention of contrast-induced nephropathy and treatment of certain hepatotoxins such as carbon tetrachloride and pennyroyal oil [2, 3].
An observed effect with the use of intravenous NAC may involve interactions with the coagulation cascade. Minor prolongations in prothrombin time (PT) have been reported with acute acetaminophen overdoses in the absence of any hepatotoxicity. This mechanism of action has been linked to the interaction of N-acetyl-p-benzoquinoneimine (NAPQI) with coagulation factors in the extrinsic pathway [4, 5]. However, there has been evidence to suggest the use of intravenous NAC itself may be responsible for the prolongation of PT [6, 7].
In the setting of an acetaminophen overdose, accurate PT measurements are clinically important in many ways. PT is a marker of the severity of liver injury and is often measured serially when evaluating a patient in fulminant hepatic failure [8]. PT is also one prognostic marker used to ascertain need for liver transplantion [9, 10]. Therefore, it is important to have an accurate measurement of the PT in the setting of an acetaminophen overdose. In a previous study, we show that NAC had a dose-dependent effect on PT [11].
This study evaluates the effect from a therapeutic NAC dose on the activity of coagulation factors II, VII, IX, and X in human plasma.
Methods
This study was an in vitro experiment using plasma samples from healthy human subjects. The study was approved by the Institutional Review Board of the University of Pittsburgh. Furthermore, this is not an industry-sponsored study.
Study Setting and Population
This study was conducted at a large, tertiary, academic emergency department. We recruited subjects from the emergency medicine residency as well as hospital staff. Exclusion criteria included patients who were less than 18 years of age, pregnancy, any known history of coagulopathy, and use of any medications that may potentially affect coagulation, such as warfarin. Patients were enrolled between March 1, 2009 and May 1, 2009. Written informed consent was obtained from all participants. We recruited ten subjects.
In Vitro Study Protocol
Venous samples were obtained using a vacutainer system in tubes with 0.129 M sodium citrate used as an anticoagulant. The ratio of blood to anticoagulant was no less than 10:1. Minimal stasis was applied to volunteer subjects with a tourniquet which was immediately released once the venous blood sample entered the first collection tube. Only a clean adequate venous sample was accepted and repeated on the other arm if an inadequate venous sample was obtained. Plasma was obtained from each venous blood sample by centrifugation at 2,000× g and 21°C for 30 min. Immediately following centrifugation, we removed red cells by carefully pipetting the layer off to avoid the platelet layer. The plasma was immediately frozen at −20°C for later analysis. Testing on stored samples occurred within a 2-week time period.
In the treatment of acetaminophen toxicity, NAC is typically administered with an initial bolus (150 mg/kg) administered over 15 min–1 h, followed by 50 mg/kg given over 4 h, and finally, 100 mg/kg given over 16 h [1]. Based upon kinetic studies by Prescott and Selden, we simulated serum NAC concentrations achieved after the initial bolus: 1,000 mg/L [12, 13]. We then thawed and divided each subject's plasma sample into two 1-mL aliquots. The first aliquot served as a control with no NAC added. To the second aliquot, we added 5 μL of NAC (as Acetadote™, [Cumberland Pharmaceuticals, Nashville, TN]) concentrations (20 %). This produced 1,000 mg of NAC per L of plasma in our second aliquot. We maintained the pH of each samples without need for buffering. We maintained constant volume with a maximum dilution of 0.5 %.
We incubated the two samples for each subject (control and 1,000 mg/L) at 37°C for 1 h and measured the activity of coagulation factors II, VII, IX, and X. The freezing and thawing of the plasma and the subsequent incubation of NAC-containing plasma does not adversely affect the laboratory results but simulates the physiologic administration of NAC to humans [14]. This was repeated for each subject's plasma sample.
Since intravenous NAC contains 0.5 mg/mL of disodium edetate (EDTA) as a diluent, we already previously evaluated the effect of EDTA on PT. We found there was no change in PT due to the diluent so we did not expect EDTA to have any effect on specific coagulation activity. Exact information on the assays used for this study can be found on http://www.usa.siemens.com.
Statistical Analysis
We designed the study to have 80 % power to detect a 10 % difference in coagulation activity from baseline. We set alpha at 0.05 and assumed the standard deviation of the difference to be 0.33 in the context of a two-sided, paired t test. We predetermined ten subjects were necessary to achieve the desired statistical power.
We compared the activity of coagulation factors II, VII, IX, and X in human plasma before and after administration of a therapeutic NAC dose. The activity of each factor was normally distributed and fit all assumptions necessary for parametric tests. We carried out paired t tests to evaluate the magnitude and significance of change in concentration with NAC administration. All analyses were completed using two-sided hypotheses, assuming a type I error rate of 0.05.
We report the mean and corresponding 95 % confidence intervals for the factors' activity before and after NAC administration. We conducted all analyses using Stata v.11.2 (Stata, Inc., College Station, Texas) [15, 16].
Results
Participants included ten healthy subjects; six males, four females, median age 31 years. Mean values of the control samples for factors II, VII, IX, and X were 134 (CI 119–149), 126 (CI 90–163), 137 (CI 117–157), and 170 (CI 144–196) %, respectively. Mean values of the NAC-containing samples for factors II, VII, IX, and X were 90 (CI 79–100), 66 (CI 51–80), 74 (CI 63–85), 81 (CI 71–90) %, respectively. All samples containing NAC had significantly lower coagulation factor activity level than their controls with a p < 0.001.
Discussion
In a previous study, we were able to demonstrate that NAC had a dose-dependent effect on PT [11]. In this study, we compared activity level of factors II, VII, IX, and X at baseline and samples that received NAC. The activity of all coagulation factors tested had a significant decrease with the addition of NAC. This fall in factor activity is not explained by the dilution of adding NAC to the test samples.
An elevated PT is often an indication of hepatic dysfunction in the setting of fulminant hepatic failure. Two studies have evaluated PT after acetaminophen toxicity in the absence of hepatic failure, reporting a decrease in prothrombin index (equivalent to an increase in prothrombin time) in patients given IV NAC without any evidence of liver injury [7, 17]. However, these studies were retrospective, observational, and complicated by the presence of supra-therapeutic doses of acetaminophen. Jepsen et al, investigated the influence of NAC on PT in six healthy subjects both in vivo and in vitro. The authors observed an in vivo decrease in the prothrombin index (increase in prothrombin time). In the in vitro portion of the study, the authors used large supra-physiologic doses of NAC unlikely to be present in clinical practice [17]. Our relatively large controlled series verifies the effect of clinically relevant NAC levels on PT without the confounding effects of hepatic failure or large concentrations of acetaminophen.
PT elevations associated with NAC use in surgical patients has also been reported. Since NAC therapy has been suggested as an organ-protective treatment during vascular procedures, Niemi and colleagues studied the significance NAC can have on coagulation during aortic aneurysm repair [18]. In this study, NAC was associated with impaired hemostasis without any significantly worsened blood loss. Yet, this study reinforces the idea that neither acetaminophen nor liver disease is a prerequisite for impaired coagulation with NAC use.
The mechanism for NAC-associated PT elevations remains elusive. Intravenous NAC may interfere with the sulfhydryl groups of coagulation factors [19]. While some speculate that disodium edetate may cause an artificial elevation in PT by chelating calcium, we confirmed that 0.5 mg/mL of disodium edetate (the amount in standard Acetadote™) did not affect PT values in a previous study [1]. While we designed our study to evaluate standard NAC concentrations upon PT used in clinical practice, NAC concentrations rapidly decrease over the 21-h infusion (Fig. 1). However, PT tends to increase much later during the NAC infusion—when plasma NAC concentrations are at their lowest. This observation suggests other mediating mechanisms potentially interacting between NAC and PT. Further study is needed to elucidate the exact mechanisms of NAC on PT.
Fig. 1.
Coagulation factors inhibited by N-acetylcysteine. Mean Differences (95 % CI); Factor II: 45 % (39–50 %); Factor VII: 61 % (37–85 %); Factor IX: 63 % (50–77 %); Factor X: 89 % (71–108 %)
This study suggests that mild PT perturbations may occur during NAC treatment. The authors do not suggest ignoring the PT when evaluating an acetaminophen toxic patient receiving NAC therapy because PT alteration is an important sign of hepatic dysfunction. However, caution must be exercised when interpreting subtle changes in the PT during a course of IV NAC. A PT of 100 s is one prognostic marker in the Kings College Criteria for liver transplantation. The PT elevations observed in this study were modest in comparison and below those expected during fulminant hepatic failure.
Limitations
There are several limitations to this study. We used healthy young volunteers; we may have observed different results with a more heterogeneous population with medical comorbidities. While our in vitro study does not fully reflect the action of NAC in vivo, our design was necessary to separate NAC's anticoagulative effects from confounding factors. Another limitation is we did not test whether acetaminophen itself could affect coagulation factors. There is some evidence to suggest an acetaminophen overdose may result in alterations in PT in the absence of hepatic dysfunction. Whether this is from acetaminophen itself or one of its metabolite such as NAPQI remains unverified. Finally it is not entirely clear what the relationship is between factor activity levels and actual change in PT measurement. Since factor activity levels may decline without any apparent initial effect on PT, a possible explanation as to why the PT seems to rise when NAC concentrations are lowest is because it takes time for the factor levels to decrease sufficiently to affect PT measurement.
Conclusion
We are able to demonstrate a significant decrease in the activity of coagulation factors II, VII, IX, and X with the addition of NAC. This may be the mechanism by which PT increased in our previous study.
Acknowledgments
This study is supported in part by grant 5UL1RR029893 from the National Center for Research Resources, National Institutes of Health, the American Academy of Clinical Toxicology Research Award (Jang DH), and the Pittsburgh Emergency Medicine Foundation (PEMF).
Contributor Information
David H. Jang, Phone: +1-646-5097251, Email: Jangd01@nyumc.org
Matthew D. Weaver, Phone: +1-412-6478283, FAX: +1-412-6478225, Email: weavermd@upmc.edu
Anthony F. Pizon, Phone: +1-412-6478283, FAX: +1-412-6478225, Email: pizonaf@umpc.edu
References
- 1.Acetadote package insert. Nashville, TN, Cumberland Pharmaceuticals, Inc., March 2004
- 2.Hoffmann U, Fischereder M, Kruger B. The value of N-acetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol. 2004;15:407–410. doi: 10.1097/01.ASN.0000106780.14856.55. [DOI] [PubMed] [Google Scholar]
- 3.Meredith TJ, Ruprah M, Liddle A, et al. Diagnosis and treatment of acute poisoning with volatile substances. Hum Toxicol. 1989;8(4):277–286. doi: 10.1177/096032718900800405. [DOI] [PubMed] [Google Scholar]
- 4.Whyte IM, Buckley NA, Reith DM, et al. Acetaminophen causes an increased international normalized ratio by reducing functional factor VII. Ther Drug Monit. 2000;22:742–748. doi: 10.1097/00007691-200012000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Thijssen HH, Soute BA, Vervoort LM, et al. Paracetamol (acetaminophen) warfarin interaction: NAPQI, the toxic metabolite of paracetamol, is an inhibitor of enzymes in the vitamin K cycle. Thromb Haemost. 2004;92:797–802. doi: 10.1160/TH04-02-0109. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt LE, Knudsen TT, Dalhoff KP, et al. Effect of N-acetylcysteine on prothrombin index in patients with uncomplicated paracetamol poisoning. Lancet. 2002;360(9340):1151–1152. doi: 10.1016/S0140-6736(02)11194-9. [DOI] [PubMed] [Google Scholar]
- 7.Lucena MI, Lopez-Torres E, Verge C. The administration of N-acetylcysteine causes a decrease in prothrombin time in patients with paracetamol overdose but without evidence of liver impairment. Eur J Gastroenterol Hepatol. 2005;17:59–63. doi: 10.1097/00042737-200501000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Harrison PM, O'Grady JG, Keays RT, et al. Serial prothrombin time as prognostic indicator in paracetamol-induced fulminant hepatic failure. BMJ. 1990;301:964–966. doi: 10.1136/bmj.301.6758.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernal W, Wendon J, Rela M, et al. Use and outcome of liver transplantation in acetaminophen-induced acute liver failure. Hepatology. 1998;27:1050–1055. doi: 10.1002/hep.510270421. [DOI] [PubMed] [Google Scholar]
- 10.Riordan SM, Williams R. Use and validation of selection criteria for liver transplantation in acute liver failure. Liver Transpl. 2000;6:170–173. doi: 10.1002/lt.500060221. [DOI] [PubMed] [Google Scholar]
- 11.Pizon AF, Jang DH, Wang HE. The in vitro effect of N-acetylcysteine on prothrombin time in plasma samples from healthy subjects. Acad Emerg Med. 2011;18(4):351–354. doi: 10.1111/j.1553-2712.2011.01041.x. [DOI] [PubMed] [Google Scholar]
- 12.Prescott LF, Donovan JW, Jarvie DR, et al. The disposition and kinetics of intravenous N-acetylcysteine in patients with paracetamol overdosage. Eur J Clin Pharmacol. 1989;37(5):501–506. doi: 10.1007/BF00558131. [DOI] [PubMed] [Google Scholar]
- 13.Selden BS, Curry SC, Clark RF, et al. Transplacental transport of N-acetylcysteine in an ovine model. Ann Emerg Med. 1991;20(10):1069–1072. doi: 10.1016/S0196-0644(05)81354-X. [DOI] [PubMed] [Google Scholar]
- 14.Thorsen S, Teisner A, Jensen SA, et al. Effect of N-acetylcysteine on the accuracy of the prothrombin time assay of plasma coagulation factor II+VII+X activity in subjects infused with the drug. Influence of time and temperature. Scand J Clin Lab Invest. 2009;69(6):643–650. doi: 10.3109/00365510902943262. [DOI] [PubMed] [Google Scholar]
- 15.Frison L, Pocock SJ. Repeated measures in clinical trials: analysis using mean summary statistics and its implications for design. Stat Med. 1992;11:1685–1704. doi: 10.1002/sim.4780111304. [DOI] [PubMed] [Google Scholar]
- 16.Geisser S, Greenhoiuse S. An extension of Box’s results on the use of the F distribution in multivariate analysis. Ann Math Stat. 1958;29:885–891. doi: 10.1214/aoms/1177706545. [DOI] [Google Scholar]
- 17.Jepsen S, Hansen AB. The influence of N-acetylcysteine on the measurement of prothrombin time and activated partial thromboplastin time in healthy subjects. Scand J Clin Lab Invest. 1994;54:543–547. doi: 10.3109/00365519409088566. [DOI] [PubMed] [Google Scholar]
- 18.Niemi TT, Munsterhjelm E, Poyhia R, et al. The effect of N-acetylcysteine on blood coagulation and platelet function in patients undergoing open repair of abdominal aortic aneurysm. Blood Coagul Fibrinolysis. 2006;17(1):29–34. doi: 10.1097/01.mbc.0000195922.26950.89. [DOI] [PubMed] [Google Scholar]
- 19.Beckett GJ, Donovan JW, Hussey AJ, et al. Intravenous N-acetylcysteine, hepatotoxicity and plasma glutathione S-transferase in patients with paracetamol overdosage. Hum Exp Toxicol. 1990;9(3):183–186. doi: 10.1177/096032719000900311. [DOI] [PubMed] [Google Scholar]