Case Presentation
A 39-year-old man presented to the emergency department after intentionally ingesting 50 tablets of metformin 1,000 mg. The reported time of ingestion was 6 h prior to arrival. Vital signs were: temperature, 37.4 °C; pulse, 94 beats per minute; blood pressure, 117/74 mmHg; respirations, 16/min; and oxygen saturation, 96 % on room air. Gastric lavage was performed, and a single dose of activated charcoal was given. His initial serum lactate was 4.3 mmol/L (normal = 0.67–1.8 mmol/L). The patient had a negative urine drug screen, and acetaminophen and salicylate levels were below detection limits. The patient was admitted for monitoring of lactic acidosis.
Twenty hours after arrival, the patient became hypotensive with a blood pressure of 85/45 mmHg. Arterial blood gas analysis revealed a pH of 7.20, pCO2 19 mmHg, and bicarbonate HCO3 of 6 mmol/L. A repeat serum lactate at this time was 20 mmol/L, and a basic metabolic panel showed sodium of 134 mmol/L, potassium 3.8 mmol/L, chloride 98 mmol/L, bicarbonate 16 mmol/L, BUN 32 mg/dL, and creatinine 1.3 mg/dL; the calculated anion gap was 20 mmol/L. A 1-L normal saline bolus was administered, and serum alkalinization was initiated with a sodium bicarbonate drip.
The nephrology service was consulted to perform emergent hemodialysis (HD). The patient subsequently underwent two HD treatments of 4-h duration each. Following dialysis, the serum pH continued to decrease to 6.7. The patient became unresponsive and was intubated for airway protection. The hypotension persisted despite fluid resuscitation and bicarbonate therapy. Norepinephrine and vasopressin drips were started in conjunction with continuous veno-venous hemofiltration (CVVH). With introduction of CVVH, the bicarbonate drip was discontinued. The lactate level increased to greater than 30 mmol/L (upper limit of the lab’s reporting range). Forty-eight hours after arrival, the serum pH was 7.0, and the patient remained hypotensive despite continued vasopressor drips and CVVH.
What Is the Therapeutic Mechanism of Metformin?
Metformin belongs to a class of drugs called biguanides, which are oral agents used in the management of non-insulin-dependent diabetes mellitus (NIDDM). The biguanide class consists of phenformin, buformin, and metformin. Biguanides were initially introduced to the market in the 1950s [1]; however, metformin was not introduced in the USA until 1995 [2]. Metformin is considered the first-line option for NIDDM and accounts for one third of all orally active diabetes drugs prescribed in the USA [3, 4]. Metformin has also been used in the treatment of polycystic ovary syndrome (PCOS). In PCOS, metformin increases uptake of glucose, limits gluconeogenesis in the ovaries, and may result in weight loss [5].
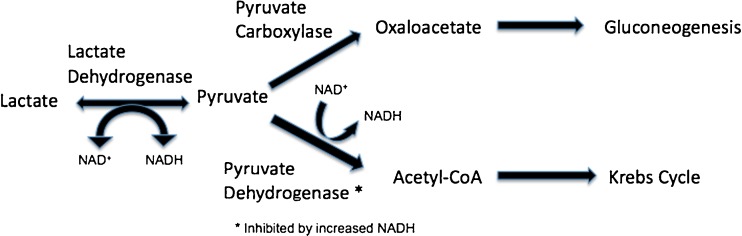
Metformin has multiple mechanisms of action, which have not been completely elucidated. Metformin reduces gluconeogenesis, increases peripheral uptake of glucose, and decreases fatty acid oxidation [1, 6]. Under normal conditions, gluconeogenesis consumes pyruvate through pyruvate carboxylase. Biguanides inhibit pyruvate carboxylase, thereby causing pyruvate to accumulate (Fig. 1). Increased amounts of pyruvate are then converted to lactate via lactate dehydrogenase. Conversely, biguanides also inhibit conversion of lactate back to pyruvate for use in gluconeogenesis [7].
Fig. 1.
Pyruvate metabolism
Tyrosine kinase activity is limited in NIDDM. The decrease in tyrosine kinase activity results in decreased glucose transporters on cellular membranes. Metformin increases tyrosine kinase activity and therefore increases the presence and activity of glucose transporters on cell membranes, leading to improved peripheral glucose uptake [1, 6]. Metformin has been shown to increase lipogenesis as well as uptake and utilization of glucose by adipose tissue. There is also an interaction with the Randle cycle, also known as the glucose–fatty acid cycle, where the increased peripheral uptake of glucose in adipose tissue and muscles leads to inhibition of fatty acid release and metabolism [1, 8].
Metformin is available only as an oral preparation and reaches peak serum concentration approximately 2 h after ingestion. Steady-state levels are achieved within 24 to 48 h [9]. The half-life ranges between 2.5 and 4.9 h [10]. Metformin is excreted predominantly unchanged by the kidneys. The elimination half-life is approximately 17 h, with 90 % of the dose ingested being renally cleared within 24 h [9]. The drug has high water solubility, minimal plasma protein binding, and a large volume of distribution, being concentrated primarily in the intestinal wall, salivary glands, kidneys, and liver [1, 3, 10, 11].
While metformin is the first-line choice for the treatment of NIDDM, there are multiple contraindications to consider. The relative contraindications to metformin use include renal or hepatic disease, alcohol abuse, history of lactic acidosis, intravenous radiographic contrast agents, pregnancy, cardiac or respiratory insufficiency, or conditions that lead to decreased peripheral perfusion (Table 1) [1, 12]. There are no absolute contraindications; however, renal dysfunction appears to be the strictest relative contraindication to metformin use. At least 50 % of people who are currently taking metformin have at least one of these contraindications [13, 14].
Table 1.
| Contraindications to metformin use |
|---|
| Renal Insufficiency |
| Creatinine cut-off of 1.4 mg/dL in women and 1.5 mg/dL in men |
| Liver dysfunction |
| Alcoholic liver disease |
| Acute hepatic toxicity |
| Intravenous radiographic contrast agents |
| Pregnancy |
| Cardiac insufficiency |
| Respiratory insufficiency |
| Conditions leading to decreased peripheral perfusion |
| Any hypoxic condition |
| History of lactic acidosis |
How Does Metformin Lead to the Development of Lactic Acidosis in Overdose?
There are two situations in which metformin may contribute to the development of lactic acidosis. The first is among patients consuming therapeutic doses. Metformin levels gradually increase when a person develops a medical condition that compromises the body’s ability to excrete the drug. The second situation describes those who intentionally ingest a large amount of metformin, acutely saturating clearance mechanisms.
There continue to be contradictory reports regarding the prevalence of metformin-associated lactic acidosis (MALA). MALA is well described in case reports and case series throughout the literature. However, by most accounts, the risk of lactic acidosis with therapeutic metformin use is considered minimal. A 2010 Cochrane review of prospective and observational cohort studies concluded that there was no evidence to support an increased risk of lactic acidosis from therapeutic metformin use [14].
Lactic acidosis is defined as a metabolic acidosis with a pH of less than 7.35 and a lactate concentration greater than 5 mmol/L [15–17]. The Cohen–Woods classification system divides lactic acidosis into types A and B. Type A results from poor perfusion, hypoxia, or increased lactate production. Etiologies include septic shock, global hypoxic states, hypoperfusion, increased anaerobic activity, or compromise of the oxygen delivery system.
Type B lactic acidosis is defined by lack of tissue hypoperfusion and arises from metabolic derangements. Such conditions may include inborn errors of metabolism, excessive catecholamine stimulation, or poisoning. Type B can be further broken down into three subgroups. The first subtype, B1, is attributable to an underlying disease state. Subtype B2 refers to a metabolic state induced by toxins. Finally, the third subtype, B3 is a result of inborn errors of metabolism [11, 15, 18, 19]. Initially, MALA is considered a type B lactic acidosis. However, there is a designation change to incorporate type A as well, after multi-organ dysfunction ensues and tissue hypoperfusion develops [3, 16, 20–22].
Metformin increases lactic acid production by inhibiting gluconeogenesis, decreasing intestinal glucose production, increasing peripheral glucose utilization, and decreasing fatty acid oxidation [2]. Metformin causes an intracellular shift in oxidation–reduction reactions from aerobic to anaerobic metabolism, which results to lactic acid accumulation [3, 7, 10, 18, 23, 24]. The developing lactic acidosis reduces intracellular pH, which continues a cycle of diminished activity of pyruvate carboxylase and decreased lactate uptake by the liver [11, 24]. Biguanides, in general, have been associated with negative inotropic effects, decreasing cardiac output and hepatic blood flow and thereby decreasing lactate clearance [24].
Most commonly, MALA occurs due to renal compromise and reduced metformin excretion. Additionally, renal insufficiency decreases hydrogen excretion by the kidneys and the development of acidosis independent of metformin [25]. An increase of hydrogen ions and lower pH further inhibit pyruvate carboxylase and dehydrogenase, causing more lactate accumulation (Fig. 1). Inhibition of pyruvate carboxylase leads to decreased gluconeogenesis as previously mentioned. Inhibition of pyruvate dehydrogenase leads to decreased production of acetyl coenzyme A, which is used in the Krebs cycle and in fatty acid synthesis (Fig. 1).
Metformin remains the mainstay of treatment for NIDDM because of significant benefit. This remains true even among those patients with relative contraindications, although use of metformin in this group should be done with caution (Table 1) [4]. While the incidence of MALA is low, its mortality is reported to approach 50 % [2, 12, 15, 16, 19, 24, 26]. The estimated incidence of lactic acidosis in chronic users of metformin is between three and nine cases per 100,000 patients-years [1, 3, 10, 13, 15, 27]. However, a 2011 study found a MALA incidence of 47 cases per 100,000 patient-years—at least five times higher than the previously reported rates [13].
How Is Metformin-Associated Lactic Acidosis from Acute Intentional Overdose Different from Inadvertent Metformin Toxicity? Are They the Same Phenomenon or Different, and Why?
As previously described, metformin inhibits gluconeogenesis, increases peripheral glucose uptake, and decreases intestinal glucose uptake and fatty acid oxidation. These pharmacologic changes inhibit mitochondrial cellular respiration and lead to elevated lactate levels [2, 18, 28]. The pathways leading to metabolic derangement and MALA are similar between inadvertent and intentional metformin toxicity, even though the etiology for metformin accumulation differs.
Inadvertent toxicity is an insidious, gradual process that may allow the body more time to adjust to lactate accumulation. The lactic acidosis that develops is likely multifactorial and more a result of comorbidities than from metformin itself. MALA may occur in chronic therapeutic dosing when an acute event affects a patient’s ability to excrete metformin. Such events include renal insufficiency, dehydration, dosage increase, sepsis, shock, or acidosis [4]. Infection, ischemia, or hypotension can also contribute to additional lactate production [7].
In acute overdose, metformin induces the initial insult via multiple processes discussed previously. Kidney function is typically normal; however, rapid drug absorption and tissue accumulation overwhelm clearance capabilities. Clinical symptoms and mortality do not correlate well with metformin levels [16, 19]. While significantly elevated lactate levels are associated with adverse outcomes, a well-defined correlation to predict morbidity and mortality based on lactate level alone has not been shown [4].
What Dose of Metformin Is Sufficient to Cause Toxicity? What Are the Indications for Hemodialysis?
The amount of metformin required to induce toxicity in acute metformin overdose has yet to be established. Reported ingestions treated with hemodialysis have been as high as 145 g [15]. Patients may present along a clinical spectrum, and metformin levels do not correlate well to symptoms, although higher metformin levels tend to be associated with more severe lactic acidosis [7].
When symptomatic, a patient may exhibit nausea, vomiting, abdominal pain, malaise, myalgias, mental status changes, renal insufficiency, and cardiovascular compromise. Though the mechanism is not well described, hypoglycemia and, less commonly, hyperglycemia have also been reported in severe poisonings [29]. Metformin-associated lactic acidosis with therapeutic dosing has up to a 50 % mortality rate [10, 26]. However, the lactic acidosis resulting from intentional acute ingestions is less well understood. In previous studies, lactate levels have demonstrated a sensitivity of 84 % to predict fatality in general poisoning [30]. Lactate levels in metformin toxicity have not been specifically correlated with predicting morbidity and mortality in the literature at this point.
A daily therapeutic metformin dose may be as high as 2.5 g daily [1], so it is not difficult for a patient to obtain enough metformin to comprise a toxic dose. Fortunately, the majority of single-agent ingestions with metformin never develop appreciable toxicity. Soyral et al. describe two cases of intentional ingestions: the first is a 50-g ingestion, and the second, 80 g. Both cases had documented elevated lactate levels. The two patients each underwent HD with bicarbonate dialysate and survived to discharge [31]. Bicarbonate therapy alone may not be sufficient to restore the acid–base derangement from lactic acidosis and multi-organ compromise [32].
In acute metformin ingestions, the threshold at which a patient qualifies for HD is subjective. Treatment with traditional HD or CVVH for acute ingestion should be directed toward accomplishing two goals. The first is restoration of an acceptable acid–base status, and the second is removal of metformin. Because of the severity of illness and a poorly understood disease process, strict criteria for recommending HD have not been clarified. However, precedents from the medical literature provide some rough guidance (see Table 2) [33].
Table 2.
Indications for HD/CVVH [33]
| Indications for HD/CVVH |
|---|
| Significant comorbidities |
| Critically ill |
| pH < 7.1 |
| Failure of supportive care |
| Renal insufficiency |
| Fluid overload state |
Is Hemodialysis a Proven Treatment for MALA? What Other Treatments Options Are There?
As yet, hemodialysis has not been proven to be beneficial in the management of MALA. Peters et al. failed to demonstrate decreased mortality with HD among patients who met criteria for MALA for both chronic and acute ingestions [34]. However, the severity of illness among their dialyzed subjects was higher. Since the mortality was similar in the dialysis versus non-dialysis groups, some benefit may be inferred from this study.
Metformin is highly water soluble and minimally protein bound and has molecular weight less than 500 Da. These factors all contribute to metformin’s ability to be effectively dialyzed to enhance elimination. However, metformin also has a large volume of distribution (654 L). An intentional acute poisoning is, therefore, likely to benefit most from early dialysis before the drug redistributes into the tissues [20]. Additionally, bicarbonate dialysate may increase metformin clearance [24]. Despite a fatal outcome, Bareutto et al. reported successful removal of metformin by dialysis [20]. Nguyen et al. reported the percentage of removal of metformin to be as high as 60 % with HD based on pre- and post-hemodialysis metformin blood levels [33].
Appropriate early patient selection is challenging. Some will remain asymptomatic, while others will progress to a severe lactic acidosis. Lactate levels may help to risk-stratify patients requiring earlier intervention such as HD, CVVH, or bicarbonate therapy [30]. Unfortunately, dialysis may be most effective before metformin distributes in significant, enough quantities to elevate the lactate. It has been suggested that due to a two-compartment model of distribution, metformin may be more amenable to prolonged dialysis (duration greater than 4 h) [3].
Administration of activated charcoal should be considered to decrease medication absorption in the gut from acute ingestions. Bicarbonate infusion without HD or CVVH has been described, but there is no clear advantage. Moreover, there is at least a theoretical concern with intravenous bicarbonate for exacerbation of intracellular acidosis and a leftward shift of the oxyhemoglobin disassociation curve [35]. While the acid–base derangement may improve with bicarbonate alone, simultaneous HD or CVVH can also remove the inciting agent. One abstract reporting the administration of a 20 % intravenous fat emulsion for metformin overdose did not show benefit [36].
One of the greatest challenges facing providers is the lack of a predictive model. At this time, there is no way to know who will become symptomatic following an acute intentional metformin overdose. Lactate levels may not become elevated for 6 h after ingestion. Recommendations for effective therapies are limited. The mainstay of therapy continues to be gastrointestinal decontamination, if appropriate, followed by supportive care with intravenous hydration and consideration of CVVH or standard HD with bicarbonate dialysate [15, 37].
What About the Other Biguanides? Is Phenformin Really that Much Worse?
In addition to metformin, the biguanides include phenformin and buformin. All three have similar mechanisms of action including inhibition of hepatic gluconeogenesis [17]. Biguanides inhibit oxidation of lactate and increase lactate release from skeletal muscle [12]. Additionally, biguanides have been shown to increase lactate levels in diabetics as well as non-diabetics [24].
Therapeutic half-lives differ between the three drugs. Phenformin has a half-life of 12 h; buformin’s half-life is 4 h, and metformin has a half-life of 1.5 h [24]. Buformin and phenformin are metabolized more extensively by the liver, while metformin is largely excreted unchanged by the kidneys. Unlike metformin, phenformin inhibits oxidative phosphorylation, thereby allowing for accumulation of excessive NADH. Increased NADH further inhibits pyruvate dehydrogenase (Fig. 1). The additional pyruvate is then converted to lactate [12].
Phenformin contains a long lipophilic side chain that binds to the mitochondrial membrane. Binding inhibits the electron transport chain, interfering with aerobic metabolism. Phenformin also decreases hepatic uptake and consumption of lactate [28, 38]. Phenformin is metabolized by CYP2D6 to 4-hydroxyphenformin. A polymorphism has been reported that may cause 5–10 % of Caucasians to have decreased metabolism, contributing to a greater risk of lactic acidosis [17, 39].
Metformin has a greater toxicity threshold than phenformin [12, 40]. Metformin is less lipophilic and does not demonstrate the same affinity for binding to the mitochondrial membrane. The improved safety profile of metformin may also be related to its shorter half-life, less accumulation within tissues, and renal excretion without the need for hepatic metabolism [17].
There continues to be a question of how significant of a correlation between lactic acidosis and biguanides exists. Aguilar et al. found that patients over the age of 70 with coronary artery disease and renal insufficiency (creatinine 1.5–3 mg/dL) had higher occurrences of lactic acidosis [41]. The study concluded that therapeutic doses of biguanides, especially metformin, were not associated with an increased risk of lactic acidosis [41]. However, Luft et al. reported occurrences of lactic acidosis with all three biguanide drugs at normal therapeutic doses [26].
Phenformin was the first biguanide to be approved in the USA for the treatment of NIDDM in the 1950s. The University Group Diabetes Program showed excessive mortality associated with phenformin in 1975 [39]. In 1977, phenformin was withdrawn from the market due to an elevated risk of lactic acidosis and associated mortality [1, 16, 27]. Prior to withdrawal, the reported incidence of lactic acidosis was 40–64 cases per 100,000 person-years, a 10–20 % higher incidence than metformin [1, 16, 17, 27, 40]. It is important to note that many countries outside the USA continue to successfully use phenformin as treatment for NIDDM.
Case Conclusion
Sixty hours after arrival, the patient was continued on norepinephrine, vasopressin, and CVVH. He developed renal insufficiency, elevated transaminases, and acute respiratory distress syndrome. Multiple boluses of epinephrine and bicarbonate were required overnight during day 3 of hospital admission. At 60 h, the pH was 7.2 with a lactate of 17 mmol/L. Finally, about 72 h into the hospital visit, the patient had a cardiac arrest; resuscitation attempts were unsuccessful, and the patient expired.
References
- 1.Bailey CJ. Metformin. N Engl J Med. 1996;334(9):574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 2.Lalau J-D. Lactic acidosis induced by metformin incidence, management and prevention. Drug Saf. 2010;33(9):727–740. doi: 10.2165/11536790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Guo PY, Storsley LJ, Finkle SN. Severe lactic acidosis treated with prolonged hemodialysis: recovery after massive overdoses of metformin. Semin Dial. 2006;19(1):80–83. doi: 10.1111/j.1525-139X.2006.00123.x. [DOI] [PubMed] [Google Scholar]
- 4.Scheen A. Metformin and lactic acidosis. Acta Clin Belg. 2011;66(5):329–331. doi: 10.2143/ACB.66.5.2062583. [DOI] [PubMed] [Google Scholar]
- 5.Badawy A, Elnashar A. Treatment options for polycystic ovary syndrome. Int J Womens Health. 2011;3:25–35. doi: 10.2147/IJWH.S11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333(9):550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 7.Perrone J, Phillips C, Gaieski D. Occult metformin toxicity in three patients with profound lactic acidosis. J Emerg Med. 2011;40(3):271–275. doi: 10.1016/j.jemermed.2007.11.055. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe RR. Metabolic interactions between glucose and fatty acids in humans. Am J Clin Nutr. 1998;67:519s–526s. doi: 10.1093/ajcn/67.3.519S. [DOI] [PubMed] [Google Scholar]
- 9.Bristol-Myers Squibb Company. (2009) Glucophage (metformin hydrochloride) tablets. Package Insert
- 10.Prikis M, Mesler EL, Hood VL, Weise WJ. When a friend can become an enemy! Recognition and management of metformin-associated lactic acidosis. Kidney Int. 2007;72(9):1157–1160. doi: 10.1038/sj.ki.5002346. [DOI] [PubMed] [Google Scholar]
- 11.Hofkens P. Metformin-associated lactic acidosis (MALA): case report. Acta Clin Belg. 2011;66(5):390–392. doi: 10.2143/ACB.66.5.2062595. [DOI] [PubMed] [Google Scholar]
- 12.Kwong SB, Brubacher J. Phenformin and lactic acidosis: a case report and review. The J Emerg Med. 1998;16(6):881–886. doi: 10.1016/S0736-4679(98)00103-6. [DOI] [PubMed] [Google Scholar]
- 13.Van Berlo-Van De Laar IR, Vermeij CG, Doorenbos CJ. Metformin associated lactic acidosis: incidence and clinical correlation with metformin serum concentration measurements. J Clin Pharm Ther. 2011;36(3):376–382. doi: 10.1111/j.1365-2710.2010.01192.x. [DOI] [PubMed] [Google Scholar]
- 14.Salpeter SR et al. (2010) Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev (1):CD002967 [DOI] [PubMed]
- 15.Akoglu H, Akan B, Piskinpasa S, et al. Metformin-associated lactic acidosis treated with prolonged hemodialysis. Am J Emerg Med. 2011;29(5):573–575. doi: 10.1016/j.ajem.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Lalau JD, Race JM. Lactic acidosis in metformin-treated patients. Prognostic value of arterial lactate levels and plasma metformin concentrations. Drug Saf. 1999;20(4):377–384. doi: 10.2165/00002018-199920040-00006. [DOI] [PubMed] [Google Scholar]
- 17.Bando K, Ochiai S, Kunimatsu T, et al. Comparison of potential risks of lactic acidosis induction by biguanides in rats. Regul Toxico Pharmacol. 2010;58(1):155–160. doi: 10.1016/j.yrtph.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Harvey B, Hickman C, Hinson G, Ralph T, Mayer A. Severe lactic acidosis complicating metformin overdose successfully treated with high-volume venovenous hemofiltration and aggressive alkalinization. Pediatr Crit Care Med. 2005;6(5):598–601. doi: 10.1097/01.PCC.0000162451.47034.4F. [DOI] [PubMed] [Google Scholar]
- 19.Gura M, Devrim S, Sagiroglu AE, Orhon Z, Sen B. (2010) Severe metformin intoxication with lactic acidosis in an adolescent: a case report. BMC Anesthesiol. 27(2)
- 20.Barrueto F. Clearance of metformin by hemofiltration in overdose. J Clin Toxicol. 2002;40(2):177–180. doi: 10.1081/CLT-120004407. [DOI] [PubMed] [Google Scholar]
- 21.Lalua JD. Role of metformin accumulation in metformin-associated lactic acidosis. Diabetes Care. 1995;18(6):779–784. doi: 10.2337/diacare.18.6.779. [DOI] [PubMed] [Google Scholar]
- 22.Guariglia A, Gonzi GL, Regolisti G, Vinci S. Treatment of biguanide-induced lactic acidosis. Ann Ital Med Int. 1994;9(1):35–39. [PubMed] [Google Scholar]
- 23.Lheureux PE, Lheureux OF, Penaloza-Baeza A. Metformin toxicity. Eur J Emerg Med. 2009;16(6):348–349. doi: 10.1097/MEJ.0b013e32832b168d. [DOI] [PubMed] [Google Scholar]
- 24.Gan SC, Barr J, Arieff AI, Pearl RG. Biguanide-associated lactic acidosis. Case report and review of the literature. Arch Intern Med. 1992;152:2333–2336. doi: 10.1001/archinte.1992.00400230129023. [DOI] [PubMed] [Google Scholar]
- 25.Lalua JD. Consequences of metformin intoxication. Diabetes Care. 1998;21(11):2036–2037. doi: 10.2337/diacare.21.11.2036. [DOI] [PubMed] [Google Scholar]
- 26.Luft D. Lactic acidosis in biguanide-treated diabetics. Diabetologia. 1978;14:75–87. doi: 10.1007/BF01263444. [DOI] [PubMed] [Google Scholar]
- 27.Kruse JA. Metformin-associated lactic acidosis. J Emerg Med. 2001;20(3):267–272. doi: 10.1016/S0736-4679(00)00320-6. [DOI] [PubMed] [Google Scholar]
- 28.Misbin R. Phenformin-associated lactic acidosis: pathogenesis and treatment. Ann Intern Med. 1977;87:591–595. doi: 10.7326/0003-4819-87-5-591. [DOI] [PubMed] [Google Scholar]
- 29.Suchard JR, Grotsky TA. Fatal metformin overdose presenting with progressive hyperglycemia. West J Emerg Med. 2008;9(3):160–164. [PMC free article] [PubMed] [Google Scholar]
- 30.Manini AF, Kumar A, Olsen D, Vlahov D, Hoffman RS. Utility of serum lactate to predict drug-overdose fatality. J Clin Toxicol. 2010;48(7):730–736. doi: 10.3109/15563650.2010.504187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soyoral YU, Begenik H, Emre H, Aytemiz E, Ozturk M, Erkoc R. Dialysis therapy for lactic acidosis caused by metformin intoxication: presentation of two cases. Hum Exp Toxicol. 2011;30(12):1995–1997. doi: 10.1177/0960327111403177. [DOI] [PubMed] [Google Scholar]
- 32.Rifkin SI, Mcfarren C, Juvvadi R, Weinstein SS. Prolonged hemodialysis for severe metformin intoxication. Ren Fail. 2011;33(4):459–461. doi: 10.3109/0886022X.2011.568132. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen H. Metformin intoxication requiring dialysis. Hemodial Int. 2011;15:S68–S71. doi: 10.1111/j.1542-4758.2011.00605.x. [DOI] [PubMed] [Google Scholar]
- 34.Peters N, Jay N, Barraud D, et al. Metformin-associated lactic acidosis in an intensive care unit. Crit Care. 2008;12(6):R149. doi: 10.1186/cc7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heaney D, Majid A, Junor B. Bicarbonate haemodialysis as a treatment of metformin overdose. Nephrol Dial Transplant. 1997;12(5):1046–1047. doi: 10.1093/ndt/12.5.1046. [DOI] [PubMed] [Google Scholar]
- 36.Miller S. Abstract: failure of lipid emulsion therapy to treat a metformin overdose. J Clin Toxicol. 2011;49:515–627. doi: 10.3109/15563650.2011.598695. [DOI] [Google Scholar]
- 37.Pan LT. Continuous venovenous haemodiafiltration for metformin-induced lactic acidosis. Anaesth Intensive Care. 2009;37:830–832. doi: 10.1177/0310057X0903700520. [DOI] [PubMed] [Google Scholar]
- 38.Radziuk J, Zhang Z, Wiernsperger N, Pye S. Effects of metformin on lactate uptake and gluconeogenesis in the perfused rat liver. Diabetes. 1997;46(9):1406–1413. doi: 10.2337/diab.46.9.1406. [DOI] [PubMed] [Google Scholar]
- 39.Lu HC, Parikh PP, Lorber DL. Phenformin-associated lactic acidosis due to imported phenformin. Diabetes Care. 1996;19(12):1449–1450. doi: 10.2337/diacare.19.12.1449. [DOI] [PubMed] [Google Scholar]
- 40.Ko GT, Chan JC, Chow CC, Yeung VT, Li YK, Cockram CS. Phenformin-induced lactic acidosis: an almost forgotten complication of treatment with biguanides. Br J Hosp Med. 1995;54(9):469–470. [PubMed] [Google Scholar]
- 41.Aguilar C. Biguanide related acidosis: incidence and risk factors. Arch Med Res. 1992;23(1):19–24. [PubMed] [Google Scholar]