Abstract
Abuse of psychogenic substances sold as “bath salts” and “plant food” has escalated in recent years in the United States (USA). Previous reports suggest regional differences in the primary active β-keto phenylalkylamines found in these products and the corresponding signs and symptoms reported after exposure. Currently, there are only limited studies describing the clinical effects associated with reported “bath salts” exposure in the USA. This study describes the clinical effects associated with “bath salt” and “plant food” exposures as reported to the poison center serving the state of North Carolina (Carolinas Poison Center). We performed a retrospective review of the Carolinas Poison Center database for all cases of reported human exposure to “bath salt” and “plant food” products from 2010 to 2011 with specific attention to clinical effects and routes of exposure. Additionally, we reviewed therapies used, trended the volume of exposure cases reported over the study period, and evaluated the distribution of calls within state counties using descriptive statistics. Carolinas Poison Center received 485 total calls and 409 reported exposure calls regarding “bath salt” or “plant food” products between January of 2010 and December of 2011. The peak of reported exposures occurred in May of 2011. Clinical effects commonly reported in the exposure cases generated from these calls included tachycardia (53.3 %, n = 218), agitated/irritable (50.4 %, n = 206), hallucination/delusions (26.7 %, n = 109), and hypertension (25.2 %, n = 103). In addition to intravenous fluids, common therapies included benzodiazepines (46.0 %, n = 188), sedation (13.4 %, n = 55), alkalinization (3.90 %, n = 16), antihistamine (4.16 %, n = 17), and intubation (3.67 %, n = 15). Haloperidol was the antipsychotic agent used most often to treat agitation (n = 40). Serious complications associated with reported exposure to “bath salt” and “plant food” products included rhabdomyolysis, renal failure, excited delirium syndrome, and death. While treatments have not been empirically determined, sedation with benzodiazepines, aggressive cooling for hyperthermic patients, and use of small doses of antipsychotics for choreoathetoid movements not controlled with benzodiazepines are not likely to be harmful.
Keywords: Designer drug; 3,4-Methylenedioxypyrovalerone; 4-Methylmethcathinone; MDPV; Mephedrone; Clinical effects
Introduction
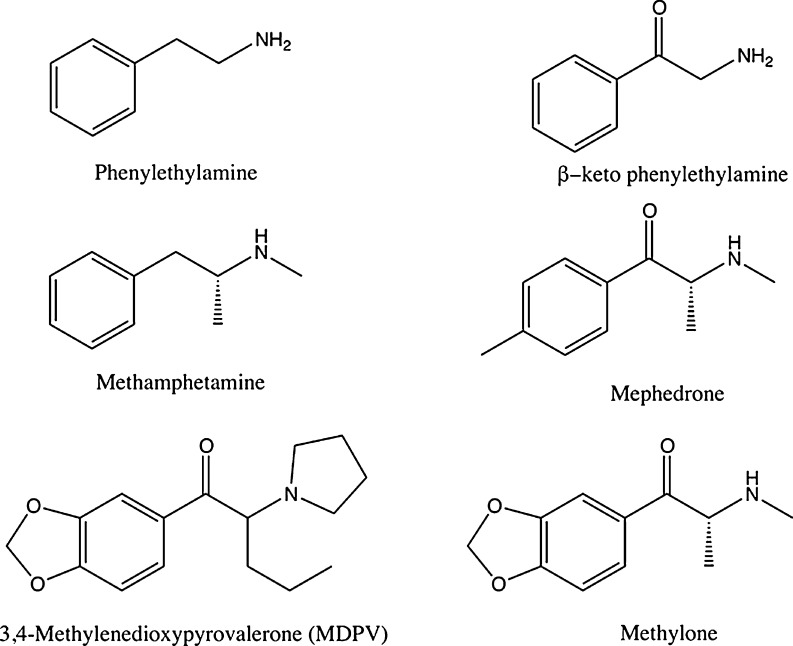
Recreational abuse of products referred to as “bath salts” or “plant food” escalated in the United States (USA) from mid-2010 through 2011. These products predominantly contain β-keto phenylalkylamines such as 4-methylmethcathinone (mephedrone), 3,4-methylenedioxypyrovalerone (MDPV), and methylone (Fig. 1). The active ingredients in these products vary by region with most European countries documenting mephedrone as the primary ingredient; however, Finland has reported a higher predominance of MDPV [1–3]. A recent study reported the detection of MDPV in the majority of patient samples tested, as well as the “bath salt” products obtained in the southeastern USA [4]. Clinical effects appear to vary with exposure to different β-keto phenylalkylamines.
Fig. 1.
Chemical structures of selected “bath salt” and “plant food” products
Carolinas Poison Center (CPC) is a regional poison center with an annual call volume of approximately 105,000 calls. This poison center primarily serves the state of North Carolina and fields roughly 73,000 calls regarding human exposures annually. We examined the clinical effects associated with “bath salt” or “plant food” exposure as reported to this regional poison center. To date, this represents the largest collection of patients with reported “bath salts” exposures from one geographic area in the USA.
Methods
This project was reviewed by the host institution’s Institutional Review Board and was deemed exempt from further oversight. Carolinas Poison Center records were searched for cases documenting calls that represented a human exposed to a “bath salts” or “plant food” substance from January 1, 2010 through December 31, 2011 using Poisindex® substance codes for “bath salts” [7042239], 4-methylmethcathinone [7030060], 3,4-methylenedioxypyrovalerone [7038436], synthetic cathinone derivatives [7057009], and synthetic cathinones [7038535], as well cases that had “plant food” listed in the substance verbatim field (free text describing the substance). Cases were excluded if it was clear from the case notes that it actually represented a case of plant fertilizer ingestion. Cases originating from calls for information on “bath salts” or “plant food”, and cases representing exposed animals were excluded. When they were available, hospital records and medical examiner’s reports were also reviewed.
Data abstracted from the poison center cases included the specific data fields of county of caller, age, sex, clinical effects, route of administration, therapies, and deaths. When hospitals or physicians provided them, hospital records were reviewed. When patient age was only coded as a broad category such as 6–12 years, teen, 20s, 30s, or unknown, it was excluded from the age analysis. The cases included in the analysis of route of exposure were only included if there was documentation of the route in the free text, not just using the coded route of exposure field. Clinical effects are the signs, symptoms, and abnormal laboratory values reported for the patient; there are 131 individual clinical effects that may be coded. Therapies are also individually coded; there are 58 individual therapies. The authors reviewed all case notes to ensure coding accuracy of clinical effects, route of administration, and therapies. The clinical effect, “Miscellaneous, Other” is used when a patient has a clinical effect that is not described by the other 130 discrete clinical effects. Standard CPC operating procedure dictates that when a case has the clinical effect of “Miscellaneous, Other” coded, the sign or symptom this indicates should be documented in the free text field followed by the phrase, “(other)”. All exposure cases where “Miscellaneous, Other” was coded were manually reviewed to determine what sign or symptom the code referred to. If the sign or symptom that was coded as “Miscellaneous, Other” was more accurately captured using an alternative clinical effect, the exposure case coding was corrected; otherwise, these signs/symptoms coded as “Miscellaneous, Other” were individually tabulated. The same process was applied to exposure cases where therapies were coded as “Other” or “Sedation, Other,” as antipsychotics do not have a specific NPDS code.
Ten percent (n = 42) of the poison center exposure cases were reviewed by two authors (C.M. and A.R.D.); and the kappa value as a marker for interrater agreement was calculated. Microsoft Excel 2003 (Microsoft®, Redmond, WA) was used in data analysis with simple descriptive statistics.
Results
Poison Center Data
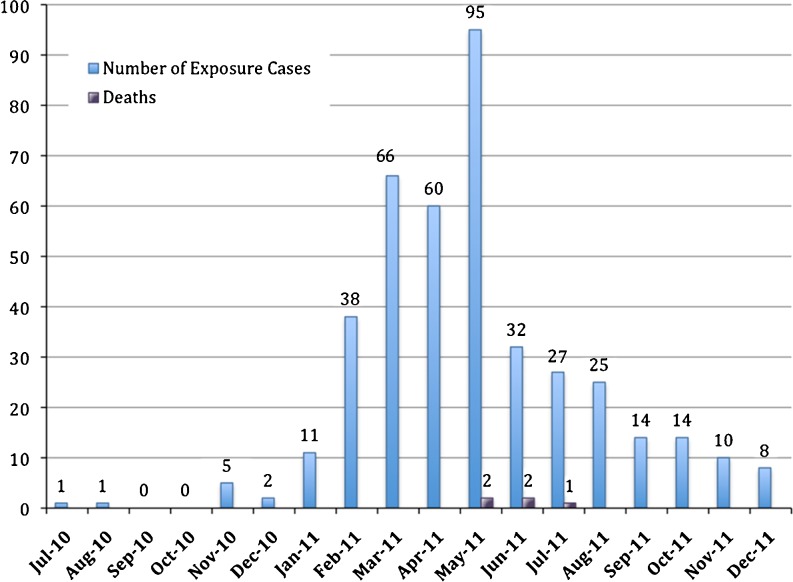
From January 1, 2010 through December 31, 2011, there were a total of 486 calls to the CPC regarding these substances of abuse with 409 cases reporting human exposures. Four hundred six of the exposure cases originated from North Carolina with one exposure case originating from the states of Virginia, Maryland, and South Carolina. Male patients represented 277 (67.7 %) of the exposure cases. Of the 380 exposure cases in which age was captured, the mean age was 28.8 years (range 21 months to 68 years); the median age was 27 years. Route of exposure was documented in the text 55.2 % of the time (n = 226) and included insufflation (71.7 %, n = 162), ingestion (19.5 %, n = 44), and parenteral (15.9 %, n = 36). The first reported exposure occurred in July of 2010 and exposures peaked in May of 2011 with 95 reported exposures that month (Fig. 2). Five deaths were reported to the CPC as related to “bath salts” during this time period; three had analysis for and detection of MDPV in blood.
Fig. 2.
Number of all reported exposure cases and deaths with reported exposure by month
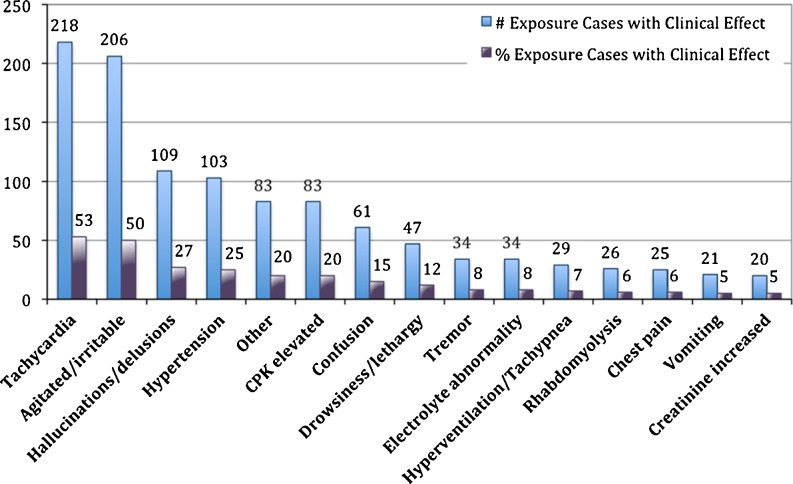
The most common clinical effects reported were tachycardia (53.3 %, n = 218), agitated/irritable (50.4 %, n = 206), hallucination/delusions (26.7 %, n = 109), and hypertension (25.2 %, n = 103) (Fig. 3). The clinical effect “Miscellaneous, Other” was coded in 83 exposure cases (20.3 %). Paranoia was the most commonly documented reason for “Miscellaneous, Other” as it was reported in 21 % calls with this clinical effect code (n = 18) (Table 1).
Fig. 3.
Predominant clinical effects documented from 409 reported exposure cases to “bath salt” or “plant food” products
Table 1.
Most prevalent clinical effects coded as “Miscellaneous, Other” after reported exposure
| Clinical effect coded as “Miscellaneous, Other” | Number of exposure cases with reported effect | Percentage of total (n = 409) exposure cases |
|---|---|---|
| Paranoia | 18 | 4.41 % |
| Not explained | 16 | 3.91 % |
| Insomnia | 13 | 3.18 % |
| Rapid speech | 5 | 1.22 % |
| Chills | 3 | 0.73 % |
Common therapies included intravenous (IV) fluids (57.9 %, n = 237), benzodiazepines (46.0 %, n = 188), other (14.7 %, n = 60), sedation (13.4 %, n = 55), alkalinization (3.90 %, n = 16), antihistamine (4.16 %, n = 17), and intubation (3.67 %, n = 15). When cases with therapy codes “Other” and “Sedation, Other” were manually reviewed (28.1 %, n = 115), the antipsychotics used specifically to control agitation included haloperidol (n = 40), olanzapine (n = 11), risperidone (n = 4), ziprasidone (n = 3), chlorpromazine (n = 1), and promethazine (n = 1). Fifty-four patients (13.2 %) received at least one antipsychotic agent. Dexmedetomidine was used in three patients as a treatment for agitation. Intubated patients (n = 16) were sedated with propofol (n = 9), midazolam (n = 4), fentanyl (n = 2), lorazepam (n = 2), and ketamine (n = 1). Maintenance sedation in intubated patients was not recorded in three patients, and more than one agent was used to maintain sedation in four of the intubated patients.
Calls were received from 62 of 100 counties in North Carolina (NC). Nineteen percent (n = 50) of all exposure cases (n = 267) reported during the early escalation of reported “bath salt” product use between July 2010 and May 2011 originated from the five counties within NC that house military instillations. Specifically, 33.3 % (n = 3) of all exposure cases in 2010, 73 % (n = 8) of exposure cases in January 2011, 18 % (n = 7) of exposure cases in February 2011, and 25 % (n = 17) of exposure cases in March of 2011 were from these counties. The percentage of exposure cases originating from these counties decreased in April 2011 (12 %, n = 7) and May 2011 (14 %, n = 13) when reports of exposure to “bath salt” products became more widespread (Table 2). There was an increase in the number of exposures reported from the 13 of 18 counties bordering South Carolina after June 1, 2011 (Table 2).
Table 2.
Reported exposures (total = 406) from all North Carolina (NC) counties by month compared to exposure cases reported from NC counties bordering South Carolina (SC), a state without a similar ban on “bath salt” products after June 1, 2011
| Month | Number of NC counties reporting exposures | Number of exposure cases from All NC counties | Number of exposure cases from counties bordering SC | Percentage of total exposure cases from counties bordering SC |
|---|---|---|---|---|
| 2010 | ||||
| July | 1 | 1 | 0 | 0 |
| August | 1 | 1 | 0 | 0 |
| September | 0 | 0 | 0 | 0 |
| October | 0 | 0 | 0 | 0 |
| November | 5 | 5 | 1 | 20 % |
| December | 2 | 2 | 1 | 50 % |
| 2011 | ||||
| January | 5 | 11 | 0 | 0 |
| February | 23 | 38 | 8 | 21 % |
| March | 30 | 66 | 9 | 14 % |
| April | 33 | 60 | 12 | 20 % |
| May | 44 | 95 | 15 | 16 % |
| June | 22 | 32 | 11 | 34 % |
| July | 15 | 27 | 11 | 41 % |
| August | 17 | 23 | 9 | 39 % |
| September | 9 | 14 | 8 | 57 % |
| October | 9 | 13 | 4 | 31 % |
| November | 8 | 10 | 5 | 50 % |
| December | 3 | 8 | 5 | 63 % |
When findings of urine drug screens were reported (n = 118), 18 patients (15.3 %) tested positive for cocaine and nine patients (7.56 %) tested positive for phencyclidine. Interrater reliability of chart review between the two reviewers of 10 % of cases was excellent, with perfect agreement on all charts (kappa = 1.0).
Review of Hospital Records
In 11 of the exposure cases, hospital records or medical examiner’s reports were obtained and reviewed beyond the poison center case notes. Only two of the hospital records reviewed were obtained from the hospital where the CPC toxicologists worked. Four exposure cases were selected to present in detail due to unique presentation.
Case 1
A 50-year-old man injected a “bath salt” product intravenously four times, 3 days prior to presentation. Past medical history was significant for aortic valve replacement, colon resection, depression, anxiety, chronic back pain, acid reflux, and substance abuse (cocaine). Routine medications included warfarin, lisinopril, and fluoxetine. His initial complaint on arrival was back pain and depression. He noted increased anxiety and inability to urinate for roughly 48 h. On physical examination, he did not appear acutely intoxicated and had minimal erythema noted around the injection site on his antecubital fossa. Vital signs were: blood pressure 139/86 mmHg, heart rate 85 beats/min, respiratory rate 20 breaths/min, oxygen saturation on room air 98 %, and oral temperature 95.6 °F. Initial laboratory studies included a white blood cell count 17.3 × 103 U/L; hemoglobin 11.4 g/dL; and platelets 104 × 103 U/L. Electrolytes were normal with the exception of a serum bicarbonate of 11 mmol/L; blood urea nitrogen (BUN) of 83 mg/dL; and creatinine of 6.0 mg/dL. Aspartate aminotransferase was 4,054 U/L, alanine transaminase was 757 U/L, international normalized ratio (INR) was 3.72, and creatinine kinase was 238,400 U/L. He was given 2 units of fresh frozen plasma for his elevated INR, which continued to rise to 7.04 nine hours after presentation. No active bleeding was documented in his records. On hospital day 1, he was transferred to a tertiary care facility. A sodium bicarbonate infusion was started, and his BUN and creatinine continued to rise over the next several days (BUN 111 mg/dL and creatinine 15.07 mg/dL on hospital day 9). On hospital day 11, a dialysis catheter was placed for continued oliguria with elevation of BUN (111 mg/dL) and creatinine (15.9 mg/dL). Daily hemodialysis was initiated but discontinued on hospital day 18 after the patient’s urine output increased to 200 mL/h and creatinine decreased to 6.72 mg/dL. During the remainder of his hospitalization, the patient’s urine output returned to normal, and his BUN and creatinine continued to improve. He was discharged home on hospital day 30 with a BUN of 36 mg/dL and creatinine of 2.61 mg/dL. Confirmatory testing was not available at the time this case was managed.
Case 2
A 40-year-old male insufflated and injected “bath salts” and became extremely agitated. He was shocked with an electronic control device and brought to the hospital where he went into cardiac arrest and was found to be hyperthermic. He was resuscitated but developed disseminated intravascular coagulopathy, rhabdomyolysis, and brain death. Toxicology laboratories performed found MDPV in his serum (82 ng/mL) and urine (670 ng/mL). His death was attributed to excited delirium syndrome from MDPV intoxication. This case was recently published in detail [5].
Case 3
A previously healthy 22-year-old male who reported snorting a product called “eight ballz” multiple times daily over the preceding 5 days. He was on no routine medications. During this 5-day period (time of “bath salt” use), he had not slept in part due to desired drug effect and in part because when he closed his eyes he had visual disturbances that induced fear and prevented sleep. On the fourth day of using this product, he was noted by a friend to be pacing continually and having some twitching movements of his arms and tongue. This friend verified the history to the examining physician. He continued to use this product and these movements became uncontrollable over the next 12–16 h prompting his presentation to the emergency department. The patient also admitted of visual and auditory hallucinations of the presence of people not in the room. Vital signs on arrival were: blood pressure 156/77 mmHg, pulse 140 beats/min, respiratory rate 22 breaths/min, and oral temperature 98.4 °F. On physical examination, the patient was lying in bed with choreoathetoid movements of his arms and legs, as well as writhing movements of his tongue but no fasciculations. His pupils were enlarged at 6 mm but reactive, skin was mildly diaphoretic, and there was increased warmth to touch noted. He had obvious interaction with hallucinations during the examination. Initial control of his movements was attempted unsuccessfully with 2 mg of IV lorazepam. Subsequently the patient was given 4 mg of lorazepam IV and 2.5 mg of haloperidol IV with complete resolution of his movements and induction of sleep. His electrolytes, lactate, venous blood gas, hemoglobin, and platelets were normal. Creatinine kinase (990 U/L) and white blood cells (18.9 × 109/L) were elevated. A qualitative urine drug screen for amphetamines, benzodiazepines, barbiturates, cocaine, marijuana, and opiates was negative. The patient had brought the purchased product with him to the hospital and this, as well as serum and urine, was sent for analysis to NMS Laboratories (Forensic Science Department, Willow Grove, PA) where further testing confirmed that the product contained MDPV and benzocaine. The patient’s serum level of MDPV was 72 ng/mL and urine level was 2,400 ng/mL. On repeat evaluation the next day, the patient was completely back to baseline with no lingering choreoathetoid movements of his extremities or tongue. He had slept continuously for several hours and felt like he had “lost a day.” Further questioning revealed the patient had previously used cocaine as a teenager and that this product had very similar desired effects but lasted longer than cocaine. The patient was contacted several days after discharge by phone and reported no cravings or recurrent nightmares.
Case 4
A 38-year-old man was reported to have difficulty breathing but was found partially naked and in asystole on EMS arrival. A bottle of “Q bath salts” was found in the home. Postmortem toxicology reports demonstrated 7-aminoclonazepam (0.083 mg/L), benzyoylecgonine (0.031 mg/L), fluoxetine (0.89 mg/L), norfluoxetine (0.33 mg/L), tramadol (0.83 mg/L), and MDPV (0.22 mg/L) in serum. Hepatic tissue contained fluoxetine (36 mg/kg) and norfluoxetine (11 mg/kg). Cause of death was considered accidental and related to MDPV toxicity with cocaine and fluoxetine toxicity as contributing agents.
Discussion
When new substances of abuse emerge, clinical recommendations are difficult to make when there is little in the medical literature to guide practitioners. In the case of the β-keto phenylalkylamines, there is almost no animal data and clinical experiences have been reported only recently with products in the USA; these reports differ when compared to the experiences in the United Kingdom (UK) with similarly labeled products [2, 6]. In an effort to better assist physicians treating “bath salt” and “plant food” exposures, we evaluated the presenting symptoms of reported exposure cases along with some of the treatment experience. Our review found that most exposure cases presented with tachycardia, agitation, confusion, hallucinations, and hypertension. Benzodiazepines and IV fluids were the most common therapies provided. Practitioners used antipsychotics, antihistamines, intubation, and dexmedetomidine to control agitation. Intubated patients were predominantly sedated with propofol or benzodiazepines.
The pattern of calls from counties in our state to report “bath salt” and “plant food” exposures was intriguing to us. A large number of our initial calls came from counties that contained military instillations. As the volume of calls to report exposures increased, the origins of calls within North Carolina became more widespread. Once state legislation banned these products (June 1, 2011), there was a marked decline in the total number of calls received, but there also appeared to be an increase in the percentage of calls from counties bordering other states without similarly strict bans on these products. It is difficult to say if this increase was due to increased use, continued availability, or a combination of both.
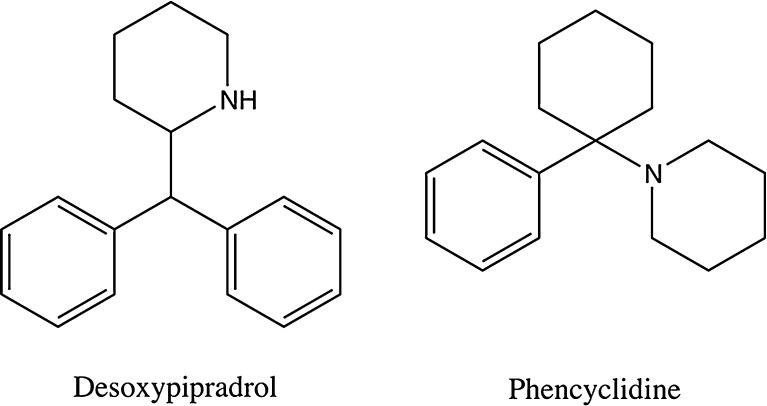
As many patients reported using these products as a legal alternative or cheaper substitute for cocaine, we were not surprised to see several reports of cocaine use concomitantly or urine drug screens positive for cocaine. The finding of phencyclidine (PCP) on drug screens may have been related to cross-reactivity with dextromethorphan, as some patients reported ingesting these products with cough and cold medication. It is unlikely this was a reflection of true PCP exposure, as its use has declined markedly in the USA and it is relatively uncommon in our area. A UK report that desoxypipradrol (a piperidine) was found in “bath salt” products that had previously contained MDPV may actually indicate these PCP-positive drug screens were the result of cross-reactivity between the assay for PCP and desoxypipradol due to structural similarities (see Fig. 4); further research in this area is warranted [7].
Fig. 4.
Chemical structures of selected piperidine derivatives
Based on a review of the deaths reported to CPC, excited delirium syndrome (ExDS) may cause some “bath salt”- or “plant food”-related deaths. Excited delirium is a well-described syndrome involving delirium with severe agitation that includes paranoia, violence towards other, excessive physical strength, and hyperthermia [8]. Symptoms often culminate with abrupt cessation of movement followed by respiratory and cardiac arrest [8]. Stimulant use often precipitates ExDS, and there is evidence that dopamine excess may be a pathophysiologic trigger [8]. In the five CPC deaths, we reviewed both the medical examiner’s report and the hospital records in one case and medical examiners’ reports for two other cases; these three patients had MDPV exposure as evidenced by the presence of MDPV in the blood. Additionally, one of the medical examiners ruled the patient’s cause of death as “due to excited delirium syndrome as a result of MDPV exposure.” Two of the deaths reported described sudden cardiac arrest and death of extremely agitated male patients almost immediately after physical restraint and administration of ziprasidone; both patients were suspected to have used “bath salts” prior to hospitalization, and it is possible that these patients also experienced ExDS [4, 5]. Unfortunately, despite multiple attempts, we were unable to obtain hospital records for further review of these cases. Of note, ziprasidone has been used in multiple patients with reported “bath salt”-related agitation/delirium without adverse effects, thus this association is unlikely causal.
A review of our limited patients with confirmatory tests, as well as the experience of Spiller et al., suggest that MDPV may be a key ingredient in “bath salt” products found in the southern and southeastern regions of the USA. Spiller’s group reported agitation, combative violent behavior, tachycardia, and hallucinations, which are very similar to the clinical effects reported in this study [4]. Clinical effects reported differed from a UK study of 205 reported mephedrone users, where the adverse effects most commonly reported included bruxism, paranoia, sore nasal passages, and hot flashes [2]. A difference in the primary active β-keto phenylalkylamines contained in these products may explain the difference in clinical effects that were reported in the UK and the clinical effects we report, as mephedrone was initially more prevalent in similar products found in the UK.
Limitations of this study include lack of timely and efficient confirmatory laboratories, self-reporting of effects, clinical effects from co-ingestants, potential physician presumed use of “bath salt” or “plant food” products based on symptoms and media focus, incomplete capturing of all exposure cases by the poison center, and the limitations of charting that come with poison center chart review. Additionally, while existing laboratory testing can detect MDPV, mephedrone is unstable and there may be cases where it was also present but not detected. The laboratory where most civilian samples are sent for testing in our region has not performed testing for methylone to date; therefore, we cannot comment on methylone’s contribution to the symptoms documented above. The majority of hospital records were not available for review to confirm clinical effects, therapies, and route of use documented in our poison center records.
Conclusions
Patients presenting after exposure to “bath salt” or “plant food” products containing β-keto phenylalkylamines experience symptoms similar to that seen with stimulant intoxication. The most commonly reported clinical effects were tachycardia, agitation, confusion, hallucinations, and hypertension. Rhabdomyolysis, renal failure, excited delirium syndrome, and death may be serious complications. Treatment recommendations would include sedation with benzodiazepines, aggressive cooling for hyperthermic patients, and use of small doses of antipsychotics for choreoathetoid movements not controlled with benzodiazepines. Further correlation of specific β-keto phenylalkylamines and clinical effects is needed but is very difficult based on current testing availability.
Acknowledgments
Conflicts of Interest
There are no conflicts to declare.
References
- 1.Wood DM, Davies S, Greene SL, et al. Case series of individuals with analytically confirmed acute mephedrone toxicity. Clin Toxicol (Phila) 2010;48:924–927. doi: 10.3109/15563650.2010.531021. [DOI] [PubMed] [Google Scholar]
- 2.Dargan PI, Albert S, Wood DM. Mephedrone use and associated adverse effects in school and college/university students before the UK legislation change. Q J Med. 2010;103:875–879. doi: 10.1093/qjmed/hcq134. [DOI] [PubMed] [Google Scholar]
- 3.Kriikku P, Wilhelm L, Schwarz O, Rintatalo J. New designer drug of abuse: 3,4-methylenedioxypyrovalerone (MDPV). Findings from apprehended drivers in Finland. Forensic Sci Int. 2011;210:195–200. doi: 10.1016/j.forsciint.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- 5.Murray BL, Murphy CM, Beuhler MC (2012) Death following recreational use of designer drug “bath salts” containing 3,4-methylenedioxypyrovalerone (MDPV). J Med Toxicol. doi:10.1007/s13181-011-0196-9 [DOI] [PMC free article] [PubMed]
- 6.Wood DM, Greene SL, Dargan PI (2010) Clinical pattern of toxicity associated with the novel synthetic cathinone mephedrone. Emerg Med J. doi:10.1136/emj.2010.092288 [DOI] [PubMed]
- 7.Important ban on psychoactive drug. UK Government Home Office (2010) http://www.homeoffice.gov.uk/media-centre/news/drug-import-ban. Accessed 2 Feb 2012
- 8.Takeuchi A, Ahern T, Henderson S. Excited delirium. West J Emerg Med. 2011;12(1):77–83. [PMC free article] [PubMed] [Google Scholar]