Abstract
Multiple mating by females is widely thought to encourage post-mating sexual selection and enhance female fitness. We show that whether polyandrous mating has these effects depends on two conditions. Condition 1 is the pattern of sperm utilization by females; specifically, whether, among females, male mating number, m (i.e. the number of times a male mates with one or more females) covaries with male offspring number, o. Polyandrous mating enhances sexual selection only when males who are successful at multiple mating also sire most or all of each of their mates' offspring, i.e. only when Cov♂(m,o), is positive. Condition 2 is the pattern of female reproductive life-history; specifically, whether female mating number, m, covaries with female offspring number, o. Only semelparity does not erode sexual selection, whereas iteroparity (i.e. when Cov♀(m,o), is positive) always increases the variance in offspring numbers among females, which always decreases the intensity of sexual selection on males. To document the covariance between mating number and offspring number for each sex, it is necessary to assign progeny to all parents, as well as identify mating and non-mating individuals. To document significant fitness gains by females through iteroparity, it is necessary to determine the relative magnitudes of male as well as female contributions to the total variance in relative fitness. We show how such data can be collected, how often they are collected, and we explain the circumstances in which selection favouring multiple mating by females can be strong or weak.
Keywords: polyandrous mating, sperm competition, post-mating sexual selection, opportunity for sexual selection, female life-history, cryptic female choice
1. Introduction
We have three goals in writing this article: (i) to identify the circumstances in which multiple mating by females influences post-mating sexual selection; (ii) to describe how these circumstances can be most effectively measured; and (iii) to document how often current researchers actually collect these data. In the context of our paper, we define sexual selection as a sex difference in the variance in relative fitness resulting from differential parentage among the individuals of one sex [1]. Our definition follows Darwin [2, p. 205] in focusing on ‘the advantage certain individuals have over others of the same sex and species in exclusive relation to reproduction’.
We begin by reviewing the current literature on how multiple mating by females is thought to affect male fitness variance in the context of post-mating sexual selection. We next describe the condition necessary for post-mating sexual selection to occur, in particular, whether the pattern of sperm utilization by females allows male mating number, m (i.e. the number of times a male mates with one or more females; the average mating number is known as ‘mating number promiscuity’ ([1]; see §1c(iv)) to covary with male offspring number, o. We argue that polyandrous mating enhances sexual selection only when males who are successful at multiple mating also sire most or all of each of their mates' offspring, i.e. only when Cov♂(m,o), is positive. We describe the parameters necessary to quantify the pattern of sperm utilization by females, and using a worked example, we explain how this condition affects the strength of post-mating sexual selection on males. We then summarize in tabular form how often these results are reported in the current literature. Our review focuses on gonochoristic animals because sexual selection, when it exists, is likely to be stronger and is less complicated to quantify in these species [1,3]. However, we illustrate our method for quantifying the opportunity for post-mating sexual selection using an excellent dataset from hermaphroditic snails [4], because this study is one of the few sufficiently detailed to contain the parameters we require.
We next review the current literature on how the pattern of female reproductive life-history, i.e. the number of times females mate and produce offspring, is thought to affect female fitness variance. Although multiple mating by females is widely thought to enhance female fitness indirectly (i.e. through enhanced offspring fitness), we argue that the direct fitness benefits females gain from polyandrous mating are sufficient to explain this behaviour. We explain that polyandrous mating by females enhances female fitness only when females who mate repeatedly produce the most offspring, i.e. when the covariance between female mating number, m, and offspring number, o, Cov♀(m,o), is positive. We describe the parameters necessary to document this covariance, as well as how simultaneously considering its effects in both sexes provides a means for understanding when post-mating sexual selection will be strong or weak. We explain how variation in female life-history can enhance or diminish the strength of sexual selection, and we describe how such variance may be partitioned and explored experimentally. We summarize in tabular form how often these results for females are reported in the current literature, and lastly, we explain how well the data for females, as well as those for males, coincide with current views of post-mating sexual selection and polyandrous mating.
Overall, we find the widespread perception that multiple mating by females can simultaneously encourage sexual selection in males, as well as enhance female fitness, is unsupported; these are usually mutually exclusive conditions. Furthermore, we show that although multiple mating may be common, direct, quantitative evidence of post-mating sexual selection is surprisingly scarce (i.e. the necessary positive covariance between mating number and fertilization success among males, Cov♂(m,o), [1,5]), in most cases, is either unmeasured or does not exist. We also show that post-mating sexual selection, occurring by sperm competition and/or cryptic female choice (see §2a), is seldom accurately measured because most studies do not include non-mating males in their estimates of selection, or simply fail to measure selection at all. The majority of researchers focus instead on the predicted outcomes of an evolutionary history of sperm competition or, less often, on measurements of fitness variance within the class of mating males, the class usually representing the smaller of the two sources of the total variance in male offspring numbers (figure 1). While numerous studies report fitness gains accrued by females who mate repeatedly, the scale of these gains remains unknown because their relative contribution to total selection's strength is undocumented. Our goal is not to find fault with existing studies; the body of this literature is impressive in size, scope and detail. Rather, we seek to reveal where additional studies could more effectively substantiate, or more convincingly refute, the hypothesis that post-mating sexual selection is ubiquitous and strong [6–8]. We hope that our findings may stimulate re-analysis of past studies, as well as inspire future studies of multiple mating and post-mating sexual selection that emphasize the quantification of the actual evolutionary processes shaping male and female phenotypes.
Figure 1.
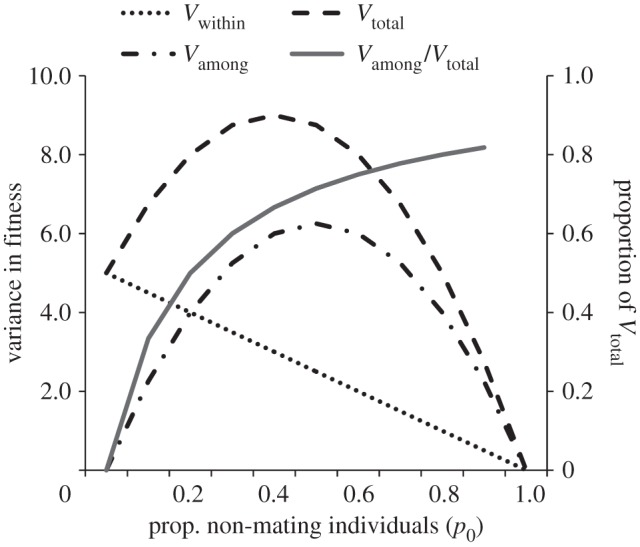
Partitioning the variance in offspring numbers for a simulated population of 100 males and 100 females as the proportion of non-mating individuals in the population, p0, increases using equation (2.3); in this population the mean and variance in offspring numbers are randomly distributed (mean ± s.d. = 5 ± 2.23); note that as p0 increases, the fraction of the total variance in offspring numbers, Vtotal, that lies among the classes of mating and non-mating males, Vamong/Vtotal (solid line), steadily increases.
2. Sexual selection and the pattern of sperm utilization by females
(a). Current literature
Sperm competition and cryptic female choice are recognized as significant sources of male fitness variance in a wide diversity of taxa (reviews in [3–10]). These two processes are presumed to be distinct; in sperm competition, male ejaculates or sperm vie for fertilization, either within or outside the female's body, whereas in cryptic female choice, females encourage or disfavour particular sperm or ejaculates in accomplishing syngamy, again either within or outside the female reproductive tract. In fact, the fitness outcomes of each process for males are identical, and are measurable in the same way, specifically, by identifying, at the population level, whether males who are successful in fertilizing ova represent a subset of the males who successfully mate. Because these processes at present are quantitatively indistinguishable, we will refer to the fitness outcomes of both sperm competition and cryptic female choice under the general heading of ‘post-mating sexual selection’.
Existing studies of post-mating sexual selection are of six types including: (i) positive correlations between particular male traits and male mating or fertilization success (although in most cases these results are obtained from experiments in which each female in the experimental population is allowed to mate with only two males, who each mate with no other females, followed by a search by researchers for patterns in fertilization success within the sample of experimental threesomes; e.g. last male mating precedence [8,11,12]); (ii) positive correlations between particular sperm traits and male fertilization success, again generally using pairs of males for each experimental female as described above [6–8,13–17]; (iii) positive correlations between testis mass or ejaculate volume and the presumed or experimentally manipulated level of sperm competition (reviews in [18–21]; (iv) evidence of differential insemination success or ejaculate retention induced within particular females by particular males [22]; and (v) evidence of mixed paternity within the clutches of individual females [23–25]. Until recently, relatively few studies have attempted to measure (vi) the variance in relative fitness arising from post-mating competition among males, or among hermaphroditic individuals emphasizing male function (i.e. the opportunity for sexual selection [4,26–31]). These and other results are summarized in table 1.
Table 1.
Existing studies of indicating post-mating sexual selection.
| taxon/common name (species) | positive correlation between male traits and mating or fertilization success (pairs of males per female) | positive correlation between sperm traits and mating or fertilization success (pairs of males per female) | positive correlation between testis mass/ejaculate volume and level of sperm competition | differential insemination success/ejaculate retention | mixed paternity within clutches | p0♂ | sire ID within clutches | Cov♂(m,o) | I♂ | Imates |
|---|---|---|---|---|---|---|---|---|---|---|
| Annelida | ||||||||||
| sessile polychaetes (Galeolaria caespitosa) [32] | x | x | (–) | |||||||
| Mollusca | ||||||||||
| freshwater snails (Biomphalaria glabra) [3] | x | x | (+) | x | ||||||
| freshwater snails (Physa acuta) [4] | x | x | x | x | (+) | x | ||||
| Hexapoda | ||||||||||
| bushcrickets (Kawanaphila nartee) [33] | x | |||||||||
| bushcrickets (Requena verticalis) [34] | x | |||||||||
| field cricket (Gryllus integer) [35] | x | |||||||||
| ground crickets (Allonemobius socius) [36] | x | (+) | ||||||||
| field cricket (Gryllus integer) [37] | x | x | x | |||||||
| harvester ants (Pogonomyrmex inermis) [38] | x | |||||||||
| harvester ants (Pogonomyrmex pronotalis) [38] | x | |||||||||
| neotropical ants (Pachycondyla inversa) [39] | x | |||||||||
| neotropical ants (Pachycondyal villosa) [39] | x | |||||||||
| bean beetles (Callosobruchis chinensis) [40] | ||||||||||
| flour beetles (T. castaneum) [41] | x | |||||||||
| red flour beetle (T. castaneum) [42] | x | (–) | ||||||||
| seed beetle (Stator limbatus) [43] | x | (–) | ||||||||
| firefly (Photinus spp.) [44] | x | |||||||||
| moths (Plodia interpunctella) [45] | x | |||||||||
| seaweed fly (Coelopa frigida) [46] | x | |||||||||
| fruitflies (Drosophila serrata) [47] | x | x | ||||||||
| fruitflies (Drosophila melanogaster) [48] | x | |||||||||
| fruitflies (D. melanogaster) [49] | (–) | |||||||||
| fruitflies (D. melanogaster) [28] | ||||||||||
| fruitflies (D. melanogaster) [50] | x | x | x | x | (+) | |||||
| fruitflies (D. melanogaster) [51] | x | x | x | (+) | x | x | ||||
| fruitflies (Drosophila virilis) [51] | x | x | x | (+) | x | x | ||||
| fruitflies (Drosophila lummei) [51] | x | x | x | 0 | x | x | ||||
| fruitflies (Drosophila bifurca) [51] | x | x | x | 0 | x | x | ||||
| Crustacea | ||||||||||
| American lobster (Homarus americanus) [52] | x | x | ||||||||
| blue crab (Callinectes sapidus) [53] | x | x | x | |||||||
| copepods (Lepeophtheirus salmonis) [54] | x | x | ||||||||
| Chelicerata | ||||||||||
| orb web spider [55] | ||||||||||
| pseudoscorpions (Cordylochernes scorpioides) [56] | (–) | |||||||||
| Echinodermata | ||||||||||
| sea star (Asterias sp.) [57] | (–) | |||||||||
| sea urchin (Heliocidaris erythrogramma) [58] | (–) | |||||||||
| Urochordata | ||||||||||
| ascidians (Pyura stolonifera) [59] | (+) | |||||||||
| ascidians (Styela plicata) [60] | x | |||||||||
| Pisces | ||||||||||
| Atlantic salmon (Salmo salar) [61] | x | x | ||||||||
| cichlid (Opthamotilapia verntralis) [62] | x | 0 | ||||||||
| guppies (Poecilia reticulatla) [63] | x | x | x | (+) | ||||||
| lingcod (Ophidion elongatus) [64] | x | x | (+) | |||||||
| lemon sharks (Negaprion brevirostris) [65] | x | x | x | |||||||
| rainbow darter (Etheostoma caeruleum) [66] | x | x | (–) | |||||||
| sandbar sharks (Carcharhinus plumbeus) [29] | x | x | (–) | x | x | |||||
| zebrafish (Danio rerio) [67] | x | (–) | ||||||||
| African cichlid fish (29 species) [68] | x | |||||||||
| Amphibia | ||||||||||
| frog (Crinia georgiana) [69] | x | |||||||||
| toads (Bufo bufo) [70] | x | |||||||||
| Reptiles | ||||||||||
| adder (Vipera berus) [71] | x | |||||||||
| Amazon river turtle (Podocnemis unifilis) [72] | x | |||||||||
| common lizard (Laecerta vivipara) [73] | x | |||||||||
| green turtles (Chelonia mydas) [74] | x | x | 0 | |||||||
| water snakes (Nerodia sipedon) [75] | x | x | x | x | (+) | |||||
| tuatara (Sphenodon punctatus) [76] | x | x | x | x | ||||||
| water snakes (Nerodia sipedon) [77] | x | x | ||||||||
| Aves | ||||||||||
| coal tit (Parus ater) [78] | x | |||||||||
| dunnocks (Prunella modularis) [79] | x | x | x | x | (+) | |||||
| Galapagos hawk (Buteo galapagoensis) [80] | x | x | ||||||||
| parrots (Eclectus sp.) [81] | x | x | ||||||||
| passerines (42 species) [82] | x | |||||||||
| scrubwren (Sericornis frontalis) [83] | x | x | x | x | ||||||
| stripe-backed wren [84] | ||||||||||
| tree swallow (Tachycineta bicolor) [85] | x | x | ||||||||
| red jungle fowl (Gallus gallus) [86] | x | x | x | (+) | x | |||||
| Mammalia | ||||||||||
| Arctic fox (Alopex lagopus) [87] | x | |||||||||
| Australian marsupials (Antechinus stuartii) [88] | x | x | x | (+) | ||||||
| Australian marsupials (Antechinus agilis) [89] | x | |||||||||
| dwarf mongoose (Helogale parvula) [90] | x | x | ||||||||
| golden lion tamarin (Leontopithecus rosalia) [91] | x | x | ||||||||
| wood mice (Apodemus sylvaticus) [24] | x | x | x | x | (+) | |||||
| striped field mouse (Apodemus agrarius) [92] | x | |||||||||
| wood mice (A. sylvaticus) [92] | x | |||||||||
| wood mice (A. sylvaticus) [93] | x |
Only studies of type (vi) constitute direct, quantitative evidence of post-mating sexual selection because they measure either the opportunity for sexual selection [1], or selection intensity acting directly on mate numbers (via Bateman gradients, [94,95]) or on traits such as body size ([3,4,31]; see also §2b). However, even in this last category, most studies summarized in table 1 use genetic paternity data to document the existence of multiple mating or to measure the variance in offspring numbers within the class of successfully mating males (but see [4,94]). While indicating the potential for post-mating sexual selection, as shown in figure 1, such estimates tend either to overestimate or underestimate the variance in male fitness, sometimes severely [96]. Thus, while indirect or correlational evidence of post-mating sexual selection is abundant, such studies can imply the existence of strong sexual selection when such selection is actually weak (less than 2% of total selection [94]). Without direct, quantitative evidence of the magnitude of post-mating sexual selection intensity at the population level, understanding of this phenomenon must be considered incomplete.
(b). Direct evidence of post-mating sexual selection
As Darwin [2] first observed, in order for sexual selection to occur, certain males within a population must experience proportionate reproductive success. Concomitantly, when some males are disproportionately successful in siring offspring, other males must be excluded from siring any offspring at all [1,96]. This same principle holds for post-mating sexual selection. Thus, among the males who are successful at mating, some males must be disproportionately successful at fertilizing ova. This condition requires that certain successfully mating males are unsuccessful at fertilizing any ova at all [1]. Stated differently, in order for sexual selection to intensify by either sperm competition or cryptic female choice, male fitness variance post-mating must exceed that which exists pre-mating. If the reverse condition is true, post-mating processes will tend to reduce the intensity of sexual selection overall.
Abundant evidence suggests that within individual females, particular males' sperm do experience differential fertilization success [97–99]. However, these circumstances are not always sufficient to generate measurable sexual selection within a population. We assert that to reach the conclusion that post-mating sexual selection occurs, researchers must obtain three kinds of data. First, to demonstrate the existence of such sexual selection, it is necessary to document the proportions of males within a population (not simply between pairs of males (cf. [8,13–17])) who are successful, as well as males who are unsuccessful, in siring offspring [1]. This is necessary because the variance in male fitness consists of two components: the variance in fitness within the class of successful males, and the variance in fitness between the classes of successful and unsuccessful males [26,27]. As sexual selection intensifies, the latter component of fitness variance represents the larger of the two components of total selection (figure 1). Documentation of only the fitness of males who successfully fertilize ova does not permit the estimation of the between-male (also called the among-male) component of fitness [100]. Similarly, studies involving pairs of males who each mate with a particular female, but do not mate with any other females [13–17], do not provide a means for estimating the among-male component of fitness variance; such results reveal only the relative success of the two males who mate with a particular female. While the results obtained from experimental threesomes may be replicated within an experiment, and while they may elegantly simulate the common phenomenon among insects of sperm displacement, wherein the last male to mate removes or inactivates nearly all of the sperm of previously mating males [6–9], even robust replication of this method within an experiment cannot reveal the relative fertilization success that any one male within the research population experiences, relative to other males mating with different females within the same population (but see [31]). Such experiments, like those focusing only on successful males, do not reveal the subset of males who successfully mate, yet fail to fertilize ova within the population of mated females. As a result, such results cannot reveal the actual magnitude of post-mating sexual selection in nature.
Second, in order to document the existence of disproportionate fertilization success among the mating males, it is necessary to document the magnitude and sign of the covariance between mating number, m, and offspring number, o, among males (Cov♂(m,o)) [1,101], as well as the magnitude and sign of the covariance between mating number, m, and offspring number, o, among females, Cov♀(m,o) (see §3c(ii)). Estimating this covariance for males is necessary because, as stated above, multiple mating by females enhances sexual selection only when a subset of the successfully mating males are differentially successful in siring young. Estimating this covariance for females is necessary as well because multiple mating by females enhances female fitness only when this covariance is positive [1]. Moreover, a sex difference in the sign of this covariance provides a quantitative measure of sexual conflict, as well as explicit predictions about the relative propensities of each sex to seek or not to seek multiple mating, independent of sex differences in gametic investment [101]. Different mating systems can influence the magnitude and sign of this covariance for both sexes. Thus, understanding the possible outcomes of these covariances provides a means for predicting when post-mating sexual selection can be strong or weak.
Third, to determine how much of total sexual selection can be attributed to post-mating competition among the ejaculates of successfully mating males, it is necessary to (i) identify the fraction of males who fail to mate, as well as the fraction of males who mate successfully but fail to sire offspring; (ii) document the mean and variance in offspring numbers for males and females; (iii) partition total sexual selection into its pre- and post-mating components; (iv) document the total opportunity for selection on males and females; and (v) estimate the sex difference in the opportunity for selection (i.e. the opportunity for sexual selection [1,26,101,102]). These steps are necessary because, although post-mating sexual selection is generally assumed to be strong, the actual strength of such selection relative to selection in other contexts has rarely been measured. Where it has been measured, the strength of such selection appears to be small (less than 2% [31]).
(c). Identifying the appropriate parameters for males
(i). Fundamental parameters
If the parentage of all offspring produced in each generation can be accurately assigned, estimation of all of the parameters mentioned above is straightforward. Methods for estimating the proportions of mating and non-mating individuals within each sex, pS, and p0, respectively (where [pS + p0] = 1), are available in [1], as are methods for estimating the average and variance in offspring numbers for each sex, O and VO, respectively, as well as the opportunity for selection within each sex, as I = VO/O2.
(ii). Variance in male fitness and the opportunity for sexual selection
Wade [26] showed that when males differ in mate numbers, the total variance in male offspring numbers can be determined if the mean and variance in offspring numbers for females, O♀, and VO♀, are known. This is true because the average number of offspring per male equals RO♀, where R is the average number of mates per male, N♀/N♂ (i.e. the sex ratio (cf. [1])). Also, the total variance in male fitness, in terms of offspring numbers, VO♂total, can be partitioned into two components, VO♂within, the average of the variances in offspring number within the classes of mating males, and VO♂among, the variance in the average number of offspring between the mating and non-mating male classes, such that VO♂total = VO♂within + VO♂between. As shown elsewhere [1,26,102], when the total variance in male fitness is divided by the squared average in male fitness, (RO♀)2, the opportunity for selection on males, I♂, equals the opportunity for selection on females, I♀, adjusted by the sex ratio, R, plus the opportunity for selection due to differences in mate numbers among males, Imates (= Is [27,103]), or I♂ = 1/R (I♀) + Imates. When the sex ratio, R, equals unity, this expression can be rearranged to show that I♂ − I♀ = Imates.
The opportunity for sexual selection thus equals the sex difference in the opportunity for selection [1,26,27]. Strong sexual selection indicates that variance in relative fitness for males, I♂, is greater than the variance in relative fitness for females, I♀. Similarly, when the variance in relative fitness for females increases, it comes at the expense of sexual selection (a relationship that is not clear in [103] because the opportunity for selection in each sex is called Is). To account for conventional and sex role-reversed species, Shuster & Wade [2] called the sex difference in the opportunity for selection ΔI, which is positive in species with conventional sex roles, and negative in species that are sex-role reversed.
(iii). The opportunity for post-mating sexual selection
With accurate paternity assignment, the fraction of mating males can be further subdivided to reveal the opportunity for selection due to the effects of post-mating sexual selection. When considering only successful and unsuccessful males, Wade & Shuster [104,105] showed that the total variance in male fitness can be partitioned into two quantities, the variance in fitness within the class of individuals who successfully reproduce, V♂within, and the variance in fitness between the successfully and unsuccessfully reproducing classes, V♂between.
When members of the male population can be identified as belonging to successful, pS♂, and unsuccessful, p0♂, classes (where pS♂ = [1 − p0♂]), and when the variance in offspring numbers among the successful males, VO♂, is known, the within-group variance in male fitness, VO♂within, is equal to the average of the variance in fitness for each of the reproducing classes; that is, the variance in fitness for each class weighted by its population frequency. Since there are only two classes, successful and unsuccessful, this expression can be written as
| 2.1 |
where VO♂ is the variance in offspring numbers among the males who secure mates.
When the average in offspring numbers among the successful males, O♂, is known, the component of the variance in male fitness that exists between the classes of successfully and unsuccessfully reproducing individuals, VO♂between, is equal to the difference in the average number of offspring between the two classes, squared, or (O♂ − 0)2, multiplied by the variance between the fitness categories, (p0♂)(1 − p0♂), or
| 2.2 |
The total variance in male fitness in terms of offspring numbers, VO♂total, is the sum of these two variance components, or
| 2.3 |
Consider again that when post-mating sexual selection occurs, the male population consists of not just two, but three fractions: (i) the fraction of males that fails to mate, p0♂, (ii) the fraction of males that mates but fails to sire offspring, pSm0♂ and (iii) the fraction of males that mates and sires offspring because they possess competitive or preferred sperm, pS♂. Each of these fractions of the population sum to unity, or
| 2.4 |
If we first use (p0♂ + pSm0♂) to estimate the unsuccessful fraction of the male population, p0♂, in equation (2.3), we obtain the total variance in fitness in terms of offspring numbers for the reproducing and non-reproducing males, VO♂total. Rewritten, equation (2.3) now equals
| 2.5 |
If we next use only p0♂ [instead of (p0♂ + pSm0♂)] to estimate the unsuccessful fraction of the male population in equation (2.3), we obtain the variance in male fitness that is due to all sources of selection except that which is caused by differences among mating males in their ability to sire young. We call this fraction, the variance in male mating success due to pre-mating fitness components, VO♂pre. The total variance in male fitness is equal to the sum of pre-mating and post-mating fitness components, or
| 2.6 |
By subtracting VO♂pre from both sides of equation (2.6), we have,
| 2.7 |
wherein VO♂post equals the variance in male fitness that is due to the effects of post-mating sexual selection. By dividing equation (2.7) by the squared average fitness for males, O♂2, we obtain the opportunity for selection among males that is due to post-mating sexual selection, I♂post = IO♂total − IO♂pre. The relative contribution of post-mating sexual selection to other sources of selection can then be expressed as the ratio of these parameters. For example, the fraction of the total opportunity for selection on males due to post-mating sexual selection can be estimated as I♂post/I♂total.
A recent dataset obtained from hermaphroditic snails, Physa acuta, provides a worked example for this approach: Pélissié et al. [4] report the mating success (cMS) and the reproductive success (gMS) for 120 snails mating and producing offspring via male and female function. Using their figure 1, we identified all of the variables mentioned in equation (2.5) for individuals reproducing as functional males and as functional females (table 2). Although we did not estimate all of the variables mentioned in [4], we verified our estimates of the mean and variance in mating numbers and offspring numbers, and the opportunity for selection for both sexes from their table. When we partitioned the total variance in fitness into the fitness components identified in equations (2.1)–(2.7) for each sex, the total variance in relative fitness (Itotal) equalled 0.45 and 0.44 for males and females, respectively. Using equation (2.7), we found the proportion of the total variance in relative fitness due to post-mating sexual selection equal to 0.29 and 0.26 for males and females, respectively (table 2). The lack of confidence limits for the results in [4], as well as in ours, limits the conclusions possible from both analyses. However, our results are consistent with the conclusions of the authors in [4] who argue that pre-mating sexual selection is stronger than post-mating sexual selection in this species.
Table 2.
Estimating the opportunity for selection due to pre-mating and post-mating sexual selection in hermaphroditic freshwater snails (P. acuta) using equations (2.1)–(2.7) and data from Pélissié et al. [4]; parameters explained in text; asterisk (*) values for Is match those reported in [4], indicating that our estimates of the mean and the variance in cMS and gMS from [4] for each sexual function are correct.
| males |
females |
|||
|---|---|---|---|---|
| parameter | ||||
| M (cMS) | 2.13 | 2.13 | ||
| VM | 0.66 | 0.94 | ||
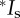
|
0.15 | 0.21 | ||
| O (gMS) | 1.48 | 1.47 | ||
| Vo | 0.80 | 0.77 | ||
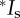
|
0.37 | 0.36 | ||
| po | 0.03 | 0.05 | ||
| pSm0 | 0.16 | 0.14 | ||
| pS | 0.82 | 0.81 | ||
| VOtotal = (pS)Vo + O2(p0 + pSm0)(pS) | ||||
| VOwithin | 0.65 | 0.62 | ||
| VOamong | 0.33 | 0.33 | ||
| VOtotal | 0.98 | 0.95 | ||
| VOpre = (pS)VO + O2(p0)(pS) | ||||
| VOwithin | 0.65 | 0.62 | ||
| VOamong | 0.04 | 0.09 | ||
| VOtotal | 0.70 | 0.71 | ||
| VOpost = VOtotal − VOpre | % of Itotal | % of Itotal | ||
| VOwithin | 0.98 | 0.95 | ||
| VOamong | 0.70 | 0.71 | ||
| VOtotal | 0.28 | 0.25 | ||
| Itotal | 0.45 | 0.44 | ||
| Ipre | 0.32 | 71 | 0.33 | 74 |
| Ipost | 0.13 | 29 | 0.11 | 26 |
(iv). The covariance between mating numbers and offspring numbers for males
The covariance between male mating number and the number of offspring sired by males equals
| 2.8 |
where mioi is the product of individual mating number and offspring number for each ith male; here, mating number, m, is the number of matings per male counting each mating, whether with the same or with a different partner, as a single event. N♂ is the total number of males, O♂ is the average number of offspring per male and P♂ is the average male mating number promiscuity, i.e. the average number of matings per male [1,101]. Whether Cov♂(m,o) is positive, negative or zero can be understood by considering whether winners in post-mating sexual selection fertilize all, none or a random number of progeny with each of their mates. Although this expression may appear unfamiliar, estimates of the covariance between mate numbers and offspring numbers have a long history; this expression equals the numerator of the standardized covariance between mate numbers and offspring numbers, also known as the Bateman gradient [4,94,95,103].
When ‘winners fertilize all’ (WFA) [1] or even fertilize most of their mate's offspring, Cov♂(m,o) is positive and post-mating sexual selection is likely because only particular mating males are successful in siring young. Although other males may be successful in mating, their sperm are out-competed by or are less attractive than those of successful males; thus only a subset of the mating males are successful in producing offspring [5].
When male fertilization success is randomly distributed across females (‘winners fertilize some’, WFS [1], Cov♂(m,o) is zero, and post-mating sexual selection (particularly sperm competition) works much like a lottery, wherein male success overall is randomly distributed but males who allocate the most sperm to individual females are likely to be most successful in siring offspring [8,10]. Again, clustered ejaculates and sperm competition may exist, but the number of ejaculates each male assigns to a particular female, or whether individual males are able to limit the number of other ejaculates a given female receives, is what determines a male's overall success. Post-mating sexual selection is possible in such situations, but only to the degree that males are able to deliver disproportionate volumes of sperm to multiple females.
The common assumption is that males are able to produce as much sperm as is necessary to accomplish this task [106], but examples of sperm limitation for both sexes are remarkably common, given the importance of large sperm volumes to sperm competition by fair or loaded lottery [8]. Most outcrossing plants and broadcast-spawning animals routinely experience pollen or sperm limitation, causing sexual selection to be relatively weak [1,107]. Moreover, sperm limitation in species in which males may breed often is documented in a wide range of species, including nematodes, fruitflies, crabs, birds and sheep (review in [108]). Models of sperm competition [8] routinely assume a trade-off between the number of mates males obtain and the number of sperm they allocate per mate, such that males must ‘optimize’ ejaculate size or sperm number. The more limited individual males are in their ability to transfer large volumes of sperm or ejaculate to multiple females, the weaker post-mating sexual selection must be.
When males sire most or all the offspring of one of their mates, but sire few or no offspring with their other mates (‘winners fertilize one’, WFO), Cov♂(m,o) is negative, and post-mating sexual selection is impossible. Here, the success of each male depends on the female with whom he mates and fertilization success tends to be equitably distributed among males. Although ejaculates may be clustered within certain females, and although sperm competition or cryptic female choice may be responsible for the tendency for one male to sire the offspring of each female, a negative covariance indicates that no single male is more successful among females than another, i.e. post-mating sexual selection is nil [49,98]. This pattern of sperm utilization is typical of situations interpreted as ‘genetic complementarity [109,110]’.
(d). Measuring the appropriate parameters for males
Table 1 summarizes the current literature claiming to document post-mating sexual selection resulting from multiple mating by females. While the development of molecular techniques that allow the assignment of parentage has made estimating the above parameters easier [10,111,112], several persistent problems remain even with the most accurate assignments of parentage to offspring. In particular, documenting the existence of multiple paternity within broods is not equivalent to documenting the existence of fitness variance among males, the condition necessary to establish post-mating sexual selection. Although genetic markers detected multiple sires within families in 64 per cent of the studies reported (47/74, table 1), such markers were often insufficiently variable to allow unambiguous paternity assignment except under the most controlled experimental conditions. Exclusion analyses usually allow neither the number of successful males, pS♂, nor the number of unsuccessful males in a population, p0♂, to be accurately identified ([85,113,114], table 1); thus only 15 per cent of the 74 studies documented p0♂ (table 1). In some cases, attempts at paternity assignment simply failed, in cases where certain progeny were not assigned to any of the males within the genotyped population [47,65,87,88,93,115].
This does not mean that incomplete estimates of parentage are useless; it simply means that the power of inference for such studies decreases as the proportion of unidentified parents and offspring increases. When data are available on the average number of males per male or per female (H = harem size), the proportion of non-mating males within the population, p0♂, can be estimated using the equation, p0♂ = 1 − (R/H), wherein R equals the sex ratio [101]. However, in general, the current limitations of genetic paternity data prevent accurate estimates of the proportions of mating and non-mating males within the population. Perhaps not surprisingly, estimates of I♂ and Imates (i.e. Is vis [103]) are few, and only about one-third of studies report results indicating or inferring the sign of Cov♂(m,o) (28/74; table 1). However, consistent with our predictions, among these studies, male traits associated with sperm competition appear in 11/14 studies in which Cov♂(m,o) is positive, whereas no such traits are reported in 10/14 studies in which Cov♂(m,o) is zero or negative (Fisher's exact test, p = 0.021; table 1).
3. Sexual selection and the pattern of female life-history
(a). Current literature
Multiple mating by females is widely thought to enhance female fitness, either directly, by increasing the number of offspring females produce [116–120], or indirectly, by allowing females to increase the survivorship of their offspring via genes or resources they receive from males [85,87,88,121,122]. While multiple mating by females has been shown in diverse taxa to enhance female fitness directly (30–70% in 122 species [119]), indirect benefits females gain from multiple mating are less clear because of confusion about the data necessary to document this effect.
Results to date suggest that direct effects on female fitness are impressively large (table 3). Primarily for this reason, we choose here to consider only the direct fitness benefits females may gain from mates and mating. As noted by Arnqvist & Nilsson [119], direct effects of female fitness appear so pervasive that presumed indirect effects on female fitness are unnecessary to account for the widespread occurrence of polyandrous mating. However, our choice is also substantiated by Wolf & Wade [134], who identified additional reasons why indirect effects on female fitness gained through offspring are likely to have weak, inconsistent or intractable effects on parental fitness. These authors note that because offspring are related to each parent only by one-half, selection on parental genes affecting offspring fitness is only half as strong as selection on zygotically expressed genes with the same fitness effects [135]. Thus, assigning offspring fitness directly to parents can overestimate the evolutionary outcome of ‘good genes’ or other resources that offspring may receive from parents. Conditional or sex-limited exposure of such genes to selection during mate choice or parental care can weaken indirect selection still further, and further exaggerate the evolutionary importance of ‘genetic quality’.
Table 3.
Existing studies indicating direct fitness advantages gained by females through multiple mating.
| variance components of female life-history |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| taxon/common name (species) | parasite avoidance | harassment prevention | predation avoidance | replenish sperm | increased life span | Vclutches | Vcs,clutch | Vcs,sires | Vcs,females | Vmultiplemates | p0♀ | Cov♀(m,o) | I♀ |
| Annelida | |||||||||||||
| sessile polychaetes (Galeolaria caespitosa) [32] | (+)/(–) | ||||||||||||
| Mollusca | |||||||||||||
| freshwater snails (Biomphalaria glabra) [3] | x | (+) | x | ||||||||||
| freshwater snails (Physa acuta) [4] | x | x | (+) | (+) | x | ||||||||
| Insects | |||||||||||||
| field crickets (Gryllus veletis) [123] | (+) | x | x | (+) | |||||||||
| field crickets (Grylloides sigalltus) [123] | (+) | x | x | (+) | |||||||||
| field cricket (Gryllus integer) [35] | x | x | x | ||||||||||
| two-eyed field crickets (Gryllus bimaculatus) [116] | 0 | ||||||||||||
| decorated crickets (Gryllus sigillatus) [124] | x | x | x | (+) | |||||||||
| ground crickets (Allonemobius socius) [36] | x | x | (+) | ||||||||||
| water strider (Aquarius paludum) [125] | x | x | x | x | (–) | ||||||||
| bumble-bee (Bombus terrestris L) [126] | x | ||||||||||||
| bean beetles (Callosobruchis chinensis) [40] | x | (+) | |||||||||||
| seed beetle (Callosobruchis maculatus) [12] | (–) | ||||||||||||
| cabbage beetles (Colaphellus bowringi) [127] | (–) | (–) | |||||||||||
| seed beetle (Stator limbatus) [43] | (+) | ||||||||||||
| red flour beetle (Tribolium castaneum) [42] | x | x | (–) | ||||||||||
| flour beetles (Tribolium castaneum) [41] | (–) | ||||||||||||
| mealworm beetles (Tenebrio molitor) [128] | x | x | (+) | ||||||||||
| armyworms (Pseudaletia unipuncta) [129] | (+) | x | x | (+) | |||||||||
| almond moth (Cadra cutella) [130] | (–) | (–) | |||||||||||
| seaweed fly (Coelopa frigida) [46] | (+) | ||||||||||||
| fruitflies (Drosophila melanogaster) [48] | (–) | ||||||||||||
| fruitflies (D. melanogaster) [49] | (–) | ||||||||||||
| fruitflies (D. melanogaster) [50] | x | x | x | x | x | x | 0 | ||||||
| fruitflies (D. melanogaster) [51] | x | x | 0 | x | |||||||||
| fruitflies (Drosophila virilis) [51] | x | x | 0 | x | |||||||||
| fruitflies (Drosophila lummei) [51] | x | x | 0 | x | |||||||||
| fruitflies (Drosophila bifurca) [51] | x | x | 0 | x | |||||||||
| Crustacea | |||||||||||||
| American lobster (Homarus americanus) [52] | x | x | (–) | ||||||||||
| blue crab (Callinectes sapidus) [53] | x | ||||||||||||
| copepods (Lepeophtheirus salmonis) [54] | x | ||||||||||||
| Chelicerata | (+) | ||||||||||||
| cellar spider (Pholcus phalangiodes) [131] | |||||||||||||
| orb web spider [55] | |||||||||||||
| pseudoscorpions (C. scorpioides) [110] | (+) | ||||||||||||
| pseudoscorpions (C. scorpioides) [56] | (+) | ||||||||||||
| Echinodermata | |||||||||||||
| sea star (Asterias sp.) [57] | (–) | ||||||||||||
| sea urchin (Heliocidaris erythrogramma) [58] | (–) | ||||||||||||
| ascidians (Pyura stolonifera) [132] | x | x | x | (+) | |||||||||
| Pisces | |||||||||||||
| cichlid (Opthamotilapia verntralis) [62] | (–) | ||||||||||||
| guppies (Poecilia reticulatla) [63] | x | x | x | x | x | (+) | |||||||
| lemon sharks (Negaprion brevirostris) [65] | x | x | (+) | ||||||||||
| sandbar sharks (Carcharhinus plumbeus) [29] | x | (+) | x | ||||||||||
| zebrafish (Danio rerio) [67] | x | x | x | x | (–) | ||||||||
| Amphibia | |||||||||||||
| salamander (Ambystoma maculatum) [133] | x | x | (+) | ||||||||||
| toads (Bufo bufo) [70] | |||||||||||||
| treefrog (Chiromantis xeramepalina) [114] | x | x | |||||||||||
| Reptiles | |||||||||||||
| common lizard (Laecerta vivipara) [73] | x | (+)/(−) | |||||||||||
| green turtles (Chelonia mydas) [74] | x | 0 | |||||||||||
| water snakes (Nerodia sipedon) [75] | x | x | (+) | ||||||||||
| tuatara (Sphenodon punctatus) [76] | x | ||||||||||||
| Aves | |||||||||||||
| passerines (42 species) [82] | |||||||||||||
| stripe-backed wren [84] | |||||||||||||
| Mammalia | |||||||||||||
| Australian marsupials (Antechinus stuartii) [88] | (+) | ||||||||||||
| wood mice (Apodemus sylvaticus) [24] | x | x | x | x | (+) | ||||||||
Wolf & Wade [134] also argued that while offspring survival may indirectly influence parental fitness, offspring survival directly influences offspring fitness. Because offspring fitness belongs to offspring themselves, assigning offspring fitness to parents without experimentally disentangling parental and offspring influences on total fitness confounds these estimates across generations, leading to erroneous evolutionary inferences about how parental traits may respond to selection. Furthermore, because parental and offspring traits are often genetically correlated, selection may act on these traits via pleiotropy, linkage or non-random mating in unpredictable ways, particularly if genetic correlations are negative, as they often are [134]. Without advance knowledge of the genetic variance/covariance structure of maternal and offspring traits, as well as the influences these traits have on the relative fitnesses of parents and offspring, it is impossible to predict how selection on offspring performance may influence maternal phenotype. For these reasons, we exclude from this review articles that identify fitness benefits to polyandrously mating females in any terms other than offspring numbers.
(b). Direct evidence of fitness advantages to multiple mating by females
Why is it important to consider the effects of multiple mating on female fitness when considering sexual selection? The short explanation is that increases in the variance in fitness among females tend to erode the opportunity for sexual selection on males [100]. As explained in [1], when a female mates once and produces only one clutch of offspring, she assigns her entire reproductive output to a single male. When a female mates more than once, she divides her clutch into several sub-clutches, equal in number to the number of sires. The production of multiple clutches either by multiple mating or iteroparity increases the variance in female fitness, and it allows more males to mate. Both changes decrease the variance in fitness among males and so decrease the opportunity for sexual selection [1].
What constitutes direct evidence that females increase their fitness by multiple mating, and what evidence exists that such gains influence sexual selection on males? While abundant evidence exists that multiple mating by females may enhance female fitness directly, few studies have placed their results within an evolutionary context except by inference. Furthermore, while theoretical evidence exists that multiple mating may reduce the intensity of sexual selection [1,100], few examples of this effect are known [1,86,136]. We assert therefore that three kinds of data are required to argue that selection favours multiple mating by females.
First, to demonstrate the existence of significant variance in female fitness associated with multiple mating, it is necessary to document the proportion of females in the population who are unsuccessful in producing offspring [1,96]. This is necessary because, just as in males, the variance in female fitness consists of two components: the variance in fitness within the class of successfully reproducing females, and the variance in fitness between the successful and unsuccessful females [26]. As reproduction becomes increasingly disproportionate among females, the latter component of fitness variance represents the larger fraction of the two components of total selection (figure 1). The larger the fraction of total fitness female fitness represents, the more sexual selection is weakened [26,27].
Second, to demonstrate the existence of differential reproduction by females due to differences in mate numbers, it is necessary to document the magnitude and sign of the covariance between mating number, m, and offspring number, o, among females, or Cov♀(m,o). If this covariance is positive, it indicates that multiple mating by females enhances female fitness, with the magnitude of the covariance proportional to the strength of such selection. This condition is necessary for multiple mating by females to enhance female fitness. However, another important reason to document whether or not multiple mating by females enhances offspring numbers is that, depending on the sign and magnitude of the covariance between mating number and offspring number for males, Cov♂(m,o), the sign and magnitude of Cov♀(m,o) for females mating with such males can lead to mating system dynamics not predicted by considering each of these covariances separately, or by not considering these covariances at all (see §3c(iii)).
Third, to determine whether the fitness advantages females gain by multiple mating are likely to lead to significant evolutionary change, it is necessary to scale these potential fitness benefits to the total opportunity for selection on females, as well as to the total opportunity for selection on both sexes (methods in [1]). To examine the opportunity for selection on females, I♀, it is necessary to partition the total variance in female fitness, VO♀, into three components: (i) the variance in fitness due to the effects of different clutch sizes among females, (ii) the variance in female fitness due to the effects of matings with different males, and (iii) the variance in female fitness due to the effects of different numbers of clutches per female [1]. These components of the variance in fitness for females can be converted to opportunities for selection by dividing each by the squared average in female fitness, O♀2 (i.e. O♂2), and can then be considered as a fraction of the opportunity for selection on females, as well as its contribution to the total opportunity for selection, where I♂ = 1/R (I♀) + Imates (see [26,27] and §2c(ii)).
(c). Identifying the appropriate parameters for females
(i). Fundamental parameters
As explained above, the proportions of successful and unsuccessfully breeding females, pS♀, and p0♀, the mean and variance in female offspring numbers, O♀ and VO♀, and the opportunity for selection on females, I♀, can all be easily estimated from molecular parentage data, provided that all parents and offspring in the population can be identified [1]. Also, as explained above, the quality of such data will determine the power of the inferences possible from such analyses. As with males, identification of the non-breeding class of females can be exceptionally difficult, and while some data are better than none, the smaller the proportion of the population included in the sample, the weaker inferences about selection can be.
(ii). The covariance between mating numbers and offspring numbers for females
The covariance between female mating number and the number of offspring produced by females equals
| 3.1 |
where mioj is the product of individual matings and offspring numbers for each jth female, N♀ is the total number of females, O♀ is the average number of offspring per female, and P♀ is the average female mating number promiscuity [1], i.e. the average number of matings per female, wherein each mating, whether with the same or with a different partner, is counted as a single event.
(iii). Combining Cov♀(m,o) with Cov♂(m,o)
As explained in Wade & Shuster [101], whether Cov♀(m,o) is positive, negative or zero determines whether females gain fitness, lose fitness or have no fitness consequences associated with multiple mating. However, additional information not explained in [101] may be obtained by considering whether females with these possible values for Cov♀(m,o) happen to mate with males who have corresponding or different values for Cov♂(m,o). Several ‘zones’ of combinations arise when Cov♀(m,o) and Cov♂(m,o) have positive, negative or zero values (figure 2a,b). The largest zone appears when either Cov♂(m,o) and/or Cov♀(m,o) is zero (figure 2a). Fertilization success within this zone is randomly distributed across mating pairs, either because females use sperm at random or because male fertilization success is randomly distributed. This zone identifies WFS post-mating competition as described in §2c(iv) [1]. While post-mating sexual selection within this zone may behave like a lottery [8,10], lottery-like conditions are likely to occur only when Cov♀(m,o) is zero and Cov♂(m,o) is positive, because only in this cell are males likely to gain by producing large amounts of sperm. This cell identifies the single scenario within this zone in which WFA post-mating sexual selection may exist.
Figure 2.
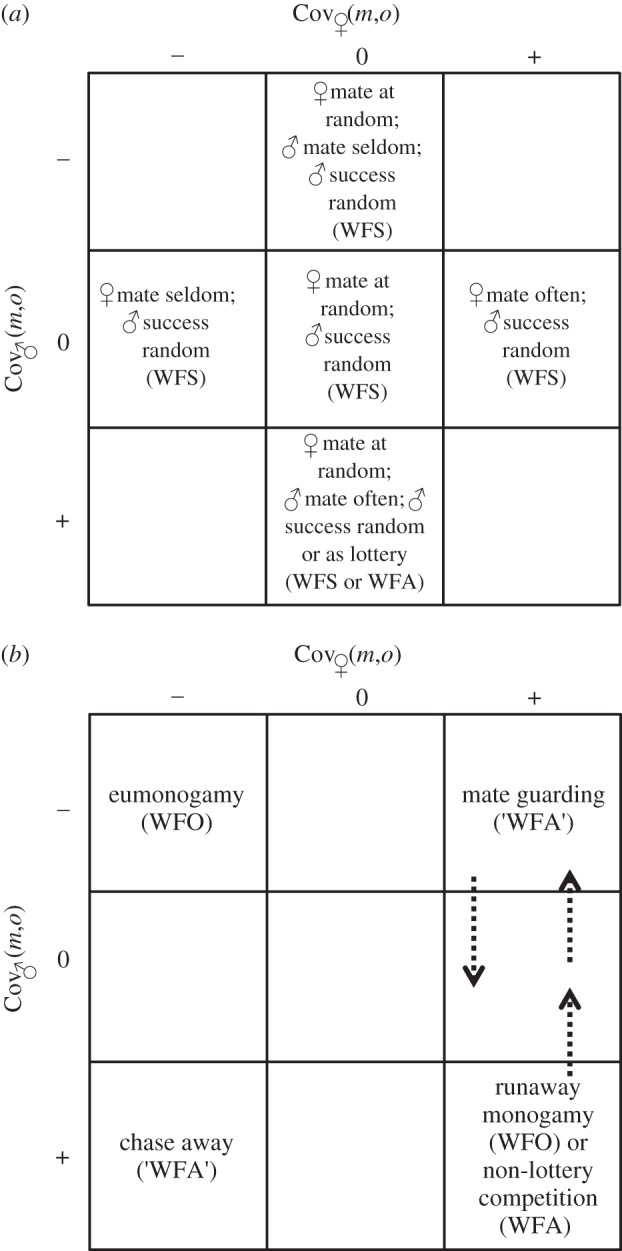
Possible mating systems resulting from simultaneous consideration of negative, zero and positive values of Cov♀(m,o) and Cov♂(m,o); (a) when fertilization success is randomly distributed across mating pairs, either because females use sperm at random or because male fertilization success is randomly distributed, post-mating sexual selection is possible only when Cov♂(m,o) is positive because males are likely to gain fertilizations by producing large amounts of sperm; this cell represents ‘winner fertilize all’ (WFA) post-mating competition, whereas all other labelled cells represent ‘winner fertilize some’ (WFS) post-mating competition [1]; (b) when fertilization success is contingent on positive or negative values of Cov♀(m,o) and Cov♂(m,o), post-mating sexual selection is again possible only when Cov♂(m,o) is positive, and only when females mate with multiple males; ‘WFO’ indicates ‘winner fertilize one’ post-mating competition; ‘WFA’ indicates situations in which ‘winners fertilize all of their mates' ova but not because post-mating sexual selection occurs; see text for details.
When Cov♂(m,o) and Cov♀(m,o) are both negative, males as well as females generate all of their offspring as mated pairs, mating only as often as is necessary to produce offspring (figure 2b). Post-mating sexual selection either by sperm competition or cryptic female choice is impossible in such eumonogamous mating systems, and mutually negative covariances between mating numbers and offspring numbers are likely to be self-reinforcing. This zone identifies a scenario that could be called WFO; a mating system, which, as mentioned, describes situations now known as genetic complementarity [49,109,110].
When Cov♀(m,o) is positive and Cov♂(m,o) is negative, post-mating sexual selection is likely to be weak because females will seek multiple matings but males will not (figure 2b). Multiple mating by females could lead to multiple sires per clutch if sperm precedence is incomplete, or if males are unable to guard their mates. However, if males are successful at either endeavour, this zone identifies another cell in which ‘WFA’ [1], and if spermatic success in the latter case is accomplished by mate guarding, post-mating sexual selection cannot occur here. If sperm precedence is incomplete, Cov♂(m,o) may become zero. However, sexual selection in any case is likely to be reduced because males are unlikely to seek multiple matings (Cov♂(m,o) is negative), and males successful at mate guarding will limit their mate numbers, increasing the number of males in the population able to sire young.
When both Cov♀(m,o) and Cov♂(m,o) are positive, males as well as females who engage in multiple matings gain high fitness (figure 2b). This combination could reinforce a genetic correlation between male and female tendencies to mate multiple times within mated pairs [137,138], and if repeatedly mating pairs experience the highest fitness, this mating system could represent ‘runaway monogamy’. This zone too identifies a situation in which ‘WFA’, but again, post-mating sexual selection by any mechanism is unlikely because males who mate repeatedly with only their partners do not obtain multiple mates.
If females tend not to cluster their mating with particular males, but instead change partners with each mating, post-mating sexual selection could increase in this scenario, but only if, among the males who mate repeatedly with different partners, particular males fertilize most or all of their mates' ova. If this condition exists, sexual selection can intensify, but stronger sexual selection means that fitness variance among females becomes proportionally weaker [1]. This mating system could also represent ‘non-lottery’ sperm competition wherein particular sperm traits rather than large ejaculate volumes are favoured, but, although predicted in theory [6,7], whether such traits are widespread in nature is somewhat unclear. Genetic correlations between sperm traits and various traits in female reproductive tracts are known [132], but co-evolution of genetically correlated traits need not involve direct competition among males (e.g. via sperm competition) and are unlikely to directly benefit female fitness [1]. Modifications of sperm traits in response to ovum density are known to exist, but evidence in broadcast spawners suggests that these traits evolve in response to female adaptations to avoid polyspermy rather than in response to sperm competition [60]. Note: An opportunity to quantitatively distinguish sperm competition from cryptic female choice may exist in such systems. Longer, faster sperm appear to correlate with greater promiscuity in cichlid fish [68], but such data appear to be rare across taxa [60,68], and could also be a response to female adaptations to avoid polyspermy or sperm limitation when the egg density is high. If females mate promiscuously and sperm precedence is incomplete, or if males in this scenario become depleted of sperm, this system will change to one in which Cov♂(m,o) is zero (WFS), where post-mating sexual selection is unlikely, or possibly one that is negative (WFO), wherein mate-guarding males ensure their paternity. In either case, neither sperm competition nor cryptic female choice are likely to be sources of sexual selection (figure 2b).
When Cov♂(m,o) is positive and Cov♀(m,o) is negative, males gain by multiple mating but females do not (figure 2b). Here, sexual selection could become extremely strong because males will attempt to mate with multiple females and females will tend to avoid multiple mating. This zone identifies what has been called ‘chase-away’ sexual selection [48] (but also see [1]) and it too identifies ‘WFA’. However, in this case too, females tend to mate rarely and, therefore, post-mating sexual selection is unlikely to occur despite the fact that sexual selection itself is likely to be more powerful than any of the above scenarios; here, males may be highly variable in their mate numbers but females are not.
Figure 3 summarizes possible relative values of the opportunity for sexual selection, Imates, based on the above discussion of different combinations of the covariance between mating numbers and offspring numbers for each sex, Cov♂(m,o) and Cov♀(m,o). In general, this diagram reveals 2/9 scenarios in which post-mating sexual selection is possible, but even in these cases only under particular circumstances: (i) where Cov♂(m,o) is positive, Cov♀(m,o) is zero, and certain males are capable of producing abundant sperm, and (ii) where both Cov♂(m,o) and Cov♀(m,o) are positive, females change partners with each mating, and ‘non-lottery’ sperm competition is possible. Note that the opportunity for post-mating sexual selection in each of these scenarios is expected to be less intense than when Cov♂(m,o) is positive and Cov♀(m,o) is negative, a situation in which sexual selection is likely to be strong, but post-mating sexual selection is unlikely to occur.
Figure 3.
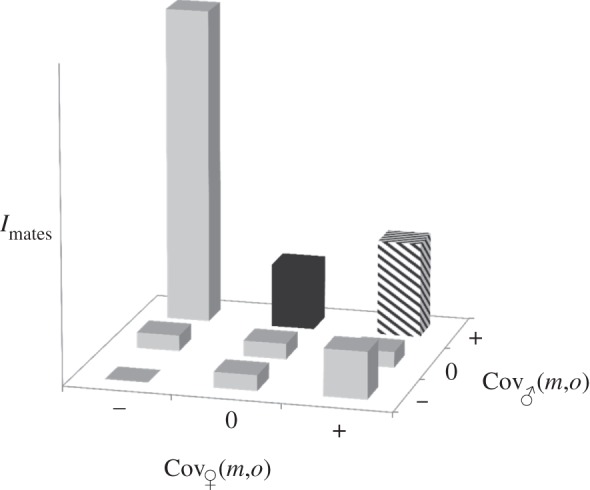
Possible relative values for the sex difference in the opportunity for selection (i.e. the opportunity for sexual selection), Imates, arising from multiple mating and post-mating sexual selection, based on the sign of the covariance in mating numbers and offspring numbers for each sex; grey bars indicate the likely values of Imates due to processes other than post-mating sexual selection; black bars indicate where post-mating sexual selection is likely, i.e. where Cov♂(m,o) is positive, Cov♀(m,o) is zero, and if certain males are capable of producing abundant sperm; hatched bars indicate where post-mating sexual selection is possible, where both Cov♂(m,o) and Cov♀(m,o) are positive, females change partners with each mating and if ‘non-lottery’ sperm competition occurs.
(iv). The variance in fitness caused by female life-history traits
The above circumstances describe how mating behaviour by females enhances or diminishes the strength of sexual selection, but females may influence sexual selection in other ways as well, through variation in (i) the number of times they mate and (ii) the number of reproductive events in a female's lifetime, which may depend on the duration of female reproductive competency [1]. Accordingly, females who mate once in their lifetime are monandrous, whereas females who mate more than once are polyandrous (the term ‘polyandry’ is frequently used to describe the latter condition, but we subscribe to the genetic, rather than the social definition of the mating system [1], wherein polyandry describes a genetic mating system in which females are variable in mate numbers, but males are not, with males having no more than one mate within their lifetime). Females who produce a single clutch of offspring are semelparous, whereas females who produce multiple clutches of offspring are iteroparous. Among iteroparous females, the longer the duration of female reproductive competency, the more variation in the pattern of female reproduction within and among seasons is possible, and simultaneously, the more variable the identities of males serving as mates for such females can be.
Wade [100] considered multiple mating and iteroparity by females similar in their effects on the opportunity for selection to the effects of multiple mating by males. He identified the term Iclutches to describe the variance in clutch number among females, Vclutches, divided by the squared average number of clutches per female, C2, noting that when females mate more than once, they divide each clutch into sub-clutches, equal in number to the number of sires. Wade [100] argued that multiple mating and iteroparity among females interchangeably decrease the variance in fitness among males and so increase the variance in fitness among females such that I♂ − I♀ = Imates − Iclutches. As explained above, the production of multiple clutches either by multiple mating or by iteroparity increases the variance in female fitness, and it allows more males to mate. Both tendencies decrease the variance in fitness among males and so decrease the opportunity for sexual selection [1].
Shuster & Wade [1] showed that when females mate more than once and produce multiple clutches of offspring, the total variance in offspring numbers for females, VO♀, is influenced by three distinct effects arising from variation in female clutch size (cs): (i) the variance in offspring numbers averaged across all females, Vcs,clutch, a value expected to become larger as females produce multiple clutches; (ii) the variance in offspring numbers due to the effects of mating with different sires on clutch size, Vcs,sires, a value expected to become larger as females mate more than once; and (iii) the variance in offspring numbers due to differences in the average clutch size that females produce, Vcs,females. The first two components of variance in female fitness are equivalent to the variance in offspring numbers within females, thus, VO♀within = Vcs,clutch + Vcs,sires. The third female fitness component is equivalent to the variance in offspring number among females, or VO♀among = Vcs,females.
As in the study of Wade [100], this approach provides a means for identifying the relative influences of multiple sires and multiple clutches on the total variance in offspring numbers for females. However, unlike Wade [100], this approach focuses not on how the number of clutches or sub-clutches females have may influence VO♀, but rather on how individual female fitness may become variable due to the effects of multiple males and multiple reproductive events. This approach [1] identifies VO♀within as the component of variance in offspring numbers affected by different numbers of sires and clutches, because the effect of multiple mating and multiple clutch numbers on the total variance in female offspring numbers arises from within individual females. Thus, to explore these effects, individual females must have life histories that include matings with multiple mates and the production of multiple clutches, or researchers must create these circumstances experimentally. When divided by the squared average in female offspring numbers, O♀2, the relationship explained above becomes, I♀ = (Ics,clutch + Ics,sires) + Ics,females.
To explore the effects of multiple mating on the among-female component of variance in female offspring numbers, females must be divided into k classes that identify the number of mates (or matings) females experience for each clutch of offspring they produce. The total variance in female fitness, in terms of offspring numbers, VO♀total, can once again be partitioned into two components, but this time VO♀within is the average of the variances in offspring number within the classes of females who mate with different numbers of males, and VO♀among is the variance in the average number of offspring among the mating and non-mating female classes, where VO♀total = VO♀within + VO♀among, or, by substitution,
| 3.2 |
where k represents each class of females based on their number of mates, pk represents the proportion of females belonging to each kth class, K equals the average number of mates or matings per female (i.e. N♂/N♀ = 1/R), c equals the average number of clutches per female, and k(O♀) and k(VO♀) represent the mean and variance in offspring numbers, respectively, for females within each kth mate or mating class, and where O♀ and VO♀ equal the mean and variance in offspring numbers per female. Collecting terms and assuming R = 1, we have
| 3.3 |
which, when divided by the squared average in female fitness, (KO♀)2, equation (3.3) becomes
| 3.4 |
Each of the three approaches above captures a different aspect of the effects of multiple mating on females. The first approach (cf. [100]) identifies the effects of variation in clutch size and clutch number on total variance in female offspring numbers. The second approach (cf. [1]) identifies the effects that multiple mates and multiple reproductive events may have on the variance in clutch sizes within individual females. The third approach identifies the effects that variation in mating number, either with the same or with different males, has on the total variance in female offspring numbers. Which approach is used depends on the experimental question asked. As explained above, the relative contribution of each of the variance components can be compared by dividing each by the squared average in female fitness, as well as compared in ratio form to I♀, which can be examined relative to I♂ and ΔI [1].
(d). Measuring the appropriate parameters for females
As explained above for males, while each of these parameters can be calculated explicitly if the numbers of successful and unsuccessful individuals in the population are known, these data can be extremely difficult to collect and so often go unrecorded. Table 3 summarizes the extent to which the above parameters have been investigated in studies to date. Abundant information appears to exist on the sources of variance in female offspring numbers, as well as on the apparent values for Cov♀(m,o), although in most cases values can only be inferred from experimental results rather than actual data. In contrast, the proportion of unsuccessful females, p0♀, and the opportunity for selection on females, I♀, are seldom quantified.
We emphasize again that such information is crucial to actual measurements of the opportunity for selection on females as well as the covariance between mating numbers and offspring number for both sexes. To show that either males or females benefit by multiple mating, it is necessary to show that a covariance between mate numbers and offspring numbers exists. Yet without examining the magnitude of these values, as explained above, it is impossible to conclude that post-mating sexual selection is strong or weak. Moreover, without measuring these values for females and comparing them to the magnitude of values collected for males, it is impossible to say whether females gain significant fitness from multiple mating relative to the total opportunity for selection.
4. Discussion
We present four major results. First, we suggest that when post-mating sexual selection is strong, i.e. when particular males gain from multiple mating, the fitness advantages females gain from males through multiple mating are likely to be proportionately weak. This relationship exists because when fewer males secure mates, the among-group component of the male variance in relative fitness becomes extremely large (figure 1), and so becomes a proportionately larger fraction of total selection [1,26,27,100]. Because the opportunity for sexual selection equals the sex difference in the opportunity for selection, ΔI = I♂ − I♀ [1], when sexual selection is strong, I♀ must be proportionately small. Conversely, when females engage in mating with multiple males and produce multiple clutches, the increased variance in female fitness is likely to erode the intensity of sexual selection most severely. Thus, when female fitness variance is proportionately large, the sex difference in the opportunity for selection must be proportionately small. This relationship has recently been documented experimentally in junglefowl, Gallus gallus [86] (see also [136]), and is expected to exist regardless of how sexual selection occurs [1].
Second, we provide a method for estimating the variance in fitness and the opportunity for selection resulting from post-mating sexual selection. Several researchers have recently used ANOVA to partition out this effect as part of their experimental design [3,4,31,86]. Our method complements this approach but has the advantage of not requiring that offspring be assigned to parents in a probabilistic way; our method explicitly assigns offspring to each parent. Our approach is also useful for examining existing datasets for which the proportions of individuals who mate and successfully produce offspring, who mate and fail to produce offspring, and who fail to mate at all, are known [3,4]. As we show, our approach is useful for gonochoristic as well as hermaphroditic species.
Our third major result is our finding that post-mating sexual selection occurs in only two of the nine possible mating systems in which multiple mating by females can occur: (i) when females have multiple mates and males are able to produce large volumes of sperm; and (ii) when females have multiple males and males have particular adaptations that allow their sperm to be competitive. There are other situations in which females may mate with multiple males, but because Cov(m,o) is zero or negative in these scenarios for one or both sexes, post-mating sexual selection is either unlikely or impossible. We acknowledge that the circumstances we identify in which post-mating sexual selection is likely to occur, are those in which most of the current evidence for sperm competition already exists, a condition that seems to be frequently observed in insects. However, the current literature on sperm competition, in our view, implies that such conditions are ubiquitous and strong in a large number of taxa. We argue that they may not be. Therefore, it is our goal to show that, while possibly unusually common in insects, this particular scenario actually represents a fraction of the possible conditions in which post-mating sexual selection could occur, and a highly contingent set of circumstances at that. We assert that if you look for sperm competition under such circumstances, you are likely to find it. We also assert, and we think justifiably, that such conditions overall may be comparatively rare. We hope that future research considers these scenarios more closely so that research on post-mating sexual selection may become more focused as well.
A fourth major result of our analyses is that we have found few recorded studies in which the overall strength of post-mating sexual selection and the presumed benefits females gain by multiple mating have been considered together in terms of the sex difference in the opportunity for selection ([1,3,4]; tables 1–3). The approach allows the relative contributions of these two consequences of polyandrous mating to be considered simultaneously. This result places some doubt on the conclusions of many presumed examples of the evolution of male traits by sperm competition or evolution of female traits by multiple mating; not because these experiments are not carefully executed, but rather because the relative intensity of selection in these contexts, as well as the context of selection itself, is yet unknown.
We have proposed a framework for measuring post-mating sexual selection, requiring highly specific information that is extremely difficult to obtain. In particular, we encourage researchers to document the classes of non-reproducing individuals in each sex because this allows the sex difference the opportunity for selection to be quantified, because it allows the specific effects of post-mating competition to be isolated and because it expands the focus of fitness measurement beyond the class of successfully reproducing individuals. We acknowledge that this procedure in some cases may confound sexual selection with other selective contexts. However, if one sex experiences greater fitness variance than the other for any reason, it will generate sex-specific selection, which, at least in gonochoristic species, is likely to lead to sexual dimorphism. In our view, such selection, even if operating in reproductive contexts not clearly identifiable as male combat or female choice, can be considered sexual selection [1,2]. We also advocate quantification of the covariance between mating number and offspring number for both sexes, Cov(m,o), primarily because this parameter provides an estimate of the strength of selection favouring multiple mating in each sex in the form of Bateman gradients ([94,95], tables 1 and 3). However, an additional unappreciated advantage of examining this covariance for both sexes simultaneously is that it provides a means for predicting mating systems in which post-mating sexual selection is possible and in which it is not.
We have proposed that estimates of sexual selection on males are incomplete without estimates of how multiple mating and multiple reproductive events by females may erode the strength of sexual selection [1]. We advocate three quantitative approaches for investigating the variance in fitness among females due to differences in female life-history that can be tailored to the experimental systems available. These approaches allow quantification of the effects of multiple matings, multiple sires and multiple reproductive events on the opportunity for selection on females. With this information, it becomes possible not only to scale the evolutionary effects of female reproductive life-history relative to sexual selection, but also to examine the magnitude of selection on multiple mating by females relative to total selection. We appear to be asking researchers to conform only to our specific criteria; but that is neither our intent nor what our suggestions amount to in practice. Our suggestions as well as those of others who emphasize a quantitative approach conform to standard evolutionary genetic theory and results. Our goal is to encourage current research to align itself with the principles it claims to promote. We argue that to understand the process of sexual selection, in this or any context, it is necessary to consider the role each sex plays in the process. Without such conjoined information, it is likely that the resulting incomplete answers will be misguided or wrong. In summary, our results suggest that existing theory and data on post-mating sexual selection are incomplete. We hope our analysis will be greeted, not with disdain, but rather with enthusiasm for investigating when post-mating sexual selection occurs, when it does not, and why.
Acknowledgements
We are grateful to Michael J. Wade for his fundamental contributions to the ideas discussed above as well as for his comments on an earlier draft of this manuscript. We are also grateful to Tommaso Pizzari and two anonymous reviewers, whose comments and suggestions greatly improved this manuscript.
References
- 1.Shuster S, Wade M. 2003. Mating systems and strategies. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Darwin CR. 1874. The descent of man and selection in relation to sex, 2nd edn. New York, NY: Rand, McNally and Co. [Google Scholar]
- 3.Anthes N, et al. 2010. Bateman gradients in hermaphrodites: an extended approach to quantify sexual selection. Am. Nat. 173, 249–263 10.1086/655218 (doi:10.1086/655218) [DOI] [PubMed] [Google Scholar]
- 4.Pélissié B, Jarne P, David P. 2012. Sexual selection without sexual dimorphism: Bateman gradients in a simultaneous hermaphrodite. Evolution 66, 66–81 10.1111/j.1558-5646.2011.01442.x (doi:10.1111/j.1558-5646.2011.01442.x) [DOI] [PubMed] [Google Scholar]
- 5.Webster M, Pruett-Jones S, Westneat D, Arnold SJ. 1995. Measuring the effects of pairing success, extra-pair copulations and mate quality on the opportunity for sexual selection. Evolution 49, 1147–1157 10.2307/2410439 (doi:10.2307/2410439) [DOI] [PubMed] [Google Scholar]
- 6.Pitnick S, Hosken DJ, Birkhead TR. 2009. Sperm morphological diversity. In Sperm biology: an evolutionary perspective. (eds Birkhead TR, Hosken DJ, Pitnick S.), pp. 69–149 Burlington, MA: Academic Press [Google Scholar]
- 7.Pizzari T, Parker GA. 2009. Sperm competition and the sperm phenotype. In Sperm biology: an evolutionary perspective (eds Birkhead TR, Hosken DJ, Pitnick S.), pp. 207–245 Burlington, MA: Academic Press [Google Scholar]
- 8.Parker GA, PIzzari T. 2010. Sperm competition and ejaculate economics. Biol. Rev. 85, 897–934 10.1111/j.1469-185X.2010.00140.x (doi:10.1111/j.1469-185X.2010.00140.x) [DOI] [PubMed] [Google Scholar]
- 9.Smith RL. 1984. Sperm competition and the evolution of animal mating systems. San Diego, CA: Academic Press [Google Scholar]
- 10.Birkhead T, Møller AP. 1998. Sperm competition and sexual selection. San Diego, CA: Academic Press [Google Scholar]
- 11.Levine L. 1967. Sexual selection in mice. IV. Experimental demonstration of selective fertilization. Am. Nat. 101, 289–294 10.1086/282491 (doi:10.1086/282491) [DOI] [Google Scholar]
- 12.Hotzy C, Arnqvist G. 2009. Sperm competition favors harmful males in seed beetles. Curr. Biol. 19, 404–407 10.1016/j.cub.2009.01.045 (doi:10.1016/j.cub.2009.01.045) [DOI] [PubMed] [Google Scholar]
- 13.Cheng KM, Siegel PB. 1990. Quantitative genetics of multiple mating. Anim. Behav. 40, 406–407 10.1016/S0003-3472(05)80939-X (doi:10.1016/S0003-3472(05)80939-X) [DOI] [Google Scholar]
- 14.Birkhead T. 1999. Reproductive biology: distinguished sperm in competition. Nature 400, 406–407 10.1038/22650 (doi:10.1038/22650) [DOI] [PubMed] [Google Scholar]
- 15.Gage MJG, Macfarlane CP, Yeates S, Ward RG, Searle JB, Parker GA. 2004. Spermatozoal traits and sperm competition in Atlantic salmon: relative sperm velocity is the primary determinant of fertilization success. Curr. Biol. 14, 44–47 10.1016/j.cub.2003.12.028 (doi:10.1016/j.cub.2003.12.028) [DOI] [PubMed] [Google Scholar]
- 16.García-González F, Simmons LW. 2005. The evolution of polyandry: intrinsic sire effects contribute to embryo viability. J. Evol. Biol. 18, 1097–1103 10.1111/j.1420-9101.2005.00889.x (doi:10.1111/j.1420-9101.2005.00889.x) [DOI] [PubMed] [Google Scholar]
- 17.Vladić TV, Järvi T. 2001. Sperm quality in the alternative reproductive tactics of Atlantic salmon: the importance of the loaded raffle mechanism. Proc. R. Soc. Lond. B 268, 2375–2381 10.1098/rspb.2001.1768 (doi:10.1098/rspb.2001.1768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Short RV. 1979. Sex determination and differentiation. Brit. Med. Bull. 35, 121–127 [DOI] [PubMed] [Google Scholar]
- 19.Hosken D, Ward P. 2001. Experimental evidence for testis size evolution via sperm competition. Ecol. Lett. 4, 10–13 10.1046/j.1461-0248.2001.00198.x (doi:10.1046/j.1461-0248.2001.00198.x) [DOI] [Google Scholar]
- 20.Wedell N, Gage MJG, Parker GA. 2002. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 17, 313–320 10.1016/S0169-5347(02)02533-8 (doi:10.1016/S0169-5347(02)02533-8) [DOI] [Google Scholar]
- 21.Gage MJG, Freckleton RP. 2003. Relative testis size and sperm morphometry across mammals: no evidence for an association between sperm competition and sperm length. Proc. R. Soc. Lond. B 270, 625–632 10.1098/rspb.2002.2258 (doi:10.1098/rspb.2002.2258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pizzari T, Birkhead TR. 2000. Female feral fowl eject sperm of subdominant males. Nature 405, 787–789 10.1038/35015558 (doi:10.1038/35015558) [DOI] [PubMed] [Google Scholar]
- 23.Foster K, Seppä P, Ratnieks FLW, Thorén PA. 1999. Low paternity in the hornet Vespa crabro indicates that multiple mating by queens is derived in vespine wasps. Behav. Ecol. Sociobiol. 46, 252–257 10.1007/s002650050617 (doi:10.1007/s002650050617) [DOI] [Google Scholar]
- 24.Bartmann S, Gerlach G. 2001. Multiple paternity and similar variance in reproductive success of male and female wood mice (Apodemus sylvaticus) housed in an enclosure. Ethology 107, 889–899 10.1046/j.1439-0310.2001.00723.x (doi:10.1046/j.1439-0310.2001.00723.x) [DOI] [Google Scholar]
- 25.Dean MD, Ardlie KG, Nachman MW. 2006. The frequency of multiple paternity suggests that sperm competition is common in house mice (Mus domesticus). Mol. Ecol. 15, 4141–4151 10.1111/j.1365-294X.2006.03068.x (doi:10.1111/j.1365-294X.2006.03068.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wade MJ. 1979. Sexual selection and variance in reproductive success. Am.Nat. 114, 742–747 10.1086/283520 (doi:10.1086/283520) [DOI] [PubMed] [Google Scholar]
- 27.Wade MJ, Arnold SJ. 1980. The intensity of sexual selection in relation to male sexual behaviour, female choice, and sperm precedence. Anim. Behav. 28, 446–461 10.1016/S0003-3472(80)80052-2 (doi:10.1016/S0003-3472(80)80052-2) [DOI] [Google Scholar]
- 28.Bjork A, Pitnick S. 2006. Intensity of sexual selection along the road to isogamy. Nature 441, 742–745 10.1038/nature04683 (doi:10.1038/nature04683) [DOI] [PubMed] [Google Scholar]
- 29.Portnoy DS, Piercy AN, Musick JA, Burgess GH, Graves JE. 2007. Genetic polyandry and sexual conflict in the sandbar shark, Carcharhinus plumbeus, in the western North Atlantic and Gulf of Mexico. Mol. Ecol. 16, 187–197 10.1111/j.1365-294X.2006.03138.x (doi:10.1111/j.1365-294X.2006.03138.x) [DOI] [PubMed] [Google Scholar]
- 30.Møller AP, Ninni P. 1998. Review. Sperm competition and sexual selection: a meta-analysis of paternity studies of birds. Behav. Ecol. Sociobiol. 43, 345–358 10.1007/s002650050501 (doi:10.1007/s002650050501) [DOI] [Google Scholar]
- 31.Pischedda A, Rice WR. 2012. Partitioning sexual selection into its mating success and fertilization success components. Proc. Natl Acad. Sci. USA 109, 2049–2053 10.1073/pnas.1110841109 (doi:10.1073/pnas.1110841109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall DJ, Evans JP. 2005. The benefits of polyandry in the free-spawning polychaete Galeolaria caespitosa. J. Evol. Biol. 18, 735–741 10.1111/j.1420-9101.2004.00873.x (doi:10.1111/j.1420-9101.2004.00873.x) [DOI] [PubMed] [Google Scholar]
- 33.Simmons LW, Kvarnemo C. 1997. Ejaculate expenditure by male bushcrickets decreases with sperm competition intensity. Proc. R. Soc. Lond. B 264, 1203–1208 10.1098/rspb.1997.0166 (doi:10.1098/rspb.1997.0166) [DOI] [Google Scholar]
- 34.Simmons LW, Craig M, Llorens T, Schinzig M, Hosken D. 1993. Bushcricket spermatophores vary in accord with sperm competition and parental investment theory. Proc. R. Soc. Lond. B 251, 183–186 10.1098/rspb.1993.0027 (doi:10.1098/rspb.1993.0027) [DOI] [Google Scholar]
- 35.Backus V, Cade WH. 1986. Sperm competition in the field cricket Gryllus integer (Orthoptera: Gryllidae). Fla. Entomol. 69, 722–728 10.2307/3495220 (doi:10.2307/3495220) [DOI] [Google Scholar]
- 36.Fedorka K, Mousseau TA. 2002. Material and genetic benefits of female multiple mating and polyandry. Anim. Behav. 64, 361–367 10.1006/anbe.2002.3052 (doi:10.1006/anbe.2002.3052) [DOI] [Google Scholar]
- 37.Bretman A, Newcombe D, Tregenza T. 2009. Promiscuous females avoid inbreeding by controlling sperm storage. Mol. Ecol. 18, 3340–3345 10.1111/j.1365-294X.2009.04301.x (doi:10.1111/j.1365-294X.2009.04301.x) [DOI] [PubMed] [Google Scholar]
- 38.Pol RG, Casenave JL, Feldhaar H, Milesi FA, Gadau J. 2008. Polyandry in two South American harvester ants. Insect. Soc. 55, 91–97 10.1007/s00040-007-0975-0 (doi:10.1007/s00040-007-0975-0) [DOI] [Google Scholar]
- 39.Kellner K, Trindl A, Heinze J, D'Ettorre P. 2007. Polygyny and polyandry in small ant societies. Mol. Ecol. 16, 2363–2369 10.1111/j.1365-294X.2007.03297x (doi:10.1111/j.1365-294X.2007.03297x) [DOI] [PubMed] [Google Scholar]
- 40.Sakurai G, Kasuya E. 2008. Different female mating rates in different populations do not reflect the benefits the females gain from polyandry in the adzuki bean beetle. J. Ethol. 26, 93–98 10.1007/s10164-007-0036-1 (doi:10.1007/s10164-007-0036-1) [DOI] [Google Scholar]
- 41.Tregenza T, Attia F, Bushaiba SS. 2009. Repeatability and heritability of sperm competition outcomes in males and females of Tribolium castaneum. Behav. Ecol. Sociobiol. 63, 817–823 10.1007/s00265-009-0716-7 (doi:10.1007/s00265-009-0716-7) [DOI] [Google Scholar]
- 42.Lewis SM, Austad SN. 1990. Sources of intraspecific variation in sperm precedence in red flour beetles. Am. Nat. 135, 351–359 10.1086/285050 (doi:10.1086/285050) [DOI] [Google Scholar]
- 43.Moya-Larano J, Fox CW. 2006. Ejaculate size, second male size, and moderate polyandry increase female fecundity in a seed beetle. Behav. Ecol. 17, 940–946 10.1093/beheco/arl029 (doi:10.1093/beheco/arl029) [DOI] [Google Scholar]
- 44.Demary KC, Lewis SM. 2007. Male reproductive allocation in fireflies (Photinus spp.). Invertebr. Biol. 126, 74–80 10.1111/j.1744-7410.2007.00078.x (doi:10.1111/j.1744-7410.2007.00078.x) [DOI] [Google Scholar]
- 45.Cook PA, Gage MJG. 1995. Effects of risks of sperm on the numbers competition of eupyrene and apyrene sperm ejaculated by the moth Plodia interpunctella (Lepidoptera: Pyralidae). Behav. Ecol. Sociobiol. 36, 261–268 10.1007/BF00165835 (doi:10.1007/BF00165835) [DOI] [Google Scholar]
- 46.Dunn DW, Sumner JP, Goulson D. 2005. The benefits of multiple mating to female seaweed flies, Coelopa frigida (Diptera: Coelpidae). Behav. Ecol. Sociobiol. 58, 128–135 10.1007/s00265-005-0922-x (doi:10.1007/s00265-005-0922-x) [DOI] [Google Scholar]
- 47.Frentiu FD, Chenoweth SF. 2008. Polyandry and paternity skew in natural and experimental populations of Drosophila serrata. Mol. Ecol. 17, 1589–96 10.1111/j.1365-294X.2008.03693.x (doi:10.1111/j.1365-294X.2008.03693.x) [DOI] [PubMed] [Google Scholar]
- 48.Holland B, Rice WR. 1999. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc. Natl Acad. Sci. USA 96, 5083–5088 10.1073/pnas.96.9.5083 (doi:10.1073/pnas.96.9.5083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark AG, Begun DJ, Prout T. 1999. Female×male interactions in drosophila sperm competition. Science 283, 217–220 10.1126/science.283.5399.217 (doi:10.1126/science.283.5399.217) [DOI] [PubMed] [Google Scholar]
- 50.Pitnick S, Brown WD, Miller GT. 2001. Evolution of female remating behaviour following experimental removal of sexual selection. Proc. R. Soc. Lond. B 268, 557–563 10.1098/rspb.2000.1400 (doi:10.1098/rspb.2000.1400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bateman AJ. 1948. Intra-sexual selection in Drosophila. Heredity 2, 349–368 10.1038/hdy.1948.21 (doi:10.1038/hdy.1948.21) [DOI] [PubMed] [Google Scholar]
- 52.Gosselin T, Sainte-Marie B, Bernatchez L. 2005. Geographic variation of multiple paternity in the American lobster, Homarus americanus. Mol. Ecol. 14, 1517–1525 10.1111/j.1365-294X.2005.02498.x (doi:10.1111/j.1365-294X.2005.02498.x) [DOI] [PubMed] [Google Scholar]
- 53.Jivoff P. 1997. The relative roles of predation and sperm competition on the duration of the post-copulatory association between the sexes in the blue crab, Callinectes sapidus. Behav. Ecol. Sociobiol. 40, 175–185 10.1007/s002650050331 (doi:10.1007/s002650050331) [DOI] [Google Scholar]
- 54.Todd CD, Stevenson RJ, Reinardy H, Ritchie MG. 2005. Polyandry in the ectoparasitic copepod Lepeophtheirus salmonis despite complex precopulatory and postcopulatory mate-guarding. Mar. Ecol. Prog. Ser. 303, 225–234 10.3354/meps303225 (doi:10.3354/meps303225) [DOI] [Google Scholar]
- 55.Elgar MA, Bruce MJ, Crespigny FECD, Cutler AR, Cutler CL, Gaskett AC, Herberstein ME, Ramamurthy S, Schneider JM. 2003. Male mate choice and patterns of paternity in the polyandrous, sexually cannibalistic orb-web spider, Nephila plumipes. Aust. J. Zool. 51, 357–365 10.1071/ZO02079 (doi:10.1071/ZO02079) [DOI] [Google Scholar]
- 56.Zeh JA, Newcomer SD, Zeh DW. 1998. Polyandrous females discriminate against previous mates. Proc. Natl Acad. Sci. USA 95, 13 732–13 736 10.1073/pnas.95.23.13732 (doi:10.1073/pnas.95.23.13732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harper FM, Hart MW. 2005. Gamete compatibility and sperm competition affect paternity and hybridization between sympatric Asterias sea stars. Biol. Bull. 209, 113–126 10.2307/3593129 (doi:10.2307/3593129) [DOI] [PubMed] [Google Scholar]
- 58.Evans JP, Marshall DJ. 2005. Male-by-female interactions influence fertilization success and mediate the benefits of polyandry in the sea urchin Heliocidaris erythrogramma. Evolution 59, 106–112 10.1111/j.0014-3820.2005.tb00898.x (doi:10.1111/j.0014-3820.2005.tb00898.x) [DOI] [PubMed] [Google Scholar]
- 59.Marshall DJ, Evans JP. 2007. Context-dependent genetic benefits of polyandry in a marine hermaphrodite. Biol. Lett. 3, 685–688 10.1098/rsbl.2007.0438 (doi:10.1098/rsbl.2007.0438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crean AJ, Marshall DJ. 2008. Gamete plasticity in a broadcast spawning marine invertebrate. Proc. Natl Acad. Sci. USA 105, 13 508–13 513 10.1073/pnas.0806590105 (doi:10.1073/pnas.0806590105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gage MJG, Stockley P, Parker GA. 1995. Effects of alternative male mating strategies on characteristics of sperm production in the Atlantic salmon (Salmo salar): theoretical and empirical investigations. Proc. R. Soc. Lond. B 350, 391–399 10.1098/rstb.1995.0173 (doi:10.1098/rstb.1995.0173) [DOI] [Google Scholar]
- 62.Immler S, Taborsky M. 2009. Sequential polyandry affords post-mating sexual selection in the mouths of cichlid females. Behav. Ecol. Sociobiol. 63, 1219–1230 10.1007/s00265-009-0744-3 (doi:10.1007/s00265-009-0744-3) [DOI] [Google Scholar]
- 63.Bechert SA, Magurran AE. 2004. Multiple mating and reproductive skew in Trinidadian guppies. Proc. R. Soc. Lond. B 271, 1009–1014 10.1098/rspb.2004.2701 (doi:10.1098/rspb.2004.2701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.King JR, Withler RE. 2005. Male nest site fidelity and female serial polyandry in lingcod (Ophiodon elongatus, Hexagrammidae). Mol. Ecol. 14, 653–660 10.1111/j.1365-294X.2005.02438.x (doi:10.1111/j.1365-294X.2005.02438.x) [DOI] [PubMed] [Google Scholar]
- 65.DiBattista JD, Feldheim KA, Gruber SH, Hendry AP. 2008. Are indirect genetic benefits associated with polyandry? Testing predictions in a natural population of lemon sharks. Mol. Ecol. 17, 783–95 10.1111/j.1365-294X.2007.03623.x (doi:10.1111/j.1365-294X.2007.03623.x) [DOI] [PubMed] [Google Scholar]
- 66.Fuller RC. 1998. Sperm competition affects male behaviour and sperm output in the rainbow darter. Proc. R. Soc. Lond. B 265, 2365–2371 10.1098/rspb.1998.0585 (doi:10.1098/rspb.1998.0585) [DOI] [Google Scholar]
- 67.Spence R, Smith C. 2006. Mating preference of female zebrafish, Danio rerio, in relation to male dominance. Behav. Ecol. 17, 779–783 10.1093/beheco/arl016 (doi:10.1093/beheco/arl016) [DOI] [Google Scholar]
- 68.Fitzpatrick JL, Montgomerie R, Desjardins JK, Stiver KA, Kolm N, Balshine S. 2009. Female promiscuity promotes the evolution of faster sperm in cichlid fishes. Proc. Natl Acad. Sci. USA 106, 1128–1132 10.1073/pnas.0809990106 (doi:10.1073/pnas.0809990106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roberts J, Standish R, Byrne P, Doughty P. 1999. Synchronous polyandry and multiple paternity in the frog Crinia georgiana (Anura: Myobatrachidae). Anim. Behav. 57, 721–726 10.1006/anbe.1998.1019 (doi:10.1006/anbe.1998.1019) [DOI] [PubMed] [Google Scholar]
- 70.Sztatecsny M, Jehle R, Burke T, Hödl W. 2006. Female polyandry under male harassment: the case of the common toad (Bufo bufo). J. Zool. 270, 517–522 10.1111/j.1469-7998.2006.00120.x (doi:10.1111/j.1469-7998.2006.00120.x) [DOI] [Google Scholar]
- 71.Stille B, Madsen T, Niklasson M, Nikiasson M. 1986. Multiple paternity in the adder, Vipera berus. Oikos 47, 173–175 10.2307/3566042 (doi:10.2307/3566042) [DOI] [Google Scholar]
- 72.Fantin C, Viana LS, Monjeló LADS, Farias IP. 2008. Polyandry in Podocnemis unifilis (Pleurodira; Podocnemididae), the vulnerable yellow-spotted Amazon River turtle. Amphibia-Reptilia 29, 479–486 10.1163/156853808786230361 (doi:10.1163/156853808786230361) [DOI] [Google Scholar]
- 73.Eizaguirre C, Laloi D, Massot M, Richard M, Federici P, Clobert J. 2007. Condition dependence of reproductive strategy and the benefits of polyandry in a viviparous lizard. Proc. R. Soc. B 274, 425–430 10.1098/rspb.2006.3740 (doi:10.1098/rspb.2006.3740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee PLM, Hays GC. 2004. Polyandry in a marine turtle: females make the best of a bad job. Proc. Natl Acad. Sci. USA 101, 6530–6535 10.1073/pnas.0307982101 (doi:10.1073/pnas.0307982101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kissner KJ, Weatherhead PJ, Gibbs HL. 2005. Experimental assessment of ecological and phenotypic factors affecting male mating success and polyandry in northern watersnakes, Nerodia sipedon. Behav. Ecol. Sociobiol. 59, 207–214 10.1007/s00265-005-0026-7 (doi:10.1007/s00265-005-0026-7) [DOI] [Google Scholar]
- 76.Moore JA, Nelson NJ, Keall SN, Daugherty CH. 2008. Implications of social dominance and multiple paternity for the genetic diversity of a captive-bred reptile population (tuatara). Conserv. Genet. 9, 1243–1251 10.1007/s10592-007-9452-6 (doi:10.1007/s10592-007-9452-6) [DOI] [Google Scholar]
- 77.Prosser MR, Weatherhead PJ, Gibbs HL, Brown GP. 2002. Genetic analysis of the mating system and opportunity for sexual selection in northern water snakes (Nerodia sipedon). Behav. Ecol. 13, 800–807 10.1093/beheco/13.6.800 (doi:10.1093/beheco/13.6.800) [DOI] [Google Scholar]
- 78.Dietrich V, Schmoll T, Winkel W, Epplen J, Lubjuhn T. 2004. Pair identity: an important factor concerning variation in extra-pair paternity in the coal tit (Parus ater). Behaviour 141, 817–835 10.1163/1568539042265644 (doi:10.1163/1568539042265644) [DOI] [Google Scholar]
- 79.Burke T, Davies NB, Bruford MW, Hatchwell BJ. 1989. Parental care and mating related to paternity by DNA fingerprinting related to paternity by DNA fingerprinting. Nature 338, 249–251 10.1038/338249a0 (doi:10.1038/338249a0) [DOI] [Google Scholar]
- 80.Faaborg J, Parker PG, DeLay L, de Vries T, Bednarz JC, Paz SM, Naranjo J, Waite TA. 1995. Confirmation of cooperative polyandry in the Galapagos hawk (Buteo galapagoensis). Behav. Ecol. Sociobiol. 36, 83–90 10.1007/BF00170712 (doi:10.1007/BF00170712) [DOI] [Google Scholar]
- 81.Heinsohn R, Ebert D, Legge S, Peakall R. 2007. Genetic evidence for cooperative polyandry in reverse dichromatic Eclectus parrots. Anim. Behav. 74, 1047–1054 10.1016/j.anbehav.2007.01.026 (doi:10.1016/j.anbehav.2007.01.026) [DOI] [Google Scholar]
- 82.Kleven O, Fossøy F, Laskemoen T, Robertson RJ, Rudolfsen G, Lifjeld JT. 2009. Comparative evidence for the evolution of sperm swimming speed by sperm competition and female sperm storage duration in passerine birds. Evolution 63, 2466–2473 10.1111/j.1558-5646.2009.00725.x (doi:10.1111/j.1558-5646.2009.00725.x) [DOI] [PubMed] [Google Scholar]
- 83.Whittingham LA, Dunn PO, Magrath RD. 1997. Relatedness, polyandry and extra-group paternity in the cooperatively-breeding white-browed scrubwren (Sericornis frontalis). Behav. Ecol. Sociobiol. 40, 261–270 10.1007/s002650050341 (doi:10.1007/s002650050341) [DOI] [Google Scholar]
- 84.Piper WH, Slater G. 1993. Polyandry and incest avoidance in the cooperative stripe-backed wren of Venezuela. Behaviour 3, 227–247 10.1163/156853993X00597 (doi:10.1163/156853993X00597) [DOI] [Google Scholar]
- 85.Dunn PO, Robertson RJ, Michaud-Freeman D, Boag PT. 1994. Extra-pair paternity in tree swallows: why do females mate with more than one male? Behav.Ecol. Sociobiol. 35, 273–281 10.1007/BF00170708 (doi:10.1007/BF00170708) [DOI] [Google Scholar]
- 86.Collet J, Richardson DS, Worley K, Pizzari T. 2012. Sexual selection and the differential effect of polyandry. Proc. Natl Acad. Sci. USA 109, 8641–8645 10.1073/pnas.1200219109 (doi:10.1073/pnas.1200219109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carmichael LE, Szor G, Berteaux D, Giroux MA, Cameron C, Strobeck C. 2007. Free love in the far north: plural breeding and polyandry of arctic foxes (Alopex lagopus) on Bylot Island, Nunavut. Can. J. Zool. 85, 338–343 10.1139/Z07-014 (doi:10.1139/Z07-014) [DOI] [Google Scholar]
- 88.Fisher DO, Double MC, Blomberg SP, Jennions MD, Cockburn A. 2006. Post-mating sexual selection increases lifetime fitness of polyandrous females in the wild. Nature 444, 89–92 10.1038/nature05206 (doi:10.1038/nature05206) [DOI] [PubMed] [Google Scholar]
- 89.Kraaijeveld-Smit F, Ward S, Smith PT. 2002. Multiple paternity in a field population of a small carnivorous marsupial, the agile antechinus, Antechinus agilis. Behav. Ecol. Sociobiol. 52, 84–91 10.1007/s00265-002-0485-z (doi:10.1007/s00265-002-0485-z) [DOI] [Google Scholar]
- 90.Keane B, Waser PM, Creel S, Creel NM, Elliott JF, Minchella DJ. 1994. Subordinate reproduction in dwarf mongooses. Anim. Behav. 47, 65–75 10.1006/anbe.1994.1008 (doi:10.1006/anbe.1994.1008) [DOI] [Google Scholar]
- 91.Baker A, Dietz JM, Kleiman DG. 1993. Behavioral evidence for monopolization of paternity in multi-male groups of golden lion tamarins. Anim. Behav. 46, 1091–1103 10.1006/anbe.1993.1299 (doi:10.1006/anbe.1993.1299) [DOI] [Google Scholar]
- 92.Baker RJ, Makova KD, Chesser RK. 1999. Microsatellites indicate a high frequency of multiple paternity in Apodemus (Rodentia). Mol. Ecol. 8, 107–111 10.1046/j.1365-294X.1999.00541.x (doi:10.1046/j.1365-294X.1999.00541.x) [DOI] [PubMed] [Google Scholar]
- 93.Booth W, Montgomery WI, Prodöhl PA. 2007. Polyandry by wood mice in natural populations. J. Zool. 273, 176–182 10.1111/j.1469-7998.2007.00312.x (doi:10.1111/j.1469-7998.2007.00312.x) [DOI] [Google Scholar]
- 94.Arnold SJ. 1994. Bateman's principles and the measurement of sexual selection in plants and animals. Am. Nat. 144, S126–S149 10.1086/285656 (doi:10.1086/285656) [DOI] [Google Scholar]
- 95.Arnold SJ, Duvall D. 1994. Animal mating systems: a synthesis based on selection theory. Am. Nat. 143, 317–348 10.1086/285606 (doi:10.1086/285606) [DOI] [Google Scholar]
- 96.Shuster S. 2009. Sexual selection and mating systems. Proc. Natl Acad. Sci. USA 106, 10 009–10 016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Parker G. 1970. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. Cambr. Philos. Soc. 45, 525–567 10.1111/j.1469-185X.1970.tb01176.x (doi:10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 98.Lewis S, Austad S. 1994. Sexual selection in flour beetles: the relationship between sperm precedence and male olfactory attractiveness. Behav. Ecol. 5, 223–224 10.1093/beheco/5.2.223 (doi:10.1093/beheco/5.2.223) [DOI] [Google Scholar]
- 99.Clark AG, Aguade M, Prout T, Harshmad LG, Langley CH. 1995. Variation in sperm displacement and its association with accessory gland protein loci in Drosophila melanogaster. Genetics 139, 189–201 10.1073/pnas.0901132106 (doi:10.1073/pnas.0901132106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wade M. 1987. Measuring sexual selection. In Sexual selection: testing the alternatives (eds Bradbury JW, Andersson MB.), pp. 197–207 New York, NY: John Wiley and Sons [Google Scholar]
- 101.Wade MJ, Shuster SM. 2005. Don't throw Bateman out with the bathwater! Integr. Comp. Biol. 45, 945–51 10.1093/icb/45.5.945 (doi:10.1093/icb/45.5.945) [DOI] [PubMed] [Google Scholar]
- 102.Crow JF. 1958. Some possibilities for measuring selection intensities in man. Hum. Biol. 30, 1–13 [PubMed] [Google Scholar]
- 103.Jones AG. 2009. On the opportunity for sexual selection, the Bateman gradient and the maximum intensity of sexual selection. Evolution 63, 1673–1684 10.1111/j.1558-5646.2009.00664.x (doi:10.1111/j.1558-5646.2009.00664.x) [DOI] [PubMed] [Google Scholar]
- 104.Wade MJ, Shuster SM. 2004. Sexual selection: harem size and the variance in male reproductive success. Am. Nat. 164, E83–E89 10.1086/424531 (doi:10.1086/424531) [DOI] [PubMed] [Google Scholar]
- 105.Shuster SM. 2010. Alternative mating strategies. In Evolutionary behavioral ecology (eds Fox C, Westneat DF.), pp. 434–450 Cambridge, UK: Cambridge University Press [Google Scholar]
- 106.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man (ed. Campbell B.), pp. 136–179 Chicago, IL: Aldine Press [Google Scholar]
- 107.Levitan DR, Petersen CW. 1995. Sperm limitation in the sea. Trends. Ecol. Evol. 10, 228–231 10.1016/S0169-5347(00)89071-0 (doi:10.1016/S0169-5347(00)89071-0) [DOI] [PubMed] [Google Scholar]
- 108.Weddell N, Gage MJG, Parker GA. 2002. Sperm competition, male prudence and sperm-limited females. Trends. Ecol. Evol. 17, 313–320 10.1016/S0169-5347(02)02533-8 (doi:10.1016/S0169-5347(02)02533-8) [DOI] [Google Scholar]
- 109.Mays HL, Albrecht T, Liu M, Hill GE. 2008. Female choice for genetic complementarity in birds: a review. Genetica 134, 147–58 10.1007/s10709-007-9219-5 (doi:10.1007/s10709-007-9219-5) [DOI] [PubMed] [Google Scholar]
- 110.Newcomer SD, Zeh JA, Zeh DW. 1999. Genetic benefits enhance the reproductive success of polyandrous females. Proc. Natl. Acad. Sci. USA 96, 10236–10241 10.1073/pnas.96.18.10236 (doi:10.1073/pnas.96.18.10236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eberhard WG. 1996. Females in control: sexual selection by cryptic female choice. Princeton, NJ: Princeton University Press [Google Scholar]
- 112.Jones AG, Ardren WR. 2003. Methods of parentage analysis in natural populations. Mol. Ecol. 12, 2511–2523 10.1046/j.1365-294X.2003.01928.x (doi:10.1046/j.1365-294X.2003.01928.x) [DOI] [PubMed] [Google Scholar]
- 113.Bernasconi G, Keller L. 2001. Female polyandry affects their sons’ reproductive success in the red flour beetle Tribolium castaneum. J. Evol. Biol. 14, 186–193 10.1046/j.1420-9101.2001.00247.x (doi:10.1046/j.1420-9101.2001.00247.x) [DOI] [PubMed] [Google Scholar]
- 114.Byrne PG, Whiting MJ. 2008. Simultaneous polyandry increases fertilization success in an African foam-nesting treefrog. Anim. Behav. 76, 1157–1164 10.1016/j.anbehav.2008.05.019 (doi:10.1016/j.anbehav.2008.05.019) [DOI] [Google Scholar]
- 115.Byrne PG, Keogh JS. 2009. Extreme sequential polyandry insures against nest failure in a frog. Proc. R. Soc. B 276, 115–120 10.1098/rspb.2008.0794 (doi:10.1098/rspb.2008.0794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Foltz ADW, Schwagmeyer PL. 1989. Sperm competition in the thirteen-lined ground squirrel: differential fertilization success under field conditions. Am. Nat. 133, 257–265 10.1086/284914 (doi:10.1086/284914) [DOI] [Google Scholar]
- 117.Kaitala A, Wiklund C. 1994. Polyandrous female butterflies forage for matings. Behav. Ecol. Sociobiol. 35, 385–388 10.1007/BF00165840 (doi:10.1007/BF00165840) [DOI] [Google Scholar]
- 118.Tregenza T, Wedell N. 1998. Benefits of multiple mates in the cricket Gryllus bimacculatus. Evolution 52, 1726–1730 10.2307/2411345 (doi:10.2307/2411345) [DOI] [PubMed] [Google Scholar]
- 119.Arnqvist G, Nilsson T. 2000. The evolution of polyandry: multiple mating and female fitness in insects. Anim. Behav. 60, 145–164 10.1006/anbe.2000.1446 (doi:10.1006/anbe.2000.1446) [DOI] [PubMed] [Google Scholar]
- 120.Pearse DE, Janzen FJ, Avise JC. 2002. Multiple paternity, sperm storage, and reproductive success of female and male painted turtles (Chrysemys picta) in nature. Behav. Ecol. Sociobiol. 51, 164–171 10.1007/s00265-001-0421-7 (doi:10.1007/s00265-001-0421-7) [DOI] [Google Scholar]
- 121.Murie JO. 1995. Mating behavior of Columbian ground squirrels. I. Multiple mating by females and multiple paternity. Can. J. Zool. 73, 1819–1926 10.1139/z95-214 (doi:10.1139/z95-214) [DOI] [Google Scholar]
- 122.Simmons LW. 2001. The evolution of polyandry: an examination of the genetic incompatibility and good-sperm hypotheses. J. Evol. Biol. 14, 585–594 10.1046/j.1420-9101.2001.00309.x (doi:10.1046/j.1420-9101.2001.00309.x) [DOI] [Google Scholar]
- 123.Burpee D, Sakaluk SK. 1993. Repeated matings offset costs of reproduction in female crickets. Evol. Ecol. 7, 240–250 10.1007/BF01237742 (doi:10.1007/BF01237742) [DOI] [Google Scholar]
- 124.Ivy TM, Sakaluk SK, Url S. 2005. Polyandry promotes enhanced offspring survival in decorated crickets. Evolution 59, 152–159 10.1111/j.0014-3820.2005.tb00902.x (doi:10.1111/j.0014-3820.2005.tb00902.x) [DOI] [PubMed] [Google Scholar]
- 125.Ronkainen K, Kaitala A, Kivelä SM. 2010. Polyandry, multiple mating, and female fitness in a water strider Aquarius paludum. Behav. Ecol. Sociobiol. 64, 657–664 10.1007/s00265-009-0883-6 (doi:10.1007/s00265-009-0883-6) [DOI] [Google Scholar]
- 126.Baer B, Schmid-Hempel P. 1999. Experimental variation in polyandry affects parasite loads and fitness in a bumble-bee. Nature 397, 151–154 10.1038/16451 (doi:10.1038/16451) [DOI] [Google Scholar]
- 127.Liu X-P, He H-M, Kuang X-J, Xue F-S. 2010. A comparison of female fitness between monogamy and polyandry in the cabbage beetle, Colaphellus bowringi. Anim. Behav. 79, 1390–1395 10.1016/j.anbehav.2010.03.019 (doi:10.1016/j.anbehav.2010.03.019) [DOI] [Google Scholar]
- 128.Drnevich JM, Papke RS, Rauser CL, Rutowski RL. 2001. Material benefits from multiple mating in female mealworm beetles (Tenebrio molitor L.). J. Insect Behav. 14, 215–230 10.1023/A:1007889712054 (doi:10.1023/A:1007889712054) [DOI] [Google Scholar]
- 129.Svard L, McNeil JN. 1994. Female benefit, male rick: polyandry in the true armyworm Pseudaletia unipuncta. Behav. Ecol. Sociobiol. 35, 319–326 10.1016/S0022-1910(98)00137-1 (doi:10.1016/S0022-1910(98)00137-1) [DOI] [Google Scholar]
- 130.McNamara KB, Elgar MA, Jones TM. 2008. A longevity cost of re-mating but no benefits of polyandry in the almond moth, Cadra cautella. Behav. Ecol. Sociobiol. 62, 1433–1440 10.1007/s00265-008-0573-9 (doi:10.1007/s00265-008-0573-9) [DOI] [Google Scholar]
- 131.Uhl G, Schmitt S, Schäfer MA. 2005. Fitness benefits of multiple mating versus female mate choice in the cellar spider (Pholcus phalangioides). Behav. Ecol. Sociobiol. 59, 69–76 10.1007/s00265-005-0010-2 (doi:10.1007/s00265-005-0010-2) [DOI] [Google Scholar]
- 132.Martin OY, Demont M. 2008. Reproductive traits: Evidence for sexually selected sperm. Curr. Biol. 18, R79–R81 10.1016/j.cub.2007.11.031 (doi:10.1016/j.cub.2007.11.031) [DOI] [PubMed] [Google Scholar]
- 133.Myers EM, Zamudio KR. 2004. Multiple paternity in an aggregate breeding amphibian: the effect of reproductive skew on estimates of male reproductive success. Mol. Ecol. 13, 1951–1963 10.1111/j.1365-294X.2004.02208.x (doi:10.1111/j.1365-294X.2004.02208.x) [DOI] [PubMed] [Google Scholar]
- 134.Wolf JB, Wade MJ. 2001. On the assignment of fitness to parents and offspring: whose fitness is it and when does it matter? J. Evol. Biol. 14, 347–356 10.1046/j.1420-9101.2001.00277.x (doi:10.1046/j.1420-9101.2001.00277.x) [DOI] [Google Scholar]
- 135.Wade M. 1998. The evolutionary genetics of maternal effects. In Maternal effects as adaptations (eds Mousseau T, Fox C.), pp. 5–21 Oxford, UK: Oxford University Press [Google Scholar]
- 136.Jones AG, Walker D, Kvarnemo C, Lindström K, Avise JC. 2001. How cuckoldry can decrease the opportunity for sexual selection: data and theory from a genetic parentage analysis of the sand goby, Pomatoschistus minutus. Proc. Natl Acad. Sci. USA 98, 9151–9156 10.1073/pnas.171310198 (doi:10.1073/pnas.171310198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shuker DM, Phillimore AJ, Burton-Chellew MN, Hodge SE, West SA. 2007. The quantitative genetic basis of polyandry in the parasitoid wasp, Nasonia vitripennis. Heredity 98, 69–73 10.1038/sj.hdy.6800897 (doi:10.1038/sj.hdy.6800897) [DOI] [PubMed] [Google Scholar]
- 138.Halliday T, Arnold S. 1987. Multiple mating by females: a perspective from quantitative genetics. Anim. Behav. 35, 939–941 10.1016/S0003-3472(87)80138-0 (doi:10.1016/S0003-3472(87)80138-0) [DOI] [Google Scholar]


