Abstract
Obligate eusociality with distinct caste phenotypes has evolved from strictly monogamous sub-social ancestors in ants, some bees, some wasps and some termites. This implies that no lineage reached the most advanced form of social breeding, unless helpers at the nest gained indirect fitness values via siblings that were identical to direct fitness via offspring. The complete lack of re-mating promiscuity equalizes sex-specific variances in reproductive success. Later, evolutionary developments towards multiple queen-mating retained lifetime commitment between sexual partners, but reduced male variance in reproductive success relative to female's, similar to the most advanced vertebrate cooperative breeders. Here, I (i) discuss some of the unique and highly peculiar mating system adaptations of eusocial insects; (ii) address ambiguities that remained after earlier reviews and extend the monogamy logic to the evolution of soldier castes; (iii) evaluate the evidence for indirect fitness benefits driving the dynamics of (in)vertebrate cooperative breeding, while emphasizing the fundamental differences between obligate eusociality and cooperative breeding; (iv) infer that lifetime commitment is a major driver towards higher levels of organization in bodies, colonies and mutualisms. I argue that evolutionary informative definitions of social systems that separate direct and indirect fitness benefits facilitate transparency when testing inclusive fitness theory.
Keywords: commitment, conflict, cooperation, eusociality, cooperative breeding, monogamy
1. Introduction
Most plants and animals are promiscuous, which implies that mate choice can be viewed as a fluid parentage market. Darwin [1] was the first to realize that the dynamics of this market are ultimately driven by paternity interests, which prevail or fail depending on male–male competition or female choice. About a century later, seminal contributions by Parker, Trivers and Eberhard [2–4] initiated a neo-Darwinian synthesis of sexual selection studies. The massive work that followed in the wake of these pioneering conceptual and empirical studies has significantly advanced our understanding of the forces that shape the diversity of mating systems. However, we are still short of a general explanation of female promiscuity/polyandry, as novel insights into the direct (resource-related) and indirect (good genes-related) benefits of female promiscuity have generated at least as many new questions as those that became answered. We are left with the notion that there is a limited set of relevant principles, but endless variation in how they combine into specific scenarios of male–female cooperation and conflict, each with their own fitness rewards to the sexes involved [5–12].
The eusocial insects with true worker castes are exceptional in having much less mating system variation, because they do not have re-mating promiscuity [13–15]. This is a remarkable feat, because obligate eusociality evolved independently in the ants, bees, wasps and termites, and yet all these lineages are characterized by mating pairs that commit for life without exception, something that is highly unusual in other organisms. Even more peculiar, mate-choice behaviour is not part of social life: it normally takes place after reproductives (prospective queens and kings/drones) have left the colony in which they hatched and it is completed before they found a new generation of colonies. As it turns out, the simplest form of partner commitment, strict lifetime monogamy, appears to have been a universally necessary, although not sufficient, condition for allowing the evolution of differentiated eusocial worker castes [14–17]. A review of polyandry in the eusocial insects thus has to take the opposite of promiscuity as its starting point and explore evolutionarily derived convergent elaborations of ancestral sexual commitment for life.
Contrasts such as this tend to inhibit intellectual exchange between fields, as they often result in semantic inconsistencies that need to be made explicit before any synthesis is possible. For example, the special nature of polyandry in the eusocial insects has implied that some researchers are reluctant to use the term and prefer the more passive ‘multiple queen-mating’, which does not carry the implicit suggestion of re-mating promiscuity. Another terminology issue worth noting is that direct and indirect benefits mean different things in sexual selection and kin selection arguments. Instead of emphasizing benefits that females receive from mates (see first paragraph), social evolution uses direct benefits when referring to gene copies in future generations obtained by personal reproduction, whereas indirect benefits refer to gene copies that come about via the reproductive success of relatives. Inclusive fitness is the sum of these two components [18,19], but it remains essential to consider them separately. This is because altruistic traits can evolve only when indirect benefits are significant because relatedness is positive, whereas mutualism characterizes cooperation between non-relatives, i.e. between interactants with zero relatedness [20–26].
The absence of the usual dynamics of re-mating has the advantage that sexual selection predictions in the eusocial domain are easier to formulate from first principles and often straightforward to test by comparing mating system characteristics across lineages in which multiple queen-mating has evolved. The concepts used are in many ways complementary to the logic of inclusive fitness theory [18–20,27] and sex allocation theory [28–30], which also predict adaptive evolutionary endpoints without explicitly considering short-term dynamics or possible constraints that may need to be overcome [31]. The simplicity emanating from lifetime commitment in eusocial mating therefore offers interesting perspectives on both sexual selection and social evolution, provided one can get one's head around the idea that eusocial polyandry does not create paternity markets, but permanent chimaeras of nestmates sharing maternal but not paternal genes.
An explicit focus on commitment makes it also transparent that reproductive conflicts are either about whether or not to make a commitment (e.g. which egg and sperm combine to become a zygote or which female and male end up breeding together), or about monopolizing or biasing the results of an irreversible commitment (e.g. imprinted genes affecting offspring provisioning, maternally transmitted genes/symbionts killing male offspring or dominant breeders coercing helpers). Much research on eusocial Hymenoptera in recent decades has used inclusive fitness theory to explain intracolonial conflicts of the biasing kind [20,32–40], whereas simultaneous studies of vertebrate cooperative breeders concentrated primarily on dominant individuals of both sexes competing for opportunities to breed while offering subordinates opportunities to help [8,41–47], i.e. on the kind of breeding commitments to be made and for how long. My present focus on constrained promiscuity in the most advanced forms of social breeding emphasizes how the absence of conflict over parental commitment conflict has helped to forge harmonious cooperation between parents and offspring.
Eusociality has traditionally been defined as (sub-social) cooperative brood care between a mother and her offspring in nests where individuals belong to reproductive castes [48]. This restricted eusociality to the vespine wasps, corbiculate bees, ants and (as we now know, foraging) termites, i.e. to lineages where there is no intracolonial conflict over the breeder role [49], consistent with Wheeler's [50, p. 23] original definition, which he used to emphasize the striking analogies between eusocial colonies and metazoan bodies [15,20,25]. The term eusociality was loosened up to include all forms of cooperative division of labour that affect lifetime reproductive success by Wilson [51], a trend that culminated in the idea of a ‘eusociality continuum’ where degree of eusociality was pragmatically defined by reproductive skew ([52]; reviewed by Costa & Fitzgerald [53]). This continuum approach was criticized by Crespi & Yanega [54], who argued that the definition of eusociality should be precise and evolutionarily informative, and thus necessarily be based on the distinct lifetime trajectories in behaviour and reproduction that accompany the evolution of irreversible castes. Crespi and Yanega defined reproductive totipotency (the default of solitary breeding) as the potential to express the full behavioural repertoire needed to independently (without helpers) produce offspring with the same abilities, and divided eusocial systems into: (i) ‘facultatively eusocial’ where the more reproductive caste has retained totipotency and the less reproductive caste has not, and (ii) ‘obligatorily eusocial’ where neither caste has retained totipotency so that all individuals have irreversible complementary roles. Their obligate and facultative eusociality terminology is largely consistent with ‘advanced’ and ‘primitive’ eusociality [55] and with ‘complex’ and ‘simple’ eusociality [20,56]. However, the advanced/primitive classification becomes ambiguous in evolutionarily-derived simplifications (e.g. various ponerine ants) and renders the bumble-bees primitively eusocial in spite of having lost all reproductively totipotent individuals [57]. The complex/simple categorization, based on colony size driving multiple aspects of social complexity, allows large epiponine wasp societies to rank above small ant societies in spite of a fundamental difference in caste commitment.
In earlier reviews [14,15], I have elaborated the conceptual framework of Crespi and Yanega to underline that irreversible evolution of a worker caste is the defining hallmark of obligate eusociality and that the acquisition of this state is a major evolutionary transition into a domain of social breeding that is distinct from solitary, cooperative and facultatively eusocial breeding combined. In the same reviews, I connected that transition to strict and lasting lifetime monogamy of colony parents as that condition was apparently necessary for the evolution of obligatorily eusocial and physically differentiated workers [16]. This resolved the critique by Beekman et al. [58], who argued that the Crespi & Yanega definition failed to acknowledge that reproductive dominance owing to permanent morphological caste differences is fundamentally different from reversible behavioural dominance. In the same reviews, I also used the term permanent eusociality as a synonym to emphasize the need for all individuals to adopt caste roles for life and for the last totipotent individuals to disappear before any transition to obligate eusociality was completed. The monogamy approach does not logically require the complexity of eusociality to be correlated with either reproductive skew or large colony size [20].
To further illustrate the generality of merging the concept of lifetime commitment with Crespi & Yanega's [54] evolutionary informative definition of eusociality, I will explore three complementary angles in this review. First, I will demonstrate how peculiar the independent, evolutionarily-derived eusocial insect mating systems are in comparison with what we normally find in animals. The bottom line of this section will be that one cannot just consider eusocial mating systems as endpoints of promiscuity gradients of which the overall principles are already generally known from theory and data obtained for other animals. Second, I will update arguments on why strict lifetime monogamy is so tightly connected to the evolution of sterile workers, and I will develop an analogous general rationale for the evolution of soldier castes in facultatively eusocial systems, the fortress defenders sensu [59]. Third, I will review how mating-commitment logic has allowed novel insights into cooperatively breeding vertebrates, where promiscuity is ubiquitous but functions as a constraint for helpers at the nest to obtain indirect fitness benefits. Fourth, I will explore some implications of the commitment/promiscuity approach for understanding more general principles of multicellular life at different levels of organization and emphasize the general need for evolutionary informed definitions of eusociality and cooperative breeding. A glossary of the main terminology that I use is provided in table 1.
Table 1.
Glossary of the main mating system and social evolution terminology used. Sources: Boomsma [14,15], Crespi & Yanega [54], Cockburn [44], Clutton-Brock [45], Davies [60], Emlen [41], Russell [47] supplemented and updated by various Wikipedia articles.
| promiscuity: having structured or casual sexual relationships with more than one other individual. The term is also used in non-sexual contexts, always retaining a meaning related to non-random mixing of elements. Examples are predictable or haphazard horizontal gene transfer in micro-organisms and the regular or occasional willingness and ability to absorb influences from multiple cultural backgrounds. |
| re-mating promiscuity: sexual promiscuity with serial mates, so that some but not all ejaculates may compete for immediate or later egg fertilizations after storage. The emphasis on social insects in the present review makes it necessary to define this temporal variance component explicitly, because it is absent when queens of eusocial Hymenoptera mate with multiple males in quick succession and store multiple ejaculates jointly in a specialized ‘spermatheca’ to never mate again later in life. |
| chimaerism: promiscuity without a temporal component resulting in the permanent coexistence of conspecific elements that normally occur alone. Examples are individuals with more than a single genetic population of cells owing to mergers of two fertilized eggs, the fertilization of a single egg by multiple sperm (strictly speaking a mosaic), the merger of sibling placentas, or the asexual merger of genetically different haplotypes. |
| polyandry: an animal mating system in which females typically mate with several or many males in the course of their lifetime. It almost always implies re-mating promiscuity, but longer-term relationships between a single female and several specific male partners are known in some vertebrate and human populations and, particularly, in the multiple-mating lineages of obligatorily eusocial Hymenoptera where queen-polyandry with a specific number of mates lasts for life because re-mating promiscuity is lacking. |
| polygyny: an animal mating system where single males mate with several to many females, usually in direct competition with each other through displays of physical strength or secondary sexually selected ornaments. Otherwise similar to polyandry, but virtually absent in the eusocial insects, where the term is used for colonies that have multiple egg-laying queens. |
| polygynandry: an animal mating system characterized by recurrent sexual relationships between multiple males and multiple females. Both co-breeding males and co-breeding females may be relatives, but inbreeding is rare. Sometimes referred to as communal breeding, or (together with polyandry and polygyny) as polygamous breeding. |
| cooperative breeding: a breeding system characterized by dominant breeders and subordinate helpers providing alloparental care. Helpers are either older siblings gaining indirect (kin-selected) fitness benefits, or unrelated adults who might earn direct fitness benefits by increasing their future probability of breeding, either independently after dispersing or by inheriting the residential nest from a more dominant breeder. |
| facultatively eusocial breeding: a cooperative breeding system where reproductive and helping roles are lifelong determined for a substantial fraction of the colony membership, but where a subset of offspring retains reproductive totipotency in spite of being part of a helper cohort, so they may later inherit the nest as dominant breeder or disperse to become dominant elsewhere. Caste roles are mostly behavioural and characterized by minor and overlapping distributions of body size and matedness, but some lifelong subordinates may belong to a physically distinct soldier caste. When a soldier caste is absent, there is no sharp distinction between advanced cooperative and facultatively eusocial breeding, as both combine obligate colony life with the retention of reproductive totipotency for some fraction of the subordinates. |
| obligatorily eusocial breeding: a breeding system in which all individuals are either designated breeders or unmated workers/soldiers for life, and where castes are always physically distinct and differentially adapted to a specific subset of social tasks so that colony growth and reproduction always require the complementary efforts of all castes. This implies that no caste has retained reproductive totipotency. The evolution of a specialized worker caste of unmated individuals is the ultimate defining character of obligate eusociality, no matter whether a worker caste evolves after (termites) or before (Hymenoptera) soldiers. |
2. Eusocial mating separates sex and society and establishes unusual adaptive syndromes
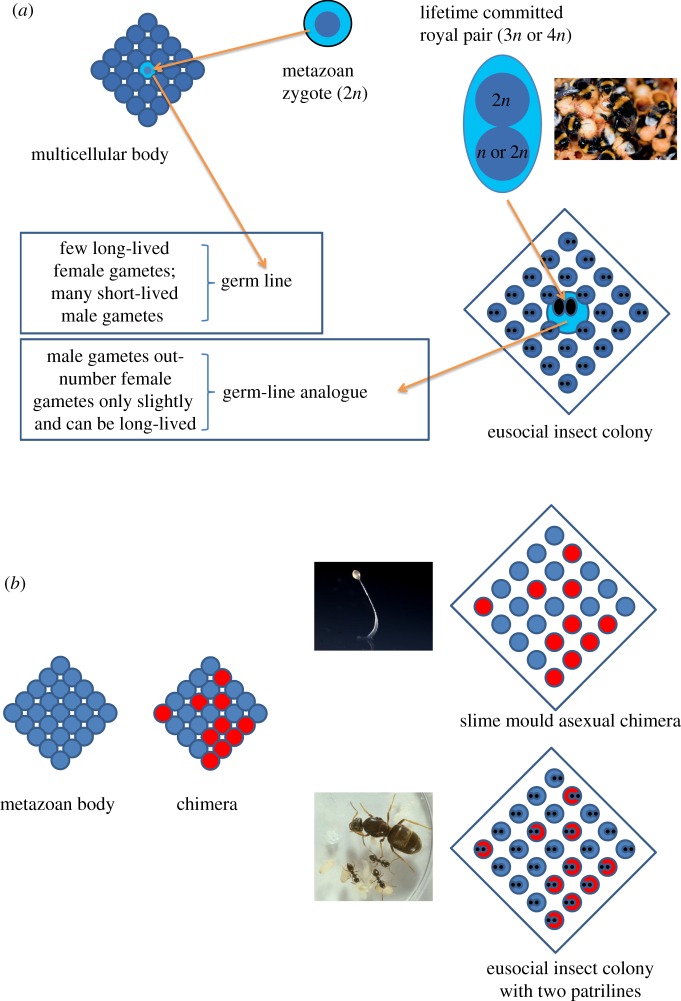
The TV comedy-drama ‘Sex and the City’ aptly illustrates that issues of mate choice in our own societies always overlap with other social interactions. It is therefore intuitively easier to relate to animals where sex has a social context than to imagine the lives of ants, bees, wasps and termites where issues of mate choice and society-building are separated in time and space. All clades that convergently entered the obligatorily eusocial domain have absolute lifetime pair-bonding, after a brief period of mate choice between dispersing from the natal nest and founding a colony (or sometimes joining one as a secondary evolutionary elaboration). It cannot be emphasized enough how exceptional this form of monogamy is: it is as strong as the commitment of a female and male gamete to a zygote—it implies that once you find a partner (s)he will be your only one and even death will not part you. A lifetime-united founding pair of diploid higher (i.e. foraging/mound-building) termites is thus analogous with a tetraploid zygote, whereas a standard colony-founding ant, bee or wasp queen is a triploid analogy, owing to the sperm of the lifetime mate in her specialized storage organ being a haploid clone rather than an ejaculate of haploid sperm related by 0.5 because of meiosis (figure 1a; see also [15]). Just like a sperm cell is unable to fertilize yet another egg, and an egg lacks the capacity to team up with another sperm after fertilization, so does the commitment of an obligatorily eusocial queen and king/drone preclude further sexual activity forever. Where multicellular eukaryote bodies produce clonal adhering cell copies and sequester germ-lines to produce new gametes, the founding ‘royalty’ of an obligatorily eusocial colony assumes a comparable role in taking the lion's share of producing new cohorts of dispersing reproductives (winged virgin queens and drones) after their ‘somatic’ colony has grown to maturity (figure 1a; see [15] for details on worker male production that make the germ-line analogy only approximately valid). Sister lineages of obligatorily eusocial clades either lack this extreme single-zygote-like colony-founding or they are unable to universally maintain that form of lifetime commitment later in the colony life cycle (see §3).
Figure 1.
Gametes, zygotes, bodies, chimaeras and colonies. (a) The somatic cells of metazoan bodies are clonal copies of a single zygote to which a female and male gamete have committed for life upon fertilization (top left), whereas standard colonies of obligatorily eusocial insects are founded by a lifetime-committed royal pair that contributes three haplotypes in the haplodiploid Hymenoptera and four in the diploid termites (top right; see [15] for details). Such colonies consist of individuals rather than cells (symbolized by eyespots). Queen and worker castes develop from totipotent larvae, similar to somatic cells differentiating from totipotent stem cells, after which both castes and somatic cells become irreversibly committed to their specialized complementary functions. The lifetime-committed royal pair is analogous to a metazoan germ line, but lineages that practise obligate eusociality have remarkable (inversed) patterns of population-wide availability and longevity of gametes (text boxes). The inset picture of the bumble-bee Bombus terrestris (photo credit: Matthias Fürst) exemplifies an obligatorily eusocial species with colonies that are always headed by a single, once-mated queen [61]. (b) Clonal metazoan bodies (left square with blue circles) only very rarely combine multiple cell lineages derived from more than two parents in a chimaera (or mosaic when strictly of single zygote origin; blue and red circles) (table 1). However, Dictyostelium slime moulds (photo credit Owen Gilbert) are characterized by non-trivial frequencies of chimaeras when they produce asexual fruiting bodies [62] and eusocial insect colonies have repeatedly evolved chimaeric structures when queens became multiply mated. The inset picture of Lasius niger exemplifies an ant species whose colonies are always headed by a single queen, but where some fraction mates with two or three males rather than with a single male [63], giving a chimaeric colony kin-structure of full-sibling patrilines that are half-siblings to each other.
(a). Eusociality equalizes sex-specific variances in reproductive success and maximizes sperm quality
Outside the eusocial insects, strict lifetime monogamy requires that a male physically merges with the body of a female who then stops being receptive for life, which is very unusual and only known or suspected from a few disparate lineages such as some of the angler fish [64] and parasitic barnacles [65]. When such strict parental commitments induce insect offspring to become altruistic helper castes rather than independent breeders, their relatedness can be accurately predicted because they are either full-siblings or a chimaeric combination of patrilines (table 1) that are half-sisters to each other (figure 1b). This predictability has been the prime reason for eusocial insects becoming excellent test systems for inclusive fitness theory, because these relatednesses determine the later (potential and realized) biasing-type conflicts between colony members about reproductive allocations [20,37,59,66].
In non-social organisms, the open-market characteristics of promiscuous mating are major drivers of evolutionary innovation, but also involve considerable waste in a utilitarian sense. This not only concerns the evolution of male ornaments in species where males offer no other contributions to breeding efforts than sperm [67], but also the massive numerical overproduction of sperm relative to eggs, combined with a suite of manipulative sperm traits for outcompeting rival sperm (reviewed in Simmons [68]) and manipulating female physiology, e.g. [69]. The males of eusocial insects universally lack ornamental traits, which seems a paradox because they appear to contribute only sperm, but lifetime partner commitment resolves this as it implies that both sexes ‘put all their gametes in the same basket’ after ultra-brief courtship [13]. This likely explains why termites have tandem-running as their main if not sole potential mechanism of premating sexual selection, a process that will tend to assort couples according to general physical condition, because the sexes have equal interests in avoiding an inferiorly endowed partner [70,71]. How little room predation pressure will leave for termite partners to steer coupling away from being purely random remains to be established (but see [70]), but the same overall logic would explain that most mate choice in ants, bees and wasps appears to be based on flight stamina and male receptiveness to queen pheromones [13,72], i.e. on indices of quality that cannot be faked [73].
When lifetime partner commitment is based on stored sperm obtained early in life, there must be strong selection for high sperm viability, high sperm longevity in storage, and prudent sperm use. While promiscuous mating systems also have their limitations in sperm production [12,74], male gametes always vastly outnumber female gametes. However, lifetime monogamy equalizes sex-specific variances in reproductive success and fundamentally changes the numbers of female and male gametes that circulate in a population (figure 1a), even though every female remains under selection to secure the best possible sperm on the single day when she picks her lifetime mate as a young virgin. All sperm that survive the brief mating window are locked away in a permanently committed production unit (termite king) or a specialized female storage organ (Hymenoptera) so that eggs continue to be fertilized with minimal waste. When multiple queen-mating evolves as a secondary elaboration, several males breeding with the same female implies that male variance in reproductive success drops below female variance in reproductive success, representing another reversal of common sexual selection practice that is also found among the vertebrate cooperative breeders with the highest reproductive skew [8,75,76] (see also §4).
Both long-lived queens of Atta leaf-cutting ants and Apis honeybees have males with high sperm viability and spermathecal fluids that actively enhance sperm preservation [77,78], and both use very few sperm to fertilize an average egg [79,80]. Higher (mound-building) termites have a founding queen and king living side by side in a lifetime monogamous relationship based on the regular transfer of aflagellate sperm that has lost the capacity to move independently [71]. It will be interesting to see whether further studies will show more variation in sperm morphology across termite lineages, but the currently available data seem to offer compelling indirect evidence for strict lifetime monogamy, as sperm lacking tails could never have evolved, let alone go to fixation, as long as even a very low probability of sperm competition would have remained (as in many lower termites—see §3).
Termite queens are unable to test the quality of their mate's sperm when committing for life, but queens of eusocial Hymenoptera will normally have an extra round of selection to work with, as their first mate-choice commitment coincides with males depositing sperm in the female bursa copulatrix [71,77,78] from where it normally needs to actively move towards final storage in the spermatheca. There will thus be an unambiguous male fitness benefit in delivering highly viable sperm with optimal motility and a consistent queen fitness benefit in evolving chemical gradients that make sperm run the gauntlet while being underway to the final (lifetime) storage organ, so she can retain the best possible fraction [13,78,79]. It would therefore be virtually impossible for eusocial hymenopteran sperm to lose tails as queens are expected to have evolved means to discard the least motile sperm. The extent to which such selection processes will make a difference depends on factors such as the size of ejaculates relative to spermathecal storage capacity, average viability of sperm and the cost of sperm storage [13,78–81]. Lifetime commitment between paternal and maternal gametes is therefore not decided at insemination or fertilization, but at the final storage of sperm, provided sperm is used randomly after storage (see §2b).
(b). Three mating system syndromes, constrained sperm competition and female control
In eusocial insects the distinction between pre- and post-copulatory sexual selection is applicable only with significant modification. There obviously is a pre-copulatory mate-choice phase, but it involves a very restricted set of mate quality cues (see §2a), and the entire process ends with a monogamous commitment, in termites even well before the first copulation takes place [70,71]. However, in the eusocial Hymenoptera, there are two very distinct post-copulatory phases, separated by sperms' irreversible entry into the spermatheca. As long as lifetime monogamy prevails, there will be no sexual selection in either of these, as sperm of haploid males are clonal so that any differential storage can be based only on non-genetic criteria [13,79]. Ejaculates of different males will interact only in those hymenopteran lineages where multiple queen-mating evolved as a secondary elaboration (cf. [16]). A logical corollary of the monogamy hypothesis is, therefore, that during the evolution of a lifetime-committed worker caste there was never any mixing of sperm, so that all mechanisms of ejaculate conflict between insemination and final sperm storage had to evolve de novo and independently after secondary switches to facultative or obligate multiple mating had occurred [15].
The window for ejaculate competition is brief and very distinct; so the null hypothesis to be rejected is that we expect females to have evolved control over sperm competition. Once permanently stored, there will normally not be opportunities for preferential sperm use during egg fertilization many months or even years later, as such biasing would either require empowering mechanisms for clones of stored sperm derived from specific males (patrilines) or female fitness incentives for allowing anything else than ‘fair raffle’ sperm use when fertilizing eggs [13,79,82]. Selfish patrilines [83,84] might have evolved ways to overcome this form of queen control, but otherwise one would expect to see complete sperm mixing if queen fitness increases with increasing genetic diversity of workers [85]. Thus, when paternity differences across worker cohorts in the same colony appear to occur, it is important to check whether this might reflect differential larval growth rather than temporary variable sperm use owing to incomplete sperm mixing [82,83,86].
The increasingly convincing evidence for the secondary nature of multiple queen-mating across the obligatority eusocial Hymenoptera [16,85,87,88] also reinforced that facultative and obligate multiple-mating appear to be mutually exclusive lineage-specific syndromes forming chimaera colonies (table 1 and figure 1b). Each of these mating systems tends to be typical for entire genera or higher-level clades [85,87] rather than being shifting alternatives in some mating-frequency continuum. Thus, ants, eusocial bees and eusocial wasps tend to have either 100 per cent single mating, or some mixture of colonies headed by a monandrous or mildly polyandrous queen, or 100 per cent (usually high) polyandry of queens [16,85,87] (figure 2). There is now reasonable consensus about the evolution of obligate multiple mating having been driven by benefits related to enhanced genetic diversity among workers (proposed by [89,90] and reviewed by [85,87,91–93]), but what has driven the convergent evolution of facultative multiple mating has remained enigmatic. It is tempting to speculate that this mating system evolved to allow females to correct suboptimal first inseminations, but considerable research effort will be required to unravel the interaction between sperm transfer, mating plug efficiency and female sperm storage responses, which seems a tall order as almost no eusocial species with facultative multiple mating are known to mate under laboratory conditions.
Figure 2.
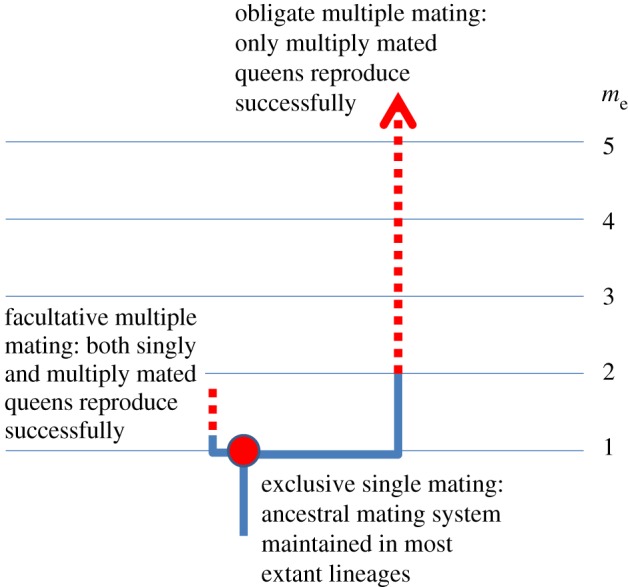
An illustration of the categorical, rather than continuous, variation in mating systems of facultatively and obligatorily eusocial insects. Most extant species appear to have retained the ancestral exclusive single mating habit [16,85,87,88]. Facultative and obligate multiple mating are distinct secondary evolutionary endpoints that have convergently evolved in multiple lineages (often entire genera or higher order clades) of ants, vespine wasps and corbiculate bees, but apparently never in the termites. Frequency distributions of the genetically effective number of matings (me) across species (the standard used by social insect researchers as it is inversely related to nest-mate relatedness; [87]) are therefore not unimodal but trimodal (illustrated by the non-overlapping ranges of red dots). Facultative multiple mating is likely to represent a mixed evolutionarily stable strategy as both singly and multiply mated queens can successfully found colonies and reproduce. Obligate multiple mating implies that mature full-sibling colonies are essentially never found, which must imply that directional selection quickly took these lineages through an inevitable ancestral phase of facultative multiple mating, presumably driven by genetic diversity benefits [85,87]. The selection factors that stably maintain facultative multiple mating are unknown. Obligate multiple mating is only found in the obligatorily eusocial Hymenoptera, but facultative multiple mating occasionally occurs in facultatively eusocial lineages, and re-evolved in a number of obligatorily eusocial clades [16,85,87,88].
Because re-mating promiscuity (table 1) is absent, all brood cohorts throughout a polyandrous queen's life will be fertilized by the same set of fathers that managed to get their sperm stored on the single day that they mated with her in quick succession. This implies that any competition between ejaculates for storage would have to take place in the provisional storage phase, as continued competition after storage will affect lifetime fecundity of queens and thus be selected against [13,94]. Recent work has shown that seminal fluid proteins play a key role in hostile interactions between ejaculates during this phase [77,78,95], as accessory gland secretions of monandrous fungus-growing ants and bumble-bees were as supportive to own sperm and alien sperm (as expected when ejaculates have no evolutionary history of ever interacting), whereas respective polyandrous sister lineages of leaf-cutting ants and honeybees appear to have a combination of generally supportive and specifically hostile seminal fluid interactions with alien sperm [78].
As inseminated ejaculates are highly viable and new inseminations become impossible soon after the first because queens lose receptivity, eusocial sperm competition is expected to be a closed endgame where males have little power, and the queen decides both the number of ejaculate participants at the start (when she stops mating) and the completion of sperm admission into the spermatheca (when she discards the remaining sperm). Queens thus provide a bursa copulatrix arena to let a fixed number of ‘gladiator ejaculate armies’ perform a self-thinning process before the survivors are admitted to potential reproductive status in her storage sanctuary. It thus seems almost unimaginable that queens would not have close to 100 per cent control over sperm competition, a situation that is highly unusual in promiscuous mating systems where ejaculates enter serially. In such cases, ejaculates can be adjusted by males depending on the likely reproductive value of current versus future matings, and accessory gland proteins can affect female physiology in more selfish ways because males will not breed with the same female again [69,96,97]. Given that eusocial polyandry evolved convergently in multiple lineages of ants, corbiculate bees and vespine wasps [15,16,85], and each time from strictly monogamous ancestors, it will be interesting to see whether the molecular mechanisms that mediate seminal fluid hostility in different genomic backgrounds also have elements of functional convergence.
Once sperm of different males has become permanently stored, all male manipulation is expected to cease, because from now on the Monty Python ‘Meaning of Life’ logic that ‘every sperm is sacred’ will apply [13]. This is because mother queens of mature colonies are more likely to be sperm limited [94] in their total lifetime reproductive success than egg limited, because they can continue to lay eggs as long as they have workers to feed the hatching larvae, but they cannot continue to fertilize eggs to replace short-lived workers when their spermatheca is empty. Recent work has shown that sperm-limited lifetime reproductive success seems indeed likely for queens of Atta colombica leaf-cutting ants [80] and that spermathecal fluid of these ants somehow terminates hostile interactions between seminal fluid and genetically different sperm, as expected when queens ‘consider’ permanently stored sperm as an invaluable commodity that is no longer to be depreciated by any form of male–male competition [78]. This encouraging match between expectations and first empirical analyses deserves further testing in other polyandrous ants, bees and wasps to see whether complications in the general logic of these evolutionary inferences might emerge.
As internal fertilization is a universal trait in many vertebrate and invertebrate lineages, there are many ways in which sperm must have been selected to avoid being attacked by the female immune system. As long as sperm tenure after insemination is transient because females frequently re-mate, one would expect female immune defences to contribute to the gauntlet-running test-bed were that to enhance the probability of the most suitable sperm reaching the eggs. However, queens of the eusocial Hymenoptera, and long-lived ant queens in particular, are lifetime pregnant with a large clump of non-self sperm, and selection should thus have ensured that not even a little harm is done to these sperm cells once they have entered the spermatheca. It would be interesting to know how the transcriptomes and proteomes of known immune genes in spermathecal fluid differ from those in control tissues. Termites could offer an interesting parallel test, as their queens have sperm storage organs that are regularly refilled by re-mating with the same colony king so that storage remains brief [71] and female immune genes could thus impose adaptive ejaculate thinning before queens use sperm.
3. Lifetime monogamy as universal ancestral state for obligate eusociality
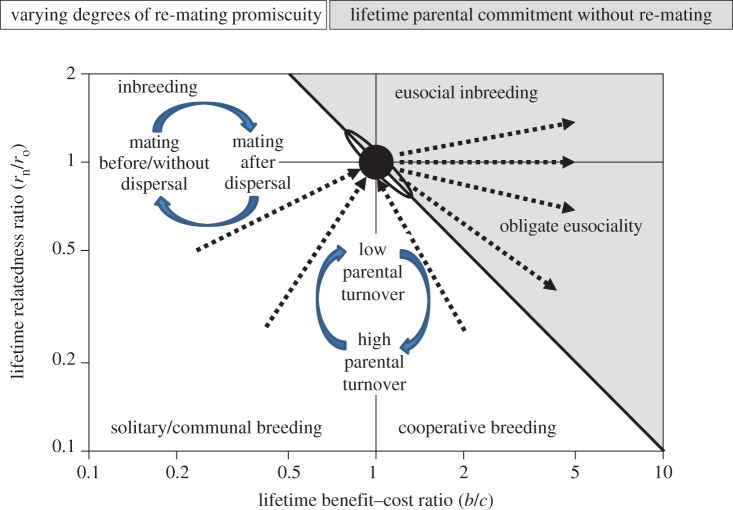
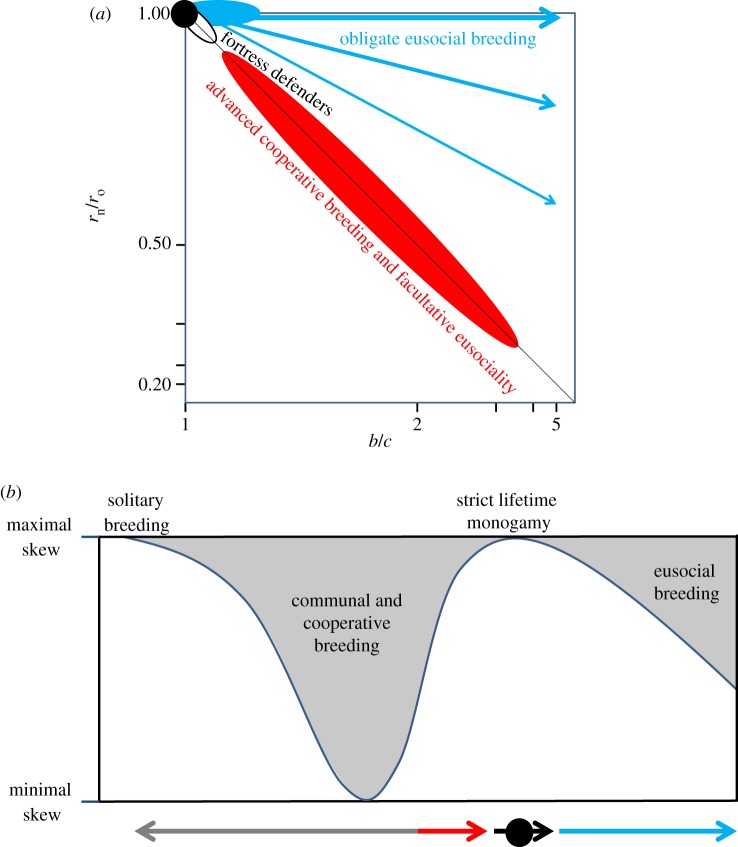
In a previous review [14], I summarized the arguments for strict lifetime monogamy being the most obvious general factor to facilitate rare irreversible transitions to obligate eusociality in a diagram that I reproduce here in a more precise version (figure 3). The parsimony inference was that if the establishment of irreversible caste phenotypes is most likely to happen in a long and gradual evolutionary process of infinitesimally small steps, then there would be no better general facilitating condition than the strict lifetime monogamy that we know has remained the commonest form of family organization throughout extant clades of the obligatorily eusocial insects (figure 2). Eusociality is favoured most effectively if the product of the benefits of lifetime helping (b) and average lifetime relatedness to nest-mates (rn) exceeds the product of the lifetime costs of helping (c) and relatedness to offspring (ro). Hamilton's rule thus reduces to b/c > 1 when both relatednesses are always 0.5 on average, whereas the necessary b/c-ratio threshold will always have to be higher when parental promiscuity reduces sibling relatedness to values below 0.5 on average. Hence, very slight efficiency benefits will allow the transition to obligate eusociality under lifetime monogamy as long as they are long-term consistent (which will rarely be the case), but less so under even low degrees of serial monogamy or promiscuity. The prediction has now been formally substantiated by comparative data [16], confirming that all known independent evolutionary developments towards obligate eusociality appear to have been realized by ancestors going through a prolonged lifetime monogamy window (straight arrows in figure 3) and not by crossing the white/grey diagonal elsewhere, even though that would be allowed by Hamilton's [18,19] rule (see also [14]). However, facultative eusociality plays out along the diagonal, but in separate regions depending on whether it is derived from lifetime monogamy (possibly including a soldier caste; §3b), or from advanced cooperative breeding where colony life is based on recurrent turnover of dominant female and male breeders (§4). The cooperative breeding triangle is not formally distinct from the other white areas towards the left as social systems move in and out of these over evolutionary time.
Figure 3.
The monogamy window (filled black circle in the centre) towards obligate eusociality in the parameter space provided by Hamilton's [18,19] rule as it plays out over the entire lives of altruistic helpers, with the log ratio of relatedness to nest-mates (rn) versus offspring (ro) on the y-axis and the log ratio of benefits (b) versus costs (c) of helping on the x-axis. The grey triangle represents all parameter combinations where Hamilton's condition for reproductive altruism (brn > cro; here plotted as log(rn/ro) >−log(b/c)) is fulfilled across the lifetime of offspring, so that permanent worker castes are expected to evolve and be maintained. Breeding systems are written in the different parts of the diagram and the white/grey presence or absence of promiscuity is highlighted in the text boxes at the top. Straight arrows illustrate that both inclusive fitness logic [14] and available evidence [16] indicate that transitions towards obligate eusociality were achieved via the narrow monogamy window (black circle) in the centre of the diagram, and not by crossing the white/grey diagonal elsewhere. Bent arrows illustrate that non-eusocial mating systems are expected to be solitary or communal when parental turnover is high and cooperatively breeding when parental turnover is low. Similarly, mating before (possible) dispersal or after dispersal will determine whether a breeding system will be inbred or outbred, but no social systems based on inbreeding are known to have produced obligate eusociality. The ellipse overlapping with the black circle approximates the combination of rn, b and c values that likely apply in fortress defenders that have often evolved altruistic soldier morphs, but rarely true worker castes. Figure modified after Boomsma [14] and Cornwallis et al. [98].
Strict lifetime monogamy as a necessary condition for making the transition to obligate eusociality with altruistic (true) workers is fully consistent with defining obligate eusociality based on all individuals having lost reproductive totipotency [14,54,58] (table 1). However, necessity does not imply sufficiency. There are a number of animal lineages with lifetime monogamy that never had life cycles or ecologies that would make the b/c ratio in Hamilton's rule favourable for offspring to become sterile workers rather than (at least potentially) independent breeders. What the monogamy hypothesis posits is that those sub-social lineages that have managed to make the transition had lost re-mating promiscuity before any b/c benefits could vector them over into the obligatorily eusocial domain. This is consistent with many primitively eusocial systems approaching monogamy (cf. [16]) but without having achieved this 100 per cent, as for example the halictid bees maintaining low frequencies of facultative multiple mating and replacement of founder queens by daughters, the wood-dwelling lower termites being unable to avoid colony mergers, and the paper wasps where auxillary foundresses occur in many species (see §3a,b).
The monogamy hypothesis works the same way for haplodiploidy and diploidy, because 0.75 relatedness to diploid supersisters and 0.25 relatedness to haploid brothers gives an average relatedness of 0.5 similar to diploid full-sibling relatedness under ancestral 50/50 sex allocation [14,15]. This is convenient as Trivers & Hare [99] decisively refuted the notion that haplodiploidy would have given the Hymenoptera a higher likelihood of evolving eusociality just because of 0.75 relatedness to full sisters. Recent years have seen a renewed interest in modelling the possible effects of haplodiploidy without ignoring the compensating 0.25 relatedness to brothers. The results have been mixed [100,101], so for now it seems most reasonable to assume that haplodiploidy can either be favourable or unfavourable for the evolution of eusocial castes, depending on assumptions.
(a). Major transitions require irreversibly completed developments: principle and examples
The monogamy hypothesis is incompatible with obligate eusociality being the tail end of some eusociality continuum [52]. Extant species are either obligatorily eusocial or they are not in the original meaning of the term (truly social). If they are, they have physically distinct queen and worker castes with complementary social roles without which a colony can never grow to reproduce [50,54,58], or they are derived from ancestors that must have had such obligate castes (e.g. workerless inquiline ants and ants with gamergate reproductives, see §3a(ii)). Almost having made a transition to obligate eusociality therefore means not having made it, as a genetic trait that has not gone to fixation remains easily reversible. To realize the transition, both lifetime monogamy and (slight) b/c benefits needed to be long-term consistent, so that there was enough evolutionary time for gene complexes which normally secure unrestricted expression of reproductive totipotency to accumulate so many deleterious mutations and deletions when expressed in a worker phenotype that reversal becomes a practical impossibility. This is why becoming obligatorily eusocial is a major evolutionary transition, but living in societies or colonies is not [14,15]. The logic that an evolutionary transition completes a development without being part of it is similar to, and complements, Gardner & Grafen's [102] argument that there is a fundamental difference between group selection and group adaptation, because adaptations for the exclusive benefit of the group do not arise gradually, but only after lower-level selection has all but disappeared. Defining eusociality based on morphologically distinct castes [14,53,54,58] (table 1) is thus more evolutionarily informative than using pragmatic definitions.
(i). Polistine wasps: obligate eusociality remains to be demonstrated
Recent studies have underlined the fundamental significance of the distinction between eusociality and cooperative breeding. Leadbeater et al. [103] confirmed (for a review of earlier evidence, see [15]) that helpers of Polistes wasps do not always irreversibly commit to their behavioural caste roles. They may gain considerable indirect fitness benefits when the dominant breeder is a relative, but females of the same cohort in the same population may also help non-relatives, because such nests offer a favourable probability of obtaining direct fitness benefits by acquiring breeder status later in life. These alternative tactics coexist side by side, showing on the one hand that there are significant indirect fitness benefits for the average helper in the population, and on the other hand that many helpers are likely to obtain direct fitness benefits as later breeders. The same study showed that a small minority of offspring females opted out by mating and overwintering early to join the pool of spring breeders rather than completing their life cycle in the same season, i.e. they continued to behave as univoltine, reproductively totipotent solitary breeders. Phenotypic plasticity of this kind is characteristic for cooperative breeders, but inconsistent with hard-wired obligate eusociality where worker-helpers will never obtain the direct fitness of a dispersing breeder. It seems likely that similar social dynamics apply in all Ropalidini and related lineages of independent-founding paper wasps [104,105].
The Epiponini are the sister clade of the Polistini and have from several to many mated and egg-laying females per nest, considerable body size variation in some species, and colony founding by swarming (reviewed by Hunt [105] and Jeanne [106]). Although relatedness is high when female reproductives are produced [107,108], this complex type of sociality seems to challenge the monogamy hypothesis, as this scenario would require a clade ancestor with 100 per cent colony founding by a single once-mated queen. However, the enigma would disappear if it could be shown that epiponine worker roles have remained reversible as a study by Strassmann et al. [109] indicates (making them advanced cooperative breeders similar to Polistes), or that the basal branches of their phylogeny would have lifetime monogamous parents and solitary colony founding (which might make them an independent transition to obligate eusociality if worker castes in at least some species would prove to be hard wired). The social evolution status of the crown group of the vespid wasp phylogeny thus remains ambiguous. This is not because lineages do not all have obligate colony life, but because it remains to be proved that caste phenotypes outside the obligatorily eusocial vespine (yellowjacket) wasps are distinct for all individuals, rather than phenotypically plastic with reproductive totipotency always being one of the available options for some fraction of each cohort. Hunt [105] provides an update review on our current knowledge of wasp social biology, emphasizing various intriguing forms of phenotypic plasticity in polistine wasps, but neglecting the key question of whether any of these wasps has become obligatorily eusocial in the sense of permanent caste commitment. As long as some fraction of each cohort are ‘false workers’ in the termite sense of remaining uncommitted as young adults, the transition towards obligate eusociality has not been made, and polistine wasps would thus remain cooperative breeders or facultatively eusocial in the sense of [54].
(ii). Ants with cooperative breeder traits: distinct from non-eusocial cooperative breeders
The ants are monophyletic and obligatorily eusocial throughout [110,111], but some basal lineages seem to have reverted to forms of cooperative breeding [49,112]. However, careful inspection of the details shows that this is not really the case in spite of a number of suggestive convergent analogies. It appears that these ants are all derived elaborations of obligate eusociality that emerged via secondary selection against independent colony founding by winged, newly mated and dispersing queens while favouring alternative modes of reproduction based on the division of existing colonies [113–115]. Such evolutionary developments have happened in all subfamilies of ants [115] and normally start with some form of coexistence between independent and dependent colony founding where, respectively, queens mate during dispersal or before/after colony division [113]. Further evolutionary change may then result in the complete loss of the ancestral state so that obligate colony division by fission or budding remains and lineages become fully characterized by ergatoid queens lacking flight muscles and wings [115].
In three subfamilies of ants (Amblyoponinae, Ponerinae, Ectatomminae), selection against having only queens that disperse on the wing occurred in species where the workers had not irreversibly lost their spermathecae (i.e. they had retained the potential to express spermatheca genes in a morphologically distinct worker caste phenotype [116]). In some species belonging to these lineages, the winged queen caste was completely lost and replaced by gamergates (sexually reproducing workers), consistent with the idea that once selection for colony division as an alternative mode of reproduction starts, cheaper (i.e. smaller, leaner and non-flying) female reproductives evolve as replacements [115]. These derived lineages may ultimately restore single-female (monogynous) breeding, even though the ancestors that initiated the loss of flying queens could only do so by having multiple gamergates per nest [114]. Gamergates are much less fecund than ergatoid queens, so colonies headed by gamergates are small, particularly in the most derived lineages that tend to converge on a single gamergate breeder per colony [114]. A major general difference between ergatoid queens and gamergates is that the former retain the exclusive caste-specific monopoly of mating and reproduction, whereas in gamergate species, the workers must establish dominance hierarchies to regulate who ends up mating and laying eggs. Gamergate societies have thus secondarily acquired the cooperative breeder trait that a single ant phenotype can change caste role behaviourally by mating and advancing to breeder status. However, they never re-evolved other key traits that characterize cooperative breeding, such as re-mating promiscuity (vertebrates) and the ability to found colonies alone after long distance dispersal (polistine wasps). Gamergates can only change to breeder roles early in adult life [115], whereas such changes tend to be associated with later adulthood in vertebrate cooperative breeders. These evolutionarily-derived eusocial breeders thus never regained reproductive totipotency.
(iii). Ancestral versus derived inbreeding: termites, ambrosia beetles and social spiders
Recent phylogenies suggest that there have likely been two or three independent origins of true workers in the termites and that these transitions were always associated with the adoption of central place foraging from one-piece-nesting (wood-dwelling) ancestors [117–119]. However, only one of these lineages—the higher termites—realized a significant radiation and ecological footprint, indicating that making the transition to obligate eusociality does not always start a major lineage development. Further work will be needed to clarify the constraints that prevented the foraging Mastotermitidae and Hodotermididae from carving out their own major eusocial niche space comparable to the Termitidae. That non-foraging lower termites are essentially cooperative breeders (albeit with a differentiated soldier caste, see §3b) has recently been underlined by evidence that larvae and nymphs of several genera provide little indirect-fitness-driven brood care, but rely on the likelihood of later advancement to breeder status, either in the same nest or after dispersal to find a mate and found a new colony [120–122], not unlike the Polistes wasps discussed above. Few species have been investigated, but it appears that these lower termites still have some sperm motility, consistent with experiencing a non-zero probability of promiscuity later in life [71,123–125]. All this fits the term ‘false workers’ that some authors have used to characterize these, at best, conditionally altruistic workers [126,127].
A further notion worth emphasizing is that association of relatives, either as outbred siblings with recent co-ancestry or as inbred offspring of a local group of parents, appears not to have ever produced a single obligatorily eusocial lineage (hence no straight arrow in the top left of figure 3; see also [14]). Recent work on ambrosia beetles [128] has shown that inbred offspring help, both as larvae and adults, but (sib)mate and predominantly disperse to found new burrows, illustrating that high sibling relatedness combined with monogamy does not have to produce eusociality. Although some (dead wood) burrows of ambrosia beetles may last for two or three generations, most deteriorate sooner, precluding indirect fitness gains for later offspring that would fail to disperse after some variable period of helping—quite similar to wood-dwelling lower termites running out of food. Tellingly, the only known ambrosia beetle that has apparently evolved true workers is diploid and digs its burrows in live Eucalyptus trees, so they can last for many years [129]. Another African species of apparently outbred platypodine ambrosia beetles is known to found colonies biparentally in live trees and may represent earlier stages of eusociality that can be compared with sympatric sister lineages living in dead wood where burrows are shorter-lived [130,131]. Analogous arguments for inbred social spiders, which never produced obligatorily eusocial lineages, have been given by Boomsma [14] (see also [132] for a recent review of their comparative biology). It thus appears that all known obligatorily eusocial lineages have arisen sub-socially from lifetime-committed outbred parents.
Both in the lower and the higher termites, sib-mating offspring of founders may inherit nests or become the reproductives of nest fragments that bud off [118,127,133]. However, it is important to note that such incestuous replacement breeders do not violate the non-promiscuity rule, as no fresh blood enters the colony, so they merely recombine the genes of their lifetime monogamous colony-founding parents [14,15]. The same is true when queen succession happens by automictic parthenogenesis as is known to occur in Reticulitermes (Rhinotermitidae) [134]. This underlines that termites with true workers could apparently never evolve secondary elaborations of obligate eusociality such as multiple mating and adoption of offspring queens mated to unrelated males, as the eusocial Hymenoptera did. This suggests that any form of genetic chimaerism to secondarily diversify the founding tetraploid zygote analogue (table 1 and figure 1b) would likely have corrupted established true worker pathways. This may well be because the termites lack pupal metamorphosis to developmentally (and irreversibly) connect differential larval growth trajectories with specific adult caste phenotypes (see also §3b). In ants, obligate or predominant inbreeding only evolved in a few evolutionarily-derived lineages [115]. In sum, inbreeding appears to be a severe constraint for monogamous breeding systems to evolve obligate eusociality (see also §3b), but it can evolve as a derived condition once obligate eusociality has become established. The upper part of the small ellipse through the monogamy window and the arrow in the eusocial inbreeding rectangle of figure 3 illustrate this distinction.
(iv). Swarming and the possibility of worker interference during mating
As in the epiponine wasps discussed in §3a(i), swarming and colony fission have also (convergently) evolved in obligatorily eusocial clades such as honeybees, stingless bees [57], army ants [93] and a few other lineages of obligatorily eusocial insects (reviewed by Cronin et al. [135]). These developments are always derived, i.e. are elaborations on already advanced forms of obligate eusocial life. They thus represent secondary losses of the ability of single queens to found colonies independently [113,115,136]—one of the hallmarks characterizing the origin of obligate eusociality where sex and society became separated. This makes these swarms fundamentally different from the swarms in epiponine wasps that always contain multiple egg layers [106]. Although the later evolution of multiple mating and adoption of non-sib-mated daughter queens (including the ergatoid and gamergate elaborations of this type of colony kin structure) did not restore re-mating promiscuity, so swarm-founding did not remove the principle of queens mating alone and without the interference of workers (e.g. honeybees and stingless bees). The army ants are probably the exception that proves the rule, as queens are permanently wingless and mate with multiple unrelated males in their own nest, after the old queen and approximately half of the workers have left the colony [93,137]. This is fully comparable to honeybee colony fission and virgin-queen mating shortly thereafter, except that the mating swarm has been moved ‘indoors’ [93,138]. Attempts to re-mate old queens later in life can be artificially induced, but without leading to sperm transfer [137], confirming that lifetime commitment between initial mating partners prevails.
The army ants are one of the few evolutionarily-derived eusocial mating systems where society (i.e. workers) may affect mating success of virgin queens [138], similar to Cardiocondyla and some unicolonial ants [13,139]. However, there is no evidence that workers do in fact actively and independently interfere with their sisters' mate choice. Tellingly, the only known case of male-killing by workers affecting sister mate-choice in Cardiocondyla ants needs chemical marking by rival males of the male victims to occur [140], which makes the behaviour an extended phenotype of the competing males themselves. The separation of sex and society that appears to be the leading principle of the obligatorily eusocial domain would suggest that it is most likely that the virgin army ant queen herself decides which of the available males she will mate with (i.e. that workers would not prevent specific copulations), but that the worker collective might function as a gauntlet-running matrix decreasing the likelihood that less fit or too genetically similar males may reach the virgin queen. Such a process would then be reminiscent of female reproductive tracts providing physiological hurdles for sperm on their way to eggs or storage organs. It is a pity that the field biology of army ants makes it very difficult to test hypotheses of this kind.
(v). Obligatorily eusocial bumble-bees and facultatively eusocial halictids and allodapines
The recent literature on bees suggests that obligate eusociality has evolved twice rather than once in the corbiculate bees [57], with the honeybees and the bumble-bees plus stingless bees representing separate origins. This is gratifying as it reconciles many ambiguities (discussed in [57]) and also clearly separates the clades in terms of mating systems, with the honeybees having obligate multiple mating throughout and the bumble-bees and stingless bees having single mating or sometimes facultative multiple mating (figure 2; [16,85]). While bee researchers tend to classify bumble-bees, some halictid bees and some allodapine bees as primitively eusocial [57,141], the evolutionarily informative definitions advocated here (table 1) separate these clades, emphasizing that the differences between bumble-bees and stingless bees evolved in the obligatorily eusocial domain as they are both derived from a single ancestor that passed through the monogamy window towards obligate eusociality, whereas none of the halictine and allodapine bees has done so.
Some allodapine bees have morphological differentiation reminiscent of queen and worker castes [142], but neither of the two studied species has lost their last totipotent individuals, indicating that their sociality has remained facultative. Their remarkable social systems evolved in dry habitats where the lifespan of limiting nest sites came to exceed individual lifespan, so that nest inheritance became a major kin-selected force allowing offspring to become larger replacement queens. The tendency towards facultative eusociality in halictid bees evolved ca 35 Ma ago [141], but sister lineages often reversed to solitary breeding, as expected when eusociality has not become obligate, similar to the most advanced allodapine bees having sister species with simple colonies [142]. This is very different in the bumble-bees that only lost the worker caste in some lineages that secondarily became social parasites—similar to the vespine wasps that are always categorized as advanced (i.e. obligate) eusocial.
(b). The evolution of soldiers
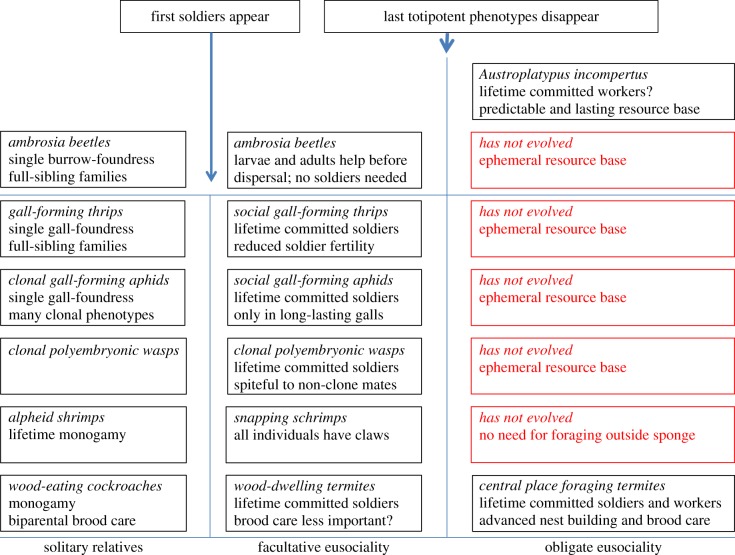
Previous versions of the monogamy hypothesis [14,15] have remained incomplete by hardly addressing the evolution of soldier castes. This is not a serious omission in eusocial clades such as ants where soldiers always arise as derived additional worker castes and typically in lineages with large colonies and substantial size dimorphism between queens and standard-size workers [143–145]. Morphologically distinct soldier castes have not been documented for any facultatively or obligatorily eusocial vespid wasps [105,106], consistent with worker–queen dimorphism being small; cf. [143]), and the only example in eusocial (stingless) bees appears to be associated with defence against an unusually effective robber-bee [146]. However, soldier castes in termites have evolved before true workers [147], and a number of other invertebrate lineages have in recent years been singled out as eusocial based on the existence of altruistic soldiers (figure 4). To be consistent with the monogamy hypothesis, such soldier castes should have evolved in single foundress families with, at least early in family life, maximal relatedness among offspring, but in ecological settings that will never select for true (foraging) workers (figure 4).
Figure 4.
The evolution of defence altruism in invertebrate clades that live in their food and where fortress-defence requirements [59] have produced soldier morphs, but only rarely true workers. The ambrosia beetles are either diploid or haplodiploid and may or may not be inbred [128]. Obligate eusociality was not selected for, except in a single Australian species that nests in live wood and which represents an independent lineage not closely related to the cooperatively breeding ambrosia beetles [128,129]. The fixed diameter of entrance tunnels would never select for soldier morphs because normal sized individuals can block tunnels. In the haplodiploid gall-forming thrips soldiers appear to be irreversible phenotypes, but remain able to reproduce [148,149]. In the gall-forming aphids, clonality makes direct/indirect fitness distinctions irrelevant, similar to germ-line and somatic cells in metazoan bodies having identical interests, and many alternative phenotypes already exist in the non-social aphid lineages that have produced social species [150]. In the polyembryonic wasps, soldiers evolved to eliminate offspring of unrelated cofoundresses and clonality ensures that their evolution was relatively unconstrained [151,152]. Snapping shrimp feed on the sponge they live in or on food items carried by water currents, so there has never been selection for specialized foragers or nest builders [153,154]. The termites have irreversible soldier phenotypes in the wood-dwelling lineages and lifetime-committed workers (in addition to soldiers) in derived lineages that no longer live in their food [120,122].
As outlined by previous authors [59,117,127,148,155,156], nesting within an abundant food resource is a powerful predictor for the initiation of sociality outside the Hymenoptera. No indication has been obtained that any of these fortress defenders [59] would not have monogamous colony-founding, and in the better studied species strict lifetime monogamy (or clonality as in aphids and polyembryonic wasps) appears to be upheld (see references in figure 4). The question therefore is why most of these lineages have not evolved true workers after evolving soldiers, and the answer appears to be that there has never been selection for specialized phenotypes operating outside the nest [156]. The aphids and thrips have nothing to gain from foraging unless they can gain access to a neighbouring gall, which would compromise genetic homogeneity and the inclusive fitness of residents and thus solicit vicious defence. They are also constrained in expanding their galls, so the number of broods remains low [148,150], similar to the ambrosia beetles nesting in dead wood [128]. Snapping shrimp obtain their food from the sponge tissues around their nests or passively via water flowing through [157]. Food is therefore not a limiting factor, so that foraging outside the nest never pays off in any of these lineages. Similar logic would appear to apply for the false worker castes in termites. Living inside the food resource, referred to as ‘bonanzas’ by previous reviews [156,158], implies that immatures can largely feed themselves, so that prolonged provisioning of younger siblings by older nest-mates may not be a major driver of social evolution. This appears to be consistent with recent findings that brood care is mostly focused on prophylactic hygiene measures, both in lower termites [122] and in wood-dwelling ambrosia beetles [128] where larval social behaviour appears focused on waste concentration, whereas adults are waste disposers and defensive gallery blockers.
The basal termite lineages have strictly monogamous colony founding, but face competition later in life from conspecific founding pairs colonizing the same log [120,122,123]. As the full-sibling larvae of these founding pairs start eating out cavities and gallery systems, colonies are bound to meet and compete for feeding and nesting resources. While this often allows adjusted coexistence in nests that remain separate, colonies may also merge, which will end with one of the breeding pairs being killed (usurpation) or one of each pair being eliminated, resulting in re-mating promiscuity of the surviving breeders [125,126]. It is this higher than zero statistical likelihood of experiencing unrelated (after having been usurped) or half-sibling (after one surviving parent re-mates) nest-mates later in the colony cycle that the monogamy hypothesis predicts to be a non-starter for transition towards obligate eusociality with a true worker caste. However, as strict monogamy is always intact early in the colony cycle, there is no reason why altruistic soldiers should not evolve and be maintained by kin selection when pressure from natural enemies is substantial. Phylogenetic data [119] are consistent with the non-zero probability of re-mating promiscuity in lower termites precluding that true workers could evolve until termites stopped living in their food, spaced out sufficiently to avoid colony mergers, and thus secured continuing full-sib relatedness [117,120,147].
Overall, it appears that the evolution of defence castes in termites is generally compatible with the monogamy hypothesis. Lower termite soldiers tend to be relatively few and be produced early enough to maximize the likelihood that full-siblings are protected when colonies are most vulnerable because they are still small [120,126,127]. Soldiers have to be sclerotized to be effective, which comes at the expense of losing the ability to moult back into an uncommitted nymphal stage [120]. They thus represent irreversibly altruistic individuals, but their social systems are not obligatorily eusocial as long as the pool of large immatures remains false workers, i.e. transient developmental phenotypes that can be abandoned when conditions change [120]. Major questions that need to be addressed in termites are whether the soldiers of lower termites are primarily meant to defend colonies against predators or competing conspecifics in the same log, and whether their production is mostly a function of relatedness or the level of imminent threat.
The Synalpheus sponge-dwelling shrimps are yet another invertebrate example of fortress colony defence (figure 4). A recent study by Duffy & Macdonald [154] has shown that the alpheid shrimp clade in which complex social family structures evolved is monogamous throughout, but exceptional in having small body-size and non-dispersing larvae that hatch directly into crawling juveniles, rather than into swimming dispersers. Life-history and phylogeny data for these shrimp are thus consistent with monogamy being a necessary condition for transitions towards advanced social organization. It is important to note, however, that the snapping shrimp have no permanently differentiated castes, as all individuals have fighting claws and the female breeders merely tend to become the largest individuals in the sponge as colonies grow [153], similar to naked mole rats (see §4) and some allodapine bees [142]. As argued above, there will never be selection for worker foragers leaving the nest, so snapping shrimp will remain advanced cooperative breeders for whom defence of nest sponges is essential but where future research may show that there is little brood care altruism similar to the lower termites so far investigated [120,122]. The social spiders (§3b(iii)) passively acquire food similar to the snapping shrimp and they inbreed as do some of the ambrosia beetles, but have never evolved specialized castes for either defence or foraging [132].
A final example is found in the polyembryonic wasps, where soldier morphs eliminate non- or lesser relatives to secure the fitness gains that their mothers intended when laying an egg in a host of fixed size [151,152]. These wasps are particularly interesting because a modelling study [159] has indicated that female soldiers (which tend to be the majority) are primarily adaptive for mediating sex ratio conflicts, whereas male soldiers eliminate competing unrelated individuals, suggesting that selection forces during the origin and later elaboration of soldier morphs may have been different.
The arguments of this section are mostly an update of previous attempts to functionally classify categories of social organization in insects and vertebrates [49,59,118], but I believe that the monogamy criterion adds useful insights into the most likely evolutionary pathways that produced fortress defending life histories and constrained their further social evolution. As illustrated by the ellipse superimposed on the monogamy window in figure 3, most fortress defenders have been unable to evolve away from the log(rn/ro) = −log(b/c) diagonal to irreversibly enter the obligatorily eusocial domain in spite of monogamous parents. The approach taken here makes it explicit that ‘fortress defenders’ and ‘life insurers’ (sensu [59]) almost never share direct common ancestry. The comparative data (figure 4) suggest that the termites are the only major exception to this rule, and even in this lineage the emergence of obligate eusociality was a rare event with only a single major radiation with true workers. It is tempting to speculate that evolving obligate eusociality from fortress defending ancestors was facilitated by the termites decomposing wood and other organic matter (with the help of endosymbionts), so that the resources they could obtain outside their fortresses were not too radically different from what the walls of their fortresses used to provide. The other likely exception is the platypodid ambrosia beetles that never needed morphologically distinct soldiers to block their burrows, but have likely progressed to obligate eusociality in one, and possibly a few more, species that independently specialized on exploiting live trees (figure 4).
4. Vertebrates were never monogamous enough to have evolved obligatorily eusocial lineages
The monogamy hypothesis was developed from the idea that promiscuous mating reduces, all else being equal, the indirect fitness benefits that older siblings obtain from adopting roles as helper at the nest. Following the first comparative analysis by Griffin & West [160], this topic was briefly explored for vertebrates in Boomsma [14], suggesting that cooperative breeding should, all else being equal, be characterized by lower degrees of parental re-mating promiscuity than solitary breeding. Recent comparative analyses have shown that parental re-mating indeed explains a large proportion of the variation in cooperative and solitary breeding in birds and mammals. Cornwallis et al. [98] assembled a dataset of 267 bird species with known breeding systems and showed that the likelihood of cooperative breeding is higher when parents are more monogamous, both across and within extant species, and that the evolution of monogamy normally preceded the evolution of cooperative breeding, whereas the loss of cooperative breeding followed rather than induced higher parental promiscuity. Similar results were obtained for mammals by Lukas & Clutton-Brock [161], analysing comparative data from 57 species.
(a). Interpreting the comparative data
The consistent support for monogamy affecting the probability of cooperative breeding across the birds and mammals is remarkable because mammalian helper roles differ in many ways from those in birds. The analyses confirm that relatedness incentives are a crucial selection force for cooperative breeding when all three Hamiltonian variables (r, b, c) vary continuously and can compensate each other, in contrast to the obligatorily eusocial insects that evolved from ancestors where average relatedness to siblings was always 0.5, so that the r-term cancelled out of Hamilton's rule, i.e. stopped being a variable determining helper commitment (figure 5a; see also §2a and [14]). The results obtained [98,161] reinforce that cooperative breeding is not a distinct domain of social evolution by itself, as lineages enter and leave this form of sociality over evolutionary time (the bent arrows to and from the triangle in figure 3) and some fraction of the helpers always retain reproductive totipotency [15,54].
Figure 5.
The fundamentally different Hamiltonian parameter space in which cooperative breeding, facultative eusociality and obligate eusociality operate. (a) As before (the present figure amplifies the bottom right of figure 3), the axes represent the ratios of lifetime relatednesses to younger nest-mates (rn) versus offspring (ro) and the efficiency benefits (b) and costs (c) associated with offspring staying to help parents reproduce. Advanced cooperative and facultatively eusocial breeders have condition-dependent helping for a substantial fraction of subordinates. They remain close to the diagonal and cannot move beyond this line as their breeding populations retain a number of subordinate individuals whose ability to become dominant breeders later on (i.e. moving back to the other side of the diagonal) is actively maintained by selection (red ellipse). The origin of the insect clades that evolved obligate eusociality after passing through the strict monogamy window (figure 3) is now represented by the filled black circle at the top left. In practice, this circle does not overlap with the red ellipse because a lasting transition from minimal promiscuity by serial monogamy to strict lifetime monogamy is very difficult to make and thus is not part of continuous variation over ecological time (see text). As in figure 3, the fortress defenders of figure 4 (note this panel only covers the outbred ones) can be conceptualized as a small black ellipse extending downwards along the diagonal. The obligatorily eusocial insects have evolved irreversibly ‘rewired’ developmental pathways for at least two distinct caste trajectories. In this process, they lost the last reproductively totipotent individuals while retaining relatedness ratios (rn/ro) of exactly unity so they could slowly consolidate the benefits of eusocial life (i.e. the b/c ratio becoming larger as illustrated by the blue ellipse). Once no single individual no longer had the possibility to change caste later in life (i.e. all individuals had become lifetime committed to a single caste), secondary elaborations such as polyandry (multiple queen-mating) and polygyny (adopting newly mated daughter queens back in the nest) could, but often did not, evolve to further increase the b/c ratio. This reduced the relatedness ratio, but without there being an obvious overall correlation between relatedness and b/c ratios (blue arrows). Measuring b/c ratios is impossible in the obligatorily eusocial domain, because there is no longer a joint currency of independent personal reproduction, as there is in the cooperative and facultatively eusocial breeders who remain under selection to continuously evaluate the Hamiltonian inequality and adjust their individual commitment decisions accordingly [103,162,163]. (b) Possible variation in reproductive skew when evolution proceeds from solitary to cooperative breeding and vice versa, and in some lineages to eusocial breeding as an irreversible transition. Colours are as in figure 5a, but now given below the x-axis. Reproductive skew is maximal (=1) per definition both in solitary breeders and during the origin of obligate eusociality. This implies that lower degrees of reproductive skew (the grey space between the curve and the maximal skew = 1 line) in the obligatorily eusocial and cooperative breeding domains have evolved in fundamentally different social contexts (with and without sterile workers; without and with promiscuity; without and with the option for long distance dispersal when fitness options in other groups are superior) and thus cannot be directly compared (see text for details).
Monogamy in birds and mammals is never as absolute as in the eusocial domain. At its vertebrate extreme, long-lasting serial monogamy without cuckoldry all but eliminates promiscuity in the sense of sperm competition, but yet maintains some long-term unpredictability of parenthood at established nests. While obligate eusociality arose in insects that die with the only sexual partner with which they ever mate on a single day early in life, serial monogamy implies that individuals will have some likelihood of producing half-sibling families over their lifetime rather than only full-sibling families. This difference between strict and serial monogamy explains that no bird or mammal lineage has ever crossed the white–grey diagonal towards the obligate eusocial domain in figure 3 or the gap between the red ellipse and the black dot in figure 5a. The continued presence of some degree of promiscuity will retain the standard market dynamics of mate choice that the eusocial domain has principally abandoned and replaced by lifetime commitment (even when polyandry evolved later). Thus, the Hamiltonian condition for helping is not completely fulfilled for even the most advanced cooperative breeders, as there will always be some initially subordinate individuals who will change reproductive roles during their lives to become dominant breeders that are themselves dependent on helpers. This implies that part of the red ellipse of figure 5a always remains below the diagonal, precluding that individuals become permanent members of castes that behaviourally and morphologically cement reproductive division of labour instead of keeping it plastic. In a companion paper, Lukas & Clutton-Brock [164] show that mammalian monogamy has led only to kin-selected cooperative breeding when there was a realistic possibility for increasing litter size of the dominant breeding female. This is an interesting finding as studies of Seychelles warblers that normally lay a single egg per clutch have shown that helpers rely primarily on direct rather than indirect fitness benefits [165].
Both Cornwallis et al. [98] and Lukas & Clutton-Brock [161] explicitly excluded communal and other forms of social breeding (table 1) that either do not offer indirect fitness benefits to helpers at the nest or make such benefits ambiguous because alternative direct fitness interpretations are possible. This indicates that traditional broad definitions of cooperative breeding are at best partly evolutionarily informative, similar to pragmatic definitions of eusociality, and that clarity would be gained by considering vertebrate communal and polygamous breeding systems (polyandry, polygyny, polygynandry; table 1) separately when they lack indirect fitness incentives for offspring to stay as helpers at the nest. The comparative studies by Lukas & Clutton-Brock [161,164] further emphasize that cooperative breeding in mammals always involves a single dominant female and that transitions towards cooperative breeding have never occurred in lineages where groups of multiple females breed communally, often with a single dominant male. Communal (polygynandrous) breeding tends to create positive matrifilial relatedness, because males disperse and compete for access to multigenerational groups of females. However, this mating system needs continuous reinforcement by the promiscuity market forces of sexual selection and will inevitably imply that subsequent offspring of a focal female are at least partly half-siblings rather than full-siblings. Cooperative breeding in mammals being based on commitment between a single dominant female and her mate(s) is gratifying as it offers a conceptual connection across the dividing line between the cooperative and eusocial breeders in figure 3, and across the gap between the red ellipse and the black dot in figure 5a—but only as long as one realizes that the transition from serial- to lifetime monogamy is very difficult to make.
(b). Inbreeding, reproductive skew, domain comparisons and modelling
The bird and mammal data seem to provide little support for inbreeding having been an important driving factor in vertebrate social evolution, suggesting that the most social of all mammals, the naked mole rat, is a special rather than a generally representative case. Its ecology has similarities with the fortress defenders living in their food [59,156] (figure 4), suggesting that inbreeding is a derived trait to secure nest inheritance for some generations, rather than a precursor of further social evolution, consistent with the Damaraland mole rat having independently evolved outbred cooperative breeding but with smaller colonies [166]. Categorizing the naked mole rat as a specialized (obligate) cooperative breeder is consistent with a scheme proposed by Clutton-Brock [45]. The mole rats and other specialized cooperative breeders such as the Kalahari meerkats would also fit my definition of facultative eusociality (table 1), but that is merely a semantic issue as facultative eusociality and advanced cooperative breeding are both part of an evolutionary continuum (the white areas in figure 3). The most cooperative edge of that continuum is characterized by cooperative breeding being obligate (i.e. no reproductive success can be realized by individuals that are not part of a social group), but that is fundamentally different from all individuals having distinct lifetime caste roles. The crucial point is that the mole rats are not obligatorily eusocial, just like the Polistes wasps where obligate colony life has not allowed the evolution of permanent individual caste fates either. Both for caste/helper roles and for inbreeding, we thus see coherent general patterns emerge. Across the vertebrates and the invertebrates, there is a fundamental distinction between variably committed ‘helpers’ (cooperative and facultatively eusocial breeding) and the lifetime-committed ‘workers’ that allow further caste evolution beyond adult phenotypic plasticity (obligate eusociality only). Similarly, we know of no examples, in either vertebrates or invertebrates, of inbreeding (often combined with female biased sex allocation) having vectored lineages across the point of no return towards obligate eusociality with true workers (figure 4), independently of there being inbreeding depression or outbreeding depression [132,167–169].
Reproductive skew is a useful measure of biased reproductive allocation in communal/cooperative and eusocial breeders [170,171], but the ways in which skews are achieved in these two domains are fundamentally different, because there was maximal skew per definition in the monogamy window separating them (figure 5b). This underlines once more that attempts to capture all variation in a eusociality continuum [52] cannot be conceptually defended. It is interesting to note that advanced vertebrate cooperative breeders with high reproductive skew may have more than a single male breeding with the dominant female [8,75], which implies that the overall variance in reproductive success among females may become higher than among males, similar to what happens when queens of eusocial Hymenoptera evolve multiple mating (§2).
Separating obligate eusociality, on the one hand, and solitary, communal, cooperative and facultatively eusocial breeding, on the other hand, makes it easier to see both the commonalities and the fundamental differences between these domains, which Costa & Fitzgerald [53] refer to as merely ‘eusocial’ and ‘social’. The crucial difference is that the former have irreversibly evolved away from the diagonals in figure 3 and figure 5a, and the latter have not because they retained some fraction of reproductively totipotent individuals. The obligate eusocial domain offers interesting questions about skew in paternity [172] and maternity (reviewed in [170,171]), but options to leave the nest to become an independent breeder are absent, while they remain possible in cooperative breeding and facultative eusociality. Acknowledging these domain differences appears more fundamental than clade-specific differences within domains. For example, cooperative breeding in vertebrates is rare outside the mammals and birds, but the fishes offer some exceptions of which the cooperatively breeding cichlids are best studied. Helping in cichlids is driven by defence against predators and by a fluid combination of direct and indirect fitness benefits [173–175]. It is straightforward to acknowledge the breeder/helper similarities with Polistes wasps or fairy wrens that can help at parental and alien nests [103,176] while taking into account that cichlid predation risks are exceptionally high. However, similar comparisons with queens of ants or workers of honeybees, stingless bees or bumble-bees are less meaningful because reproductive totipotency no longer applies.
Only few explicit attempts have been made to formally model the idea that monogamy enhances social evolution, either in the form of eusocial workers or as facultative helpers at the nest. The first such model was produced by Charnov [177] and specifically addressed the likelihood of male helpers. More recently, Nonacs [178] suggested that the effect of monogamy was at best minor and could also easily be opposite, i.e. promiscuity favouring the evolution of helping, even suggesting that inclusive fitness logic is inadequate for making predictions about the origin of eusociality. However, a model by Gardner et al. [101] confirmed the importance of monogamy and a combination of inclusive fitness, and individual-based modelling by Leggett et al. [17] was also generally supportive, similar to a model by Fromhage & Kokko [100]. These somewhat mixed results are not surprising as the assumptions of the models matter, consistent with the idea that monogamy is a necessary but not sufficient condition for the evolution of altruistic helping [14,15]. For example, it would seem essential that models consider conditions that would favour altruistic helping to become fixed, as the complete loss of totipotency is decisive for making the transition towards obligate eusociality, not the frequency of helping genes in a socially polymorphic population. Furthermore, nest inheritance should either be excluded or allowed to be lost, as this direct fitness benefit does not apply during the hypothetical monogamy window towards obligate eusociality and only re-evolved in some lineages as a later elaboration of obligate eusociality.
5. Promiscuity across the domains of social evolution
The monogamy hypothesis makes an explicit connection between paternity uncertainty of fathers and the probability of indirect fitness for offspring. Its logic and the tests currently available explain that: (i) major evolutionary transitions towards obligate eusociality could be made only when there was no paternity uncertainty at all, (ii) the eusocial domain has a number of very peculiar adaptations that make sense only in the light of re-mating promiscuity having been completely abandoned, (iii) the frequency of re-mating promiscuity is an important driver for cooperative breeding outside the obligate eusocial domain, determining in considerable measure the position of species in a continuum between solitary (including communal and polygamous) breeding and advanced cooperative breeding or facultative eusociality (see table 1 for definitions). The former tends to generate little or no indirect fitness benefits from social interactions, whereas the latter normally has such indirect fitness benefits but without precluding the realization of substantial direct fitness benefits later in life and (iv) provisioning castes (workers) that collectively and irreversibly forego mating to express morphologically specialized phenotypes are restricted to the separate, obligatorily eusocial domain, because the presence of this caste is the defining trait for obligate eusociality (table 1) [14,50,54,58].
The evolutionarily informative definition of eusociality used here allows a direct and explicit connection between lifetime commitment of mating partners and the evolution of eukaryote multicellularity from single zygotes [15,20,25,50,179], a type of conceptual coherence that pragmatic definitions of eusociality lack. Drawing this analogy emphasizes that there are only a handful of independent major adaptive radiations for each of these transition categories (plants, animals, fungi and two lineages of algae versus ants, higher termites, vespine wasps and two lineages of corbiculate bees), but considerably higher numbers of sister lineages that have remained ‘facultative’ [15,180]. Facultative and obligate eusociality belonging to different domains of social evolution captures this fundamental difference, whereas implicit [51] and explicit [52] definitions based on reproductive skew do not. The approach pursued here follows Bourke [20] in acknowledging that processes of social evolution have origins (formations), elaborations (maintenance) and transitions (transformations) to higher levels of integration, but differs in emphasizing the irreversibility (sensu [181]) of known transitions towards eusociality and eukaryote multicellularity from the moment that these states became obligate.
Using commitment criteria helps to generalize social evolution theory building on the major transition groundwork laid by Buss [182], Maynard Smith & Szathmáry [183] and Queller [25,184]. The major transitions approach emphasizes that there are clearly separated domains of social evolution, where the same inclusive fitness principles apply [20] but where the outcomes are different. In this view, major transitions are singularities [14], i.e. significant discontinuities in the omnipresent gradients in which life manifests itself. Such discontinuities give different meaning to established processes, and promiscuity is an example case in point: it is about unpredictable parentage in eukaryotes, about horizontal gene flow in prokaryotes, mostly about gene–culture interactions in humans, and it is absent in the obligatorily eusocial domain (table 1). Singularities also reconcile apparent contradictions: at the dawn of eukaryote multicellularity, the sequestering of germ-lines can both be interpreted as enforced [182] and altruistic [25], but clonality makes these mechanisms two sides of the same coin. At the origin of obligate eusociality, the evolution of worker castes may be interpreted as parental manipulation or altruism, but lifetime monogamy makes these explanations equivalent [136,185].
Extensions towards obligate mutualistic cooperation between species appear to follow suit. They are egalitarian transitions [25,184] and the most successful and irreversible ones appear to be based on lifetime commitment between the minimal possible number of lineages required for a specific symbiosis (see also [23,24,182]). We see this in multicellular eukaryotes having a single lineage of mitochondria and plasmids, and in the most conflict-free ectosymbioses being based on rearing single clones of symbionts per host or host compartment, in spite of ample population-wide genetic variation. An illustrative example concerns fungus farming by insect societies, where colonies of attine ants acquire their fungal symbiont by vertical transmission and actively protect it against secondary competition with similar but not identical clones [186–188]. Colonies of fungus-growing termites (except for a few derived exceptions with vertical transmission) acquire their lifetime association with a single fungus-garden clone via horizontal transmission and positive frequency-dependent propagation of strains inside colonies so that only one of them prevails and becomes impossible to invade [189]. These convergent farming mutualisms have lifetime commitment in common, not transmission mode, type of promiscuity threats or evolutionary history. Also in mutualistic interactions, it might therefore be useful to distinguish between systems where lifetime commitment happens without exception and with considerable complementarity of function after the mutual loss of reproductive totipotency (analogous to the black dot and blue ellipse in figure 5a) and a separate gradient of other commitments (reminiscent of the red ellipse in figure 5a and thus varying in promiscuity and mutual benefits). Frank [190], Sachs et al. [191], Foster & Wenseleers [21], Leigh [24] and Scheuring & Yu [192] offer general reviews and models to address the stability of the latter types of mutualism.
Perhaps not surprisingly, the emphasis on commitment rather than promiscuity reinforces an explicitly ‘female’ perspective on the propagation of life with an egg committing to a single sperm, a queen committing to a single mate, and insect colonies hosting single clones of particular symbionts. All of these are principally life-long symbioses between unrelated genetic elements that create complex chimaeric (super)organisms. The committed unions are egalitarian in the sense of Queller [184], but driven by ‘female’ enforcement of exclusive ‘male’ commitments so that interests become joined and symmetrical. In all cases, a larger number of possible ‘male’ elements compete for becoming part of such commitment: many sperm per egg, multiple males per female, several fungal clones per social insect colony. The ultimate success criterion of commitment is the absolute exclusion of other ‘male’ elements from the symbiosis—a process with which we are familiar when thinking about sexual selection, but much less so when considering polyspermy [193] or host–symbiont conflict over symbiont mixing [194–196]. The exclusion of promiscuity is almost universally successful in fertilization, but only partially so across interspecific symbioses, and rarely in mating systems. Here, promiscuity normally prevails so that individuals operate in fluid markets [197], where commitments last only until ‘male’ elements are displaced by competitors or ‘females/hosts’ can pick better alternatives.
Lifetime commitment or promiscuous lack thereof is also of relevance when considering the extent to which social constructions can be considered to have (super)organismal properties. In a recent review, Queller & Strassmann [198] arranged a representative selection of widely varying forms of sociality along two axes, one representing the extent of cooperation and the other the degree of conflict, to identify which of these are most organismal, i.e. combine high degrees of cooperation with low conflict. It is interesting to see that their organismal quadrats for multicellular individuals and symbioses mostly contain examples of lifetime-committed partnership, whereas their respective ‘society’ quadrats of high cooperation combined with high conflict tend to coincide with cooperative or advanced communal breeders and promiscuous symbioses. Their comparative approach exposes some of the inconsistencies in the way the field has traditionally used terms such as ‘society’ and ‘superorganism’, but the correspondence of their results with the lifetime commitment approach advocated here is encouraging. It confirms that a productive way to conceptualize colonies of obligatorily eusocial species is to acknowledge that they are ‘organismal’ per definition and underway to ‘full’ or ‘super’ organismality as possible endpoint (see also [20,23,66]). It would seem a logical general hypothesis to expect that promiscuity, either in mating or in symbiont acquisition, will preclude two-partner interactions from crossing an organismality threshold when hosts have unitary growth (metazoans), but that hosts offering modular compartments for symbiont colonization might realize lifetime-committed chimaeras with multiple lineages of the same symbiont without jeopardizing overall organismal integrity [199–202].
Acknowledgements
I thank Boris Baer, Peter Biedermann, Susanne den Boer, Andrew Bourke, Tim Clutton-Brock, Charlie Cornwallis, Andy Gardner, Jürgen Heinze, Judith Korb, Christian Peeters, Tommaso Pizzari, Dustin Rubenstein, Michael Schwarz, Shannon Smith, Michael Taborsky and Stu West for discussion and comments on earlier versions of the manuscript, and the Danish National Research Foundation for funding.
References
- 1.Darwin C. 1871. The descent of man and selection in relation to sex. London, UK: John Murray [Google Scholar]
- 2.Parker GA. 1970. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45, 525–567 10.1111/j.1469-185X.1970.tb01176.x (doi:10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 3.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man, 1871–1971 (ed. Campbell B.), pp. 136–172 Chicago, IL: Aldine-Atherton [Google Scholar]
- 4.Eberhard WG. 1985. Sexual selection and animal genitalia. Cambridge, MA: Harvard University Press [Google Scholar]
- 5.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 6.Arnqvist G, Rowe L. 2005. Sexual conflict. Princeton, NJ: Princeton University Press [Google Scholar]
- 7.Birkhead TR, Pizzari T. 2002. Postcopulatory sexual selection. Nat. Rev. Gen. 3, 262–273 10.1038/nrg774 (doi:10.1038/nrg774) [DOI] [PubMed] [Google Scholar]
- 8.Clutton-Brock TH. 2007. Sexual selection in males and females. Science 318, 1882–1885 10.1126/science.1133311 (doi:10.1126/science.1133311) [DOI] [PubMed] [Google Scholar]
- 9.Parker GA, Pizzari T. 2010. Sperm competition and ejaculate economics. Biol. Rev. 85, 897–934 [DOI] [PubMed] [Google Scholar]
- 10.Shuster SM. 2009. Sexual selection and mating systems. Proc. Natl Acad. Sci. USA 106, 10 009–10 016 10.1073/pnas.0901132106 (doi:10.1073/pnas.0901132106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slatyer RA, Mautz BS, Backwell PRY, Jennions MD. 2011. Estimating genetic benefits of polyandry from experimental studies: a meta-analysis. Biol. Rev. 87, 1–33 10.1111/j.1469-185X.2011.00182.x (doi:10.1111/j.1469-185X.2011.00182.x) [DOI] [PubMed] [Google Scholar]
- 12.Wedell N, Gage MJG, Parker GA. 2002. Sperm competition, male prudence and sperm limited females. Trends Ecol. Evol. 17, 313–320 10.1016/S0169-5347(02)02533-8 (doi:10.1016/S0169-5347(02)02533-8) [DOI] [Google Scholar]
- 13.Boomsma JJ, Baer B, Heinze J. 2005. The evolution of male traits in social insects. Annu. Rev. Entomol. 50, 395–420 10.1146/annurev.ento.50.071803.130416 (doi:10.1146/annurev.ento.50.071803.130416) [DOI] [PubMed] [Google Scholar]
- 14.Boomsma JJ. 2007. Kin selection versus sexual selection: why the ends do not meet. Curr. Biol. 17, R673–R683 10.1016/j.cub.2007.06.033 (doi:10.1016/j.cub.2007.06.033) [DOI] [PubMed] [Google Scholar]
- 15.Boomsma JJ. 2009. Lifetime monogamy and the evolution of eusociality. Phil. Trans. R. Soc. B 364, 3191–3207 10.1098/rstb.2009.0101 (doi:10.1098/rstb.2009.0101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes WOH, Oldroyd BP, Beekman M, Ratnieks FLW. 2008. Ancestral monogamy shows kin selection is key to the evolution of eusociality. Science 320, 1213–1216 10.1126/science.1156108 (doi:10.1126/science.1156108) [DOI] [PubMed] [Google Scholar]
- 17.Leggett HG, El Mouden C, Wild G, West SA. 2011. Promiscuity and the evolution of cooperative breeding. Proc. R. Soc. B 279, 1405–1411 10.1098/rspb.2011.1627 (doi:10.1098/rspb.2011.1627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton WD. 1964. The genetical evolution of social behaviour, I & II. J. Theor. Biol. 7, 1–52 10.1016/0022-5193(64)90038-4 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 19.Hamilton WD. 1972. Altruism and related phenomena, mainly in social insects. Annu. Rev. Ecol. Syst. 3, 193–232 10.1146/annurev.es.03.110172.001205 (doi:10.1146/annurev.es.03.110172.001205) [DOI] [Google Scholar]
- 20.Bourke AFG. 2011. Principles of social evolution. Oxford, UK: Oxford University Press [Google Scholar]
- 21.Foster KR, Wenseleers T. 2006. A general model for the evolution of mutualisms. J. Evol. Biol. 19, 1283–1293 10.1111/j.1420-9101.2005.01073.x (doi:10.1111/j.1420-9101.2005.01073.x) [DOI] [PubMed] [Google Scholar]
- 22.Lehmann L, Keller L. 2006. The evolution of cooperation and altruism: a general framework and a classification of models. J. Evol. Biol. 19, 1365–1376 10.1111/j.1420-9101.2006.01119.x (doi:10.1111/j.1420-9101.2006.01119.x) [DOI] [PubMed] [Google Scholar]
- 23.Leigh EG. 2010. The group selection controversy. J. Evol. Biol. 23, 6–19 10.1111/j.1420-9101.2009.01876.x (doi:10.1111/j.1420-9101.2009.01876.x) [DOI] [PubMed] [Google Scholar]
- 24.Leigh EG. 2010. The evolution of mutualism. J. Evol. Biol. 23, 2507–2528 10.1111/j.1420-9101.2010.02114.x (doi:10.1111/j.1420-9101.2010.02114.x) [DOI] [PubMed] [Google Scholar]
- 25.Queller DC. 2000. Relatedness and the fraternal major transitions. Phil. Trans. R. Soc. Lond. B 355, 1647–1655 10.1098/rstb.2000.0727 (doi:10.1098/rstb.2000.0727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West SA, Griffin AS, Gardner A. 2007. Evolutionary explanations for cooperation. Curr. Biol. 17, R661–R672 10.1016/j.cub.2007.06.004 (doi:10.1016/j.cub.2007.06.004) [DOI] [PubMed] [Google Scholar]
- 27.West-Eberhard MJ. 1975. The evolution of social behaviour by kin selection. Q. Rev. Biol. 50, 1–33 10.1111/j.1469-185X.1975.tb00988.x (doi:10.1111/j.1469-185X.1975.tb00988.x) [DOI] [Google Scholar]
- 28.Charnov EL. 1982. The theory of sex allocation. Princeton, NJ: Princeton University Press [Google Scholar]
- 29.Hamilton WD. 1967. Extraordinary sex ratios. Science 156, 477–488 10.1126/science.156.3774.477 (doi:10.1126/science.156.3774.477) [DOI] [PubMed] [Google Scholar]
- 30.West SA. 2009. Sex allocation. Monographs in population biology. Princeton, NJ: Princeton University Press [Google Scholar]
- 31.Grafen A. 1991. Modelling in behavioural ecology. In Behavioural ecology: an evolutionary approach (eds Krebs JR, Davies NB.), pp. 5–31, 3rd edn Oxford, UK: Blackwell Scientific [Google Scholar]
- 32.Boomsma JJ, Grafen A. 1991. Colony-level sex ratio selection in the eusocial Hymenoptera. J. Evol. Biol. 4, 383–407 10.1046/j.1420-9101.1991.4030383.x (doi:10.1046/j.1420-9101.1991.4030383.x) [DOI] [Google Scholar]
- 33.Bourke AFG, Ratnieks FLW. 1999. Kin conflict over caste determination in social Hymenoptera. Behav. Ecol. Sociobiol. 46, 287–297 10.1007/s002650050622 (doi:10.1007/s002650050622) [DOI] [Google Scholar]
- 34.Keller L, Chapuisat M. 1999. Cooperation among selfish individuals in insect societies. Bioscience 49, 899–909 10.2307/1313649 (doi:10.2307/1313649) [DOI] [Google Scholar]
- 35.Meunier J, West SA, Chapuisat M. 2008. Split sex ratios in the social Hymenoptera: a meta-analysis. Behav. Ecol. 19, 382–390 10.1093/beheco/arm143 (doi:10.1093/beheco/arm143) [DOI] [Google Scholar]
- 36.Ratnieks FLW. 1988. Reproductive harmony via mutual policing by workers in eusocial Hymenoptera. Am. Nat. 132, 217–236 10.1086/284846 (doi:10.1086/284846) [DOI] [Google Scholar]
- 37.Ratnieks FLW, Foster KR, Wenseleers T. 2006. Conflict resolution in insect societies. Annu. Rev. Entomol. 51, 581–608 10.1146/annurev.ento.51.110104.151003 (doi:10.1146/annurev.ento.51.110104.151003) [DOI] [PubMed] [Google Scholar]
- 38.Strassmann JE, Queller DC. 2007. Insect societies as divided organisms: the complexities of purpose and cross-purpose. Proc. Natl Acad. Sci. USA 104, 8619–8626 10.1073/pnas.0701285104 (doi:10.1073/pnas.0701285104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sundström L, Boomsma JJ. 2001. Conflicts and alliances in insect families. Heredity 86, 515–521 10.1046/j.1365-2540.2001.00884.x (doi:10.1046/j.1365-2540.2001.00884.x) [DOI] [PubMed] [Google Scholar]
- 40.Ratnieks FLW, Helantera H. 2009. The evolution of extreme altruism and inequality in insect societies. Phil. Trans. R. Soc. B 364, 3169–3179 10.1098/rstb.2009.0129 (doi:10.1098/rstb.2009.0129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emlen ST. 1991. Evolution of cooperative breeding in birds and mammals. In Behavioural ecology: an evolutionary approach (eds Krebs JR, Davies NB.), pp. 301–337, 3rd edn. Oxford, UK: Blackwell Scientific [Google Scholar]
- 42.Emlen ST, Wrege PH, Demong NJ. 1995. Making decisions in the family: an evolutionary perspective. Am. Sci. 83, 148–157 [Google Scholar]
- 43.Cockburn A. 1998. Evolution of helping behavior in cooperatively breeding birds. Ann. Rev. Ecol. Syst. 29, 141–177 10.1146/annurev.ecolsys.29.1.141 (doi:10.1146/annurev.ecolsys.29.1.141) [DOI] [Google Scholar]
- 44.Cockburn A. 2004. Mating systems and sexual conflict. In Ecology and evolution of cooperative breeding in birds (eds Koenig WD, Dickinson JL.), pp. 81–101 Cambridge, UK: Cambridge University Press [Google Scholar]
- 45.Clutton-Brock TH. 2006. Cooperative breeding in mammals. In Cooperation in primates and humans: mechanisms and evolution (eds Kappeler PM, van Schaik CP.), pp. 173–190 Berlin, Germany: Springer [Google Scholar]
- 46.Clutton-Brock TH. 2009. Structure and function in mammalian societies. Phil. Trans. R. Soc. B 364, 3229–3242 10.1098/rstb.2009.0120 (doi:10.1098/rstb.2009.0120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell AF. 2004. Mammals: comparisons and contrasts. In Ecology and evolution of cooperative breeding in birds (eds Koenig WD, Dickinson JL.), pp. 210–227 Cambridge, UK: Cambridge University Press [Google Scholar]
- 48.Batra SWT. 1966. Nest and social behavior of halictine bees of India. Indian J. Entomol. 28, 375–393 [Google Scholar]
- 49.Hart AG, Ratnieks FLW. 2005. Crossing the taxonomic divide: conflict and its resolution in societies of reproductively totipotent individuals. J. Evol. Biol. 18, 383–395 10.1111/j.1420-9101.2004.00832.x (doi:10.1111/j.1420-9101.2004.00832.x) [DOI] [PubMed] [Google Scholar]
- 50.Wheeler WM. 1928. The social insects. New York, NY: Harcourt, Brace & Co [Google Scholar]
- 51.Wilson EO. 1975. Sociobiology: the new synthesis , 2nd edn. Cambridge, MA: Belknap Press [Google Scholar]
- 52.Sherman PW, Lacey EA, Reeve HK, Keller L. 1995. The eusociality continuum. Behav. Ecol. 6, 102–108 10.1093/beheco/6.1.102 (doi:10.1093/beheco/6.1.102) [DOI] [Google Scholar]
- 53.Costa JT, Fitzgerald TD. 2005. Social terminology revisited: where are we ten years later? Ann. Zool. Fennici 42, 559–564 [Google Scholar]
- 54.Crespi BJ, Yanega D. 1995. The definition of eusociality. Behav. Ecol. 6, 109–115 10.1093/beheco/6.1.109 (doi:10.1093/beheco/6.1.109) [DOI] [Google Scholar]
- 55.Michener C. 1974. The social behavior of the bees: a comparative study. Cambridge, MA: Harvard University Press [Google Scholar]
- 56.Bourke AFG. 1999. Colony size, social complexity and reproductive conflict in social insects. J. Evol. Biol. 12, 245–257 10.1046/j.1420-9101.1999.00028.x (doi:10.1046/j.1420-9101.1999.00028.x) [DOI] [Google Scholar]
- 57.Cardinal S, Danforth BN. 2011. The antiquity and evolutionary history of social behavior in bees. PLoS ONE 6, e21086 10.1371/journal.pone.0021086 (doi:10.1371/journal.pone.0021086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beekman M, Peeters C, O'Riain MJ. 2006. Developmental divergence: neglected variable in understanding the evolution of reproductive skew in social animals. Behav. Ecol. 17, 622–627 10.1093/beheco/ark006 (doi:10.1093/beheco/ark006) [DOI] [Google Scholar]
- 59.Queller DC, Strassmann JE. 1998. Kin selection and social insects. Bioscience 48, 165–175 10.2307/1313262 (doi:10.2307/1313262) [DOI] [Google Scholar]
- 60.Davies NB. 1991. Mating systems. In Behavioural ecology: an evolutionary approach (eds Krebs JR, Davies NB.), pp. 263–299, 3rd edn. Oxford, UK: Blackwell Scientific [Google Scholar]
- 61.Schmid-Hempel R, Schmid-Hempel P. 2000. Female mating frequencies in Bombus spp. from Central Europe. Insectes Soc. 47, 36–41 10.1007/s000400050006 (doi:10.1007/s000400050006) [DOI] [Google Scholar]
- 62.Strassmann JE, Queller DC. 2007. Altruism among amoebas. Nat. History 116, 24–29 [Google Scholar]
- 63.Corley M, Fjerdingstad EJ. 2011. Mating strategies of queens in Lasius niger ants: is environment type important? Behav. Ecol. Sociobiol. 65, 889–897 10.1007/s00265-010-1089-7 (doi:10.1007/s00265-010-1089-7) [DOI] [Google Scholar]
- 64.Pietsch T. 2005. Dimorphism, parasitism, and sex revisited: modes of reproduction among deep-sea ceratoid anglerfishes (Teleostei: Lophiiformes). Ichty. Res. 52, 2007–2236 [Google Scholar]
- 65.Høeg JT, Ritchie LE. 1985. Male Cypris settlement and its effects on juvenile development in Lernaeodiscus porcellanae Müller (Crustacae: Cirripedia: Rhizocephala). J. Exp. Mar. Biol. Ecol. 87, 1–11 10.1016/0022-0981(85)90187-X (doi:10.1016/0022-0981(85)90187-X) [DOI] [Google Scholar]
- 66.Ratnieks FLW, Reeve HK. 1992. Conflict in single-queen hymenopteran societies: the structure of conflict and processes that reduce conflict in advanced eusocial species. J. Theor. Biol. 158, 33–65 10.1016/S0022-5193(05)80647-2 (doi:10.1016/S0022-5193(05)80647-2) [DOI] [Google Scholar]
- 67.Cronin H. 1991. The ant and the peacock. Cambridge, UK: Cambridge University Press [Google Scholar]
- 68.Simmons LW. 2001. Sperm competition and its evolutionary consequences in the insects. Princeton, NJ: Princeton University Press [Google Scholar]
- 69.Gioti A, Wigby S, Wertheim B, Schuster E, Martinez P, Pennington CJ, Partridge L, Chapman T. 2012. Sex peptide of Drosophila melanogaster males is a global regulator of reproductive processes in females. Proc. R. Soc. B 279, 4423–4432 10.1098/rspb.2012.1634 (doi:10.1098/rspb.2012.1634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shellman-Reeve JS. 1999. Courting strategies and conflicts in a monogamous, biparental termite. Proc. R. Soc. Lond. B 266, 137–144 10.1098/rspb.1999.0613 (doi:10.1098/rspb.1999.0613) [DOI] [Google Scholar]
- 71.Hartke TR, Baer B. 2011. The mating biology of termites: a comparative review. Anim. Behav. 82, 927–936 10.1016/j.anbehav.2011.07.022 (doi:10.1016/j.anbehav.2011.07.022) [DOI] [Google Scholar]
- 72.Hölldobler B, Bartz SH. 1985. Sociobiology of reproduction in ants. In Experimental behavioral ecology and sociobiology (eds Hölldobler B, Lindauer M.), pp. 237–272 Stuttgart, Germany: Gustav Fisher [Google Scholar]
- 73.Maynard Smith J, Harper D. 2003. Animal signals. Oxford, UK: Oxford University Press [Google Scholar]
- 74.Snook RR. 2005. Sperm in competition: not playing by the numbers. Trends Ecol. Evol. 20, 46–53 10.1016/j.tree.2004.10.011 (doi:10.1016/j.tree.2004.10.011) [DOI] [PubMed] [Google Scholar]
- 75.Heinsohn R, Legge S. 2003. Breeding biology of the reverse-dichromatic, co-operative parrot Eclectus roratus. J. Zool. Lond. 259, 197–208 10.1017/S0952836902003138 (doi:10.1017/S0952836902003138) [DOI] [Google Scholar]
- 76.Rubenstein DR, Lovette IJ. 2009. Reproductive skew and selection on female ornamentation in social species. Nature 462, 786–789 10.1038/nature08614 (doi:10.1038/nature08614) [DOI] [PubMed] [Google Scholar]
- 77.Den Boer SPA, Boomsma JJ, Baer B. 2009. Honey bee males and queens use glandular secretions to enhance sperm viability before and after storage. J. Insect Physiol. 55, 538–543 10.1016/j.jinsphys.2009.01.012 (doi:10.1016/j.jinsphys.2009.01.012) [DOI] [PubMed] [Google Scholar]
- 78.Den Boer SPA, Baer B, Boomsma JJ. 2010. Seminal fluid mediates ejaculate competition in social insects. Science 327, 1506–1509 10.1126/science.1184709 (doi:10.1126/science.1184709) [DOI] [PubMed] [Google Scholar]
- 79.Baer B. 2005. Sexual selection in Apis bees. Apidologie 36, 187–200 10.1051/apido:2005013 (doi:10.1051/apido:2005013) [DOI] [Google Scholar]
- 80.Den Boer SPA, Baer B, Dreier S, Aron S, Nash DR, Boomsma JJ. 2009. Prudent sperm use by leaf-cutter ant queens. Proc. R. Soc. B 276, 3945–3953 10.1098/rspb.2009.1184 (doi:10.1098/rspb.2009.1184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baer B, Armitage SAO, Boomsma JJ. 2006. Sperm storage induces an immunity cost in ants. Nature 441, 872–875 10.1038/nature04698 (doi:10.1038/nature04698) [DOI] [PubMed] [Google Scholar]
- 82.Holman L, Stürup M, Trontti K, Boomsma JJ. 2011. Random sperm use and genetic effects on worker caste fate in Atta colombica leaf-cutting ants. Mol. Ecol. 20, 5092–5102 10.1111/j.1365-294X.2011.05338.x (doi:10.1111/j.1365-294X.2011.05338.x) [DOI] [PubMed] [Google Scholar]
- 83.Hughes WOH, Boomsma JJ. 2008. Genetic royal cheats in leaf-cutting ant societies. Proc. Natl Acad. Sci. USA 105, 5150–5153 10.1073/pnas.0710262105 (doi:10.1073/pnas.0710262105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sundström L, Boomsma JJ. 2000. Reproductive alliances and posthumous fitness enhancement in male ants. Proc. R. Soc. Lond. B 267, 1439–1444 10.1098/rspb.2000.1161 (doi:10.1098/rspb.2000.1161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boomsma JJ, Kronauer DJC, Pedersen JS. 2009. The evolution of social insect mating systems. In Organization of insect societies: from genomes to socio-complexity (eds Gadau J, Fewell J.), pp. 3–25 Cambridge, MA: Harvard University Press [Google Scholar]
- 86.Wiernasz DC, Cole BJ. 2010. Patriline shifting leads to apparent genetic caste determination in harvester ants. Proc. Natl Acad. Sci. USA 107, 12 958–12 962 10.1073/pnas.1003299107 (doi:10.1073/pnas.1003299107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boomsma JJ, Ratnieks FLW. 1996. Paternity in eusocial Hymenoptera. Phil. Trans. R. Soc. Lond. B 351, 947–975 10.1098/rstb.1996.0087 (doi:10.1098/rstb.1996.0087) [DOI] [Google Scholar]
- 88.Strassmann JE. 2001. The rarity of multiple mating by females in the social Hymenoptera. Insectes Soc. 48, 1–13 10.1007/PL00001737 (doi:10.1007/PL00001737) [DOI] [Google Scholar]
- 89.Hamilton WD. 1987. Kinship, recognition, disease, and intelligence: constraints of social evolution. In Animal societies: theories and facts (eds Itô Y, Brown JL, Kikkawa J.), pp. 81–102 Tokyo, Japan: Japan Science Society Press [Google Scholar]
- 90.Sherman PW, Seeley TD, Reeve HK. 1988. Parasites, pathogens, and polyandry in social Hymenoptera. Am. Nat. 131, 602–610 10.1086/284809 (doi:10.1086/284809) [DOI] [PubMed] [Google Scholar]
- 91.Hughes WOH, Ratnieks FLW, Oldroyd BP. 2008. Multiple paternity or multiple queens: two routes to greater intracolonial genetic diversity in the eusocial Hymenoptera. J. Evol. Biol. 21, 1090–1095 10.1111/j.1420-9101.2008.01532.x (doi:10.1111/j.1420-9101.2008.01532.x) [DOI] [PubMed] [Google Scholar]
- 92.Keller L, Reeve HK. 1994. Genetic variability, queen number, and polyandry in social Hymenoptera. Evolution 48, 694–704 10.2307/2410479 (doi:10.2307/2410479) [DOI] [PubMed] [Google Scholar]
- 93.Kronauer DJC, Johnson RA, Boomsma JJ. 2007. The evolution of multiple mating in army ants. Evolution 61, 413–422 10.1111/j.1558-5646.2007.00040.x (doi:10.1111/j.1558-5646.2007.00040.x) [DOI] [PubMed] [Google Scholar]
- 94.Cole BJ. 1983. Multiple mating and the evolution of social behaviour in the Hymenoptera. Behav. Ecol. Sociobiol. 12, 191–201 10.1007/BF00290771 (doi:10.1007/BF00290771) [DOI] [Google Scholar]
- 95.Baer B, Heazlewood JL, Taylor NL, Eubel H, Millar AH. 2009. The seminal fluid proteome of the honeybee Apis mellifera. Proteomics 9, 2085–2097 10.1002/pmic.200800708 (doi:10.1002/pmic.200800708) [DOI] [PubMed] [Google Scholar]
- 96.Cornwallis CK, O'Connor EA. 2009. Sperm : seminal fluid interactions and the adjustment of sperm quality in relation to female attractiveness. Proc. R. Soc. B 276, 3467–3475 10.1098/rspb.2009.0807 (doi:10.1098/rspb.2009.0807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wigby S, Sirot LK, Linklater JR, Buehner N, Calboli FCF, Bretman A, Wolfner MF, Chapman T. 2009. Seminal fluid protein allocation and male reproductive success. Curr. Biol. 19, 751–757 10.1016/j.cub.2009.03.036 (doi:10.1016/j.cub.2009.03.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cornwallis CK, West SA, Davis KE, Griffin AS. 2010. Promiscuity and the evolutionary transition to complex societies. Nature 466, 969–972 10.1038/nature09335 (doi:10.1038/nature09335) [DOI] [PubMed] [Google Scholar]
- 99.Trivers RL, Hare H. 1976. Haplodiploidy and the evolution of the social insects. Science 191, 249–263 10.1126/science.1108197 (doi:10.1126/science.1108197) [DOI] [PubMed] [Google Scholar]
- 100.Fromhage L, Kokko H. 2011. Monogamy and haplodiploidy act in synergy to promote the evolution of eusociality. Nat. Commun. 2, 397 10.1038/ncomms1410 (doi:10.1038/ncomms1410) [DOI] [PubMed] [Google Scholar]
- 101.Gardner A, Alpedrinha J, West SA. 2012. Haploidy and the evolution of eusociality: split sex ratios. Am. Nat. 179, 240–256 10.1086/663683 (doi:10.1086/663683) [DOI] [PubMed] [Google Scholar]
- 102.Gardner A, Grafen A. 2009. Capturing the superorganism: a formal theory of group adaptation. J. Evol. Biol. 22, 659–671 10.1111/j.1420-9101.2008.01681.x (doi:10.1111/j.1420-9101.2008.01681.x) [DOI] [PubMed] [Google Scholar]
- 103.Leadbeater E, Carruthers JM, Green JP, Rosser NS, Field J. 2011. Nest inheritance is the missing source of direct fitness in a primitively eusocial insect. Science 333, 874–876 10.1126/science.1205140 (doi:10.1126/science.1205140) [DOI] [PubMed] [Google Scholar]
- 104.Gadagkar R. 1991. Belonogaster, Mischocyttarus, Parapolybia, and independent-founding Ropalidia. In The social biology of wasps (eds Ross KG, Matthews RW.), pp. 149–190 Ithaca, NY: Cornell University Press [Google Scholar]
- 105.Hunt JH. 2012. A conceptual model for the origin of worker behaviour and adaptation of eusociality. J. Evol. Biol. 25, 1–19 10.1111/j.1420-9101.2011.02421.x (doi:10.1111/j.1420-9101.2011.02421.x) [DOI] [PubMed] [Google Scholar]
- 106.Jeanne RL. 1991. The swarm-founding Polistinae. In The social biology of wasps (eds Ross KG, Matthews RW.), pp. 191–231 Ithaca, NY: Cornell University Press [Google Scholar]
- 107.Queller DC, Strassmann JE, Solis CR, Hughes CR, DeLoach DM. 1993. A selfish strategy of social insect workers that promotes social cohesion. Nature 365, 639–641 10.1038/365639a0 (doi:10.1038/365639a0) [DOI] [Google Scholar]
- 108.Hastings MD, Queller DC, Eischen F, Strassmann JE. 1998. Kin selection, relatedness, and worker control of reproduction in a large-colony epiponine wasp, Brachygastra mellifica. Behav. Ecol. 9, 573–581 10.1093/beheco/9.6.573 (doi:10.1093/beheco/9.6.573) [DOI] [Google Scholar]
- 109.Strassmann JE, Sullender BW, Queller DC. 2002. Caste totipotency and conflict in a large-colony social insect. Proc. R. Soc. Lond. B 269, 263–270 10.1098/rspb.2001.1880 (doi:10.1098/rspb.2001.1880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brady SG, Schultz TR, Fisher BL, Ward PS. 2006. Evaluating alternative hypotheses for the early evolution and diversification of ants. Proc. Natl Acad. Sci. USA 103, 18 172–18 177 10.1073/pnas.0605858103 (doi:10.1073/pnas.0605858103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moreau C, Bell C, Vila R, Archibald S, Pierce N. 2006. Phylogeny of the ants: diversification in the age of angiosperms. Science 312, 101–104 10.1126/science.1124891 (doi:10.1126/science.1124891) [DOI] [PubMed] [Google Scholar]
- 112.Hölldobler B, Wilson EO. 2008. The super-organism. New York, NY: Norton [Google Scholar]
- 113.Peeters C, Ito F. 2001. Colony dispersal and the evolution of queen morphology in social Hymenoptera. Annu. Rev. Entomol. 46, 601–630 10.1146/annurev.ento.46.1.601 (doi:10.1146/annurev.ento.46.1.601) [DOI] [PubMed] [Google Scholar]
- 114.Monnin T, Peeters C. 2008. How many gamergates is an ant queen worth? Naturwissenschaften 95, 109–116 10.1007/s00114-007-0297-0 (doi:10.1007/s00114-007-0297-0) [DOI] [PubMed] [Google Scholar]
- 115.Peeters C. 2012. Convergent evolution of wingless reproductives across all subfamilies of ants, and sporadic loss of winged queens (Hymenoptera: Formicidae). Myrmecol. News 16, 75–91 [Google Scholar]
- 116.Gobin B, Ito F, Billen J, Peeters C. 2008. Degeneration of sperm reservoir and the loss of mating ability in worker ants. Naturwissenschaften 95, 1041–1048 10.1007/s00114-008-0420-x (doi:10.1007/s00114-008-0420-x) [DOI] [PubMed] [Google Scholar]
- 117.Higashi M, Yamamura N, Abe T, Burns TP. 1991. Why don't all termite species have a sterile worker caste? Proc. R. Soc. Lond. B 246, 25–30 10.1098/rspb.1991.0120 (doi:10.1098/rspb.1991.0120) [DOI] [PubMed] [Google Scholar]
- 118.Thorne BL, Traniello JFA. 2003. Comparative social biology of basal taxa of ants and termites. Ann. Rev. Entomol. 48, 283–306 10.1146/annurev.ento.48.091801.112611 (doi:10.1146/annurev.ento.48.091801.112611) [DOI] [PubMed] [Google Scholar]
- 119.Inward DJG, Vogler AP, Eggleton P. 2007. A comprehensive phylogenetic analysis of termites (Isoptera) illuminates key aspects of their evolutionary biology. Mol. Phylogenet. Evol. 44, 953–967 10.1016/j.ympev.2007.05.014 (doi:10.1016/j.ympev.2007.05.014) [DOI] [PubMed] [Google Scholar]
- 120.Korb J. 2008. The ecology of social evolution in termites. In Ecology of social evolution (eds Korb J, Heinze J.), pp. 151–174 Berlin, Germany: Springer [Google Scholar]
- 121.Myles TG. 1988. Resource inheritance in social evolution from termites to man. In The ecology of social behavior (ed. Slobodchikoff CN.), pp. 379–423 New York, NY: Academic Press [Google Scholar]
- 122.Korb J, Buschmann M, Schafberg S, Liebig J, Bagnères A-G. 2012. Brood care and social evolution in termites. Proc. R. Soc. B 279, 2662–2671 10.1098/rspb.2011.2639 (doi:10.1098/rspb.2011.2639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thorne BL, Breisch NL, Muscedere ML. 2003. Evolution of eusociality and the soldier caste in termites: influence of intraspecific competition and accelerated inheritance. Proc. Natl Acad. Sci. USA 100, 12 808–12 813 10.1073/pnas.2133530100 (doi:10.1073/pnas.2133530100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Korb J, Schneider K. 2007. Does kin structure explain the occurrence of workers in a lower termite? Evol. Ecol. 21, 817–828 10.1007/s10682-006-9153-5 (doi:10.1007/s10682-006-9153-5) [DOI] [Google Scholar]
- 125.Johns PM, Howard KJ, Breisch NL, Rivera A, Thorne BL. 2009. Nonrelatives inherit colony resources in a primitive termite. Proc. Natl Acad. Sci. USA 106, 17 452–17 456 10.1073/pnas.0907961106 (doi:10.1073/pnas.0907961106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Korb J. 2009. Termites: an alternative road to eusociality and the importance of group benefits in social insects. In Organization of insect societies: from genomes to socio-complexity (eds Gadau J, Fewell J.), pp. 128–147 Cambridge, MA: Harvard University Press [Google Scholar]
- 127.Korb J, Hartfelder K. 2008. Life history and development: a framework for understanding developmental plasticity in lower termites. Biol. Rev. 83, 295–313 10.1111/j.1469-185X.2008.00044.x (doi:10.1111/j.1469-185X.2008.00044.x) [DOI] [PubMed] [Google Scholar]
- 128.Biedermann PHW, Taborsky M. 2011. Larval helpers and age polyethism in ambrosia beetles. Proc. Natl Acad. Sci. USA 108, 17 064–17 069 10.1073/pnas.1107758108 (doi:10.1073/pnas.1107758108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kent DS, Simpson JA. 1992. Eusociality in the beetle Austroplatypus incompertus (Coleoptera: Curculionidae). Naturwissenschaften 79, 86–87 10.1007/BF01131810 (doi:10.1007/BF01131810) [DOI] [Google Scholar]
- 130.Roberts H. 1960 Trachyostus ghanaensis Schedl, (Col., Platypodidae) an ambrosia beetle attacking wawa, Triplochiton scleroxylon K. Schum. Tech. Bull. W. Afr. Timb. Borer Res. Unit no. 3. [Google Scholar]
- 131.Roberts H. 1968. Notes on the biology of ambrosia beetles of the genus Trachyostus Schedl (Coleoptera: Platypodidae) in West Africa. Bull. Entomol. Res. 58, 325–352 10.1017/S000748530005687X (doi:10.1017/S000748530005687X) [DOI] [Google Scholar]
- 132.Bilde T, Lubin Y. 2011. Group living in spiders: cooperative breeding and coloniality. In Behaviour and ecology of spiders (ed. Herberstein M.), pp. 275–306 Cambridge, UK: Cambridge University Press [Google Scholar]
- 133.Vargo EL, Husseneder C. 2011. Genetic structure of termite colonies and populations. In Biology of termites: a modern synthesis (eds Bignell DE, Roisin Y, Lo N.), pp. 321–348, 2nd edn Berlin, Germany: Springer [Google Scholar]
- 134.Matsuura K. 2011. Sexual and asexual reproduction in termites. In Biology of termites: a modern synthesis (eds Bignell DE, Roisin Y, Lo N.), pp. 255–277, 2nd edn Berlin, Germany: Springer [Google Scholar]
- 135.Cronin AL, Molet M, Doums C, Monnin T, Peeters C. 2013. Recurrent evolution of dependent colony foundation across eusocial insects. Annu. Rev. Entomol. 58, 37–55 [DOI] [PubMed] [Google Scholar]
- 136.Bourke AFG, Franks NR. 1995. Social evolution in ants. Princeton, NJ: Princeton University Press [Google Scholar]
- 137.Kronauer DJC, Boomsma JJ. 2007. Do army ant queens re-mate later in life? Insectes Soc. 54, 20–28 10.1007/s00040-007-0904-2 (doi:10.1007/s00040-007-0904-2) [DOI] [Google Scholar]
- 138.Franks NR, Hölldobler B. 1987. Sexual competition during colony reproduction in army ants. Biol. J. Linn. Soc. 30, 229–243 10.1111/j.1095-8312.1987.tb00298.x (doi:10.1111/j.1095-8312.1987.tb00298.x) [DOI] [Google Scholar]
- 139.Heinze J, Hölldobler B. 1993. Fighting for a harem of queens: physiology of reproduction in Cardiocondyla male ants. Proc. Natl Acad. Sci. USA 90, 8412–8414 10.1073/pnas.90.18.8412 (doi:10.1073/pnas.90.18.8412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yamauchi K, Kawase N. 1992. Pheromonal manipulation of workers by a fighting male to kill his rival males in the ant Cardiocondyla wroughtonii. Naturwissenschaften 79, 274–276 10.1007/BF01175395 (doi:10.1007/BF01175395) [DOI] [Google Scholar]
- 141.Gibbs J, Brady SG, Kanda K, Danforth BN. 2012. Phylogeny of halictine bees supports a shared origin of eusociality for Halictus and Lasioglossum (Apoidea: Anthophila: Halictidae). Mol. Phylogenet. Evol. 65, 926–939 [DOI] [PubMed] [Google Scholar]
- 142.Schwarz MP, Tierney SM, Rehan SM, Chenoweth LB, Cooper SJB. 2011. The evolution of eusociality in allodapine bees: workers began by waiting. Biol. Lett. 7, 277–280 10.1098/rsbl.2010.0757 (doi:10.1098/rsbl.2010.0757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fjerdingstad EJ, Crozier RH. 2006. The evolution of worker caste diversity in social insects. Am. Nat. 167, 390–400 10.1086/499545 (doi:10.1086/499545) [DOI] [PubMed] [Google Scholar]
- 144.Molet M, Wheeler DE, Peeters C. 2012. Evolution of novel mosaic castes in ants: modularity, phenotypic plasticity, and colonial buffering. Am. Nat. 180, 328–341 10.1086/667368 (doi:10.1086/667368) [DOI] [PubMed] [Google Scholar]
- 145.Rajakumar R, et al. 2012. Ancestral developmental potential facilitates parallel evolution in ants. Science 335, 79–82 10.1126/science.1211451 (doi:10.1126/science.1211451) [DOI] [PubMed] [Google Scholar]
- 146.Grüter C, Menezes C, Imperatriz-Fonseca VL, Ratnieks FLW. 2012. A morphologically specialized soldier caste improves colony defense in a neotropical eusocial bee. Proc. Natl Acad. Sci. USA 109, 1182–1186 10.1073/pnas.1113398109 (doi:10.1073/pnas.1113398109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Roisin Y, Korb J. 2011. Social organisation and the status of workers in termites. In Biology of termites: a modern synthesis (eds Bignell DE, Roisin Y, Lo N.), pp. 133–164, 2nd edn. Berlin, Germany: Springer [Google Scholar]
- 148.Crespi BJ, Mound LA. 1997. Ecology and evolution of social behavior among Australian gall thrips and their allies. In The evolution of social behavior in insects and arachnids (eds Choe JC, Crespi BJ.), pp. 166–180 Cambridge, UK: Cambridge University Press [Google Scholar]
- 149.Chapman TW, Kranz BD, Bejah KL, Morris DC, Schwarz MP, Crespi BJ. 2002. The evolution of soldier reproduction in social thrips. Behav. Ecol. 13, 519–525 10.1093/beheco/13.4.519 (doi:10.1093/beheco/13.4.519) [DOI] [Google Scholar]
- 150.Stern DL, Foster WA. 1997. The evolution of sociality in aphids: a clone's-eye view. In Social behavior in insects and arachnids (eds Choe JC, Crespi BJ.), pp. 150–165 Cambridge, UK: Cambridge University Press [Google Scholar]
- 151.Giron D, Dunn DW, Hardy ICW, Strand MR. 2004. Aggression by polyembryonic wasp soldiers correlates with kinship but not resource competition. Nature 430, 676–679 10.1038/nature02721 (doi:10.1038/nature02721) [DOI] [PubMed] [Google Scholar]
- 152.Giron D, Ross KG, Strand MR. 2006. Presence of soldier larvae determines the outcome of competition in a polyembryonic wasp. J. Evol. Biol. 20, 165–172 10.1111/j.1420-9101.2006.01212.x (doi:10.1111/j.1420-9101.2006.01212.x) [DOI] [PubMed] [Google Scholar]
- 153.Tóth E, Duffy JE. 2005. Coordinated group response to nest intruders in social shrimp. Biol. Lett. 1, 49–52 10.1098/rsbl.2004.0237 (doi:10.1098/rsbl.2004.0237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Duffy JE, Macdonald KS. 2009. Kin structure, ecology and the evolution of social organization in shrimp: a comparative analysis. Proc. R. Soc B 277, 575–584 10.1098/rspb.2009.1483 (doi:10.1098/rspb.2009.1483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Alexander RD, Noonan KM, Crespi BJ. 1991. The evolution of eusociality. In The biology of the naked mole rat (eds Sherman PW, Jarvis JUM, Alexander RD.), pp. 3–44 Princeton, NJ: Princeton University Press [Google Scholar]
- 156.Korb J, Heinze J. 2008. The ecology of social life: a synthesis. In Ecology of social evolution (eds Korb J, Heinze J.), pp. 245–259 Berlin, Germany: Springer [Google Scholar]
- 157.Duffy JE. 1996. Eusociality in a coral-reef shrimp. Nature 381, 512–514 10.1038/381512a0 (doi:10.1038/381512a0) [DOI] [Google Scholar]
- 158.Korb J. 2010. Social insects, major evolutionary transitions and multilevel selection. In Behaviour: evolution and mechanisms (ed. Kappeler P.), pp. 179–212 Berlin, Germany: Springer [Google Scholar]
- 159.Gardner A, Hardy ICW, Taylor PD, West SA. 2007. Spiteful soldiers and sex ratio conflict in polyembryonic wasps. Am. Nat. 169, 519–533 10.1086/512107 (doi:10.1086/512107) [DOI] [PubMed] [Google Scholar]
- 160.Griffin AS, West SA. 2003. Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science 302, 634–636 10.1126/science.1089402 (doi:10.1126/science.1089402) [DOI] [PubMed] [Google Scholar]
- 161.Lukas D, Clutton-Brock TH. 2012. Cooperative breeding and monogamy in mammalian societies. Proc. R. Soc. B 279, 2151–2156 10.1098/rspb.2011.2468 (doi:10.1098/rspb.2011.2468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cant MA, Field J. 2001. Helping effort and future fitness in cooperative animal societies. Proc. R. Soc. Lond. B 268, 1959–1964 10.1098/rspb.2001.1754 (doi:10.1098/rspb.2001.1754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yagi N, Hasegawa E. 2012. A halictid bee with sympatric solitary and eusocial nests offers evidence for Hamilton's rule. Nat. Commun. 3, 939 10.1038/ncomms1939 (doi:10.1038/ncomms1939) [DOI] [PubMed] [Google Scholar]
- 164.Lukas D, Clutton-Brock TH. 2012. Life histories and the evolution of cooperative breeding in mammals. Proc. R. Soc. B 279, 4065–4070 10.1098/rspb.2012.1433 (doi:10.1098/rspb.2012.1433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Richardson DS, Burke T, Komdeur J. 2002. Direct benefits and the evolution of female-biased cooperative breeding in Seychelles warblers. Evolution 56, 2313–2321 [DOI] [PubMed] [Google Scholar]
- 166.Burland TM, Bennett NC, Jarvis JUM, Faulkes CG. 2004. Colony structure and parentage in wild colonies of co-operatively breeding Damaraland mole-rats suggest incest avoidance alone may not maintain reproductive skew. Mol. Ecol. 13, 2371–2379 10.1111/j.1365-294X.2004.02233.x (doi:10.1111/j.1365-294X.2004.02233.x) [DOI] [PubMed] [Google Scholar]
- 167.Calleri DV, II, McGrail Reid E, Rosengaus RB, Vargo EL, Traniello JFA. 2006. Inbreeding and disease resistance in a social insect: effects of heterozygosity on immunocompetence in the termite Zootermopsis angusticollis. Proc. R. Soc. B 273, 2633–2640 10.1098/rspb.2006.3622 (doi:10.1098/rspb.2006.3622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Peer K, Taborsky M. 2005. Outbreeding depression, but no inbreeding depression in haplodiploid ambrosia beetles with regular sibling mating. Evolution 59, 317–323 [PubMed] [Google Scholar]
- 169.Ross-Gillespie A, O'Riain J, Keller LF. 2007. Viral epizootic reveals inbreeding depression in a habitually inbreeding mammal. Evolution 61, 2268–2273 10.1111/j.1558-5646.2007.00177.x (doi:10.1111/j.1558-5646.2007.00177.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nonacs P, Hager R. 2011. The past, present and future of reproductive skew theory and experiments. Biol. Rev. 86, 271–298 10.1111/j.1469-185X.2010.00144.x (doi:10.1111/j.1469-185X.2010.00144.x) [DOI] [PubMed] [Google Scholar]
- 171.Reeve HK, Keller L. 2001. Tests of reproductive-skew models in social insects. Annu. Rev. Entomol. 46, 347–385 10.1146/annurev.ento.46.1.347 (doi:10.1146/annurev.ento.46.1.347) [DOI] [PubMed] [Google Scholar]
- 172.Jaffe R, Garcia-Gonzalez F, Den Boer SPA, Simmons LW, Baer B. In press. Patterns of paternity skew among polyandrous social insects: what can they tell us about the potential for sexual selection? Evolution. 10.1111/j.1558-5646.2012.01721.x (doi:10.1111/j.1558-5646.2012.01721.x) [DOI] [PubMed] [Google Scholar]
- 173.Taborsky M. 1994. Sneakers, satellites, and helpers – parasitic and cooperative behaviour in fish reproduction. Adv. Study Behav. 3, 1–100 10.1016/S0065-3454(08)60351-4 (doi:10.1016/S0065-3454(08)60351-4) [DOI] [Google Scholar]
- 174.Dierkes P, Heg D, Taborsky M, Skubic E, Achmann R. 2005. Genetic relatedness in groups is sex specific and declines with age of helpers in a cooperatively breeding cichlid. Ecol. Lett. 8, 968–975 10.1111/j.1461-0248.2005.00801.x (doi:10.1111/j.1461-0248.2005.00801.x) [DOI] [PubMed] [Google Scholar]
- 175.Bruintjes R, Taborsky M. 2011. Size-dependent task specialization in a cooperative cichlid in response to experimental variation of demand. Anim. Behav. 81, 387–394 10.1016/j.anbehav.2010.10.004 (doi:10.1016/j.anbehav.2010.10.004) [DOI] [Google Scholar]
- 176.Dunn PO, Cockburn A, Mulder RA. 1995. Fairy-wren helpers often care for young to which they are unrelated. Proc. R. Soc. Lond. B. 259, 339–343 10.1098/rspb.1995.0050 (doi:10.1098/rspb.1995.0050) [DOI] [Google Scholar]
- 177.Charnov EL. 1981. Kin selection and helpers at the nest: effects of paternity and biparental care. Anim. Behav. 29, 631–632 10.1016/S0003-3472(81)80130-3 (doi:10.1016/S0003-3472(81)80130-3) [DOI] [Google Scholar]
- 178.Nonacs P. 2011. Monogamy and high relatedness do not preferentially favour the evolution of cooperation. BMC Evol. Biol. 11, 9 10.1186/1471-2148-11-9 (doi:10.1186/1471-2148-11-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wolpert L, Szathmáry E. 2002. Multicellularity: evolution and the egg. Nature 420, 745 10.1038/420745a (doi:10.1038/420745a) [DOI] [PubMed] [Google Scholar]
- 180.Grosberg RK, Strathmann RR. 2007. The evolution of multicellularity: a minor major transition? Annu. Rev. Ecol. Evol. Syst. 38, 621–654 10.1146/annurev.ecolsys.36.102403.114735 (doi:10.1146/annurev.ecolsys.36.102403.114735) [DOI] [Google Scholar]
- 181.Bull JJ, Charnov EL. 1985. On irreversible evolution. Evolution 39, 1149–1155 10.2307/2408742 (doi:10.2307/2408742) [DOI] [PubMed] [Google Scholar]
- 182.Buss LW. 1987. The evolution of individuality. Princeton, NJ: Princeton University Press [Google Scholar]
- 183.Maynard Smith J, Szathmáry E. 1995. The major transitions in evolution. Oxford, UK: Freeman [Google Scholar]
- 184.Queller DC. 1997. Cooperators since life began. Q. Rev. Biol. 72, 184–188 10.1086/419766 (doi:10.1086/419766) [DOI] [Google Scholar]
- 185.Charnov EL. 1978. Evolution of eusocial behavior: offspring choice or parental parasitism? J. Theor. Biol. 75, 451–465 10.1016/0022-5193(78)90356-9 (doi:10.1016/0022-5193(78)90356-9) [DOI] [PubMed] [Google Scholar]
- 186.Bot ANM, Rehner SA, Boomsma JJ. 2001. Partial incompatibility between ants and symbiotic fungi in two sympatric species of Acromyrmex leaf-cutting ants. Evolution 55, 1980–1991 [DOI] [PubMed] [Google Scholar]
- 187.Poulsen M, Boomsma JJ. 2005. Mutualistic fungi control crop diversity in fungus-growing ants. Science 307, 741–744 10.1126/science.1106688 (doi:10.1126/science.1106688) [DOI] [PubMed] [Google Scholar]
- 188.Ivens ABF, Nash DR, Poulsen M, Boomsma JJ. 2008. Caste-specific symbiont policing by workers of Acromyrmex fungus-growing ants. Behav. Ecol. 20, 378–384 10.1093/beheco/arn150 (doi:10.1093/beheco/arn150) [DOI] [Google Scholar]
- 189.Aanen DK, De Fine Licht HH, Debets AJM, Kerstes NAG, Hoekstra RF, Boomsma JJ. 2009. High symbiont relatedness stabilizes cooperation in fungus-growing termites. Science 326, 1103–1106 10.1126/science.1173462 (doi:10.1126/science.1173462) [DOI] [PubMed] [Google Scholar]
- 190.Frank SA. 1994. Genetics of mutualism: the evolution of altruism between species. J. Theor. Biol. 170, 393–400 10.1006/jtbi.1994.1200 (doi:10.1006/jtbi.1994.1200) [DOI] [PubMed] [Google Scholar]
- 191.Sachs JL, Mueller UG, Wilcox TP, Bull JJ. 2004. The evolution of cooperation. Q. Rev. Biol. 79, 135–160 10.1086/383541 (doi:10.1086/383541) [DOI] [PubMed] [Google Scholar]
- 192.Scheuring I, Yu D. 2012. How to assemble a beneficial microbiome in three easy steps. Ecol. Lett. 15, 1300–1307 10.1111/j.1461-0248.2012.01853.x (doi:10.1111/j.1461-0248.2012.01853.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Snook RR, Hosken DJ, Karr TL. 2011. The biology and evolution of polyspermy: insights from cellular and functional studies of sperm and centrosomal behavior in the fertilized egg. Reproduction 142, 779–792 10.1530/REP-11-0255 (doi:10.1530/REP-11-0255) [DOI] [PubMed] [Google Scholar]
- 194.Eberhard WG. 1980. Evolutionary consequences of intracellular organelle competition. Q. Rev. Biol. 55, 231–249 10.1086/411855 (doi:10.1086/411855) [DOI] [PubMed] [Google Scholar]
- 195.Cosmides L, Tooby J. 1981. Cytoplamatic inheritance and intragenomic conflict. J. Theor. Biol. 89, 83–129 10.1016/0022-5193(81)90181-8 (doi:10.1016/0022-5193(81)90181-8) [DOI] [PubMed] [Google Scholar]
- 196.Frank SA. 1996. Host-symbiont conflict over the mixing of symbiont lineages. Proc. R. Soc. Lond. B 263, 339–344 10.1098/rspb.1996.0052 (doi:10.1098/rspb.1996.0052) [DOI] [PubMed] [Google Scholar]
- 197.Noe R, Hammerstein P. 1995. Biological markets: supply-and-demand determine the effect of partner choice in cooperation, mutualism and mating. Behav. Ecol. Sociobiol. 35, 1–11 10.1007/BF00167053 (doi:10.1007/BF00167053) [DOI] [Google Scholar]
- 198.Queller DC, Strassmann JE. 2009. Beyond society: the evolution of organismality. Phil. Trans. R. Soc. B 364, 3143–3155 10.1098/rstb.2009.0095 (doi:10.1098/rstb.2009.0095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Herre EA. 1999. Laws governing species interactions? Encouragement and caution from figs and their associates. In Levels of selection in evolution (ed. Keller L.), pp. 209–237 Princeton, NJ: Princeton University Press [Google Scholar]
- 200.Jander KC, Herre EA. 2010. Host sanctions and pollinator cheating in the fig tree–fig wasp mutualism. Proc. R. Soc. B 277, 1481–1488 10.1098/rspb.2009.2157 (doi:10.1098/rspb.2009.2157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kiers ET, Rousseau RA, West SA, Denison RF. 2003. Host sanctions and the legume–rhizobium mutualism. Nature 425, 78–81 10.1038/nature01931 (doi:10.1038/nature01931) [DOI] [PubMed] [Google Scholar]
- 202.Kiers ET, et al. 2011. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333, 880–882 10.1126/science.1208473 (doi:10.1126/science.1208473) [DOI] [PubMed] [Google Scholar]