Abstract
Mating with more than one pollen donor, or polyandry, is common in land plants. In flowering plants, polyandry occurs when the pollen from different potential sires is distributed among the fruits of a single individual, or when pollen from more than one donor is deposited on the same stigma. Because polyandry typically leads to multiple paternity among or within fruits, it can be indirectly inferred on the basis of paternity analysis using molecular markers. A review of the literature indicates that polyandry is probably ubiquitous in plants except those that habitually self-fertilize, or that disperse their pollen in pollen packages, such as polyads or pollinia. Multiple mating may increase plants' female component by alleviating pollen limitation or by promoting competition among pollen grains from different potential sires. Accordingly, a number of traits have evolved that should promote polyandry at the flower level from the female's point of view, e.g. the prolongation of stigma receptivity or increases in stigma size. However, many floral traits, such as attractiveness, the physical manipulation of pollinators and pollen-dispensing mechanisms that lead to polyandrous pollination, have probably evolved in response to selection to promote male siring success in general, so that polyandry might often best be seen as a by-product of selection to enhance outcross siring success. In this sense, polyandry in plants is similar to geitonogamy (selfing caused by pollen transfer among flowers of the same plant), because both polyandry and geitonogamy probably result from selection to promote outcross siring success, although geitonogamy is almost always deleterious while polyandry in plants will seldom be so.
Keywords: multiple paternity, pollen competition, polyandry, pollen dispersal, pollination, plant–pollinator interaction
1. Introduction
Plants are sessile and employ animals, water or wind to disperse their pollen. Accordingly, they have rather less control over whom they mate with than do many animals. Pollen-dispersing individuals may sire progeny on many mothers, and mothers are likely to produce progeny sired by more than one father. The great majority of outcrossing plant populations are thus probably best described as polygamous. Nevertheless, inasmuch as the seed producers of a population receive pollen from more than one pollen donor, they can profitably be regarded as polyandrous: it is then interesting to ask, first, what advantages or disadvantages there could be for an individual to mate with more than one male; and second, to what extent plants could in fact ever choose to mate, or avoid mating, with more than one male, given an overall cost or benefit.
It is worth recalling from the start that mating in plants technically always occurs between haploid gametophytes, which produce sperm and egg cells by mitosis. In taxa in which the gametophytes are independent life stages, such as bryophytes and ferns, sperm from more than one gametophyte can end up competing to fertilize the eggs of a single common partner, i.e. polyandry is possible among gametophytes. In seed plants, by contrast, the female gametophyte, i.e. the ovule, is only ever fertilized by sperm delivered by a single male gametophyte, i.e. the pollen grain, so that polyandry is technically not possible at the gametophytic stage. Narrowly viewed, there is thus no possibility, for example, of sperm competition in seed plants. Nonetheless, it is useful to consider mating in these taxa in terms of interactions among sporophytes, and to view the dispersal of pollen grains from more than one sporophyte to the stigma(s) of another as ‘polyandry’. This usage allows comparison on a functional basis with other organisms that engage in multiple mating. Sperm competition in animals, for example, is then in many respects functionally analogous to pollen competition in seed plants [1], which takes place before sperm are liberated into the ovule.
Another characteristic of plants that influences how we view the mating system and polyandry is their frequent hierarchical modularity, with floral parts nested in flowers, flowers nested in inflorescences, inflorescences nested in branches or ramets, which, in turn, are potentially part of a larger genotype that might be spatially extensive. The number, phenology and spatial disposition of parts at all these levels can influence the mating system and have presumably been shaped by natural selection. It is thus important to bear in mind that reproductive success will be determined by the coordination of the reproductive strategy at all levels in the modular hierarchy, and that selection on traits at one level can influence patterns of mating at another. The size of the floral display (number and size of inflorescences), for example, can influence both the number of pollinators visiting individual flowers and the mix of pollen they deposit on stigmas [2,3]. Similarly, the order in which flowers in a hermaphrodite inflorescence express their male and female functions can affect the selfing rate within individual fruits as a result of the transfer of pollen among the flowers of the same genet (geitonogamy) [4,5].
Modularity in angiosperms extends also to the within-flower level. In general, flowers have more than one stamen and more than one carpel. The possession of multiple carpels within a flower is of particular note. In species with primitive flowers, such as magnolias and water lilies, carpels are independent of one another within the flower [6], and pollen deposited on one stigma competes to fertilize the ovules in the corresponding ovary, with no access to other ovaries. In these species, considerations of phenomena such as pollen competition and pollen limitation (and perhaps polyandry) ought to be directed at the level of individual carpels within the flower, taking due account of competition for space and resources. In species with evolutionarily derived flowers, by contrast, the different carpels within a flower tend to be fused [6], so that pollen tubes must travel down a common style to fertilize the ovules (sometimes from separate stigmas). In these cases (the vast majority of flowering plants), there is thus a single functional unit representing female function in flowers, although flowers benefit from the attractiveness of the entire inflorescence and are not necessarily independent in terms of the allocation of resources. Interactions among pollen grains, and between pollen grains and the female tissues, in flowers with fused carpels therefore probably ought to be analysed at the floral level.
Whether it makes more sense to consider polyandry at the level of the individual genet, the flower or, in cases where carpels are relatively independent, the carpel itself, will depend on the specific question being addressed. From the perspective of the genet, individuals that produce many flowers are likely to receive pollen from more than one donor, even if individual flowers were to receive pollen from only a single donor. The likelihood of polyandry and multiple paternity at the plant level is thus likely to be uniformly high for plants with multiple flowers, and perhaps beyond the control of individual plants. Because plants tend to disperse their seeds locally [7], the mix of fathers siring seeds on a particular individual will affect the genetic mix of progeny that establish in its vicinity, and thus influence the genetic structure of a population [8]. Polyandry at the plant level could therefore be an important factor shaping within-population genetic structure. This will, however, not be our chief focus here. Rather, in this article, most of our attention will be directed to polyandry within fruits, because it is at this level that pollen–style and pollen–pollen interactions will tend to occur, and thus where natural selection on traits that influence multiple mating could act. It is also the level at which the most profitable comparisons can be drawn between plants and animals.
A key question concerns the extent to which plants are able to control their mating system at all, even at the within-fruit level. Although it is probably true that plants exercise less control over their mating than do animals, they do in fact influence their mating in a number of ways. These include: determining when they flower; how attractive they are to pollinators; where in the flower (and inflorescence) their anthers and stigmas are positioned (and when); when their anthers open and pollen is dispersed (and how much pollen is liberated during each pollinator's visit); when their stigmas are receptive (and for how long), and even which pollen grains are allowed access to the ovary after they have been deposited on the stigma. All of these processes, taken together, constitute a plant's floral syndrome, which will have evolved in response to selection to optimize reproductive success through both male and female sexual functions. The question, then, is not whether plants control whom they mate with, but rather how well, by what means and to what end.
A great deal of attention over the past couple of decades has been devoted to understanding the occurrence of ‘mixed mating’, where selfing rates are intermediate due to the deposition onto stigmas of a mix of self and outcross pollen. Much of this work has been stimulated by models that predicted that intermediate selfing rates would be evolutionarily unstable (reviewed in [9]). Although mixed mating is a special case of polyandry, particularly in animal-pollinated plants [10], its intensive study has perhaps drawn attention away from polyandrous mating in plants more generally. Given its extensive treatment elsewhere [9], we will not be considering it in our review here.
Another important question concerns whether the adaptations we see in flowers are shaped directly by selection through the female function of plants to regulate the number of their potential mates, or are instead chiefly the outcome of selection on the male function to increase siring success. If females do regulate their mate number, we need to know why, i.e. what benefits might they receive by doing so. These questions apply to dioecious species (with separate sexes), but they are particularly pertinent to hermaphrodite plants because of the possibility of conflict that occurs between the male and female functions. Resolving this conflict, i.e. optimizing both the male and female components of reproductive success, has probably been a major theme in the evolution of floral strategies [11,12].
In this article, we review the occurrence of polyandry in plants and consider its potential functional significance. We begin by assessing the frequency of polyandry among plants and ask whether there are certain traits that are particularly associated with multiple mating. We then consider the extent to which plants might benefit from, or be compromised by, mating with more than one individual from the female's point of view. In the subsequent section, we contrast this possibility with the proposition that polyandry might be the result of simple random mating, modified by selection on plants to increase their male component of fitness through improved siring success. If the possible benefits of multiple mating to plants through their female function are just an incidental outcome of selection for increased siring success, this would suggest that the study of polyandry in plants could be seen as a relatively unprofitable detour in attempts to understand the evolution of flowers and plant mating.
2. How common is polyandry in plants?
The dynamics of pollination probably mean that we should expect polyandry to be the rule in plants rather than the exception. Although we focus in this article on angiosperms, where most of the relevant work has been done, polyandry is of course also possible in other plant groups. In bryophytes and ferns, sperm swim through water from potentially more than one source to fertilize eggs produced by a single female. In some homosporous ferns with separate sexes, spores germinate and develop, by default, into female or hermaphrodite gametophytes that control their mating prospects by the release of hormones into their environment [13]; these hormones cause nearby spores, potentially from unrelated sporophytes, to germinate and develop as males, thereby increasing the level of polyandry and sperm competition [13,14]. Mating in gymnosperms is similar to that in wind-pollinated angiosperms (where polyandry is also possible), except that sperm take much longer to reach their target in gymnosperms [15].
Both biotic and abiotic pollinations can result in polyandry. In animal-pollinated plants, which comprise about 80 per cent of angiosperms [16], polyandrous pollination can occur in two ways: either when a single visitor deposits a mix of pollen from more than one pollen donor because some of their pollen grains were carried over during the preceding sequence of flower visits [17–20]; or via the successive visits of different pollinators that each deposit a small amount of pollen from a different pollen donor over a short period [21]. Both mechanisms are not mutually exclusive and can occur together, i.e. with sequential visitations each depositing a mixed-donor pollen load [22,23]. Because pollinators tend to visit more than one flower and may be abundant at a site, individual flowers will often receive multiple visits by animals that have visited more than one flower in their foraging bouts [24]; mixed-donor pollination is therefore likely to be very common in animal-pollinated plants [25]. But polyandry should be common in wind-pollinated plants too, because stigmas often receive more than one pollen grain, haling from different donors, particularly in dense populations [26,27].
Almost everything we know about the incidence of multiple mating in plants comes from marker-assisted paternity inference. These estimates of polyandry will tend to be conservative, because they ignore those mates that delivered pollen to stigmas but failed to sire seeds. Nonetheless, estimates of multiple paternity probably give a reasonable handle on the incidence of multiple mating. A large number of such studies have now been conducted, and multiple paternity has been found in all cases in which evidence for it has been sought [28]. Such studies have characterized multiple paternity either directly through the identification of all putative fathers in a progeny array (i.e. using seed paternity analysis [28]), or in terms of the coefficient of correlated paternity within a fruit [29,30]). These methods have allowed an estimation of the degree of multiple paternity at both the fruit and the whole plant levels for a number of plant species.
Figure 1 summarizes data from several studies in which the extent of polyandry has been inferred using molecular markers. For these species, the numbers of sires range up to about nine within individual fruits, with estimates of the effective number of sires within fruits (calculated by down-weighting those sires that contribute less to paternity) tending to be lower than this, as one would expect. Estimates of the effective number of sires among fruits on a plant are of course higher than within fruits, reaching values greater than 20. A search for patterns among the data reveals no specific feature that might allow us to predict the occurrence of multiple paternity. Most studies have been of insect-pollinated hermaphrodite species, but multiple paternity has also been found in dioecious species [33] and those pollinated by birds [47] and by bats [40]: it occurs in flowers with few [18,19,25,36,44,48] to many ovules or seeds [23,33–35]; in self-incompatible [18,19,32,33,40,48] and self-compatible species [23,25,34–36,41,44,47]; in species that differ in their floral morphologies (actinomorphy [18,19,33,48] versus zygomorphy [23,34]), and inflorescence architecture (complex inflorescence [32] versus solitary flowers [23]). Nor are there any discernible patterns in the occurrence of multiple paternity among species that vary in other traits related to pollinator attraction (such as coloration), or pollen uptake and deposition (anthers and stigma position and protrusion). Only in a few studies has the mechanism of pollen deposition (i.e. pollen carry-over versus sequential visits) been empirically determined [19,23,34,35]. These studies used a combination of pollinator observations (noting the time interval between visits, the time spent on each flower and the number of visited flowers) and manipulation of the pollen load on test flowers for which seed paternity [49] or the proportion of fertilized ovules were measured [20].
Figure 1.
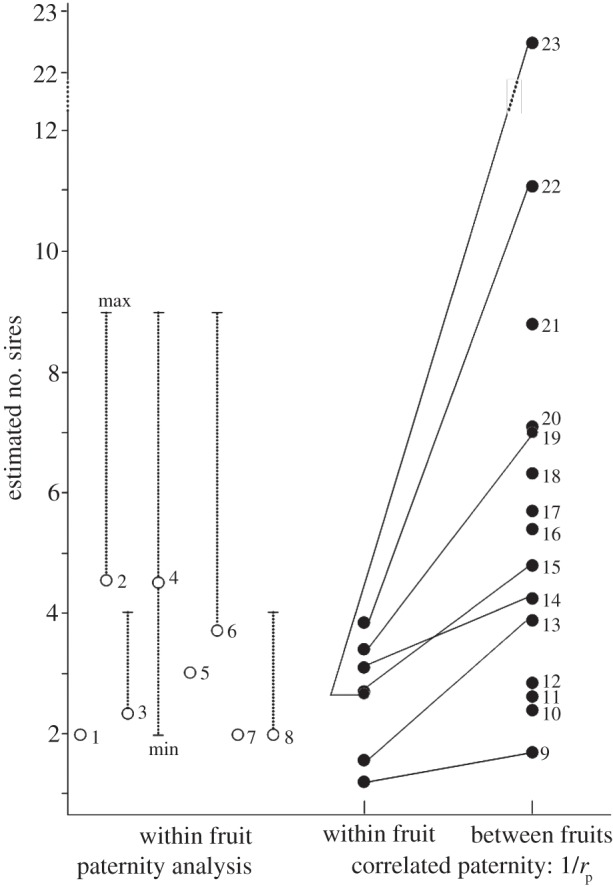
Mean number of sires within fruits and among fruits on a same plant, based on paternity assignment or estimation of the effective number of sires, Nep, on the basis of 1/rp, where rp is the probability that two individuals randomly chosen in a same progeny array are full-sibs (sibling-pair model [31]). Numbers beside data points refer to species and publications, listed here with sample sizes. 1: Ascelpias exaltata; 55 plants and 103 fruits [32]; 2: Ipomopsis aggregata; 12 plants, 28 fruits and 2–14 seeds/fruit [18]; 3: Raphanus sativus; nine plants, four to eight fruits/plant (total 59 fruits) and three to seven seeds/fruit [19]; 4: Silene latifolia; four populations, 15 plants/population, one fruit/plant and 20 seeds/fruit [33]; 5: Mimulus guttatus; three populations, 30 plants, two capsules/plant and 15 seeds/capsule [23]; 6: Mimulus rigens; 144 ramets, 204 fruits (total) and 10 seeds/fruit [34]; 7: Phaseolus vulgaris; year 1: 57 plants, 44 fruits/plant and five to seven seeds/fruit; year 2: 111 plants, 33 fruits/plant and five to seven seeds/fruit [25]; 8: Yucca filamentosa: 10 plants, three fruits/plant and 12 seeds/fruit [35]; 9: Glycine argyrea: 20 plants, two fruits/plant and six seeds/fruit [36]; 10: Grevillea iaspicula: average Nep over five populations, six to eight plants/population and 8–15 seeds/plant [37]; 11: Lambertia orbifolia; average Nep over four populations, 13–18 plants/population and 11.5–20.5 seeds/plant [38]; 12: Albizia julibrissin: 15 plants and 60 seeds/plant, [39]; 13: Pachira quinata: 15 plants, 20 fruits/plant and four seeds/fruit, [40]; 14: Eichhornia paniculata: 36 plants, 4.1±1.1 fruit/plant and five seeds/fruit [41]; 15: Mimulus guttatus: average Nep over two populations, two to three fruits/plant and 24 fruits/population [31]; 16: Centaurea corymbosa: 47 plants, eight seeds from single fruit/plant [42]; 17: Daviesia mimosoides: average Nep over five populations, 14–15 plants/population, 14–16 fruits/plant and one seed/fruit [43]; 18: Daviesia suaveolens: average Nep over three populations, 14–15 plants/population, 14–16 fruits/plant and one seed/fruit [43]; 19: Sorbus torminalis: 14 plants [44]; 20: Caryocar brasiliense: average Nep over four populations: 10 plants and 16 seeds/plant in each population [45]; 21: Centaurea solstitialis: average Nep over eight populations, 40 plants/population and 12 seeds from a single fruit head/plant [46]; 22: Eucalyptus rameliana: 31 plants, three to four fruits/plant and 33–39 seedlings/plant [47]; 23: Arabidopsis halleri: 22 plants, five fruits/plant and 26.7±21.9 s.d. seeds/plant [48].
Of particular interest are species that disperse their pollen grains in aggregate, either packaged in tetrads or polyads (where pollen arising from the product of a single or several meiotic events is aggregated and dispersed as a unit) or pollinia (an aggregate of all the pollen grains from an anther that is dispersed by animal pollinators as a single unit). For example, in Asclepia exalta, a species with pollinia, only 2 per cent of the 103 fruits sampled by Broyles & Wyatt [32] were found to contain seeds sired by more than one father. In Acacia melanoxylon, a species with pollen grains dispersed in polyads of 16 grains, only about 10 per cent of the fruits sampled showed multiple paternity [50]. These low values probably reflect the fact that such pollen packages contain enough pollen grains to fertilize all available ovules (e.g. there are 14 ovules in the ovaries of Acacia melanoxylon). Nevertheless, it is revealing that despite aggregated pollen dispersal, there is still some degree of multiple paternity shown by these species. Unsurprisingly, multiple paternity is low in species that habitually self-fertilize (e.g. Phaseolus vulgaris [25] and Glycine argyrea [36]).
3. The selection of polyandry through female function: costs, benefits and mechanisms
The possibility that polyandry might affect, and therefore be selected through, female fitness has prompted much research on animal species, not least because multiple mating by females may be costly [51–53]. What might the costs and benefits of polyandry be for the female function of plants? Almost certainly, one of the costs faced by animals will also apply to plants: the increased risk of becoming infected by disease as a result of mating with more than one male [54,55]. Work on the dioecious perennial herb Silene latifolia has shown that increased pollinator visits to female flowers is positively associated with the probability of infection by the anther-smut fungus, Microbotryum violaceum, which causes both males and females to produce sterile staminate (male) flowers that disperse only fungal spores [56,57]. Visitation by large numbers of pollinating animals can also cause physical damage to flowers and plants, just as multiple mating by female animals can reduce their viability [58]. For example, the female fitness of Yucca spp. may be reduced with increased visitation of the pollinating yucca moths, because each successive moth lays more parasitizing eggs in its flowers, and these reduce fruit set through fruit abortion [59,60]. How such costs trade off against the potential benefits of multiple mating is still poorly understood, and more work would be valuable.
The potential benefits of polyandry to plants through their female function may be either direct or indirect. In animals, direct benefits include the provision by males of food to their mating partners, whether in the form of semen that is digested rather than used for fertilization, or in the form of nuptial food gifts, including the case where males offer themselves as a food reward after mating [58,61]. Similar benefits are unlikely to be available to plants. Rather, a plant's female function might benefit from multiple mating either if mating with more than one male increases pollen receipt to levels required for the fertilization of all available ovules, or if there are genetic benefits of large and diverse pollen loads. We can define three broad classes of such genetic benefits, each of which has also been proposed as explanations for the occurrence of polyandry in animals [51,62,63]: the production of progeny that are more genetically diverse; the promotion of competition among male gametophytes of differing genetic quality; and the possibility of direct female choice among progeny that differ in quality and/or compatibility. In the following subsections, we first consider the possible benefits of multiple mating in terms of increasing the amount of pollen received (thus alleviating pollen limitation, i.e. the benefits of producing more progeny), and then consider the three potential genetic benefits. We end this section by asking which traits might evolve to influence the level of polyandry from the female point of view in response to selection promoting or disfavouring it.
(a). Benefits of producing more progeny
It is often supposed that Bateman's principle applies to plants as much as it does to animals, i.e. the idea that reproductive success by males and females will be limited by access to mates and resources to provision progeny, respectively [64]. An important corollary of Bateman's principle, when applied to plants, is that fruit and seed set should not be pollen-limited. In fact, experiments in which extra pollen is applied to stigmas in natural populations often give rise to increased seed set [65–67]. These experiments thus suggest that a basic assumption of Bateman's principle is violated and point to the possibility that selection through a plant's female function might favour increased rates of pollen deposition on stigmas [68]. But how reliable are these experiments?
Ashman et al. [66] reviewed the literature on pollen limitation and suggested that the pollen-addition experiments that have found evidence for it might be inadequate for two reasons. First, experiments that supplement the pollen on only a fraction of the flowers may find false evidence for pollen limitation, because it is the seed production of the whole plant that matters, and resources can be allocated among fruits of the same plant. Similarly, increased seed set in response to pollen supplementation in one season may cause reduced seed set in subsequent seasons through among-season trade-offs [66]. Experiments thus really need to add pollen to all flowers and to account for between-season allocation trade-offs.
Second, pollen-addition experiments to test for pollen limitation have most typically involved the addition of outcross pollen to one or more stigmas on manipulated individuals, but pollinators deliver a mix of outcross and self pollen, particularly if they visit more than one flower on the same individual, as is typical [67]. Because fertilization by self pollen often leads to lower seed set (as a result of early acting inbreeding depression during seed development), open pollination in the wild could thus yield lower seed set than experimental pollination using only outcross pollen. Fitness through female function might then be limited not by a paucity of pollinators, but by the tendency of pollinators to deliver an insufficient proportion of outcross pollen relative to self pollen or pollen otherwise incompatible with a plant's ovules [67]. In other words, seed set might be limited by its ‘quality’, i.e. the extent to which it is genetically compatible with the plant's ovules [67].
Even so, it is important to note that pollen limitation, where it occurs, could be overcome by mating multiple times with the same male or pollen donor, i.e. polyandry is not essential. In accordance with this, several manipulative studies of single-donor pollen supplementation have demonstrated increased female reproductive success not only through an obvious increase of the pollen : ovule ratio and thus the avoidance of pollen limitation [69], but also through density-dependent chemical and hormonal mechanisms that enhance pollen tube germination and growth rate [70,71] as well as seed and fruit mass (reviewed in [72]). Ultimately, pollen quantity limitation is perhaps most easily alleviated by traits that increase autogamous selfing [73–75] and should be selected as long as the resulting progeny do not suffer too much through inbreeding depression [9].
(b). Benefits of producing genetically more variable progeny
Polyandry may increase female fitness by enhancing the adaptive ability and ecological complementarity associated with genetically diverse progeny. Recently, Aguirre & Marshall [76] tested this hypothesis for animals; using ascidians as a model, they empirically separated the effect of sexual selection on female reproductive success from that of offspring genetic diversity, and found greater performance of populations comprising half-sib families when compared with those comprising full-sibs sired by the best male. Similar effects may be expected in plants [77], not least because individual plants have less control over which habitat they are destined for after dispersal. In species that disperse their seeds locally, for instance, where siblings are likely to end up growing together, a genetically more diverse progeny population might be less susceptible to herbivores [78,79], and their progeny, in turn, should be less prone to biparental inbreeding and the associated inbreeding depression [53,80].
(c). Benefits of promoting pollen competition
By increasing the amount and diversity of pollen deposited on stigmas, multiple mating may promote pollen competition and allow post-pollination selection for males with the fastest-growing pollen tubes. There are two possible advantages that such pollen competition might have. First, to the extent that pollen tube growth rates are heritable, females fertilized by fast pollen grains will produce offspring that themselves are more likely to sire offspring in the next generation [81]. Schlichtling et al. [82] and Lankinen et al. [83] have found evidence for heritable variation in pollen-tube growth rates, but more work needs to be done.
Pollen competition might also be advantageous if those sporophytes fertilized by sperm from fast-growing pollen grains are more vigorous, i.e. if traits conferring competitiveness in the gametophyte generation are transferred to the sporophyte generation, either because they are genetically heritable or because of maternal (or paternal) effects [84,85]. This possibility has also been proposed as an advantage of sperm competition in animals, but it would seem to be even more plausible for plants, given that 60 per cent (or more) of a plant's genes are expressed in the pollen tube (gametophyte generation) [86,87].
Evidence for the effect of pollen competition on offspring vigour has been provided by studies manipulating the pollen load on stigmas (reviewed in [1,72,88]). These studies have given mixed results and have been criticized either because the range of pollen loads applied have been inappropriate [89], because offspring have been monitored at inappropriate life stages or growing environments [90], or because differences in progeny vigour that arise from large versus small pollen loads might be due to maternal effects of seed provisioning and seed size rather than genetic quality [85,91]. Nevertheless, comparison of offspring vigour arising from a large pollen load of mixed- versus single-donor pollen has highlighted a positive effect of pollen diversity in several studies [92,93].
Another advantage of mixed pollen loads is that they may allow selection (through competition) against pollen that carries meiotic drivers, i.e. alleles that favour their own transmission [94]. In single-donor pollen loads, this possibility would confer a large advantage to the driver allele, even if it reduced the viability or fertility of the resulting offspring (and thus the fitness of the mother; reviewed in [1,95]). However, as Haig & Bergstrom [94] pointed out, the advantages to driver alleles would be diluted under competition between pollen grains from multiple fathers, because ‘genes expressed in pollen would have little to gain by sabotaging other pollen grains within their own anther’ (p. 272). In the dioecious plant S. latifolia, single-donor pollinations by some males gave rise to female-biased sex ratios, presumably because of a driving X chromosome, and these males tended to have low siring success in multiple-donor pollen loads [95]. The putative driver alleles responsible are perhaps maintained in S. latifolia because multiple paternity is relatively low, at least in its introduced range in North America (unpublished results cited in [95]). In species that have reduced multiple paternity as a result of the dispersal of pollen in polyads or pollinia (see §2), pollen grains within a dispersal unit develop synchronously even after meiosis [96], and this should further mitigate against meiotic drivers [94].
Although pollen competition resulting from large mixed pollen loads may confer some benefits on a female fitness, in certain cases it can also be disadvantageous. For example, in dioecious species with sex chromosomes, genetic degeneration of the Y chromosome can mean that Y-bearing pollen tubes grow more slowly so that progeny sex ratios become female-biased [97]. This effect is seen in the dioecious species Rumex nivalis, where sex ratios become increasingly female-biased as pollen competition intensifies with greater pollen loads [98,99]. In R. nivalis, which is wind-pollinated, pollen load on stigmas and progeny female frequencies correlate positively with local patch density [99]. Although these effects are not typical (because most plants are hermaphroditic and most dioecious species do not have degenerate sex chromosomes; [100]), they provide compelling evidence that pollen competition can have important phenotypic effects on the sporophytic generation in ways that influence maternal fitness.
(d). Benefits of allowing females to choose among diverse male gametophytes
Although a signal of male genetic quality or compatibility can help female animals in their pre-mating choice [101,102], it is difficult to see how such signals could be easily available to female plants, or how they could exercise much control over the identity of the pollen deposited on their stigma. (The reciprocal placement of stigmas and anthers in heterostylous species, whereby pollen is preferentially deposited on compatible stigmas, provides an unusual example of the latter [103].) Nonetheless, females could in principle influence the paternity of their seeds though post-pollination mechanisms that may occur both before ovule fertilization and during seed development (i.e. as cryptic female choice). While pollen competition should sort among the potential compatible fathers by their intrinsic genetic qualities, maternal effects might also actively control siring success and favour some males over others, as has been found, for example, in crickets [62]. The possibility of female choice among potential sires through post-zygotic mechanisms, such as selective investment in seeds and seed abortion, has been sought in a number of plant species, but there is still very little direct evidence for it (reviewed in [104–106]). Indeed, it is difficult to distinguish the role played in non-random paternity and seed abortion by female choice from that played by paternal effects or competition among embryos for resources owing to differing sink strengths. The incidence of active female mate choice in plants is thus still speculative [88,104].
While variation in progeny vigour can be due to the direct additive effects on fitness of alleles derived from different fathers, they might also be the result of variation in compatibility between maternal and paternal genes, such as segregation distorters, female post-zygotic distorters or genomic imprinting (reviewed in [1,51,107]). The importance of such genetic interactions is suggested by the fact that the paternity of seeds can depend on both the genotype of the potential sires depositing pollen on the stigma and the genotype of the mother [108]. Perhaps the most common example of such non-additive genetic incompatibility is provided by the expression of deleterious recessive alleles that come together as homozygotes in inbred progeny, i.e. the effect of inbreeding depression [109,110]. The evolution of mechanisms that avoid selfing and promote outcrossing, such as self-incompatibility (SI), is likely in itself to lead to multiple mating.
Molecular SI mechanisms are found widely among angiosperms, with modes of action that may be determined either by the maternal sporophyte (sporophytic SI) or by the pollen gametophytes themselves (gametophytic SI) [111]. Although these modes differ in important ways, they generally prevent the fertilization of their ovules both by self pollen grains and by pollen grains from other individuals in the population carrying the same SI alleles. Because strong negative frequency-dependent selection favours the maintenance of large numbers of SI alleles in plant populations (because pollen grains carrying rare alleles are compatible with most of the rest of the population), the probability that a single outcross pollen donor will be compatible with any given maternal genotype will typically be high [112]. In such situations, although polyandry might still be important for allowing male–male competition or female choice among genetically different pollen grains, it is probably not a critical factor for ensuring the receipt of an adequate quantity of pollen that is compatible at the SI locus itself.
In small populations of SI species, by contrast, in which the number of S-alleles may be low due to their loss by drift, a sizable fraction of potential mates might be incompatible at the SI locus (reviewed in [113]). Polyandrous pollination in such populations should tend to alleviate compatible pollen limitation and might thus be selected not only to allow for male–male competition or female choice among pollen grains differing in their genetic quality, but also to mitigate against low seed set per se. Self-incompatible populations with different levels of mate availability due to variation in their number of S-alleles therefore present cases in which the benefits of polyandry are likely to differ, too. In populations (or species) in which mate availability has been compromised by the loss of S-alleles [114,115], we might thus expect to see evidence for selection of traits that increase the incidence of polyandry. To our knowledge, this hypothesis has not been tested.
(e). Traits that regulate the incidence of polyandry
If polyandry entails specific costs or benefits to a plant's female function, what traits might evolve in response to the implied selection for or against it? There are essentially two classes of such traits: those that increase pollinator visitation and effective pollen pick-up from pollinators' bodies; and those that increase female choice or male–male competition. The latter is likely to be particularly important, because little will be gained from polyandry if the first pollen grains that arrive on a stigma are always the most successful, regardless of their genetic quality or compatibility [116–118].
Attractiveness is one obvious way in which plants can affect the number of their pollinators and mates. There are obvious benefits of being attractive, but attractiveness also entails costs, because pollinators are more likely to remain on attractive plants and to move among their flowers, effecting self-pollination via geitonogamy [119,120]. Because geitonogamy is detrimental to a plant's male and female components of reproductive success, traits that allow increased attractiveness while keeping geitonogamy in check are thus likely to be particularly favourable. Indeed, many of the exquisite adaptations displayed by flowers and inflorescences can be interpreted along these lines [11].
Several traits should alter the scope for pollen competition or female choice (reviewed in [88]). For example, pollen competition can be enhanced by producing large stigmatic areas [121,122] and long styles [123,124], as well as by processes that influence the germination and growth of pollen tubes down the style [125,126]. Prolonging the period of stigma receptivity can increase the opportunities for pollen from different donors to be picked up on their stigmas [127], although prolonged stigma receptivity can only be beneficial if plants are able to prevent the immediate success of the first pollen that arrives on the stigma, which often enjoys a siring advantage [118,128,129]. One way of reducing the advantages of first-arriving pollen is for plants to allow pollen to accumulate on their stigmas while preventing its immediate germination, thus removing the advantages of first-arriving pollen [130–132]. However, a large number of plants in fact actively shut down stigma receptivity and allow petals to collapse as soon as compatible pollen has been delivered [116]. This process, which occurs particularly in plants with protandrous flowers once they have served their purpose in terms of pollen dispersal, is likely a response to selection to save on the costs of maintaining flowers [133]; certainly, its occurrence argues against the advantages of polyandry as a result of pollen accumulated sequentially in these species.
4. Polyandry as a result of selection among males to increase siring success
From the foregoing, it should be clear that plants might benefit from, or be compromised by, mating with more than one male, and that there are at least certain ways in which they could exercise control over their mating system. However, the extent to which they actually do exercise control to reap the benefits is still largely unknown and in need of much more research. Being sessile and requiring the intercession of biotic or abiotic vectors to transport their pollen means that plants undoubtedly have less control over their mating partners than do many animals. We are thus left with the possibility that the degree of multiple mating in plant populations is often simply an outcome of a random union of gametes (or gametophytes), influenced by the spatial structure of a population and the strategies employed by males to disperse their pollen (rather than the strategies of females to receive it).
The intricacies of the angiosperm flower and the wonderful diversity in floral form and inflorescence display are a testament to the power of natural selection to bring about morphological change in structures that affect mating in plants. Indeed, much of the oeuvre of pollen biology and the analysis of plant sexual and mating systems support the hypothesis that variation in floral form and display affects mating prospects through both sexual functions. While plant ecologists for a long time viewed flowers as seed-producing organs, it is now widely appreciated that selection on flowers also operates through the male function. Indeed, an extreme view is that the intricate traits displayed by flowers are due almost entirely to selection through a plant's male function, i.e. flowers are male [134]. There are two reasons for this.
The first is Bateman's principle, i.e. the proposition that only males are limited in their reproductive success by their access to mates (and that female reproduction is limited by resources). We have already pointed to experiments that suggest that seed set is in fact often pollen-limited, and that a key assumption of Bateman's principle may thus not be valid. Certainly, experiments such as these have given some authors reason to doubt the relevance of Bateman's principle for plants [65]. However, as noted earlier, more recent work has called experiments on pollen limitation into question, so the idea that males are more limited by the availability of mates than are females cannot yet be ruled out. If it applies, then we would expect selection to act on floral strategies principally through the male function. The fact that fruit : flower ratios are often low (i.e. that flowers often contribute nothing directly to female reproductive success) would seem to support this view.
The second reason for holding that flowers function more as male than female organs derives from considerations of the likely relative shapes of the male versus female fitness gain curves in hermaphroditic plants, i.e. the return upon investment in their male versus female functions [135]. In animal-pollinated plants, particularly those with specialist pollinators, it is widely thought that the fitness gained through male function flattens off as a function of investment more rapidly than does that through female function, because increasing investment in pollen dispersal soon leads either to the saturation of pollen on pollinators' bodies, or to competition among pollen grains from the same donor to fertilize a finite pool of ovules (local mate competition) [136–138]. We still have very little idea of what the shapes of the fitness gain curves really are [138]. However, if the male curve does typically saturate more quickly than the female one, then this would explain the common strategy employed by flowers of placing pollen on pollinators' bodies so that it is less easily removed by grooming, or of dispensing pollen little by little to consecutive pollinator visitors rather than all at once [139]. For example, they may expose and open their anthers sequentially over a period of time rather than all together, or (in the case of buzz-pollinated flowers) pollen may be dispensed little by little through small pores analogous to those of a pepper-shaker [140,141]. It is precisely this sort of strategy that should increase the levels of polyandry.
Although some floral traits that increase attractiveness may increase the transfer to a stigma of both outcross and self pollen from other flowers, plants have also evolved mechanisms that favour pollen export to other individuals in the population while keeping geitonogamous pollen transfer in check. Such mechanisms, which include both the timing of development and the arrangement of flowers within inflorescences [4], as well as polymorphisms such as heterostyly and enantiostyly [142], are also likely to increase the number of a potential sires contributing pollen to individual stigmas. Importantly, mechanisms that reduce geitonogamous selfing are also found in self-incompatible species (where the detrimental effects of selfing for female function are prevented), suggesting that they have evolved in response to selection for increased male fitness rather than female fitness [143]. Therefore, irrespective of the validity of Bateman's principle for plants, the influence on levels of polyandry of both pollen-dispensing mechanisms and of traits that limit geitonogamy (particularly in self-incompatible species) can probably be attributed to responses to selection through a plant's male function.
5. Concluding remarks
Polyandry is ubiquitous and probably all but inevitable in outcrossing plants. Although there may be several benefits of polyandry to plants through their female function, to a large extent polyandry is likely to be the incidental outcome of selection to increase outcross siring success. Indeed, it is difficult to envisage selection that acts to promote outcrossing that would not at the same time increase multiple-donor pollination—except when pollen is dispersed in polyads or pollinia. Plants that restrict the period over which stigmas are receptive or flowers are open might reduce the possibility of polyandry via sequential visitation of different pollinators, but such strategies risk missing out on mating opportunities and would not necessarily reduce pollen carry-over by pollinators that visit more than one pollen donor in sequence.
Although studies to discern the female benefits of polyandry have had somewhat mixed results, there is nonetheless evidence that some plants benefit from enhancing competition among the pollen grains they receive. Thus, it seems plausible that a syndrome of polyandry is not only the incidental outcome of selection for outcrossing, but may also be promoted in its own right through selection on a plant's female function. The evolution of traits that enhance the potential for pollen competition (such as stigma size and extended receptivity) is consistent with this possibility.
Research on plant mating systems has focused on documenting and understanding selfing-rate variation and the maintenance of mixed mating, which is a special case of polyandry. This focus is understandable: mixed mating poses a clear enigma, because simple models predict its evolutionary instability [9], and both the selfing rate itself and the key parameters of evolutionary models that might account for its maintenance are easily measured. In contrast, the description of polyandry in terms of the full distribution of a plant's mating partners is more challenging, and the likely costs and benefits of multiple mating are more subtle. Moreover, botanists' intuition, based on observations of the messy business of pollination by biotic or abiotic vectors among plants that produce large numbers of flowers, that plants have only limited control over the mix of their mating partners is plausible. Accordingly, research on multiple mating in plants is still in its infancy, and few of the ideas surrounding it that have been considered by zoologists studying multiple mating in animals have made their way into studies of plants.
To advance our understanding of polyandry in plants, we ultimately need assessments of the relative costs and benefits of polyandry under field conditions, alongside those of monandrous outcrossing, geitonogamy, autogamy and other patterns of mating. Fertile ground for such research might be offered by self-incompatible species (allowing a focus on the mix of outcross mating partners) or populations that differ in their mate availability (e.g. by possessing different numbers of S-alleles), species that display mixed mating where selfing occurs via geitonogamy, and dioecious species where floral traits (e.g. those influencing attractiveness) can evolve to some extent independently in males and females. It would also be useful to know more about the extent to which polyandry occurs via pollen carry-over versus sequential visitation in species with contrasting floral and inflorescence traits.
Acknowledgements
We are grateful to Tom Pizzari and two anonymous referees for their very helpful comments on the manuscript and to the Swiss National Science Foundation for funding.
References
- 1.Bernasconi G, et al. 2004. Evolutionary ecology of the prezygotic stage. Science 303, 971–975 10.1126/science.1092180 (doi:10.1126/science.1092180) [DOI] [PubMed] [Google Scholar]
- 2.Schmid-Hempel P, Speiser B. 1988. Effects of inflorescence size on pollination in Epilobium angustifolium. Oikos 53, 98–104 10.2307/3565669 (doi:10.2307/3565669) [DOI] [Google Scholar]
- 3.Karron JD, Mitchell RJ. 2012. Effects of floral display size on male and female reproductive success in Mimulus ringens. Ann. Bot. 109, 563–570 10.1093/aob/mcr193 (doi:10.1093/aob/mcr193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harder LD, Barrett SCH. 1995. Mating cost of large floral displays in hermaphrodite plants. Nature 373, 512–515 10.1038/373512a0 (doi:10.1038/373512a0) [DOI] [Google Scholar]
- 5.de Jong TJ, Waser NM, Klinkhamer PGL. 1993. Geitonogamy: the neglected side of selfing. Trends Ecol. Evol. 8, 321–325 10.1016/0169-5347(93)90239-L (doi:10.1016/0169-5347(93)90239-L) [DOI] [PubMed] [Google Scholar]
- 6.Judd WS, Campbell CS, Kellogg EA, Stevens P. 2008. Plant systematics: a phylogenetic perspective. Sunderland, MA: Sinauer [Google Scholar]
- 7.Nathan R, Muller-Landau HC. 2000. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol. Evol. 15, 278–285 10.1016/S0169-5347(00)01874-7 (doi:10.1016/S0169-5347(00)01874-7) [DOI] [PubMed] [Google Scholar]
- 8.Ingvarsson PK, Giles BE. 1999. Kin-structured colonization and small-scale genetic differentiation in Silene dioica. Evolution 53, 605–611 10.2307/2640796 (doi:10.2307/2640796) [DOI] [PubMed] [Google Scholar]
- 9.Goodwillie C, Kalisz S, Eckert CG. 2005. The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annu. Rev. Ecol. Evol. Syst. 36, 47–79 10.1146/annurev.ecolsys.36.091704.175539 (doi:10.1146/annurev.ecolsys.36.091704.175539) [DOI] [Google Scholar]
- 10.Vogler DW, Kalisz S. 2001. Sex among the flowers: the distribution of plant mating systems. Evolution 55, 202–204 [DOI] [PubMed] [Google Scholar]
- 11.Barrett SCH. 2002. The evolution of plant sexual diversity. Nat. Rev. Genet. 3, 274–284 10.1038/nrg776 (doi:10.1038/nrg776) [DOI] [PubMed] [Google Scholar]
- 12.Lloyd DG. 1984. Gender allocation in outcrossing cosexual plants. In Perspectives on plant population ecology (eds Dirzo R, Sarukhan J.), pp. 277–300 Sunderland, MA: Sinauer [Google Scholar]
- 13.Banks JA. 1997. Sex determination in the fern Ceratopteris. Trends Plant Sci. 2, 175–180 10.1016/S1360-1385(97)85223-5 (doi:10.1016/S1360-1385(97)85223-5) [DOI] [Google Scholar]
- 14.Haig D, Westoby M. 1988. Sex expression in homosporous ferns: an evolutionary perspective. Evol. Trends Plant 2, 111–120 [Google Scholar]
- 15.Fernando DD, Lazzaro MD, Owens JN. 2005. Growth and development of conifer pollen tubes. Sexual Plant Reprod. 18, 149–162 10.1007/s00497-005-0008-y (doi:10.1007/s00497-005-0008-y) [DOI] [Google Scholar]
- 16.Ollerton J, Winfree R, Tarrant S. 2011. How many flowering plants are pollinated by animals? Oikos 120, 321–326 10.1111/j.1600-0706.2010.18644.x (doi:10.1111/j.1600-0706.2010.18644.x) [DOI] [Google Scholar]
- 17.Thomson JD, Plowright RC. 1980. Pollen carryover, nectar rewards, and pollinator behavior with special reference to Diervilla lonicera. Oecologia 46, 68–74 10.1007/BF00346968 (doi:10.1007/BF00346968) [DOI] [PubMed] [Google Scholar]
- 18.Campbell DR. 1998. Multiple paternity in fruits of Ipomopsis aggregata (Polemoniaceae). Am. J. Bot. 85, 1022–1027 10.2307/2446369 (doi:10.2307/2446369) [DOI] [PubMed] [Google Scholar]
- 19.Ellstrand NC. 1984. Multiple paternity within the fruits of the wild radish, Raphanus sativus. Am. Nat. 123, 819–828 10.1086/284241 (doi:10.1086/284241) [DOI] [Google Scholar]
- 20.Labouche AM, Bernasconi G. 2010. Male moths provide pollination benefits in the Silene latifolia-Hadena bicruris nursery pollination system. Func. Ecol. 24, 534–544 10.1111/j.1365-2435.2009.01658.x (doi:10.1111/j.1365-2435.2009.01658.x) [DOI] [Google Scholar]
- 21.Addicott JF, Tyre AJ. 1995. Cheating in an obligate mutualism: how often do yucca moths benefit yuccas? Oikos 72, 382–394 10.2307/3546124 (doi:10.2307/3546124) [DOI] [Google Scholar]
- 22.Karron JD, Mitchell RJ, Bell JM. 2006. Multiple pollinator visits to Mimulus ringens (Phrymaceae) flowers increase mate number and seed set within fruits. Am. J. Bot. 93, 1306–1312 10.3732/ajb.93.9.1306 (doi:10.3732/ajb.93.9.1306) [DOI] [PubMed] [Google Scholar]
- 23.Dudash MR, Ritland K. 1991. Multiple paternity and self-fertilization in relation to floral age in Mimulus guttatus (Scrophulariaceae). Am. J. Bot. 78, 1746–1753 10.2307/2444854 (doi:10.2307/2444854) [DOI] [Google Scholar]
- 24.Engel EC, Irwin RE. 2003. Linking pollinator visitation rate and pollen receipt. Am. J. Bot. 90, 1612–1618 10.3732/ajb.90.11.1612 (doi:10.3732/ajb.90.11.1612) [DOI] [PubMed] [Google Scholar]
- 25.Ibarra-Perez FJ, Ellstrand NC, Waines JG. 1996. Multiple paternity in common bean (Phaseolus vulgaris L, Fabaceae). Am. J. Bot. 83, 749–758 10.2307/2445852 (doi:10.2307/2445852) [DOI] [Google Scholar]
- 26.Friedman J, Barrett SCH. 2011. The evolution of ovule number and flower size in wind-pollinated plants. Am. Nat. 177, 246–257 10.1086/657954 (doi:10.1086/657954) [DOI] [PubMed] [Google Scholar]
- 27.Friedman J, Barrett SCH. 2009. Wind of change: new insights on the ecology and evolution of pollination and mating in wind-pollinated plants. Ann. Bot. 103, 1515–1527 10.1093/aob/mcp035 (doi:10.1093/aob/mcp035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernasconi G. 2003. Seed paternity in flowering plants: an evolutionary perspective. Perspect. Plant Ecol. 6, 149–158 10.1078/1433-8319-00075 (doi:10.1078/1433-8319-00075) [DOI] [Google Scholar]
- 29.Hardy OJ, Vekemans X. 2002. SPAGEDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol. Ecol. Notes 2, 618–620 10.1046/j.1471-8286.2002.00305.x (doi:10.1046/j.1471-8286.2002.00305.x) [DOI] [Google Scholar]
- 30.Ritland K. 2002. Extensions of models for the estimation of mating systems using n independent loci. Heredity 88, 221–228 10.1038/sj.hdy.6800029 (doi:10.1038/sj.hdy.6800029) [DOI] [PubMed] [Google Scholar]
- 31.Ritland K. 1989. Correlated matings in the partial selfer Mimulus guttatus. Evolution 43, 848–859 10.2307/2409312 (doi:10.2307/2409312) [DOI] [PubMed] [Google Scholar]
- 32.Broyles SB, Wyatt R. 1990. Paternity analysis in a natural population of Asclepias exalta: multiple paternity, functional gender, and the pollen donation hypothesis. Evolution 44, 1454–1468 10.2307/2409329 (doi:10.2307/2409329) [DOI] [PubMed] [Google Scholar]
- 33.Teixeira S, Bernasconi G. 2007. High prevalence of multiple paternity within fruits in natural populations of Silene latifolia, as revealed by microsatellite DNA analysis. Mol. Ecol. 16, 4370–4379 10.1111/j.1365-294X.2007.03493.x (doi:10.1111/j.1365-294X.2007.03493.x) [DOI] [PubMed] [Google Scholar]
- 34.Mitchell RJ, Karron JD, Holmquist KG, Bell JM. 2005. Patterns of multiple paternity in fruits of Mimulus ringens (Phrymaceae). Am. J. Bot. 92, 885–890 10.3732/ajb.92.5.885 (doi:10.3732/ajb.92.5.885) [DOI] [PubMed] [Google Scholar]
- 35.Massey LK, Hamrick JL. 1999. Breeding structure of a Yucca filamentosa (Agavaceae) population. Evolution 53, 1293–1298 10.2307/2640832 (doi:10.2307/2640832) [DOI] [PubMed] [Google Scholar]
- 36.Brown AHD, Grant JE, Pullen R. 1986. Outcrossing and paternity in Glycine argyrea by paired fruit analysis. Biol. J. Linnean Soc. 29, 283–294 10.1111/j.1095-8312.1986.tb00280.x (doi:10.1111/j.1095-8312.1986.tb00280.x) [DOI] [Google Scholar]
- 37.Hoebee SE, Young AG. 2001. Low neighbourhood size and high interpopulation differentiation in the endangered shrub Grevillea iaspicula McGill (Proteaceae). Heredity 86, 489–496 10.1046/j.1365-2540.2001.00857.x (doi:10.1046/j.1365-2540.2001.00857.x) [DOI] [PubMed] [Google Scholar]
- 38.Coates DJ, Hamley VL. 1999. Genetic divergence and the mating system in the endangered and geographically restricted species, Lambertia orbifolia Gardner (Proteaceae). Heredity 83, 418–427 10.1038/sj.hdy.6885760 (doi:10.1038/sj.hdy.6885760) [DOI] [PubMed] [Google Scholar]
- 39.Irwin AJ, Hamrick JL, Godt MJW, Smouse PE. 2003. A multiyear estimate of the effective pollen donor pool for Albizia julibrissin. Heredity 90, 187–194 10.1038/sj.hdy.6800215 (doi:10.1038/sj.hdy.6800215) [DOI] [PubMed] [Google Scholar]
- 40.Quesada M, Fuchs EJ, Lobo JA. 2001. Pollen load size, reproductive success, and progeny kinship of naturally pollinated flowers of the tropical dry forest tree Pachira quinata (Bombacaceae). Am. J. Bot. 88, 2113–2118 10.2307/3558436 (doi:10.2307/3558436) [DOI] [PubMed] [Google Scholar]
- 41.Morgan MT, Barrett SCH. 1990. Outcrossing rates and correlated mating within a population of Eichhornia paniculata (Pontederiaceae). Heredity 64, 271–280 10.1038/hdy.1990.33 (doi:10.1038/hdy.1990.33) [DOI] [Google Scholar]
- 42.Hardy OJ, González-Martínez SC, Colas B, Fréville H, Mignot A, Olivieri I. 2004. Fine-scale genetic structure and gene dispersal in Centaurea corymbosa (Asteraceae). II. Correlated paternity within and among sibships. Genetics 168, 1601–1614 10.1534/genetics.104.027714 (doi:10.1534/genetics.104.027714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young AG, Brown AHD. 1998. Comparative analysis of the mating system of the rare woodland shrub Daviesia suaveolens and its common congener D. mimosoides. Heredity 80, 374–381 10.1046/j.1365-2540.1998.00304.x (doi:10.1046/j.1365-2540.1998.00304.x) [DOI] [Google Scholar]
- 44.Oddou-Muratorio S, Klein EK, Demesure-Musch B, Austerlitz F. 2006. Real-time patterns of pollen flow in the wild-service tree, Sorbus torminalis (Rosaceae). III. Mating patterns and the ecological maternal neighborhood. Am. J. Bot. 93, 1650–1659 10.3732/ajb.93.11.1650 (doi:10.3732/ajb.93.11.1650) [DOI] [PubMed] [Google Scholar]
- 45.Collevatti RG, Grattapaglia D, Hay JD. 2001. High resolution microsatellite based analysis of the mating system allows the detection of significant biparental inbreeding in Caryocar brasiliense, an endangered tropical tree species. Heredity 86, 60–67 10.1046/j.1365-2540.2001.00801.x (doi:10.1046/j.1365-2540.2001.00801.x) [DOI] [PubMed] [Google Scholar]
- 46.Sun M, Ritland K. 1998. Mating system of yellow starthistle (Centaurea solstitialis), a successful colonizer in North America. Heredity 80, 225–232 10.1046/j.1365-2540.1998.00290.x (doi:10.1046/j.1365-2540.1998.00290.x) [DOI] [Google Scholar]
- 47.Sampson JF. 1998. Multiple paternity in Eucalyptus rameliana (Myrtaceae). Heredity 81, 349–355 10.1046/j.1365-2540.1998.00404.x (doi:10.1046/j.1365-2540.1998.00404.x) [DOI] [Google Scholar]
- 48.Llaurens V, Castric V, Austerlitz F, Vekemans X. 2008. High paternal diversity in the self-incompatible herb Arabidopsis halleri despite clonal reproduction and spatially restricted pollen dispersal. Mol. Ecol. 17, 1577–1588 10.1111/j.1365-294X.2007.03683.x (doi:10.1111/j.1365-294X.2007.03683.x) [DOI] [PubMed] [Google Scholar]
- 49.Ellstrand NC, Marshall DL. 1985. Interpopulation gene flow by pollen in wild radish, Raphanus sativus. Am. Nat. 126, 606–616 10.1086/284442 (doi:10.1086/284442) [DOI] [Google Scholar]
- 50.Muona O, Moran GF, Bell JC. 1991. Hierarchical patterns of correlated mating in Acacia melanoxylon. Genetics 127, 619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeh JA, Zeh DW. 2001. Reproductive mode and the genetic benefits of polyandry. Anim. Behav. 61, 1051–1063 10.1006/anbe.2000.1705 (doi:10.1006/anbe.2000.1705) [DOI] [Google Scholar]
- 52.Birkhead TR, Pizzari T. 2002. Postcopulatory sexual selection. Nat. Rev. Genet. 3, 262–273 10.1038/nrg774 (doi:10.1038/nrg774) [DOI] [PubMed] [Google Scholar]
- 53.Cornell SJ, Tregenza T. 2007. A new theory for the evolution of polyandry as a means of inbreeding avoidance. Proc. R. Soc. B 274, 2873–2879 10.1098/rspb.2007.0926 (doi:10.1098/rspb.2007.0926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antonovics J. 2005. Plant venereal diseases: insights from a messy metaphor. New Phytol. 165, 71–80 10.1111/j.1469-8137.2004.01215.x (doi:10.1111/j.1469-8137.2004.01215.x) [DOI] [PubMed] [Google Scholar]
- 55.Stephenson AG. 2012. Safe sex in plants. New Phytol. 193, 827–829 10.1111/j.1469-8137.2011.04035.x (doi:10.1111/j.1469-8137.2011.04035.x) [DOI] [PubMed] [Google Scholar]
- 56.Shykoff JA, Bucheli E, Kaltz O. 1996. Flower lifespan and disease risk. Nature 379, 779 10.1038/379779a0 (doi:10.1038/379779a0) [DOI] [Google Scholar]
- 57.Kaltz O, Shykoff JA. 2001. Male and female Silene latifolia plants differ in per-contact risk of infection by a sexually transmitted disease. J. Ecol. 89, 99–109 10.1046/j.1365-2745.2001.00527.x (doi:10.1046/j.1365-2745.2001.00527.x) [DOI] [Google Scholar]
- 58.Arnqvist G, Nilsson T. 2000. The evolution of polyandry: multiple mating and female fitness in insects. Anim. Behav. 60, 145–164 10.1006/anbe.2000.1446 (doi:10.1006/anbe.2000.1446) [DOI] [PubMed] [Google Scholar]
- 59.Thompson JD. 1994. The coevolutionary process. Chicago, IL: University of Chicago Press [Google Scholar]
- 60.Pellmyr O, Huth CJ. 1994. Evolutionary stability of mutualism between yuccas and yucca moths. Nature 372, 257–260 10.1038/372257a0 (doi:10.1038/372257a0) [DOI] [Google Scholar]
- 61.Gwynne DT. 2008. Sexual conflict over nuptial gifts in insects. In Annual review of entomology, pp. 83–101 Palo Alto, CA: Annual Reviews; [DOI] [PubMed] [Google Scholar]
- 62.Tregenza T, Wedell N. 2002. Polyandrous females avoid costs of inbreeding. Nature 415, 71–73 10.1038/415071a (doi:10.1038/415071a) [DOI] [PubMed] [Google Scholar]
- 63.Hosken DJ, Stockley P. 2003. Benefits of polyandry: a life history perspective. Evol. Biol. 33, 173–194 [Google Scholar]
- 64.Bateman AJ. 1948. Intra-sexual selection in Drosophila. Heredity 2, 349–368 10.1038/hdy.1948.21 (doi:10.1038/hdy.1948.21) [DOI] [PubMed] [Google Scholar]
- 65.Wilson P, Thomson JD, Stanton ML, Rigney LP. 1994. Beyond floral Batemania: gender biases in selection for pollination success. Am. Nat. 143, 283–296 10.1086/285604 (doi:10.1086/285604) [DOI] [Google Scholar]
- 66.Ashman TL, et al. 2004. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85, 2408–2421 10.1890/03-8024 (doi:10.1890/03-8024) [DOI] [Google Scholar]
- 67.Aizen MA, Harder LD. 2007. Expanding the limits of the pollen-limitation concept: effects of pollen quantity and quality. Ecology 88, 271–281 10.1890/06-1017 (doi:10.1890/06-1017) [DOI] [PubMed] [Google Scholar]
- 68.Haig D, Westoby M. 1988. On limits to seed production. Am. Nat. 131, 757–759 10.1086/284817 (doi:10.1086/284817) [DOI] [Google Scholar]
- 69.Brown E, Kephart S. 1999. Variability in pollen load: implications for reproduction and seedling vigor in a rare plant, Silene douglasii var. oraria. Int. J. Plant Sci. 160, 1145–1152 10.1086/314198 (doi:10.1086/314198) [DOI] [PubMed] [Google Scholar]
- 70.Niesenbaum RA. 1999. The effects of pollen load size and donor diversity on pollen performance, selective abortion, and progeny vigor in Mirabilis jalapa (Nyctaginaceae). Am. J. Bot. 86, 261–268 10.2307/2656941 (doi:10.2307/2656941) [DOI] [PubMed] [Google Scholar]
- 71.Kron P, Husband BC. 2006. The effects of pollen diversity on plant reproduction: insights from apple. Sexual Plant Reprod. 19, 125–131 10.1007/s00497-006-0028-2 (doi:10.1007/s00497-006-0028-2) [DOI] [Google Scholar]
- 72.Delph LF, Havens K. 1998. Pollen competition in flowering plants. In Sperm competition and sexual selection (eds Birkhead T, Møller A.), pp. 149–173 San Diego, CA: Academic Press [Google Scholar]
- 73.Darwin C. 1876. The effects of cross- and self-fertilization in the vegetable kingdom. London, UK: John Murray [Google Scholar]
- 74.Jain SK. 1976. The evolution of inbreeding in plants. Annu. Rev. Ecol. Syst. 7, 469–495 10.1146/annurev.es.07.110176.002345 (doi:10.1146/annurev.es.07.110176.002345) [DOI] [Google Scholar]
- 75.Jarne P, Charlesworth D. 1993. The evolution of the selfing rate in functionally hermaphrodite plants and animals. Annu. Rev. Ecol. Syst. 24, 441–466 10.1146/annurev.es.24.110193.002301 (doi:10.1146/annurev.es.24.110193.002301) [DOI] [Google Scholar]
- 76.Aguirre JD, Marshall DJ. 2012. Does genetic diversity reduce sibling competition? Evolution 66, 94–102 10.1111/j.1558-5646.2011.01413.x (doi:10.1111/j.1558-5646.2011.01413.x) [DOI] [PubMed] [Google Scholar]
- 77.Karron JD, Marshall DL. 1990. Fitness consequences of multiple paternity in wild radish, Raphanus sativus. Evolution 44, 260–268 10.2307/2409405 (doi:10.2307/2409405) [DOI] [PubMed] [Google Scholar]
- 78.Janzen DH. 1970. Herbivores and the number of tree species in tropical forests. Am. Nat. 104, 501–528 10.1086/282687 (doi:10.1086/282687) [DOI] [Google Scholar]
- 79.Schmitt J, Antonovics J. 1986. Experimental studies of the evolutionary significance of sexual reproduction. IV. Effect of neighbor relatedness and aphid infestation on seedling performance. Evolution 40, 830–836 10.2307/2408467 (doi:10.2307/2408467) [DOI] [PubMed] [Google Scholar]
- 80.Griffin CAM, Eckert CG. 2003. Experimental analysis of biparental inbreeding in a self-fertilizing plant. Evolution 57, 1513–1519 [DOI] [PubMed] [Google Scholar]
- 81.Knowlton N, Greenwell SR. 1984. Male sperm competition avoidance mechanisms: the influence of female interests. In Sperm competition and the evolution of animal mating systems (ed. Smith RL.), pp. 61–84 Orlando, FL: Academic Press [Google Scholar]
- 82.Schlichting CD, Stephenson AG, Small LE. 1990. Pollen loads and progeny vigor in Cucurbita pepo: the next generation. Evolution 44, 1358–1372 10.2307/2409295 (doi:10.2307/2409295) [DOI] [PubMed] [Google Scholar]
- 83.Lankinen A, Maad J, Armbruster WS. 2009. Pollen-tube growth rates in Collinsia heterophylla (Plantaginaceae): one-donor crosses reveal heritability but no effect on sporophytic-offspring fitness. Ann. Bot. 103, 941–950 10.1093/aob/mcp014 (doi:10.1093/aob/mcp014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kress WJ. 1981. Sibling competition and evolution of pollen unit, ovule number, and pollen vector in angiosperms. Syst. Bot. 6, 101–112 10.2307/2418541 (doi:10.2307/2418541) [DOI] [Google Scholar]
- 85.Delph LF, Weinig C, Sullivan K. 1998. Why fast-growing pollen tubes give rise to vigorous progeny: the test of a new mechanism. Proc. R. Soc. Lond. B 265, 935–939 10.1098/rspb.1998.0381 (doi:10.1098/rspb.1998.0381) [DOI] [Google Scholar]
- 86.Honys D, Twell D. 2004. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol. 5, R85 10.1186/gb-2004-5-11-r85 (doi:10.1186/gb-2004-5-11-r85) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chibalina MV, Filatov DA. 2011. Plant Y chromosome degeneration is retarded by haploid purifying selection. Curr. Biol. 17, 1475–1479 10.1016/j.cub.2011.07.045 (doi:10.1016/j.cub.2011.07.045) [DOI] [PubMed] [Google Scholar]
- 88.Skogsmyr I, Lankinen A. 2002. Sexual selection: an evolutionary force in plants. Biol. Rev. 77, 537–562 10.1017/S1464793102005973 (doi:10.1017/S1464793102005973) [DOI] [PubMed] [Google Scholar]
- 89.Mitchell RJ. 1997. Effects of pollen quantity on progeny vigor: evidence from the desert mustard Lesquerella fendleri. Evolution 51, 1679–1684 10.2307/2411219 (doi:10.2307/2411219) [DOI] [PubMed] [Google Scholar]
- 90.Kalla SE, Ashman TL. 2002. The effects of pollen competition on progeny vigor in Fragaria virginiana (Rosaceae) depend on progeny growth environment. Int. J. Plant Sci. 163, 335–340 10.1086/338395 (doi:10.1086/338395) [DOI] [Google Scholar]
- 91.Charlesworth D. 1988. Evidence for pollen competition in plants and its relationship to progeny fitness: a comment. Am. Nat. 132, 298–302 10.1086/284852 (doi:10.1086/284852) [DOI] [Google Scholar]
- 92.Paschke M, Abs C, Schmid B. 2002. Effects of population size and pollen diversity on reproductive success and offspring size in the narrow endemic Cochlearia bavarica (Brassicaceae). Am. J. Bot. 89, 1250–1259 10.3732/ajb.89.8.1250 (doi:10.3732/ajb.89.8.1250) [DOI] [PubMed] [Google Scholar]
- 93.Schemske DW, Pautler LP. 1984. The effects of pollen composition on fitness components in a neotropical herb. Oecologia 62, 31–36 10.1007/BF00377369 (doi:10.1007/BF00377369) [DOI] [PubMed] [Google Scholar]
- 94.Haig D, Bergstrom CT. 1995. Multiple mating, sperm competition and meiotic drive. J. Evol. Biol. 8, 265–282 10.1046/j.1420-9101.1995.8030265.x (doi:10.1046/j.1420-9101.1995.8030265.x) [DOI] [Google Scholar]
- 95.Taylor DR, Saur MJ, Adams E. 1999. Pollen performance and sex-ratio evolution in a dioecious plant. Evolution 53, 1028–1036 10.2307/2640808 (doi:10.2307/2640808) [DOI] [PubMed] [Google Scholar]
- 96.Heslop-Harrison J. 1968. Pollen wall development. Science 161, 230 10.1126/science.161.3838.230 (doi:10.1126/science.161.3838.230) [DOI] [PubMed] [Google Scholar]
- 97.Charlesworth D, Charlesworth B, Marais G. 2005. Steps in the evolution of heteromorphic sex chromosomes. Heredity 95, 118–128 10.1038/sj.hdy.6800697 (doi:10.1038/sj.hdy.6800697) [DOI] [PubMed] [Google Scholar]
- 98.Field DL, Pickup M, Barrett SCH. 2012. The influence of pollination intensity on fertilization success, progeny sex ratio, and fitness in a wind-pollinated, dioecious plant. Int. J. Plant Sci. 173, 184–191 10.1086/663164 (doi:10.1086/663164) [DOI] [Google Scholar]
- 99.Stehlik I, Friedman J, Barrett SCH. 2008. Environmental influence on primary sex ratio in a dioecious plant. Proc. Natl Acad. Sci. USA 105, 10 847–10 852 10.1073/pnas.0801964105 (doi:10.1073/pnas.0801964105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Charlesworth D, Mank JE. 2010. The birds and the bees and the flowers and the trees: lessons from genetic mapping of sex determination in plants and animals. Genetics 186, 9–31 10.1534/genetics.110.117697 (doi:10.1534/genetics.110.117697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zahavi A. 1975. Mate selection: selection for a handicap. J. Theor. Biol. 53, 205–214 10.1016/0022-5193(75)90111-3 (doi:10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 102.Andersson M. 1982. Sexual selection, natural selection and quality advertisement. Biol. J. Linnean Soc. 17, 375–393 10.1111/j.1095-8312.1982.tb02028.x (doi:10.1111/j.1095-8312.1982.tb02028.x) [DOI] [Google Scholar]
- 103.Barrett SCH. 1992. Heterostylous genetic polymorphisms: model systems for evolutionary analysis. In Evolution and function of heterostyly (ed. Barrett SCH.), pp. 1–39 New York, NY: Springer [Google Scholar]
- 104.Marshall DL, Folsom MW. 1991. Mate choice in plants: an anatomical to population perspective. Annu. Rev. Ecol. Syst. 22, 37–63 10.1146/annurev.es.22.110191.000345 (doi:10.1146/annurev.es.22.110191.000345) [DOI] [Google Scholar]
- 105.Marshall DL, Shaner MGM, Oliva JP. 2007. Effects of pollen load size on seed paternity in wild radish: the roles of pollen competition and mate choice. Evolution 61, 1925–1937 10.1111/j.1558-5646.2007.00167.x (doi:10.1111/j.1558-5646.2007.00167.x) [DOI] [PubMed] [Google Scholar]
- 106.Korbecka G, Klinkhamer PGL, Vrieling K. 2002. Selective embryo abortion hypothesis revisited: a molecular approach. Plant Biol. 4, 298–310 10.1055/s-2002-32331 (doi:10.1055/s-2002-32331) [DOI] [Google Scholar]
- 107.Jennions MD, Petrie M. 2000. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. 75, 21–64 10.1017/S0006323199005423 (doi:10.1017/S0006323199005423) [DOI] [PubMed] [Google Scholar]
- 108.Marshall DL, Avritt JJ, Shaner M, Saunders RL. 2000. Effects of pollen load size and composition on pollen donor performance in wild radish, Raphanus sativus (Brassicaceae). Am. J. Bot. 87, 1619–1627 10.2307/2656738 (doi:10.2307/2656738) [DOI] [PubMed] [Google Scholar]
- 109.Charlesworth D, Willis JH. 2009. Fundamental concepts in genetics: the genetics of inbreeding depression. Nat. Rev. Genet. 10, 783–796 10.1038/nrg2664 (doi:10.1038/nrg2664) [DOI] [PubMed] [Google Scholar]
- 110.Charlesworth D, Charlesworth B. 1987. Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Syst. 18, 273–288 10.1146/annurev.es.18.110187.001321 (doi:10.1146/annurev.es.18.110187.001321) [DOI] [Google Scholar]
- 111.Hiscock SJ, McInnis SM. 2003. The diversity of self-incompatibility systems in flowering plants. Plant Biol. 5, 23–32 10.1055/s-2003-37981 (doi:10.1055/s-2003-37981) [DOI] [Google Scholar]
- 112.Wright S. 1939. The distribution of self-sterility alleles in populations. Genetics 24, 538–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Castric V, Vekemans X. 2004. Plant self-incompatibility in natural populations: a critical assessment of recent theoretical and empirical advances. Mol. Ecol. 13, 2873–2889 10.1111/j.1365-294X.2004.02267.x (doi:10.1111/j.1365-294X.2004.02267.x) [DOI] [PubMed] [Google Scholar]
- 114.Young AG, Brown AHD, Murray BG, Thrall PH, Miller CH. 2000. Genetic erosion, restricted mating and reduced viability in fragmented populations of the endangered grassland herb Rutidosis leptorrhynchoides. In Genetics, demography and viability of fragmented populations (eds Young AG, Clarke GM.), pp. 335–359 Cambridge, UK: Cambridge University Press [Google Scholar]
- 115.Young AG, Pickup M. 2010. Low S-allele numbers limit mate availability, reduce seed set and skew fitness in small populations of a self-incompatible plant. J. Appl. Ecol. 47, 541–548 10.1111/j.1365-2664.2010.01798.x (doi:10.1111/j.1365-2664.2010.01798.x) [DOI] [Google Scholar]
- 116.Primack RB. 1985. Longevity of individual flowers. Annu. Rev. Ecol. Syst. 16, 15–37 10.1146/annurev.es.16.110185.000311 (doi:10.1146/annurev.es.16.110185.000311) [DOI] [Google Scholar]
- 117.Spira TP, Snow AA, Puterbaugh MN. 1996. The timing and effectiveness of sequential pollinations in Hibiscus moscheutos. Oecologia 105, 230–235 [DOI] [PubMed] [Google Scholar]
- 118.Burkhardt A, Internicola A, Bernasconi G. 2009. Effects of pollination timing on seed paternity and seed mass in Silene latifolia (Caryophyllaceae). Ann. Bot. 104, 767–773 10.1093/aob/mcp154 (doi:10.1093/aob/mcp154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Klinkhamer PGL, Dejong TJ, Metz JAJ. 1994. Why plants can be too attractive: a discussion of measures to estimate male fitness. J. Ecol. 82, 191–194 10.2307/2261399 (doi:10.2307/2261399) [DOI] [Google Scholar]
- 120.Eckert CG. 2000. Contributions of autogamy and geitonogamy to self-fertilization in a mass-flowering, clonal plant. Ecology 81, 532–542 10.1890/0012-9658(2000)081[0532:COAAGT]2.0.CO;2 (doi:10.1890/0012-9658(2000)081[0532:COAAGT]2.0.CO;2) [DOI] [Google Scholar]
- 121.Armbruster WS. 1996. Evolution of floral morphology and function: an integrative approach to adaptation, constraint and compromise in Dalechampia (Euphorbiaceae). In Floral biology (eds Harder LD, Barrett SCH.), pp. 241–272 New York, NY: Chapman and Hall [Google Scholar]
- 122.Rodrigo J, Herrero M, Hormaza JI. 2009. Pistil traits and flower fate in apricot (Prunus armeniaca). Ann. Appl. Biol. 154, 365–375 10.1111/j.1744-7348.2008.00305.x (doi:10.1111/j.1744-7348.2008.00305.x) [DOI] [Google Scholar]
- 123.Mulcahy D. 1983. Models of pollen tube competition Geranium maculatum. In Pollination biology (ed. Real L.), pp. 151–161 Orlando, FL: Academic Press [Google Scholar]
- 124.Ruane LG. 2009. Post-pollination processes and non-random mating among compatible mates. Evol. Ecol. Res. 11, 1031–1051 [Google Scholar]
- 125.Herrero M, Hormaza JI. 1996. Pistil strategies controlling pollen tube growth. Sexual Plant Reprod. 9, 343–347 10.1007/BF02441953 (doi:10.1007/BF02441953) [DOI] [Google Scholar]
- 126.Herrero M. 2003. Male and female synchrony and the regulation of mating in flowering plants. Phil. Trans. R. Soc. Lond. B 358, 1019–1024 10.1098/rstb.2003.1285 (doi:10.1098/rstb.2003.1285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Castro S, Silveira P, Navarro L. 2008. Effect of pollination on floral longevity and costs of delaying fertilization in the out-crossing Polygala vayredae Costa (Polygalaceae). Ann. Bot. 102, 1043–1048 10.1093/aob/mcn184 (doi:10.1093/aob/mcn184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marshall DL, Ellstrand NC. 1985. Proximal causes of multiple paternity in wild radish, Raphanus sativus. Am. Nat. 126, 596–605 10.1086/284441 (doi:10.1086/284441) [DOI] [Google Scholar]
- 129.Epperson BK, Clegg MT. 1987. First pollination primacy and pollen selection in the morning glory, Ipomoea purpurea. Heredity 58, 5–14 10.1038/hdy.1987.2 (doi:10.1038/hdy.1987.2) [DOI] [Google Scholar]
- 130.Galen C. 1986. Floral morphology and pollen receipt in Polemonium viscosum: the shape of things to come. Am. J. Bot. 73, 667 [Google Scholar]
- 131.Lankinen A, Madjidian JA. 2011. Enhancing pollen competition by delaying stigma receptivity: pollen deposition schedules affect siring ability, paternal diversity, and seed production in Collinsia heterophylla (Plantaginaceae). Am. J. Bot. 98, 1191–1200 10.3732/ajb.1000510 (doi:10.3732/ajb.1000510) [DOI] [PubMed] [Google Scholar]
- 132.Dahl AE, Fredrikson M. 1996. The timetable for development of maternal tissues sets the stage for male genomic selection in Betula pendula (Betulaceae). Am. J. Bot. 83, 895–902 10.2307/2446267 (doi:10.2307/2446267) [DOI] [Google Scholar]
- 133.Ashman TL, Schoen DJ. 1996. Floral longevity: fitness consequences and resource costs. In Studies on floral evolution in animal-pollinated plants (eds Lloyd DG, Barrett SCH.), pp. 112–138 New York, NY: Chapman and Hall [Google Scholar]
- 134.Bell G. 1982. The materpiece of nature: the evolution and genetics of sexuality. Berkely, CA: University of California Press [Google Scholar]
- 135.Charnov EL, Maynard Smith J, Bull JJ. 1976. Why be an hermaphrodite? Nature. 263, 125–126 10.1038/263125a0 (doi:10.1038/263125a0) [DOI] [Google Scholar]
- 136.Charnov EL. 1982. The theory of sex allocation. Princeton, NJ: Princeton University Press [Google Scholar]
- 137.Charlesworth D. 1999. Theories of the evolution of dioecy. In Gender and sexual dimorphism in flowering plants (eds Geber MA, Dawson TE, Delph LF.), pp. 33–60 Heidelberg, Germany: Springer [Google Scholar]
- 138.Perry LE, Dorken ME. 2011. The evolution of males: support for predictions from sex allocation theory using mating arrays of Satittaria latifolia (Alismataceae). Evolution 65, 2782–2791 10.1111/j.1558-5646.2011.01344.x (doi:10.1111/j.1558-5646.2011.01344.x) [DOI] [PubMed] [Google Scholar]
- 139.Harder LD, Wilson WG. 1994. Floral evolution and male reproductive success: optimal dispensing schedules for pollen dispersal by animal-pollinated plants. Evol. Ecol. 8, 542–559 10.1007/BF01238257 (doi:10.1007/BF01238257) [DOI] [Google Scholar]
- 140.Harder LD, Barclay RMR. 1994. The functional significance of poricidal anthers and buzz pollination: controlled pollen removal from Dodecatheon. Func. Ecol. 8, 509–517 10.2307/2390076 (doi:10.2307/2390076) [DOI] [Google Scholar]
- 141.Kawai Y, Kudo G. 2009. Effectiveness of buzz pollination in Pedicularis chamissonis: significance of multiple visits by bumblebees. Ecol. Res. 24, 215–223 10.1007/s11284-008-0500-6 (doi:10.1007/s11284-008-0500-6) [DOI] [Google Scholar]
- 142.Barrett SCH, Jesson LK, Baker AM. 2000. The evolution and function of stylar polymorphisms in flowering plants. Ann. Bot. 85, 253–265 10.1006/anbo.1999.1067 (doi:10.1006/anbo.1999.1067) [DOI] [Google Scholar]
- 143.Lloyd DG, Webb CJ. 1992. The evolution of heterostyly. In Evolution and function of heterostyly (ed. Barrett SCH.), pp. 151–178 Berlin, Germany: Springer [Google Scholar]


