Abstract
Background
An estimated 1 million needlestick injuries (NSIs) occur in Europe each year. The Council Directive 2010/32/EU on the prevention of NSIs describes minimum requirements for prevention and calls for the implementation of local, national and Europe-wide reporting systems. The Directive is to be implemented by all EU member states by 11 May 2013. The purpose of this study was to assess (and improve) the procedures for the reporting and treatment of needlestick injuries in a German tertiary-care hospital.
Methods
We carried out a prospective observational study of the NSI reporting system in the hospital over a period of 18 months and determined the incidence of NSIs, the prevalence of blood-borne pathogens among index patients, the rate of initiation of post-exposure prophylaxis, and the rate of serological testing of the affected health care personnel.
Results
519 instances of NSI were reported to the accident insurance doctor over the period of the study, which consisted of 547 working days. 86.5% of the index patients underwent serological study for hepatitis B and C (HBV and HCV) and for the human immune deficiency virus (HIV); this resulted in two initial diagnoses (one each of active hepatitis B and hepatitis C) in the index patient. 92 of 449 index patients, or one in five, was infected with at least one blood-borne pathogen. HIV post-exposure prophylaxis was initiated in 41 health care workers. One case of hepatitis C virus transmission arose and was successfully treated. Other than that, no infection was transmitted.
Conclusion
Complete reporting of NSIs is a prerequisite for the identification of risky procedures and to ensure optimal treatment of the affected health care personnel. The accident insurance doctor must possess a high degree of interdisciplinary competence in order to treat NSI effectively.
The European Agency for Safety and Health at Work (EU-OSHA) estimates that approximately 1 million needlestick injuries (NSIs) occur in Europe each year (1). NSIs pose a serious risk of the bloodborne infections hepatitis B (HBV), hepatitis C (HCV), and HIV (2– 4). Follow-up examinations after NSIs are important for both the health care personnel affected and the patients they treat, in order to prevent any nosocomial infections or identify transmission of infections as early as possible.
EU Directive 2010/32/EU, published in June 2010, contains regulations intended to prevent needlestick and sharp injuries in hospitals and health care and sets minimum requirements for prevention. It must be incorporated into national and local legislation in all EU countries by May 11, 2013 (5). The new EU guidelines require member states to make changes to existing laws and regulations. In Germany, for example, the Ordinance on Safety and Health Protection at Work Involving Biological Agents and the Technical Biological Agents Regulations (TRBA 250) are to be reviewed (6). The EU guidelines require implementation of local, national, and Europe-wide reporting systems, for instance, in order to improve epidemiological reporting and evaluation of NSIs. Because NSIs are considerably underreported (underreporting rates of between 20% and 90% have been described in the literature [3]), major improvements in prevention are expected from an improved reporting system.
The aim of this study was to evaluate NSIs reported to accident insurance doctors and to establish their frequency, in order to obtain an overview of the infection-related and epidemiological factors in the disease burden resulting from NSIs (7).
In Germany, accident insurance doctors are physicians authorized by employers’ liability insurance associations and accident insurance providers to treat occupational and commuting accidents and who manage treatment with a specific obligation as representatives of an accident insurance company. Reporting procedures at Frankfurt University Hospital were evaluated in light of the new EU directive during the observation period, with regard to the duration of the reporting process and introduction of postexposure measures.
Methods
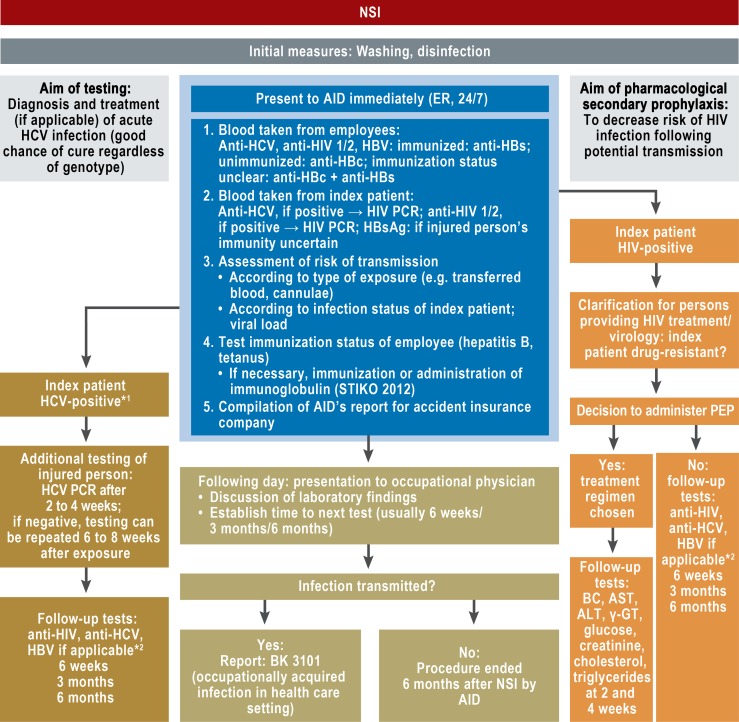
Frankfurt am Main University Hospital is a hospital that provides essential emergency care. It has 1187 beds, 4223 employees, and 3775 medical and dentistry students. In October 2010, new regulations were introduced for medical aftercare of employees following NSIs at the University Hospital. From this point onwards, there was interdisciplinary collaboration between the accident insurance doctor and occupational health service, leading to joint evaluation of NSIs (Figure 1). Where needed, the hospital’s infectious disease specialists were involved in consultation on HIV postexposure prevention (PEP), and the Institute of Medical Virology in consultation on the evaluation of virological laboratory test values.
Figure 1.
Frankfurt am Main University Hospital’s procedure for reporting to accident insurance doctor and follow-up care for needlestick injuries (NSIs)
*1HCV: if positive for anti-HCV, perform HCV PCR test to determine viral load of index patient
*2HBV: anti-HBs test at 6 weeks if affected employee’s HBV immunity is insufficient at time of NSI and booster immunization therefore administered
AID: accident insurance doctor; ER: emergency room; HCV: hepatitis C virus; HBV: hepatitis B virus; PCR: polymerase chain reaction; PEP: postexposure prophylaxis; STIKO: Standing Committee on Vaccination Recommendations (Ständige Impfkommission); BC: Blood count; AST: aspartate aminotransferase; ALT: alanine aminotransferase
A prospective observational study was conducted. It was based on accident insurance doctors’ reports and occupational follow-up examinations on the incidence of NSIs, whether index patients were infectious, employees’ hepatitis B immunization status, and the initiation of postexposure measures. Accident insurance doctors’ work was judged on the basis of the duration of individual steps in treatment. The observation period lasted 18 months (mid-October 2010 to mid-April 2012). Records of accidents were classified according to severity of injury by two study authors:
Category 1: deep scalpel or cannula cuts
Category 2: superficial injuries: puncture wounds caused by needles, dental scalers, etc. (not by cannulae)
Category 3: contamination of the mucous membranes or contact with nonintact skin.
In the event of a lack of consensus, a third examiner was consulted.
Employees receiving HIV PEP (PEP: post exposure prophylaxis) presented at the Occupational Health Service at 14 days and after the end of their HIV PEP (day 28). When they presented, subjective findings were summarized using a standardized questionnaire. The study had been granted a positive vote by Frankfurt University Hospital’s data protection officer and the Ethics Committee of the J.W. Goethe University Faculty of Medicine (Reference No. 106/12).
Definition
Needlestick injuries (NSIs) are puncture wounds, cuts, or scratches inflicted by medical instruments intended for cutting or puncturing (cannulae, lancets, scalpels, etc.) that may be contaminated with a patient’s blood or other bodily fluids. Contact of blood with nonintact skin and contact with mucous membranes (eye, mouth, nose) are also subsumed under the term “needlestick injury.”
Statistical analyses
Statistical analyses of frequency distributions were performed using Pearson’s chi-square test. A p-value <0.05 was taken to be statistically significant. Calculations of significance and 95% confidence intervals (95% CIs) were performed using the program BiAS for Windows 9.04 (Epsilon, Hochheim Darmstadt 2009).
Results
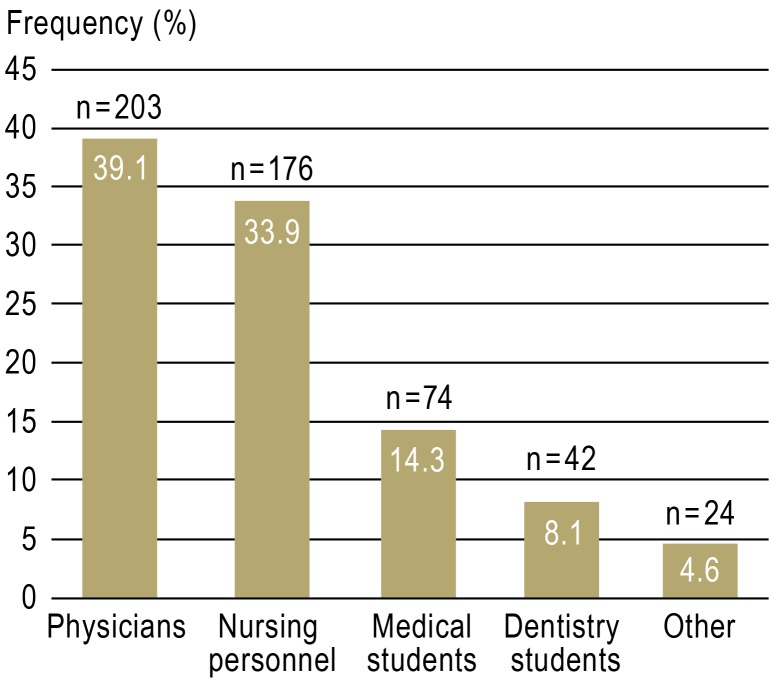
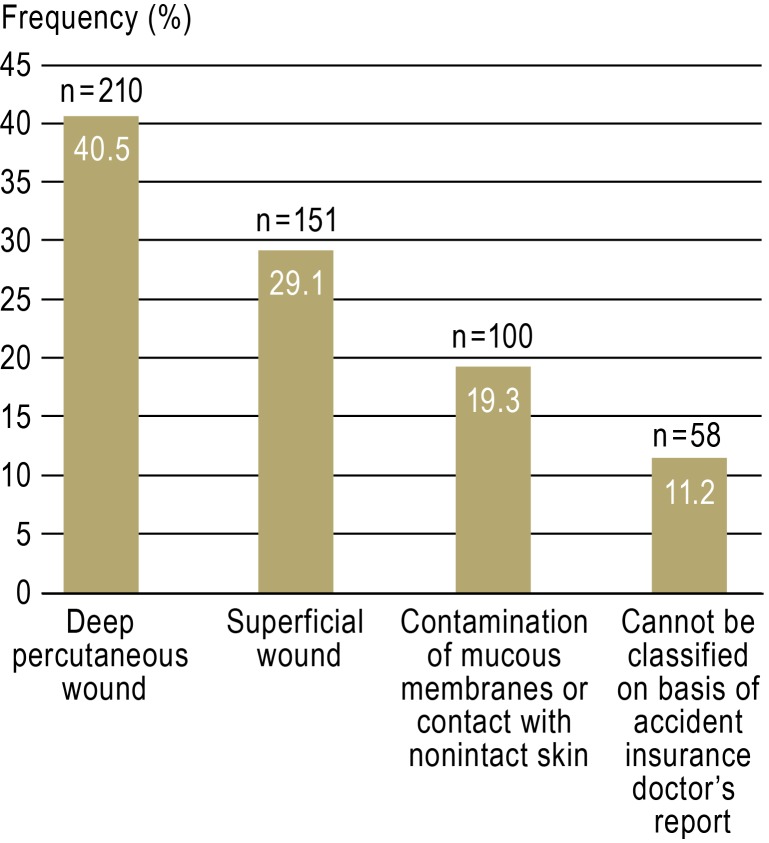
A total of 519 NSIs were reported to the accident insurance doctor over 547 working days. This corresponds to 29.2 NSIs per 100 beds per year and almost one NSI per working day. Distribution of NSIs by professional group, specialty, and severity of injury are shown in Figures 2 to 4.
Figure 2.
Distribution of reported needlestick injuries (n = 519) by professional group
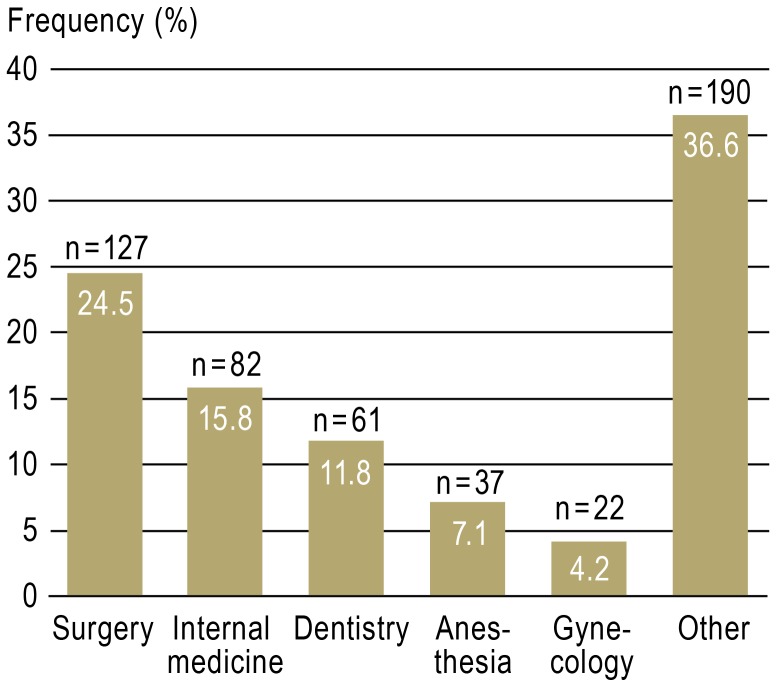
Figure 4.
Distribution of reported needlestick injuries (n = 519) by category of injury
Index patients
The index patients for 86.5% of NSIs (449 of 519) underwent serum testing for HBV, HCV, and HIV. For the remaining 13.5% of NSIs (70 of 519) the index patient was either unknown (e.g. for NSIs caused by cannulae of unknown index patients during waste disposal, e.g. with an overfull waste container, or during tidying or cleaning work) or blood testing was refused (e.g. by outpatients). Only one index patient refused blood testing. A total of 20.5% of the tested index patients (92 of 449) tested positive for at least one bloodborne infection. 11.4% of the index patients (51 of 449) were HIV-positive, 9.8% (44 of 449) had positive HCV PCR tests (PCR: polymerase chain reaction), and 3.6% (16 of 449) tested positive for active HBV infection. Of this group, 4.2% (19 of 449) had a concomitant infection, usually HCV or HIV (15 of 19).
Rare infections diagnosed in index patients included one Trypanosoma rhodesiense infection and viremic cytomegaly, Epstein–Barr, and parvovirus infections.
During the study period, HCV was transmitted to one physician. It was diagnosed 15 days after the NSI, using PCR testing (8, 9). Due to the low spontaneous cure rate of acute hepatitis C and good response to antiviral treatment in the early phase of infection, antiviral therapy was indicated (10). HCV treatment was successful, and after the end of treatment there was no longer any evidence of HCV ribonuclein acid (RNA). No further transmissions of infection were found during the follow-up period (as of October 28, 2012).
HIV postexposure prophylaxis
HIV postexposure prophylaxis (PEP) was prescribed for 41 employees. In a total of 19 index patients who tested positive to HIV RNA PCR tests, the viral load was between 20 and 7 360 000 copies/mL, and in 16 HIV-positive index patients the viral load when the NSI occurred was 20 copies/mL or fewer. Six employees who had suffered NSIs and for whom the index patient was unknown received HIV PEP due to the severity of their injuries. A total of 75.6% of HIV PEP treatments (31 of 41) involved a daily dose of tenofovir 300 mg/emtricitabine 200 mg and lopinavir 800 mg/ritonavir 200 mg, according to the recommendations of the German AIDS Society (DAIG, Deutsche AIDS-Gesellschaft) (11).
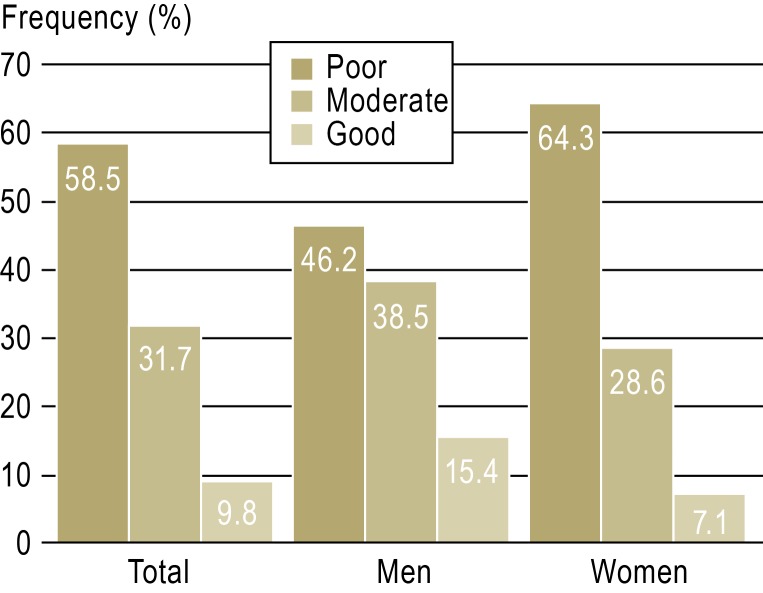
Because HIV resistance was observed in the index patient or a drug combination mentioned above was intolerable, appropriate antiviral therapy was administered to 10 employees. A total of 58.5% of the employees (24 of 41) reported that they had tolerated PEP poorly (quotes: “It was a disaster,” “I felt like I was being driven over by a bus the whole time.”); 31.7% (13 of 41) rated PEP tolerability as “moderate”; only four employees reported that they had tolerated PEP well (Figure 5).
Figure 5.
Tolerability of HIV postexposure prophylaxis in 41 employees following work-related blood contact
A total of six employees were unable to work for between one and four weeks during PEP. The most common adverse effects described were gastrointestinal symptoms (nausea, vomiting, diarrhea) and dermatological complaints (rashes, generalized pruritus). Two employees suffered scleral icterus with increased liver enzymes, and one suffered hematemesis. A total of 58.5% of those treated (24 of 41) took PEP on 28 consecutive days. 41.5% (17 of 41) interrupted treatment due to adverse effects.
The average time between NSI and the beginning of PEP was 75 minutes. The shortest time to the beginning of PEP was 10 minutes after NSI. Two employees did not present to the accident insurance doctor until the day after their NSIs, resulting in a delay in the beginning of treatment.
Hepatitis B immunization status
At the time of their NSIs, 81.7% of those affected (424 of 519) had anti-HBs =100 IU/L, and 14.5% (75 of 519) had anti-HBs between >10 IU/L and <100 IU/L. Only 3.9% (20 of 519) had anti-HBs below 10 IU/L. With the exception of one employee who refused HBV immunization, all employees with anti-HBs below 100 IU/L received HBV booster immunization within 48 hours of their NSIs.
Of the 16 employees who had suffered NSIs and for whom the index patient was HBsAg-positive, 12 had anti-HBs ≥100 IU/L and three had anti-HBs between >10 IU/L and <100 IU/L. One employee was anti-HBs-negative at the time of her NSI, following initial HBV immunization in 2003. Check-ups showed adequate response to booster immunization, and no HBV transmission was observed.
Time taken by the accident insurance doctor’s work
It was possible to analyze the time taken by the accident insurance doctor’s work for a total of 94.4% of the affected employees (490 of 519). Employees presented to the emergency room 145.3 ± 278.2 minutes (median: 39 minutes) after an NSI. The subsequent waiting time before seeing the accident insurance doctor was 52.4 ± 57.2 minutes (median: 34 minutes; minimum waiting time: 5 minutes; maximum waiting time: 560 minutes).
The length of the waiting time before seeing the accident insurance doctor varied significantly during the observation period. While the average waiting time (mean) at the beginning of the observation period was 60.7 ± 59.5 minutes, at the end of the observation period it was 31.9 ± 19.3 minutes (p <0.001).
When index patients were known to be infectious, those affected by NSIs did not present for medical care sooner; no statistically significant correlation was found between whether the index patient was infectious and time to presentation (infectious index patient versus unknown infection status: 152.4 ± 308.9 minutes versus 143.8 ± 271.9 minutes, p = 0.799).
Discussion
Approximately one in every five index patients at Frankfurt University Hospital tested positive for a bloodborne infection. Although this number of infections seems unexpectedly high at first glance (the seroprevalence of HIV, HCV, and HBV in the “normal” German population is 0.05% to 0.7% [12, 13]), it is in line with figures reported in the international literature. Data from Switzerland indicate that 12.3% of tested index patients were HCV-positive, 6.5% were HIV-positive, and 2.2% were HBV-positive (14). In a university hospital in the USA, 26% of trauma patients and 24% of nontrauma patients were HIV-positive (15). Similar figures were documented in an American emergency room as early as 1992: 24% of the tested patients tested positive for at least one bloodborne infection (16).
An earlier study by our working group had already documented higher infection rates for patients at Frankfurt University Hospital than in the “normal” population (17). Nevertheless, the infection rates it found (HIV: 4.1%; HCV: 5.8%) were significantly below the figures found in this study. An HCV seroprevalence study in Frankfurt University Hospital’s emergency room found similar figures, with an HCV prevalence rate of 3.5% (18).
Interestingly, we found a somewhat higher HBV prevalence rate (5.3%) in our seroprevalence study in 2005 to 2007 (17) than in the index patients tested here (3.6%). The increasing hepatitis B immunization coverage of the population may have contributed to this.
NSIs with index patients known to be infectious or with high-risk patients are certainly reported more frequently (19), but even NSIs with index patients known to be infectious are often not reported (20). Thus an American multicenter study found that 99% of surgeons had suffered at least one NSI during their continuing education. For 53% of these NSIs the patients were high-risk, and a total of 16% of blood contacts were not reported (19). Although no reliable figures on reporting behavior are available, it can be assumed that NSIs for which patients are known to be infectious are reported more consistently. For example, our own data from 2006/2007 showed that more than half of employees did not report their NSIs (21). This is a rate approximately three times higher than the underreporting rate for infectious index patients (16%), which is then reflected in the infection rates of index patients.
Index patients themselves may also benefit from their blood tests (22). During the study period, two patients were given initial diagnoses of hepatitis virus infection. Both received appropriate treatment for the infection.
Interestingly, in this study NSIs were not reported sooner when patients were known to be infectious than when patients’ infection status was unknown or negative. Employees did not present to the accident insurance doctor until an average of 2.5 hours after their NSIs. However, HIV PEP is only effective during a particular postexposure time window, which is limited but cannot be precisely defined. Experimental tests show HIV virus adsorption to the host cell within two hours of exposure (11). In general, HIV PEP should be performed as soon as possible (11, 23– 25).
The tolerability of antiretroviral medication during PEP in HIV-negative employees differs in an intriguing way from tolerability during HIV treatment (26). Fewer than 10% of employees in this study group reported that they had tolerated PEP well. Similar figures had already been published in 2000, by an Italian working group. More than 70% of health care personnel receiving HIV PEP complained of adverse effects; in contrast, only 11.1% of HIV-positive patients treated with the same drug combination reported adverse effects (27).
Female employees in this study population tolerated PEP worse than their male co-workers, but the difference was not statistically significant (Figure 5). A French study (26) came to a similar conclusion. However, good tolerability is required for PEP to be taken for the recommended 28 consecutive days. Older model calculations state an 81% reduction in the risk of HIV transmission as a result of HIV PEP (95% CI: 48% to 94%) (28). Case reports of HIV infections following work-related blood contact despite consistent PEP have been described (28, 29). However, no systematic analysis containing original data from large study populations has yet been published.
Limitations
Although we can draw conclusions on the basis of a large number of NSIs, this study does have some limitations. In our analysis, we refer only to NSIs reported to the accident insurance doctor; we are unable to provide figures on unreported NSIs and can only estimate the extent of underreporting or refer to previous studies (3, 14, 17). In addition, employees’ reporting behavior may have led to an overestimate of infection rates among index patients.
Summary
Despite some limitations, these data do give rise to a range of measures that are relevant to practice. Comprehensive treatment of NSIs must be given high priority. The following procedures were optimized:
Clear definition of responsibility for individual stages of work
Development of a standard operating procedure (SOP)
Interdisciplinary collaboration
Intensive communication with interfaces
Regular discussion among all those involved
Communication using the hospital’s internal media (intranet, e-mail, hospital journal).
This achieved a significant reduction of the waiting time before treatment (from 60.7 ± 59.5 minutes before the beginning of the observation period to 31.9 ± 19.3 minutes at the end of the observation period [p <0.001]).
However, the gathered data also show that employees must be given even more information and training. In particular, the need to report NSIs immediately so that HIV PEP can be started as quickly as possible if indicated must be communicated emphatically.
Statistically, the risk of transmission of infection is not high; however, the consequences of virus transmission are serious. All hospitals should therefore have an adequate, easily-accessible reporting and treatment system that is available 24 hours a day, 365 days a year. The adoption of EU Directive 2010/32/EU will lead to improved occupational health protection for medical professionals.
Figure 3.
Distribution of reported needlestick injuries (n = 519) by specialty
Key Messages.
A total of 20.5% of index patients at Frankfurt University Hospital tested positive for a bloodborne infection.
More than 90% of employees receiving HIV postexposure prophylaxis tolerated antiretroviral medication “moderately” to “poorly.”
Clear responsibility with a standardized treatment procedure and interdisciplinary collaboration allows for optimum treatment of employees.
Acknowledgments
We would like to thank Heike Kämmerer for her unfailing support in the care of affected employees.
Footnotes
Conflict of interest statement
Prof. Rabenau has received lecture fees from Mölnlycke Health Care.
PD Dr. Wicker has received reimbursement of travel and accommodation expenses and received lecture fees from B. Braun, BD, BV-Med, and pfm.
The remaining authors declare that no conflict of interest exists.
References
- 1.Europäische Agentur für Sicherheit und Gesundheitsschutz am Arbeitsplatz (OSHA-EU) Vermeidung von Verletzungen durch scharfe/spitze Instrumente am Arbeitsplatz. Available at. http://osha.europa.eu/de/sector/healthcare/prevention-sharp-injuries-workplace. Last accessed on 27 October 2012.
- 2.Henderson DK. Management of needlestick injuries: a house officer who has a needlestick. JAMA. 2012;307:75–84. doi: 10.1001/jama.2011.1828. [DOI] [PubMed] [Google Scholar]
- 3.Wicker S, Gottschalk R, Rabenau HF. Risk of needlestick injuries from an occupational medicine and virological viewpoint. Dtsch Arztebl. 2007;104(45):A 3102–A 3107. [Google Scholar]
- 4.Ridzon R, Gallagher K, Ciesielski C, et al. Simultaneous transmission of human immunodeficiency virus and hepatitis C virus from a needle-stick injury. N Engl J Med. 1997;336:919–922. doi: 10.1056/NEJM199703273361304. [DOI] [PubMed] [Google Scholar]
- 5.Richtlinie 2010/32/EU des Rates vom 10. Mai 2010 zur Durchführung der vom HOSPEEM und EGÖD geschlossenen Rahmenvereinbarung zur Vermeidung von Verletzungen durch scharfe/spitze. Amtsblatt der Europäischen Union L134/66. Instrumente im Krankenhaus- und Gesundheitssektor. [Google Scholar]
- 6.Technische Regel für Biologische Arbeitsstoffe 250. Biologische Arbeitsstoffe im Gesundheitswesen und in der Wohlfahrtspflege (TRBA 250). November 2003 edition, last revised and updated: GMBl No. 15-20, 25. April 2012, p. 380-382. Available at: www.baua.de/de/Themen-von-A-Z/Biologische-Arbeitsstoffe/TRBA/TRBA-250.html. Last accessed on 16 August 2012.
- 7.World Health Organization (WHO) Health statistics and health information systems: About the global burden of disease (GDB) project. Available at: www.who.int/healthinfo/global_burden_disease/about/en/index.html. Last accessed on 16 August 2012.
- 8.Berger A, Stürmer M, Doerr HW. Case report: risk of virus infection after accidental blood inoculation from a multi-infected AIDS patient. J Med Virol. 2012;84:897–900. doi: 10.1002/jmv.23286. [DOI] [PubMed] [Google Scholar]
- 9.Himmelreich H, Sarrazin C, Stephan C, Rabenau HF, Marzi I, Wicker S. Frühzeitige Diagnose einer Hepatitis C-Übertragung nach Nadelstichverletzung. Der Unfallchirurg, Epub ahead of print: 8 September. 2012 doi: 10.1007/s00113-012-2261-5. [DOI] [PubMed] [Google Scholar]
- 10.Sarrazin C, Berg T, Ross RS, et al. Update der S 3-Leitlinie Prophylaxe, Diagnostik und Therapie der Hepatitis-C-Virus(HCV)-Infektion, AWMF-Register-Nr: 021/012. Z Gastroenterol. 2010;48:289–351. doi: 10.1055/s-0028-1110008. [DOI] [PubMed] [Google Scholar]
- 11.Deutsche AIDS-Gesellschaft Österreichische AIDS-Gesellschaft. Postexpositionelle Prophylaxe der HIV-Infektion Deutsch-Österreichische Empfehlungen. Dtsch Med Wochenschr. 2009;134:16–33. doi: 10.1055/s-0028-1123966. [DOI] [PubMed] [Google Scholar]
- 12.Robert Koch-Institut. Virushepatitis B, C und D im Jahr 2011. Epidemiologisches Bulletin. 2012;38:371–385. [Google Scholar]
- 13.Robert Koch-Institut. HIV-Infektionen und AIDS-Erkrankungen in Deutschland. Epidemiologisches Bulletin. 2012;28:255–274. [Google Scholar]
- 14.Voide C, Darling KEA, Kenfak-Foguena A, Erad V, Cavassini M, Lazor-Blanchet C. Underreporting of needlestick and sharps injuries among healthcare workers in a Swiss university hospital. Swiss Med Wkly. 2012;142 doi: 10.4414/smw.2012.13523. [DOI] [PubMed] [Google Scholar]
- 15.Weiss ES, Cornwell EE, Wang T, et al. Human immunodeficiency virus and hepatitis testing and prevalence among surgical patients in an urban university hospital. Am J Surg. 2007;193:55–60. doi: 10.1016/j.amjsurg.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Kelen GD, Green GB, Purcell RH, et al. Hepatitis B and hepatitis C in emergency department patients. N Engl J Med. 1992;326:1399–1404. doi: 10.1056/NEJM199205213262105. [DOI] [PubMed] [Google Scholar]
- 17.Wicker S, Cinatl J, Berger A, Doerr HW, Gottschalk R, Rabenau HF. Determination of risk of infection with bloodborne pathogene following a needlestick injury in hospital workers. Ann Occup Hyg. 2008;52:615–622. doi: 10.1093/annhyg/men044. [DOI] [PubMed] [Google Scholar]
- 18.Vermehren J, Schlosser B, Domke D, et al. High prevalence of anti-HCV antibodies in two metropolitan emergency departments in Germany: a prospective screening analysis of 28,809 patients. PLoS one 7(7) doi: 10.1371/journal.pone.0041206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makary MA, Al-Attar A, Holzmueller CG, et al. Needlestick injuries among surgeons in training. N Engl J Med. 2007;356:2693–2699. doi: 10.1056/NEJMoa070378. [DOI] [PubMed] [Google Scholar]
- 20.Rabaud C, Zanea A, Mur JM, et al. Occupational exposure to blood: Search for a relation between personality and behaviour. Infect Control Hosp Epidemiol. 2000;21:564–574. doi: 10.1086/501805. [DOI] [PubMed] [Google Scholar]
- 21.Wicker S, Jung J, Allwinn R, Gottschalk R, Rabenau HF. Prevalence and Prevention of needlestick injuries among HCWs in a German university hospital. Int Arch Occup Environm Health. 2008;81:347–354. doi: 10.1007/s00420-007-0219-7. [DOI] [PubMed] [Google Scholar]
- 22.Upjohn LM, Stuart RL, Korman TM, Woolley IJ. New HIV diagnosis after occupational exposure screening: the importance of reporting needlestick injuries. Intern Med J. 2012;42:202–204. doi: 10.1111/j.1445-5994.2011.02616.x. [DOI] [PubMed] [Google Scholar]
- 23.Panlilio AL, Cardo DM, Grohskopf LA, Heneine W, Ross CS. Updated US. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis .MMWR Recomm Rep. 2005;54:1–17. [PubMed] [Google Scholar]
- 24.Puro V, Cicalini S, DeCarli G, et al. Post-exposure prophylaxis of HIV in healthcare workers: recommendations for the European setting. Eur J Epidemiol. 2004;19:577–584. doi: 10.1023/b:ejep.0000032349.57057.8a. [DOI] [PubMed] [Google Scholar]
- 25.Gerberding JL. Occupational exposure to HIV in health care settings. N Engl J Med. 2003;348:826–833. doi: 10.1056/NEJMcp020892. [DOI] [PubMed] [Google Scholar]
- 26.Tosini W, Muller P, Prazuck T, et al. Tolerability of HIV postexposure prophylaxis with tenofovir/emtricitabine and lopinavir/ritonavir tablet formulation. AIDS. 2010;24:2375–2380. doi: 10.1097/QAD.0b013e32833dfad1. [DOI] [PubMed] [Google Scholar]
- 27.Quirino T, Niero F, Ricci E, et al. HAART tolerability: post-exposure prophylaxis in healthcare workers versus treatment in HIV-infected patients. Antivir Ther. 2000;5:195–197. [PubMed] [Google Scholar]
- 28.Cardo DM, Culver DH, Ciesielski Ca, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. N Engl J Med. 1997;337:1485–1490. doi: 10.1056/NEJM199711203372101. [DOI] [PubMed] [Google Scholar]
- 29.Camacho-Ortiz A. Failure of HIV postexposure prophylaxis after a work-related needlestick injury. Infect Control Hosp Epidemiol. 2012;33:646–647. doi: 10.1086/665718. [DOI] [PubMed] [Google Scholar]