Summary
Endovascular treatment of wide neck intracranial aneurysms is technically difficult and leads to less favorable treatment results and long term outcome. We participated in a multicenter prospective study to evaluate the safety and performance of a new self-expandable nitinol micro stent (Neuroform) in stent assisted coil occlusion of wide neck intracranial aneurysms. Eighteen patients were enrolled in the study in a single center. The anatomy of the target aneurysm and the parent vessel, technical details of the procedure, device functionality, anatomic and clinical results were evaluated. All enrolled aneurysms were either wide necked or showed an unfavorable neck-to-fundus ratio. In 16 out of 18 patients the Neuroform device allowed stent assisted coil occlusion of the aneurysm. The occlusion rate was 95% in eight patients and 100% in eight patients. The two failures were both due to anatomic reasons. Flexibility of the stent, behavior during deployment and subsequent ability to retain coils within the aneurysmal sac were considered as good as or better than the properties of previous balloon expandable stents. No device-related adverse events were encountered. Procedure-related clinical complications occurred in seven patients but caused no severe permanent neurological deficit. The Neuroform neurovascular stenting system is a safe and effective adjunct for the stent-assisted coil occlusion of wide necked intracranial aneurysms. The major advantages of this device are its self-expanding property and very high flexibility which allows safe navigation, easy sizing, as well as accurate positioning of the stent while providing sufficient bridging of the aneurysm neck for subsequent coil placement.
Key words: intracranial aneurysm, electrolytically detachable coil, intracranial stent, nitinol, embolization, internal carotid artery, vertebral artery, basilar artery
Introduction
During the last decade the endovascular occlusion of saccular intracranial aneurysms with electrolytically detachable platinum coils developed to a safe and effective treatment alternative14,31. In aneurysms with a neck diameter of more than 4 mm or with a neck to fundus ratio ≥ 1:2 both the immediate risk of thromboembolic complications or coil herniation into the parent vessel during the procedure is increased and the probability of permanent occlusion of the aneurysm is reduced15,36. Coil occlusion of these wide necked aneurysms can be facilitated either by temporary balloon occlusion of the parent vessel at the site of aneurysm origin during coil insertion (remodeling)22, or by the use of coils with a spherical configuration (3D)3, or by using the dual catheter technique in order to stabilize the coil pack inside the aneurysm with a coil retention device (TriSpan)30.
A different concept is based on the creation of an artificial boundary between the parent vessel and the aneurysm by bridging the aneurysm origin with a porous stent and subsequently filling the aneurysm with coils5. Until now balloon expandable stainless steel stents were the only available option for this purpose. This method proved to function well in selected cases8,9,11,15-17,25,34.The expansion of a balloon in an intracranial vessel is, however, associated with a considerable risk of dissection and/or rupture of the parent vessel35. Even in the case of accurate sizing of balloon and stent according to the diameter of the vessel concerned· displacement and straightening of this vessel during balloon inflation can hardly be avoided. The potential hazard related to sizing and inflation injury can theoretically be avoided by the use of a self-expandable micro stent.
Until now self-expandable nitinol stents were available for peripheral· coronary and supra-aortic vessels. Recently, a self-expandable microstent using a microcatheter based delivery system was developed and became available for multicentered clinical trials after extensive bench top and animal studies. Here we report on our first clinical experiences with this self-expandable microstent for the treatment of wide necked intracranial aneurysms.
Device and Procedure
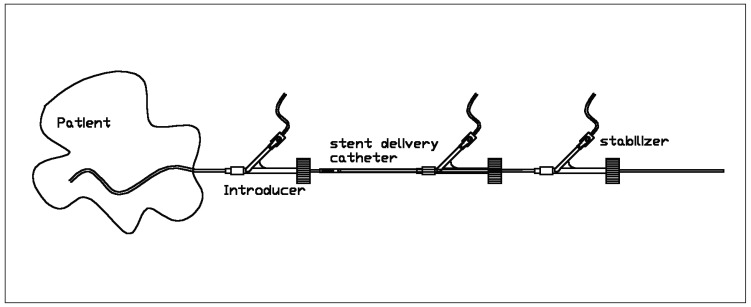
The Neuroform neurovascular stenting system has been developed and produced by SMART Therapeutics (San Leandro, California, USA). The device consists of three components: the self-expanding neurovascular stent, a 3F stent delivery microcatheter, and an over-the-wire stabilizer (figure 1).
Figure 1.
Schematic drawing of the Neuroform stent device.
The stent is comprised of six to eight connected cells. These cells are designed in a way that exploits a shape-memory-effect. The stent is currently available in 3 (3.75), 3.5 (4.25), 4 (4.75) and 4.5 (5.25) mm nominal diameters (numbers in parentheses indicate the maximum diameter of the stent in mm in an unrestrained condition) and in 15 mm as well as 20 mm nominal lengths. The stent struts are essentially radiolucent. The stent has four radio-opaque markers on each end (distal and proximal). In the unexpanded state, these markers denote the position of the stent within the microcatheter delivery system. As the stent is deployed, the markers move apart. When the stent is fully expanded, the markers denote the outer diameter of the expanded stent within the parent vessel. The self-expanding stent is packaged preloaded into the microcatheter delivery system. The stent delivery catheter has an outer diameter of 0.39” and an inner diameter of 0.26”. The distal tip of the stent catheter carries a radioopaque marker. The stent delivery catheter is compatible with .050” ID and larger introducing catheters. It has a see-through distal tip and is packaged with a peel away micro sleeve positioned within the central lumen of the stent for aiding in the passage of a guidewire through the stent. The stabilizer is essentially a second microcatheter with an outer diameter of 0.25” and an inner diameter of 0.17”. The stabilizer has one radio-opaque marker at the distal tip. The resulting inner diameter of the whole system (i.e., mounted stent, stent catheter and stabilizer) accepts a 0.014 inch guide wire. Both stent catheter and stabilizer are equipped with a luer lock hub, which allows continuous flushing of both lumina during insertion of the system.
The device is assembled in the sterile field prior to use by inserting the stabilizer into the proximal end of the microcatheter containing a preloaded stent. The stent loaded microcatheter and stabilizer assembly is positioned in the intra-cranial vasculature over an exchange length .014” guidewire. The peel-away introducer sleeve facilitates back loading the assembly over the guidewire. Prior to deployment, the stabilizer's distal tip is positioned adjacent to the proximal end of the stent. The microcatheter is then retracted, while the stabilizer holds the stent stationary, relative to the patients' anatomy. This maneuver deploys the device across the target lesion, by unsheathing the self-expanding stent.
Material and Methods
A multicenter prospective study was designed to evaluate the safety and performance of this self expanding micro-stent during the stent-assisted coil occlusion of wide necked intracranial aneurysms. The inclusion criteria required the target lesion to be a wide necked,ruptured or unruptured saccular intracranial aneurysm. A wide neck was defined as a dome-to-neck ratio of < 2 and/or a neck length of ≥ 4 mm, not exceeding a neck diameter of 12 mm. The diameter of the parent vessel was required to be in the range of 1.5 - 5.5 mm. All measurements were based on pretreatment digital subtraction angiograms.
The German institutional review boards of Freiburg and Essen approved the study protocol. All patients signed informed consent. From August through November 2001, we enrolled 18 patients with wide necked saccular intracranial aneurysms. There were one male and 17 female patients; the age range was 35 to 79 years. Demographic data, clinical presentation, and procedural details are shown in table 1.
Table 1.
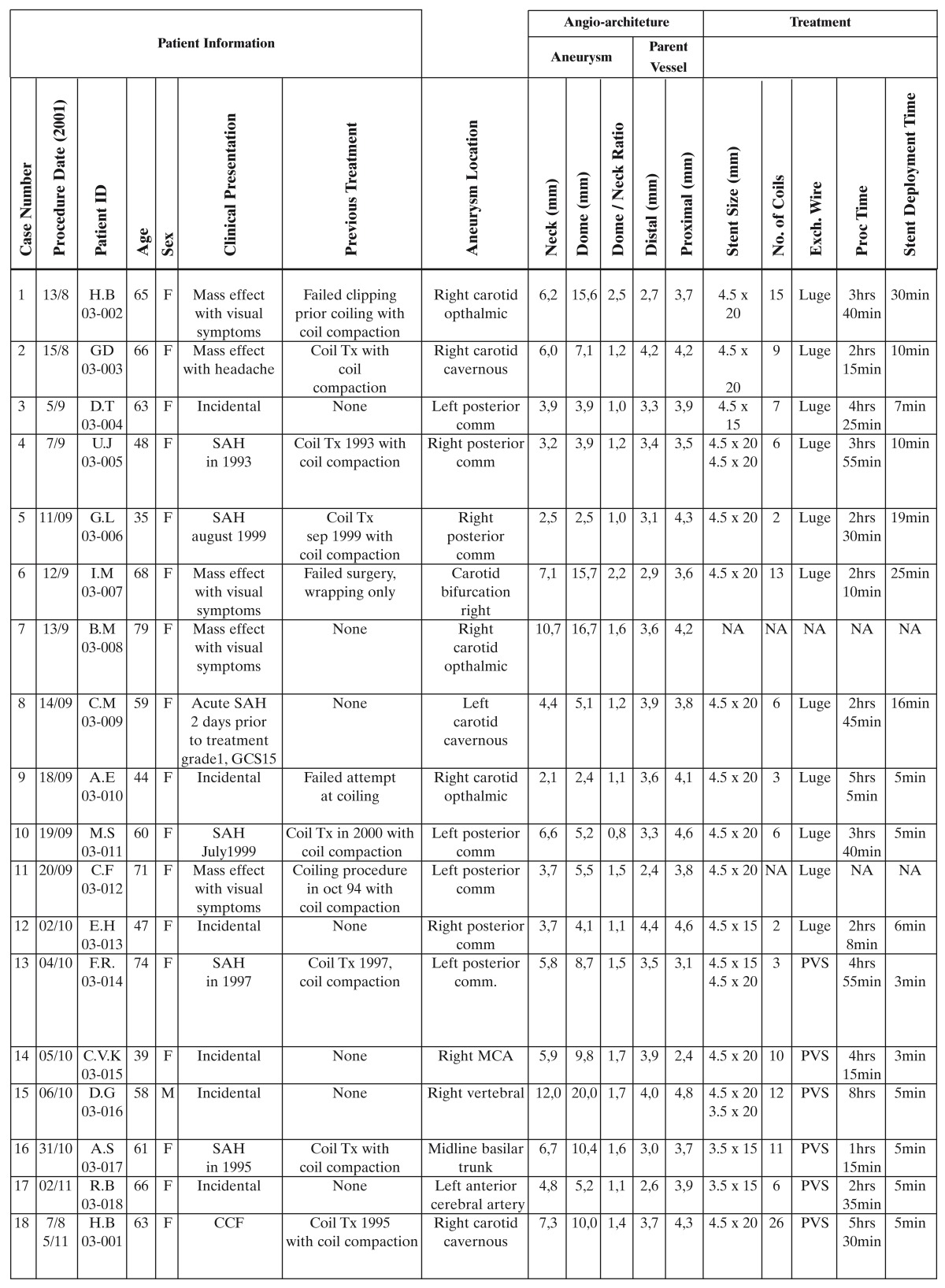
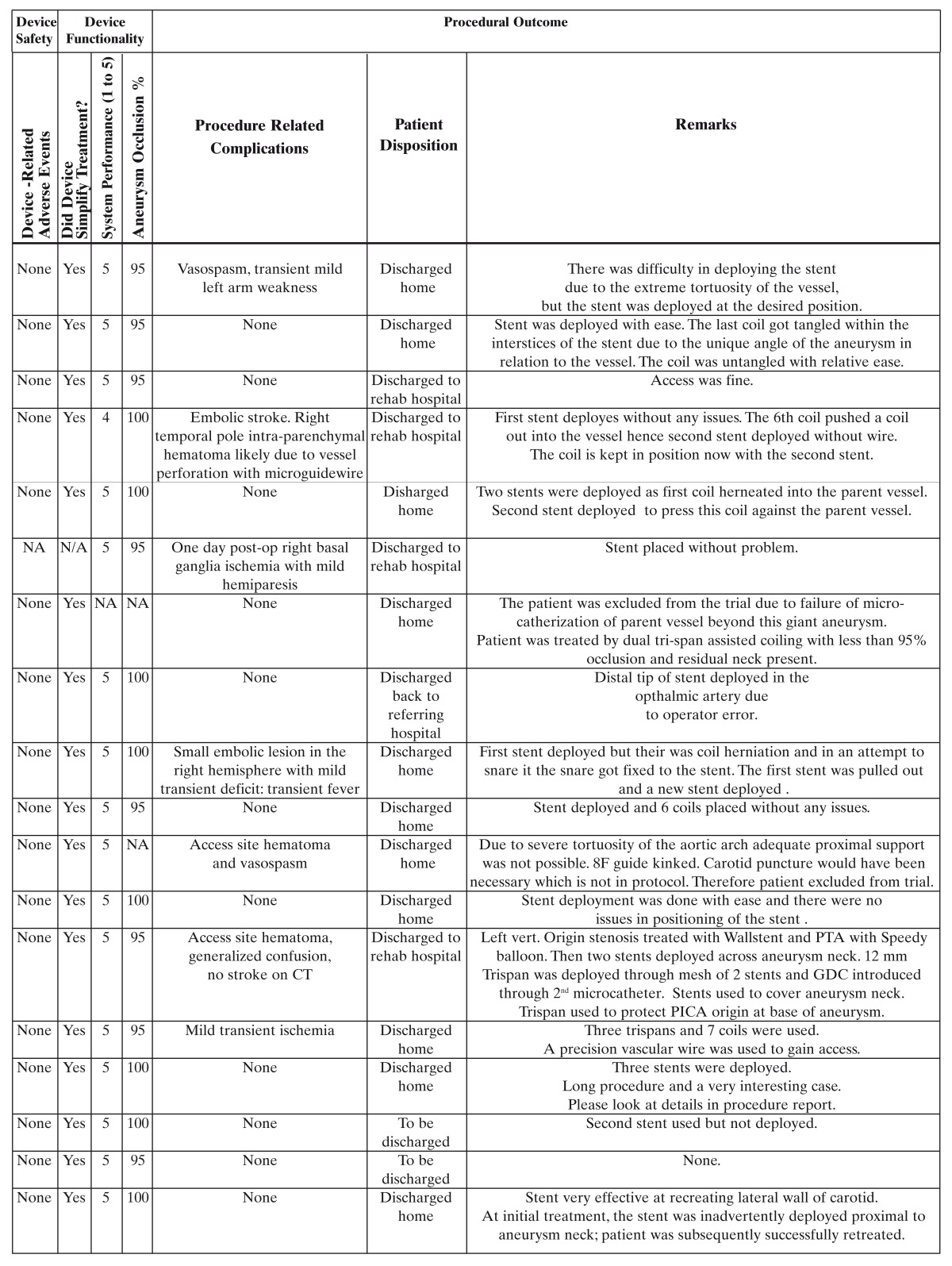
Treatment Methods
Medical pretreatment was initiated at least one day prior to the procedure with 300 - 500 mg of aspirin and 225 mg of clopidogrel or 500 mg of ticlopidine. All patients were treated under general anesthesia. A bolus of 5,000 IU of heparin was administered after the insertion of a 90 cm 6 F introducer sheath and 1,000 IU of heparin was given every hour during the procedure.
We used a biplane DSA system (Neurostar, Siemens) and defined two working projections, one which best portrayed the parent vessel for stent placement and one which optimally showed the aneurysm neck for coil placement. Through the 6 F guiding catheter a 0.014” microcatheter was placed coaxially beyond the location of the aneurysm. Subsequently the microcatheter was exchanged over a 300 cm 0.014” guidewire. Then the stent delivery system was introduced over this guidewire with continuous pressurized flushing with heparinized saline solution. The stent delivery system was advanced until the aneurysm neck was bridged by the stent as defined by the position of its radio-opaque distal and proximal markers. In case of tortuous parent vessels the stent was deployed by advancing the delivery system beyond the aneurysm and subsequently slowly pulling back while unsheathing the stent to bridge the aneurysm neck. After full expansion of the distal marker bands further unsheathing continued from a distal to proximal direction in order to ensure that the stent conformed ideally to the contour of the parent vessel.
After stent deployment the delivery system and guidewire were removed. Subsequently a dual marker microcatheter was introduced over a guidewire to the aneurysm neck. Attention was paid to ensure an atraumatic crossing of the proximal stent markers. The aneurysm fundus was accessed through the stent interstices in order to allow the introduction of electrolytically detachable coils. Initial basket formation was achieved by using a variety of coil geometries including GDC 3D (Target Therapeutics, San Jose, CA, USA) as well as TriSpan coil configurations (Target Therapeutics, San Jose, CA, USA). EDC supersoft and fibered coils (Dendron, Bochum, Germany) were used to optimize a dense coil packing. We attempted to completely fill the aneurysm sac by coiling down to the aneurysm neck, which was defined by the interface created by the stent within the parent vessel.
After immediate post-treatment control angiography, instrumention was removed and hemostasis achieved. Post-procedure, patients were maintained on full heparinization for at least 24 hours. Antiplatelet therapy was continued for 30 days using both aspirin and clopidogrel or ticlopidine. Thereafter only aspirin was administered.
In the current protocol, follow-up clinical and angiographic evaluations are scheduled at six months post treatment.
Stent Sizing and Guidewire Selection
Unlike balloon expandable stents, accurate diameter sizing of the parent vessel is not critical to the safe deployment of the self-expanding micro-stent Neuroform. In general the self-expanding micro-stent diameter chosen should be at least 0.5 - 1 mm larger than the target vessel. The ability to access the target location with the stent delivery system was relatively independent of stent length and diameter. Therefore, the selection of stent length ensured a minimum of 5 mm proximal and distal overlap of the aneurysm neck.
The combination of this overlap and the self-expanding nature of the stent were adequate to anchor the device properly at the target location. To facilitate access to the target location with the stent delivery system, the exchange length micro-guidewire characteristics should minimize distal stiffness and maximize flexibility. High support exchange guidewire systems actually hinder smooth delivery by straightening the parent vessel and adding friction to the system.
Evaluation Procedure
The treatment results were evaluated on the basis of pre-treatment digital subtraction angiograms, procedural technical device performance, as well as post-treatment control digital subtraction angiograms.
Aneurysm and parent vessel dimensions were established based on calibrated external fiducial markers. Device performance evaluation was based on a discrete grading scale from 1 - 5 of the following parameters: ability of the stent delivery system to track over the guidewire, maneuver through tortuous vessel anatomy, stent positioning accuracy at target location, ease and accuracy of stent deployment, ease of placement of the microcatheter through the stent interstices, ease of coil deployment into the aneurysm, ability of the stent to prevent coil herniation into the parent vessel, overall system reliability and performance, and the ability of the stent to simplify aneurysm treatment.
Post-treatment angiograms were evaluated for degree of aneurysm occlusion, presence of stent associated thrombus, and presence of parent vessel dissection or coil herniation. The determination of the degree of aneurysm occlusion was based on a three-tiered scale composed of a rating of 100% indicating complete occlusion, 95-99% indicating subtotal occlusion with residual neck or dome remnant or minor contrast opacification of coil interstices, less than 95% indicating only partial aneurysm occlusion. An independent interventional neuroradiologist double read all anatomic measurements, post-treatment angiographic evaluations and determination of percent aneurysm occlusion.
Device Sources
Guiding catheters used were 6 F, 90 cm Cordis Envoy (Cordis Neurovascular, Miami Lakes, FL, USA). Microguidewires used were 0.014”, 300 cm Choice PT extra support (Boston Scientific Corporation, Natick, MA, USA), 0.014”, 300 cm Luge moderate support (Boston Scientific Corporation, Natick, MA, USA), 0.014”, 300 cm Synchro (Precision Vascular Systems, West Valley City, UT, USA). Microcatheters used were Excel14 and Excelsior 14 (Target / Boston Scientific Corporation, Natick, MA, USA), Prowler14 (Cordis Neurovascular, Miami, FL, USA). Intracranial stents used were Neuroform neurovascular stenting system (Smart Therapeutics Inc., San Leandro, CA, USA). Electrolytically detachable coils used were mainly EDC I and II systems (Dendron GmbH, Bochum, Germany) as well as GDC 3D and TriSpan Coils (Target Therapeutics / Boston Scientific Corporation, Natick, MA, USA).
Results
The clinical characteristics and procedural outcome data of each patient, details of angioarchitecture, treatment, safety and functionality of the Neuroform stent device are summarized in table 1.
Six out of 18 treated aneurysms were incidental findings, five aneurysms had caused clinical symptoms due to their space occupying effect and seven aneurysms had previously ruptured. Only one of the ruptured aneurysms was treated during the acute phase after subarachnoid hemorrhage.
Stent assisted coil occlusion was the first treatment in seven aneurysms. Eleven patients had failed prior treatment attempts: previous coil occlusion was followed by coil compaction and recurrent contrast opacification of the aneurysm requiring retreatment in eight patients; failed clipping with subsequent coil occlusion and coil compaction in one patient, failed clipping with subsequent wrapping in one patient, and failed coiling attempt in one patient.
The 18 treated aneurysms were located as follows: carotid cavernous (three), carotid paraophthalmic (three), carotid / posterior communicating artery (seven), carotid bifurcation (one), anterior cerebral artery (one), middle cerebral artery (one), vertebral artery (one), basilar artery (one). The neck diameter was ≥ 4 mm in 12 aneurysms. In those six aneurysms with a neck width of less than 4 mm the dome-to-neck ratio was ≤ 1.5. The diameter of the parent vessel ranged between 2.4 and 4.8 mm. According to the diameter of the parent vessel and the aneurysm neck characteristics, stents of the following sizes were deployed: 3.5 × 15 (2), 3.5 × 20 (1), 4.5 × 20 (15), 4.5 × 15 (3).
Two patients were enrolled but excluded from the study. In one patient there was a wide necked paraophthalmic aneurysm of the convex curve of the ICA in continuation of the axis of the cavernous segment. This anatomy prevented safe distal catheterization of the parent vessel even prior to stent insertion. The other patient had extreme tortuosity of the aortic arch, which resulted in recurrent kinking of the guide catheter and hampered the navigation of the stent delivery catheter, and direct puncture of the internal carotid artery was not allowed in the study protocol. Three patients had more than one Neuroform stent deployed in the parent vessel. Additional measures after Neuroform stent deployment such as balloon-expandable stents and balloon remodeling and parent vessel occlusion were not necessary in any enrolled patient.
The total procedure time and to a lesser extent the stent deployment time were largely influenced by anatomical details, the shortest total procedure time being 75 minutes. Endovascular coil insertion after stent deployment achieved an occlusion rate of 95% in eight patients and 100% in eight patients. The number of coils necessary to occlude the aneurysm ranged from two to 26. It was observed that the stent deployment significantly facilitated the aneurysm treatment in all 16 patients.
In 11 patients no technical or clinical complications occurred. No adverse event directly related to the Neuroform stent was encountered in the study. Three patients experienced adverse events related to guidewire manipulation, two patients experienced vasospasm, one of which had associated transient arm weakness, another patient suffered a parenchymal hematoma. Distal embolism evident from diffusion weighted MRI was encountered in three patients, associated with a hemiparesis in one patient and transient focal deficits in two patients. One patient experienced prolonged post-procedural confusion without evidence of cerebral infarction.
The following cases illustrate the use of the Neuroform neurovascular stenting system in stent assisted coiling of wide neck aneurysms.
Illustrative Cases
Case 1 RB-03-018
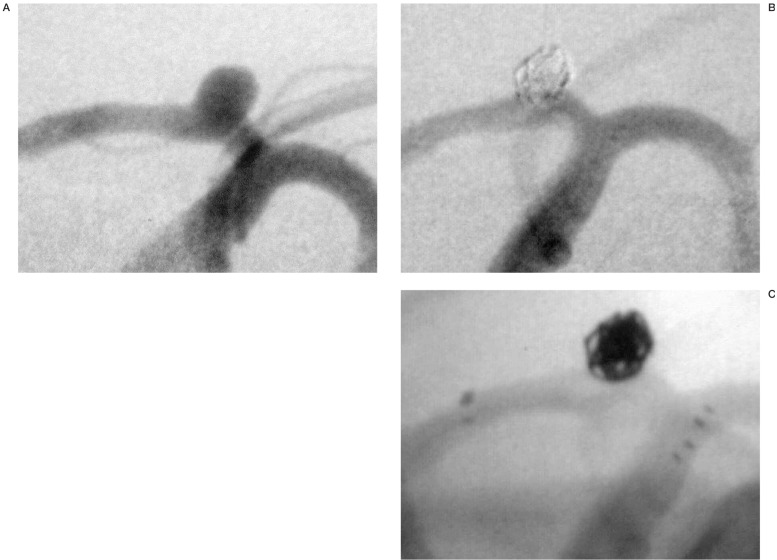
During the work-up for recurrent headache, an incidental aneurysm arising from the left A1 segment (fundus and neck about 5 mm) was found in this 66 year-old-patient (figure 2A). Under general anesthesia a 90 cm 6F Envoy guiding catheter was inserted into the left ICA. The left ACA was catheterized with a microcatheter (RapidTransit) and a Synchro 0.014” microguidewire was exchanged into the distal ACA. Over this wire a Neuroform stent (3.5 mm × 15 mm) was introduced and deployed from the A1 segment to the distal segment of the ICA. Leaving the guidewire in place in order to stabilize the deployed stent, the stent catheter and the stabilizer were removed. Through the same guiding catheter, a Prowler-10 microcatheter was introduced. The aneurysm was subsequently catheterized without difficulty and occluded with six electrolytically detachable coils. The initial coil was a 3 mm GDC 3D Tracker-10. With the aneurysm about 95% occluded (figures 2B,C), the patient was discharged asymptomatic.
Figure 2.
Wide-necked, incidental aneurysm of the left A1 segment (A). The aneurysm was bridged with a Neuroform stent and subsequently occluded with coils (B). The unsubtracted image (C) shows the position of the radio-opaque stent markers in the ICA bifurcation and in the A1 segment.
Case 2 CVK-03-015
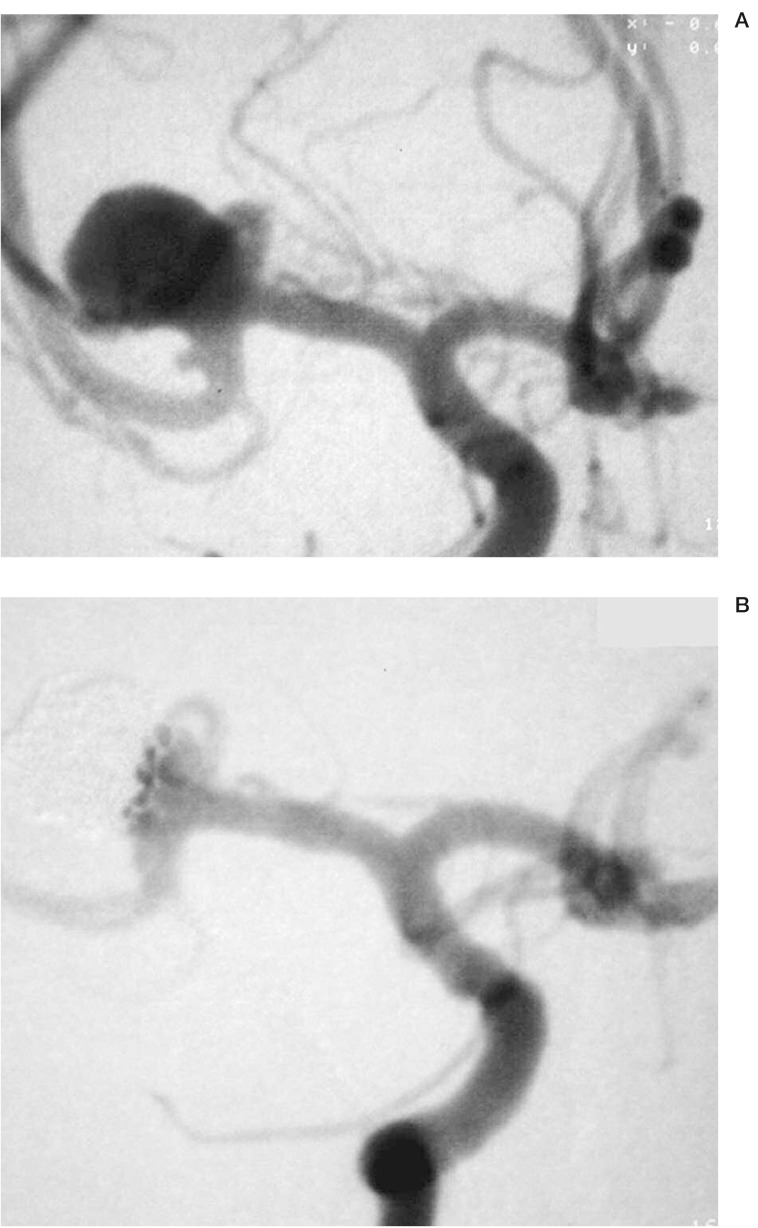
This 39-year-old woman had an incidental aneurysm of the right MCA bifurcation with a 10 mm fundus and a 6 mm neck. The patient refused surgical clipping and was therefore referred for endovascular treatment. Under general anesthesia the right ICA was catheterized with a 90 cm 6F Envoy. On the inferior division of the MCA, a second small aneurysm with 1-2 mm diameter was found. A Synchro 0.014” micro guidewire was positioned distally within the MCA and a Neuroform stent was deployed from the inferior division of the MCA distally to the M1 segment proximally. A RapidTransit microcatheter was used to catheterize the aneurysm. During the subsequent insertion and electrolytic detachment of seven EDC II coils, herniation of coil loops towards the superior division of the MCA, which was not protected by a stent, was noted. A total of three TriSpan coils were used to reposition these displaced coil loops. To reposition coil loops away from the MCA bifurcation, the microcatheter was pulled back to the level of the Neuroform stent and the TriSpan coil was inserted and opened in this position. With the TriSpan wings fully open, the microcatheter together with the TriSpan was advanced forward pushing the coil loops away from the origin of the superior division of the MCA. Intermingling of the displaced coil loops and the TriSpan wings allowed for proper repositioning of these loops. During this part of the procedure it was evident that the stent in the inferior MCA division provided significant stability for the TriSpan device. The final degree of aneurysm occlusion was rated as 95%. The small aneurysm on the inferior MCA division was no longer opacified after stent deployment into the parent vessel. Immediately after the treatment, a mild left sided hemiparesis was observed, which resolved completely within the following 12 days.
Case 3 HB-03-001
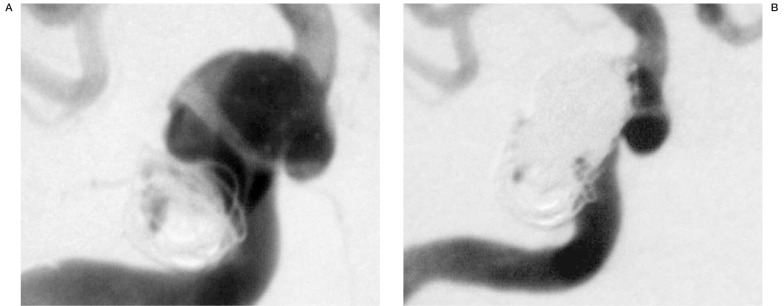
This 63-year-old woman presented in 1995 with chemosis and ocular bruit consistent with a carotid-cavernous sinus fistula. Further evaluation revealed a ruptured aneurysm, originating from the cavernous segment of the right ICA. At the time of the first treatment, the arteriovenous fistula was already thrombosed. The remaining aneurysm had a fundus diameter of about 12 mm and a neck width of 5 mm. Due to the broad aneurysm neck incorporating the lateral wall of the ICA in the cavernous segment, densely packed coil occlusion of the aneurysm was not possible. The patient did well after initial treatment. Follow-up angiography showed recurrent filling of the aneurysm due to coil compaction (Fig. 4a). Stent-assisted coil-occlusion was attempted to achieve better aneurysm occlusion.
Figure 3.
Wide-necked aneurysm of the right MCA bifurcation and a small aneurysm originating from the inferior division of the same vessel (A). Stent deployment from the M1 segment into the inferior division of the MCA and subsequent stent and TriSpan assisted coil occlusion of the MCA bifurcation aneurysm resulted in complete occlusion of the small M2 aneurysm and in subtotal occlusion of the MCA bifurcation aneurysm (B).
Figure 4.
Cavernous aneurysm of the right ICA with recurrent filling due to coil packing six years after initial treatment (A). Stent deployment over the aneurysmal neck facilitated significantly the coil occlusion of the aneurysm. Complete occlusion of the aneurysm was possible despite the complex geometrical relationship between aneurysm and parent vessel. Prior endovascular treatments were restricted by the severe tortuosity of the proximal ICA. This vessel was straightened by coaxial placement of two self expanding Carotid Wallstents.
Under general anesthesia a 90 cm 6F Envoy guiding catheter was introduced into the right ICA. During insertion of a microcatheter into the right MCA, significant difficulty was encountered due to the severe tortuosity of the ICA. A Choice PT extra support 0.014” microguidewire was finally brought to the MCA. A Neuroform stent (4.5 mm × 20 mm) was navigated into the cavernous segment. Stent deployment was hindered due to the very tortuous ICA and the selection of too stiff a microguidewire. The stent was deployed just proximal to the aneurysm origin. It was decided to wait several weeks until a second treatment attempt in order to allow for endothelialization of the malpositioned stent.
Based on the experience from the use of balloon expandable stents, it was decided to straighten and enforce the proximal segment of the right ICA, prior to stent assisted coiling, by deploying self-expandable Carotid Wallstents. Through an 8F BriteTip guiding catheter, a Choice PT extra support 0.014” guidewire was inserted into the petrous segment of the ICA. A first Carotid Wallstent (7 mm × 50 mm) and distal to this with some overlap a second Carotid Wallstent (5 mm × 30 mm) were deployed into the ICA. Subsequently, a Synchro 0.014” microguidewire was inserted into the right MCA. Over this wire a Neuroform stent (4.5 mm × 15 mm) was deployed without difficulty across the aneurysm origin. The aneurysm was then catheterized with a Prowler-10 microcatheter and completely occluded with 26 elec-trolytically detachable coils (EDC II) (figure 4B). The lateral wall of the ICA in the cavernous segment was reconstructed by the mesh of the Neuroform stent and served as an effective barrier preventing coil herniation into the ICA and enabling dense coil packing of the aneurysm volume. The procedure was well tolerated and the patient was discharged home four days after treatment.
Discussion
Possible indications for the deployment of microstents in intracranial vessels are the balloon dilation of atherosclerotic stenoses 6,11,26, endovascular occlusion of arteriovenous fistulae 35, and stent assisted coil occlusion of wide necked aneurysms 19,20,28,29,32,33. Rare conditions treatable by stent deployment are vessel dissections1,18,21 and herniations of coil loops into the parent vessel during coil occlusion of intracranial aneurysms4,12. Stent-assisted coil occlusion of wide necked intracranial aneurysm will be the most frequently required procedure among the above mentioned indications. Recent studies have shown a relatively high frequency of neck remnants and recanalization of the aneurysmal sac adjacent to the neck after endovascular coil occlusion of wide necked aneurysms 7.
Balloon remodeling 22 (i.e., temporary occlusion of the parent vessel and the aneurysm neck with a non-detachable balloon during aneurysm coiling through a second microcatheter) has the obvious advantage of avoiding a permanent intravascular implant and the associated antiplatelet medication. The lowpressure inflation of a soft balloon during the remodeling procedure may cause less injury to the vessel wall than balloon deployment of a stent (4-6 atm). However, the risks associated with transient interruption of blood flow within the parent vessel during balloon remodeling and the benefits of endovascular reconstruction at the site of aneurysm origin are strong arguments in favor of stent assisted coil occlusion.
Some authors achieved good results with balloon expandable stent assisted coil occlusion of intracranial aneurysms11, while others encountered significant complications related to these devices 35, mostly due to dissection or rupture of the parent vessel. Other concepts for the treatment of wide necked intracranial aneurysms based on the use of liquid embolic agents 23,27 or covered stents 24 have been proposed, but are still experimental procedures.
The hemodynamic effect of a porous stent bridging the neck of an aneurysm is insufficient to alone induce thrombosis of the aneurysmal sac11. This is due in part to the high porosity of available stents and required use of anticoagulation as well as antiplatelet medication. However, stent-assisted coiling may allow for more dense filling of an aneurysm with coils and a stent mesh across the neck of an aneurysm may change blood flow in such a way that coil compaction is prevented. Clearly, these remain theoretical considerations until long term outcome data become available. With high porosity stents however, the risk of stent induced occlusion of lateral branches and perforating vessels seems to be small6,10. Intimal hyperplasia within several months 2 of balloon expandable stent placement is rare but seems possible in the intracranial vasculature 11.
Self-expandable microstents represent a new alternative for the stent-assisted treatment of wide necked aneurysms in order to prevent recanalization and to facilitate retreatment of recurrent aneurysms. From the results of our initial experiences with the Neuroform stent it appears that deployment and self-expansion do not harm the wall of the parent vessel. The flexibility of the Neuroform device enables access to distal intracranial vessels, even in cases of tortuous anatomy. In selected cases, straightening of tortuous supra-aortic vessels with selfexpandable carotid stents is of additional help. In contrast to balloon expandable intracranial stents, the maneuvering of the Neuroform stent is excellent especially with soft and flexible micro guidewires. In addition, the use of the Neuroform stent does not require far distal advancement of the guidewire. We encountered three guidewire-related complications (one hematoma, vasospasm in two patients) in the beginning of the study, before we recognized this advantage of the Neuroform stent and delivery system. Based on the current experience the Neuroform stent can safely be deployed as distally as the MCA bifurcation over a soft, flexible exchange length microguidewire.
The deployment of the Neuroform stent by unsheathing a pliable self-expanding mesh allows exact placement even in tight vessel loopswithout the risks related to vessel straightening seen with balloon expandable stents. The design of the Neuroform stent with thin struts and relatively wide interstices avoids inadvertent compromise of small side branches and perforators adjacent to the aneurysm neck and provides an elastic, low profile implant that should be better tolerated by the host vessel.
The advantages of the Neuroform stent design in terms of flexibility, maneuverability, and low surface coverage represents a theoretical compromise in radial force and rigidity of the stent during catheterization of the aneurysm and coil packing. We observed stent displacement or coil protrusion in two patients. Leaving the exchange wire in place after stent delivery can minimize displacement of the stent during catheterization of the aneurysm. Another solution is the bridging of the aneurysm with more than one stent. This strategy proved successful in two patients, where the aneurysm was located at the convexity of tight vessel curves. Coil protrusion through the interstices of the stent in small aneurysms can also be avoided by selecting a first coil with a helical diameter of 3 mm or more and by selecting the softest possible coils for packing close to the aneurysm neck.
The regimen of medical pre- and post-treatment for this study was adopted from previous studies using balloon expandable stents. It remains to be evaluated whether long-term antiplatlet medication is also necessary for the Neuroform stent design. The implantation of such a low profile filigree device in non-atherosclerotic vessels might well be tolerated without long term antiplatlet therapy.
Conclusions
The ideal neurovascular stent to assist coil occlusion of intracranial aneurysms should meet certain critical requirements. The profile and flexibility of the stent and delivery system should be sufficient to allow access to the target site even through the most tortuous vascular anatomy. The stent deployment should be possible with a maximum of accuracy and predictability, avoiding any injury to the vessel wall. Sufficient radio-opacity is required to determine the position of the stent and the plane of interface between parent vessel and aneurysm neck. Proven biocompatibility, high resistance to corrosion and mechanical fatigue as well as low thrombogenicity is required from any long-term neurovascular implant. Stent deployment and long term implantation should result in minimal intimal hyperplasia at the implant site.
Stent bridging of the aneurysm neck resulting in hemodynamic isolation of the aneurysm sac from the circulation without occlusion of adjacent normal vessels would be the most physiologic treatment. As a compromise, a deployed stent should create a border between parent vessel and aneurysm which is firm enough to keep detachable coils within the aneurysm.
Since measurement of the diameter of intracranial vessels with sub millimeter accuracy is difficult to achieve, sizing of the implant should be as simple and forgiving as possible, with an one-size-fits-all concept as an ideal solution. Sizing of an ideal stent should address the possibility that the parent vessel at the target site may vary in its diameter.
Overall, the neuro-endovasular implant should aim to reconstruct the parent vessel at the site of parent vessel pathology, i.e. the aneurysm neck. In addition, the device profile, geometry, and mechanical characteristics should enable the device to conform to the local angio-architecture; simultaneously, the device should be sufficiently supple and elastic to optimize its anatomic and physiologic acceptance by the host vessel.
The self-expandable Neuroform stent for the stent-assisted coil occlusion of intracranial aneurysms is the first step towards such an optimized implant. Compared with the balloon expandable stents available to date, the Neuroform stent is superior in terms of flexibility, ease of sizing, safety of deployment and radioopacity.
The ability to keep coils within an aneurysm seems equal for both types of stents. The ability to achieve stent characteristics of low profile, high flexibility and branch vessel perfusion are at odds with the ability to maximize stent mesh density across the aneurysm neck. For this reason both balloon-expandable and self-expandable stents always will be subject to a compromise regarding these competing characteristics. The data presented in this prospective study demonstrate the acute safety and technical feasibility of self expanding Neuroform microstent assisted coiling of a variety of intracranial wide necked aneurysms.
References
- 1.Lot G, Houdart E, et al. Combined management of intracranial aneurysms by surgical and endovascular treatment. Modalities and results from a series of 395 cases. Acta Neurochir. 1999;141:557–562. doi: 10.1007/s007010050343. [DOI] [PubMed] [Google Scholar]
- 2.Vinuela F, Duckwiler G, et al. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86:475–482. doi: 10.3171/jns.1997.86.3.0475. [DOI] [PubMed] [Google Scholar]
- 3.Assali AR, Sdringola S, et al. Endovascular repair of traumatic pseudoaneurysm by uncovered self-expandable stenting with or without transstent coiling of the aneurysm cavity. Cathet Cardiovasc Intervent. 2001;53:253–258. doi: 10.1002/ccd.1160. [DOI] [PubMed] [Google Scholar]
- 4.Bai H, Masuda J, et al. Neointima formation after vascular stent implantation. Spatial and chronological distribution of smooth muscle cell proliferation and phenotypic modulation. Arterioscler Throm. 1994;14:1846–1853. doi: 10.1161/01.atv.14.11.1846. [DOI] [PubMed] [Google Scholar]
- 5.Cloft HJ, Joseph GJ, et al. Use of three-dimensional Guglielmi detachable coils in the treatment of widenecked cerebral aneurysms. Am J Neuroradiol. 2000;21:1312–1314. [PMC free article] [PubMed] [Google Scholar]
- 6.Fessler RD, Ringer AJ, et al. Intracranial stent placement to trap an extruded coil during endovascular aneurysm treatment: technical note. Neurosurgery. 2000;46:248–253. [PubMed] [Google Scholar]
- 7.Geremia G, Haklin M, et al. Embolization of experimentally created aneurysms with intravascular stent devices. Am J Neuroradiol. 1994;15:1223–1231. [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez CR, Misra VK, et al. Elective stenting of symptomatic basilar artery stenosis. Stroke. 2000;31:95–99. doi: 10.1161/01.str.31.1.95. [DOI] [PubMed] [Google Scholar]
- 9.Hayakawa M, Murayama Y, et al. Natural history of the neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. J Neurosurg. 2000;93:561–568. doi: 10.3171/jns.2000.93.4.0561. [DOI] [PubMed] [Google Scholar]
- 10.Higashida RT, Smith W, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery. Case report and review of the literature. J Neurosurg. 1997;87:944–949. doi: 10.3171/jns.1997.87.6.0944. [DOI] [PubMed] [Google Scholar]
- 11.Horowitz MB, Levy EI, et al. Transluminal stent-assisted coil embolization of a vertebral confluence aneurysm. Surg Neurol. 2001;55:291–296. doi: 10.1016/s0090-3019(01)00421-9. [DOI] [PubMed] [Google Scholar]
- 12.Lanzino G, Fessler RD, et al. Angioplasty and stenting of basilar artery stenosis: technical case report. Neurosurgery. 1999;45:404–407. doi: 10.1097/00006123-199908000-00047. [DOI] [PubMed] [Google Scholar]
- 13.Lanzino G, Wakhloo AK, et al. Efficacy and current limitations of intravascular stents for intracranial internal carotid, vertebral, and basilar artery aneurysms. J Neurosurg. 1999;91:538–546. doi: 10.3171/jns.1999.91.4.0538. [DOI] [PubMed] [Google Scholar]
- 14.Lavine SD, Larsen DW, et al. Parent vessel Guglielmi detachable coil herniation during wide-necked aneurysm embolization: treatment with intracranial stent placement. Two technical case reports. Neurosurgery. 2000;46:1013–1017. [PubMed] [Google Scholar]
- 15.Lempert TE, Malek AM, et al. Endovascular treatment of ruptured posterior circulation cerebral aneurysm: clinical and angiographic outcomes. Stroke. 2000;31:100–110. doi: 10.1161/01.str.31.1.100. [DOI] [PubMed] [Google Scholar]
- 16.Lownie SP, Pelz DM, et al. Endovascular treatment of a large vertebral artery aneurysm using stent and coils. Can J Neurol Sci. 2000;27:162–165. [PubMed] [Google Scholar]
- 17.Lylyk P, Ceratto R, et al. Treatment of vertebral dissecting aneurysm with stents and coils: technical case report. Neurosurgery. 1998;43:385–388. doi: 10.1097/00006123-199808000-00132. [DOI] [PubMed] [Google Scholar]
- 18.Lylyk P, Cohen JE, et al. Combined endovascular treatment of dissecting vertebral artery aneurysms by using stents and coils. J Neurosurg. 2001;94:427–432. doi: 10.3171/jns.2001.94.3.0427. [DOI] [PubMed] [Google Scholar]
- 19.Malek AM, Higashida RT, et al. Tandem intracranial stent deployment for treatment of an iatrogenic, flowlimiting, basilar artery dissection: technical case report. Neurosurgery. 1999;45:919–924. doi: 10.1097/00006123-199910000-00042. [DOI] [PubMed] [Google Scholar]
- 20.Massoud TF, Turjman F, et al. Endovascular treatment of fusiform aneurysms with stents and coils: technical feasibility in a swine model. Am J Neuroradiol. 1995;16:1953–1963. [PMC free article] [PubMed] [Google Scholar]
- 21.Mericle RA, Lanzino G, et al. Stenting and secondary coiling of intracranial internal carotid artery aneurysm: technical case report. Neurosurgery. 1998;43:1229–1233. doi: 10.1097/00006123-199811000-00130. [DOI] [PubMed] [Google Scholar]
- 22.Miyachi S, Ishiguchi T, et al. Endovascular stenting of a traumatic dissecting aneurysm of the extracranial internal carotid artery - case report. Neurol Med Chir. 1997;37:270–274. doi: 10.2176/nmc.37.270. [DOI] [PubMed] [Google Scholar]
- 23.Moret J, Cognard C, et al. Reconstruction technique in the treatment of wide-neck intracranial aneurysms: long-term angiographic and clinical results - Apropos of 56 cases. J Neuroradiol. 1997;24:30–44. [PubMed] [Google Scholar]
- 24.Murayama Y, Vinuela F, et al. Endovascular treatment of experimental aneurysms by use of a combination of liquid embolic agents and protective devices. Am J Neuroradiol. 2000;21:1726–1735. [PMC free article] [PubMed] [Google Scholar]
- 25.Redekop G, Marotta T, et al. Treatment of traumatic aneurysms and arteriovenous fistulas of the skull base by using endovascular stents. J Neurosurg. 2001;95:412–419. doi: 10.3171/jns.2001.95.3.0412. [DOI] [PubMed] [Google Scholar]
- 26.Sekhon LHS, Morgan MK, et al. Combined endovascular stent implantation and endosaccular coil placement for the treatment of a wide-necked vertebral artery aneurysm: technical case report. Neurosurgery. 1998;43:380–384. doi: 10.1097/00006123-199808000-00127. [DOI] [PubMed] [Google Scholar]
- 27.Storey GS, Marks MP, et al. Vertebral artery stenting following percutaneous transluminal angioplasty. J Neurosurg. 1996;84:883–887. doi: 10.3171/jns.1996.84.5.0883. [DOI] [PubMed] [Google Scholar]
- 28.Szikora I, Guterman LR, et al. Endovascular treatment of experimental aneurysms with liquid polymers. The protective potential of stents. Neurosurgery. 1996;38:339–347. doi: 10.1097/00006123-199602000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Szikora I, Guterman LR, et al. Combined use of stents and coils to treat experimental wide-necked carotid aneurysms: preliminary results. Am J Neuroradiol. 1994;15:1091–1102. [PMC free article] [PubMed] [Google Scholar]
- 30.Turjman F, Massoud TF, et al. Combined stent implantation and endosaccular coil placement for treatment of experimental wide-necked aneurysms: a feasibility study in swine. Am J Neuroradiol. 1994;15:1087–1090. [PMC free article] [PubMed] [Google Scholar]
- 31.Turk AS, Rappe AH, et al. Evaluation of the TriSpan neck bridge device for the treatment of wide-necked aneurysms. An experimental study in canines. Stroke. 2001;32:492–497. doi: 10.1161/01.str.32.2.492. [DOI] [PubMed] [Google Scholar]
- 32.Wakhloo AK, Lanzino G, et al. Stents for intracranial aneurysms: the beginning of a new endovascular era? Nerosurgery. 1998;43:377–379. doi: 10.1097/00006123-199808000-00126. [DOI] [PubMed] [Google Scholar]
- 33.Wakhloo AK, Schellhammer F, et al. Self-expanding and balloon expandable stents in the treatment of carotid aneurysms: an experimental study in the canine model. Am J Neuroradiol. 1994;15:493–502. [PMC free article] [PubMed] [Google Scholar]
- 34.Weber W, Henkes H, et al. Stentimplantation in die A. basilaris zur Unterstützung der endovaskulären Aneurysmabehandlung. Nervenarzt. 2000;71:843–848. doi: 10.1007/s001150050674. [DOI] [PubMed] [Google Scholar]
- 35.Weber W, Henkes H, et al. Wirksamkeit and Sicherheit der Implantation ballon-expandierbarer Stents in intrakranielle Gefäße. Klin Neuroradiol. 2001;11:157. [Google Scholar]
- 36.Zubillaga AF, Guglielmi G, et al. Endovascular occlusion of intracranial aneurysms with electrically detachable coils: correlation of aneurysm neck size and treatment results. Am J Neuroradiol. 1994;15:815–820. [PMC free article] [PubMed] [Google Scholar]