Summary
Head and neck paragangliomas are highly vascular tumors with variable clinical behavior. The goal of this paper is to determine specific clinical and radiological findings and extract from these findings a treatment algorithm.
Twenty-three patients with paragangliomas were referred from different surgical centers for angiography and pre-operative embolization. Clinical records were analyzed retrospectively, and focused on impairment of cranial nerves. Angiographic features of paragangliomas, such as arterial supply, tumor flow characteristics, and venous drainage, were evaluated to find characteristic angioarchitectural patterns.
All but one patient presented with a single tumor. All eight jugular and four of five vagal paragangliomas caused a lower cranial nerve deficit. Tympanic paragangliomas presented with hearing loss and tinnitus. The ascending pharyngeal artery was the main feeder and contributed to the supply in every tumor. Jugular and vagal paragangliomas typically compromise the patency of the jugular vein with resulting antegrade or retrograde flow through collateral venous systems.
Surgical resection of vagal and jugular paragangliomas was especially performed when unifocal paragangliomas were present. In all of these patients, the tumor caused a cranial nerve deficit. The supply from an enlarged ascending pharyngeal artery is typical for paragangliomas. The venous drainage pattern of jugular and vagal paragangliomas allows differentiation from other vascular lesions at the skull base.
Key words: paragangliomas, head & neck, angiography
Introduction
Paragangliomas of the head and neck are benign but locally invasive highly vascular tumors that originate from paraganglionic tissue distributed in the middle ear cavity (tympanic paragangliomas), the jugular fossa (temporal paragangliomas), and the extracranial parasympathetic ganglia of the vagus nerve (vagal paragangliomas) and carotid body (carotid body tumors). Clinical symptoms are caused by local tumor growth in the parapharyngeal space, skull base and posterior cranial fossa and may lead to cranial nerve deficits1-3. The decision to treat is not only dependent on clinical symptomatology, but should also take into consideration age and general health of the patient, and the possible multifocality of these tumors4,5. Growth rate is another important determinant in the decision to intervene surgically6.
Once it has been decided that an operation is indicated, detailed information on the vascular supply of the tumors is required. On information on the vascular supply of paragangliomas in combination with the clinical symptomatology, a decision can be made on whether such a lesion can be embolized pre-operatively in the same session7.
The purpose of this study was twofold: first to analyze the specific symptoms and the angio-architectural features, including specific arterial supply, tumor flow characteristics, and venous drainage pattern, for each paraganglioma localization. Second, to extract from these findings, a treatment algorithm and compare this with treatment proposals given in literature.
Material and Methods
From 1995 to 1999, 23 consecutive patients (14 females, nine males, median age 51 years, range 31-76 years) with suspected paragangliomas underwent angiography and embolization prior to surgical resection. All clinical records and angiographic examinations were analyzed retrospectively.
Paragangliomas were divided into four groups (carotid, vagal, jugular and tympanic paragangliomas) to allow correlation of specific symptomatology with tumor localization. Although paragangliomas can consist of multiple compartments8, even in a single tumor localization, these tumoral compartments were not analyzed separately.
The clinical symptoms analyzed were pulsatile tinnitus, hearing loss, vertigo, otic discharge, VII nerve palsy, IX nerve neuralgia, hypoglossal neuralgia, a IX, X, XI th cranial nerve paralysis (jugular foramen syndrome) or a cervical mass.
The arterial supply, the intensity of the parenchymal blush in the arterial phase, with either a faint or a dense staining, and the first opacification of the draining veins were determined. Furthermore, the venous drainage pattern was analyzed according to the direction of flow: antegrade venous flow through the internal jugular vein or cervical veins or retrograde flow into the inferior petrous sinus or into the transverse sinus. The patency of the jugulo-sigmoid junction and internal jugular vein, either thrombosed, invaded or compressed, was assessed.
Results
Twenty-four lesions, including four carotid body tumors, five vagal, eight jugular and five tympanic paragangliomas were identified. Two patients were diagnosed elsewhere as having jugular paragangliomas. Both lesions caused a pulsatile tinnitus.
In both patients the diagnosis of jugular paraganglioma was changed to osteodural arteriovenous malformations based on angiographic findings.
Only one patient presented with two paragangliomas, a symptomatic jugular paraganglioma and an asymptomatic carotid body tumor (not treated). The other patients were considered to have single tumors especially in those cases where vagal paragangliomas extended cranially or where jugular paragan-gliomas extended caudally through the jugular foramen.
Carotid Body Tumors
Four carotid body tumors were diagnosed. Mass effect was the presenting symptom in three of four patients. A vocal cord paralysis, due to a vagal nerve palsy was present in one patient (table 1). Supply was from musculospinal branches from the ascending pharyngeal artery (table 2).
Table 1.
Symptomatology for each separate lesion
| Carotid PGGL (n=4) |
Vagal PGGL (n=5) |
Jugular PGGL (n=8) |
Tympanic PGGL (n=5) |
Osteodural AVM (n=2) |
|
| Mass effect | 3 | 2 | - | - | - |
| Cranial nerve deficit (VII) | - | - | 4 | - | - |
| Cranial nerve deficit (IX, X, XI) | 1 | 3 | 2 | - | - |
| Vertigo | - | 1 | - | - | - |
| Tinnitus | - | - | 2 | 4 | 2 |
| Hearing loss | - | 1 | 6 | 2 | - |
| Otic discharge | - | - | 2 | 1 | - |
| PGGL= paraganglioma, AVM= arteriovenous malformation | |||||
Table 2.
Specific arterial supply for each separate lesion
| Carotid PGGL (n=4) |
Vagal PGGL (n=5) |
Jugular PGGL (n=8) |
Tympanic PGGL (n=5) |
Osteodural AVM (n=2) |
|
| Internal carotid artery | - | 1 | 5 | - | - |
| Ascending pharyngeal artery | 4 | 5 | 7 | 4 | 3a |
| Occipital artery | 1 | 3 | 6 | 2 | 1 |
| Posterior auricular artery | - | 2 | 3 | - | - |
| Superior laryngeal artery | 1 | - | - | - | - |
| Middle meningeal artery | - | 1 | 3 | 3 | - |
| Branch vertebral artery | - | 3 | 3 | - | 1 |
| Posterior cervical artery | - | - | 2 | - | - |
|
PGGL= paraganglioma, AVM= arteriovenous malformation a ipsiand contra-lateral ascending pharyngeal artery | |||||
Vagal Paragangliomas
Five patients were diagnosed as having a vagal paraganglioma. Three tumors with intracranial extension through the jugular foramen caused a jugular foramen syndrome. Detailed information on the vascular supply is given in table 2.
In three tumors, supply was seen from the vertebral artery through the C3 anastomosis towards the odontoid arch, reaching the ascending pharyngeal artery (figure 1). The internal jugular vein was compressed by the tumor in all cases, without compromising the normal venous drainage (table 3).
Table 3.
Lesion size and flow characteristics for each separate localization
| Carotid PGGL (n=4) |
Vagal PGGL (n=5) |
Jugular PGGL (n=8) |
Tympanic PGGL (n=5) |
Osteodural AVM (n=2) |
|
| Dense parenchymal blush | 2 | 5 | 7 | 2 | - |
| Rapid venous filling | 1 | - | 7 | 2 | 2 |
| Antegrade drainage | 4 | 5 | 3a | 5 | 2 |
| Retrograde drainage | - | 5 | - | - | |
|
PGGL= paraganglioma, AVM= arteriovenous malformation a through collateral veins, occlusion jugular vein | |||||
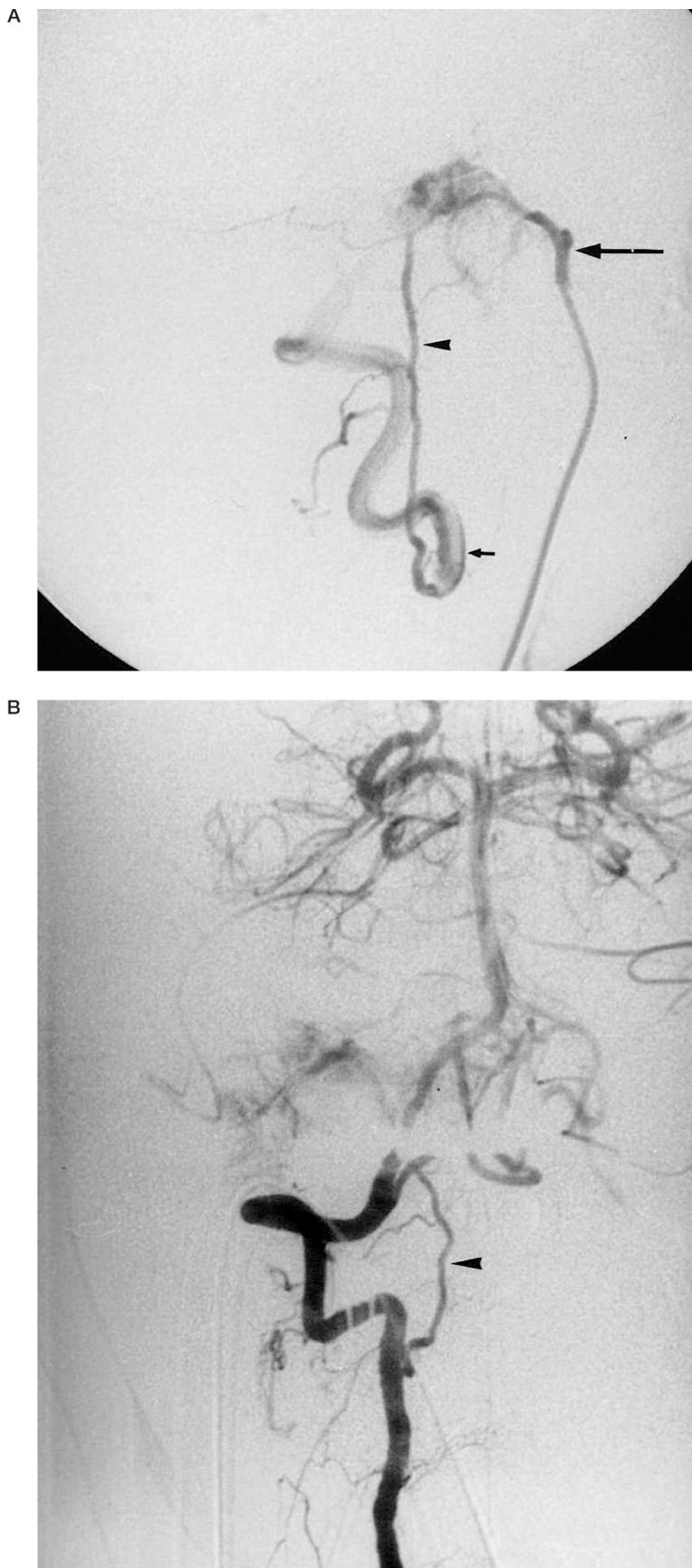
Figure 1.
65-year-old woman with a right sided vagal paraganglioma. Because a complete jugular foramen syndrome is present, no additional risk of cranial nerve deficit is present when embolizing in the region of the jugular foramen.
A) Selective injection of the right ascending pharyngeal artery (arrow) in lateral projection shows filling of a small tumoral compartment. The vertebral artery (small arrow) is filled retrogradely through the odontoid arcade (arrowhead). B) Selective injection of the right vertebral artery in AP projection shows same tumor opacification through the anastomotic channel (arrowhead).
Jugular Paragangliomas
The eight patients with jugular paragangliomas most frequently presented with a cranial nerve deficit (table 1). A jugular foramen syndrome was only seen when tumor extended caudally through the jugular foramen. Detailed information on the vascular supply is given in table 2. The ascending pharyngeal artery, through its neuromeningeal trunk supplied seven of eight tumors. The only exception was a patient in whom the neuromeningeal trunk originated from the occipital artery (figure 2). Two petrous branches from the internal carotid artery and three posterior clival arteries added to the supply but none of them were embolized (table 3). Invasion and secondary occlusion of the internal jugular vein caused retrograde venous drainage.
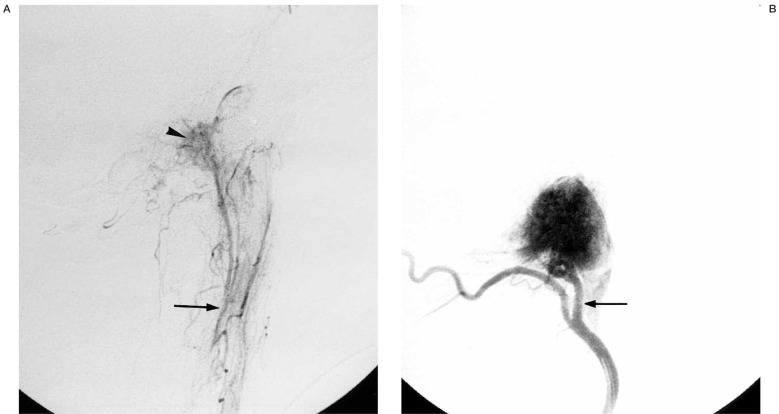
Figure 2.
56-year-old man with a right sided jugular paraganglioma. A) Selective injection of the ascending pharyngeal artery (arrow) in lateral projection shows only slight tumor opacification (arrowhead). B) Selective injection of the occipital artery in lateral projection shows the enlarged neuromeningeal trunk (arrow) giving the main supply to the lesion.
Tympanic Paragangliomas
All five tympanic paragangliomas were below 1 cm in diameter. Tinnitus was the most frequent presenting symptom (table 1). The tympanic branch, from the ascending pharyngeal artery supplied all five tumors (figure 3). The stylomastoid branch from the occipital artery and the anterior tympanic branch from the middle meningeal artery added to the vascularization in three patients (table 2).
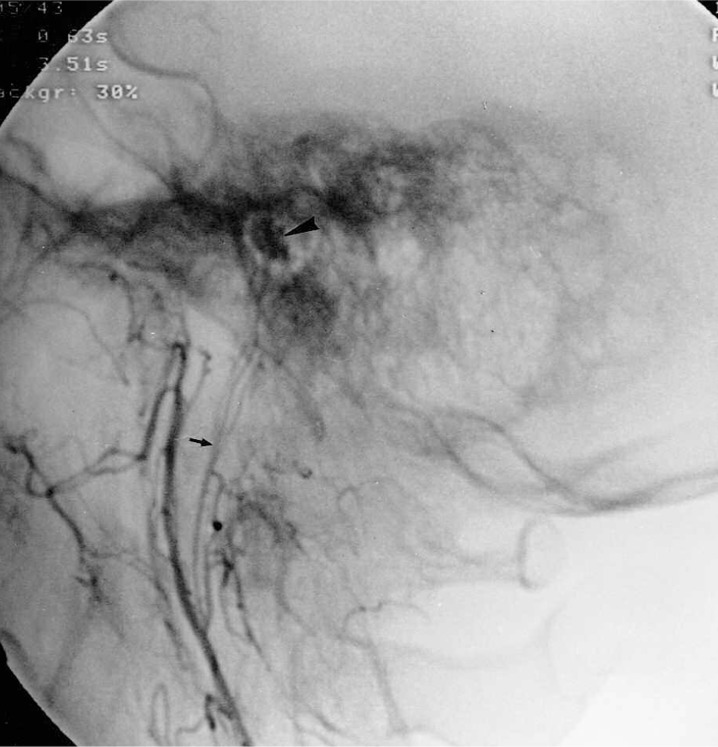
Figure 3.
46-year-old woman with a right-sided tympanic paraganglioma. Selective injection of the ascending pharyngeal artery in lateral projection shows the tympanic branch (small arrow) feeding a small tympanic paraganglioma (arrowhead).
Osteodural Arteriovenous Malformations
The first patient was operated but no tumor could be found. Repeat angiography at our institution showed a vascular lesion with high flow, but no signs of a real parenchymal blush, and drainage through a patent ipsilateral jugular vein (figure 4). The other patient underwent pre-operative particle embolization. However, because the pulsatile tinnitus had disappeared after embolization, she refused further treatment. MR imaging after four years did not show a mass in the jugular region nor did a control angiography show a vascular lesion.
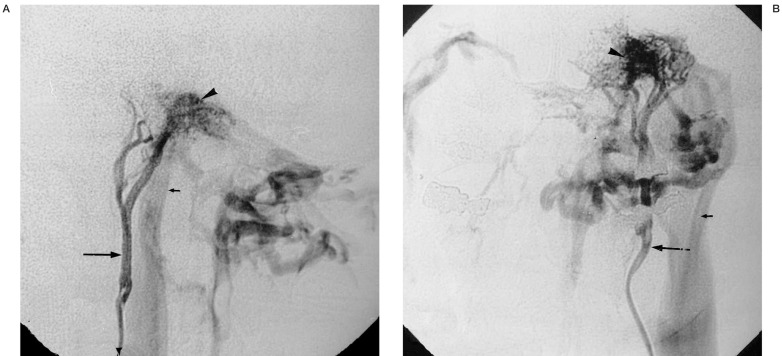
Figure 4.
59-year-old man with suspected right-sided jugular paraganglioma. A) Selective injection of the ascending pharyngeal artery (arrow) in lateral projection shows a highly vascular lesion in the left temporal bone (arrowhead). No real parenchymal stain is seen. Rapid venous drainage in a patent jugular vein is present (small arrow). B) Same in antero-posterior projection, the venous drainage pattern suggests an osteodural arteriovenous malformation.
Discussion
The symptomatology caused by head and neck paragangliomas is mainly dependent on tumor localization. Despite the location of tympanic paragangliomas in the middle ear, hearing loss was only present in two of our five cases (40%), whereas a pulsatile tinnitus was more frequently present (80%), as found in two other studies 3, 9. Probably because tympanic lesions do not fill the whole tympanic cavity, their influence on hearing function is not that profound. In our group of jugular paragangliomas, as opposed to findings in two other clinical studies 1, 9, hearing loss was always present and a pulsatile tinnitus was less common. Extension of jugular and vagal paragangliomas in the jugular foramen can lead to a partial or complete jugular foramen syndrome in which the ninth, tenth, and eleventh cranial nerves are impaired. This can result in hoarseness, uvular displacement, or sternocleidomastoid and trapezoid muscle paralysis2,9. Carotid body tumors usually present as a cervical mass and less frequently cause a cranial nerve deficit10.
An angiographic study can confirm the diagnosis and gives information on the specific vascular supply of the tumor. For analysis of this supply it is recommended to work according to an angiographic protocol 15.
Most important are selective injections of the ascending pharyngeal artery. The ascending pharyngeal artery can be considered “the artery of the paraganglioma”, because its branches can vascularize tympanic, jugular, vagal, carotid, and even laryngeal paragangliomas. Apart from its dominant role in the supply of paragangliomas, the ascending pharyngeal artery plays an important role in the anastomotic pathways between the external carotid artery and the internal carotid and vertebral arteries (figure 1).
In the differential diagnosis of paragangliomas, other lesions in the head and neck region causing similar symptomatology, such as neurinomas, metastatic disease and hemangiopericytomas should be considered. MR imaging allows differentiation in most cases. However, sometimes angiography is still necessary to make the diagnosis. This is especially true for osteodural arteriovenous malformations close to the jugular foramen, mimicking jugular paragangliomas. Clinically, these lesions also cause a pulsatile tinnitus. Angiographic features allow differentiation because these dural lesions lack a real tumor stain as opposed to paragangliomas. Furthermore, a patent jugular vein is seen with osteodural malformations, as opposed to invasion and secondary occlusion of the jugular vein in jugular paragangliomas.
Finally, when the contra-lateral ascending pharyngeal artery adds to the vascularization, the diagnosis of osteodural malformation is more likely because of the extension and richness of the vascular supply in these lesions. This bilateral supply is only seen in large paragangliomas with midline extension.
When carotid body tumors are suspected, proximally located internal carotid artery aneurysms should be considered in the differential diagnosis. The MR appearance of these aneurysms can mimic a carotid body tumor. Angiography, however, clearly reveals the true nature of the lesion.
When the diagnosis of paraganglioma is made, it has to be decided whether or not the patient should be treated. The treatment strategy for paragangliomas is dependent on multiple parameters such as clinical symptomatology, tumor localization, and (intracranial-) tumor extension. In addition, multifocal disease in patients with hereditary head and neck paraganglioma can change treatment. The growth rate of paragangliomas is another determining parameter when treatment of paragangliomas is considered5,6. MR imaging plays a key role in the assessment of these parameters11,12. Finally, patient age is important to determine if the tumor is expected to cause serious morbidity in the future. Because of the multitude of factors, indications for treatment are highly variable.
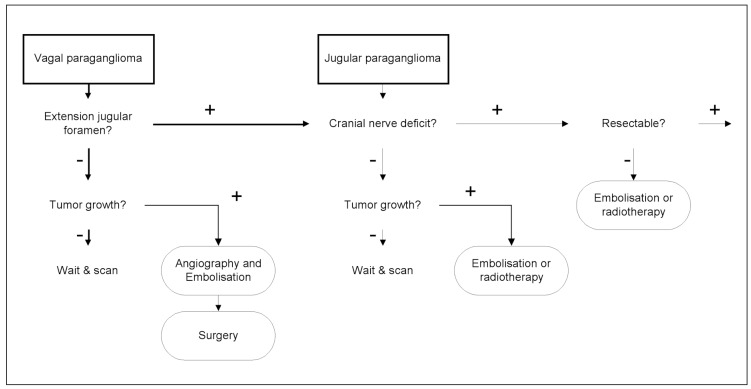
The patients in this study represent a highly selected group, who all underwent angiography prior to surgical resection. Although they were referred from different surgical centers, almost all had single lesions and the majority presented with a cranial nerve deficit. Our data indicate that unilateral lesions, causing a cranial nerve deficit positively influenced the decision to intervene surgically. This is in concordance with the recommendation by Jackson 5 to treat the symptomatic patient with progressive impairment of cranial nerves. Pre-existing cranial nerve impairment allows more radical surgical resection and makes pre-operative embolization less restricted. Extracted from both the findings in this study as well as in literature, a treatment algorithm can be proposed for jugular and vagal paragangliomas (figure 5). A second group of patients in whom resection is recommended are patients with carotid paragangliomas because resection carries a much lower risk of surgically induced cranial nerve impairment10.
Figure 5.
Surgery is less well tolerated when bilateral vagal or jugulotympanic tumors are present, because these patients can expect bilateral cranial nerve impairment in the future, which is a serious assault on quality of life. In these patients a more conservative “wait and see” approach is justified 5, 13. Radiotherapy can play a role in stabilizing tumor growth14, especially in those patients in whom the expected surgical morbidity is unacceptably high. It is not yetclear whether embolization alone can influence tumor growth on the long term.
In the majority of cases, a diagnostic pre-operative angiography will be combined with embolization. The aim of embolization is to devascularize the capillary bed of the tumor to reduce per-operative blood loss. Complications related to embolization are most often attributed to passage of the embolic agent through anastomotic channels into the intracranial circulation. Special care of these anastomoses is mandatory as they are always present but functionally inactive and especially become patent during embolization. The increased resistance caused by the more distal tumor embolization will result in rerouting of the injected material towards the more proximally located anastomotic channels. These anastomoses are not a contra-indication for embolization but only limit the use of specific embolic agents. A second source of morbidity is caused by obliteration of the arterial supply to the cranial nerves. Especially liquid embolic agents and particles with a size from 50 to 150 micron will more easily penetrate these small capillaries. The vascularization of the cranial nerves at the jugularforamen (IX, X, XI) or hypoglossal canal (XII), through their respective ascending pharyngeal artery branches, needs special attention.
Even after embolization, surgical resection of paragangliomas in the region of the jugular foramen can be complicated by hemorrhage. Large jugular and vagal paragangliomas can cause invasion and occlusion of the jugular bulb, impairing the normal venous drainage, resulting in venous reflux towards the inferior petrous sinus.
This venous congestion is the main cause of the continuous, hard to handle venous hemorrhage during surgery. Occlusion of the inferior petrous sinus is advocated by certain authors to reduce this venous hemorrhage (Valavanis A, personal communication).
Conclusions
Surgical resection of paragangliomas is especially justified when solitary paragangliomas are causing a cranial nerve deficit. The most characteristic angiographic feature of paragangliomas is the enlarged ascending pharyngeal artery that always plays a dominant role in the supply of the tumor. Paragangliomas located in the region of the skull base typically cause rerouting of venous drainage because of jugular vein compression.
References
- 1.Green D, Brackmann DE, et al. Surgical management of previously untreated glomus jugulare tumors. Laryngoscope. 1994;104:917–921. doi: 10.1288/00005537-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Netterville JL, Jackson CG, et al. Vagal paraganglioma. Arch Otolaryngol Head Neck Surg. 1998;124:1133–1140. doi: 10.1001/archotol.124.10.1133. [DOI] [PubMed] [Google Scholar]
- 3.O’Leary MJ, Shelton C, et al. Glomus tympanicum tumors: a clinical perspective. Laryngoscope. 1991;101:1038–1043. doi: 10.1288/00005537-199110000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Sillars HA, Fagan PA. The management of multiple paraganglioma of the head and neck. J Laryngol Otol. 1993;107:538–542. doi: 10.1017/s0022215100123643. [DOI] [PubMed] [Google Scholar]
- 5.Jackson CG. Neurotologic skull base surgery for glomus tumors. Diagnosis for treatment planning and treatment options. Laryngoscope. 1993;103:17–22. doi: 10.1002/lary.1993.103.s60.17. [DOI] [PubMed] [Google Scholar]
- 6.Jansen J, Van den Berg R, et al. Estimation of growth rate in patients with head and neck paragangliomas influences the treatment proposal. Cancer. 2000;88:2811–2816. [PubMed] [Google Scholar]
- 7.Lasjaunias P, Berenstein A. Dangerous vessels. Surgical Neuroangiography 1. Berlin Heidelberg New York London Paris Tokyo: Springer Verlag; 1987. pp. 239–244. [Google Scholar]
- 8.Valavanis A. Embolization of intracranial and skull base tumors. In: Valavanis A, editor. Interventional neuroradiology. Berlin Heidelberg New York London Paris: Springer-Verlag; 1993. pp. 63–92. [Google Scholar]
- 9.Woods CI, Strasnick B, Jackson CG. Surgery for glomus tumors: the otology group experience. Laryngoscope. 1993;103:65–72. doi: 10.1002/lary.1993.103.s60.65. [DOI] [PubMed] [Google Scholar]
- 10.Netterville JL, Reilly KM. Carotid body tumors: a review of 30 patients with 46 tumors. Laryngoscope. 1995;105:115–126. doi: 10.1288/00005537-199502000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Olsen WL, Dillon WP, et al. MR imaging of paragangliomas. Am J Roentgenol. 1987;148(1):201–204. doi: 10.2214/ajr.148.1.201. [DOI] [PubMed] [Google Scholar]
- 12.Vogl TJ, Brüning R, et al. Paragangliomas of the jugular bulb and carotid body: MR imaging with short sequences and Gd-DTPA enhancement. Am J Neuroradiol. 1989;10:823–827. doi: 10.2214/ajr.153.3.583. [DOI] [PubMed] [Google Scholar]
- 13.Van der Mey AGL, Frijns JH, et al. Does intervention improve the natural course of glomus tumors? A series of 108 patients seen in a 32-year period. Ann Otol Rhinol Laryngol. 1992;101(8):635–642. doi: 10.1177/000348949210100802. [DOI] [PubMed] [Google Scholar]
- 14.Powell S, Peters N, Harmer C. Chemodectoma of the head and neck: results of treatment in 84 patients. Int J Radiat Oncol Biol Phys. 1992;22(5):919–924. doi: 10.1016/0360-3016(92)90788-j. [DOI] [PubMed] [Google Scholar]
- 15.Lasjaunias P, Berenstein A. Surgical Neuroangiography 1. Berlin Heidelberg New York London Paris Tokyo: Springer Verlag; 1987. pp. 341–405. [Google Scholar]