Summary
With the rapidly developing applications of GDC endovascular aneurysm embolization, the recognition and treatment of potential intra-procedural complications is crucial to reducing the morbidity and mortality of this procedure. Thromboembolic complications occur with an incidence of 2-11 % with endovascular aneurysm coiling. We describe five cases in which the intraarterial use of thrombolytics was applied to disrupt a fresh clot and recanalize the occluded vessels with variable angiographic and clinical success.
Five cases are presented in which thromboembolic complications occurred during or shortly after GDC endovascular aneurysm occlusion. The complication was recognized while depositing coils in two cases, on post-embolization angiogram in one, and a few hours following embolization in two cases in which a new neurologic deficit developed in the ICU.
In those cases recognized while the microcatheter was near the aneurysm site, immediate thrombolysis was performed at the site of occlusion. The patients who developed a new neurologic deficit were returned to the endovascular suite and the site of occlusion was noted to be distal to the coiled aneurysm. Clot disruption was performed with the microcatheter before delivering intraarterial thrombolytics.
Thromboembolic complications of GDC aneurysm embolization are fortunately rare and can be managed with delivery of thrombolytic therapy at the site of occlusion. Intraarterial thrombolysis of fresh clot caused by GDC aneurysm occlusion can successfully open the occluded vessels but not without serious risk of hemorrhage.
Key words: intraarterial thrombolysis, coiling complications, thromboemboli
Introduction
With the approval of Guglielmi Detachable Coils (GDC) by the FDA in 1995, the techniques of endovascular aneurysm occlusion have rapidly developed. Several large clinical series have shown that such techniques are effective in preventing aneurysmal hemorrhage and in providing angiographic evidence of complete aneurysm occlusion8,11,15,17. This less invasive approach prevents some ischemic complications associated with surgical clipping such as cerebral infarct due to excessive brain retraction or inadvertent clip occlusion of distal arterial branches. There is still a risk, however, of thromboembolic cerebral infarct due to catheter-related trauma to the parent vesselwall or distal embolic either showering during coil placement within the aneurysm or forming later on the coil and migrating to a distal vessel. The incidence of this complication ranges from 2-11%. Aneurysm location seems to influence this rate as middle cerebral artery aneurysms are more frequently associated with thromboembolic events 5,6,17. Vinuela et Al found these events were more common in wide-necked aneurysms with post-treatment small residual necks and that they were often recognized within 48 hours of the coiling procedure17. Cognard et Al reported that superselective infusion of urokinase reduced the morbidity associated with this complication· however a fatal hemorrhage could occur following thrombolytic therapy5. We describe five cases in which intraarterial thrombolytic agents were used to treat acute vascular occlusion associated with endovascular coiling of intracranial aneurysms.
Case Reports
Case 1
An 80-year-old woman presented with a Hunt Hess grade 3 / Fisher grade 4 subarachnoid hemorrhage filling the basilar cisterns. An MRI/MRA revealed a lesion suspicious for an anterior communicating artery aneurysm. CT angiography and cerebral angiogram revealed a bilobed 6 mm anterior communicating artery aneurysm with spasm of the ipsilateral A1 segment and bilateral A2 segments.
An external ventricular drain was placed and the patient was started on systemic heparin before performing endovascular coiling on post bleed day one. Once the microcatheter was in the aneurysm via the left anterior cerebral artery· an attempt to place a 5 mm GDC T18 (Target Therapeutics, Fremont, CA) failed as the coil herniated into the parent vessel. This coil was removed and a repeat attempt to place a 4 mm GDC coil was successful. Three more coils were deposited and an immediate post-coil angiogram showed no aneurysm filling. A repeat control angiogram five minutes later showed poor filling of the both anterior cerebral arteries despite no movement of the coils. Urokinase (500,000 units) was then infused via microcatheter in the left ICA over one hour with successful restoration of flow into both anterior cerebral arteries and a small amount of aneurysm filling. However, ten minutes later a repeat angio showed occlusion of the right ACA distal to the AComm. Papaverine (300 mg) was infused which successfully opened the right ACA with minimal residual filling of the aneurysm at the conclusion of the case. Papaverine was used at this point as we had noted vasospasm involving the ACA's prior to beginning the case and hoped papaverine induced vessel dilatation might improve collateral flow. We were reluctant to infuse additional urokinase in the face of a recently ruptured aneurysm. Upon transport to the ICU profuse bloody drainage was noted from the EVD and the patient died of a presumed hemorrhage before imaging studies could be obtained.
Case 2
A 40-year-old woman presented with a Hunt-Hess grade 3 / Fisher grade 4 subarachnoid hemorrhage from an 11 mm posterior communicating artery aneurysm (figure 1A). Once the microcatheter was within the aneurysm a series of 29 GDC coils (T18 hard and soft, T10 soft, sizes 2-10 mm diameter and 10-30 mm length) were deposited. Post-embolization angiography revealed patent blood flow through the parent vessels with a completely occluded neck and slight filling of the fundus (figures 1B,C). The patient's systemic anticoagulation with heparin was reversed with protamine after the final coil was detached to induce complete aneurysm thrombosis. The patient was transferred to the ICU without neurologic deficit.
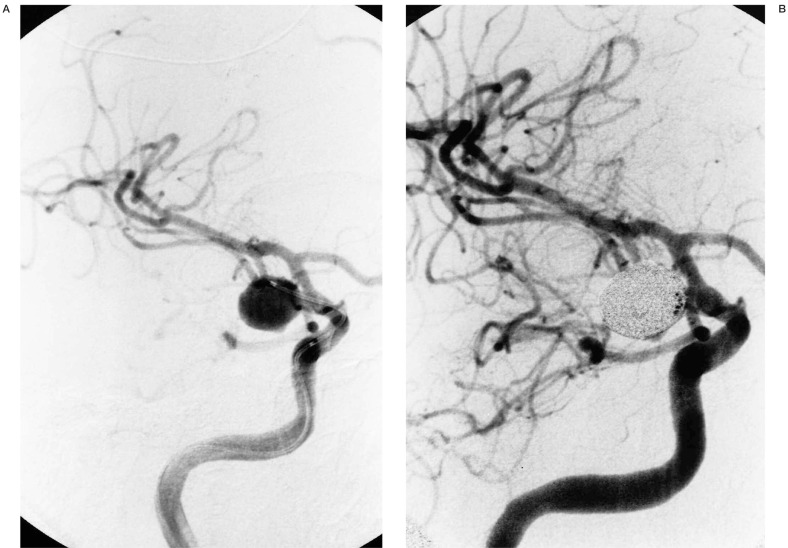
Figure 1.
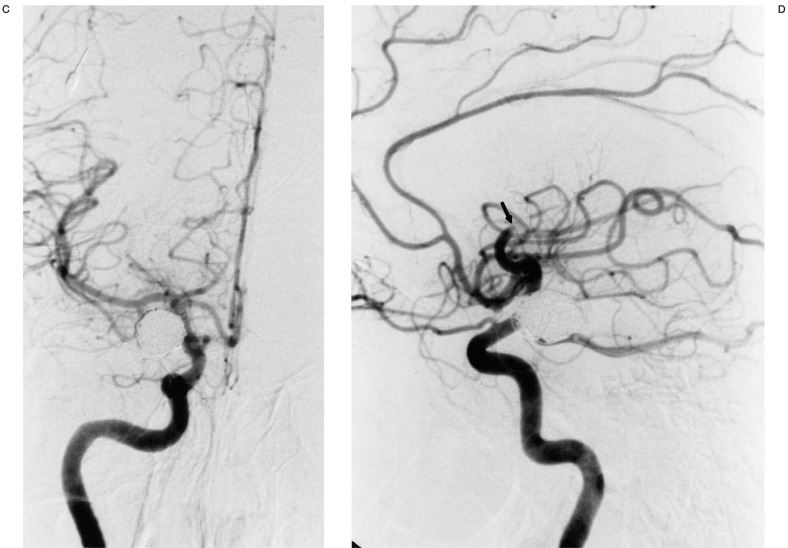
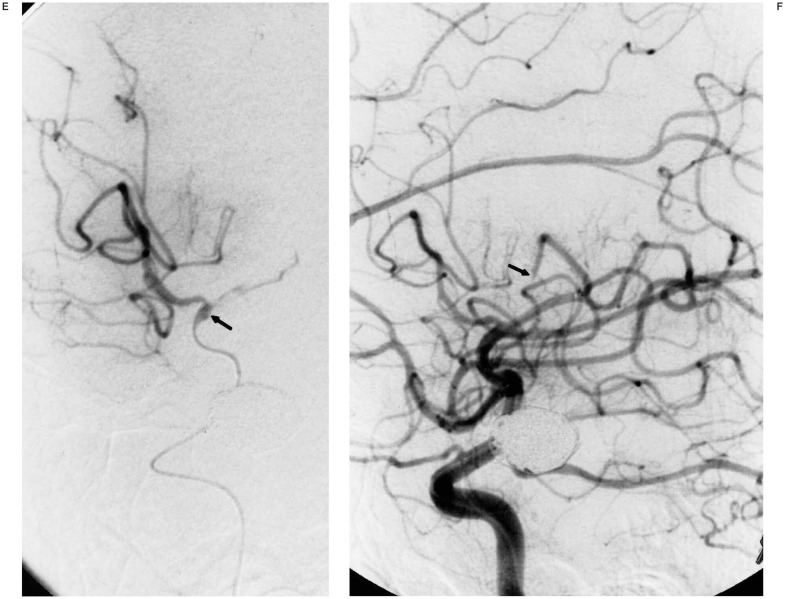
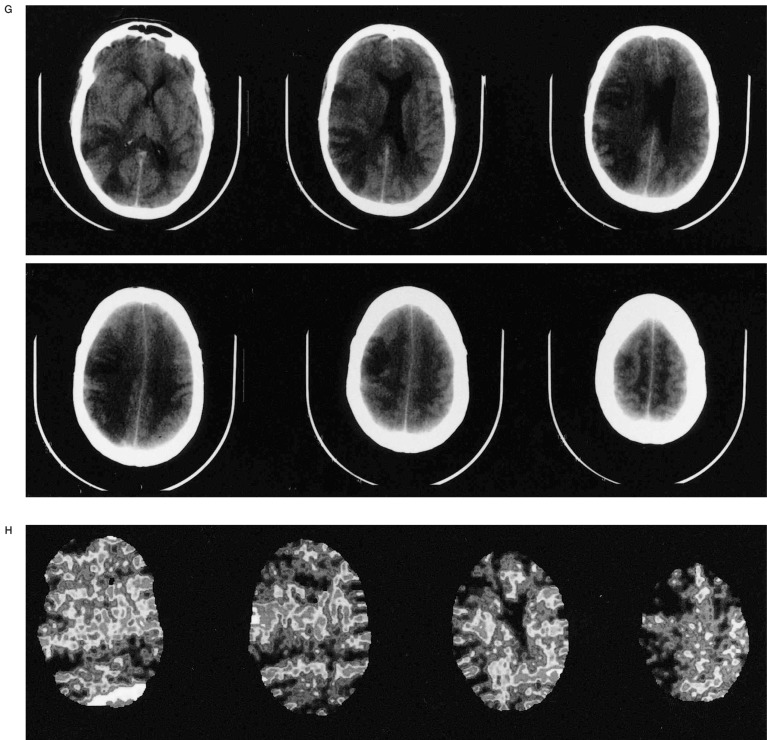
A) Oblique view of right ICA injection demonstrating an 11-mm posterior communicating artery aneursym. B,C) Multiple views of right ICA injection immediately following GDC embolization showing complete aneurysm neck occlusion and minimal residual fundus with patent distal ACA and MCA vessels. D) Lateral right ICA arteriogram following sudden onset of hemiparesis four hours after embolization demonstrating occluded anterior and middle MCA M2 branches (arrow at occlusion site). E) Superselective right MCA angiogram with catheter at occlusion site (arrow). Anterior MCA branches are already refilling following 2 mg tPA administration. F) Right ICA angiogram post-tPA infusion. The MCA is now open except for a M4 occlusion (arrow). G,H) Post-thrombolysis CT and Xenon CT demonstrating evolving infarct and diminished blood flow to portions of the right MCA territory.
Approximately four hours later, the patient developed left hemiplegia with a right gaze preference. The patient was taken back to the endovascular suite where angiography revealed an occlusion of the superior M2 branch of the right MCA and a completely thrombosed right PComm aneurysm (figure 1D). tPA (22 mg over 45 minutes) was infused at the site of the occlusion with catheter repositioning once more distally into an M4 branch that was the only remaining site of occlusion following thrombolysis (figures 1E-F). A Xenon CT scan following thrombolysis showed severely diminished blood flow in the region of the right MCA and follow-up CT scan three days post thrombolysis revealed an evolving infarct in this region (figure 1G-H). The patient was transferred to rehab with resolution of her gaze preference and persistence of moderate weakness in her right upper extremity. Her weakness resolved at follow-up two months following discharge at which time fine motor strength was normal.
Case 3
A 62-year-old woman presented for GDC embolization with a Hunt-Hess grade 3 and Fisher grade 3 SAH from a recently rupturedanterior communicating artery aneurysm. The patient was placed under general endotracheal anesthesia, systemically heparinized, and complete aneurysm occlusion was obtained using GDC T18 soft coils with patent flow of the parent vessels observed on the post-embolization angiogram before heparin was discontinued. The patient awoke in the ICU with right hemiparesis and was taken back to the endovascular suite where a left ICA angiogram revealed occlusion of the left A2 segment at its origin. As the ACT had normalized, the patient was given systemic heparin and the left A1 segment was catheterized.
Multiple attempts to dislodge the thrombus were unsuccessful before 750,000 units of Urokinase were delivered at the site of the occlusion over 90 minutes. The vessel remained occluded despite the attempts to fragment and thrombolyse the clot. The patient remained hemiparetic on the right side at six-month follow-up.
Case 4
A 67-year-old woman with known unruptured bilateral ICA aneurysms was taken to the endovascular suite under general endotracheal anesthesia for GDC embolization of a 9 mm unruptured superior hypophyseal artery aneurysm.
During placement of the third GDC 18 soft coil, the coil unraveled despite 95% of the coil length being packed into the aneurysm fundus. An attempt to use balloon-remodeling technique to pack the displaced portion of coil into the fundus was unsuccessful and thus the unraveled coil was removed. The proximal third of the coil, however, would not exit the aneurysm and angiogram showed thrombus completely occluding the aneurysm but also extending into the cavernous ICA and distally into the left ACA. The microcatheter was advanced to the site of intraluminal thrombus and 1 mg of tPA was delivered. This re-established flow through ICA but a filling defect remained in the left A1 segment. The microcatheter was advanced distally into the A1 segment where the thrombus was fragmented and 0,4 mg of tPA was given with successful thrombolysis and complete aneurysm occlusion on final angiogram.
The patient returned to the ICU neurologically intact when she developed nausea, vomiting, and right hemiparesis. Emergent angiography was performed to evaluate a source of bleeding following evidence of a large frontal lobe ICH and diffuse SAH on CT scan. This revealed contrast extravasation from the left ICA aneurysm. Subsequent aneurysm recoiling with ICA sacrifice was performed as the patient had a large anterior communicating vessel for collateral flow.
The patient remained in poor neurologic condition and went on to have a craniotomy for hematoma evacuation causing mass effect and aneurysm clipping due to persistent retrograde filling of the dome despite proximal ICA occlusion. She remained in poor neurologic condition at follow-up examination.
Case 5
A 70-year-old woman was taken to the endovascular suite under general endotracheal anesthesia for GDC embolization of an unruptured left carotid bifurcation aneurysm. During placement of the third GDC T18 soft coil, two coil loops protruded into the ICA lumen and difficulty was encountered when an attempt to remove the coil was made. Contrast injection at this time revealed acute thrombus extending from this portion of the ICA into the left MCA causing near complete occlusion of flow into the MCA.
An attempt to use the balloon remodeling technique to stabilize the coils in the fundus failed and the third coil remained stuck in the thrombus. Approximately 1 mg of tPA was then delivered first through the guiding catheter in the ICA and then through the balloon catheter directly at the site of thrombus. This allowed the protruding coil to be removed completely and the microcatheter was advanced distally within the M1 segment thrombus where 1,6 mg of additional tPA was delivered with successful return of flow through the MCA. ReoPro and heparin were infused systemically and final angiography revealed patent flow through the left ICA and MCA with residual filling of the aneurysm neck.
Six-month follow-up revealed that the patient was neurologically intact. Her angiogram at six months post-embolization showed the residual neck and complete fundus obliteration were stable while the MCA and ACA vessels were completely patent.
Discussion
Current endovascular techniques have revolutionized the team approach to cerebral aneurysm treatment. Though coil embolization is a safe and effective method to occlude these lesions, the intravascular manipulation of both the aneurysm and the intimal layer of parent arteries creates the risk of thromboembolic complications. The intraarterial introduction of a foreign body (catheter and coil) with a goal of creating intrafundal thrombosis also adds to this risk. Current literature suggests a 2-11% incidence of thromboemboli though little focus has been given to the use of thrombolytic agents in this situation. The efficacy of intraarterial thrombolytic therapy is reported in a variety of situations including embolic stroke during carotid endartectomy, post partum basilar artery thrombosis, and acute embolic stroke from a mechanical heart valve 2,4,10. Stroke research shows recanalization with intraarterial urokinase and clot disruption when compared to medical therapy or transluminal angioplasty alone has improved survival rates and quality of outcome3,7,9,14,16. It has been a useful therapy in some cases as far as 48 hours out from onset of symptoms 1. A meta-analysis of outcomes following intra-arterial thrombolysis of acute vertebrobasilar occlusion showed complete recanalization, distal clot location, and lack of coma at presentation were statistically significant factors for increased survival 13.
The application of intra-arterial thrombolysis using tPA or urokinase infusion techniques to treat acute thromboemboli resulting a consequence of aneurysm embolization has become an option in a number of endovascular cases. Kwan et Al reported a case of balloon occlusion of a large basilar tip aneurysm complicated by thrombosis of a vertebral artery and distal basilar artery 12. This was recognized three hours following the initial procedure. The patient was treated with a combination of urokinase and tPA and ultimately went on to recover complete neurological function. Cronqvist et Al reviewed their experience with intraarterial urokinase fibrinolysis of thromboemboli that occurred in 5,6% of 352 coil embolization cases 6. They observed complete recanalization in 53% and partial recanalization in 47% of cases with a better clinical outcome following complete recanalization. Their results suggest clot fragmentation preceding delivery of urokinase improved the rate of recanalization. They report hemorrhage following thrombolysis in three of the 19 cases. Two aneurysms re-bled and were re-embolized with minimal morbidity in these patients. One intraparenchymal hematoma representing hemorrhagic transformation of ischemic tissue was managed conservatively and the patient expired.
Our clinical and angiographic outcomes support the above data as successful recanalization predicted a good clinical outcome. We achieved resolution of neurologic deficits in both patients where complete resolution of flow was established.
The patient with persistent hemiparesis had an unsuccessful attempt at thrombolysis and clot fragmentation. Our experience suggests that the endovascular surgeon must carefully weigh the risk of hemorrhage associated with thrombolytics before using these agents following thromboemboli after coil embolization. Significant morbidity and mortality occurred when hemorrhage complicated thrombolysis in two of our cases. Case 1 was complicated by the fact that the parent vessels were vasospastic at the time of aneurysm embolization, however, there was clearly recanalization following urokinase. We will never know whether the patient's hemorrhage was from aneurysm rebleed or a cerebral hematoma as she quickly expired before repeat imaging was obtained and autopsy was not performed.
All of our patients undergo systemic heparinization at the time of microcatheter insertion into the guide catheter but prior to aneurysm catheterization. In the past, our tendency was to reverse the heparin with protamine at the procedure's conclusion in the hope of promoting aneurysm thrombosis. Due to thromboembolic complications, however, we have now initiated a protocol in which all patients remain heparinized for 12-24 hours following the procedure unless clear contraindications (such as intraprocdural rupture) exist. Using this protocol our incidence of thromboembolic complications has been reduced to 3%. Regardless of the etiology of a thrombo-embolic complication we recommend complete aneurysm occlusion with coils before performing thrombolysis to minimize the risk of a rebleed. Cerebral blood flow studies can be a useful adjunct to determining whether perfusion suggests the tissue distal to thrombolytic delivery is salvageable or already necrotic. Such information can minimize the risk of reperfusion hemorrhages.
Conclusions
Thromboembolic complications of GDC aneurysm embolization are fortunately rare. When they do occur, however, immediate delivery of thrombolytic therapy at the site of occlusion is possible. In our small experience, angiographic recanalization was achieved in four of five patients.
We observed clinical improvement in two of five patients. Two patients experienced hemorrhage which caused death in one and severe morbidity in the second. In conclusion, intraarterial thrombolysis of fresh clot associated with GDC therapy can successfully open occluded vessels but not without serious risk of hemorrhage.
References
- 1.Barnwell SL, Clark WM, et al. Safety and efficacy of delayed intraarterial urokinase therapy with mechanical clot disruption for thromboembolic stroke. Am J Neuroradiol. 1994;15:1817–1822. [PMC free article] [PubMed] [Google Scholar]
- 2.Barr JD, Horowitz MB, et al. Intraoperative urokinase infusion for embolic stroke during carotid endartectomy. Neurosurgery. 1995;36:606–611. doi: 10.1227/00006123-199503000-00024. [DOI] [PubMed] [Google Scholar]
- 3.Becker KJ, Monsein LH, et al. Intraarterial thrombolsis in vertebrobasilar occlusion. Am J Neuroradiol. 1996;17:255–262. [PMC free article] [PubMed] [Google Scholar]
- 4.Cincotta RB, Davis SM, et al. Thrombolytic therapy for basilar artery thrombosis in the puerperium. Am J Obstet Gynecol. 1995;173:967–969. doi: 10.1016/0002-9378(95)90382-8. [DOI] [PubMed] [Google Scholar]
- 5.Cognard C, Weill A, et al. Intracranial berry aneurysms: Angiographic and clinical results after endovascular treatment. Radiology. 1998;206:499–510. doi: 10.1148/radiology.206.2.9457205. [DOI] [PubMed] [Google Scholar]
- 6.Cronqvist M, Pierot L, et al. Local intrarterial fibrinolysis of thromboemboli occurring during endovascular treatment of intracerebral aneurysm: A comparison of anatomic results and clinical outcome. Am J Neuroradiol. 1998;19:157–165. [PMC free article] [PubMed] [Google Scholar]
- 7.Cross DT, Moran CJ, et al. Relationship between clot location and outcome after basilar artery thrombolysis. Am J Neuroradiol. 1997;18:1221–1228. [PMC free article] [PubMed] [Google Scholar]
- 8.Eskridge JM, Song JK. Endovascular embolization of 150 basilar tip aneurysms with Guglielmi detachable coils: Results of the Food and Drug Administration multicenter clinical trial. J Neurosurg. 1991;75:8–14. doi: 10.3171/jns.1998.89.1.0081. [DOI] [PubMed] [Google Scholar]
- 9.Hacke W, Zuener H, et al. Intraarterial thrombolytic therapy improves outcome in patients with acute vertebrobasilar occlusive disease. Stroke. 1988;19:1216–1222. doi: 10.1161/01.str.19.10.1216. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi J, Oguma F, et al. Direct thrombolytic revascularization of the occluded basilar artery. Cardiovasc Surg. 1993;1:547–549. [PubMed] [Google Scholar]
- 11.Kuether TA, Nesbit GM, Barnwell SL. Clinical and angiographic outcomes, with treatment data, for patients with cerebral aneurysms treated with Guglielmi detachable coils. Neurosurgery. 1998;43:1016–1025. doi: 10.1097/00006123-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Kwan ESK, Kwon OJ, Borden JA. Successful thrombolysis in the vertebrobasilar artey after endovascular occlusion of a recently ruptured large basilar tip aneurysm. Am J Neuroradiol. 1995;16:847–851. [PMC free article] [PubMed] [Google Scholar]
- 13.Levy EI, Firlik AD, et al. Factors affecting survival rates for acute vertebrobasilar artery occlusions treated with intrarterial thrombolytic therapy: A meta-analytical approach. Neurosurgery. 1999;45:539–548. doi: 10.1097/00006123-199909000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Mori E, Tabuchi M, et al. Intracarotid urokinase with thromboembolic occlusion of the middle cerebral artery. Stroke. 1988;19:802–812. doi: 10.1161/01.str.19.7.802. [DOI] [PubMed] [Google Scholar]
- 15.Raymond J, Roy D. Safety and efficacy of endovascular treatment of acutely ruptured aneurysms. Neurosurgery. 1997;41:1235–1246. doi: 10.1097/00006123-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Ueda T, Hatakeyama T, et al. Endovascular treatment for acute thrombotic occlusion of the middle cerebral artery: Local intraarterial thrombolysis combined with percutaneous transluminal angioplasty. Neuroradiology. 1997;39:99–104. doi: 10.1007/s002340050374. [DOI] [PubMed] [Google Scholar]
- 17.Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: Perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86:475–482. doi: 10.3171/jns.1997.86.3.0475. [DOI] [PubMed] [Google Scholar]