Summary
CD4+ T‐helper subsets are lineages of T cells that have effector function in the lung and control critical aspects of lung immunity. Depletion of these cells experimentally or by drugs or human immunodeficiency virus (HIV) infection in humans leads to the development of opportunistic infections as well as increased rates of bacteremia with certain bacterial pneumonias. Recently, it has been proposed that CD4+ T‐cell subsets may also be excellent targets for mucosal vaccination to prevent pulmonary infections in susceptible hosts. Here, we review recent findings that increase our understanding of T‐cell subsets and their effector cytokines in the context of pulmonary infection.
Keywords: CD4+T cells, pneumonia, mucosal vaccination
This article is a part of a series of reviews covering CD4+ T‐cell Subsets appearing in Volume 252 of Immunological Reviews.
Introduction
CD4+ T‐helper (Th) cells are critical components of the adaptive immunity in the lung (Fig. 1). The development of these cells arises after priming with antigen presented by class II major histocompatibility complexes (termed signal 1). Further activation occurs after the engagement of costimulatory molecules and their receptors expressed on both antigen‐presenting cells (APCs) and T cells (termed signal 2). This second signal is critical for both generating antigen‐specific effector T cells as well as memory cells. Presentation of antigen in the absences of costimulation can result in T‐cell anergy. Final effector function is due to cytokine/growth factor‐directed CD4+ T‐cell differentiation (signal 3). This latter aspect of differentiation is driven by lineage‐specific transcription factors as well as changes in chromatin remodeling. The critical role of CD4+ T cells in lung immunity and pulmonary host defenses was clearly demonstrated by the high incidence of pulmonary infections as a complication of human immunodeficiency virus (HIV) infections/acquired immunodeficiency syndrome 1, 2, 3.
Figure 1.
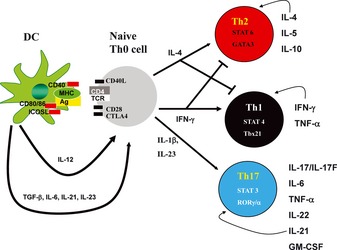
CD 4 + T‐helper cell differentiation. Antigen is presented by dendritic cells (DCs) by class II major histocompatibility complex (MHC) to naive CD4+ Th0 cells. Full T‐cell activation requires a second signal consisting of the upregulation and expression of costimulatory molecules such as inducible costimulatory ligand (ICOSL), CD28, and cytotoxic T‐lymphocyte antigen‐4 (CTLA‐4). T‐cell differentiation is instructed by cytokine/growth factor signals. T‐bet can be activated by STAT1 as well as bind to the IFNγ locus followed by induction of IL‐12βR2. IL‐12 activates STAT4, which can further drive Th1 development. IFNγ can act in an autocrine manner to further augment Th1 differentiation. IL‐4 induces Gata3, which further induces IL‐4 and supports the differentiation of Th2 cells. Th2 cells produce IL‐4, IL‐5, and IL‐13 as their effector cytokines. TGFβ and IL‐6 can induce RORγt expression as well as activation of STAT3. This leads to induction of the IL‐23 receptor, rendering these cells responsive to IL‐23, which is required for terminal differentiation of Th17 cells. Th17 cells produce the cytokines IL‐17/IL‐17F, IL‐22, IL‐21. IL‐21 can act in an autocrine fashion to further the differentiation of the Th17 lineage.
CD4+ T‐helper subsets
Th2 cells
Th2 CD4+ T cells differentiate from naive precursors under the direction of the transcription factor GATA3 and signal transducer and activator of transcription 6 (STAT6) 4. Effector cytokines produced by Th2 cells include interleukin‐4 (IL‐4), IL‐5, and IL‐13. GATA3 binds directly to the Il4 locus, and IL‐4 and signaling via STAT6 is critical for further Th2 proliferation and lineage commitment 4. Th2 effector cytokines mediate immunity against infections with helminths (Fig. 2). Deletion of Gata3 in mice results is embryonic lethal, but conditional deletion of Gata3 in T cells confirms the essential role of this transcription factor in Th2 differentiation and the expulsion of helminths from the gastrointestinal tract 5. IL‐5 is an essential growth factor for eosinophilopoiesis 6, 7. Mice with homozygous deletion of Il5 have substantial reduction in both peripheral and bone marrow eosinophils 6, 7. In contrast, overexpression of IL‐5 protein results in substantial eosinophilia in blood and tissues 8. IL‐13 signals through a receptor complex of IL13RA1 and IL4Rα and activates STAT6 signaling. These receptors are expressed on airway epithelium as well as airway smooth muscle. In bronchial epithelium, IL‐13 is a major factor in mucous production and goblet cell differentiation in the airway 9, 10. Moreover, IL‐13 signaling via STAT6 in airway smooth muscle and in airway epithelium leads to airways hyperresponsiveness to methacholine 9, 10. These cells and effector cytokines have been implicated in diseases such as allergic rhinitis, atopic dermatitis, and asthma 11. Anti‐IL‐5 has been investigated in asthma and although initial studies did not show clear cut efficacy 12, but subgroups of patients with high sputum eosinophilia respond to IL‐5 blockade 12. Similarly, initial studies with IL‐13 blockade were also negative, but recently, studies suggest that by stratifying patients with IL‐13 driven asthma (by assessing the serum level of periostin, an IL‐13 regulated gene in lung epithelium) can identify subgroups who respond to anti‐IL‐13 13. In addition to asthma, IL‐13 has been implicated in fibrotic processes in the lung in response to drugs such as bleomycin 14, 15, 16.
Figure 2.
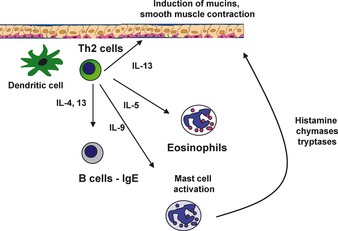
Th2 cells and immunity at the mucosa. Allergen parasites or helminthic infection can induce TSLP and IL‐25 in the lung epithelium. These cytokines can drive early IL‐4 production leading to the differentiation of Th2 cells. IL‐4 and IL‐13 can drive the induction of IgE as well as stimulate epithelial cells to increase mucous production. IL‐5 is a critical regulator of eosinophilopoiesis. Binding of IgE to FcRε on mast cells leads to their degranulation and release of chymases, tryptases, and histamines.
Th2 cells also facilitate B‐cell differentiation and antibody responses to T‐cell‐dependent protein antigens 17, including the development of an anti‐immunoglobulin E (IgE) response. It has been recently recognized that Th2 cell differentiation is not only regulated by IL‐4 but also several cytokines produced by the lung epithelium, including thymic stromal lymphopoietin (TSLP), IL‐25, and IL‐33 17. It has been shown recently that polymorphism in both IL‐33 and its receptors ST2 is associated with asthma, strongly implicating Th2 cells and specifically the IL‐33 ST2 signaling pathway in this disease.
Th1 cells
Th1 cells, initially described by Mossman and Coffman 18, were defined by their ability to express interferon‐γ (IFN‐γ). Differentiation of CD4+ Th1 cells is controlled by the transcription factors T‐bet 19 and STAT4. Differentiation is controlled by IL‐12p70, which is a heterodimer of IL‐12p35 and IL‐12p40 subunits 20. However, both in vitro and in vivo Th1 differentiation can be independent of IL‐12. One critical IL‐12‐independent pathway is through the induction of type I interferons that can facilitate Th1 differentiation in certain situations 21. IFN‐γ which signals via a receptor complex consisting of two IFN‐γR1 and two IFN‐γR2 chains can signal in an autocrine paracrine fashion to further amplify Th1 differentiation and lineage commitment. IFN‐γ receptors are expressed on a wide variety of cells including myeloid cells including macrophages and dendritic cells (DCs), as well as structural cells in the lung such as epithelial cells and fibroblasts 22. IFN‐γR1 and IFN‐γR2 activate Janus‐associated kinases 1 and 2 (JAK1/2), which phosphorylates STAT1. STAT1 undergoes homodimerization and translocation to the nucleus and binds to DNA‐encoded γ‐activated sequences that ultimately control gene transcription 22.
IFN‐γ is critical for mediating immunity and host resistance to many intracellular infections including Mycobacterium tuberculosis, Listeria moncytogenes, and Salmonella typhimurium. Patients with mutations IL‐12p40, IFN‐γ, or receptors for IL‐12 or IFN‐γ have increased susceptibility to intracellular infections due to these organisms 23, 24. Patients with IFN‐γ receptor mutations can develop disseminated infection with bacillus Calmette‐Guerin (BCG) that is resistant to antibiotics and IFN‐γ therapy 23, 25. Patients with IL‐12p40 mutations can also develop BCG or S. typhimurium infection, but theoretically can respond to IFN‐γ 24. Thus, there is strong evidence that this pathway is essential for human control of these intracellular pathogens.
Th17 cells
Th17 cells are recently described effector lineage of T‐helper cells that produces IL‐17A, IL‐17F, IL‐21, IL‐22, and IL‐26 (the latter expressed in human cells). Th17 cells differentiate under control of the transcription factors retinoid orphan receptor‐γ (RORγ), ROR‐α, and STAT3 26, 27. It was initially believed that one of the critical instructional cytokines for Th17 differentiation was IL‐23 28; however, IL‐23 receptors are not expressed on naive CD4+ T cells. Landmark studies published in 2005 showed that these cells develop independently of STAT4 or STAT6 as well as T‐bet or GATA3 29, 30. These data implicated that Th17 cells were a distinct CD4+ T‐cell lineage 29, 30. Th17 differentiation can occur with stimulation with transforming growth factor‐β (TGF‐β) and IL‐6 31, 32, 33. Moreover, this cytokine/growth factor combination induces the expression of IL‐23R 31, 32, 33. Signaling via IL‐23 allows terminal differentiation and expansion of Th17 cells 34. Another critical effector cytokine produced by Th17 cells is IL‐22, which is controlled by IL‐23 as well as the transcription factor the arylhydrocarbon receptor (Ahr) 35. IL‐21 36, 37 can function in an autocrine manner to further expand Th17 differentiation (Fig. 1). It has been recently shown in vivo that TGFβ activation and thus differentiation of Th17 cells requires activation of latent TGFβ by αv integrins 38, 39. This pathway is not required for IL‐17 production by γδ T cells 38, 39.
T‐follicular helper cells
T‐follicular helper (Tfh) cells are a subgroup of CD4+ T cells that are located in the B‐cell follicle region of secondary lymphoid tissues including lymph nodes and bronchial‐associated lymphoid tissues in the lung. These cells regulate T‐cell‐dependent B‐cell activation through the expression of CD40L and IL‐21 40. These cells develop under the control of the transcription factor Bcl‐6 40 and are also regulated by inducible costimulatory (ICOS).
T‐regulatory cells
Treg differentiation is controlled by the transcription factors Foxp3 and STAT5 41. These cells are essential for mediating tolerance to inhaled antigen in the lung 41. Deletion of these cells or abrogating their effector molecules, which include IL‐10 and TGFβ, prevent airways sensitization to allergen as well as allergic inflammation 41. These cells can suppress the effector activity of many T‐helper subsets and can be thymically derived (natural Tregs) or induced in the periphery (iTregs). An exhaustive review of these cells is beyond the scope of this chapter, but the reader is referred to excellent thorough reviews 41, 42 of these cells if they seek a more in‐depth description of these cells.
Non‐CD4+ T‐helper cell sources of Th1/Th2/Th17 effector cytokines in the lung
Other cells in the lung exist that produce many of the same effector cytokines as Th1, Th2, or Th17 cells including innate lymphoid cells, natural killer (NK) cells, and γδ T cells. γδ T cells are resident in mucosal sites including the lung and can produce IFN‐γ, IL‐4, IL‐17, and IL‐22 43. These cells are a major source of early IL‐17 after pulmonary infection, and IL‐17 production by these cells is regulated by IL‐23 and IL‐1β 44, 45, 46, 47, 48. γδ T‐cell production of IL‐10 has been shown to play a key counter‐inflammatory role in some pulmonary infections such as Pneumocystis infection 49.
NK cells can also produce IL‐4, IFNγ, and IL‐17 50 in the lung in response to infection, allergen, or ozone. One population that has been extensively studied is a population that expresses an invariant T‐cell receptor (iNKT cells) that recognizes a galactolipid Sphingomonas, α‐galactosylceramide 51, 52. These cells are elevated in the bronchial alveolar lavage fluid of patients with asthma 53, 54. NKT cells produce IFNγ in response to S. pneumoniae pulmonary infection 55 and IL‐17 in response to Escherichia coli lipopolysaccharide (LPS) 50. These cells also produce IL‐17, and this response is critical for airways hyperresponsiveness to ozone 56. NK cells can develop under the control of IL‐15 and express antiviral molecules such as IFN‐γ as well as cytotoxic molecules 57.
Innate lymphoid cells are also important sources of effector cytokines in the lung. These cells are defined by the lack of lineage markers and T‐cell receptors, but they require IL‐7 signaling for their development. Thus, these cells are present in recombination‐activating gene 1 (RAG1) or RAG2−/− mice, but are lacking in RAG2, common γ chain (γc) double deleted mice 58, 59. Retinoid orphan receptor γt (RORγt)‐expressing cells are critical for the formation of secondary lymphoid tissues (via regulation of lymphotoxin expression) and play critical roles in mucosal immunology in the gastrointestinal tract through the production of IL‐17 and IL‐22 60. Type 2 ILCs produce IL‐5 and IL‐13 and participate in the clearance of helminths from the gastrointestinal tract 61. These cells appear to be regulated by IL‐25 (IL‐17E) as well as IL‐33, a member of the IL‐1 family. Recently, it has been demonstrated that a population of ILCs produce IL‐13 in response to IL‐33 induced by viral infection, and these cells mediate in part viral induced exacerbation of allergic disease in the lung 62. These cells can also be activated by protease allergens to drive eosinphilic airways inflammation as well as airways hyperresponsiveness 58 under control of IL‐33 and TSLP. Thus, these cells recapitulate many aspects of CD4+ T‐cell immunity in that there are subsets that express similar effector molecules, yet these cells are activated early and their activation is independent of TCR stimulation.
CD4+ T‐cell effector cytokines in the lung
Type 2 effectors
Both IL‐4 and IL‐13 activates STAT6 signaling in a variety of lung cells including alveolar macrophages, fibroblasts, airway smooth muscle, and airway epithelium. In macrophages, activation of STAT6 leads to what is termed ‘alternative macrophage activation’ which is characterized by the expression of arginase 1, YM1, YM2, and the macrophage mannose receptor 63. It has been shown that IL‐4 treatment of macrophages can reduce their phagocytic ability, but IL‐4 stimulation of macrophages can augment the clearance of apoptotic neutrophils 63. Alternatively, activated macrophages (AAMs) play regulatory roles in helminth infection and can reduce immunopathology 63. It has also been shown that AAMs have augmented dectin‐1 expression as well as the macrophage mannose receptor. Given that both of these receptors are critical in recognizing the fungal carbohydrates β1,3 glucan and mannan, respectively, AAMs may have greater fungicidal activity. Induction of AAMs may also be exploited by pathogens to allow their survival. For example, Francisella tularensis, a virulent pathogen in the lung, can prolong its intracellular survival via induction of AAMs 64.
As mentioned earlier, IL‐13 induction of STAT6 signaling induces several mucin genes in airway epithelium including Muc5ac and Muc5b as well as inducing goblet cell hyperplasia 65. These signaling effects are critical for host defenses against helminths such as Nippostrongylus brasiliensis 66. During viral infection, airways mucins can prevent viral spread; however, in infants this can also contribute to airway obstruction. The role of IL‐13 in viral infection is complex, and IL‐13 is not necessarily beneficial to the host. For example, IL‐13 has recently been shown to increase the susceptibility of epithelial cells to infection with rhinovirus 67.
Type 1 effectors
There are several mechanisms by which IFN‐γ controls lung immunity to intracellular pathogens. IFN‐γ can prime macrophages to enhance their intracellular microbiocidal activity 68 in a process termed classical activation of macrophages 69. IFN‐γ priming augments Toll‐like receptor (TLR) signaling 70. IFN‐γ also increases microbiocidal activity via the induction of inducible nitric oxide synthase, which regulates the production of reactive nitrogen intermediates 71, 72. IFN‐γ can also increase the production of reactive oxygen species. These activities may explain the therapeutic benefit of IFN‐γ in patients with chronic granulomatous disease due to mutations in NADPH oxidase 73, 74, 75.
IFN‐γ signaling also upregulates class II major histocompatibility complex molecules and costimulatory molecules such as CD80 and CD86, which can augment antigen presentation to naive T cells. IFN‐γ's first observed activity was its ability to suppress viral replication in many target cells including macrophages, fibroblasts, and lung epithelial cells 76. This occurs in part through the induction of many antiviral genes such as MxA 77. However, other respiratory viruses including severe acute respiratory syndrome coronavirus are controlled by IFNγ through mechanisms that are independent of MxA 78.
IFN‐γ signaling in lung structural cells including fibroblasts and epithelium cells induces several chemokine ligands for CXCR3, including CXCL9, CXCL10, and CXCL11. CXCR3 is expressed on Th1 cells, and thus, the induction of these chemokines by IFN‐γ may be a critical mechanism to increase the recruitment of Th1 cells. Moreover, these chemokines are critical for granuloma formation, which is essential for control of many intracellular pathogens such as M. tuberculosis 79, 80. Systemic and aerosolized IFN‐γ has been investigated for the potential adjunctive treatment of tuberculosis. A recent meta‐analysis showed that IFN‐γ was well tolerated and associated with higher sputum sterilization rates 81; however, definitive randomized control trials are lacking to make firm conclusions on the efficacy of this cytokine.
Type 17 effectors
IL‐17 can signal in human bronchial epithelium (HBE) to induced chemokine ligands for CXCR2, such as CXCL8, CXCL1, and the granulopoietic growth factor G‐CSF 82, 83. Like other cell systems, HBE responses to IL‐17 or IL‐17F are augmented by TNF‐α 82, 84. HBE cells express IL‐17RA, IL‐17RC, as well as IL‐22R and IL‐10R2, the receptors for IL‐22 82, 83 (Fig. 3). As IL‐17A and IL‐17F can be coexpressed in the same cell, it has been reported that these two IL‐17 family members can form three cytokines including IL‐17A homodimers, IL‐17A/F heterodimers, which have intermediate activity compared with IL‐17A homodimers, and IL‐17F homodimers, which have the least potent activity 85. One mechanism by which IL‐17 increases the production of chemokines is through increasing mRNA stability of this transcript 86, 87 resulting in augmented protein production. A similar mechanism has also been reported for IL‐17‐mediated increases in G‐CSF production 88.
Figure 3.
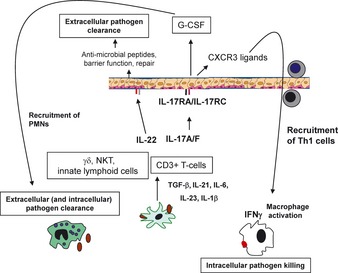
Th17 cytokines in mucosal immunity. Many infections, including those caused by fungi and bacteria, can activate dendritic cells and macrophages to produce interleukin‐6 (IL‐6), IL‐23, and IL‐1β. IL‐23 and IL‐1β can drive IL‐17 production by both innate lymphoid cells and γδ T cells. Differentiation of Th17 cells requires TGFβ, and a critical mechanism of activation of TGFβ in the lung is through the activation of this growth factor by αv integrins. IL‐17 and IL‐22 can signal to the epithelium to augment G‐CSF as well as ligands for CXCR2 that mediate the recruitment of neutrophils. These two cytokines also induce the expression of anti‐microbial proteins such as lipocalin‐2 and β‐defensins. IL‐22 can also augment epithelial repair. After vaccination, Th17 cells through the production of IL‐17 can also induce ligands for CXCR3 that increase the recruitment of IFNγ‐producing Th1 cells, which can also help control intracellular pathogen growth.
IL‐17RA is ubiquitously expressed on many cells including myeloid cells; however, these cells express very little IL‐17RC. Thus, IL‐17A and IL‐17F have limited activity on myeloid cells. It has been reported that IL‐17 can enhance IL‐12p70 in alveolar macrophages 89 as well CCL2, CCL3, GM‐CSF, IL‐1β, and IL‐9 in CD4+ T cells 90. Th17 cells express IL‐17RA and IL‐17RE, which form a receptor complex for IL‐17C 91, 92, 93. IL‐17C can increase the production of IL‐17 by these cells 91, 92, 93. IL‐17C can be expressed in lung epithelium (unpublished observations) and thus can serve as a feed forward mechanism by which the epithelium could influence interstitial T‐cell responses. In addition to regulating neutrophil recruitment in the lung 94, IL‐17 augments apical bicarbonate transport in HBE cells 95. Carbonate anion regulates the activity of β defensins 96, and this may be an important mechanism of IL‐17's net anti‐microbial effect in the lung mucosa.
IL‐17RA is required for host resistance to the extracellular pathogens K. pneumoniae 94, and in this model IL‐17RA regulates the local production of CXCR2 ligands as well granulopoiesis through the regulation of G‐CSF. IL‐17RA is dispensable for the control of intracellular pathogen M. tuberculosis 83, but is required for the control of the intracellular pathogen F. tularensis 89. In this latter model, IL‐17 regulates IL‐12p70 production by macrophages, which subsequently generates the Th1 CD4+ T‐cell response ultimately controls the pathogen 89.
IL‐22 signals through STAT3 in HBE cells. IL‐22 increases the clonogenic potential of HBE cells in colony assays 83 and also augments repair to puncture injury in confluent HBE cells 97 (Fig. 3). IL‐17 and IL‐22 stimulation of HBE and mouse tracheal epithelial cells induces several anti‐microbial genes in lung epithelium including lipocalin 2 83 and regenerating islet‐derived protein 3‐γ 98. Neutralization of IL‐22 during experimental K. pneumoniae lung infection results in rapid bacteremia, which substantially increases mortality in this model. IL‐22 is regulated by IL‐23 and recombinant IL‐22 can rescue IL‐23‐deficient mice 83. A complication pneumonia is acute lung injury which can be exacerbated by mechanical ventilation also called ventilation‐induced lung injury (VILI). In a model of VILI, recombinant IL‐22 has also been shown to decrease lung leak and improve lung fluid dynamics 99. These data support a potential therapeutic role of IL‐22 in diseases such as severe pneumonia or acute respiratory distress syndrome.
In primary infection, early sources of IL‐17 and IL‐22 can be from innate lymphoid cells 100, NK or NKT cells 100, 101, or γδ T cells 44, 45, 102. However, CD4+ Th17 cells can be elicited in the lung by vaccination. Vaccine‐induced Th17 responses have been shown to play protective roles in a diverse set of organisms including both intracellular and extracellular bacteria as well as fungi. For example, Th17 cells induced by vaccination with the antigen from M. tuberculosis ESAT‐6 induce a population of Th17 cells that augment the local production of ligands for CXCR3 in the lung and result in substantial enhanced recruitment of protective Th1 cells 103. Fungal‐specific Th17 cells have also been shown to be critical for vaccine‐induced protection against Coccidioides posadasii, Histoplasma capsulatum, and Blastomyces dermatitidis infection 104. In this setting, the protection against fungal challenge was dependent on IL‐17 regulation of neutrophil recruitment.
Vaccination of whole‐cell polysaccharides of S. pneumoniae has been shown to induce IL‐17 in the lung, and this IL‐17 response has been shown to mediate serotype‐independent immunity 105. Chen et al. 48 have also shown that Th17 cells elicited by K. pneumoniae vaccination recognize conserved outer membrane proteins in the cell wall of the bacteria and these antigens could also provide serotype‐independent immunity. Th17 cells conferred heterologous protection against multiple serotypes of the organism 48. Thus, in two models of important human extracelluar pathogens, Th17 cells are capable of mediating serotype‐independent immunity which may advance vaccine approaches against these pathogens. It still remains to be defined which specific aspects of Th17 function that are required for protection. There needs to be better understanding of factors important in generating mucosal Th17 cells in the lung such as adjuvants, factor regulating proliferation, homing, and survival. More research is needed to also define the contributions of IL‐22, IL‐17F, and IL‐17A/F heterodimers.
Conclusions
CD4+ T cells play critical roles in lung immunity, and these cells are impacted by many drugs as well as by HIV infection. CD4+ T cells are critical targets to achieve therapeutic vaccines against both bacterial and fungal pathogens. Future work will be required to understand the induction and survival of these cells in the lung and how they can be manipulated therapeutically.
Acknowledgement
Funding for this project was provided, in part, by R37HL079142 and P50HL084932 form the National Institutes of Health. The author has no conflicts of interest to declare.
Immunological Reviews 2013. Vol.252: 156–163 23405903
References
- 1. Centers for Disease Control and Prevention . Pneumocystis pneumonia–Los Angeles. Morb Mortal Wkly Rep 1981;30:250–252. [PubMed] [Google Scholar]
- 2. Gottlieb MS, et al. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med 1981;305:1425–1431. [DOI] [PubMed] [Google Scholar]
- 3. Masur H, et al. An outbreak of community‐acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N Engl J Med 1981;305:1431–1438. [DOI] [PubMed] [Google Scholar]
- 4. Paul WE. What determines Th2 differentiation, in vitro and in vivo? Immunol Cell Biol 2010;88:236–239. [DOI] [PubMed] [Google Scholar]
- 5. Zhu J, et al. Conditional deletion of Gata3 shows its essential function in T(H)1‐T(H)2 responses. Nat Immunol 2004;5:1157–1165. [DOI] [PubMed] [Google Scholar]
- 6. Kopf M, et al. IL‐5‐deficient mice have a developmental defect in CD5+ B‐1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity 1996;4:15–24. [DOI] [PubMed] [Google Scholar]
- 7. Fallon PG, et al. IL‐4 induces characteristic Th2 responses even in the combined absence of IL‐5, IL‐9, and IL‐13. Immunity 2002;17:7–17. [DOI] [PubMed] [Google Scholar]
- 8. Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med 1990;172:1425–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wills‐Karp M, et al. Interleukin‐13: central mediator of allergic asthma. Science 1998;282:2258–2261. [DOI] [PubMed] [Google Scholar]
- 10. Grunig G, et al. Requirement for IL‐13 independently of IL‐4 in experimental asthma. Science 1998;282:2261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barnes PJ. Role of GATA‐3 in allergic diseases. Curr Mol Med 2008;8:330–334. [DOI] [PubMed] [Google Scholar]
- 12. Corren J. Anti‐interleukin‐5 antibody therapy in asthma and allergies. Curr Opin Allergy Clin Immunol 2011;11:565–570. [DOI] [PubMed] [Google Scholar]
- 13. Corren J, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med 2011;365:1088–1098. [DOI] [PubMed] [Google Scholar]
- 14. Belperio JA, et al. Interaction of IL‐13 and C10 in the pathogenesis of bleomycin‐induced pulmonary fibrosis. Am J Respir Cell Mol Biol 2002;27:419–427. [DOI] [PubMed] [Google Scholar]
- 15. Liu T, et al. Regulation of found in inflammatory zone 1 expression in bleomycin‐induced lung fibrosis: role of IL‐4/IL‐13 and mediation via STAT‐6. J Immunol 2004;173:3425–3431. [DOI] [PubMed] [Google Scholar]
- 16. Jakubzick C, et al. Therapeutic attenuation of pulmonary fibrosis via targeting of IL‐4‐ and IL‐13‐responsive cells. J Immunol 2003;171:2684–2693. [DOI] [PubMed] [Google Scholar]
- 17. Willart M, Hammad H. Lung dendritic cell‐epithelial cell crosstalk in Th2 responses to allergens. Curr Opin Immunol 2011;23:772–777. [DOI] [PubMed] [Google Scholar]
- 18. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 1986;136:2348–2357. [PubMed] [Google Scholar]
- 19. Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T‐bet, directs Th1 lineage commitment. Cell 2000;100:655–669. [DOI] [PubMed] [Google Scholar]
- 20. Wolf SF, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol 1991;146:3074–3081. [PubMed] [Google Scholar]
- 21. Longhi MP, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med 2009;206:1589–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gough DJ, Levy DE, Johnstone RW, Clarke CJ. IFNgamma signaling‐does it mean JAK‐STAT? Cytokine Growth Factor Rev 2008;19:383–394. [DOI] [PubMed] [Google Scholar]
- 23. Dorman SE, et al. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet 2004;364:2113–2121. [DOI] [PubMed] [Google Scholar]
- 24. Picard CJ, et al. Inherited interleukin‐12 deficiency: IL12B genotype and clinical phenotype of 13 patients from six kindreds. Am J Hum Genet 2002;70:336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sologuren I, et al. Partial recessive IFN‐gammaR1 deficiency: genetic, immunological and clinical features of 14 patients from 11 kindreds. Hum Mol Genet 2011;20:1509–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang XO, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity 2008;28:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL‐17+ T helper cells. Cell 2006;126:1121–1133. [DOI] [PubMed] [Google Scholar]
- 28. Aggarwal S, Ghilardi N, Xie MH, De Sauvage FJ, Gurney AL. Interleukin 23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin 17. J Biol Chem 2003;278:1910–1914. [DOI] [PubMed] [Google Scholar]
- 29. Harrington LE, et al. Interleukin 17‐producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005;6:1123–1132. [DOI] [PubMed] [Google Scholar]
- 30. Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005;6:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL‐17‐producing T cells. Immunity 2006;24:179–189. [DOI] [PubMed] [Google Scholar]
- 32. Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006;441:235–238. [DOI] [PubMed] [Google Scholar]
- 33. Mangan PR, et al. Transforming growth factor‐b induces development of the TH17 lineage. Nature 2006;441:231–234. [DOI] [PubMed] [Google Scholar]
- 34. McGeachy MJ, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17‐producing effector T helper cells in vivo. Nat Immunol 2009;10:314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Veldhoen M, et al. The aryl hydrocarbon receptor links T(H)17‐cell‐mediated autoimmunity to environmental toxins. Nature 2008;453:106–109. [DOI] [PubMed] [Google Scholar]
- 36. Nurieva R, et al. Essential autocrine regulation by IL‐21 in the generation of inflammatory T cells. Nature 2007;448:480–483. [DOI] [PubMed] [Google Scholar]
- 37. Korn T, et al. IL‐21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 2007;448:484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Melton AC, Bailey‐Bucktrout SL, Travis MA, Fife BT, Bluestone JA, Sheppard D. Expression of alphavbeta8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J Clin Invest 2010;120:4436–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Acharya M, et al. alphav Integrin expression by DCs is required for Th17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. J Clin Invest 2010;120:4445–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol 2011;29:621–663. [DOI] [PubMed] [Google Scholar]
- 41. Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 2012;30:531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ray A, Khare A, Krishnamoorthy N, Qi Z, Ray P. Regulatory T cells in many flavors control asthma. Mucosal Immunol 2010;3:216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bonneville M, O'Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol 2010;10:467–478. [DOI] [PubMed] [Google Scholar]
- 44. Lockhart E, Green AM, Flynn JL. IL‐17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol 2006;177:4662–4669. [DOI] [PubMed] [Google Scholar]
- 45. Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL‐17 production. J Immunol 2007;178:4466–4472. [DOI] [PubMed] [Google Scholar]
- 46. Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin‐1 and IL‐23 induce innate IL‐17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 2009;31:331–341. [DOI] [PubMed] [Google Scholar]
- 47. Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin‐17‐producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 2009;31:321–330. [DOI] [PubMed] [Google Scholar]
- 48. Chen K, et al. Th17 cells mediate clade‐specific, serotype‐independent mucosal immunity. Immunity 2011;35:997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Steele CR, Oppenheim DE, Hayday AC. Gamma(delta) T cells: non‐classical ligands for non‐classical cells. Curr Biol 2000;10:R282–R285. [DOI] [PubMed] [Google Scholar]
- 50. Michel ML, et al. Identification of an IL‐17‐producing NK1.1neg iNKT cell population involved in airway neutrophilia. J Exp Med 2007;204:995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brossay L, et al. CD1d‐mediated recognition of an alpha‐galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med 1998;188:1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Burdin N, et al. Selective ability of mouse CD1 to present glycolipids: alpha‐galactosylceramide specifically stimulates V alpha 14+ NK T lymphocytes. J Immunol 1998;161:3271–3281. [PubMed] [Google Scholar]
- 53. Akbari O, et al. CD4+ invariant T‐cell‐receptor+ natural killer T cells in bronchial asthma. N Engl J Med 2006;354:1117–1129. [DOI] [PubMed] [Google Scholar]
- 54. Pettersson B, Curvall M, Enzell C. The inhibitory effect of tobacco smoke compound on ciliary activity. Eur J Respir Dis 1985;139(Suppl):89–92. [PubMed] [Google Scholar]
- 55. Nakamatsu M, et al. Role of interferon‐gamma in Valpha14+ natural killer T cell‐mediated host defense against Streptococcus pneumoniae infection in murine lungs. Microbes Infect 2007;9:364–374. [DOI] [PubMed] [Google Scholar]
- 56. Pichavant M, et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL‐17. J Exp Med 2008;205:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Steel JC, Waldmann TA, Morris JC. Interleukin‐15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci 2012;33:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of th2 cell‐type cytokines in protease allergen‐induced airway inflammation. Immunity 2012;36:451–463. [DOI] [PubMed] [Google Scholar]
- 59. Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol 2012;30:647–675. [DOI] [PubMed] [Google Scholar]
- 60. Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008;28:454–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Neill DR, McKenzie AN. Nuocytes and beyond: new insights into helminth expulsion. Trends Parasitol 2011;27:214–221. [DOI] [PubMed] [Google Scholar]
- 62. Kim HY, et al. Innate lymphoid cells responding to IL‐33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol 2012;129:216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 2010;32:593–604. [DOI] [PubMed] [Google Scholar]
- 64. Shirey KA, Cole LE, Keegan AD, Vogel SN. Francisella tularensis live vaccine strain induces macrophage alternative activation as a survival mechanism. J Immunol 2008;181:4159–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rose MC, et al. Model systems for investigating mucin gene expression in airway diseases. J Aerosol Med 2000;13:245–261. [DOI] [PubMed] [Google Scholar]
- 66. Price AE, et al. Systemically dispersed innate IL‐13‐expressing cells in type 2 immunity. Proc Natl Acad Sci USA 2010;107:11489–11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lachowicz‐Scroggins ME, Boushey HA, Finkbeiner WE, Widdicombe JH. Interleukin‐13‐induced mucous metaplasia increases susceptibility of human airway epithelium to rhinovirus infection. Am J Respir Cell Mol Biol 2010;43:652–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Murray HW. Interferon‐gamma, the activated macrophage, and host defense against antimicrobial challenge. Ann Int Med 1988;108:595–608. [DOI] [PubMed] [Google Scholar]
- 69. Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003;3:23–35. [DOI] [PubMed] [Google Scholar]
- 70. Schroder K, Sweet MJ, Hume DA. Signal integration between IFNgamma and TLR signalling pathways in macrophages. Immunobiology 2006;211:511–524. [DOI] [PubMed] [Google Scholar]
- 71. Xie QW, et al. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 1992;256:225–228. [DOI] [PubMed] [Google Scholar]
- 72. Xie QW, Whisnant R, Nathan C. Promoter of the mouse gene encoding calcium‐independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med 1993;177:1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Naderi BF, et al. Increased production of nitric oxide by neutrophils from patients with chronic granulomatous disease on interferon‐gamma treatment. Int Immunopharmacol 2012;12:689–693. [DOI] [PubMed] [Google Scholar]
- 74. Segal BH, Veys P, Malech H, Cowan MJ. Chronic granulomatous disease: lessons from a rare disorder. Biol Blood Marrow Transplant 2011;17:S123–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fernandez‐Boyanapalli R, et al. Impaired phagocytosis of apoptotic cells by macrophages in chronic granulomatous disease is reversed by IFN‐gamma in a nitric oxide‐dependent manner. J Immunol 2010;185:4030–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hovanessian AG, La BC, Falcoff E. Action of murine gamma (immune)interferon on beta (fibroblast)‐interferon resistant L 1210 and embryonal carcinoma cells. J Interferon Res 1980;1:125–135. [DOI] [PubMed] [Google Scholar]
- 77. Ronni T, Sareneva T, Pirhonen J, Julkunen I. Activation of IFN‐alpha, IFN‐gamma, MxA, and IFN regulatory factor 1 genes in influenza A virus‐infected human peripheral blood mononuclear cells. J Immunol 1995;154:2764–2774. [PubMed] [Google Scholar]
- 78. Spiegel M, Pichlmair A, Muhlberger E, Haller O, Weber F. The antiviral effect of interferon‐beta against SARS‐coronavirus is not mediated by MxA protein. J Clin Virol 2004;30:211–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Aly S, Laskay T, Mages J, Malzan A, Lang R, Ehlers S. Interferon‐gamma‐dependent mechanisms of mycobacteria‐induced pulmonary immunopathology: the role of angiostasis and CXCR3‐targeted chemokines for granuloma necrosis. J Pathol 2007;212:295–305. [DOI] [PubMed] [Google Scholar]
- 80. Chakravarty SD, Xu J, Lu B, Gerard C, Flynn J, Chan J. The chemokine receptor CXCR3 attenuates the control of chronic Mycobacterium tuberculosis infection in BALB/c mice. J Immunol 2007;178:1723–1735. [DOI] [PubMed] [Google Scholar]
- 81. Gao XF, Yang ZW, Li J. Adjunctive therapy with interferon‐gamma for the treatment of pulmonary tuberculosis: a systematic review. Int J Infect Dis 2011;15:e594–e600. [DOI] [PubMed] [Google Scholar]
- 82. McAllister F, et al. Role of IL‐17A, IL‐17F, and the IL‐17 receptor in regulating growth‐related oncogene‐{alpha} and granulocyte colony‐stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol 2005;175:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Aujla SJ, et al. IL‐22 mediates mucosal host defense against Gram‐negative bacterial pneumonia. Nat Med 2008;14:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jones CE, Chan K. Interleukin‐17 stimulates the expression of interleukin‐8, growth‐related oncogene‐alpha, and granulocyte‐colony‐stimulating factor by human airway epithelial cells. Am J Respir Cell Mol Biol 2002;26:748–753. [DOI] [PubMed] [Google Scholar]
- 85. Wright JF, et al. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J Biol Chem 2007;282:13447–13455. [DOI] [PubMed] [Google Scholar]
- 86. Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL‐17 enhances chemokine gene expression through mRNA stabilization. J Immunol 2007;179:4135–4141. [DOI] [PubMed] [Google Scholar]
- 87. Sun D, Novotny M, Bulek K, Liu C, Li X, Hamilton T. Treatment with IL‐17 prolongs the half‐life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing‐regulatory factor SF2 (ASF). Nat Immunol 2011;12:853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cai XY, Gommoll CPJ, Justice L, Narula SK, Fine JS. Regulation of granulocyte colony‐stimulating factor gene expression by interleukin‐17. Immunol Lett 1998;62:51–58. [DOI] [PubMed] [Google Scholar]
- 89. Lin Y, et al. Interleukin‐17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis . Immunity 2009;31:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ishigame H, et al. Differential roles of interleukin‐17A and ‐17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 2009;30:108–119. [DOI] [PubMed] [Google Scholar]
- 91. Song X, et al. IL‐17RE is the functional receptor for IL‐17C and mediates mucosal immunity to infection with intestinal pathogens. Nat Immunol 2011;12:1151–1158. [DOI] [PubMed] [Google Scholar]
- 92. Ramirez‐Carrozzi V, et al. IL‐17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol 2011;12:1159–1166. [DOI] [PubMed] [Google Scholar]
- 93. Chang SH, Reynolds JM, Pappu BP, Chen G, Martinez GJ, Dong C. Interleukin‐17C promotes Th17 cell responses and autoimmune disease via interleukin‐17 receptor E. Immunity 2011;35:611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ye P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony‐stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 2001;194:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kreindler JL, et al. Interleukin‐17A induces bicarbonate secretion in normal human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 2009;296:L257–L266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dorschner RA, et al. The mammalian ionic environment dictates microbial susceptibility to antimicrobial defense peptides. FASEB J 2006;20:35–42. [DOI] [PubMed] [Google Scholar]
- 97. Aujla SJ, Kolls JK. IL‐22: a critical mediator in mucosal host defense. J Mol Med 2009;87:451–454. [DOI] [PubMed] [Google Scholar]
- 98. Zheng Y, et al. Interleukin‐22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 2008;14:282–289. [DOI] [PubMed] [Google Scholar]
- 99. Hoegl S, et al. Protective Properties of Inhaled IL‐22 in a Model of Ventilator‐induced Lung Injury. Am J Respir Cell Mol Biol 2011;44:369–376. [DOI] [PubMed] [Google Scholar]
- 100. Crellin NK, Trifari S, Kaplan CD, Cupedo T, Spits H. Human NKp44+IL‐22+ cells and LTi‐like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J Exp Med 2010;207:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cella M, et al. A human natural killer cell subset provides an innate source of IL‐22 for mucosal immunity. Nature 2009;457:722–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Simonian PL, Roark CL, Born WK, O'Brien RL, Fontenot AP. Gammadelta T cells and Th17 cytokines in hypersensitivity pneumonitis and lung fibrosis. Transl Res 2009;154:222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Khader SA, et al. IL‐23 and IL‐17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 2007;8:369–377. [DOI] [PubMed] [Google Scholar]
- 104. Wuthrich M, et al. Vaccine‐induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J Clin Invest 2011;121:554–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Malley R, et al. Antibody‐independent, interleukin‐17A‐mediated, cross‐serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun 2006;74:2187–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]


