Abstract
Background
Advanced soft tissues sarcomas (STS) have limited therapeutic options. Sorafenib (BAY 43–9006) is a multi-targeted tyrosine kinase inhibitor of raf, VEGFR1-3, PDGFRB, flt-3, and c-kit.
Methods
We tested sorafenib at a dose of 400 mg BID in patients with advanced sarcomas of vascular derivation (VS), high grade liposarcomas and leiomyosarcomas who had received 0–1 prior regimens for advanced disease.
Results
Fifty one patients were accrued, with thirty-seven evaluable for toxicity and response. There were no unexpected side effects and no confirmed responses. Median progression free survival and overall survival were 3 months and 17 months, respectively. Six of 8 patients in the VS cohort had prolonged clinical benefit (stable disease or better), resulting in a median progression free survival of 5 months, compared with 2–3 months for liposarcoma and leiomyosarcomas respectively.
Conclusion
Sorafenib, at this dose and schedule, did not result in RECIST responses in VS, liposarcoma or leiomyosarcoma.
Keywords: Tyrosine kinase inhibitor, liposarcoma, leiomyosarcoma, angiosarcoma, solitary fibrous tumor
INTRODUCTION
Therapy for advanced soft tissue sarcomas (STS) has been limited to few effective chemotherapeutic agents: anthracyclines, ifosfamide, gemcitabine with docetaxel, and dacarbazine. In Europe, trabectedin is also available. These agents have proven activity in STS, with higher response rates for combination regimens however at the expense of greater toxicity without a clear survival advantage.
The development of targeted drugs has afforded an opportunity to evaluate new agents in advanced STS. Limited pre-clinical data in sarcomas has made selection of specific subtypes of sarcomas for therapeutic clinical trials challenging. Sorafenib (BAY 43–9006, Nexavar, Bayer Onyx), is a multi-targeted tyrosine kinase inhibitor of raf, VEGFR1-3, PDGFRB, flt-3, and c-kit, some of which may be of relevance in STSs. Sarcomas have been shown to express PDGFR (1,2) as well as VEGFR (3) with mixed data on the correlation of VEGF and VEGFR expression as a predictor of clinical outcome (4–6); KIT expression is found in GIST but not most other sarcomas (7).
We performed a multi-center phase II trial in specific sarcoma histologies to test the clinical benefit of sorafenib. Sarcomas deriving from epithelial tissues, angiosarcomas, hemangiosarcomas, and solitary fibrous tumors (previously termed hemangiopericytomas) have all been shown to express VEGFR, VEGF mRNA (8–10), as well as PDGFR (2, 11–12). We hypothesized that sorafenib may inhibit autocrine or paracrine growth stimulation in these tumors. We also included two of the most common high-grade adult STSs, leiomyosarcoma and liposarcomas, because of the known expression of VEGFR and PDGFR in these tumor histologies (1–3), as well as the potential correlation of VEGF and VEGFR expression with prognosis (4–6). In addition, there is increasing preclinical data that suggests targeting both VEGFR and PDGFR may be very effective in causing regression of tumor associated vasculature (13,14) and thus targeting these pathways may be of benefit for all three histologic groups.
We report the results of a multi-center phase II study of BAY 43–9006 in patients with advanced sarcomas.
MATERIALS AND METHODS
Study Population
Patients were eligible for participation if they had measurable residual, recurrent or metastatic vascular sarcomas (cutaneous or visceral angiosarcoma, malignant hemangiosarcoma, solitary fibrous tumor), or grade 3–4 liposarcoma or leiomyosarcoma. Patients were eligible if they were 18 or older and had a Zubrod PS 0–1. Patients were allowed to have had prior surgery, radiation therapy and one prior chemo therapy regimen for advanced disease as long as they had recovered from all toxicities, and had not had chemotherapy within the past 4 weeks (6 weeks for BCNU and mitomycin C). Normal organ and marrow function were required. Appropriate contraception while receiving therapy was also mandated. Patients with a history of brain metastases, thromboembolic disease, or uncontrolled infectious, cardiac or psychiatric illness were excluded as were patients on active anti-coagulation. Women who were pregnant or breast feeding, as well as those who were receiving combination retro-viral therapy for HIV disease, were also not candidates for trial participation.
All participants gave oral and written informed consent in accordance with institutional and federal guidelines. The protocol was approved by the Institutional Review Boards at participating institutions, and was monitored by the SWOG study coordinator, statistician and ICAS chair (EB).
Study Design
This was a phase II, open-label, multi-center study conducted through the Intergroup Coalition Against Sarcomas, and administered through the SWOG. Patients received sorafenib 400 mg orally twice daily continuously for 28 days. Toxicity was monitored and graded utilizing the NCI Common Terminology Criteria for Adverse Events, version 3.0. Patients encountering grade 3 or 4 toxicities had there dose held until resolved to grade 2 or better. Therapy was continued with a dose reduction; two dose reductions were allowed: 200 mg BID and 200 mg daily. Patients requiring more than 2 weeks to recover from a grade 4 toxicity or who required more than 2 dose reductions were removed from study. After every two 28-day cycles, patients were evaluated for tumor response utilizing RECIST criteria (15).
STATISTICAL METHODS
Patients were stratified on the basis of histology: vascular sarcoma (angiosarcoma, hemangiosarcoma, or solitary fibrous tumor) versus leiomyosarcoma versus liposarcoma (dedifferentiated and myxoid/round cell). The primary objective of the study was to assess the response probability (confirmed complete response (CR) and partial response (PR)) in these three histologic strata. This design was based upon the assumption that within each of the three strata, a response probability of 25% would be of interest and that further testing would not be pursued if the response probability was 5% or lower. Initially 15 eligible patients were to be accrued within each stratum. If one or more responses were seen within a stratum, then an additional 10 patients were to be accrued to that stratum for a total of 25 eligible patients. If four or more responses were seen within the 25 patients in the strata, sorafenib would be considered worth of further study in that cohort. This design allowed a significance level of 3% and a power of 90% within each stratum.
In addition to the within-stratum hypothesis testing, this study was also designed to look at the response probability over all histologies. Once 40 eligible patients overall strata had been accrued, the study would temporarily close. If fewer than two responses were observed in the first 40 eligible patients, the accrual for all strata would be discontinued. Otherwise, a maximum of 35 additional patients would be entered (depending on whether any individual stratum was closed). Eight or more responses out of the maximum 75 patients would be considered as evidence warranting further study of the regimen provided other factors, such as toxicity and overall survival, also appear favorable. The properties of this design were to vary based on the final sample size, true response probability and the observed frequency of patients within each stratum. If 50% patients have leiomyosarcoma, 30% have liposarcoma and 20% have VS, then this overall design had a significance level 4% (probability of falsely declaring the regimen with a 5% response probability to warrant further study) and power of 89% (probability of declaring regimen with 15% response probability overall all stratum to warrant further study).
In addition, patients were followed to assess the four-month progression-free survival rate, as well as for the frequency and severity of adverse events on the investigational therapy.
RESULTS
Patient characteristics
This study was activated in March of 2006 and closed in February of 2007, following completion of the first stage accrual. Fifty-one patients were registered to the study from twenty institutions, with the goal of having 40 eligible patients. Patient characteristics are summarized in Table 1. Thirteen patients were ineligible, 3 each in the vascular sarcoma and liposarcoma strata, and 7 from the leiomyosarcoma cohort. Six had elevated baseline lab values (primarily anticoagulation parameters), two had more than one prior chemotherapy beyond the adjuvant setting, and one had Grade 2 leiomysarcoma based on institutional pathology. Four additional patients were ineligible upon central pathology review with diagnoses of synovial sarcoma, myxofibrosarcoma, GIST and high-grade pleomorphic undifferentiated sarcoma. One patient did not receive any study drug and is not included in any of the following analyses.
Table 1.
Patient Characteristics (N=37)
| Age | Range: 24–88.5 | Median: 62.7 |
| Sex | Male: 15 (41%) | Female: 22 (59%) |
| Ethnicity | White: 33 (89%) | |
| Black: 3 (8%) | ||
| Pacific Islander: 1 (3%) | ||
| Histology | ||
| VS STS | 8 (22%) | |
| Angiosarcoma | 5 | |
| Solitary fibrous tumor | 3 | |
| Liposarcoma | 10 (27%) | |
| Dedifferentiated | 8 | |
| Myxoid/Round Cell | 2 | |
| Leiomysarcoma | 19 (51%) | |
| Uterine | 7 | |
| Extremity | 3 | |
| Other | 9 | |
| Prior Therapy | 31 (84%) | |
| Surgery | 28 (76%) | |
| Radiation | 15 (41%) | |
| Chemotherapy | 19 (51%) | |
| Performance status 0/1 | 15(41%)/22 (59%) | |
| Metastases | 35 (95%) | |
| Lung/Pleura | 21 (57%) | |
| Abdominal Organ* | 11(30%) | |
| Bone | 6 (16%) | |
| Soft Tissue | 13 (35%) | |
| Lymph Nodes | 4 (11%) | |
| Other: | 8 (22%) | |
| Cutaneous | 3 (8%) | |
| Abdomen/Pelvis | 5 (14%) | |
| Chest wall/Mediastinum | 1 (3%) | |
includes liver and mets to bowel or juxtacolonic area
Toxicity
Thirty-seven patients were evaluable for adverse events. There were no toxicities noted that had not been previously reported with sorafenib. The most common adverse events reported were fatigue in 59% (22 patients), hand-foot syndrome in 57% (21 patients), and diarrhea in 49% (18 patients), typically grades 1–2. Three grade 4 events were reported, one each for a decline in hemoglobin, elevated bilirubin with aspartate transaminase, and elevated lipase and amylase. Additional grade 3 adverse events included elevations of lipase (14%, n=5), hand foot syndrome (11%, n=4), electrolyte abnormalities (11%, n=4), diarrhea (11%, n=4), 2 cases each of fatigue, hypertension, liver function abnormalities, and rash (5%), as well as one each of nausea, vomiting, constipation, abdominal pain, pancreatitis, and platelets (3%). The most common reason for dose reduction was hand foot syndrome or rash (22%, n=8), and elevated amylase/lipase alone or with pancreatitis (11%, n=4) with additional dose reductions for diarrhea (5%, n=2), hypertension (5%, n=2), vomiting, transaminitis, and fatigue (3%, n=1 each). Seventeen patients (20%) had unplanned dose interruptions or dose reductions in at least one cycle. Nineteen patients (51%) had one dose reduction, with only 2 patients (5%) requiring a second dose reduction. One patient (3%) had drug held for thrombocytopenia which did not resolve within 14 days resulting in removal from protocol therapy. Of the patients who had no dose reductions, the median number of cycles was 3 (range 1–10). In contrast, patients that received dose reductions received a median of 4 cycles (range 2–25). A greater percentage of patients requiring dose reductions had received prior chemotherapy and radiation therapy than those without dose reductions (33 % versus 10%).
Response
No confirmed responses were observed in the 37 patients in which response was assessed (0%; 95% confidence interval 0% – 9%) [Table 2]. Due to lack of efficacy, the study was terminated without proceeding to the second stage accrual. One patient had an unconfirmed partial response. This was a 67 y/o female with metastatic uterine leiomyosarcoma diagnosed in 1996. Prior therapies included pulmonary metastatectomy 2 and 5 years later, doxorubicin, and gemcitabine with docetaxel. She was then initiated sorafenib and had an unconfirmed PR after 2 cycles. The patient progressed after 5 cycles of sorafenib.
Table 2.
Response Data by Cohort
| Leiomyosarcoma | Liposarcoma | Vascular Sarcomas | ||||
|---|---|---|---|---|---|---|
| Complete Response | 0 | 0% | 0 | 0% | 0 | 0% |
| Partial Response | 0 | 0% | 0 | 0% | 0 | 0% |
| Unconfirmed Complete Response | 0 | 0% | 0 | 0% | 0 | 0% |
| Unconfirmed Partial Response | 1 | 5% | 0 | 0% | 1 | 13% |
| Stable/No Response | 8 | 42% | 2 | 20% | 5 | 63% |
| Increasing Disease | 9 | 47% | 6 | 60% | 1 | 13% |
| Assessment Inadequate | 1 | 5% | 2 | 20% | 1 | 13% |
| Total | 19 | 100% | 10 | 100% | 8 | 100% |
A second patient with metastatic solitary fibrous tumor involving the liver and bone demonstrated significant decreases in radiographic attenuation of the metastatic lesions, as documented by a decline in Hounsfield units, despite stable target lesion measurements by RECIST (Figure 3). These findings were suggestive of tumor necrosis and the patient maintained stable disease for over 2 years on sorafenib.
Figure 3.
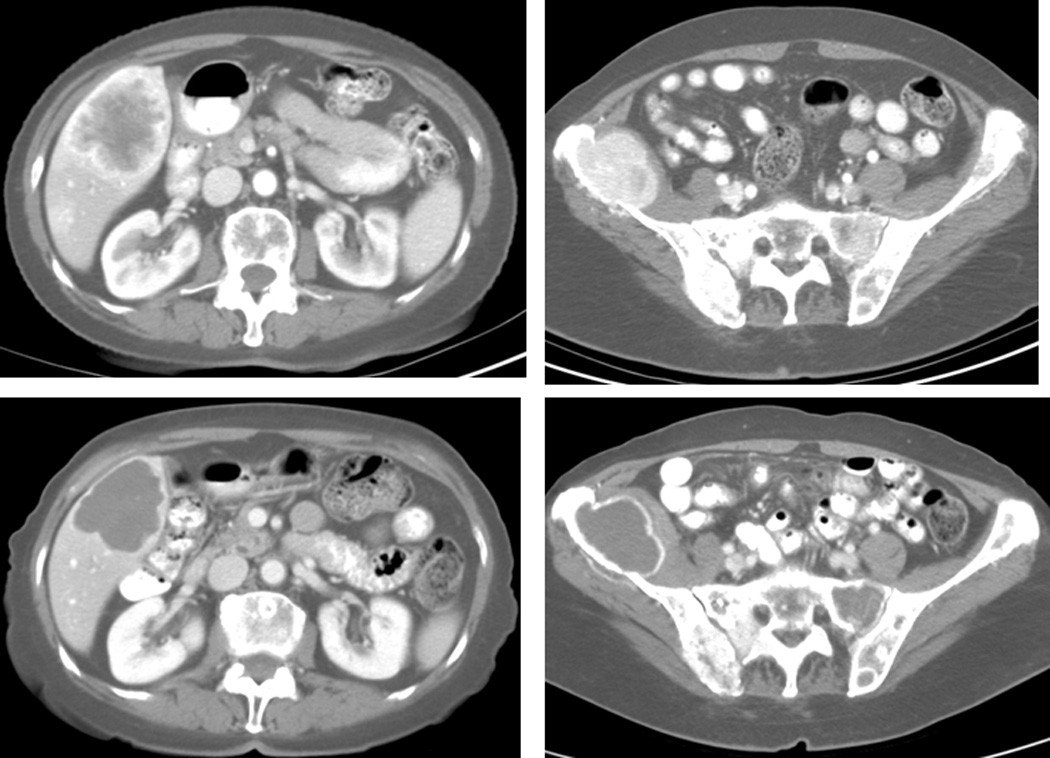
Images of patient with solitary fibrous tumor at baseline in May 2006, upper panel, and 22 months later in March 2008 demonstrating changes in tumor density with prolonged stable disease. Baseline Hounsfield units in the liver and ileum lesions were 112.8 and 174.4 respectively. On the follow-up scan they have decreased to 51.1 and 45.8 respectively.
Fourteen patients (38%) demonstrated stable disease, 5 in the vascular sarcoma cohort (63%), 8 with leiomyosarcoma (42%), and 2 (20%) with dedifferentiated liposarcoma [Table 3]. Sixteen patients had progressive disease (43%). Response in four patients was not assessable due to lack of follow-up in two cases, and due to incomplete imaging at followup in the remaining two cases.
Table 3.
PFS and OS Data by Cohort
| Total | Leiomyosarcoma | Liposarcoma | Vascular Sarcomas | |
|---|---|---|---|---|
| Median PFS | 3 mos (2–5 mos.) | 3 mos (2–9 mos) | 2 mos (1.6–2 mos) | 5 mos (3–6 mos) |
| 3-Month PFS (12 wk) | 49% (33% – 65%) | 42% (20% – 64%) | 30% (2% – 58%) | 87% (65% – 100%) |
| 4-Month PFS | 43% (27% – 59%) | 42% (20% – 64%) | 30% (2% – 58%) | 63% (29 – 96%) |
| 6-month PFS (24wk) | 32% (17% – 48%) | 37% (15% – 59%) | 20% (0% – 45%) | 38% (4% – 71%) |
| Median OS | 17 mos (13–25 mos) | 21 mos (13–27 mos) | 15 mos (5–15 mos) | 23 mos (7–38 mos) |
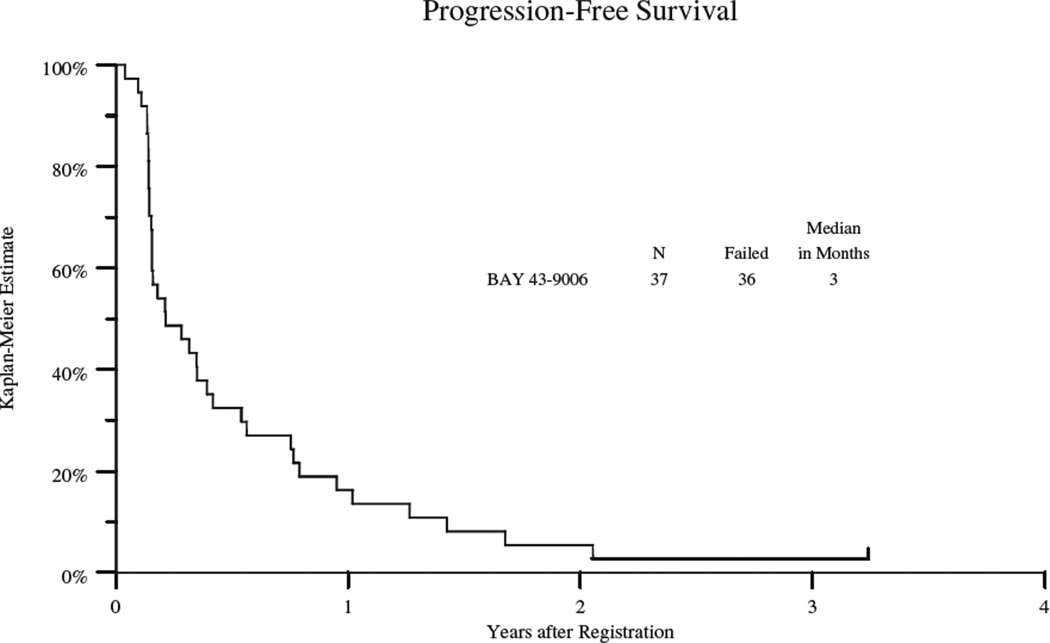
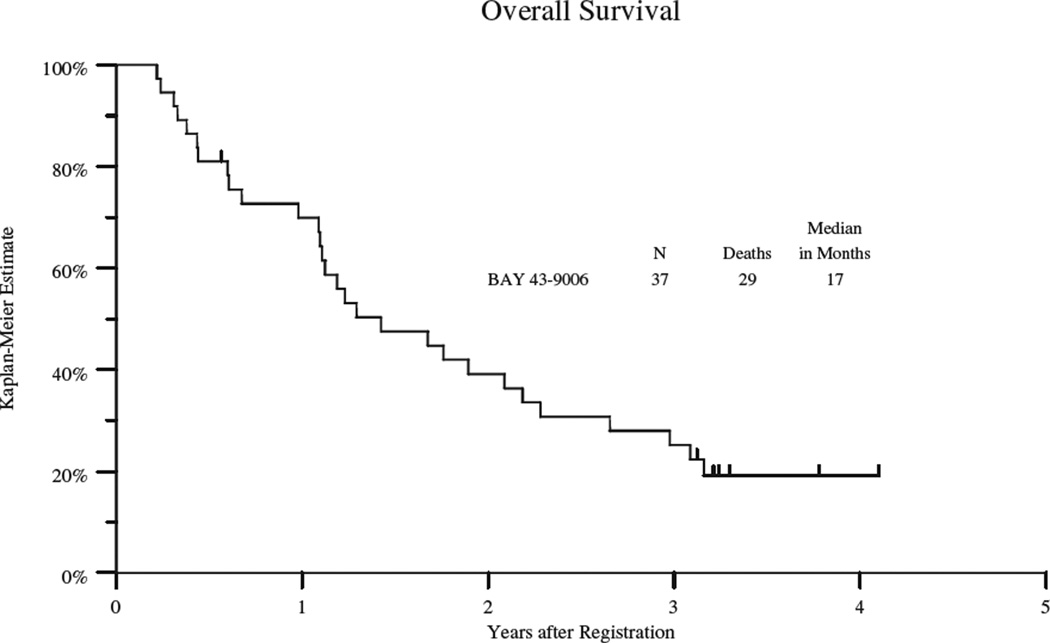
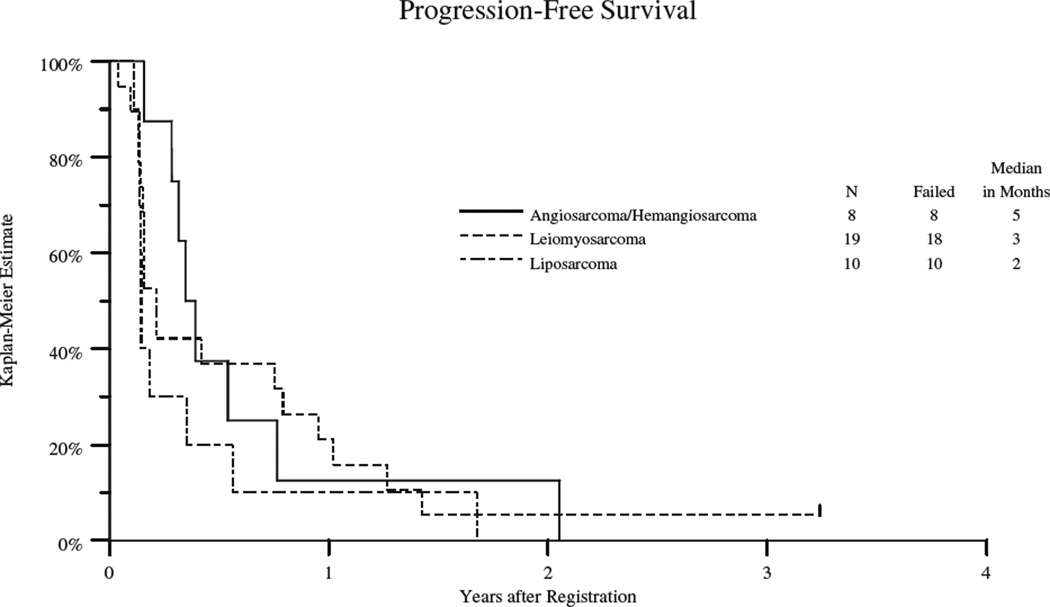
Thirty-six patients progressed with a median progression-free survival (PFS) of 3 months (95% confidence interval 2 – 5 months) and overall survival of 17 months (95% confidence interval 13 – 25 months) (see table 3, Figure 1A and 1B). PFS varied by cohort and the data is summarized in table 3 and figures 2. The VS group had the longest PFS (5 mos; 95% CI: 3–6 mos), followed by leiomyosarcoma (3 mos; 95% CI 2–9 mos); the dedifferentiated and myxoid/round cell liposarcoma group had the shortest PFS (2 mos; 95% CI: 1.6–2 mos). Twenty-nine patients have died with a median overall survival of 17 months (95% confidence interval of 13 – 25 months) (table 3 and figure 1). The VS cohort had the longest OS (23 mos; 95% CI: 7–38), followed by leiomyosarcoma (21 mos; 95% CI 13–27 mos) and shortest for the liposarcoma group (15mos; 95% CI: 5–15). The 2 patients who remained on study the longest were a patient with high grade leiomyosarcomas (18 cycles) and a patient with solitary fibrous tumor (25 cycles).
Figure 1.
A: Kaplan Meyer Plot of Progression Free Survival
B: Kaplan Meyer Plot of Overall Survival
Figure 2.
Kaplan Meyer Plot of Progression Free Survival by Cohort
DISCUSSION
In this study, sorafenib did not lead to any confirmed responses. Our study, which enrolled 51 patients, was limited by a high rate of ineligible patients, particularly in the two cohorts where some activity was noted thus reducing our statistical power to meet our endpoint. Although this regimen did not show activity with regard to RECIST-defined response, there was a suggestion of clinical benefit as 32% of patients remained on study without progressive disease for 6 months or more. These results compare favorably to the European Organization for Research and Treatment of Cancer (EORTC) soft tissue sarcoma (STS) phase II data, where a progression free rate of 14% at six months for active agents in the second line setting (16). In addition, the progression free rate at 6 months in the leiomyosarcomas was 37% compared with 40% in previously untreated patients receiving an anthracycline based regimen. The VS cohort had a progression free rate of 38%; the EORTC data does not provide a reference for angiosarcoma or other vascular tumors.
Sorafenib was tolerated without any unanticipated side effects. The rate of dose reductions for toxicity was greater than has been reported for sorafenib in the pivotal phase III renal cell carcinoma trial (17); this may have been due to stricter dose reduction guidelines or greater predisposition to experiencing toxicity in this patient population. Indeed, the phase II experience in sarcoma had a similar rate of dose reductions as in our study (18).
Maki and colleagues published the results of their phase II trial of sorafenib in sarcomas (18). Similar to our experience, the most common toxicity was dermatologic, however, fatigue or diarrhea were not reported. They also noted 4 cases of hemorrhage, one case of a deep venous thrombosis, 2 cases of perforation (one in the ileum and the other a pneumothorax), as well as one case each of congestive heart failure, transient ischemic attack and reversible posterior leukoencephalopathy. All of these have been described with anti-angiogenic agents, but were not seen in our study with the exception of grade 1 epistaxis in three patients. In addition, these authors found an inverse correlation between toxicity and height; we did not observe this correlation in our patient population (data not shown).
In Maki’s study, 37 patients with vascular tumors, primarily angiosarcoma with a few epithelioid hemangioendotheliomas, were assessable for response. They reported one complete response, 4 partial responses in therapy naïve radiation induced high grade angiosarcomas of the breast as well as previously treated high grade angiosarcomas of the head and neck. In addition, there were 8 patients with vascular tumors that had stable disease of greater than six months. The Kaplan-Meier estimates of progression free survival and overall survival for this group were 3.2 months and 14.3 months, respectively.
The other cohort noted to have response by Maki and colleagues was the leiomyosarcoma group in which there was one PR as well as 18 patients with stable disease with PFS of 3.8 months and overall survival of 22.4 months. Prolonged stable disease of greater than six months was also seen in 2 patients with leiomyosarcoma, as well as one each of malignant peripheral nerve sheath tumor, extraskeletal myxoid chondrosarcoma, conventional chondrosarcoma and fibrosarcoma. Although they only had three patients on their trial with liposarcoma, one achieved stable disease for less than 6 months, paralleling our experience.
Similar to our observations in solitary fibrous tumor, activity of VEGF-directed therapies including sorafenib, sunitinib, and bevacizumab with temozolomide against this subtype have recently been reported. Dumond and colleagues described benefit from sorafenib and sunitinib in solitary fibrous tumor (19). Other reports of benefit from sorafenib include a complete response in a non-AIDS related Kaposi’s sarcoma (20), as well as a significant partial response in a patient with metastatic synovial sarcoma involving the lung (21). Sunitinib has shown clinical benefit in desmoplastic small round cell tumor, solitary fibrous tumor, alveolar soft part sarcoma, giant cell tumor of bone, synovial sarcoma as well as chordoma (22). A phase II trial of sunitinib in solitary fibrous tumor has demonstrated prolonged stable disease with changes in tumor density (23). A phase II study of Bevacizumab has also demonstrated responses and stable disease in vascular sarcomas (25), however, the combination of bevacizumab with doxorubicin was associated with a higher risk of cardiac toxicity than is usually seen with doxorubicin alone (26). A recent report of pazopanib, demonstrated a progression free survival rate at 12 weeks of 26% in adipocytic tumors, 44% in leiomyosarcomas and 39% in a cohort of STSs that included vascular tumors.
In conclusion, our study demonstrated some activity of sorafenib in patients with vascular sarcomas, and perhaps in leiomyosarcomas based on favorable PFS. Our study results support other reported clinical trial data findings with sorafenib and other multi-targeted tyrosine kinase inhibitors in sarcomas. The limited activity in dedifferentiated and myxoid/round cell liposarcomas in our study, as well as in others, suggests this histology is less sensitive to antiangiogenic therapies. The limited number of objective responses reported in this and other studies suggests that studies combining sorafenib with chemotherapy may lead to enhanced activity; it may also reflect the difficulties of utilizing RECIST criteria for determining response with targeted therapies. There are ongoing studies evaluating sorafenib, as well as other tyrosine kinase directed therapies, with chemotherapy in STSs which may result in greater tumor shrinkage.
Acknowledgments
Funding: This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA46113, CA20319, CA68183, CA46368, CA35431, CA45807, CA04919, CA35090, CA35178, CA35176, CA45560, CA45808, CA37981, CA46136, CA14028, CA46441, CA58861, CA12644 (SWOG); CA77202 and CCSRI 021039 (NCIC-CTG); and CA031946 (CALGB)
Footnotes
Financial Disclosures: G.D. Demetri has served as a paid consultant to Bayer-Onyx; C.W. Ryan has served as a paid consultant and received honoraria and research support from Bayer-Onyx.
Results presented in part by Dr. Christopher W. Ryan at the 44th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, Il
REFERENCES
- 1.Weiner TM, Liu ET, Craven RJ, Cance WG. Expression of growth factor receptors, the focal adhesion kinase, and other tyrosine kinases in human soft tissue tumors. Ann Surg Oncol. 1994;1:18–27. doi: 10.1007/BF02303537. [DOI] [PubMed] [Google Scholar]
- 2.Franklin WA, Christison WH, Colley M, Montag AG, Stephens JK, Hart CE. In situ distribution of the beta-subunit of platelet-derived growth factor receptor in non-neoplastic tissue and in soft tissue tumors. Cancer Res. 1990;50(19):6344–6348. [PubMed] [Google Scholar]
- 3.Heymach JV. Angiogenesis and antiangiogenic approaches to sarcomas. Curr Opin Oncol. 2001;12(4):261–269. doi: 10.1097/00001622-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Saenz NC, Heslin MJ, Adsay V, et al. Neovascularity and clinical outcome in high-grade extremity soft tissue sarcomas. Ann Surg Oncol. 1998;5(1):48–53. doi: 10.1007/BF02303764. [DOI] [PubMed] [Google Scholar]
- 5.Chao C, Al-Saleem T, Brooks JJ, Rofatko A, Kraybill WG, Eisenberg B. Vascular endothelial growth factor and soft tissue sarcomas: tumor expression correlates with grade. Ann Surg Oncol. 2001;8(3):260–267. doi: 10.1007/s10434-001-0260-9. [DOI] [PubMed] [Google Scholar]
- 6.Yoon SS, Segal NH, Olshen AB, Brennan MF, Singer S. Circulating angiogenic factor levels correlate with extent of disease and risk of recurrence in patients with soft tissue sarcoma. Ann Oncol. 2004;15(8):1261–1266. doi: 10.1093/annonc/mdh309. [DOI] [PubMed] [Google Scholar]
- 7.Hornick JL, Fletcher CD. Immunohistochemical staining for KIT (CD117) in soft tissue sarcomas is very limited in distribution. Am J Clin Pathol. 2002;117(2):188–193. doi: 10.1309/LX9U-F7P0-UWDH-8Y6R. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto M, Ohsawa M, Ohnishi A, Naka N, Hirota S, Kitamura Y, Aozasa K. Expression of vascular endothelial growth factor and tis receptor mRNA in angiosarcoma. Lab Invest. 1995;73(6):296–301. [PubMed] [Google Scholar]
- 9.Amo Y, Masuzawa M, Hamada Y, Katsuoka K. Expression of vascular endothelial growth factor in a human hemangiosarcoma cell line (ISO-HAS) Arch Dermatol Res. 2001;293(6):296–301. doi: 10.1007/s004030100228. [DOI] [PubMed] [Google Scholar]
- 10.Hatva E, Bohling T, Jaaskelainen J, Persico MG, Haltia M, Alitalo K. Vascular growth factors and receptors in capillary hemangioblastomas and hemangiopericytomas. Am J Pathol. 1996;148(3):763–775. [PMC free article] [PubMed] [Google Scholar]
- 11.Palman C, Bowen-Pope DF, Brooks JJ. Platelet-derived growth factor receptor (beta-subunit) immunoreactivity in soft tissue tumors. Lab Invest. 1992;66(1):108–115. [PubMed] [Google Scholar]
- 12.Hansen T, Gaumann A, Ghalibafian M, Hoferlin A, Heintz A, Kirkpatrick CJ. Haemangiopericytoma of the thyroid gland in combination with Hashitmoto’s disease. Virchows Arch. 2004;445(3):315–319. doi: 10.1007/s00428-004-1066-5. [DOI] [PubMed] [Google Scholar]
- 13.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111(9):1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erber R, Thurnher A, Katsen AD, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18(2):338–340. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. JNCI. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Van Glabbeke M, Verweij J, Judson I, Nielsen OS. Progression-Free Rate as the principal end-point in phase II trials in soft-tissue sarcomas. Euro J Cancer. 2002;38:543–549. doi: 10.1016/s0959-8049(01)00398-7. [DOI] [PubMed] [Google Scholar]
- 17.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 18.Maki RG, D'Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27(19):3133–3140. doi: 10.1200/JCO.2008.20.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domont J, Massard C, Lassau N, Armand JP, Le Cesne A, Soria JC. Hemangiopericytoma and antiangiogenic therapy: clinical benefit of antiangiogenic therapy (sorafenib and sunitinib) in relapsed malignant haemangioperyctoma/solitary fibrous tumour. Invest New Drugs. 2010;28(2):199–202. doi: 10.1007/s10637-009-9249-1. [DOI] [PubMed] [Google Scholar]
- 20.Ardavanis A, Doufexis D, Kountourakis P, Rigatos G. A Kaposi's sarcoma complete clinical response after sorafenib administration. Ann Oncol. 2008;19(9):1658–1659. doi: 10.1093/annonc/mdn528. [DOI] [PubMed] [Google Scholar]
- 21.Brunello A, Basso U, Bertuzzi A, Santoro A. Sorafenib is active on lung metastases from synovial sarcoma. Ann Oncol. 2009;20(2):386–387. doi: 10.1093/annonc/mdn685. [DOI] [PubMed] [Google Scholar]
- 22.George S, Merriam P, Maki RG, et al. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J Clin Oncol. 2009;27(19):3154–3160. doi: 10.1200/JCO.2008.20.9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park MS, Patel SR, Ludwig JA, et al. Combination therapy with temozolomide and bevacizumab in the treatment of hemangiopericytoma/malignant solitary fibrous tumor. J Clin Oncol. 2008;26:15s. (abstr 10512). [Google Scholar]
- 24.Stacchiotti S, Tamborini E, Marrari A, et al. Response to Sunitinib Malate in advanced alveolar soft part sarcoma. Clin Cancer Res. 2009;15(3):1096–1104. doi: 10.1158/1078-0432.CCR-08-2050. [DOI] [PubMed] [Google Scholar]
- 25.Agulnik M, Okuno SH, von Mehren M, et al. An open-label multicenter phase II study of bevacizumab for the treatment of angiosarcoma. J Clin Oncol. 2009;27:15s. doi: 10.1093/annonc/mds237. (abstr 10522). [DOI] [PubMed] [Google Scholar]
- 26.D'Adamo DR, Anderson SE, Albritton K, et al. Phase II study of doxorubicin and bevacizumab for patients with metastatic soft-tissue sarcomas. J Clin Oncol. 2005;23(28):7135–7142. doi: 10.1200/JCO.2005.16.139. [DOI] [PubMed] [Google Scholar]